Abstract
Episodic memory is typically affected during the course of Alzheimer’s disease (AD). Due to the pronounced heterogeneity of functional neuroimaging studies on episodic memory impairments in mild cognitive impairment (MCI) and AD regarding their methodology and findings, we aimed to delineate consistent episodic memory-related brain activation patterns. We performed a systematic, quantitative, coordinate-based whole-brain activation likelihood estimation meta-analysis of 28 functional magnetic resonance imaging (fMRI) studies comprising 292 MCI and 102 AD patients contrasted to 409 age-matched control subjects. We included episodic encoding and/or retrieval phases, investigated the effects of group, verbal or image stimuli and correlated mean Mini-Mental-Status-Examination (MMSE) scores with the modelled activation estimates. MCI patients presented increased right hippocampal activation during memory encoding, decreased activation in the left hippocampus and fusiform gyrus during retrieval tasks, as well as attenuated activation in the right anterior insula/inferior frontal gyrus during verbal retrieval. In AD patients, however, stronger activation within the precuneus during encoding tasks was accompanied by attenuated right hippocampal activation during retrieval tasks. Low cognitive performance (MMSE scores) was associated with stronger activation of the precuneus and reduced activation of the right (para)hippocampus and anterior insula/inferior frontal gyrus. This meta-analysis provides evidence for a specific and probably disease stage-dependent brain activation pattern related to the pathognomonic AD characteristic of episodic memory loss.
Keywords: Alzheimer’s disease, Mild cognitive impairment, Episodic memory, Functional neuroimaging, Meta-analysis, Hippocampus
Introduction
Episodic memory has been defined as memory for information (‘what’) paired with a temporal (‘when’) and a spatial (‘where’) aspect (Tulving 1972; Jonides et al. 2002). It can roughly be divided into encoding and retrieval phases. Encoding presents an automatic and effortless process (Dere et al. 2010; Pause et al. 2013), whereas retrieval is a more active cognitive function. Functional neuroimaging studies have identified several encoding-related brain activation patterns including the dorsolateral and ventrolateral prefrontal cortices, intraparietal sulcus/superior parietal lobule, medial temporal lobe (MTL) and adjacent occipito-temporal areas (Spaniol et al. 2009). The MTL encompasses the hippocampal formation which is essential for long-term storage of memory (Scoville and Milner 2000; Squire et al. 2004), the parahippocampal gyrus (PHG) which plays an important role in episodic encoding and spatial navigation (Kühn and Gallinat 2013), the perirhinal and entorhinal cortices and the amygdala. In contrast, the retrieval phase, has been shown to engage the posterior parietal cortex including the inferior and posterior lateral parietal cortices and precuneus, the left dorsolateral prefrontal cortex, the anterior cingulate cortex, the left ventrolateral prefrontal cortex, the left PHG, bilateral insula and bilateral caudate (Spaniol et al. 2009). Importantly, brain activation patterns have been reported to be rather left-hemispheric for verbal stimuli while bilateral or preferably right-hemispheric for images (Buckner et al. 1996; Kelley et al. 1998; Golby et al. 2001).
In the psychopathological continuum of Alzheimer’s Disease (AD)—the world’s most common neurodegenerative disorder—episodic memory is one of the first cognitive functions impaired (Welsh et al. 1991). For the early stage, the term “mild cognitive impairment” (MCI), and specifically the amnestic subtype (aMCI), has been widely used in previous studies (Petersen 2011), especially before the revision of the definition of early or prodromal AD by Dubois et al. (2010). MCI describes a clinical condition of objective cognitive (MCI) and/or memory (aMCI) impairment without interference in daily activities and high likelihood of transition to AD. The hippocampal formation is typically affected already in early stages of AD pathology (e.g. MCI) (Braak and Braak 1991). Previous functional neuroimaging research on memory encoding and episodic retrieval in MCI reported attenuated hippocampal activation in line with findings in AD (Hanseeuw et al. 2011; Hampstead et al. 2011; Jin et al. 2012). Paradoxical results of increased encoding hippocampal activation in MCI patients (Celone et al. 2006; Kircher et al. 2007; Hämäläinen et al. 2007; Trivedi et al. 2008; Clément et al. 2010) have been discussed along with longitudinal findings of increased hippocampal activation in earlier stages of AD which later progress to attenuation in more severe stages (O’Brien et al. 2010).
Along with the hippocampus, the precuneus has recently attracted a lot of attention. It has been hypothesised to be deactivated rather than activated during memory encoding tasks (Huijbers et al. 2012). Morphological studies have reported AD-like findings (e.g. atrophy) in MCI patients (Nickl-Jockschat et al. 2012) while neuropathological studies found increasing amyloid plaque deposition over the course of the AD continuum (Braak and Braak 1991; Buckner et al. 2005). The precuneus might be prognostic to distinguish between high and low risk of conversion to AD (Schroeter et al. 2009).
The present study aimed at identifying patterns of aberrant activation during episodic memory processes that are common to MCI and/or AD using a systematic, quantitative comparison of neural correlates in patients compared to non-affected controls. Accordingly, a meta-analysis was carried out using an advanced activation likelihood estimation (ALE), which is a powerful tool to quantitatively converge different studies and to identify consistent patterns of group differences (Eickhoff et al. 2009). Two ALE meta-analyses have previously been published in the field of episodic memory in AD (Schwindt and Black 2009; Browndyke et al. 2012). However, the present study used different methodologies and provides several further insights: in contrast to work by Schwindt and Black (2009), we included only studies reporting differences between patient and control groups, rendering the results more specific. In contrast to the second meta-analysis (Browndyke et al. 2012), we chose to restrict our meta-analysis to whole-brain studies rather than including work derived from smaller fields of view, which is biased by a hypothesis-driven analysis. Following a joint analysis of AD and MCI patients, we also probed episodic memory activation in MCI and AD separately. The present work is furthermore aimed at extending the existing literature not only by (1) increasing the number of studies included, but also by (2) putting a particular emphasis on the differentiation of episodic memory for verbal and image stimuli, (3) investigating encoding as well as retrieval phases and (4) probing the relationship between altered brain activation and cognitive performance.
We hypothesised that the convergence of episodic memory-related brain regions determined by functional neuroimaging would detect aberrant brain activation patterns in brain areas affected in patients with MCI or AD. We further investigated the MCI and AD phenotypes in verbal as well as image episodic memory tasks to unravel differential stimuli dependency. Finally, we conjectured that reduced cognitive performance predicts dysfunctional brain activation.
Methods
Literature search and study selection
A systematic search was carried out for functional studies investigating MCI and AD subjects within the PubMed (http://www.ncbi.nlm.nih.gov/pubmed/; search-strings: [{(magnetic resonance imaging) OR PET} Alzheimer OR (Mild Cognitive Impairment) episodic memory] and BrainMap databases (http://brainmap.org/). We further screened the reference lists of published reviews on MCI and AD as well as of all obtained studies to identify additional studies. We selected studies according to the following seven criteria:
Functional magnetic resonance imaging (fMRI) datasets
Subjects had to be diagnosed with (a)MCI or AD (Table 1). As most studies were performed prior to the revision of diagnostic research criteria for AD and prodromal as well as preclinical stages in 2010 (Dubois et al. 2010), studies on MCI mainly used the proposed criteria by Petersen (Petersen et al. 1999; Petersen 2011). For the same reason, AD patients fulfilled diagnostic criteria proposed by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA, McKhann et al. 1984). When inspecting the diagnostic criteria of the four studies labelling their subjects as “MCI”, we found relevant episodic memory deficit characteristics. Three studies (Celone et al. 2006; Heun et al. 2007; Kircher et al. 2007) particularly described objective memory deficits as inclusion criteria in line with an aMCI diagnosis. The fourth study (Kochan et al. 2010) comprised 25 aMCI and only seven naMCI patients. In general, naMCI patients have been shown to also convert to AD, although at lower rates than aMCI patients (Jungwirth et al. 2012; Espinosa et al. 2013). However, compared to aMCI, naMCI patients also have a higher rate of progression to other dementia syndromes, such as vascular dementia or dementia with Lewy bodies.
An episodic memory task had to be performed by the subjects while undergoing functional scanning. Specifically, episodic memory was defined as memory for information that is stored regarding the time and location of occurrence (Jonides et al. 2002) (e.g. encoding word lists). For encoding, we included intentional and incidental memory from block design studies as well as encoding phases from working memory if they had been reported separately from retrieval based on an event-related design (Peters et al. 2009). Given that differential neural correlates have been proposed for episodic retrieval and working memory (Cabeza et al. 2002), we aimed at selectively including episodic retrieval. Therefore, we included only event-related and block design retrieval experiments comprising several distinct stimuli (minimum included: seven) in the encoding block, which were later retrieved. Based on this criterion, we rejected three retrieval experiments which had only one or two stimuli per condition (Gould et al. 2005; Peters et al. 2009; Bokde et al. 2010).
Direct comparison of study population and a group of age-matched controls.
Stereotactic whole-brain coordinates were reported in a standardised reference space [Talairach or Montreal Neurological Institute (MNI) space] for the location of activation differences between MCI or AD, respectively, and controls.
Independent study samples without overlap
Studies solely focusing on drug effects were excluded.
Table 1.
Summary of clinical and demographic data of included articles with memory task characteristics
| Study | Patients |
Controls |
Task and contrast | Stimuli | Memory process | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MCI/AD | n (f) | Age (years, mean ± SD) | MMSE (mean ± SD) | n (f) | Age (years, mean ± SD) | MMSE (mean ± SD) | ||||
| Belleville et al. (2011) | aMCI | 15 (11) | 70.13 ± 7.34 | 27.73 ± 1.87 | 15 (10) | 70 ± 7.26 | 29.1 ± 0.74 | Encoding > resting | Wordsa | Encoding |
| Old–new recognition judgement > resting | Wordsa | Retrieval | ||||||||
| Bokde et al. (2010) | aMCI | 8 (2) | 70.8 ± 5.3 | 26.6 ± 1.3 | 8 (3) | 66.6 ± 3.9 | 30 ± 0.0 | Successful encoding > resting | 5 Consonantsa | Encoding |
| Celone et al. (2006) | MCIb (low SB) | 15 (7) | 75.1 ± 7.1 | 29.3 ± 0.9 | 15 (8) | 75.5 ± 6.0 | 29.5 ± 0.5 | Encoding > fixation (Positive Task-related component; ICA) | Face-name pairsa, c | Encoding |
| Clément and Belleville (2010) | aMCI (higher cognition) | 13 (8) | 68.62 ± 10.3 | 28.85 ± 1.57 | 14 (8) | 67.21 ± 6.8 | 29.29 ± 1.14 | Encoding > fixation | Unrelated word pairsa | Encoding |
| Clément et al. (2010) | aMCI | 12 (9) | 67.83 ± 7.49 | 27.83 ± 1.59 | 10 (8) | 71.7 ± 7.62 | 29.1 ± 0.74 | Encoding > resting | Wordsa | Encoding |
| Old–new recognition judgement > resting | Wordsa | Retrieval | ||||||||
| Clément and Belleville (2012) | aMCI (higher cognition) | 13 (8) | 68.62 ± 10.30 | 28.85 ± 1.57 | 14 (8) | 67.21 ± 6.8 | 29.29 ± 1.14 | Correct associated or rearranged judgement > fixation | Wordsa | Retrieval |
| old-new recognition judgement > fixation | Wordsa | Retrieval | ||||||||
| Clément and Belleville (2012) | aMCI (lower cognition) | 13 (7) | 67.08 ± 6.29 | 26.46 ± 1.56 | 14 (8) | 67.21 ± 6.8 | 29.29 ± 1.14 | Old–new recognition judgement > fixation | Words | Retrieval |
| Dannhauser et al. (2008) | aMCI | 10 (5) | 72 ± 13.5 | 24.5 ± 1.5 | 10 (6) | 68 ± 13.5 | 28.3 ± 1.6 | Reading out stimulus and memorising > reading out “wait” | Wordsa | Encoding |
| Giovanello et al. (2012) | aMCI | 12 (7) | 75.2 ± 4.3 | 27.8 ± 1.7 | 12 (7) | 72.6 ± 5.9 | 29.5 ± 0.9 | Relational > item recognition | Wordsa | Retrieval |
| Item > relational recognition | Wordsa | Retrieval | ||||||||
| Golby et al. (2005) | AD | 7 (2) | 69 | 20.8 | 7 (3) | 66 | 29.4 | Novel > repeated | Scenesc | Encoding |
| Gould et al. (2005) | AD | 12 (7) | 77.3 ± 4.9 | 26.33 ± 2.06 | 12 (7) | 77.3 ± 4.8 | 29.08 ± 0.9 | Successful encoding > resting | Object-location pairsc | Encoding |
| Correct > incorrect encoding | Object-location pairsc | Encoding | ||||||||
| Grön et al. (2002) | AD | 12 (7) | 61.7 ± 5.0 | 25.9 ± 3.5 | 12 (5) | 59.8 ± 2.6 | 30 ± 0.0 | High > low familiarity recall | Abstract patternsc | Retrieval |
| Hämäläinen et al. (2007) | aMCI | 14 (10) | 72.4 ± 7.3 | 25.6 ± 3.1 | 21 (17) | 71.2 ± 4.9 | 27.7 ± 2.0 | Encoding > fixation | Word-picture pairsa, c | Encoding |
| Hämäläinen et al. (2007) | AD | 15 (10) | 73.1 ± 6.7 | 21.7 ± 3.7 | 21 (17) | 71.2 ± 4.9 | 27.7 ± 2.0 | Encoding > fixation | Word-picture pairsa, c | Encoding |
| Hampstead et al. (2011) | aMCI | 18 (n.a.) | 71.2 ± 8.5 | 26.7 ± 2.3 | 16 (n.a.) | 72.1 ± 7.3 | 27.8 ± 1.97 | Novel > repeated | Object-location pairsc | Encoding |
| Hanseeuw et al. (2011) | aMCI | 16 (5) | 72.6 ± 7.9 | 27.3 ± 1.6 | 15 (9) | 69.4 ± 4.8 | 28.7 ± 1.5 | Encoding > fixation | Item-cue paira, c | Encoding |
| Heun et al. (2007) | MCI | 20 (9) | 69.7 ± 7.1 | 26.6 ± 1.5 | 28 (11) | 67.5 ± 5.4 | 28.9 ± 1.1 | Correct recognition > fixation | Wordsa | Retrieval |
| Correct rejections > fixation | Wordsa | Retrieval | ||||||||
| Jin et al. (2012) | aMCI | 8 (3) | 60.9 ± 3.2 | 28.1 ± 1.1 | 8 (4) | 60.6 ± 8.3 | 29.6 ± 0.5 | Simple encoding > control task | Picturesc | Encoding |
| Associative encoding > control task | Face-occupation pairsa, c | Encoding | ||||||||
| Old–new recognition judgement > control task | Picturesc | Retrieval | ||||||||
| Old–new recognition judgement > control task | Face-occupation pairsa, c | Retrieval | ||||||||
| Old–new recognition judgement > control task | Wordsa | Retrieval | ||||||||
| Kircher et al. (2007) | MCI | 21 (12) | 69.7 ± 7.0 | 26.6 ± 1.4 | 29 (17) | 67.8 ± 5.4 | 28.8 ± 1.2 | Successful encoding > fixation | Wordsa | Encoding |
| Kochan et al. (2010) | MCI | 35 (21) | 77.97 ± 3.88 | 27.94 ± 1.56 | 22 (12) | 77.16 ± 3.31 | 29.32 ± 0.95 | High > low load interaction | Object-location pairsc | Encoding |
| Machulda et al. (2009) | aMCI | 29 (15) | 75 ± 7 | n.a. | 19 (8) | 73 ± 7 | n.a. | Encoding > matching picture decision | Picturesc | Encoding |
| Old–new recognition judgement > matching picture decision | Picturesc | Retrieval | ||||||||
| Mandzia et al. (2009) | aMCI | 14 (7) | 68.6 ± 7.4 | 27.7 ± 1.1 | 14 (7) | 72.2 ± 6.4 | 28.6 ± 1.1 | ‘Deep’ incidental encoding > scrambled picture | Picturesc | Encoding |
| ‘Shallow’ incidental encoding > scrambled picture | Picturesc | Encoding | ||||||||
| ‘Deep’ old–new recognition judgement > scrambled picture | Picturesc | Retrieval | ||||||||
| ‘Shallow’ old-new recognition judgement > scrambled picture | Picturesc | Retrieval | ||||||||
| Pariente et al. (2005) | AD | 12 (8) | 70.9 | 25.1 | 17 (4) | 76.5 | 29 | Correct > incorrect encoding | Face-name pairsa, c | Encoding |
| Correct encoding | Face-name pairsa, c | Encoding | ||||||||
| Correct > incorrect associative recognition | Face-name pairsa, c | Retrieval | ||||||||
| Correct associative recognition | Face-name pairsa, c | Retrieval | ||||||||
| Peters et al. (2009) | AD | 16 (13) | 77.1 ± 6.6 | 23.4 ± 1.7 | 16 (14) | 76.0 ± 6.1 | n.a. | Encoding > control task | Wordsa | Encoding |
| Petrella et al. (2006) | aMCI | 20 (8) | 75.0 ± 7.6 | 26.7 ± 1.5 | 20 (11) | 71.2 ± 4.5 | 28.4 ± 1.4 | Novel > repeated | Face-name pairsa, c | Encoding |
| Novel > repeated | Face-name pairsa, c | Retrieval | ||||||||
| Petrella et al. (2007) | AD | 13 (5) | 71.37 ± 6.8 | 24.62 ± 2.43 | 28 (14) | 71.96 ± 4.94 | 28.25 ± 1.4 | Novel > repeated | Face-name pairsa, c | Encoding |
| Rémy et al. (2005) | AD | 8 (7) | 72.2 ± 10.8 | 21.2 ± 6.4 | 11 (6) | 65.9 ± 5.7 | 29.4 ± 0.5 | Encoding > reading | Wordsa | Encoding |
| Old–new recognition judgement > reading | Wordsa | Retrieval | ||||||||
| Sperling et al. (2003) | AD | 7 (6) | 80.6 ± 6.9 | 22.6 ± 2.2 | 10 (8) | 74.1 ± 7.3 | n.a. | Novel > fixation | Face-name pairsa, c | Encoding |
| Novel > repeated | face-name pairsa, c | Encoding | ||||||||
| Trivedi et al. (2008) | aMCI | 16 (11) | 77.0 ± 8.4 | 26.3 ± 2.3 | 23 (12) | 73.1 ± 5.5 | 28.8 ± 1.2 | Encoding > reading ‘push’ | Picturesc | Encoding |
| Remembered > forgotten | Picturesc | Encoding | ||||||||
| Remembered > forgotten | Picturesc | Retrieval | ||||||||
| Correct rejections > false alarms | Picturesc | Encoding | ||||||||
| Correct rejections > hits | Picturesc | Encoding | ||||||||
| Van der Meulen et al. (2012) | aMCI | 13 (9) | 69.2 ± 8.2 | 26.7 ± 2.3 | 15 (9) | 68.1 ± 7.2 | 29.5 ± 0.8 | Encoding > instructions | Picture pairsc | Encoding |
| Familiarity > recollection | Picture pairsc | Retrieval | ||||||||
| Recollection > familiarity | Picture pairsc | Retrieval | ||||||||
Values represent numbers of subjects or mean ± SD
F female, (a)MCI (amnestic) mild cognitive impairment, AD Alzheimer’s disease, MMSE mini-mental state examination, n.a. not available
Verbal stimuli
MCI diagnosis according to clinical dementia rating with at least 0.5 in the memory box
Image stimuli
We contacted authors for missing data if the publications were otherwise suitable for our meta-analysis. This resulted in the inclusion of one additional study (Hampstead et al. 2011). To avoid omitting valuable information on spatial distribution of functionally altered brain activation patterns, we included several experiments/contrasts per study (i.e. different stimuli types). All reported clusters have at least two independent studies contributing to it. To gather all available information on spatial distribution, we decided to also include second level contrasts, for instance ‘remembered > forgotten’ or ‘high > low demand’. This meta-analysis was performed according to the ethical standards of the Medical Faculty of RWTH Aachen University and the latest version of the Declaration of Helsinki.
Study characteristics
In the first step, we screened the abstracts of 1,425 individual studies published up until January 2013. Twenty-eight fMRI studies matched our inclusion criteria and were subjected to an ALE meta-analysis. They comprised 473 foci from 56 experiments from 102 patients with AD, 292 with MCI and 409 healthy age-matched controls.
All demographic and clinical data along with descriptions of employed memory tasks and contrasts are listed in Table 1. In the following paragraph, we will briefly outline the diverse episodic memory paradigms employed. As stimuli, word lists and face-name pairs were most commonly used. Instructions for encoding phases explicitly required the subjects to intentionally memorise the stimuli with the exception of “incidental” memory (Mandzia et al. 2009), whereby the subjects performed a distraction task (i.e. decision whether picture is man-made or natural) and later received a surprise retrieval task. For retrieval tasks, we found most frequently the so-called “old-new recognition judgement”, which involves the presentation of either a formerly presented or an unknown stimulus along with an according response by the subject. Other studies employed cued recall (“Which stimulus was presented with this cue?”) (Hämäläinen et al. 2007) or associative recognition (e.g. “Have these two stimuli been presented together earlier?”). Most experiments directly compared the encoding or retrieval phases to respective control tasks, thus presented a “first-level contrast”. Often an undemanding referential condition was used as baseline (e.g. fixation cross). Some studies included second-level contrasts such as ‘correct > incorrect encoding’ which, for instance, highlighted successful memory. Finally, Pariente et al. (2005) directly compared the encoding or recognition phases of AD patients to controls without subtracting any referential conditions before.
To guarantee comparability of MCI and AD studies in the respective contrasts (encoding, retrieval), we statistically compared mean age and MMSE scores. We used the statistical package SPSS 19 (Chicago, IL, USA) for Levene’s Tests for Equality of Variances (p < 0.05) and t tests (p < 0.05). MCI compared to AD patients did not differ significantly for age in either task. In contrast, AD patients had significantly lower MMSE scores than MCI patients (encoding: MCI 27.28 ± 1.38 vs. AD 23.22 ± 1.99; retrieval: MCI 27.34 ± 0.83 vs. AD 24.58 ± 2.30). This finding was rather expected since significant cognitive problems are a discriminative criterion between MCI and AD. We then checked for comparability of encoding and retrieval studies within each patient group. MCI patients did not differ significantly for age or MMSE scores. AD patients were matched for MMSE scores for both tasks and were significantly older in the encoding (mean 73.95 ± 3.96) than in the retrieval studies (mean 66.89 ± 5.43). However, we are not convinced that age solely explains the reported differential brain activation patterns. The difference in age might be a result of the small selection of only four AD retrieval studies.
ALE algorithm
A revised version (Eickhoff et al. 2012) of the ALE approach for coordinate-based meta-analysis of neuroimaging data (Turkeltaub et al. 2002; Laird et al. 2009; Eickhoff et al. 2009) was used to test for significant convergence of episodic memory-associated brain activation in MCI and AD. The central data processing step in ALE meta-analysis is to model each focus’ suspected real location as a 3D Gaussian probability distribution. Larger sample sizes are believed to provide data closer to the real value, and the belonging Gaussian distribution is thus smaller (Eickhoff et al. 2009). The probabilities of all foci reported in a given experiment were then combined for each voxel, resulting in a modelled activation (MA) map (Turkeltaub et al. 2012). Taking the union across these MA maps yielded voxel-wise ALE scores describing the convergence of results at each particular location of the brain. To distinguish between ‘true’ convergence studies and random convergence (i.e. noise), ALE scores were compared to an analytically derived null-distribution reflecting a random spatial association between experiments (Eickhoff et al. 2012). The yielded non-parametric p values for each meta-analysis were then thresholded at a cluster-level corrected threshold of p < 0.05 (cluster-forming threshold at voxel-level p < 0.001) and transformed into Z scores for display. The extent-threshold necessary to control the cluster-level family-wise error (cFWE) rate was derived from a Monte-Carlo simulation of the excursion-set above cluster-forming threshold based on the analysis of randomly distributed foci under otherwise identical settings.
All resulting clusters of the relative activation differences were labelled using the SPM Anatomy Toolbox v1.8 (Eickhoff et al. 2005, 2007). The Anatomy Toolbox provides maximum probability maps, which represent a summary of different histological maps and allows attribution of each voxel of the reference space to the most likely cytoarchitectonic area (Eickhoff et al. 2005). Additionally, to define subregions in the hippocampus in more detail, clusters in the cornu ammonis (CA) were visually allocated by experienced raters to the subregions CA1, CA2, CA3 or CA4 by manually drawing labels according to ‘The Human Hippocampus’ (Duvernoy et al. 2005) (Fig. 1).
Fig. 1.

Hippocampal clusters and (manual) cytoarchitectonic allocation. a Axial section of increased anterior hippocampal activation (mainly CA2-4) in MCI patients during encoding (all stimuli). b Coronary section of reduced left-hemispheric and hippocampal activation (mainly CA1) extending to fusiform gyrus in MCI patients during image retrieval. c Axial section showing decreased hippocampal activation (SUB, CA1) in AD patients during retrieval (all stimuli). All clusters are family-wise error cluster corrected at p < 0.05. MCI mild cognitive impairment, AD Alzheimer’s disease, R right hemisphere, L left hemisphere, x, y, z display Montreal Neurological Institute (MNI) coordinates, CA cornu ammonis, DG dentate gyrus, SUB subiculum, PHG parahippocampal gyrus
Contrasts and subgroup-analyses
Encoding and retrieval phases were analysed separately. First, co-localisations between the four contrasts published by our included studies were calculated (‘MCI > Controls’, ‘Controls > MCI’, ‘AD > Controls’ or ‘Controls > AD’). Assuming MCI to be part of the continuum from healthy ageing to AD (Buckner et al. 2005), we searched for convergent clusters within MCI and AD patients compared to controls by generating encoding and retrieval maps for the MCI and AD groups pooled together for the published contrasts [‘MCI > Controls’ AND ‘AD > Controls’] and [‘Controls > MCI’ AND ‘Controls > AD], further referred to as “MCI + AD > Controls” and “Controls > MCI + AD”, respectively. To address the hypothesis of a common pathophysiology of MCI and AD, we computed conjunction analyses (cFWE p < 0.05). However, the main results (Table 2) for each contrast in the MCI vs. controls group revealed entirely different regions than the AD vs. controls group in the respective contrasts. Hence, a quantitative conjunction analysis could and did not yield any results at the same statistical threshold.
Table 2.
Significant clusters of convergence in the ALE meta-analyses
| Contrast | Task | Volume (n) | MNI coordinates |
R/L | Macroanatomical and cytoarchitectonic region | Computed experiments | Main contributing studies | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| MCI > Controls | Encoding all | 66 | 24 | −16 | −14 | R | Hippocampus (maximum: CA) Distribution: 82 % in CA, 6 % in SUB CA manually: CA2, CA3, CA4 |
10 | Celone et al. (2006), Trivedi et al. (2008) |
| Encoding verbal | 8 | ||||||||
| Encoding images | 88 | 25 | −15 | −14 | R | Hippocampus (maximum: CA) Distribution: 75 % in CA, 8 % in amygdala (CM), 6 % in SUB CA manually: CA2, CA3, CA4 |
5 | Celone et al. (2006), Trivedi et al. (2008) | |
| Retrieval all | 13 | ||||||||
| Retrieval verbal | 10 | ||||||||
| Retrieval images | 4 | ||||||||
| Controls > MCI | Encoding all | 19 | |||||||
| Encoding verbal | 7 | ||||||||
| Encoding images | 14 | ||||||||
| Retrieval all | 14 | ||||||||
| Retrieval verbal | 94 | 32 | 24 | −4 | R | Anterior insula lobe, inferior frontal gyrus | 8 | Petrella et al. (2006), Giovanello et al. (2012) | |
| Retrieval images | 92 | −32 | −12 | −22 | L | Fusiform gyrus, hippocampus (maximum: CA) | 8 | Petrella et al. (2006), Trivedi et al. (2008), Jin et al. (2012) | |
| Distribution: 75 % in CA | |||||||||
| CA manually: CA1, CA4 | |||||||||
| AD > Controls | Encoding all | 140 | 12 | −60 | 38 | R | Precuneus | 9 | Sperling et al. (2003), Pariente et al. (2005), Petrella et al. (2007) |
| Encoding verbal | 187 | 12 | −60 | 38 | R | Precuneus | 7 | Sperling et al. (2003), Pariente et al. (2005), Petrella et al. (2007) | |
| 116 | −14 | −64 | 32 | L | Precuneus | 7 | Sperling et al. (2003), Petrella et al. (2007) | ||
| Encoding images | 140 | 12 | −60 | 38 | R | Precuneus | 8 | Sperling et al. (2003), Pariente et al. (2005), Petrella et al. (2007) | |
| 85 | −14 | −66 | 32 | L | Precuneus | 8 | Sperling et al. (2003), Petrella et al. (2007) | ||
| Retrieval all | 4 | ||||||||
| Retrieval verbal | 3 | ||||||||
| Retrieval images | 3 | ||||||||
| Controls > AD | Encoding all | 10 | |||||||
| Encoding verbal | 7 | ||||||||
| Encoding images | 8 | ||||||||
| Retrieval all | 156 | 26 | −18 | −20 | R | Hippocampus (maximum: SUB) | 4 | Grön et al. (2002), Pariente et al. (2005), Rémy et al. (2005) | |
| Distribution: 50 % in CA, 47 % in SUB | |||||||||
| CA manually: CA1 | |||||||||
| Retrieval verbal | 3 | ||||||||
| Retrieval images | 155 | 26 | −18 | −20 | R | PHG, hippocampus (maximum: SUB) | 3 | Grön et al. (2002), Pariente et al. (2005) | |
| Distribution: 42 % in CA, 40 % in SUB, 10 % in EC, 7 % in amygdala (LB) | |||||||||
| CA manually: CA1 | |||||||||
n number of voxels, MNI Montreal Neurological Institute, R right, L left, MCI mild cognitive impairment, AD Alzheimer’s disease, CA cornu ammonis, SUB subiculum, CM centromedial, PHG parahippocampal gyrus, EC entorhinal cortex, LB laterobasal, DG dentate gyrus
We also performed subgroup analyses of either verbal or image stimuli (cf. Table 1). Any stimulus with letters or words was depicted as being verbal. Photographs, pictures, patterns or line drawings were categorised in the “images” subgroup. Some stimuli combined verbal and image aspects, i.e. face-name pairs and were consequently included in both subgroup analyses.
Correlation between MMSE and likelihood of altered activation
The Mini-Mental State Examination (MMSE) (Folstein et al. 1975) is still the most frequently used screening test and short neuropsychological exam in fMRI literature to asses a subject’s global cognitive performance. We aimed to investigate the dependence of the likelihood of changes revealed by functional neuroimaging and cognitive performance using mean MMSE scores for Spearman rank correlations, thresholded at a p value of p < 0.05.
Results
Table 2 summarises all significant clusters and provides volume, peak coordinates, macroanatomic as well as cytoarchitectonic regions (if available) and contributing studies. We provide more details in Table 2, including the most probable cytoarchitectonic region of the peak coordinate, spatial distribution of the cluster by giving the percentage in different sub-regions of the whole volume, as well as manual allocation to CA1-4 and finally, number of experiments included in each contrast. The remaining analyses, which are not mentioned in the following results part, failed to reach the significance threshold (cf. Table 2). Furthermore, the pooled analyses mainly replicated the group-specific comparisons. The results are shown in supplementary Table S1. They are essential to assure power of the correlational analyses.
Aberrant episodic memory-related brain activation in MCI patients compared to controls
Compared to non-affected controls, MCI patients showed consistently increased right anterior hippocampal activation (CA2-4, subiculum = SUB) (Fig. 1a) during encoding. A similar cluster in the hippocampus (CA2-4, SUB) extending to the amygdala yielded significance in the image subgroup.
During verbal retrieval, MCI patients showed decreased activation in the right anterior insula (Fig. 2), while attenuated activation was observed in the left anterior hippocampus (CA1, CA4) extending to the left fusiform gyrus (Fig. 1b) for the image stimuli subgroup.
Fig. 2.
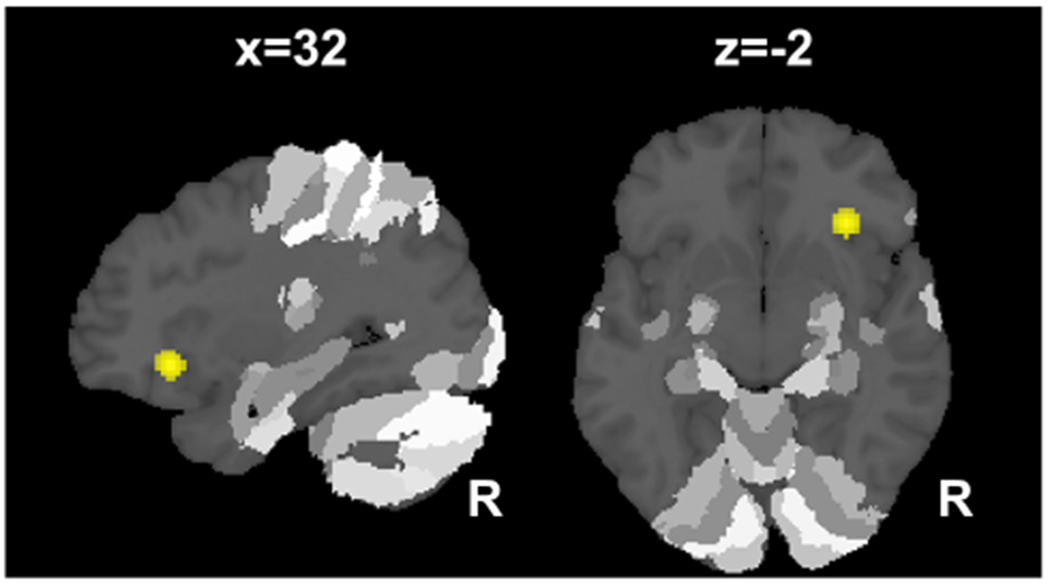
Decreased insula and inferior frontal gyrus activation during verbal retrieval tasks in patients with mild cognitive impairment. Patients with mild cognitive impairment showed less activation during verbal retrieval in the right anterior insula extending to the inferior frontal gyrus when compared to controls. All clusters are family-wise error cluster corrected at p < 0.05. R right hemisphere, x, z in Montreal Neurological Institute (MNI) coordinates
Aberrant episodic memory-related activation in AD patients compared to controls
Across all encoding paradigms (verbal and images), AD patients activated the right precuneus stronger than control subjects. During encoding of either verbal or image stimuli, the bilateral precuneus also showed stronger activation (Fig. 3).
Fig. 3.

Stronger precuneus activation and correlation with performance. A convergent finding was stronger bilateral precuneus activation in AD patients compared to controls during image encoding (blue). We furthermore report significant voxel-wise correlation with mini-mental state examination (MMSE) scores in the right precuneus in MCI + AD patients compared to controls during image encoding (magenta). All clusters are family-wise error cluster corrected at p < 0.05. R right hemisphere, x, y, z display Montreal Neurological Institute (MNI) coordinates
During episodic retrieval of all stimuli, the right anterior hippocampus (SUB, CA1) was significantly less active in AD compared to controls (Fig. 1c). Decreased activation remained significant in the image retrieval subgroup in the PHG, the hippocampus (SUB, CA1, entorhinal cortex) as well as the amygdala.
Correlation of cognitive performance with aberrant brain activation in all patient groups
A voxel-wise analysis investigating whether patients’ aberrant brain activation correlated with MMSE scores highlighted four clusters (Table 3). Specifically, lower MMSE scores, which reflect more severe cognitive impairments, are associated with a higher probability of finding altered activation in several regions. Decreased activation in the right anterior insula/inferior frontal gyrus during verbal retrieval was linked to lower MMSE scores in MCI, while AD patients with lower MMSE scores exhibited decreased right hippocampal (SUB, CA1) activation during image retrieval. Stronger right precuneus activation during encoding of images was associated with lower MMSE scores of the pooled group of MCI + AD (Fig. 3). Finally, lower right (para)hippocampal (SUB, CA1, CA3, CA4) and amygdala activation was correlated with lower MMSE performance during image retrieval in the pooled MCI + AD group. Pooling MCI and AD data focused on the continuum of MCI to AD. Given the regional diversity of brain activation patterns reported on group level, we found effects that correlate well with MMSE scores. The method confirmed the disease stage dependency of memory processing network alterations.
Table 3.
Significant correlation of lower MMSE scores with altered activation in specific brain regions
| Contrast | Task | r | R/L | MNI coordinates |
Macroanatomical and cytoarchitectonic region | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Controls > MCI | Retrieval verbal | −0.8 | R | 32 | 28 | −2 | Anterior insula lobe, inferior frontal gyrus |
| (MCI + AD) > Controls | Encoding | −0.51 | R | 10 | −58 | 36 | Precuneus |
| (MCI + AD) > Controls | Encoding images | −0.61 | R | 10 | −58 | 36 | Precuneus |
| Controls > (MCI + AD) | Retrieval images | −0.85 | R | 26 | −18 | −20 | PHG, hippocampus (maximum: SUB) |
| Distribution: 42 % in SUB, 40 % in CA, 10 % in EC, 7 % in amygdala (LB) | |||||||
| CA manually: CA1, CA3, CA4 | |||||||
r Spearman correlation coefficient, R right, L left, MNI Montreal Neurological Institute, MCI mild cognitive impairment, AD Alzheimer’s disease, PHG parahippocampal gyrus, EC entorhinal cortex, SUB subiculum, CA = cornu ammonis, LB laterobasal
Discussion
Using a quantitative meta-analysis approach, we characterised episodic memory-related alternations in brain activation patterns in patients with MCI or AD. Our study yielded four main results. First, we identified increased encoding-related brain activation in the hippocampus (mainly CA2-4, also SUB) in early disease stages (MCI) as well as decreased activation in other hippocampal areas (mainly CA1, also CA4) during retrieval. Second, we report that MCI and AD are characterised by decreased PHG activation during episodic retrieval. Third, we found encoding-related stronger activation in the precuneus in AD only. Fourth, higher probability of finding decreased right (para)hippocampal and right anterior insula/inferior frontal activation as well as stronger precuneus activation was associated with worse cognitive performance measures (MMSE scores). The results highlight both the neuropathological role of the MTL and precuneus and their association to cognitive performance and importantly verify specific and probably disease stage-dependent brain activation patterns.
Hippocampal formation
As revealed by our meta-analysis, individuals with MCI showed elevated activation during encoding of all or image stimuli in the anterior hippocampus, mainly in CA2-4, and also in the SUB. Activation in the hippocampal formation in general during encoding has been shown to be essential for long-term storage of memory (Scoville and Milner 2000; Squire et al. 2004) and has also been associated with encoding success (Spaniol et al. 2009). In MCI patients, hippocampal activation has been contradictorily described as both elevated (Celone et al. 2006; Kircher et al. 2007; Hämäläinen et al. 2007) and reduced (Hanseeuw et al. 2011; Hampstead et al. 2011; Giovanello et al. 2012) compared to age-matched healthy controls. These discrepancies may be explained by a longitudinal study suggesting that elevated hippocampal activation only occurs in early stages of the disease and precedes decline in cognition and hippocampal activation (O’Brien et al. 2010). Understanding of the underlying neuropathological processes can be augmented by studies which reported neurofibrillary tangles within the earliest stages of the disease as well as amyloid β protein (Aβ) depositions in later stages (Braak and Braak 1991; Braak et al. 2006). Neurofibrillary tangles lead to dysfunctions and disconnections accompanied by impaired function of the affected regions already in MCI patients (Bokde et al. 2006). It is hypothesised that hippocampal hyperactivation in MCI might represent an early response to AD pathology, which may be of compensative nature or predicts impending hippocampal failure and memory decline. This period of hippocampal hyperactivation is then followed by loss of hippocampal activation as the disease progresses (Fig. 4). It is suggested that the hyperactivation may be an indicator of neuronal stress or directly related to the pathophysiologic process of AD. For instance, hyperactivation was associated with aberrant sprouting of cholinergic fibres (Hashimoto and Masliah 2003), excitotoxicity from the excessive stimulation of NMDA receptors (Hynd et al. 2004) or stress from Aβ-induced neuronal alterations (e.g. excitatory activation triggers compensatory inhibitory mechanisms, which jointly contribute to network dysfunction) (Palop et al. 2007). Postmortem studies have furthermore implied that the entorhinal cortex is prominently affected, the SUB and CA1 severely involved, whereas CA3 and the DG are relatively preserved (Mueller et al. 2010; Small et al. 2011). This may explain why our observed cluster in CA2-4 presumably represents compensatory elevated activation in early stages of the disease (MCI). This hippocampal hyperactivation failed to correlate with consistent MMSE score trends, indicating that it is too rare or simply not related to specific MMSE scores.
Fig. 4.
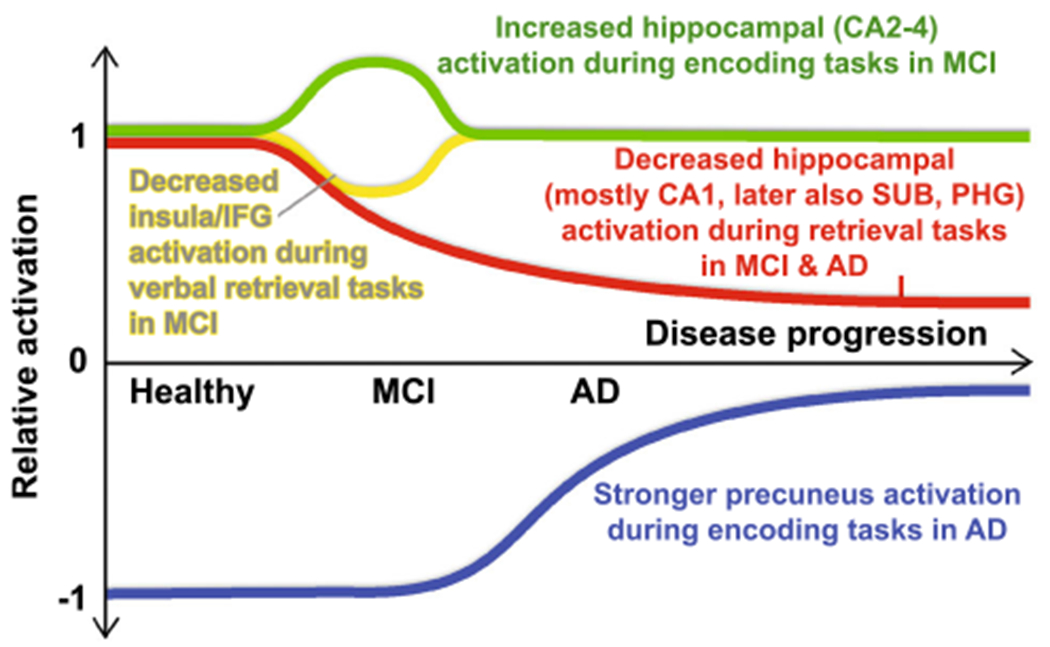
Schematic course of differential (para)hippocampal, precuneus and insula/IFG activation during episodic memory tasks over the course of the disease. Retrieval task-induced hippocampal (at first predominantly CA1, later also SUB and PHG; red line) activation declines over the course of the disease from MCI to fully expressed AD compared to controls in hippocampal sub-regions which are specifically and severely affected by AD pathology. This is accompanied by attenuated verbal retrieval task-related activation in the insula and inferior frontal gyrus (IFG) in MCI only (yellow line). Encoding task-induced activation is higher in MCI patients than in controls in hippocampal sub-regions relatively spared from AD pathology (CA2-4), probably compensatory for the loss of memory function and activation (green line). Precuneus activation is stronger during encoding conditions in severely affected AD patients than in controls. CA cornu ammonis, AD Alzheimer’s disease, MCI mild cognitive impairment, SUB subiculum, PHG parahippocampal gyrus, IFG inferior frontal gyrus
Additionally, we found that during functional neuroimaging retrieval experiments using image stimuli, MCI compared to controls showed attenuated left anterior hippocampal (mainly CA1, also CA4) activation extending to the left fusiform gyrus. In addition to the above-mentioned involvement in encoding tasks, the hippocampus has been previously associated with episodic retrieval (Hayes et al. 2004; Kühn and Gallinat 2013), especially retrieval success (Nyberg et al. 1996; Schacter et al. 1999; Hayes et al. 2004). The fusiform gyrus is anatomically and functionally adjacent to the hippocampus and has been highlighted during face recognition (McCarthy et al. 1996; Lim et al. 2011) and colour processing (Wang et al. 2013). The main contributing studies employed pictures, face-name pairs and black-and-white line drawings of real objects. In addition to the above-mentioned histopathological findings in the hippocampus in AD, the CA1 region has shown decreased metabolic responses (Salehi and Swaab 1999). Decreased activation within the hippocampal formation itself is believed to result in decreased activation of consecutive regions (Small et al. 2011) such as the fusiform gyrus. Early AD pathology revealed by histopathological as well as neuroimaging studies might explain decreased activation within the hippocampus (mainly CA1) in MCI.
In our meta-analysis of AD patients compared to controls, the right anterior hippocampus (especially the SUB and CA1) was significantly less activated during retrieval of all kinds of probed stimuli. In the subgroup of image stimuli, we found a similar cluster in the SUB and CA1 extending to the entorhinal cortex as well as the amygdala. In addition to the above-mentioned functions of the hippocampus, retrieval-related activation has been linked to the SUB (Small et al. 2011). This decreased (mainly SUB and CA1) activation during image retrieval was associated with lower MMSE scores. Taken together, reduced hippocampal (SUB, CA1) activation apparently precisely colocalises with AD pathology and is associated with impaired retrieval memory performance in AD.
Correlational analysis of the pooled analysis of MCI and AD revealed that reduced right posterior PHG and hippocampal (mostly SUB and CA) activation during retrieval of image stimuli was associated with lower MMSE scores.
Reduced PHG and hippocampal (mainly CA1 and SUB) activation during episodic retrieval tasks is the most consistent effect of AD in early as well as late stages, also related to impaired performance.
In summary, we report that early (presumably compensatory) encoding-related hippocampal hyperactivation (mainly CA2-4) in MCI patients is followed by retrieval-related declining activation in both MCI and AD patients, especially in the CA1, SUB and PHG—the sub-regions specifically affected by AD pathology.
Notably, all studies that mainly contributed to clusters in the MTL contrasted memory-related brain activation to brain activation during a demanding referential condition (e.g. ‘hits > misses’ or ‘novel > repeated’). It has been shown that undemanding referential conditions (e.g. visual fixation of a cross) involve the MTL, and especially hippocampal activation, comparable to that in memory tasks (Stark and Squire 2001). Thus to explore hippocampal activation during memory tasks, a (demanding) referential condition is needed which does not involve hippocampal activation.
Precuneus
While AD patients were characterised by stronger encoding-related activation in the precuneus, no aberrant precuneus activation was found within the 20 included studies on MCI patients compared to controls. The precuneus is presumably involved in complex behavioural tasks, especially visuo-spatial imagery, self-processing and consciousness (for a review see Cavanna and Trimble 2006). Moreover, the precuneus steadily plays a main role in the so-called ‘default-mode network’ which has the highest metabolic rates and blood perfusion during resting conditions (Gusnard and Raichle 2001; Schilbach et al. 2012). Considering the normal function of the precuneus, activation during memory tasks has been described as an encoding/retrieval flip (Huijbers et al. 2012) implying that the BOLD-signal is negative (lower than during resting baseline conditions) in encoding tasks (“deactivation”). Following this hypothesis, the relatively stronger precuneus activation found during encoding in AD patients compared to controls might reflect “less deactivation”. Nevertheless, the ideas of areal baseline and deactivations remain highly controversial. Stronger precuneus activation might also reflect a compensatory or dysfunctional increase of activation in response to amyloid deposition. In contrast, relatively increased activation compared to baseline is observed during retrieval tasks (“activation”). AD-related histopathological changes in the precuneus have mainly been associated with extracellular Aβ depositions in later stages of the disease, such as Braak IV-VI (Braak and Braak 1991; Braak et al. 2006). In vivo imaging of fibrillary amyloid depositions via Pittsburgh compound B-PET (PiB-imaging) has marked the precuneus and posterior cingulate as regions that are consistently affected rather early. Healthy subjects with elevated levels of Aβ depositions showed highly aberrant patterns of default network fMRI activation (Celone et al. 2006; Sperling et al. 2009). This link between pathology and function has been hypothesised as a result of loss of connectivity and synapses in this region (Scheff et al. 2013). Less deactivation or stronger activation during demanding encoding tasks compared to undemanding tasks in the precuneus has been suggested as prognostic for the development of AD in at-risk stages such as MCI (Petrella et al. 2007). Our observation that MCI patients were spared from precuneus dysfunctions is supported by a study reporting that cholinergic activity was maintained regardless of PiB binding and amyloid burden in postmortem brains (Ikonomovic et al. 2011). This suggests maintained function of the precuneus until severe stages of the disease (Fig. 4). We showed that precuneus dysfunctions during episodic memory tasks do not consistently occur in MCI and, thus, investigation of task-related activation in prodromal AD in the precuneus has to be more differential.
In AD, bilateral precuneus activation was significantly strongly activated in the subgroups of verbal or image stimuli. In a former meta-analysis of functional neuroimaging studies of episodic memory tasks in AD, precuneus activation has been described as both greater and less activated compared to controls, notably during both encoding and retrieval phases (Schwindt and Black 2009). In line with our findings, experimental studies reported stronger activation of the precuneus in AD compared to controls during encoding tasks (Frings et al. 2010; Browndyke et al. 2012).
The correlation analysis in the pooled group MCI + AD of MMSE scores and likelihood of finding altered activation showed significant correlation between low MMSE scores and the right precuneus during encoding (all and image subgroups). The contributing studies employed AD patients only. This supports the hypothesis that functional deficits in the precuneus follow the pathological amyloid deposition with delay, only in patients achieving MMSE scores on average of less than 26, and are most consistent during encoding of images. Stronger activation in the precuneus co-localises with AD histopathology and might underlie the memory encoding deficits.
Insula, inferior frontal gyrus
During retrieval of verbal stimuli, individuals with MCI presented attenuated activation in the right anterior insula and inferior frontal gyrus. The insula is a part of the brain believed to integrate perceptions and emotional situations with cognitive processes. The anterior part in particular is connected to the frontal lobe and specifically active during attention and memory retrieval paradigms (here only right-hemispheric) revealed by meta-analysis (Kurth et al. 2010). It is further believed to play an active role in language processing (McCarthy et al. 1993; Price 2000; Kurth et al. 2010), whilst its function was more recently described as a very basic planning of general orofacial functions independent of actual words (Price 2010). Bilateral, rather than one-sided prefrontal activation (including the inferior frontal gyrus) has been observed in older rather than younger subjects, presumably to maintain declining cognitive functions (Grady 2012), e.g. during verbal or memory tasks. Neurofibrillary tangles have been reported in this region in the latest stages IV to VI in AD (Braak et al. 2006). MCI patients showed impaired verbal memory performance (Giovanello et al. 2012) and activation likelihood in the afore-mentioned cluster was correlated with the lowest MMSE scores during retrieval of verbal stimuli. Summing up, decreased anterior insula/inferior frontal gyrus activation is phenotypic for verbal retrieval performance deficits in MCI patients.
Stimulus specificity
It has been shown that tasks with images and verbal stimuli preferably activate right-to-bilateral and left-hemispheric brain regions, respectively. Since AD pathology is asymmetric yet not lateralised (Derflinger et al. 2011), we expected disease-related brain activation alterations to be more obvious in regions of greater task involvement or stimulus dependency. Increased right hippocampal activation in MCI patients compared to controls during encoding was found with most contributions from studies employing objects and face-name pairs as stimuli. Stronger left-hemispheric activation was earlier observed during encoding of verbal stimuli, while images, faces, scenes or patterns have been described as being processed bilaterally or right-hemispherically (Kelley et al. 1998; Schacter et al. 1999; Golby et al. 2001; Rosazza et al. 2009). This right-hemispheric hippocampal hyperactivation is in line with those previous findings. We further found mostly right-hemispheric hippocampal activation during retrieval tasks in MCI and AD patients independent of stimulus material or in the image stimuli subgroups. Although the only left-hemispheric cluster of decreased activation in MCI compared to controls in the hippocampus appeared in the image retrieval subgroup, we identified clear verbal aspects in the material used. Stimuli consisted of face-name-pairs, face-occupation pairs and, as stated in the original publication (Trivedi et al. 2008), “nameable” objects. Reduced right anterior insula/inferior frontal gyrus activation in MCI patients during verbal retrieval tasks seemed to contradict the above-mentioned left-hemispheric dependency of verbal tasks at first. However, according to a recent meta-analysis on insula functions, bilateral verbal processing within the insula seems to be an exception from general left-hemispheric lateralisation in verbal tasks (Kurth et al. 2010). In further support of our findings, unilateral right anterior insula activation has been reported as being specific for memory tasks (Kurth et al. 2010).
Stimulus specificity has not yet been reported for the precuneus during encoding tasks.
Limitations
Although we used the meta-analytic approach to converge previous findings, limitations still exist in the heterogeneity of included studies such as stimuli and diagnosis of patients. First, the considered patient groups exhibit a certain level of heterogeneity as we included studies with single- and multidomain aMCI as well as very few non-aMCI as diagnostic entity. Future studies should examine more homogeneous prodromal AD groups by applying the revised diagnostic criteria by Dubois et al. (2010). It is important to note that none of the included studies reported cerebrospinal fluid (CSF) examination results or amyloid status revealed by PiB-PET. Future investigations combining this important information with task-related fMRI results for patients and controls could help to unravel the possible co-existence or co-localisation of tau and amyloid accumulations and functional impairment. Furthermore, AD patients in contrast to control groups were frequently on medication which itself has been shown to influence memory-related brain activation. Given that published papers do not typically report patient-to-patient medication, we had to ignore the potential effect of medication. Moreover, we summarised different stimuli types, variable memory tasks and time spans between encoding and retrieval phases, varying task complexity and demand and several baseline or reference conditions. Future studies may (1) employ demanding referential conditions that do not depend on MTL activation and (2) check for possible differences of short-term vs. long-term memory-related activation patterns in AD compared to controls to increase the understanding of the relevance of histopathological AD-related findings in the MTL. Finally, in the included studies’ magnetic field strength ranged from 1.5 to 4.0 T while the majority of data stemmed from 3.0 T scanners. In the future more studies using ultra high field fMRI (e.g. MTL during episodic memory tasks) may unravel the nature and exact cytoarchitectonic localisation of hippocampal activation in MCI and AD. While region of interest (ROI) based analyses may offer a higher spatial resolution, this approach requires a strong a priori hypothesis as additional relevant neural correlates might be overseen not using a whole-brain method. An additional problem in that endeavour was the high variety of chosen shapes and sizes of ROIs.
Conclusions
This quantitative meta-analysis provides evidence for a specific and probably disease stage-determined brain activation pattern linked to the pathognomonic AD feature of episodic memory loss. In this meta-analysis, cytoarchitectonic labelling of clusters revealed by neuroimaging studies was substantial for a precise understanding of brain (dys)functions. CA1 and the SUB as well as the PHG are not only relatively selectively and severely affected by AD pathology, but also display declining activation in MCI and AD patients during retrieval tasks. Notably, CA2-4 is relatively preserved from AD pathology and yet reveals (presumably compensatory) hyperactivation in early disease stages (MCI). Decreased MTL activation in AD is accompanied by stronger activation during memory encoding tasks in the precuneus, indicating compensatory or rather dysfunctional mechanisms. These key regions might operate as surrogate markers for further clinical trials.
Supplementary Material
Acknowledgments
We would like to thank B. Hampstead for sharing additional data which lead to the inclusion of one further study (Hampstead et al. 2011). We would like to furthermore thank Imis Dogan for her invaluable input during our data investigation, Ana Costa for providing knowledge and relevant literature about the different human memory systems and Melissa Chung for proofreading the manuscript. NJS and JBS are in part funded by the Helmholtz Alliance ICEMED - Imaging and Curing Environmental Metabolic Diseases, through the Initiative and Network Fund of the Helmholtz Association. SBE was funded by the Human Brain Project (R01-MH074457-01A1) and acknowledges funding by the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Systems Biology (Human Brain Model) and the IRTG 1328. SBE and KR were funded by the Excellence Initiative of the German federal and state governments.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00429-014-0744-6) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Nils Nellessen, Department of Neurology, RWTH Aachen University, Pauwelsstrasse 30, 52074 Aachen, Germany; Institute of Neuroscience and Medicine (INM-4), Forschungszentrum Jülich GmbH, Wilhelm-Johnen-Straße, 52428 Jülich, Germany; Jülich Aachen Research Alliance (JARA), Translational Brain, Medicine, Jülich, Aachen, Germany.
Claudia Rottschy, Institute of Neuroscience and Medicine (INM-1), Forschungszentrum Jülich GmbH, Wilhelm-Johnen-Straße, 52428 Jülich, Germany; Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, Pauwelsstrasse 30, 52074 Aachen, Germany.
Simon B. Eickhoff, Institute of Neuroscience and Medicine (INM-1), Forschungszentrum Jülich GmbH, Wilhelm-Johnen-Straße, 52428 Jülich, Germany; Institute of Clinical Neuroscience and Medical Psychology, Heinrich Heine University, Universitätsstr. 1, 40225 Düsseldorf, Germany
Simon T. Ketteler, Department of Neurology, RWTH Aachen University, Pauwelsstrasse 30, 52074 Aachen, Germany; Institute of Neuroscience and Medicine (INM-4), Forschungszentrum Jülich GmbH, Wilhelm-Johnen-Straße, 52428 Jülich, Germany
Hanna Kuhn, Department of Neurology, RWTH Aachen University, Pauwelsstrasse 30, 52074 Aachen, Germany; Institute of Neuroscience and Medicine (INM-4), Forschungszentrum Jülich GmbH, Wilhelm-Johnen-Straße, 52428 Jülich, Germany; Jülich Aachen Research Alliance (JARA), Translational Brain Medicine, Jülich, Aachen, Germany.
N. Jon Shah, Department of Neurology, RWTH Aachen University, Pauwelsstrasse 30, 52074 Aachen, Germany; Institute of Neuroscience and Medicine (INM-4), Forschungszentrum Jülich GmbH, Wilhelm-Johnen-Straße, 52428 Jülich, Germany; Jülich Aachen Research Alliance (JARA), Translational Brain Medicine, Jülich, Aachen, Germany.
Jörg B. Schulz, Department of Neurology, RWTH Aachen University, Pauwelsstrasse 30, 52074 Aachen, Germany; Jülich Aachen Research Alliance (JARA), Translational Brain Medicine, Jülich, Aachen, Germany
Martina Reske, Institute of Neuroscience and Medicine (INM-4), Forschungszentrum Jülich GmbH, Wilhelm-Johnen-Straße, 52428 Jülich, Germany.
Kathrin Reetz, Department of Neurology, RWTH Aachen University, Pauwelsstrasse 30, 52074 Aachen, Germany; Institute of Neuroscience and Medicine (INM-4), Forschungszentrum Jülich GmbH, Wilhelm-Johnen-Straße, 52428 Jülich, Germany; Jülich Aachen Research Alliance (JARA), Translational Brain Medicine, Jülich, Aachen, Germany.
References
- Bäckman L, Andersson JLR, Nyberg L et al. (1999) Brain regions associated with episodic retrieval in normal aging and Alzheimer’s disease. Neurology [DOI] [PubMed] [Google Scholar]
- Belleville S, Clément F, Mellah S et al. (2011) Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain 134:1623–1634. doi: 10.1093/brain/awr037 [DOI] [PubMed] [Google Scholar]
- Bokde ALW, Lopez-Bayo P, Meindl T et al. (2006) Functional connectivity of the fusiform gyrus during a face-matching task in subjects with mild cognitive impairment. Brain 129:1113–1124. doi: 10.1093/brain/awl051 [DOI] [PubMed] [Google Scholar]
- Bokde ALW, Karmann M, Born C et al. (2010) Altered brain activation during a verbal working memory task in subjects with amnestic mild cognitive impairment. J Alzheimers Dis 21:103–118. doi: 10.3233/JAD-2010-091054 [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259 [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T et al. (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404. doi: 10.1007/s00401-006-0127-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browndyke JN, Giovanello K, Petrella J et al. (2012) Phenotypic regional functional imaging patterns during memory encoding in mild cognitive impairment and Alzheimer’s disease. Alzheimer’s Dement 9:284–294. doi: 10.1016/j.jalz.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Miezin FM, Petersen SE (1996) Functional anatomic studies of memory retrieval for auditory words and visual pictures. J Neurosci 16:6219–6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ et al. (2005) Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L (2002) Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage 16:317–330. doi: 10.1006/nimg.2002.1063 [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129:564–583. doi: 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC et al. (2006) Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J Neurosci 26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément F, Belleville S (2010) Compensation and disease severity on the memory-related activations in mild cognitive impairment. Biol Psychiatry 68:894–902. doi: 10.1016/j.biopsych.2010.02.004 [DOI] [PubMed] [Google Scholar]
- Clément F, Belleville S (2012) Effect of disease severity on neural compensation of item and associative recognition in mild cognitive impairment. J Alzheimers Dis 29:109–123. doi: 10.3233/JAD-2011-110426 [DOI] [PubMed] [Google Scholar]
- Clément F, Belleville S, Mellah S (2010) Functional neuroanatomy of the encoding and retrieval processes of verbal episodic memory in MCI. Cortex 46:1005–1015. doi: 10.1016/j.cortex.2009.07.003 [DOI] [PubMed] [Google Scholar]
- Dannhauser TM, Shergill SS, Stevens T et al. (2008) An fMRI study of verbal episodic memory encoding in amnestic mild cognitive impairment. Cortex 44:869–880. doi: 10.1016/j.cortex.2007.04.005 [DOI] [PubMed] [Google Scholar]
- Dere E, Pause BM, Pietrowsky R (2010) Emotion and episodic memory in neuropsychiatric disorders. Behav Brain Res 215:162–171. doi: 10.1016/j.bbr.2010.03.017 [DOI] [PubMed] [Google Scholar]
- Derflinger S, Sorg C, Gaser C et al. (2011) Grey-matter atrophy in Alzheimer’s disease is asymmetric but not lateralized. J Alzheimers Dis 25:347–357. doi: 10.3233/JAD-2011-110041 [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C et al. (2010) Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol 9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4 [DOI] [PubMed] [Google Scholar]
- Duvernoy H, Cattin F, Naidich T et al. (2005) Sectional anatomy and magnetic resonance imaging. In: Duvernoy HM (ed) Hum. Hippocampus, 3rd edn. Springer, Heidelberg, pp 129–217 [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H et al. (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S et al. (2007) Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36:511–521. doi: 10.1016/j.neuroimage.2007.03.060 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C et al. (2009) Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926. doi: 10.1002/hbm.20718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR et al. (2012) Activation likelihood estimation meta-analysis revisited. Neuroimage 59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa A, Alegret M, Valero S et al. (2013) A longitudinal followup of 550 mild cognitive impairment patients: evidence for large conversion to dementia rates and detection of major risk factors involved. J Alzheimers Dis 34:769–780. doi: 10.3233/JAD-122002 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Frings L, Dressel K, Abel S et al. (2010) Reduced precuneus deactivation during object naming in patients with mild cognitive impairment, Alzheimer’s disease, and frontotemporal lobar degeneration. Dement Gerieatr Cogn 30:334–343. doi: 10.1159/000320991 [DOI] [PubMed] [Google Scholar]
- Giovanello KS, De Brigard F, Hennessey Ford J et al. (2012) Event-related functional magnetic resonance imaging changes during relational retrieval in normal aging and amnestic mild cognitive impairment. J Int Neuropsychol Soc 18:886–897. doi: 10.1017/S1355617712000689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golby A, Silverberg G, Race E et al. (2005) Memory encoding in Alzheimer’s disease: an fMRI study of explicit and implicit memory. Brain 128:773–787. doi: 10.1093/brain/awh400 [DOI] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Brewer JB et al. (2001) Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain 124:1841–1854 [DOI] [PubMed] [Google Scholar]
- Gould RL, Brown RG, Owen AM et al. (2005) Functional neuroanatomy of successful paired associate learning in Alzheimer’s disease. Am J Psychiatry 162:2049–2060 [DOI] [PubMed] [Google Scholar]
- Grady C (2012) The cognitive neuroscience of ageing. Nat Rev Neurosci 13:491–505. doi: 10.1038/nrn3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grön G, Bittner D, Schmitz B et al. (2002) Subjective memory complaints: objective neural markers in patients with Alzheimer’s disease and major depressive disorder. Ann Neurol 51:491–498. doi: 10.1002/ana.10157 [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME (2001) Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2:685–694 [DOI] [PubMed] [Google Scholar]
- Hämäläinen A, Pihlajamäki M, Tanila H et al. (2007) Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging 28:1889–1903. doi: 10.1016/j.neurobiolaging.2006.08.008 [DOI] [PubMed] [Google Scholar]
- Hampstead BM, Stringer AY, Stilla RF et al. (2011) Where did I put that? Patients with amnestic mild cognitive impairment demonstrate widespread reductions in activity during the encoding of ecologically relevant object-location associations. Neuropsychologia 49:2349–2361. doi: 10.1016/j.neuropsychologia.2011.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanseeuw B, Dricot L, Kavec M et al. (2011) Associative encoding deficits in amnestic mild cognitive impairment: a volumetric and functional MRI study. Neuroimage 56:1743–1748. doi: 10.1016/j.neuroimage.2011.03.034 [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Masliah E (2003) Cycles of aberrant synaptic sprouting and neurodegeneration in Alzheimer’s and dementia with Lewy bodies. Neurochem Res 28:1743–1756 [DOI] [PubMed] [Google Scholar]
- Hayes SM, Ryan L, Schnyer DM, Nadel L (2004) An fMRI study of episodic memory: retrieval of object, spatial, and temporal information. Behav Neurosci 118:885–896. doi: 10.1037/0735-7044.118.5.885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heun R, Freymann K, Erb M et al. (2007) Mild cognitive impairment (MCI) and actual retrieval performance affect cerebral activation in the elderly. Neurobiol Aging 28:404–413. doi: 10.1016/j.neurobiolaging.2006.01.012 [DOI] [PubMed] [Google Scholar]
- Huijbers W, Vannini P, Sperling RA et al. (2012) Explaining the encoding/retrieval flip: memory-related deactivations and activations in the posteromedial cortex. Neuropsychologia 50:3764–3774. doi: 10.1016/j.neuropsychologia.2012.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynd MR, Scott HL, Dodd PR (2004) Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem Int 45:583–595. doi: 10.1016/j.neuint.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Klunk WE, Abrahamson EE et al. (2011) Precuneus amyloid burden is associated with reduced cholinergic activity in Alzheimer disease. Neurology 77:39–47. doi: 10.1212/WNL.0b013e3182231419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Pelak VS, Curran T et al. (2012) A preliminary study of functional abnormalities in aMCI subjects during different episodic memory tasks. Magn Reson Imaging 30:459–470. doi: 10.1016/j.mri.2011.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Wager T, Badre DT (2002) Memory, neuroimaging. In: Ramachandran VS (ed) Encycl. Hum. Brain. Elsevier Science, San Diego, pp 797–833 [Google Scholar]
- Jungwirth S, Zehetmayer S, Hinterberger M et al. (2012) The validity of amnestic MCI and non-amnestic MCI at age 75 in the prediction of Alzheimer’s dementia and vascular dementia. Int Psychogeriatr 24:959–966. doi: 10.1017/S1041610211002870 [DOI] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB et al. (1998) Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron 20:927–936 [DOI] [PubMed] [Google Scholar]
- Kircher TT, Weis S, Freymann K et al. (2007) Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J Neurol Neurosurg Psychiatry 78:812–818. doi: 10.1136/jnnp.2006.104877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan N, Breakspear M, Slavin MJ et al. (2010) Functional alterations in brain activation and deactivation in mild cognitive impairment in response to a graded working memory challenge. Dement Geriatr Cogn 30:553–568. doi: 10.1159/000322112 [DOI] [PubMed] [Google Scholar]
- Kühn S, Gallinat J (2013) Segregating cognitive functions within hippocampal formation: a quantitative meta-analysis on spatial navigation and episodic memory. Hum Brain Mapp. doi: 10.1002/hbm.22239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT et al. (2010) A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct 214:519–534. doi: 10.1007/s00429-010-0255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F et al. (2009) ALE meta-analysis workflows via the brainmap database: progress towards a probabilistic functional brain atlas. Front Neuroinform 3:11. doi: 10.3389/neuro.11.023.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim TS, Lee HY, Barton JJS, Moon SY (2011) Deficits in face perception in the amnestic form of mild cognitive impairment. J Neurol Sci 309:123–127. doi: 10.1016/j.jns.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Machulda MM, Senjem ML, Weigand SD et al. (2009) Functional MRI changes in amnestic and non-amnestic MCI during encoding and recognition tasks. J Int Neuropsychol Soc 15:372–382. doi: 10.1017/S1355617709090523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandzia JL, McAndrews MP, Grady CL et al. (2009) Neural correlates of incidental memory in mild cognitive impairment: an fMRI study. Neurobiol Aging 30:717–730. doi: 10.1016/j.neurobiolaging.2007.08.024 [DOI] [PubMed] [Google Scholar]
- McCarthy G, Blamire AM, Rothman DL et al. (1993) Echo-planar magnetic resonance imaging studies of frontal cortex activation during word generation in humans. Proc Natl Acad Sci USA 90:4952–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Gore JC, Allison T (1996) Face-specific processing in the human fusiforrn gyms. J Cogn Neurosci 9:605–610 [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M et al. (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–944 [DOI] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Yaffe K et al. (2010) Hippocampal atrophy patterns in mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp 31:1339–1347. doi: 10.1002/hbm.20934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Kleiman A, Schulz JB et al. (2012) Neuroanatomic changes and their association with cognitive decline in mild cognitive impairment: a meta-analysis. Brain Struct Funct 217:115–125. doi: 10.1007/s00429-011-0333-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, McIntosh AR, Houle S et al. (1996) Activation of medial temporal structures during episodic memory retrieval. Nature 380:715–717. doi: 10.1038/380715a0 [DOI] [PubMed] [Google Scholar]
- O’Brien JL, O’Keefe K, LaViolette PS et al. (2010) Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology 74:1969–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED et al. (2007) Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55:697–711. doi: 10.1016/j.neuron.2007.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariente J, Cole S, Henson R et al. (2005) Alzheimer’s patients engage an alternative network during a memory task. Ann Neurol 58:870–879. doi: 10.1002/ana.20653 [DOI] [PubMed] [Google Scholar]
- Pause BM, Zlomuzica A, Kinugawa K et al. (2013) Perspectives on episodic-like and episodic memory. Front Behav Neurosci 7:33. doi: 10.3389/fnbeh.2013.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters F, Collette F, Degueldre C et al. (2009) The neural correlates of verbal short-term memory in Alzheimer’s disease: an fMRI study. Brain 132:1833–1846. doi: 10.1093/brain/awp075 [DOI] [PubMed] [Google Scholar]
- Petersen RC (2011) Mild cognitive impairment. N Engl J Med 364:2227–2234 [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC et al. (1999) Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56:303–308 [DOI] [PubMed] [Google Scholar]
- Petrella JR, Krishnan S, Slavin MJ et al. (2006) Mild cognitive impairment: evaluation with 4-T functional MR imaging. Radiology 240:177–186 [DOI] [PubMed] [Google Scholar]
- Petrella JR, Prince SE, Wang L et al. (2007) Prognostic value of posteromedial cortex deactivation in mild cognitive impairment. PLoS ONE 2:e1104. doi: 10.1371/journal.pone.0001104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ (2000) The anatomy of language: contributions from functional neuroimaging. J Anat 197:335–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ (2010) The anatomy of language: a review of 100 fMRI studies published in 2009. Ann NY Acad Sci 1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x [DOI] [PubMed] [Google Scholar]
- Rémy F, Mirrashed F, Campbell B, Richter W (2005) Verbal episodic memory impairment in Alzheimer’s disease: a combined structural and functional MRI study. Neuroimage 25:253–266. doi: 10.1016/j.neuroimage.2004.10.045 [DOI] [PubMed] [Google Scholar]
- Rosazza C, Minati L, Ghielmetti F et al. (2009) Engagement of the medial temporal lobe in verbal and nonverbal memory: assessment with functional MR imaging in healthy subjects. Am J Neuroradiol 30:1134–1141. doi: 10.3174/ajnr.A1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi A, Swaab DF (1999) Diminished neuronal metabolic activity in Alzheimer’s disease. Review article. J Neural Transm 106:955–986 [DOI] [PubMed] [Google Scholar]
- Schacter DL, Curran T, Reiman EM et al. (1999) Medial temporal lobe activation during episodic encoding and retrieval: a PET study. Hippocampus 9:575–881. doi: [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA et al. (2013) Synapse stability in the precuneus early in the progression of Alzheimer’s disease. J Alzheimers Dis 35:599–609. doi: 10.3233/JAD-122353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B et al. (2012) Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS ONE 7:e30920. doi: 10.1371/journal.pone.0030920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Stein T, Maslowski N, Neumann J (2009) Neural correlates of Alzheimer’s disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients. Neuroimage 47:1196–1206. doi: 10.1016/j.neuroimage.2009.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt GC, Black SE (2009) Functional imaging studies of episodic memory in Alzheimer’s disease: a quantitative meta-analysis. Neuroimage 45:181–190. doi: 10.1016/j.neuroimage.2008.11.024 [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B (2000) Loss of recent memory after bilateral hippocampal lesions. J Neuropsych Clin Neurosci 12:103–113 [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB et al. (2011) A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 12:585–601. doi: 10.1038/nrn3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN et al. (2009) Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia 47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028 [DOI] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF et al. (2003) fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry 74:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O’Keefe K et al. (2009) Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 63:178–188. doi: 10.1016/j.neuron.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE (2004) The medial temporal lobe. Annu Rev Neurosci 27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130 [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR (2001) When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci USA 98:12760–12766. doi: 10.1073/pnas.221462998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MA, Murphy CM, Goetz C et al. (2008) fMRI activation changes during successful episodic memory encoding and recognition in amnestic mild cognitive impairment relative to cognitively healthy older adults. Dement Geriatr Cogn Disord 26:123–137. doi: 10.1159/000148190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E (1972) Episodic and semantic memory. In: Tulving E (ed) Organ. Mem Academic Press, New York, pp 381–403 [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002) Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16:765–780. doi: 10.1006/nimg.2002.1131 [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR et al. (2012) Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp 33:1–13. doi: 10.1002/hbm.21186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meulen M, Lederrey C, Rieger SW et al. (2012) Associative and semantic memory deficits in amnestic mild cognitive impairment as revealed by functional magnetic resonance imaging. Cogn Behav Neurol 25:195–215 [DOI] [PubMed] [Google Scholar]
- Wang X, Han Z, He Y et al. (2013) Where color rests: spontaneous brain activity of bilateral fusiform and lingual regions predicts object color knowledge performance. Neuroimage 76:252–263. doi: 10.1016/j.neuroimage.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Welsh K, Butters N, Hughes J et al. (1991) Detection of abnormal memory decline in mild cases of Alzheimer’s disease using CERAD neuropsychological measures. Arch Neurol Chicago 48:278–281 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


