Abstract
Purpose of Review
Intracranial atherosclerosis (ICAS) is the most common cause of stroke throughout the world. It also increases the risk of recurrent stroke and dementia. As a complex and multifactorial disease, ICAS is influenced by multiple genetic, biological, and environmental factors. This review summarizes the candidate gene and genome-wide studies aimed at discovering genetic risk factors of ICAS.
Recent Findings
Numerous studies have focused on the association between single nucleotide polymorphisms (SNPs) of atherosclerosis-related genes and the risk of ICAS. Variants in adiponectin Q (ADIPOQ), ring finger protein 213 (RNF213), apolipoprotein E (APOE), phosphodiesterase 4D (PDE4D), methylenetetrahydrofolate reductase (MTHFR), lipoprotein lipase (LPL), α-adducin (ADD1) genes, angiotensin-converting enzyme (ACE), as well as other genes related to renin-angiotensin-aldosterone system have been associated with ICAS.
Summary
We review the available evidences on the candidate genes and SNPs associated with genetic susceptibility to ICAS, and point out future developments of this field. Genetic discoveries could have clinical implications for intracranial atherosclerotic disease.
Keywords: Intracranial atherosclerosis, Genetic factor, Genome-wide association study, Adiponectin Q, Ring finger protein 213
Introduction
Intracranial atherosclerosis (ICAS) is the most common cause of stroke in the world and a common risk factor for dementia [1–4]. ICAS is a progressive disease characterized chiefly by the accumulation of lipids and cellular and fibrous elements in the large arteries, leading to changes ranging from eccentric wall thickening and substenotic plaques to hemodynamically significant luminal narrowing (Figure 1) [5–7]. ICAS can occur in isolation, or as part of coexistent atherosclerosis in systemic arteries such as the aorta, extracranial carotids, coronary, or lower extremity arteries [8]. The most common site of ICAS is the middle cerebral artery, followed by the basilar artery, internal carotid artery, and intracranial vertebral artery [9]. In addition, ICAS may coexist with other potential stroke etiologies, e.g. small vessel disease, in which ICAS confers a worse prognosis [10].
Figure 1. Pathological example of intracranial large artery atherosclerosis, a cholesterol-mediated disease.
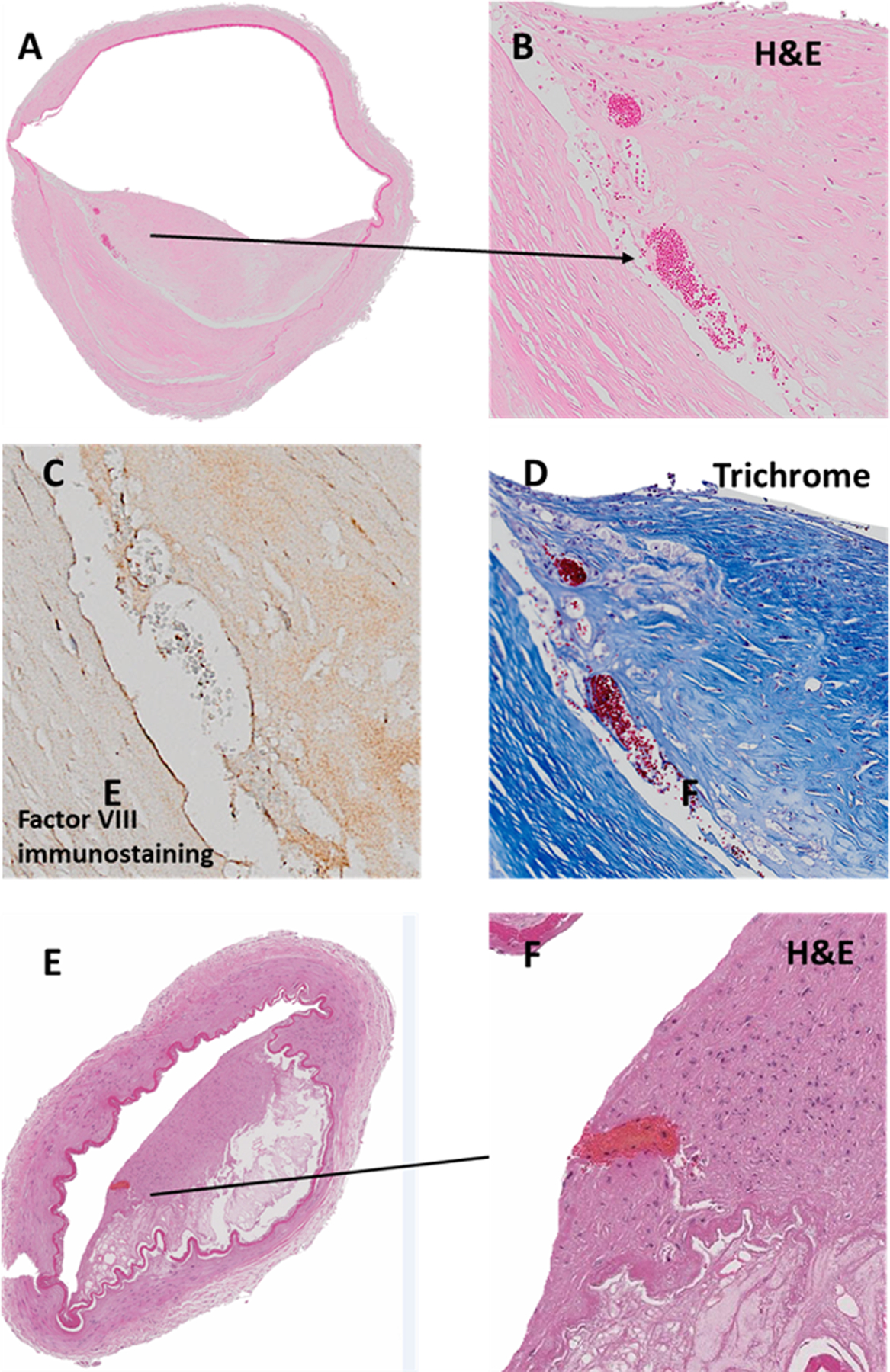
Panel A demonstrates a typical example of atherosclerosis, defined by eccentric intima thickening with a necrotic core consisting of cholesterol crystals and necrotic tissue (panel 1). One may observe neoangiogenesis in the border zone between the media and the intima (panel b-c). One of the proposed mechanisms by which atherosclerosis may cause stroke is plaque rupture, with endothelial surface denudation, thrombosis and eventual artery-to-artery embolization or luminal occlusion due to an expanding in-situ thrombus (panel d-c). Images were obtained from the Brain Arterial Remodeling Study (BARS).
The most common risk factors for ICAS include age, hypertension, diabetes mellitus, and dyslipidemia [4, 11–14]. The prevalence of ICAS has been reported higher in women, particularly in women older than 63 years compared with men within the same age group [15]. Several studies suggest that race/ethnicity is a predisposing factor, especially in combination with acquired risk factors, such as lifestyle [1, 11, 13, 16–18]. Individuals of Hispanic [11, 16, 17], African-American [11, 16–18], and Asian (Chinese, Japanese, and Korean descent) ethnic backgrounds [12, 19–22] have a significantly higher incidence and prevalence of ICAS compare to non-Hispanic whites. The difference in the incidence of ICAS among different ethnicities could be caused by differences in certain genetic predisposition or morphological characteristics of the cerebral arteries [23]. Dyslipidemia, a risk factor that are linked to coronary atherosclerosis and myocardial infarction, is also associated with ICAS [24, 25]. Smoking has been associated with ICAS [4, 26], but not consistently across studies [27]. The variability in results may be due to heterogeneous populations and methods used to assess the outcome.
Certain genetic traits are associated with ICAS, either by predisposing to vascular risks such as hypertension or diabetes mellitus, or by a direct contribution to an established atherosclerosis mechanism [28, 29]. Genome-wide association studies (GWAS) have provided new approaches for detecting novel variants and gene loci without a specific hypothesis implicating a particular molecular pathway. In this article, we review genetic traits associated with ICAS, summarize candidate gene and genome-wide studies aimed at discovering and identifying genetic risk factors of ICAS and subclinical phenotypes, and outline the future developments and challenges of ICAS genetic research.
Search strategy
We performed a MEDLINE/PubMed literature search using the MeSH term “intracranial arterial diseases” or “intracranial atherosclerosis” or “intracranial stenosis” or “cerebral atherosclerosis”. Articles published in English before October 2019 were screened for relevance to the genetic risk factor of ICAS. Studies of ≥200 participants were included. We selected studies with ICAS-related phenotypes represented as stroke caused by ICAS or studies related to ICAS physiopathology. The key findings are summarized in Table 1.
Table 1.
Genetic factors associated with ICAS.
| Gene | Gene function | Polymorphism | Study population | Sample size | Main finding | References |
|---|---|---|---|---|---|---|
| ADIPOQ | Regulate adiponectin level | g.15734A>G | Chinese | 602 (199 ICAS / 403 controls) | Increased risk of ICAS | [36] |
| American | 2847 | Related to carotid and coronary atherosclerosis in African Americans | [35] | |||
| Chinese | 2212 (1105 T2DM /1107 controls) | Closely related to hypoadiponectinemia | [42] | |||
| g.5320G>A | American | 1200 (600 MI or IS / 600 controls) | Reduced of cerebral infarction risk level | [37] | ||
| Chinese | 476 | Elevated plasma adrenomedullin levels | [43] | |||
| RNF213 | Result in vascular fragility, lead to vessels more vulnerable to hemodynamic stress | Japanese | 433 | Significant association with ICAS | [47] | |
| Japanese | 221 | Associated with anterior ICAS | [48] | |||
| c.14429G>A | Korean | 532 | Associated with vasculogenesis | [53] | ||
| APOE | Associate with high LDL cholesterol level | ɛ4 | Finnish | 700 | Associated with larger coronary and aortic atherosclerotic lesion areas in men | [58] |
| American | 720 | [59] | ||||
| Finnish | 536 (237 IS / 362 controls) | There was a link with ICAS in men | [60] | |||
| Thai | 308 | Associated with extracranial atherosclerosis | [61] | |||
| PDE4D | Has a major role in the degradation of cAMP | g.319027C>T | Indian | 336 | TT genotype was associated with ICAS | [72] |
| MTHFR | Reduce enzyme activity, decrease folate level and increase Hcy | g.14783C>T | Chinese | 929 | This variant and Hcy had interaction in the formation of cerebral atherosclerosis | [78] |
| Korean | 463 (267 atherosclerosis / 196 controls) | Hcy level and this variant did not contribute to the distribution of cerebral atherosclerosis | [79] | |||
| LPL | Involve in plasma lipoprotein metabolism and transportation | g.27496T>G | Indian | 1025 (525 IS / 500 controls) | Associated with intracranial large artery atherosclerosis | [81] |
| p.Ser447Stop | Japanese | 354 (177 CVD / 177 controls) | Reduced risk of atherothrombotic cerebral infarction | [80] | ||
| g.23608C>T, p.Ser447Ter | Chinese | 371 (185 CI / 186 controls) | Closely associated with atherothrombotic cerebral infarction | [82] | ||
| ADD1 | Associate with blood pressure levels | p.Gly460Trp | Dutch | 6471 and 1018 | Associated with atherosclerosis | [88] |
| Indian | 339 | No significant difference between ICAS and extracranial atherosclerosis | [89] | |||
| ACE | Take part in vascular remodeling and the development of atherosclerosis | Insertion/Deletion | Thai | 308 | No association between this polymorphism and ICAS | [61] |
| Chinese | 210 | [94] | ||||
| Chinese | 708 | [95] | ||||
| CYP11B2 | Involve in the aldosterone system | g.4660T>C | Indian | 797 (403 stroke / 394 controls) | Associated with intracranial large artery atherosclerosis | [98] |
CI: cerebral infarction; CVD: cerebrovascular disease; ICAS: intracranial atherosclerosis; IS: ischemic stroke; MI: myocardial infarction; T2DM: type 2 diabetes mellitus.
ADIPOQ gene
Adiponectin Q (ADIPOQ) gene regulates a variety of metabolic processes and helps inhibit the biochemical pathways that lead to metabolic syndrome. It contributes to lipids and glucose regulation, with anti-inflammatory and anti-atherogenic effect [30–33]. ADIPOQ SNPs are likely to contribute to metabolic disorders, and consequently influence atherosclerosis [34]. The g.15734A>G genotypes are associated with carotid and coronary atherosclerosis in African Americans [35] and ICAS in Chinese [36], while g.5320G>A SNP is associated with reduced risk of cerbral infaction in Caucasian [37]. Among 602 subjects (199 cases with ICAS and 403 controls) included in the Chinese study [36], authors genotyped 10 selected tag ADIPOQ SNPs associated with adiponectin levels, atherosclerosis or cardiovascular events. After adjusting for conventional vascular risks, there was a modest association with ICAS in participants with the g.15734A>G AG/AA genotype (OR = 2.2, 95% CI: 1.1–4.9, p = 0.040) and the AG/GG genotype of the g.5320G>A (OR = 1.8, 95% CI: 1.1–2.9, p = 0.017). Moreover, the haploid analysis results indicated that the A-G haplotype prevalence of the g.15734A>G and g.5320G>A was higher in the ICAS group than in the control group (p = 0.026).
The g.5320G>A and g.15734A>G belong to the first and second intron of ADIPOQ gene, respectively, and are closely associated with reduction of adiponectin levels. Previous clinical studies suggested the ADIPOQ g.5320G>A was closely associated with reduced adiponectin levels in Asian, African and Caucasian populations [38–41]. Du et al. performed a case-control study using 1105 patients with type 2 diabetes mellitus and 1107 control subjects, and found patients with genotype AG/GG of g.15734A>G had lower levels of serum adiponectin than those with the genotype AA (p = 0.044), indicating g.15734A>G may be closely related to hypoadiponectinemia in the Chinese population [42]. Therefore, these ADIPOQ SNPs may play a role in proatherosclerotic blood signals mediated by adiponectin or adrenomedullin [43]. However, the findings require testing for replication and validation in larger sample size studies and experimentation. It is uncertain whether modulating these pathways may recue or modify the risk of ICAS and ICAS-related vascular events.
RNF213 gene
Ring finger protein 213 (RNF213) encodes a protein containing 5256 amino acids, containing a C3HC4-type RING (Really Interesting New Gene) finger domain and an AAA (Adenosine triphosphatase Associated with various cellular Activities) domain, which are involved in mediating protein-protein interactions and ATPase activity, respectively [44, 45]. RNF213 genetic variants may result in arterial fragility and susceptibility to hemodynamic stress, which may increase the risk of ICAS [46].
RNF213 was initially identified as a susceptibility gene for moyamoya disease (MMD) in a community‐based GWAS [45, 44]. In Japan, Miyawaki et al. compared the prevalence of c.14576G>A variant in each phenotype group (definite MMD, unilateral MMD, ICAS not diagnosed as MMD, extracranial carotid atherosclerosis, cerebral aneurysm, or intracerebral hemorrhage) with control subjects without cerebrovascular disease [47]. The authors found that the c.14576G>A variant was associated with definite MMD (OR=144.0, 95% CI: 26.7–775.9, p < 0.001), unilateral MMD (OR=54.0, 95% CI: 7.5–386.8, p < 0.001), and non-MMD ICAS (OR=16.8, 95% CI: 3.8–74.5, p <0.001) compared to controls without cerebrovascular disease. They also investigated whether c.14576G>A variant related to anterior or posterior circulation ICAS [48]. The investigators found an association with anterior circulation ICAS (OR = 14.8, 95% CI: 3.1–71.3, p < 0.001) but no association with posterior circulation ICAS. Studies in Korea and Japan found about one fifth of non-MMD ICAS patients carry RNF213 c.14576G>A variant [47, 49, 50]. It was estimated that about 16 million people carry this genetic polymorphism in East Asian countries [51]. Although the role of RNF213 c.14576G>A variants has mostly been studied in relationship to ICAS, another variants c.14429G>A may also play a role in vasculogenesis [52, 53].
APOE gene
Apolipoprotein E (APOE) is the principal cholesterol carrier in the brain [54]. It is mainly produced by astrocytes, and transports cholesterol to neurons through ApoE receptors [55]. There are six well-known common APOE genotypes (ɛ22, ɛ32, ɛ33, ɛ42, ɛ43, and ɛ44), which are generated by a combination of two genetic variants (g.7903T>C and g.8041C>T). APOE ɛ4 is associated with high LDL cholesterol level, which is an important factor in the early stage of atherosclerosis [56], and might interact with other risk factors to affect lipid metabolism and cellular repair mechanism [57]. Autopsy studies in men reveal the APOE ɛ4+ genotype is associated with larger coronary and aortic atherosclerotic lesion areas [58, 59]. Separately, another group of investigators tested whether APOE ɛ4 genetic variants related to atherosclerosis. Men with APOE ɛ4 allele had a higher prevalence of ICAS, but the association was not found in women [60]. The association between APOE ɛ4 allele and ICAS is conflicting, however, Chutinet et al. reported APOE ɛ4 allele was associated with extracranial atherosclerosis (ECAS) (OR=2.8, 95% CI:1.3–5.9, p < 0.05) but not with ICAS (OR=1.2, 95% CI: 0.5–2.7) [61]. Given the role of APOE ɛ4 in Alzheimer dementia [62], and the fact that Alzheimer dementia is associated with ICAS [63, 64], it is uncertain whether the association of APOE ɛ4 with arterial disease may contribute partially or have a role in the increased risk of dementia attributed to APOE ɛ4, independent of its effect in amyloid pathways.
PDE4D gene
Phosphodiesterase 4D (PDE4D) variants are associated with ischemic stroke, carotid atherosclerosis and coronary artery disease [65, 66], but it is less certain if PDE4D variants relate to ICAS. PDE4D encodes cyclic adenosine monophosphate (cAMP) -specific 3′,5′-cyclic phosphodiesterase 4D, which has a major role in the degradation of cAMP [67]. The proliferation and migration of vascular smooth muscle cells and macrophages, regulated partially by cAMP, is a crucial early stage in the development of atherosclerosis [68–71]. PDE4D TT genotype frequency of g.319027C>T polymorphism in ICAS patients was significantly higher than that in non-ICAS patients (20 vs 2%, p = 0.01) [72]. Furthermore, variant in the PDE4D gene may not only relate to the prevalence of ICAS, but it may also relate to the severity of the disease as suggested by a report of worse outcomes after ICAS-related stroke. It is uncertain how variant in PDE4D gene may alter outcome, but understanding these mechanisms may open opportunities for decreasing the risk of events related to ICAS.
MTHFR gene
Methylenetetrahydrofolate reductase (MTHFR) is the key enzyme for the metabolism of circulating homocysteine (Hcy), which have a critical role in the regulation of cell signaling and homeostasis [73]. MTHFR g.14783C>T, a common variant leading to a reduction in enzyme activity that results in decreased folate level and increased Hcy level, has been associated with increased risk of stroke [74–76]. MTHFR g.14783C>T polymorphism is associated with atherosclerosis by regulating genome methylation level [77]. For example, Hcy concentration is associated with ICAS and ECAS as demonstrated in a sample of 929 hypertensive patients without stroke in China. However, this association was modified by the g.14783C>T genotype, suggesting that there was an interaction between g.14783C>T genotype and Hcy in cerebral atherosclerosis [78]. Another study evaluated the effect of Hcy level and g.14783C>T polymorphism on determining the intracranial and extracranial locations of atherosclerosis, suggesting Hcy level and MTHFR g.14783C>T was not associated with intracranial as oppose to extracranial atherosclerosis [79].
LPL gene
Lipoprotein lipase (LPL) has an essential role in plasma lipoprotein metabolism and transportation. Several studies have reported a relationship between g.27496T>G, g.23608C>T, p.Ser447Ter polymorphisms of LPL and cerebrovascular diseases [80–82]. In a case-control study of 1025 subjects (525 ischemic stroke patients and 500 control cases), the g.27496T>G variant of LPL gene was associated with ICAS in Indian population (OR=3.7, 95% CI: 1.9–7.2, p < 0.001) [81]. In another case-control study carried out in Japan, p.Ser447Stop polymorphism was associated with intracranial large artery atherosclerosis-related stroke [80]. Moreover, Xu et al. indicated that PvuII and p.Ser447Ter polymorphisms, were closely associated with atherothrombotic cerebral infarction in Chinese population [82]. A meta-analysis of 4681 ischemic stroke cases and 8516 controls from 13 studies found that the p.Ser447Ter polymorphism was more important in association with the reduced risk for ischemic stroke (OR = 0.79, 95% CI: 0.68–0.93, p = 0.005), especially atherosclerotic stroke (OR = 0.44, 95% CI: 0.32–0.62, p < 0.001), indicating p.Ser447Ter might be the protective factors for atherosclerotic stroke [83].
ADD1 gene
Adducin is a cytoskeletal protein consisting of an α- and a β-subunit. The α-adducin (ADD1) p.Gly460Trp polymorphism has been associated with blood pressure levels, salt sensitivity and the risk factor for cardiovascular events [84–87]. van Rijn et al. studied the p.Gly460Trp polymorphism in relation to atherosclerosis, myocardial infarction, and cerebrovascular disease within two cohorts, involving in 6471 and 1018 subjects respectively, revealing this ADD1 polymorphism was significantly associated with atherosclerosis, but data related to ICAS was not reported in this study [88]. Other investigators evaluated the relationship between ADD1 gene variants and ICAS versus ECAS. But according to further comparison of this variant’s effect in ICAS and ECAS, there was no significant difference between ICAS and ECAS [89].
ACE gene
Angiotensin-converting enzyme (ACE) is a major component of renin-angiotensin system, which takes part in vascular remodeling and the development of atherosclerosis [90]. Previous evidence suggests that ACE is an important candidate gene for hereditary susceptibility to vascular diseases [91, 92]. Several studies provided evidence of the association between the ACE insertion/deletion (I/D) polymorphism and stroke risk [93]. The linkage between ACE I/D polymorphism and ICAS has been studies before, but no association between I/D polymorphism and ICAS has been found. [61, 94, 95].
RAAS related genes
The renin-angiotensin-aldosterone system (RAAS) regulates blood pressure, sodium and water balance, and cardiovascular and renal homeostasis. RAAS related genes role in these processes relate them to atherosclerosis [96, 97]. Polymorphisms of RAAS related genes have high frequency in Asians. Among these genes, g.4660T>C polymorphism of aldosterone synthase (CYP11B2) was associated with ICAS in a case-control study using 403 stroke patients and 394 sex and age matched controls in India (OR=3.0, 95% CI: 1.5–5.9, p < 0.001) [98]. A case-control study of 332 patients and 250 controls investigated the association of CYP11B2 polymorphism genotype with ischemic stroke and subtypes in Chinese Han population, suggesting TT genotype of g.4660T>C polymorphism has association with ischemic stroke (OR = 1.6, 95% CI: 1.1–2.3; p = 0.014), large artery atherosclerosis (OR = 1.7, 95% CI: 1.2–2.6, p = 0.005) and small vessel disease (OR = 1.7, 95% CI: 1.1–2.7, p = 0.012) [99]. Since genetic variations in CYP11B2 gene are associated with the progression of carotid atherosclerotic plaque size, CYP11B2 polymorphism might act through carotid atherosclerosis to enhance susceptibility to the large artery atherosclerosis [100].
Shared genetic variants between large artery atherosclerosis and small vessel disease
Large artery atherosclerosis (LAA) and small vessel disease (SVD) are major subtypes of ischemic stroke. Several studies have shown a relationship between SVD and LAA [101–103]. For example, treatments for LAA (e.g. antihypertensive therapy) are also effective in SVD patients [104]. Recent studies discovered common variants shared between LAA and SVD. A meta-analysis of combined LAA and SVD phenotype was performed to detect the shared genetic SNPs, observing three SNPs (g.153898623T>A, g.153899292A>T and g.153900493C>A) at chromosome 6q25.2, ~100kb upstream of the opioid receptor μ1 (OPRM1) gene [105]. Previous study had shown that the OPRM1 gene may be associated with coronary heart disease, which commonly has an atherosclerotic etiology [106]. Another case-control study in China identified the association of two tumor necrosis factor super family member 4 (TNFSF4) SNPs, g.15225T>C and g.4236G>A, with the risk of LAA and SVD [107]. TNFSF4 encodes the costimulatory molecule, OX40 ligand (OX40L), which is involved in T cell activation and the formation of atherosclerosis [108, 109]. In addition, TNFSF4 expression is associated with an increased risk of atherosclerosis [110]. However, the biological mechanisms of these SNPs are still not clear, and more attention and targeted efforts are needed to better define the clinical significance of these SNPs.
Future developments
As a complex, multifactorial disease that is influenced by multiple pathophysiologic, genetic and environmental factors, ICAS does not have a single genetics cause, but is likely associated with the effects of multiple genes (polygenic) in combination with lifestyle and environmental factors. Environmental factors such as dietary habits have a significant role in development of atherosclerosis, while genetic factors represent consequential ICAS determinants [111]. GWAS use large case-control cohorts, including familial and sporadic cases, categorical trait definitions and up to half a million common SNPs, which could provide identification of novel genomic associations with complex diseases. Even though GWAS has initial successes, the variance of interpretation for these genetic associations is limited. The complexity of genes makes it possible to have thousands of unknown variants, each of which may provide a modest contribution to disease.
With the development of life science and technology, research on the analysis and detection of disease-related genes has advanced to clinical levels and provides a scientific basis for diagnosis and differential diagnosis of diseases. The novel technologies could help identify multiple causal variants as well as rare variants [112]. Advanced imaging technologies and genetic data can improve diagnostic accuracy and distinguish cerebral atherosclerotic from non-atherosclerotic intracranial diseases. After diagnosis, prospective genetic studies could be performed in patients with diverse ethnicities, which will facilitate the understanding of different ICAS phenotypes across ethnic and racial groups. Future studies should focus also on sample size and the synergistic effect of multiple ICAS candidate genes to further elucidate the pathogenesis of ICAS-related gene polymorphisms and cerebrovascular disease, and to provide target sites for gene techniques. The comprehensive understanding of the pathogenesis of ICAS is essential for preventing and treating it accordingly.
The next challenge is how to translate the genetic information into an improved therapeutic approach to decrease ICAS-related events. A first step may be variant assessment. It is necessary to determine variants of the reported ICAS phenotypes and how these variants affect the risk of ICAS-related events, especially because different types of variants in the same gene may be associated with distinct phenotypes or inheritance patterns [113]. The studies presented here use various definitions of ICAS, and homogenizing the phenotype and methods may allow for larger meta-analyses and reproducibility of the findings. The advent of high-resolution intracranial wall imaging to evaluate eccentric intima thickening or for a necrotic core among people with ICAS may prove valuable to increase the specificity of the atherosclerotic phenotype and distinguish atherosclerosis from other non-atherosclerotic phenotypes that may cause arterial stenosis.
Conclusions
We present an overview of the current genetic knowledge of ICAS, as it relates to variants related to ADIPOQ, RNF213, APOE, PDE4D, MTHFR, LPL, ADD1, ACE, and RAAS genes. The results discussed here should be contextualized in the setting of limitation related to the data reviewed. Most of clinical studies are cross-sectional, and therefore the quality and quantity of acquired risk factors might not be adequately studied. In addition, due to the heterogeneity in risk factor definitions, diagnostic methods, and characteristics of study subjects (such as ethnicity) or cerebral vessels (such as middle cerebral artery vs. anterior cerebral artery; asymptomatic vs. symptomatic), admixture of non-atherosclerotic intracranial arterial diseases may be partly responsible for the heterogeneity of the results. Additionally, ICAS is a slow and complex multifocal arterial disease. Consequently, polygenic interactions might not be readily apparent in a study of individual genes. Nonetheless, this review provides clues to gain a deeper understanding of the genetic aspects of ICAS, and future paths to continuing gaining understanding of one of the most common causes of stroke in the world.
Footnotes
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
DISCLOSSURES:
NIA R01 5R01AG057709-02
REFERENCES
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Ritz K, Denswil NP, Stam OC, van Lieshout JJ, Daemen MJ. Cause and mechanisms of intracranial atherosclerosis. Circulation. 2014;130(16):1407–14. doi: 10.1161/CIRCULATIONAHA.114.011147. [DOI] [PubMed] [Google Scholar]
- 2.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39(8):2396–9. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 3.Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB et al. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012;135(Pt 12):3749–56. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos D, van der Rijk MJ, Geeraedts TE, Hofman A, Krestin GP, Witteman JC et al. Intracranial carotid artery atherosclerosis: prevalence and risk factors in the general population. Stroke. 2012;43(7):1878–84. doi: 10.1161/STROKEAHA.111.648667. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet (London, England). 2014;383(9921):984–98. doi: 10.1016/s0140-6736(13)61088-0. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez J, Elkind MS, Virmani R, Goldman J, Honig L, Morgello S et al. A pathological perspective on the natural history of cerebral atherosclerosis. Int J Stroke. 2015;10(7):1074–80. doi: 10.1111/ijs.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro SD, Goldman J, Morgello S, Honig L, Elkind MSV, Marshall RS et al. Pathological correlates of brain arterial calcifications. Cardiovasc Pathol. 2019;38:7–13. doi: 10.1016/j.carpath.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee C, Chimowitz MI. Stroke Caused by Atherosclerosis of the Major Intracranial Arteries. Circ Res. 2017;120(3):502–13. doi: 10.1161/CIRCRESAHA.116.308441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. The New England journal of medicine. 2011;365(11):993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshino T, Sissani L, Labreuche J, Ducrocq G, Lavallee PC, Meseguer E et al. Prevalence of Systemic Atherosclerosis Burdens and Overlapping Stroke Etiologies and Their Associations With Long-term Vascular Prognosis in Stroke With Intracranial Atherosclerotic Disease. JAMA neurology. 2018;75(2):203–11. doi: 10.1001/jamaneurol.2017.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rincon F, Sacco RL, Kranwinkel G, Xu Q, Paik MC, Boden-Albala B et al. Incidence and risk factors of intracranial atherosclerotic stroke: the Northern Manhattan Stroke Study. Cerebrovascular diseases (Basel, Switzerland). 2009;28(1):65–71. doi: 10.1159/000219299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bang OY, Kim JW, Lee JH, Lee MA, Lee PH, Joo IS et al. Association of the metabolic syndrome with intracranial atherosclerotic stroke. Neurology. 2005;65(2):296–8. doi: 10.1212/01.wnl.0000168862.09764.9f. [DOI] [PubMed] [Google Scholar]
- 13.Sacco RL, Kargman DE, Zamanillo MC. Race-ethnic differences in stroke risk factors among hospitalized patients with cerebral infarction: the Northern Manhattan Stroke Study. Neurology. 1995;45(4):659–63. doi: 10.1212/wnl.45.4.659. [DOI] [PubMed] [Google Scholar]
- 14.Kim JS, Nah HW, Park SM, Kim SK, Cho KH, Lee J et al. Risk factors and stroke mechanisms in atherosclerotic stroke: intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke. 2012;43(12):3313–8. doi: 10.1161/strokeaha.112.658500. [DOI] [PubMed] [Google Scholar]
- 15.Pu Y, Liu L, Wang Y, Zou X, Pan Y, Soo Y et al. Geographic and sex difference in the distribution of intracranial atherosclerosis in China. Stroke. 2013;44(8):2109–14. doi: 10.1161/strokeaha.113.001522. [DOI] [PubMed] [Google Scholar]
- 16.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26(1):14–20. [DOI] [PubMed] [Google Scholar]
- 17.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111(10):1327–31. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 18.Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27(11):1974–80. [DOI] [PubMed] [Google Scholar]
- 19.•.Cai B, Peng B. Intracranial artery stenosis: Current status of evaluation and treatment in China. Chronic diseases and translational medicine. 2017;3(4):197–206. doi: 10.1016/j.cdtm.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Current evaluation and treatment on intracranial artery stenosis.
- 20.Feldmann E, Daneault N, Kwan E, Ho KJ, Pessin MS, Langenberg P et al. Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology. 1990;40(10):1541–5. doi: 10.1212/wnl.40.10.1540. [DOI] [PubMed] [Google Scholar]
- 21.Gorelick P, Wong KS, Liu L. Epidemiology. Frontiers of neurology and neuroscience. 2016;40:34–46. doi: 10.1159/000448272. [DOI] [PubMed] [Google Scholar]
- 22.Uehara T, Tabuchi M, Mori E. Frequency and clinical correlates of occlusive lesions of cerebral arteries in Japanese patients without stroke. Evaluation by MR angiography. Cerebrovascular diseases (Basel, Switzerland). 1998;8(5):267–72. doi: 10.1159/000015864. [DOI] [PubMed] [Google Scholar]
- 23.Kim JS, Kang DW, Kwon SU. Intracranial atherosclerosis: incidence, diagnosis and treatment. Journal of clinical neurology (Seoul, Korea). 2005;1(1):1–7. doi: 10.3988/jcn.2005.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JH, Hong KS, Lee EJ, Lee J, Kim DE. High levels of apolipoprotein B/AI ratio are associated with intracranial atherosclerotic stenosis. Stroke. 2011;42(11):3040–6. doi: 10.1161/strokeaha.111.620104. [DOI] [PubMed] [Google Scholar]
- 25.Qian Y, Pu Y, Liu L, Wang DZ, Zhao X, Wang C et al. Low HDL-C level is associated with the development of intracranial artery stenosis: analysis from the Chinese IntraCranial AtheroSclerosis (CICAS) study. PLoS One. 2013;8(5):e64395. doi: 10.1371/journal.pone.0064395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingall TJ, Homer D, Baker HL Jr., Kottke BA, O’Fallon WM, Whisnant JP. Predictors of intracranial carotid artery atherosclerosis. Duration of cigarette smoking and hypertension are more powerful than serum lipid levels. Archives of neurology. 1991;48(7):687–91. doi: 10.1001/archneur.1991.00530190033011. [DOI] [PubMed] [Google Scholar]
- 27.Ji R, Pan Y, Yan H, Zhang R, Liu G, Wang P et al. Current smoking is associated with extracranial carotid atherosclerotic stenosis but not with intracranial large artery disease. BMC neurology. 2017;17(1):120. doi: 10.1186/s12883-017-0873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jerrard-Dunne P, Sitzer M, Risley P, Buehler A, von Kegler S, Markus HS. Inflammatory gene load is associated with enhanced inflammation and early carotid atherosclerosis in smokers. Stroke. 2004;35(11):2438–43. doi: 10.1161/01.STR.0000144681.46696.b3. [DOI] [PubMed] [Google Scholar]
- 29.Marteau JB, Zaiou M, Siest G, Visvikis-Siest S. Genetic determinants of blood pressure regulation. J Hypertens. 2005;23(12):2127–43. doi: 10.1097/01.hjh.0000186024.12364.2e. [DOI] [PubMed] [Google Scholar]
- 30.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–69. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 31.Zhao T, Zhao J. Genetic effects of adiponectin on blood lipids and blood pressure. Clinical endocrinology. 2011;74(2):214–22. doi: 10.1111/j.1365-2265.2010.03902.x. [DOI] [PubMed] [Google Scholar]
- 32.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J et al. Disruption of adiponectin causes insulin resistance and neointimal formation. The Journal of biological chemistry. 2002;277(29):25863–6. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 33.Nawrocki AR, Scherer PE. The delicate balance between fat and muscle: adipokines in metabolic disease and musculoskeletal inflammation. Current opinion in pharmacology. 2004;4(3):281–9. doi: 10.1016/j.coph.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Muiya N, Al-Najai M, Tahir AI, Elhawari S, Gueco D, Andres E et al. The 3’-UTR of the adiponectin Q gene harbours susceptibility loci for atherosclerosis and its metabolic risk traits. BMC Med Genet. 2013;14:127. doi: 10.1186/1471-2350-14-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wassel CL, Pankow JS, Rasmussen-Torvik LJ, Li N, Taylor KD, Guo X et al. Associations of SNPs in ADIPOQ and subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis (MESA). Obesity (Silver Spring). 2011;19(4):840–7. doi: 10.1038/oby.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.••.Cui M, Zhou S, Li R, Yin Z, Yu M, Zhou H. Association of ADIPOQ single nucleotide polymorphisms with the risk of intracranial atherosclerosis. Int J Neurosci. 2017;127(5):427–32. doi: 10.1080/00207454.2016.1190716. [DOI] [PubMed] [Google Scholar]; A recently study revealed the association of ADIPOQ SNPs with the risk of intracranial atherosclerosis.
- 37.Hegener HH, Lee IM, Cook NR, Ridker PM, Zee RY. Association of adiponectin gene variations with risk of incident myocardial infarction and ischemic stroke: a nested case-control study. Clin Chem. 2006;52(11):2021–7. doi: 10.1373/clinchem.2006.074476. [DOI] [PubMed] [Google Scholar]
- 38.Tong G, Wang N, Leng J, Tong X, Shen Y, Yang J et al. Common variants in adiponectin gene are associated with coronary artery disease and angiographical severity of coronary atherosclerosis in type 2 diabetes. Cardiovascular diabetology. 2013;12:67. doi: 10.1186/1475-2840-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foucan L, Maimaitiming S, Larifla L, Hedreville S, Deloumeaux J, Joannes MO et al. Adiponectin gene variants, adiponectin isoforms and cardiometabolic risk in type 2 diabetic patients. J Diabetes Investig. 2014;5(2):192–8. doi: 10.1111/jdi.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ong KL, Li M, Tso AW, Xu A, Cherny SS, Sham PC et al. Association of genetic variants in the adiponectin gene with adiponectin level and hypertension in Hong Kong Chinese. Eur J Endocrinol. 2010;163(2):251–7. doi: 10.1530/EJE-10-0251. [DOI] [PubMed] [Google Scholar]
- 41.Wassel CL, Pankow JS, Jacobs DR Jr., Steffes MW, Li N, Schreiner PJ. Variants in the adiponectin gene and serum adiponectin: the Coronary Artery Development in Young Adults (CARDIA) Study. Obesity (Silver Spring). 2010;18(12):2333–8. doi: 10.1038/oby.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du W, Li Q, Lu Y, Yu X, Ye X, Gao Y et al. Genetic variants in ADIPOQ gene and the risk of type 2 diabetes: a case-control study of Chinese Han population. Endocrine. 2011;40(3):413–22. doi: 10.1007/s12020-011-9488-8. [DOI] [PubMed] [Google Scholar]
- 43.Wong HK, Ong KL, Leung RY, Cheung TT, Xu A, Lam TH et al. Plasma level of adrenomedullin is influenced by a single nucleotide polymorphism in the adiponectin gene. PLoS One. 2013;8(8):e70335. doi: 10.1371/journal.pone.0070335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamada F, Aoki Y, Narisawa A, Abe Y, Komatsuzaki S, Kikuchi A et al. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. Journal of human genetics. 2011;56(1):34–40. doi: 10.1038/jhg.2010.132. [DOI] [PubMed] [Google Scholar]
- 45.Liu W, Morito D, Takashima S, Mineharu Y, Kobayashi H, Hitomi T et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS One. 2011;6(7):e22542. doi: 10.1371/journal.pone.0022542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujimura M, Sonobe S, Nishijima Y, Niizuma K, Sakata H, Kure S et al. Genetics and Biomarkers of Moyamoya Disease: Significance of RNF213 as a Susceptibility Gene. J Stroke. 2014;16(2):65–72. doi: 10.5853/jos.2014.16.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyawaki S, Imai H, Shimizu M, Yagi S, Ono H, Mukasa A et al. Genetic variant RNF213 c.14576G>A in various phenotypes of intracranial major artery stenosis/occlusion. Stroke. 2013;44(10):2894–7. doi: 10.1161/strokeaha.113.002477. [DOI] [PubMed] [Google Scholar]
- 48.••.Shinya Y, Miyawaki S, Imai H, Hongo H, Ono H, Takenobu A et al. Genetic Analysis of Ring Finger Protein 213 (RNF213) c.14576G>A in Intracranial Atherosclerosis of the Anterior and Posterior Circulations. J Stroke Cerebrovasc Dis. 2017;26(11):2638–44. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.043. [DOI] [PubMed] [Google Scholar]; A recently study revealed the association between the RNF213 variant and intracranial atherosclerosis.
- 49.Miyawaki S, Imai H, Takayanagi S, Mukasa A, Nakatomi H, Saito N. Identification of a genetic variant common to moyamoya disease and intracranial major artery stenosis/occlusion. Stroke. 2012;43(12):3371–4. doi: 10.1161/STROKEAHA.112.663864. [DOI] [PubMed] [Google Scholar]
- 50.Bang OY, Ryoo S, Kim SJ, Yoon CH, Cha J, Yeon JY et al. Adult Moyamoya Disease: A Burden of Intracranial Stenosis in East Asians? PLoS One. 2015;10(6):e0130663. doi: 10.1371/journal.pone.0130663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu W, Hitomi T, Kobayashi H, Harada KH, Koizumi A. Distribution of moyamoya disease susceptibility polymorphism p.R4810K in RNF213 in East and Southeast Asian populations. Neurol Med Chir (Tokyo). 2012;52(5):299–303. doi: 10.2176/nmc.52.299. [DOI] [PubMed] [Google Scholar]
- 52.Choi EH, Lee H, Chung JW, Seo WK, Kim GM, Ki CS et al. Ring Finger Protein 213 Variant and Plaque Characteristics, Vascular Remodeling, and Hemodynamics in Patients With Intracranial Atherosclerotic Stroke: A High-Resolution Magnetic Resonance Imaging and Hemodynamic Study. Journal of the American Heart Association. 2019;8(20):e011996. doi: 10.1161/JAHA.119.011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bang OY, Chung JW, Cha J, Lee MJ, Yeon JY, Ki CS et al. A Polymorphism in RNF213 Is a Susceptibility Gene for Intracranial Atherosclerosis. PLoS One. 2016;11(6):e0156607. doi: 10.1371/journal.pone.0156607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10(3):241–52. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–18. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bennet AM, Di Angelantonio E, Ye Z, Wensley F, Dahlin A, Ahlbom A et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. Jama. 2007;298(11):1300–11. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- 57.Hogh P, Garde E, Mortensen EL, Jorgensen OS, Krabbe K, Waldemar G. The apolipoprotein E epsilon4-allele and antihypertensive treatment are associated with increased risk of cerebral MRI white matter hyperintensities. Acta Neurol Scand. 2007;115(4):248–53. doi: 10.1111/j.1600-0404.2006.00779.x. [DOI] [PubMed] [Google Scholar]
- 58.Ilveskoski E, Perola M, Lehtimaki T, Laippala P, Savolainen V, Pajarinen J et al. Age-dependent association of apolipoprotein E genotype with coronary and aortic atherosclerosis in middle-aged men: an autopsy study. Circulation. 1999;100(6):608–13. doi: 10.1161/01.cir.100.6.608. [DOI] [PubMed] [Google Scholar]
- 59.Hixson JE. Apolipoprotein E polymorphisms affect atherosclerosis in young males. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb. 1991;11(5):1237–44. [DOI] [PubMed] [Google Scholar]
- 60.Abboud S, Viiri LE, Lutjohann D, Goebeler S, Luoto T, Friedrichs S et al. Associations of apolipoprotein E gene with ischemic stroke and intracranial atherosclerosis. Eur J Hum Genet. 2008;16(8):955–60. doi: 10.1038/ejhg.2008.27. [DOI] [PubMed] [Google Scholar]
- 61.Chutinet A, Suwanwela NC, Snabboon T, Chaisinanunkul N, Furie KL, Phanthumchinda K. Association between genetic polymorphisms and sites of cervicocerebral artery atherosclerosis. J Stroke Cerebrovasc Dis. 2012;21(5):379–85. doi: 10.1016/j.jstrokecerebrovasdis.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279(10):751–5. [DOI] [PubMed] [Google Scholar]
- 63.Roher AE, Esh C, Rahman A, Kokjohn TA, Beach TG. Atherosclerosis of cerebral arteries in Alzheimer disease. Stroke. 2004;35(11 Suppl 1):2623–7. doi: 10.1161/01.STR.0000143317.70478.b3. [DOI] [PubMed] [Google Scholar]
- 64.Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer’s Coordinating Center. Neurology. 2005;64(3):494–500. doi:64/3/494 [pii] 10.1212/01.WNL.0000150886.50187.30. [DOI] [PubMed] [Google Scholar]
- 65.Yan Y, Luo X, Zhang J, Su L, Liang W, Huang G et al. Association between phosphodiesterase 4D polymorphism SNP83 and ischemic stroke. J Neurol Sci. 2014;338(1–2):3–11. doi: 10.1016/j.jns.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 66.Liao YC, Lin HF, Guo YC, Yu ML, Liu CK, Juo SH. Sex-differential genetic effect of phosphodiesterase 4D (PDE4D) on carotid atherosclerosis. BMC Med Genet. 2010;11:93. doi: 10.1186/1471-2350-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Munshi A, Kaul S. Stroke genetics--focus on PDE4D gene. Int J Stroke. 2008;3(3):188–92. doi: 10.1111/j.1747-4949.2008.00199.x. [DOI] [PubMed] [Google Scholar]
- 68.Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370(Pt 1):1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith SA, Newby AC, Bond M. Ending Restenosis: Inhibition of Vascular Smooth Muscle Cell Proliferation by cAMP. Cells. 2019;8(11). doi: 10.3390/cells8111447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim C, Wilcox-Adelman S, Sano Y, Tang WJ, Collier RJ, Park JM. Antiinflammatory cAMP signaling and cell migration genes co-opted by the anthrax bacillus. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(16):6150–5. doi: 10.1073/pnas.0800105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang JX, Hsiung TC, Weng FC, Ding SL, Wu CP, Conti M et al. Synergistic effect of phosphodiesterase 4 inhibitor and serum on migration of endotoxin-stimulated macrophages. Innate immunity. 2018;24(8):501–12. doi: 10.1177/1753425918809155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalita J, Somarajan BI, Kumar B, Kumar S, Mittal B, Misra UK. Phosphodiesterase 4 D gene polymorphism in relation to intracranial and extracranial atherosclerosis in ischemic stroke. Dis Markers. 2011;31(4):191–7. doi: 10.3233/DMA-2011-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim JO, Park HS, Ryu CS, Shin JW, Kim J, Oh SH et al. Interplay between 3’-UTR polymorphisms in the methylenetetrahydrofolate reductase (MTHFR) gene and the risk of ischemic stroke. Sci Rep. 2017;7(1):12464. doi: 10.1038/s41598-017-12668-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao M, Wang X, He M, Qin X, Tang G, Huo Y et al. Homocysteine and Stroke Risk: Modifying Effect of Methylenetetrahydrofolate Reductase C677T Polymorphism and Folic Acid Intervention. Stroke. 2017;48(5):1183–90. doi: 10.1161/STROKEAHA.116.015324. [DOI] [PubMed] [Google Scholar]
- 75.Cronin S, Furie KL, Kelly PJ. Dose-related association of MTHFR 677T allele with risk of ischemic stroke: evidence from a cumulative meta-analysis. Stroke. 2005;36(7):1581–7. doi: 10.1161/01.STR.0000169946.31639.af. [DOI] [PubMed] [Google Scholar]
- 76.Rutten-Jacobs LC, Traylor M, Adib-Samii P, Thijs V, Sudlow C, Rothwell PM et al. Association of MTHFR C677T Genotype With Ischemic Stroke Is Confined to Cerebral Small Vessel Disease Subtype. Stroke. 2016;47(3):646–51. doi: 10.1161/strokeaha.115.011545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin X, Zhang W, Lu Q, Lei X, Wang T, Han X et al. Effect of MTHFR Gene Polymorphism Impact on Atherosclerosis via Genome-Wide Methylation. Med Sci Monit. 2016;22:341–5. doi: 10.12659/msm.895296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Zhang J, Qian Y, Tang X, Ling H, Chen K et al. Association of Homocysteine with Aysmptomatic Intracranial and Extracranial Arterial Stenosis in Hypertension Patients. Sci Rep. 2018;8(1):595. doi: 10.1038/s41598-017-19125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oh SH, Kim NK, Kim HS, Kim WC, Kim OJ. Plasma total homocysteine and the methylenetetrahydrofolate reductase 677C>T polymorphism do not contribute to the distribution of cervico-cerebral atherosclerosis in ischaemic stroke patients. European journal of neurology. 2011;18(3):491–6. doi: 10.1111/j.1468-1331.2010.03188.x. [DOI] [PubMed] [Google Scholar]
- 80.Shimo-Nakanishi Y, Urabe T, Hattori N, Watanabe Y, Nagao T, Yokochi M et al. Polymorphism of the lipoprotein lipase gene and risk of atherothrombotic cerebral infarction in the Japanese. Stroke. 2001;32(7):1481–6. doi: 10.1161/01.str.32.7.1481. [DOI] [PubMed] [Google Scholar]
- 81.Munshi A, Babu MS, Kaul S, Rajeshwar K, Balakrishna N, Jyothy A. Association of LPL gene variant and LDL, HDL, VLDL cholesterol and triglyceride levels with ischemic stroke and its subtypes. J Neurol Sci. 2012;318(1–2):51–4. doi: 10.1016/j.jns.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 82.Xu E, Li W, Zhan L, Guan G, Wang X, Chen S et al. Polymorphisms of the lipoprotein lipase gene are associated with atherosclerotic cerebral infarction in the Chinese. Neuroscience. 2008;155(2):403–8. doi: 10.1016/j.neuroscience.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 83.Wang C, Sun T, Li H, Bai J, Li Y. Lipoprotein lipase Ser447Ter polymorphism associated with the risk of ischemic stroke: a meta-analysis. Thromb Res. 2011;128(5):e107–12. doi: 10.1016/j.thromres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 84.Bianchi G, Ferrari P, Staessen JA. Adducin polymorphism: detection and impact on hypertension and related disorders. Hypertension. 2005;45(3):331–40. doi: 10.1161/01.HYP.0000156497.39375.37. [DOI] [PubMed] [Google Scholar]
- 85.Beeks E, Kessels AG, Kroon AA, van der Klauw MM, de Leeuw PW. Genetic predisposition to salt-sensitivity: a systematic review. J Hypertens. 2004;22(7):1243–9. doi: 10.1097/01.hjh.0000125443.28861.0d. [DOI] [PubMed] [Google Scholar]
- 86.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S et al. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet (London, England). 1997;350(9093):1734–7. doi: 10.1016/S0140-6736(97)05189-1. [DOI] [PubMed] [Google Scholar]
- 87.Wang R, Zhong B, Liu Y, Wang C. Association between alpha-adducin gene polymorphism (Gly460Trp) and genetic predisposition to salt sensitivity: a meta-analysis. Journal of applied genetics. 2010;51(1):87–94. [DOI] [PubMed] [Google Scholar]
- 88.van Rijn MJ, Bos MJ, Yazdanpanah M, Isaacs A, Arias-Vasquez A, Koudstaal PJ et al. Alpha-adducin polymorphism, atherosclerosis, and cardiovascular and cerebrovascular risk. Stroke. 2006;37(12):2930–4. doi: 10.1161/01.STR.0000248760.67039.2b. [DOI] [PubMed] [Google Scholar]
- 89.Kalita J, Misra UK, Kumar B, Somarajan BI, Kumar S, Mittal B. ACE and ADD1 gene in extra and intracranial atherosclerosis in ischaemic stroke. Neurol Res. 2013;35(4):429–34. doi: 10.1179/1743132813Y.0000000161. [DOI] [PubMed] [Google Scholar]
- 90.Lucarini L, Sticchi E, Sofi F, Pratesi G, Pratesi C, Pulli R et al. ACE and TGFBR1 genes interact in influencing the susceptibility to abdominal aortic aneurysm. Atherosclerosis. 2009;202(1):205–10. doi: 10.1016/j.atherosclerosis.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 91.Catto A, Carter AM, Barrett JH, Stickland M, Bamford J, Davies JA et al. Angiotensin-converting enzyme insertion/deletion polymorphism and cerebrovascular disease. Stroke. 1996;27(3):435–40. [PubMed] [Google Scholar]
- 92.Sharma P Meta-analysis of the ACE gene in ischaemic stroke. J Neurol Neurosurg Psychiatry. 1998;64(2):227–30. doi: 10.1136/jnnp.64.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Z, Xu G, Liu D, Fan X, Zhu W, Liu X. Angiotensin-converting enzyme insertion/deletion polymorphism contributes to ischemic stroke risk: a meta-analysis of 50 case-control studies. PLoS One. 2012;7(10):e46495. doi: 10.1371/journal.pone.0046495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rong C, Xing Y, Jiang X, Wang J, Gao B, Zhao J et al. Angiotensin-converting enzyme gene polymorphism and middle cerebral artery stenosis in a Chinese Han population. Neural Regen Res. 2013;8(15):1410–7. doi: 10.3969/j.issn.1673-5374.2013.15.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thomas GN, Lin JW, Lam WW, Tomlinson B, Yeung V, Chan JC et al. Middle cerebral artery stenosis in type II diabetic Chinese patients is associated with conventional risk factors but not with polymorphisms of the renin-angiotensin system genes. Cerebrovascular diseases (Basel, Switzerland). 2003;16(3):217–23. doi: 10.1159/000071119. [DOI] [PubMed] [Google Scholar]
- 96.Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin-angiotensin system and cardiovascular risk. Lancet (London, England). 2007;369(9568):1208–19. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- 97.Roscioni SS, Heerspink HJ, de Zeeuw D. The effect of RAAS blockade on the progression of diabetic nephropathy. Nat Rev Nephrol. 2014;10(2):77–87. doi: 10.1038/nrneph.2013.251. [DOI] [PubMed] [Google Scholar]
- 98.Munshi A, Sharma V, Kaul S, Rajeshwar K, Babu MS, Shafi G et al. Association of the −344C/T aldosterone synthase (CYP11B2) gene variant with hypertension and stroke. J Neurol Sci. 2010;296(1–2):34–8. doi: 10.1016/j.jns.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 99.Yan G, Wang Y. Association of CYP11B2 gene polymorphism with ischemic stroke in the north Chinese Han population. Neurology India. 2012;60(5):504–9. doi: 10.4103/0028-3886.103196. [DOI] [PubMed] [Google Scholar]
- 100.Sharma R, Katz J. Preliminary studies on human aldosterone synthase (CYP11B2) gene polymorphism, matrix metalloprotease-9, apoptosis, and carotid atherosclerosis plaque size by proton magnetic resonance imaging. J Renin Angiotensin Aldosterone Syst. 2010;11(3):198–204. doi: 10.1177/1470320309358109. [DOI] [PubMed] [Google Scholar]
- 101.Ding L, Hong Y, Peng B. Association between large artery atherosclerosis and cerebral microbleeds: a systematic review and meta-analysis. Stroke and vascular neurology. 2017;2(1):7–14. doi: 10.1136/svn-2016-000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Klein IF, Lavallee PC, Mazighi M, Schouman-Claeys E, Labreuche J, Amarenco P. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: a high-resolution MRI study. Stroke. 2010;41(7):1405–9. doi: 10.1161/strokeaha.110.583534. [DOI] [PubMed] [Google Scholar]
- 103.Romero JR, Beiser A, Seshadri S, Benjamin EJ, Polak JF, Vasan RS et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke. 2009;40(5):1590–6. doi: 10.1161/strokeaha.108.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 105.Holliday EG, Traylor M, Malik R, Bevan S, Falcone G, Hopewell JC et al. Genetic overlap between diagnostic subtypes of ischemic stroke. Stroke. 2015;46(3):615–9. doi: 10.1161/strokeaha.114.007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lettre G, Palmer CD, Young T, Ejebe KG, Allayee H, Benjamin EJ et al. Genome-wide association study of coronary heart disease and its risk factors in 8,090 African Americans: the NHLBI CARe Project. PLoS genetics. 2011;7(2):e1001300. doi: 10.1371/journal.pgen.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang Y, Liu X, Du Y, Zhou S. rs1234313 and rs45454293 are risk factors of cerebral arterial thrombosis, large artery atherosclerosis, and carotid plaque in the Han Chinese population: a case-control study. BMC neurology. 2019;19(1):31. doi: 10.1186/s12883-019-1259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schonbeck U, Libby P. CD40 signaling and plaque instability. Circ Res. 2001;89(12):1092–103. doi: 10.1161/hh2401.101272. [DOI] [PubMed] [Google Scholar]
- 109.Ria M, Eriksson P, Boquist S, Ericsson CG, Hamsten A, Lagercrantz J. Human genetic evidence that OX40 is implicated in myocardial infarction. Biochemical and biophysical research communications. 2006;339(3):1001–6. doi: 10.1016/j.bbrc.2005.11.092. [DOI] [PubMed] [Google Scholar]
- 110.Olofsson PS, Soderstrom LA, Jern C, Sirsjo A, Ria M, Sundler E et al. Genetic variants of TNFSF4 and risk for carotid artery disease and stroke. Journal of molecular medicine (Berlin, Germany). 2009;87(4):337–46. doi: 10.1007/s00109-008-0412-5. [DOI] [PubMed] [Google Scholar]
- 111.Kovacic S, Bakran M. Genetic susceptibility to atherosclerosis. Stroke Res Treat. 2012;2012:362941. doi: 10.1155/2012/362941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bersano A, Zuffardi O, Pantoni L, Quaglini S, Ciccone R, Vetro A et al. Next generation sequencing for systematic assessment of genetics of small-vessel disease and lacunar stroke. J Stroke Cerebrovasc Dis. 2015;24(4):759–65. doi: 10.1016/j.jstrokecerebrovasdis.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 113.Duzkale H, Shen J, McLaughlin H, Alfares A, Kelly MA, Pugh TJ et al. A systematic approach to assessing the clinical significance of genetic variants. Clin Genet. 2013;84(5):453–63. doi: 10.1111/cge.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]


