Abstract
Background and objectives: Brain-derived neurotrophic factor (BDNF) is one of the most studied neurotrophins. Low BDNF concentrations have been noted in patients with traditional cardiovascular disease risk factors and have been associated with the increased risk of stroke/transient ischemic attack (TIA). We aimed to study the correlation of BDNF serum levels with acute stroke severity and its potential role as a biomarker in predicting functional outcome. Materials and methods: We systematically searched PubMed, Web of Science, and the Cochrane database using specific keywords. The endpoints examined were the correlation of BDNF with functional outcome, the National Institute of Health stroke scale (NIHSS) measured at the acute phase, and stroke infarct volume. We also compared serum BDNF levels between stroke patients and healthy controls. Results: Twenty-six records were included from the initial 3088 identified. Twenty-five studies reported NIHSS and BDNF levels on the first day after acute stroke. Nine studies were further meta-analyzed. A statistically significant negative correlation between NIHSS and BDNF levels during the acute phase of stroke was noted (COR: −0.3013, 95%CI: (−0.4725; −0.1082), z = −3.01, p = 0.0026). We also noted that BDNF levels were significantly lower in patients with stroke compared to healthy individuals. Due to the heterogeneity of studies, we only conducted a qualitative analysis regarding serum BDNF and functional outcome, while no correlation between BDNF levels and stroke infarct volume was noted. Conclusions: We conclude that in the acute stroke phase, stroke severity is negatively correlated with BDNF levels. Concurrently, patients with acute stroke have significantly lower BDNF levels in serum compared to healthy controls. No correlations between BDNF and stroke infarct volume or functional outcome at follow-up were noted.
Keywords: stroke, BDNF, functional outcome, acute phase, meta-analysis
1. Introduction
Stroke is the second cause of death worldwide and a leading cause of disability [1]. The recent advances in acute ischemic stroke treatment, such as intra-arterial mechanical thrombectomy, improved stroke management and reduced stroke-related disability. Nonetheless, the aging of the population and the increasing prevalence of stroke risk factors are predicted to lead to an increase in stroke survivors. In Europe, the projections forecast a rise of stroke survivors, who will reach the 4,631,050 people in 2035, a 45% increase in stroke-related deaths, and a rise of lost disability-adjusted life years lost (DALYs) by 32% [2]. The need for a potent, non-invasive biomarker which can be used to diagnose and predict the functional outcome of stroke is growing.
Neurotrophins are a family of various soluble molecules, which are involved in multiple nervous system functions, such as cell growth, differentiation, and plasticity [3]. They act through binding to two classes of transmembrane receptors: the tropomyosin-related tyrosine kinase (Trk) receptor family and the p75NT receptor (p75NTR) [4,5]. In the healthy brain, neurotrophins derive from neurons, while peripheral blood cells and cells of the immune system also produce small amounts [3,6]. Their contribution rises when the neurotrophin levels fall due to a pathological CNS process. Brain-derived neurotrophic factor (BDNF) is one of the most studied neurotrophins, the role of which has been unveiled through animal experiments. In the CNS, mature BDNF mediates in synaptic plasticity, dendritic branching, the regulation of both inhibitory and excitatory neurotransmitters, and neuronal growth, while proBDNF contributes to cell apoptosis [4,7,8]. In healthy individuals, BDNF can be detected in the serum and is mostly stored in platelets. The normal values vary depending on the method used [8]. Female individuals have higher levels of BDNF, while a gradual decrease has been reported with increasing age in both sexes. Several studies show that BDNF levels are activity dependent. Physical activity or cognitive enhancement and social activity enhance BDNF expression. On the other hand, a sedentary lifestyle and obesity can lead to decreased concentrations in BDNF [9].
Alterations of BDNF levels in serum have been reported in epilepsy, various neurodegenerative and psychiatric disorders, such as Alzheimer’s dementia, and schizophrenia [10]. Low concentrations have also been noted in patients with traditional cerebrovascular disease (CVD) risk factors and metabolic syndrome, while a negative correlation between circulating BDNF and body mass index, lipid profile and blood pressure has been observed [11]. In a Framingham sub-study, low BDNF levels in healthy individuals were associated with an increased risk of future stroke/TIA [12]. In acute stroke, low BDNF levels have been correlated with worse scores in the National Institute of Health stroke scale (NIHSS), larger infarct volume, and poor long-term functional outcome [13,14].
We conducted this systematic review and meta-analysis, aiming to study the role of BDNF as a biomarker in predicting the functional outcome of stroke when measured in the acute stroke phase. Concurrently, we tried to define the association between BDNF levels in the acute phase and stroke severity, as expressed with clinical and neuroradiological measures.
2. Materials and Methods
We conducted our systematic review and meta-analysis according to the methodological steps of PRISMA guidelines [15]. Using the search strategy: (stroke OR cerebrovascular disease OR CVD) AND (BDNF OR brain-derived neurotrophic factor OR neurotrophin), we systematically searched PubMed, Web of Science Search engines, and the Cochrane database. We imported all the references into a reference manager software [16]. After removing the duplicates, two authors (K.E. and P.A.) independently screened the titles, abstracts, and full texts of the potentially eligible articles. We resolved any arising disagreements through discussion.
2.1. Inclusion and Exclusion Criteria
The inclusion criteria were the following: (a) patients with stroke (ischemic or hemorrhagic) (b) BDNF values in serum, (c) acute phase (within 14 days after stroke), (d) participants 17 years old or older, male or female, (e) stroke severity, stroke volume infarct, and functional outcome as the primary or secondary of the study. The studies were excluded according to the following criteria: (a) animal studies, (b) review or meta-analysis, (c) study including below 5 participants, (d) study in healthy individuals.
2.2. Study Endpoints
We performed primary efficacy and sensitivity analyses to assess the correlation of serum BDNF levels in the acute stroke phase with functional outcome in patients with acute stroke.
We conducted additional analyses to assess the correlation of BDNF in the acute stroke phase with stroke severity as quantified with the National Institute of Health stroke scale (NIHSS) and infarct volume. We also performed primary efficacy and sensitivity analyses to assess serum BDNF levels in patients with stroke during the acute phase versus healthy controls.
In all cases, if the data could not be further meta-analyzed, we performed a qualitative analysis, by outlining the main findings of each study.
2.3. Managing the Missing Data
To perform a comprehensive analysis, we contacted the corresponding authors of each selected article with missing data by email. In cases where additional information was provided, we included the study in our meta-analysis.
2.4. Risk of Bias Assessment
We evaluated the risk of bias of the selected articles using the Cochrane risk of bias tool [17]. We rated the studies as low, high, or unclear risk in each of the seven domains examined: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of the outcome, incomplete outcome data, selective reporting, and other sources of bias. Studies with dropouts >10% and without intention-to-treat analysis were evaluated as high risk of bias in the “incomplete outcome data” section.
2.5. Statistical Analysis
For the purpose of the statistical analysis and the graphical representation of the data, we used the Meta library in R Studio. The statistical analysis was performed using standardized mean difference methodology, and the medians and interquartile range were converted to means and standard deviation in accordance with the methodology presented by Hozo et al. [18]. We also conducted a correlation analysis between the serum BDNF levels in the acute stroke phase with functional outcome in patients with acute stroke. We used 95% confidence interval and overall heterogeneity was assessed using Cochrane’s Q statistic and I2. Based on the heterogeneity of the data for each comparison, we used a random-effects or fixed-effects model. Publication bias was assessed using Egger’s linear regression test and the visual inspection of funnel plots, and where the distribution was not roughly symmetrical, this was suggestive of an increased risk of bias. For each outcome, we used the inverse variance-weighted average method to calculate the effect of each study on our results, while t2 was measured through the DerSimonian–Laird method.
3. Results
3.1. Study Selection—Quality Assessment
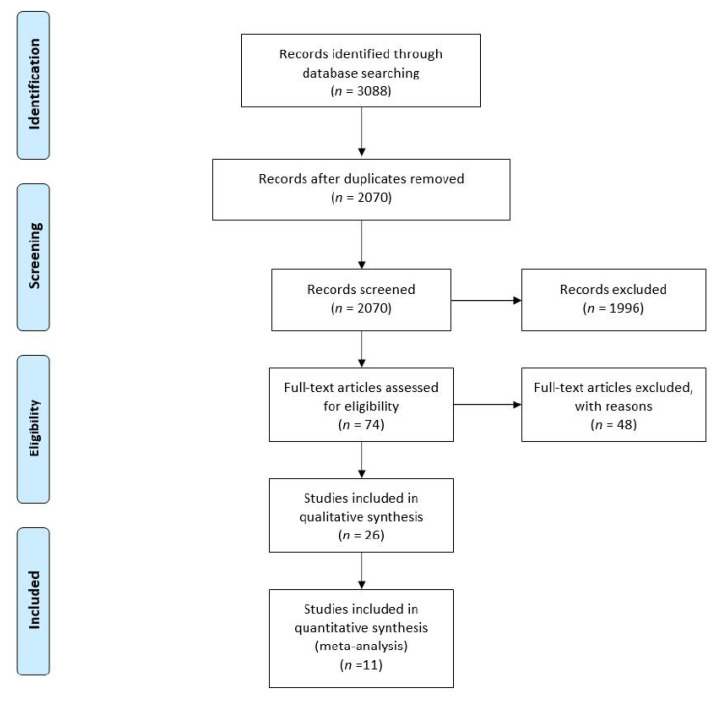
Our search engine search yielded 3088 studies. The step-by-step screening process is described in Figure 1. Twenty-six studies were selected and further assessed using the Cochrane risk of bias tool. Ten studies were assessed as low (green), one as high (red), and fifteen studies as an unclear (yellow) risk of bias (Figure 2). The basic characteristics of each study are summarized in Table 1.
Figure 1.
Prisma flow chart.
Figure 2.
Risk of bias assessment.
Table 1.
Study characteristics.
| Study | Sample | Type of Stroke | Assay | Time of Blood Collection | No of Patients | NIHSS Baseline | BDNF (ng/mL) | Age (Years) | Outcome Assessment | Time of Follow-Up | Patients Who Received rtPA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Algin 2019 | Serum | Ischemic stroke | ELISA | Within 4 h after stroke | 75 | 10.88 ± 7.72 | 3.89 ± 2.05 | 73.22 ± 11.57 | NA | No follow-up | NA |
| Asadollahi 2019 | Serum | Ischemic stroke | ELISA | 1st and 4th day after stroke | 19 saffron- treated | 11.35 (9.3–13.3) | 1.81 (1.1–2.5) | 70.16 ± 11.5 | BI | 3 months | None |
| 20 routine care | 11.26 (9.4–13) | 1.99 (1.2–2.7) | 72.25 ± 10 | ||||||||
| Chan 2015 | Serum | Ischemic stroke | ELISA | Day 1, week 1 and 3 | 75 | 3.5 (0–24) | NA | 69 (27–89) | mRS | 6 months | 6 |
| Chaturvedi 2020 | Serum | Any type | ELISA | Admission, week 2, 6th month | 104 diabetic | 9.36 ± 4.17 | 11.08 ± 3.85 | 56.29 ± 11.06 | FIM | 3 months | NA |
| 104 non-diabetic | 8.01 ± 4.14 | 8.77 ± 4.04 | 57.42 ± 10.35 | ||||||||
| Hutanu 2019 | Serum | Ischemic stroke | Xmap | Day 1and 5 | 114 | NA | 4.1 (2.73, 9.24) | 71.7 ± 10.2 | BI, mRS | No follow-up | none |
| Jimenez 2009 | Serum | Ischemic stroke | ELISA | Day 7 ± 2 and 30 ± 7 after stroke | 25 PSD | 3 (1–7) | 13.6 (9.8–20.1) | 76.6 ± 7.8 | BI, mRS | 1 month | NA |
| 109 non-PSD | 2 (1–5) | 12.9 (10.6–16.1) | 69.5 ± 9.6 | ||||||||
| Karakulova 2017 | Serum | Ischemic stroke | ELISA | Days after stroke not mentioned | 25 Cytoflavin | 6.70 ± 0.62 | 0.648 ± 0.095 | 52–74 years old | BI | 2 months | none |
| 27 routine care | 6.32 ± 0.26 | 0.598 ± 0.180 | |||||||||
| Kozak 2016 | Serum | Ischemic stroke | ELISA | On the 1st day of stroke | 11 with delirium | 10.36 ± 5.88 | 0.906 ± 0.654 | 64.63 ± 13.18 | NA | No follow-up | none |
| 49 without delirium | 6.10 ± 3.80 | 1.035 ± 0.761 | 72.91 ± 5.61 | ||||||||
| Kozak 2019 | Serum | Ischemic stroke | ELISA | On the 1st day of stroke | 17 PSD | 6.96 ± 4.75 | 0.751 ± 0.643 | 67.47 ± 11.20 | NA | No follow-up | none |
| 36 non-PSD | 0.729 ± 0.501 | 65.25 ± 13.98 | |||||||||
| Lasek-Bal 2015 | Serum | Ischemic stroke | ELISA | On the 1st day of stroke | 87 | NA | 9.96 ± 5.21 | 71.7 ± 11.8 | mRS | 90 days | none |
| Lasek-Bal 2019 | Serum | Ischemic stroke | ELISA | On the 1st day of stroke | 138 | 3 [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18] | NA | 73.11 ± 11.48 | mRS | 30days | 53 |
| Li 2014 | Serum | Ischemic stroke | ELISA | On the 1st day of stroke | 59 PSD | 8 (4–14) | 8.1 (5.6–9.4) | 72.8 (11.2) | mRS | 3 months | NA |
| 157 non-PSD | 5 (2–8) | 13.7 (10.4–16.5) | 63.6 (9.1) | ||||||||
| Lopez Cancio 2016 | Serum | Ischemic stroke | ELISA | Day 1, 7 and 3 months | 83 | 17 [12,13,14,15,16,17,18,19,20,21] | 3.3 ± 0.9 | 69.6 ± 10.9 | mRS | 3 months | NA |
| Mourao 2018 | Serum | Ischemic stroke | ELISA | Up to 24 h, 72 h and discharge | 32 (length of stay ≤ 10 days) | 5.6 ± 3.8 | 9.715 ± 2.56 | 65.5 ± 11.7 | mRS | No follow-up | NA |
| 18 (length of stay > 10 days) | 12.4 ± 7.4 | 9.737 ± 2.87 | |||||||||
| Mourao 2019 | Serum | Ischemic stroke | ELISA | Admission | 26 ASPECTS < 10 | 10.5 ± 6.4 | 9.05 ± 2.29 | 67.1 ± 12 | mRS | No follow-up | None |
| 24 ASPECTS ≥ 10 | 5.8 ± 4.9 | 10.45 ± 2.86 | 63.8 ± 11.4 | ||||||||
| Otero Ortega 2019 | Serum | Ischemic stroke | ELISA | At 24–48 h and 72–96 h | 95 Non-PSHG | 5 (3–11) | 12.3 (6.5) | 69.7 (12.5) | mRS | 72–96 h | NA |
| 79 PSHG | 4 (2–8) | 11.6 (6.6) | 72.1 (9.3) | ||||||||
| Pedard 2018 | Serum and PBMC | Ischemic stroke | ELISA | Before fibrinolysis—day 1 and 3 | 25 mRS 0–2 | 7 (4–9) | 30.4 (27.8–34.0) | 76 (65.8–83.5) | mRS | No follow-up | All |
| 15 mRS 3–6 | 8 (5–12) | 31.3 (24.6–34.8) | 81 (70–88) | ||||||||
| Qiao 2017 | Serum | Ischemic stroke | ELISA | On 1st day of stroke | 270 | 7 (4–12) | 22.1 (14.5–27.5) | 65 (56–73) | NA | No follow-up | 58 |
| Rodier 2015 | Serum | Ischemic stroke | ELISA | Day 0, 1, 7 and 90 | 14 non rtPA treated | 11.44 ± 2.25 | NA | 74.71 ± 3.55 | NA | 90 days | 24 |
| 24 rtPA treated | 11.20 ± 1.34 | 69.13 ± 3.01 | |||||||||
| Sobrino 2019 | Serum | Ischemic stroke | ELISA | Admission, 3rd months and 12 months | 351 good outcome | 8 (5, 13) | 66.9 ± 11.6 | mRS | 12 months | 198 | |
| 201 poor outcome | 18 (14, 20) | 69.6 ± 9.4 | |||||||||
| Stanne 2016 | Serum | Ischemic stroke | ELISA | Within 10 days after stroke | 491 | 3 (2, 7) | 15.4 ± 5.9 | 58 (51–63) | mRS | 3months, 2 and 7 years | NA |
| Wang 2016 | Serum | Ischemic stroke | ELISA | On 1st day of admission (until 48 h) | 204 | 6 (3–12) | 13.4 (9.2–16.9) | 64 (55–75) | mRS | 3 months | 41 |
| Wang 2019 | Serum | Ischemic stroke | ELISA | NA | 40 | 10 (5, 15) | 19.14 ± 4.87 | 63 (50–75) | NA | No follow-up | NA |
| Yang 2010 | Serum | Ischemic stroke | ELISA | 24–48 h after stroke | 37 PSD | 7 (4–9.5) | NA | 68.95 ± 9.28 | mBI | 14 days | None |
| 63 non-PSD | 3 (2–4) | 68.43 ± 11.18 | |||||||||
| Zhang 2016 | Serum | Ischemic stroke | ELISA | On the 1st day of stroke | 37 received statin | 7.87 ± 2.26 | 32.95 ± 6.14 | 65.11 ± 10.72 | mRS, BI | 6 weeks | NA |
| 38 not received statin | 8.36 ± 2.93 | 23.06 ± 5.13 | 63.34 ± 9.67 | ||||||||
| Zhou 2011 | Serum | Any type | ELISA | Within 7 days after stroke | 35 PSD | 7 (1, 24) | 29.1 ± 11.4 | 61.7 ± 8.5 | mRS, BI | 6 months | NA |
| 58 non-PSD | 5 (1, 13) | 28.1 ± 9.7 | 63.5 ± 12.5 |
Abbreviations: ASPECTS: Alberta stroke program early CT score; BDNF: brain-derived neurotrophic factor; BI: Barthel index; ELISA: enzyme-linked immunosorbent assay; FIM: functional independence measure; mRS: modified Rankin scale; NA: not available; NIHSS: National Institute of Health stroke scale; PSD: post-stroke depression; PSHG: post-stroke hyperglycemia; rtPA: recombinant tissue plasminogen activator.
3.2. Functional Outcome at Follow-Up
Twenty-one studies reported both the serum BDNF levels and measured functional outcome on follow up. Despite measuring both BDNF and functional outcome, nine studies did not examine the correlation between them [19,20,21,22,23,24,25,26,27].
Algin et al. correlated BDNF levels measured within 4 h of acute ischemic stroke with stroke mortality [28]. The authors reported that BDNF levels did not differ between stroke survivors (n = 65, BDNF = 3.80 ± 1.99 ng/mL) and non-survivors (n = 10, BDNF = 4.50 ± 2.39 ng/mL) (p = 0.296).
Chan and colleagues studied serum BDNF and the frequency of BDNF positive T cells in patients with acute ischemic stroke and controls [29]. The authors did not measure the correlation between serum BDNF and functional outcome measured with the Barthel index scale (BI) at any time-point. Nonetheless, patients with acute ischemic stroke were found to have an increase in BDNF positive T regulatory cells (Tregs) when compared to controls (p < 0.01), measured within 3 weeks after the event. Concurrently, patients with a low percentage of BDNF+ Tregs were found to be more dependent than patients with a high percentage at 6 months. Hutanu et al. examined the role of serum BDNF as a predictor of dependency in daily life activities after an acute ischemic stroke [30]. Plasma BDNF levels measured on admission were lower in patients with lower BI scores (BI ≤ 80) measured at discharge (day 5 post-stroke). An optimal cut-off of 2.33 pg/mL with 91.1% sensitivity, and 31.1% specificity for predicting a poor outcome (BI ≤ 80) was reported.
Lasek Bal and colleagues studied the role of BDNF in the acute ischemic stroke phase in correlation with functional outcome measured with modified Ranking Scale (mRS) at 90 days [31]. The authors reported that BDNF levels did not vary significantly between the groups with good outcome (mRS = 0–2) and poor outcome (mRS = 3–6).
Mourao et al. studied plasma levels of BDNF in correlation with mRS, NIHSS, and Gugging Swallowing screen scale (GUSS), all measured within the first 24 h after the ischemic stroke, at 72 h, at discharge [32]. Lower BDNF levels at 72 h were correlated with higher NIHSS, higher mRS, and higher difficulty in swallowing measured with GUSS score at the same time-points. Specifically, an adjusted R coefficient of 0.101 was reported (coefficient B = −147.126, p = 0.014, 95%CI: −262.940–31.312), leading the authors to conclude that BDNF levels are independently associated with the prognosis of acute stroke patients.
The role of BDNF in the prognosis of patients with hyperglycemia after acute stroke was examined by Otero Ortega et al. [33]. Two groups were formed: patients with post-stroke hyperglycemia (PSHG) and non-PSHG. The authors quantified the functional outcome with mRS at 90 days after an acute ischemic stroke. Blood samples were collected at 24–48 h and 72–96 h after admission. While the unadjusted analysis revealed a significant association of BDNF with poor outcome (mRS = 3–6) at follow-up (3 months after stroke), this finding was not confirmed after the adjusted analysis.
Pedard and colleagues hypothesized that the levels of BDNF containing peripheral blood mononuclear cells (PBMCs) may be connected with functional outcome after acute ischemic stroke [34]. All patients received intravenous thrombolysis (rtPA), and both serum BDNF and BDNF containing PBMCs were measured before and after the intervention. No association was found between serum BDNF and stroke outcome. Nonetheless, the authors noted that PBMCs that contained BDNF measured on the third day after stroke was increased in patients with good functional outcome. In patients with unfavorable outcome, a gradual decrease in BDNF levels in PBMCs before thrombolysis to the third day after stroke was reported (−64%, p = 0.013). Finally, after adjusting for other stroke predictors, BDNF-PBMCs predicted functional outcome (OR = 12.4, 95%CI: 1.4–112.2, p = 0.046) and the authors reported a cutoff value of 6.66 ng/mg to predict good functional outcome (mRS = 0–2) (sensitivity/specificity = 48.0%/92.9%, respectively).
Sobrino et al. explored the association between various serum biomarkers and functional outcome after ischemic stroke [35]. In this multicenter study, they included 552 patients within the first 24 h after stroke (non-lacunar etiology). Both functional outcome and BDNF levels were measured at admission, day 7, 3 months, and 12 months after stroke. BDNF levels did not differ between patients with a good functional outcome (mRS = 0–2) and those with a poor functional outcome (mRS = 3–6) at any time-point.
Stanne et al. examined the role of serum BDNF as a potential predictor of stroke outcome in patients with acute stroke followed up to seven years after the event [14]. The authors reported that BDNF levels were lowest among patients with poor functional outcome. Although, in regression analysis, BDNF levels were not correlated with the functional outcome on short-term follow-up, at 2 and 7 years after, low levels of BDNF at the acute stroke phase were correlated with poor functional outcome (mRS = 3–6). Similarly, Wang and colleagues tested whether serum BDNF can be served as a stroke outcome predictor or not [36]. The authors reported that BDNF levels measured during the acute stroke phase in patients with poor outcome (mRS = 3–6) were lower compared to those with good outcome (p < 0.001) and that BDNF was an independent stroke outcome predictor. A cutoff of 14.5 ng/mL, which yielded an 81.5% sensitivity and 70.5% specificity, predicted a poor outcome (mRS = 3–6). Concurrently, the authors reported that low BDNF levels were an independent predictor of mortality in multivariate logistic regression analysis, BDNF (OR = 4.04, 95%CI: 2.07–9.14), after adjusting for all other significant predictors.
Yang and colleagues tested the role of serum BDNF in predicting depression after stroke [37]. All outcomes were assessed on admission and fourteen days after. Lower BDNF levels on day one post-stroke were correlated with a higher incidence of post-stroke depression (OR = 0.551, 95%CI: 0.389–0.779, p = 0.001), with a cut-off value of BDNF < 5.86 ng/mL to be predictive of depression development in the first two weeks. No significant correlation of BDNF measured on day one after stroke and modified BI was noted (Spearman’s Rho = 0.128, p = 0.092).
Lastly, Zhang and colleagues examined how treatment with atorvastatin correlated with the functional outcome of stroke and BDNF levels versus the control group [38]. In all patients, linear regression analysis revealed a significant association between BDNF levels and mRS-BI. Specifically, a negative correlation between BDNF and mRS (Spearman’s rho = −0.65, p < 0.001) and a positive correlation between BDNF and BI (Spearman’s rho = 0.71, p < 0.001) was noted.
3.3. NIHSS
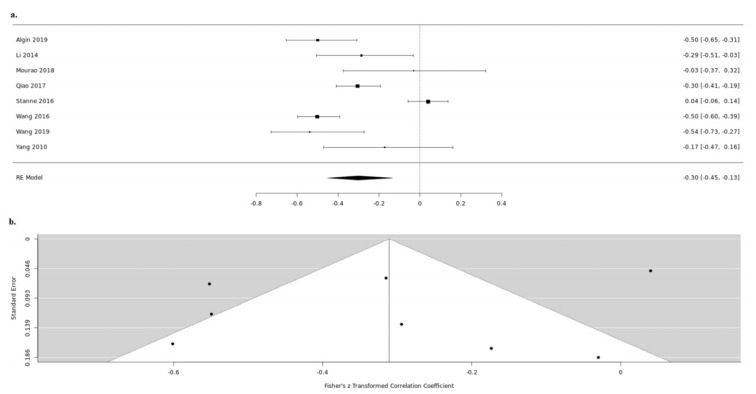
Twenty-five studies reported both the serum BDNF levels and the NIHSS during the acute stroke phase. Thirteen studies, despite measuring BDNF and scoring NIHSS, do not examine the in-between correlation [20,21,22,24,25,26,27,33,34,35,39,40,41]. Amongst the rest, nine were further meta-analyzed [14,15,23,28,32,36,37,42,43]. Due to high heterogeneity between the studies (tau2 = 0.0701, H = 3.06 (2.32; 4.05), I2 = 89.3% (81.4%; 93.9%)), we used a random-effects model. In the acute stroke phase, BDNF levels in serum were negatively correlated with NIHSS, which reached statistical significance (random-effects model COR: −0.3013, 95%CI: (−0.4725; −0.1082), z = −3.01, p = 0.0026) (Figure 3). For the detection of publication bias, we performed Egger’s regression test and the visual inspection of the funnel plot. As we detected an asymmetry in the funnel plot (t = −0.9399, df = 6, p = 0.3836), we additionally performed the rank correlation test for funnel plot asymmetry. The asymmetry detected was not statistically significant; thus, we concluded that the possibility of the publication bias to affect our results is low (Kendall’s tau = 0.2143, p = 0.5484) (Figure 3).
Figure 3.
Serum BDNF and NIHSS up (a). forest and down (b). funnel plot.
We further conducted a sensitivity analysis to assess the effects of each study to our findings. We conclude that our result is robust and is not influenced by a specific study (95%CI: −0.3011 (−0.4487; −0.1374), p = 0.0004, t2 = 0.0473, I2 = 89.3%) (Figure 4).
Figure 4.
Sensitivity analysis serum BDNF and NIHSS.
Three additional studies reported the relationship between BDNF and NIHSS during the acute stroke phase. Asadollahi and colleagues studied the effect of the aqueous extract of saffron administration in acute stroke outcome [19]. The authors formed two groups, one treated with standard treatment plus saffron extract and one receiving only standard treatment (statins, antiplatelets, antihypertensive treatment). BDNF was measured in serum on the first and fourth days after stroke. The authors reported a significant increase in BDNF concentration measured at day four post-stroke in the saffron-treated group compared to the control. Concurrently, a negative significant non-linear cubic regression between BDNF and the NIHSS score (cubic regression = 0.573, Sig = 0.0000062) was noted.
Various plasma biomarkers were tested for their efficacy in predicting stroke functional outcome by Hutanu et al. [30]. BDNF in serum collected during the first day of hospitalization was negatively associated with NIHSS measured on discharge (p = 0.014). The authors also reported that patients with NIHSS > 7 at discharge had lower BDNF levels than patients with NIHSS ≤ 7.
Lasek Bal and colleagues studied the efficacy of BDNF as a potential predictor of outcome in patients with acute ischemic stroke [31]. Both the NIHSS and BDNF were measured during the first day of stroke. The authors reported that BDNF levels in patients with mild stroke (NIHSS ≤ 4) did not differ significantly compared to patients with NIHSS > 4.
3.4. Infarct Volume
Ten studies reported both the serum BDNF levels and the infarct volume. Despite measuring both, three studies did not examine the in-between correlation [20,23,24]. Five studies were further meta-analyzed [15,28,32,36,38]. Due to high heterogeneity between the studies included (tau2 = 0.0326, H = 2.08 (1.33; 3.24), I2 = 76.8% (43.6%; 90.4%)), we used a random-effects model. No statistically significant correlation between serum BDNF and stroke volume was revealed (random-effects model COR: −0.1503, 95%CI: (−0.3286; 0.0383), z = −1.56, p = 0.1177) (Figure 5). We further proceeded to an influence analysis, which revealed that omitting one study out at a time has a significant effect on the overall result (95%CI: −0.1503(−0.3286; 0.0383), p = 0.1177, t2 = 0.0326, I2 = 76.8%) (Figure 6).
Figure 5.
Serum BDNF and infarct stroke volume up (a). forest and down (b). funnel plot.
Figure 6.
Sensitivity analysis serum BDNF and infarct stroke volume.
Mourao et al. investigated the association between the Alberta stroke program early CT score (ASPECTS) score and multiple serum biomarkers in patients with acute ischemic stroke [26]. Fifty patients were included (26 with ASPECTS < 10 and 24 with ASPECTS = 10). As expected, a significant negative correlation was reported between ASPECTS and mRS/NIHSS. Concurrently, the authors noted a significant positive correlation between ASPECTS score and BDNF levels (Spearman’s rho = 0.307, p = 0.030).
Sobrino et al. examined the correlation between multiple serum biomarkers and ischemic stroke volume measured at various time-points (admission, day 7, 3rd month, 12th month) [35]. No correlation between the two was noted at any time-point (MANOVA test, p = 0.081).
3.5. BDNF in Stroke Patients versus Controls
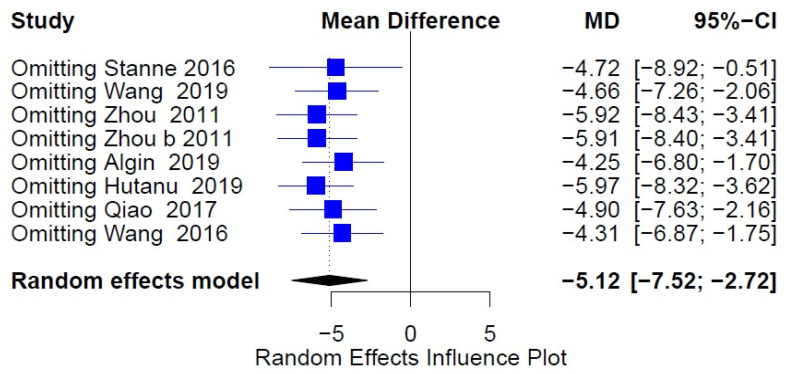
A total of seven studies measured serum BDNF and evaluated the differences between stroke patients (six studies included only ischemic stroke patients and one both ischemic and hemorrhagic) and healthy controls. All studies were further meta-analyzed [14,15,25,28,30,36,43]. Due to high heterogeneity between the studies (I2 = 99.49%), we used a random-effects model. A statistically significant difference was demonstrated (Figure 7). Specifically, we found that patients with acute stroke had significantly lower serum BDNF levels compared to healthy controls (Standard mean difference: −2.37 (−4.36, −0.38)). For the detection of publication bias, we performed weighted regression with multiplicative dispersion and visual inspection of the funnel plot (t = −0.1407, df = 6, p = 0.8927). We conclude that the possibility of the publication bias to affect our results is low (Figure 7). We further conducted a sensitivity analysis, which confirmed that our result is robust and not affected by a specific study (95%CI: −5.9084(−8.4032; −3.4136), p < 0.0001, t2 = 10.5474, I2 = 98.8%) (Figure 8).
Figure 7.
Serum BDNF in stroke patients versus controls up (a). forest and down (b). funnel plot.
Figure 8.
Sensitivity analysis serum BDNF in stroke patients versus controls.
4. Discussion
Our study confirmed a significant negative association between BDNF and NIHSS; both measured at the acute stroke phase. We also proved that BDNF levels in patients with stroke are significantly increased compared to healthy controls. No association between BDNF and infarct volume was found. Through our qualitative review, we highlighted a potential role of BDNF measured at the acute phase of stroke as a predictor of stroke outcome. We also discussed the findings of individual publications studying the role of BDNF-positive PBMCs and Tregs, in which a correlation with outcome was noted. The limited available data hindered the conduction of a meta-analysis, in which the potential role of BDNF as a predictor of functional outcome would be examined. Thus, a clear-cut association is yet to be revealed.
BDNF has been extensively used in stroke as an indicator of neural regeneration and recovery. Its role in angiogenesis, neurogenesis, brain repair, and synaptic plasticity has been unveiled through animal experiments and has established BDNF as an important component of post-stroke recovery [8,12,44]. MacLellan has also reported a positive correlation between BDNF and post-stroke rehabilitation in stroke models [45]. Nonetheless, the majority of the studies assessing the utility of BDNF derive from animal experiments. Thus, the exact role of BDNF, specifically in serum, in stroke patients is still unclear.
Stanne reported that BDNF levels measured during the acute stroke phase may not be correlated with early functional recovery (3 months post-stroke), but with late functional recovery (2 and 7 years post-stroke) [15]. The authors accredit their findings to the extended period needed for the recovery of various post-stroke deficits (3 to 6 months after stroke) [46]. From the studies we included, the period from stroke onset to follow-up was heterogeneous. The vast majority conducted a follow-up in less than three months, which varied from a few days post-stroke up to 7 years.
We assessed the role of BDNF levels in serum measured during the acute stroke phase. Di Lazzaro previously reported the stability of serum BDNF during the acute stroke phase in ten patients with first-ever acute ischemic stroke [47]. This finding has been hypothesized to be related to an early increase in BDNF in serum after stroke preceding the blood–brain barrier disruption, which follows at 3-4 h after stroke. In the few studies available with consecutive BDNF measurements after stroke, the findings are conflicting. Rodier studied 40 patients, 24 treated with rtPA, and 14 with standard care [42]. BDNF was measured at day 0, 1, 7, and 90 post-stroke. A variation of BDNF levels was indicated, especially in patients not treated with rtPA. Nonetheless, no available correlation between BDNF values was available to assess whether this variation was significant or not.
Concerning the acute stroke severity, from the twenty-three studies examined, seven studies included patients with mild stroke (NIHSS 1–4), while the vast majority of the studies included patients with mild or moderate stroke (NIHSS up to 15). Only one study included patients with moderate to severe stroke and none with severe. Concurrently, increased stroke severity, and even death by stroke were exclusion criteria in some of the studies included, especially in some assessing for depression as a primary endpoint. We conclude that the data regarding the utility of BDNF in patients with severe infarcts has not been extensively studied. Nonetheless, both stroke severity, the timing of BDNF measurement after stroke, and all the other study characteristics reported were comparable among the studies we included (Table 1).
We conducted a thorough review of all the available data concerning the relationship between BDNF, stroke outcome, and acute stroke severity. To our knowledge, this is the first time a negative correlation between acute stroke severity and BDNF and a significant decrease in BDNF levels in patients with stroke compared to controls has been reported. Our meta-analysis has some limitations. First, the differences in methodology among the included studies may have influenced our results. Nonetheless, the heterogeneity did not modify the significance of the effect in the meta-regression and the results of our sensitivity analyses were robust in most cases. Second, the findings of the studies included cannot be generalized to the population, as they derive from single-center studies. From the initial 26 studies included in our systematic review, only 11 studies were included in our meta-analysis due to insufficient data available. Finally, we restricted our search strategy in studies published in the English language.
Future studies should focus on defining the role of serum BDNF in predicting functional outcome measures from early phase up to 3–6 months after stroke. Regarding the role of BDNF as an acute stroke biomarker, the data available are insufficient, and further research is needed.
Author Contributions
Substantial contributions to conception and design by I.M., E.K. and V.P. Acquisition of data: I.M., E.K., D.K., A.P., F.P., S.C. Contributed to analysis and interpretation of data: I.M., V.P., A.C., A.-C.L., J.M., C.T. Drafted the article: I.M., E.K., V.P., A.P., S.C., A.-C.L., C.T. All authors revised the article critically for important intellectual content and final approval of the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the current data is available on request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO . The Top 10 Causes of Death. Global Health Estimates. World Health Organization; Geneva, Switzerland: 2020. [(accessed on 29 December 2020)]. Available online: www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. [Google Scholar]
- 2.Stroke Alliance for Europe . The Burden of Stroke in Europe. Stroke Alliance for Europe; London, UK: 2017. [Google Scholar]
- 3.Sato C. Releasing Mechanism of Neurotrophic Factors via Polysialic Acid. Vitam. Horm. 2017;104:89–112. doi: 10.1016/bs.vh.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Lu B., Pang P.T., Woo N.H. The Yin and Yang of Neurotrophin Action. Nat. Rev. Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 5.Berretta A., Tzeng Y.C., Clarkson A.N. Post-stroke recovery: The role of activity-dependent release of brain-derived neurotrophic factor. Expert Rev. Neurother. 2014;14:1335–1344. doi: 10.1586/14737175.2014.969242. [DOI] [PubMed] [Google Scholar]
- 6.Hohlfeld R. Neurotrophic cross-talk between the nervous and immune systems: Relevance for repair strategies in multiple sclerosis? J. Neurol. Sci. 2008;265:93–96. doi: 10.1016/j.jns.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Edelmann E., Lessmann V., Brigadski T. Pre- and Postsynaptic Twists in BDNF Secretion and Action in Synaptic Plasticity. [(accessed on 27 November 2019)]; doi: 10.1016/j.neuropharm.2013.05.043. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23791959. [DOI] [PubMed]
- 8.Panja D., Bramham C.R. BDNF mechanisms in late LTP formation: A synthesis and breakdown. Neuropharmacology. 2014;76:664–676. doi: 10.1016/j.neuropharm.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Polacchini A., Metelli G., Francavilla R., Baj G., Florean M., Mascaretti L.G., Tongiorgi E. A method for reproducible measurements of serum BDNF: Comparison of the performance of six commercial assays. Sci. Rep. 2015;5:17989. doi: 10.1038/srep17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lima G.B., Doorduin J., Klein H.C., Dierckx R.A.J.O., Bromberg E., de Vries E.F.J. Brain-Derived neurotrophic factor in brain disorders: Focus on neuroinflammation. Mol. Neurobiol. 2019;56:3295–3312. doi: 10.1007/s12035-018-1283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golden E., Emiliano A., Maudsley S., Windham B.G., Carlson O.D., Egan J.M. Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: Data from the baltimore longitudinal study of aging. PLoS ONE. 2010;5:e10099. doi: 10.1371/journal.pone.0010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pikula A., Beiser A.S., Chen T.C., Preis S.R., Vorgias D., Decarli C. Serum brain-derived neurotrophic factor and vascular endothelial growth factor levels are associated with risk of stroke and vascular brain injury framingham study. Stroke. 2013;44:2768–2775. doi: 10.1161/STROKEAHA.113.001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiao H.J., Li Z.Z., Wang L.M., Sun W., Yu J.C., Wang B. Association of lower serum Brain-derived neurotrophic factor levels with larger infarct volumes in acute ischemic stroke. J. Neuroimmunol. 2017;307:69–73. doi: 10.1016/j.jneuroim.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Stanne T.M., Aberg N.D., Nilsson S., Jood K., Blomstrand C., Andreasson U. Low circulating acute brain-derived neurotrophic factor levels are associated with poor long-term functional outcome after ischemic stroke. Stroke. 2016;47:1943–1945. doi: 10.1161/STROKEAHA.115.012383. [DOI] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. The PRISMA Group. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016;5:1–10. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J.P., Green S. Cochrane Handbook for Systematic Reviews. The Cochrane Collaboration; 2008. [DOI] [Google Scholar]
- 18.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:1–10. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asadollahi M., Nikdokht P., Hatef B., Sadr S.S., Sahraei H., Assarzadegan F. Protective properties of the aqueous extract of saffron (Crocus sativus L.) in ischemic stroke, randomized clinical trial. J. Ethnopharmacol. 2019;238:111833. doi: 10.1016/j.jep.2019.111833. [DOI] [PubMed] [Google Scholar]
- 20.Jiménez I., Sobrino T., Rodríguez-Yáñez M., Pouso M., Cristobo I., Sabucedo M. High serum levels of leptin are associated with post-stroke depression. Psychol. Med. 2009;39:1201. doi: 10.1017/S0033291709005637. [DOI] [PubMed] [Google Scholar]
- 21.Karakulova Y.V., Selyanina N.V., Zhelnin A.V., Filimonova T.A., Tsepilov S.V. Effects of Antioxidant Treatment on Neurotrophins and Rehabilitation Processes Following Stroke. Neurosci. Behav. Physiol. 2017;48:54–57. doi: 10.1007/s11055-017-0529-5. [DOI] [Google Scholar]
- 22.Lasek-Bal A., Jedrzejowska-Szypulka H., Student S., Warsz-Wianecka A., Zareba K., Puz P. The importance of selected markers of inflammation and blood-brain barrier damage for short-term ischemic stroke prognosis. J. Physiol. Pharmacol. 2019;70:209–217. doi: 10.26402/jpp.2019.2.04. [DOI] [PubMed] [Google Scholar]
- 23.Li J., Zhao Y.D., Zeng J.W., Chen X.Y., Wang R.D., Cheng S.Y. Serum Brain-derived neurotrophic factor levels in post-stroke depression. J. Affect. Disord. 2014;168:373–379. doi: 10.1016/j.jad.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 24.López-Cancio E., Ricciardi A.C., Sobrino T., Cortés J., de la Ossa N.P., Millán M. Reported prestroke physical activity is associated with vascular endothelial growth factor expression and good outcomes after stroke. J. Stroke Cerebrovasc. Dis. 2017;26:425–430. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Z., Lu T., Xu G., Yue X., Zhu W., Ma M. Decreased serum brain-derived neurotrophic factor (BDNF) is associated with post-stroke depression but not with BDNF gene Val66Met polymorphism. Clin. Chem. Lab. Med. 2011;49:185–189. doi: 10.1515/CCLM.2011.039. [DOI] [PubMed] [Google Scholar]
- 26.Mourão A.M., Vicente L.C.C., Abreu M.N.S., Sant’anna R.V.D.E., Meira F.C.A., Xavier R.M. Clinical and molecular correlates of the ASPECTS in the acute phase of stroke. [(accessed on 27 June 2020)];Arq. Neuropsiquiatr. 2020 78:262–268. doi: 10.1590/0004-282x20200001. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0004-282X2020000500262&lng=en&nrm=iso&tlng=en. [DOI] [PubMed] [Google Scholar]
- 27.Chaturvedi P., Singh A., Tiwari V., Thacker A. Diabetes mellitus type 2 impedes functional recovery, neuroplasticity and quality of life after stroke. [(accessed on 27 June 2020)];J. Fam. Med. Prim. Care. 2020 9:1035. doi: 10.4103/jfmpc.jfmpc_884_19. Available online: https://pubmed.ncbi.nlm.nih.gov/32318463/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Algin A., Erdogan M.O., Aydin I., Poyraz M.K., Sirik M. Clinical usefulness of brain-derived neurotrophic factor and visinin-like protein-1 in early diagnostic tests for acute stroke. Am. J. Emerg. Med. 2019;37:2501–2504. doi: 10.1016/j.ajem.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 29.Chan A., Yan J., Csurhes P., Greer J., McCombe P. Circulating brain derived neurotrophic factor (BDNF) and frequency of BDNF positive T cells in peripheral blood in human ischemic stroke: Effect on outcome. J. Neuroimmunol. 2015;286:42–47. doi: 10.1016/j.jneuroim.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Hutanu A., Mihaela I., Smaranda M., Rodica B.M.D. Plasma biomarkers as potential predictors of functional dependence in daily life activities after ischemic stroke: A single center study. Ann. Indian Acad. Neurol. 2019;23:496–503. doi: 10.4103/aian.AIAN_74_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lasek-Bal A., Jędrzejowska-Szypułka H., Różycka J., Bal W., Holecki M., Duława J. Low concentration of BDNF in the acute phase of ischemic stroke as a factor in poor prognosis in terms of functional status of patients. Med. Sci. Monit. 2015;31:3900–3905. doi: 10.12659/MSM.895358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mourão A.M., Vicente L.C.C., Abreu M.N.S., Vale Sant’Anna R., Vieira E.L.M., de Souza L.C. Plasma levels of brain-derived neurotrophic factor are associated with prognosis in the acute phase of ischemic stroke. J. Stroke Cerebrovasc. Dis. 2019;28:735–740. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Otero-Ortega L., Gutiérrez-Fernández M., Gutiérrez-Zúñiga. R., Madero-Jarabo R., Alonso de Leciñana M., Laso-García F. The effect of post-stroke hyperglycaemia on the levels of brain damage and repair-related circulating biomarkers: The Glycaemia in Acute Stroke Study II. Eur. J. Neurol. 2019;26:1439–1446. doi: 10.1111/ene.14010. [DOI] [PubMed] [Google Scholar]
- 34.Pedard M., Brenière C., Pernet N., Vergely C., Béjot Y., Marie C. Brain-derived neurotrophic factor in peripheral blood mononuclear cells and stroke outcome. Exp. Biol. Med. 2018;243:1207–1211. doi: 10.1177/1535370218815612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sobrino T., Rodríguez-Yáñez M., Campos F., Iglesias-Rey R., Millán M., de la Ossa N.P. Association of High Serum Levels of Growth Factors with Good Outcome in Ischemic Stroke: A Multicenter Study. Transl. Stroke Res. 2020;11:653–663. doi: 10.1007/s12975-019-00747-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J., Gao L., Yang Y.L., Li Y.Q., Chang T., Man M.H. Low Serum Levels of Brain-Derived Neurotrophic Factor Were Associated with Poor Short-Term Functional Outcome and Mortality in Acute Ischemic Stroke. Mol. Neurobiol. 2017;54:7335–7442. doi: 10.1007/s12035-016-0236-1. [DOI] [PubMed] [Google Scholar]
- 37.Yang L., Zhang Z., Sun D., Xu Z., Yuan Y., Zhang X. Low serum BDNF may indicate the development of PSD in patients with acute ischemic stroke. Int. J. Geriatr. Psychiatry. 2011;26:495–502. doi: 10.1002/gps.2552. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J., Mu X., Breker D.A., Li Y., Gao Z., Huang Y. Atorvastatin treatment is associated with increased BDNF level and improved functional recovery after atherothrombotic stroke. Int. J. Neurosci. 2017;127:92–97. doi: 10.3109/00207454.2016.1146882. [DOI] [PubMed] [Google Scholar]
- 39.Kozak H.H., Uğuz F., Kılınç İ., Uca A.U., Serhat Tokgöz O., Akpınar Z. Delirium in patients with acute ischemic stroke admitted to the non-intensive stroke unit: Incidence and association between clinical features and inflammatory markers. Neurol. Neurochir. Pol. 2017;51:38–44. doi: 10.1016/j.pjnns.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Kozak H.H., Uuz F., Kilinç I., Uca A.U., Tokgöz O.S., Güney F. A cross-sectional study to assess the association between major depression and inflammatory markers in patients with acute ischemic stroke. Indian J. Psychiatry. 2019;61:283–289. doi: 10.4103/psychiatry.IndianJPsychiatry_175_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guan Y.Z., Li J., Zhang X.W., Wu S., Du H., Cui L.Y. Effectiveness of repetitive transcranial magnetic stimulation (rTMS) after acute stroke: A one-year longitudinal randomized trial. CNS Neurosci. Ther. 2017;23:940–946. doi: 10.1111/cns.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodier M., Quirié A., Prigent-Tessier A., Béjot Y., Jacquin A., Mossiat C. Relevance of post-stroke circulating BDNF levels as a prognostic biomarker of stroke outcome. Impact of rt-PA treatment. PLoS ONE. 2015;10:e0140668. doi: 10.1371/journal.pone.0140668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J., Huang Q., Ding J., Wang X. Elevated serum levels of brain-derived neurotrophic factor and miR-124 in acute ischemic stroke patients and the molecular mechanism. [(accessed on 23 February 2020)];3 Biotech. 2019 9:386. doi: 10.1007/s13205-019-1914-2. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31656724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mamounas L.A., Altar C.A., Blue M.E., Kaplan D.R., Tessarollo L., Lyons W.E. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J. Neurosci. 2000;20:771–782. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacLellan C.L., Keough M.B., Granter-Button S., Chernenko G.A., Butt S., Corbett D. A critical threshold of rehabilitation involving brain-derived neurotrophic factor is required for poststroke recovery. Neurorehabil. Neural Repair. 2011;25:740–748. doi: 10.1177/1545968311407517. [DOI] [PubMed] [Google Scholar]
- 46.Caplan L.R. Caplan’s Stroke: A Clinical Approach. 5th ed. Cambridge University Press; Cambridge, UK: 2016. [Google Scholar]
- 47.Di Lazzaro V., Profice P., Pilato F., Dileone M., Florio L., Tonali P.A. BDNF plasma levels in acute stroke. Neurosci. Lett. 2007;422:128–130. doi: 10.1016/j.neulet.2007.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the current data is available on request from the authors.