Abstract
Marine natural products (MNPs) have been an important and rich source for antimicrobial drug discovery and an effective alternative to control drug resistant infections. Herein, we report bioassay guided fractionation of marine extracts from sponges Lendenfeldia, Ircinia and Dysidea that led us to identify novel compounds with antimicrobial properties. Tertiary amines or quaternary amine salts: aniline 1, benzylamine 2, tertiary amine 3 and 4, and quaternary amine salt 5, along with three known compounds (6–8) were isolated from a crude extract and MeOH eluent marine extracts. The antibiotic activities of the compounds, and their isolation as natural products have not been reported before. Using tandem mass spectrometry (MS) analysis, potential structures of the bioactive fractions were assigned, leading to the hit validation of potential compounds through synthesis, and commercially available compounds. This method is a novel strategy to overcome insufficient quantities of pure material (NPs) for drug discovery and development which is a big challenge for pharmaceutical companies. The antibacterial screening of the marine extracts has shown several of the compounds exhibited potent in-vitro antibacterial activity, especially against methicillin-resistant Staphylococcus aureus (MRSA) with minimum inhibitory concentration (MIC) values between 15.6 to 62.5 microg mL−1. Herein, we also report structure activity relationships of a diverse range of commercial structurally similar compounds. The structure-activity relationships (SAR) results demonstrate that modification of the amines through linear chain length, and inclusion of aromatic rings, modifies the observed antimicrobial activity. Several commercially available compounds, which are structurally related to the discovered molecules, showed broad-spectrum antimicrobial activity against different test pathogens with a MIC range of 50 to 0.01 µM. The results of cross-referencing antimicrobial activity and cytotoxicity establish that these compounds are promising potential molecules, with a favourable therapeutic index for antimicrobial drug development. Additionally, the SAR studies show that simplified analogues of the isolated compounds have increased bioactivity.
Keywords: marine natural products, tandem mass spectrometry (MS), structure—activity relationships (SAR), antimicrobial drug discovery, multidrug resistant bacteria, methicillin-resistant Staphylococcus aureus
1. Introduction
Human pathogens are associated with a variety of moderate to severe infections and the recent rise of multi-drug resistant pathogens makes treatment more difficult [1]. The last two decades have seen the emergence of methicillin-resistant Staphylococcus aureus (MRSA) strains resistant even to ‘drugs of last resort’ such as vancomycin [2], and Mycobacterium tuberculosis resistant to all first-line agents [3,4,5], highlighting the urgent need to find new effective antibiotics with distinct mechanisms of action.
Natural products continue to offer a productive source of structural diversity and bioactivity and therefore hold the potential for the discovery of new and efficacious antimicrobial drugs [6,7,8,9]. Almost 70 percent of the Earth’s surface is covered by ocean, representing a huge reserve of natural biological and chemical diversity on our planet [10]. Marine ecosystems have long been a rich source of bioactive natural products in the search for interesting molecules and novel therapeutic agents [6,7,11,12,13]. Many interesting and structurally diverse secondary metabolites have been isolated from marine sources over the last 70 years [8,9,14,15]. In addition, the preclinical pharmacology of seventy-five compounds isolated from marine organisms have been reported to have biological activities [16]. Yet the first drugs from marine natural products were only approved in the early 2000s: the cone snail peptide ziconotide (ω-conotoxin MVIIA) in 2004 to alleviate chronic pain [17], and sea squirt metabolite trabectedin in 2007 for the treatment of soft-tissue sarcoma [18]. Marine natural products (MNPs) have displayed exceptional potency and potential as anticancer therapeutics [19]. The interest in MNPs has continued to grow [8,14,15], spurred in part by the spread of antimicrobial resistant pathogens and the need for new drugs to combat them [6].
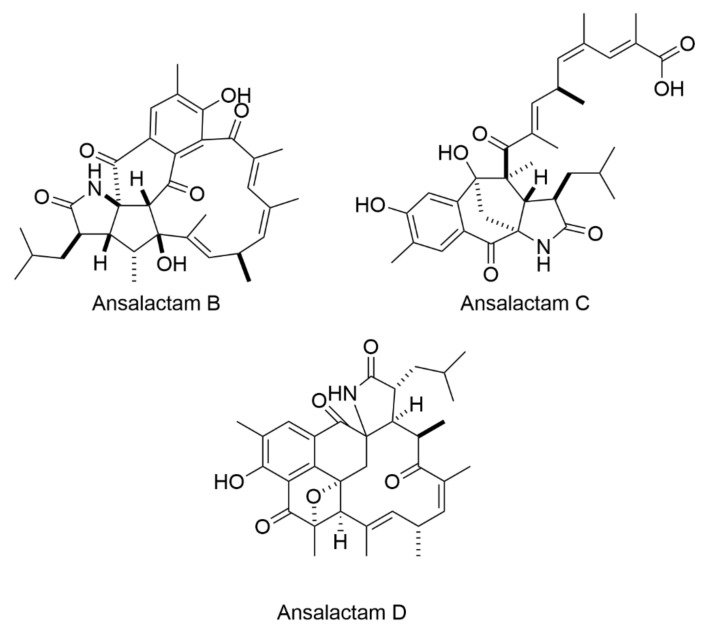
The most prolific marine organisms are sponges [6], and the oldest metazoans on earth belong to the phylum Porifera [20]. The Demospongiae are the most abundant class of Porifera, representing 83% of described species [20,21], and has the largest number of bioactive compounds [19]. The genus Lendenfeldia is known as a source of sulfated sterols [22] and metabolites from the Lendenfeldia species have anti-HIV, anti-tumor [23], anti-inflammatory, antifouling [24] activities but they lack antimicrobial activity [22]. Secondary metabolites of the genus Ircinia and Dysidea are prime candidates for further study to unveil biological metabolites with antibacterial activity (Figure 1) [15,25,26,27].
Figure 1.
Ansalactams B–D displayed moderate antibacterial activity towards MRSA [15].
In the search for new antimicrobial agents, we screened a set of marine extracts [28] to determine activity against antibiotic resistant microorganisms using a high-throughput screening (HTS) assay. The fractionation and purification of active components by high-performance liquid chromatography (HPLC), Nuclear magnetic resonance (NMR) and structural elucidation using high resolution and tandem mass spectrometry (MS) led us to a series of potential scaffolds for new, bioactive amine natural products (Figure 1 and Figures S20, S21 and S25).
Following on from these studies, we decided to screen commercially available compounds, with structural similarity to the amine scaffolds we identified, synthesised [29] and ascididemin-like compounds. Thus, we found these molecules to be an interesting potential structure for further development, focusing on increasing antimicrobial potency by investigating the structure—activity relationship (SAR). Based on these facts, in this work, small-molecule libraries of commercial analogues were studied to increase the structural variability and improving the pharmacological properties of the final drugs.
2. Results and Discussion
2.1. Identification of Active Marine Extracts
To identify marine samples with activity against MRSA, 1434 compounds from the AIMS Bioresources Library [28] (provided by the Queensland Compound Library, Brisbane, Australia [30], now called Compounds Australia [31]) were screened in a resazurin cell viability assay. Of the samples tested, 29 inhibited the growth of MRSA by greater than 50% compared to non-treated controls. The minimum inhibitory concentrations (MICs) were determined for the 23 most promising samples, representing extracts and fractions from the phyla Porifera (90%), Echinodermata (5%) and Chordata (5%) (Table 1). The five most active samples showed MICs at 31.3 µg mL−1 (all Porifera samples), while another four samples returned MICs of 62.5 µg mL−1 (also all Porifera). Cytotoxicity screens against HepG2, HEK 293, A549 and THP-1 cell lines were performed with concentrations from 500 µg mL−1 to 7.8 µg mL−1 to define the cytotoxicity profile of the most active samples. Pleasingly, all the samples most active against MRSA were also nontoxic to the cell lines tested (Table 1).
Table 1.
Summary of the nine marine samples selected for further study.
| Entry | AIMS Sample Code | QCL Sample Number | MIC (µg mL−1) | Cytotoxicity (% Cell Survival) | ||
|---|---|---|---|---|---|---|
| MRSA | Hep G2 | A549 | HEK | |||
| 1 | 19,033 | SN00733110 | 31.25 ± 0.9 | 91 ± 1.2 | 91 ± 0.3 | 98 ± 1.6 |
| 2 | 20,608 | SN00760947 | 31.25 ± 1.3 | 97 ± 1.0 | 101 ± 2.9 | 102 ± 0.7 |
| 3 | 20,608 | SN00760956 | 31.25 ± 0.4 | 100 ± 4.4 | 106 ± 1.8 | 95 ± 1.0 |
| 4 | 20,608 | SN00760958 | 62.5 ± 2.2 | 98 ± 0.6 | 108 ± 14 | 95 ± 2.5 |
| 5 | 26,051 | SN00731005 | 62.5 ± 2.8 | 101 ± 1.0 | 110 ± 2.7 | 98 ± 2.0 |
| 6 | 24,307 | SN00730755 | 31.25 ± 1.0 | 100 ± 1.0 | 106 ± 1.0 | 96 ± 4.0 |
| 7 | 25,663 | SN00732222 | 15.6 ± 1.5 | 100 ± 1.6 | 89 ± 2.0 | 97 ± 0.0 |
| 8 | 26,104 | SN00734298 | 62.5 ± 1.0 | 98 ± 0.5 | 100 ± 1.1 | 98 ± 1.1 |
| 9 | 22,565 | SN00739718 | 31.25 ± 0.4 | 100 ± 0.0 | 99 ± 0.4 | 97 ± 1.0 |
2.2. Isolation and Characterization of Bioactive Compounds
Following the primary screening of the AIMS library and selection of positive hits, HPLC was used to separate and isolate active compounds, guided by bioassays against MRSA. The extracts were fractionated by analytical HPLC (see Experimental section and Supporting Information for further details), and fractions evaluated for bioactivity. Preparative scale HPLC was carried out on each bulk sample to isolate the active component (Table S1 and Figures S1–S10), NMR and tandem mass spectrometry (MS/MS) methods used to deduce potential structures for compounds (Table 2 and Supporting Information) [32,33,34]. Insufficient quantities were obtained for positive ion high resolution mass spectrometry (HRMS) analyses.
Table 2.
Key high resolution mass spectrometry (HRMS) data for bioactive samples, and proposed structures as shown in the Supplementary Materials file 1.
| Sample | Molecular Ion (m/z) | Molecular Formula | Proposed Structures | Spectra | MS/MS | NMR Analyses |
|---|---|---|---|---|---|---|
| 19,033 † | 326.37813 [M + H]+ |
[C22H48N]+ Calc. = 326.37802 (∆m = 0.11 ppm) RDBE = 0 |
3 | Figure S1 |
Table S2
Figure S11 |
- |
| 20,608 | 332.33115 [M + H]+ |
[C23H42N]+ Calc = 332.33118 (Δm = 0.03 ppm) RDBE = 4 ‡ |
1,2 | Figure S3 |
Table S3
Figure S13 |
- |
| 26,051 | 368.42508 [M + H]+ |
[C25H54N]+ Calc. = 368.42495 (∆m = 0.13 ppm) RDBE = 0 |
4,5 | Figure S5 |
Table S4
Figure S15 |
- |
| 25,663 | 306.0635 [M+Na]+ |
[C18H9ON3] Calc. = 306.0637 (∆m = 0.8 ppm) RDBE = 16 |
6 | Figure S7 | - |
Table S5
Figure S20 |
| 25,663 | 361.9925 [M + H]+ |
[C18H8BrN3O] Calc. = 361.9923 (∆m = 0.5 ppm) RDBE = 16 |
7 | Figure S7 | - |
Table S6 Figure S21 |
| 26,104 | 467.117 [M + H]+ |
[C30H17N2O4] Calc. = 467.118 (∆m = 1.9 ppm) RDBE = 0 |
8 | Figure S9 | - |
Table S7
Figures S22–S25 |
† The same active species was observed for all five fractions SN00760947, SN00760956, SN00732222, SN00734298 and SN00760958. ‡ RDBE = ring or double bond equivalents.
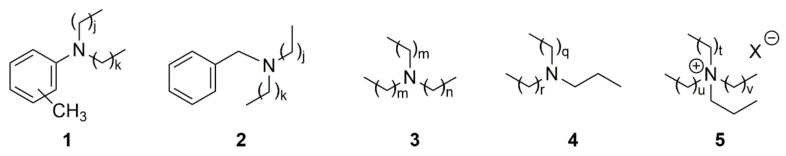
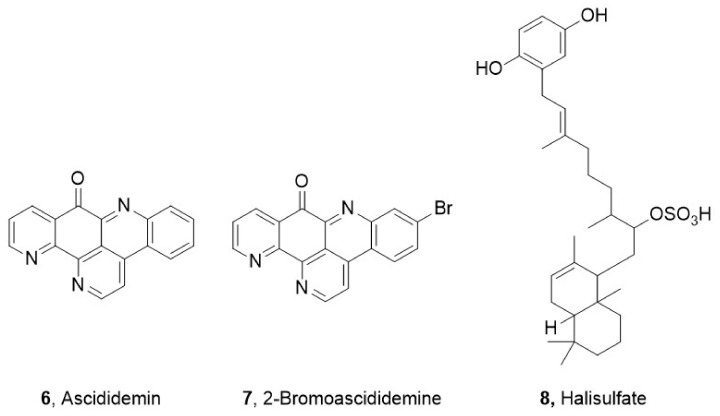
Active components were isolated and characterised for eight of the nine extracts shown in Table 1: aniline 1 and benzylamine 2 from the Lendenfeldia sp. samples (AIMS Sample Code 20608, Table 1 entries 2–4, Figure 2); tertiary aliphatic amine 3 from the Dysidea herbacea extract (AIMS Sample Code 19033, Table 1 entry 1, Figure 2); aliphatic tertiary amine 4 and quaternary amine salt 5 from Ircinia gigantea (AIMS Sample Code 26051, Table 1 entry 5, Figure 2); ascididemin 6 and 2-bromoascididemin (2-Bromoleptoclinidinone)7 from the Flavobranchia samples (AIMS Sample Code 25663, Table 1 entries 7, Figure 2 and Figures S26 and S27); and halisulfate 8 from Ircinia (AIMS Sample Code 26104, Table 1 entry 8, Figure 2 and Figures S26 and S27). The aniline/amines 1–5 have not previously been identified as natural products, nor have their antimicrobial activities been assessed.
Figure 2.
Proposed structures of bioactive amine natural products identified as new natural products compounds (1–5) and rediscovered compounds (6–8) in this study; j = 14; m = 5, n = 9; q = 20; t = 19; X = unidentified counterion.
Compounds 6, 7 [35,36,37] and 8 [38,39,40,41] have previously been reported (Figure 2), and in the current study these structures were confirmed by comparison of data with HRMS, NMR and literature values. The antimycobacterial activity of compounds 6, 7 and 8 with wide range of MIC against different microorganisms have also been reported [42,43].
2.3. Structure—Activity Relationship
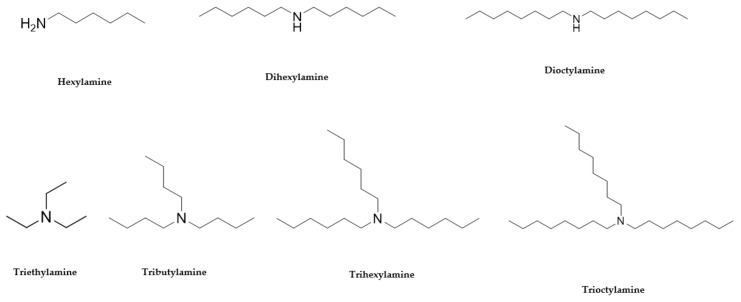
The SAR was investigated through commercially available compounds of three groups of molecules, namely the tertiary amines, benzyl substituted amines [29], and the ascididemin structural motifs. To investigate the number of carbons in the linear chains, commercially available amines compounds with structures similar to synthesised compounds 3 and 13 [29] (Figure 3), such as triethylamine, tributylamine and trihexylamine were screened; in addition, the primary and secondary amines hexylamine, dihexylamine and dioctylamine were screened. Biological activities of commercial compounds are based on the molecular weight of the proposed structures.
Figure 3.
The commercially available amines with structures similar to 3 and 13 [29].
The results show a general trend of increased activity with increasing chain length of the tertiary amines (triethylamine, tributylamine, trihexylamine and trioctylamine), as seen in the bioactivity against MRSA, E. coli and P. aeruginosa (Table 3). In addition, the trend in the primary, secondary, and tertiary hexylamines, show increased activity with an increasing number of alkyl chains; hexylamine, showed activity against MRSA, E. coli and P. aeruginosa with MICs of 50 to 100 µM, which all improve to MICs of 3 to 6.5 µM for trihexylamine (Table 3). Interestingly, trioctylamine with eight carbon alkyl chains, showed strong activity against M. tuberculosis H37Rv (MIC of 0.02 µM). Furthermore, dicyclohexylamine has poorer bioactivity than dihexylamine, suggesting potential benefits of linear chains. This trend in structural modification did not improve activity against M. tuberculosis H37Rv or alter the toxicity to cell lines except HEK 293 (Table 3).
Table 3.
Screening of commercially available amines with structures similar to 3 and 13 (The biological activities are based on the molecular weight of the proposed structures).
| Name and CAS no. | Structures | MRSA | E. coli | P. aeruginosa | M. tuberculosis | HepG2 | HEK 293 | A549 | THP-1 |
|---|---|---|---|---|---|---|---|---|---|
|
Hexylamine 111-26-2 |

|
>100 | >100 | 50 | >100 | >100 | >100 | >100 | >100 |
|
Dihexylamine 143-16-8 |
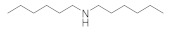
|
12.5 | 12.5 | 6.2 | >100 | >100 | >100 | >100 | >100 |
|
Dioctylamine 1120-48-5 |
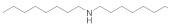
|
6.2 | 6.2 | 3 | >100 | >100 | >100 | >100 | >100 |
|
Triethylamine 121-44-8 |
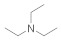
|
>100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
|
Tributylamine 102-82-9 |
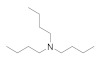
|
>100 | >100 | 50 | >100 | >100 | >100 | >100 | >100 |
|
Trihexylamine 102-86-3 |
|
3 | 3 | 6.5 | >100 | >100 | 50 | >100 | >100 |
|
Trioctylamine 1116-76-3 |
|
12.5 | 25 | 25 | 0.02 | >100 | 6 | 50 | 3.1 |
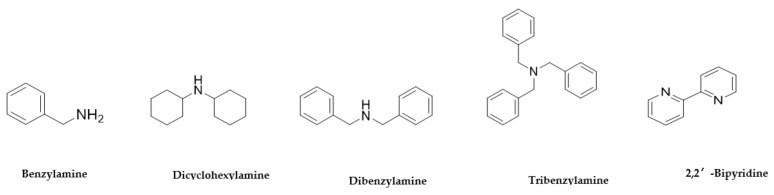
Further structures related to 1a, 1b and 2a [29] and the sequence of benzylamine, dibenzylamine, tribenzylamine, were investigated (Figure 4). Observations from this experiment correlate with the increased substitution leading to increased bioactivity against P. aeruginosa. Anti P. aeruginosa activity was improved to MIC = 6.5 with tribenzylamine (Table 4).
Figure 4.
The commercially available amines with structures similar to 1a, 1b and 2a [29].
Table 4.
Screening of commercially available amines with structures related to 1a, 1b and 2a.
| Name and CAS no. | Structures | MRSA | E. coli | P. aeruginosa | M. tuberculosis | HepG2 | HEK 293 | A549 | THP-1 |
|---|---|---|---|---|---|---|---|---|---|
|
Benzylamine 100-46-9 |
|
>100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
|
Dicyclohexylamine 101-83-7 |
|
>100 | >100 | 12.5 | >100 | >100 | >100 | >100 | >100 |
|
Dibenzylamine 103-49-1 |
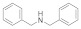
|
>100 | >100 | 12.5 | >100 | >100 | >100 | >100 | >100 |
|
Tribenzylamine 620-40-6 |
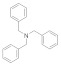
|
>100 | >100 | 6.5 | >100 | >100 | >100 | >100 | >100 |
|
2,2′-Bipyridine 366-18-7 |
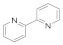
|
>100 | >100 | >100 | 0.1 | 3 | 3 | >100 | 50 |
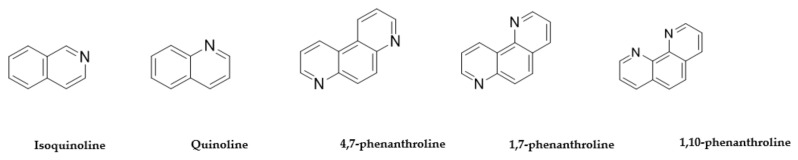
The third group of compound structures we looked at for SAR analysis are related to the structural motifs of ascididemin (Figure 5), which is a pentacyclic aromatic alkaloid, bearing similarity to motifs of quinoline and phenanthroline. Therefore, isoquinoline, quinoline, 4,7-phenanthroline, 1,7-phenanthroline, 1,10-phenanthroline and bipyridine structures (Table 4 and Table 5) were evaluated for bioactivity inspired by the ascididemin structure. The results show isoquinoline and quinoline displayed improved anti-microbial activity, especially against M. tuberculosis (Table 5). Furthermore, the fused tricyclic cores of 1,10-phenanthroline, when compared with the more flexible 2,2′-bipyridine, showed activity against M. tuberculosis with MIC = 0.1 but also displayed toxicity for 4 cell lines tested: HepG2, HEK 293 and THP-1. Interestingly, structures in which the two nitrogens were closer to each other have more activity against M. tuberculosis and P. aeruginosa while the opposite situation was seen with MRSA and E. coli activity; this can be observed by comparing the results of 1,10-phenanthroline with those observed for 4,7-phenanthroline. The toxicity assays determined that the A549 cell line is more resistant to 4,7-phenanthroline. Alongside the acceptable antimicrobial activity of 4,7-phenanthroline and 1,7-phenanthroline, also show limited toxicity against mammalian cells (Table 5). Meanwhile, 1,10-phenanthroline displayed variable toxicity and antimicrobial activity (Table 5).
Figure 5.
The commercially available compounds with structures inspired by ascididemin.
Table 5.
Screening of commercially available compounds with structures inspired by ascididemin.
| Name and CAS no. | Structures | MRSA | E. coli | P. aeruginosa | M. tuberculosis | HepG2 | HEK 293 | A549 | THP-1 |
|---|---|---|---|---|---|---|---|---|---|
| Isoquinoline 119-65-3 |
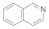
|
>100 | >100 | >100 | 0.1 | 3 | >100 | 50 | 3 |
| Quinoline 91-22-5 |
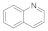
|
>100 | >100 | >100 | 0.1 | 3 | 50 | 50 | 3 |
| 4,7-phenanthroline 230-07-9 |
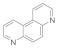
|
1.5 | 1.5 | 2.5 | 2.5 | >100 | >100 | >100 | >100 |
| 1,7-phenanthroline 230-46-6 |
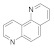
|
25 | 25 | 2.5 | >100 | >100 | >100 | >100 | >100 |
| 1,10-phenanthroline 66-71-7 |
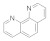
|
>100 | 50 | 0.3 | 0.1 | 0.1 | 50 | 3 | 0.1 |
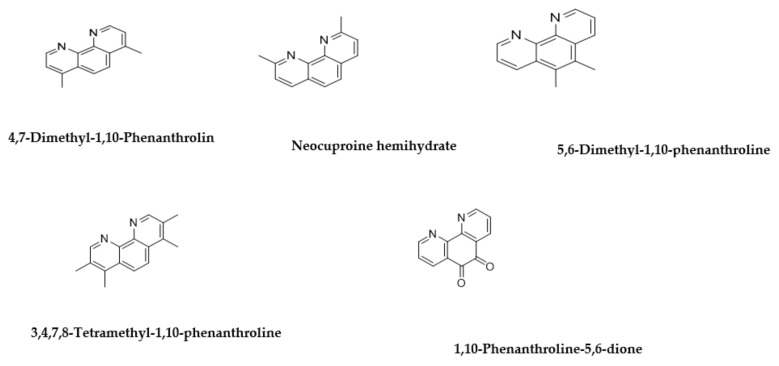
To further study the phenanthroline compounds (Figure 6) we evaluated 4,7-dimethyl-1,10-phenanthrolin, neocuproine hemihydrate, 5,6-dimethyl-1,10-phenanthroline, 3,4,7,8-tetramethyl-1,10-phenanthroline, 1,10-phenanthroline-5,6-dione, 5-nitro-1,10-phenanthroline, bathophenanthroline, 2,9-dimethyl-4,7-diphenyl-1,10-phenanthrolin, and bathocuproine disulfonic acid for bioactivity (Table 6). Modifying the three aromatic rings further, by adding methyl groups as in 4,7-dimethyl-1,10-phenanthroline, neocuproine hemihydrate and 5,6-dimethyl-1,10-phenanthroline (Figure 7) improved the anti-bacterial activity of the compounds but also increased their toxicity effect on all cell lines tested (Table 6). Maintaining one of the methyl groups in the rings as in 5,6-dimethyl-1,10-phenanthroline reduced the activity against P. aeruginosa (Table 6).
Figure 6.
The compounds with structures inspired by ascididemin which include three aromatic rings.
Table 6.
Screening of compounds with structures inspired by ascididemin which include three aromatic rings.
| Name and CAS no. | Structures | MRSA | E. coli | P. aeruginosa | TB. H37Rv | HepG2 | HEK 293 | A549 | THP-1 |
|---|---|---|---|---|---|---|---|---|---|
| 4,7-Dimethyl-1,10-Phenanthrolin 3248-05-3 |
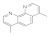
|
0.3 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Neocuproine hemihydrate 34302-69-7 |
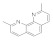
|
0.09 | 0.3 | 0.04 | 0.1 | 0.1 | 0.1 | 6.2 | 12 |
| 5,6-Dimethyl-1,10-phenanthroline 3002-81-1 |
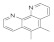
|
0.3 | 0.78 | >100 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 3,4,7,8-Tetramethyl-1,10-phenanthroline 1660-93-1 |
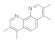
|
0.3 | 0.3 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 1,10-Phenanthroline-5,6-dione 27318-90-7 |
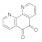
|
0.04 | 0.04 | 0.04 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
Figure 7.
The compounds with structures inspired by ascididemin which include three aromatic rings plus further modifications.
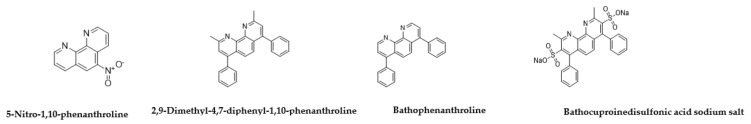
Increasing the number of methyl substituents to four (3,4,7,8-tetramethyl-1,10-phenanthroline) or incorporating carbonyl groups (1,10-phenanthroline-5,6-dione) or a nitro group (5-nitro-1,10-phenanthroline) instead of the methyl groups enhanced the activity of the compounds (Table 6 and Table 7). A different behaviour was only observed against P. aeruginosa in the presence of the nitro group. The inclusion of two additional aromatic rings as pendants on the phenanthroline core (bathophenanthroline) showed impressive antibacterial activity with MIC = 0.09 µM for MRSA, MIC = 0.019 µM for E. coli, MIC = 0.03 µM for P. aeruginosa and MIC = 0.01 µM for M. tuberculosis. Furthermore, cross-referencing the biological activity and toxicity of these molecules demonstrated they are more potent against the microorganisms than against mammalian cells. In addition, adding two methyl groups or two sulfonate groups, as in 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline and bathocuproine disulfonic acid, respectively, resulted in the reduced activity of molecules against bacteria, but also a reduction in toxicity against the A549 and THP-1 cells. However, including the sulfonate groups improved the activity against the P. aeruginosa with MIC = 0.19 µM (Table 7).
Table 7.
Screening of compounds with structures inspired by ascididemin which include three aromatic rings plus further modifications.
| Name and CAS no. | Structures | MRSA | E. coli | P. aeruginosa | TB. H37Rv | HepG2 | HEK 293 | A549 | THP-1 |
|---|---|---|---|---|---|---|---|---|---|
| 5-Nitro-1,10-phenanthroline 4199-88-6 |
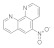
|
0.09 | 0.78 | >100 | 0.1 | 0.1 | 0.1 | 12 | 0.1 |
| Bathophenanthroline 1662-01-7 |
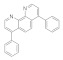
|
0.09 | 0.019 | 0.03 | 0.01 | 1.5 | 25 | 3 | 13 |
| 2,9-Dimethyl-4,7-diphenyl-1,10-phenanthroline 4733-39-5 |
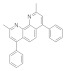
|
25 | 0.78 | 50 | 50 | 0.1 | 0.1 | >100 | >100 |
| Bathocuproinedisulfonic acid sodium salt 1257642-74-2 |
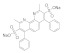
|
>100 | >100 | 0.19 | 1.2 | 50 | 0.1 | >100 | >100 |
A summary of all activities of the commercially available amines that were screened is presented in Table 8. The potential antimicrobial activity of commercial materials which are structurally related to ascididemin (Table 6 and Table 7) was identified by bioactivity screening. 1,10-phenanthroline-5,6-dione at MIC 0.04 μM was found to be a potent inhibitor of MRSA, while bathophenanthroline at MIC 0.019 μM, MIC 0.03 μM and MIC 0.01 μM showed strong activity against E. coi, P. aeruginosa and M. tuberculosis, respectively. These results reinforce the relationship between structures and anti-microbial activity for the synthesised compounds. For example, in the third group of compounds tested (Table 6 and Table 7) bioactivity was optimal for 1,10-phenanthroline-5,6-dione containing carbonyl groups, and biological activity depends on structural properties such as the length of the aliphatic linker, and the side chain of these compounds. The results of these experiments further suggest that hydrophobic compounds, such as dioctylamine (Table 3) and bathophenanthroline (Table 7), have a more pronounced effect on the inhibition of bacterial growth, as has been reported previously [41,42,43,44,45,46,47].
Table 8.
Summary of anti-bacterial activity and toxicity of compounds inspired by the natural products characterised in this project.
| Sample Name, structure and CAS no | Minimum Inhibitory/Toxicity Concentration of Drug (µM) | |||||||
|---|---|---|---|---|---|---|---|---|
| MRSA | E. coli | P. aeruginosa | M. tuberculosis H37Rv | HepG2 | HEK 293 | A549 | THP-1 | |
Hexylamine  111-26-2 111-26-2 |
>100 | >100 | 50 | >100 | >100 | >100 | >100 | >100 |
Dihexylamine 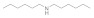 143-16-8 143-16-8 |
12.5 | 12.5 | 6.2 | >100 | >100 | 100 | >100 | >100 |
Dioctylamine 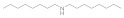 1120-48-5 1120-48-5 |
6.2 | 6.2 | 3 | >100 | >100 | >100 | >100 | >100 |
Triethylamine 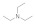 121-44-8 121-44-8 |
>100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
Tributylamine  102-82-9 102-82-9 |
>100 | >100 | 50 | >100 | >100 | >100 | >100 | >100 |
Trihexylamine  102-86-3 102-86-3 |
3 | 3 | 6.5 | >100 | >100 | 50 | 100 | >100 |
Trioctylamine  1116-76-3 1116-76-3 |
12.5 | 25 | 25 | 0.02 | >100 | 6 | 50 | 3.1 |
Benzylamine  100-46-9 100-46-9 |
>100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
Dicyclohexylamine  101-83-7 101-83-7 |
>100 | >100 | 12.5 | >100 | >100 | >100 | >100 | >100 |
Dibenzylamine 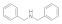 103-49-1 103-49-1 |
>100 | >100 | 12.5 | >100 | >100 | >100 | >100 | >100 |
Tribenzylamine 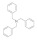 620-40-6 620-40-6 |
>100 | >100 | 6.5 | >100 | >100 | >100 | >100 | >100 |
2,2′-Bipyridyl 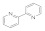 366-18-7 366-18-7 |
>100 | >100 | >100 | 0.1 | 3 | 3 | >100 | 50 |
Isoquinoline 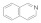 119-65-3 119-65-3 |
>100 | >100 | >100 | 0.1 | 3 | 3 | >100 | 50 |
Quinoline 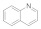 91-22-5 91-22-5 |
>100 | >100 | >100 | 0.1 | 3 | 3 | 50 | 50 |
4,7-phenanthroline 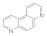 230-07-9 230-07-9 |
1.5 | 1.5 | 2.5 | 2.5 | >100 | >100 | >100 | >100 |
1,7-phenanthroline 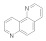 230-46-6 230-46-6 |
25 | 25 | 2.5 | >100 | >100 | >100 | >100 | >100 |
1,10 phenanthroline 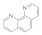 66-71-7 66-71-7 |
>100 | 50 | 0.3 | 0.1 | 0.1 | 0.1 | 50 | 3 |
4,7-DImethyl-1,10-Phenanthroline 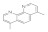 3248-05-3 3248-05-3 |
0.3 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
Neocuproine hemihydlyate 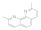 34302-69-7 34302-69-7 |
0.09 | 0.3 | 0.04 | 0.1 | 0.1 | 0.1 | 6.2 | 12 |
5,6-Dimethyl-1, 10-phenanthroline 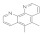 3002-81-1 3002-81-1 |
0.3 | 0.78 | >100 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
3,4,7,8-Tetramethyl-1,10-phenanthroline 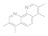 1660-93-1 1660-93-1 |
0.3 | 0.3 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
1,10-Phenanthroline-5,6-dione 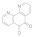 27318-90-7 27318-90-7 |
0.04 | 0.04 | 0.04 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
5-Nitro-1,10-phenanthroline 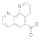 4199-88-6 4199-88-6 |
0.09 | 0.78 | >100 | 0.1 | 0.1 | 0.1 | 12 | 0.1 |
Bathophenanthroline 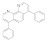 1662-01-7 1662-01-7 |
0.09 | 0.019 | 0.03 | 0.01 | 1.5 | 25 | 3 | 13 |
2,9-Dimethyl-1,7-diphenyl-1,10-phenatroline 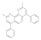 4733-39-5 4733-39-5 |
25 | 0.78 | 50 | 50 | 0.1 | 0.1 | >100 | >100 |
Bathocuproinedisulfonic acid sodium salt 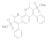 1257642-74-2 1257642-74-2 |
>100 | >100 | 0.19 | 1.2 | 50 | 0.1 | >100 | >100 |
Furthermore, the side chain length significantly affects the hydrophobicity of these compounds, and subsequently the logP values, which are important molecular descriptors in determining biological activity. The presence of aromatic rings with short pendant groups and nitrogen-containing rings in 2,2′-bipyridine significantly reduced the activity of the molecules against the Gram-positive and Gram-negative pathogens tested, however the compounds were active against mycobacteria (Table 4). This suggests that the mechanism of action may be based on targeting complex cell wall structures, such as those present in mycobacteria. Many factors influence the activity of a molecule, including side chain length, symmetry, length of the alkyl chain, the presence of charged pyridinium moiety, the presence and position of the carboxylate in the structure, the position of the methyl group, the distance between the two pyridinium moieties and the chain linker between the two pyridinium rings. Thus, the mechanism of action of these molecules may be based on the space between the rings and distance between charged moieties, which possibly manifests through interaction with membrane sites. These results are in agreement with previous reports [48,49]; however, to clarify such hypotheses and determine important factors in bioactivity further pharmacological and biophysical studies are necessary to determine the mechanisms of action.
3. Materials and Methods
3.1. General
Chemical reagents were purchased from BDH Chemicals and Sigma Aldrich (Castle Hill, Sydney, Australia) and used as supplied unless otherwise indicated. Commercial amines of >97% purity were purchased from Sigma Aldrich and were used in this study (CAS numbers are added in the SI table).
3.2. Natural Product Library
Natural product extracts were provided by the Australian Institute of Marine Science (AIMS), Townsville, Australia as part of the AIMS Bioresources Library [28], via the Queensland Compound Library, Brisbane, Australia [29], (now called Compounds Australia [30]). Crude extracts had been partially fractionated by AIMS/ QCL to generate a library of 1434 samples, supplied in DMSO (100%) solution and stored at −80 °C. Original concentrations as provided were 5 mg mL−1. Stock solutions were made by diluting these samples by a factor of 1:10 in dH2O and stored at −80 °C.
3.3. Bacterial Inhibition Assays
For screening of the AIMS library, each test sample (10 µL) was dispensed into a separate well of a 96 well microtiter plates (final sample concentration 0.5 mg mL−1) using sterile dH2O. For determination of MIC, extracts (250 to 0.5 µg mL−1) or synthesised compounds (100 to 0.0002 µM) were serially diluted in microtiter plates. Bacterial suspension (90 µL, OD600nm 0.001) was added to each well and plates were incubated at 37 °C for either 18 h (MRSA, P. aeruginosa PAO1, E. coli EC958) or 7 days for M. tuberculosis H37Rv as described previously [50]. Resazurin (10 μL; 0.05% w/v) was added and plates were incubated for 3 h or 24 h (M. tuberculosis) at 37 °C. The inhibitory activity was calculated by visual determination of colour change within wells or detection of fluorescence at 590 nm using a FLUOstar Omega microplate reader (BMG Labtech, Ortenberg, Germany). Percentage survival was calculated in comparison to the average of untreated control wells after normalising for background readings.
3.4. Evaluating
Toxicity of AIMS Extract Library. Human alveolar epithelial cells (A549) [51], Madin-Darby canine kidney epithelial cells (MDCK) [52], human leukaemia cells (THP-1) [53], human hepatocellular carcinoma cells (Hep-G2) [54] and human embryonic kidney cells 293 (HEK293) [55] were grown and differentiated in complete RPMI (Roswell Park Memorial Institute Medium) and DMEM (Dulbecco’s Modified Eagle’s medium) tissue culture media (RPMIc and DMEMc, from the Thermo Fisher Scientific, Waltham, MA, USA) by adding 10% FBS, 200 µM L-glutamine and 1 mM HEPES buffer solution. To determine toxicity of the AIMS extract library, 2 × 105 of each cell type were added to a 96-well plate and left for 48 h in a humidified 5% CO2 incubator at 37 °C to adhere. Extract samples at a final concentration of 0.5 mg mL−1 were added to the wells, then the plates incubated for 4 more days in a humidified 5% CO2 incubator at 37 °C. Then, resazurin (10 μL of 0.05% w/v) was added and after 4 h, fluorescence measured as described previously. The cell viability was calculated as percentage fluorescence relative to untreated cells.
3.5. Purification of Natural Products from Extracts and Structure Elucidation
3.5.1. High Performance Liquid Chromatography (HPLC) Purification
The samples were separated using analytical (Waters 2695 Alliance pump with Waters 2996 PDA, Sunfire reversed-phase column, and WFIII fraction collector) and preparative (Waters 600 HPLC pump, Phenomenex reversed-phase column, Waters 2487 UV detector and WFIII fraction collector) HPLC systems with UV detectors at 254 and 280 nm, employing a gradient of solvents A (dH2O) and B (acetonitrile) with trifluoroacetic acid (0.1%). Extract mixtures were kept at 4 °C until injection, then extract sample (100 μL) was injected onto an analytical Waters X-bridge C18 100 Å (4.6 × 250 mm, 5 µm) reversed-phase column on the same analytical HPLC system described above. The mobile phase was obtained using binary gradients of solvents A and B at a flow rate of 1 mL min−1 at 30 °C over 80 min. Fractions, separated every 60 s, were collected. The purified fractions were flash-frozen in liquid nitrogen then freeze-dried overnight. The resulting fractionated extracts were re-suspended in DMSO and antibacterial activity versus MRSA was determined as described above. Fractions identified as active against MRSA were further purified on the preparative HPLC unit described above, using a Phenomenex C18 100 Å (250 × 21.2 mm, 10 µm) reversed-phase column with UV detection at 254 and 280 nm, 7 mL min-1flow rate with water/acetonitrile gradient containing 0.1% trifluoroacetic acid. The gradient for AIMS extracts 19033, 20608, 26104, 25641 and 26051 was 0% B initially, increased to 40% B over 20 min, then to 100% over 40 min, held at 100% for 10 min. For AIMS extracts 25663 gradient was 0% B initially, increased to 40% B over 60 min, then to 100% over 30 min, held at 100% for 10 min. Compounds thus purified were evaluated for biological activity and analysed by MS to determine potential structures for the bioactive components.
3.5.2. Identification and Structure Elucidation
Identification and Elucidation of Purified Compounds from Sample Numbers 19033, 20608 and 26051
Purified compounds were identified and characterised using MS and NMR. High resolution ESI mass spectra (HRMS) were recorded on a Bruker Apex Qe 7T Fourier Transform ion cyclotron resonance mass spectrometer with an Apollo II ESI MTP ion source with samples (in CH3CN:H2O 1:1) infused using a Cole Palmer syringe pump at 180 µL h−1. Where required, low resolution ESI tandem MS was performed on a Bruker amaZon SL ion trap via syringe infusion or by injection into a constant flow stream with a rheodyne valve and an Alltech HPLC pump (mobile phase methanol, flow rate 0.3 mL min−1) connected to an Apollo II ESI MTP ion source in positive ion mode. Tandem mass spectra of the [M + H]+ parent ion were obtained manually up to MS5 (depending on sensitivity). Spectra were acquired in positive ion mode using a 1–4 Da isolation window, with the excitation amplitude manually optimized for each spectrum to have the selected mass at ~10% of the height of the largest fragment. Data analysis was performed for both high resolution MS and low-resolution tandem MS data using Bruker Data Analysis 4.0 with smart formula assuming C, H, N, O, Na (0-1), mass error <2 ppm, C:H ratio 3 maximum, even electron (or both for tandem MS data). The results of high-resolution MS data analysis were further refined manually by comparing isotopic fine structures of simulations where possible (resolving power >200,000) to further eliminate potential formulae within the 2ppm mass error window (particularly 15N, 18O, 2H, 13C and 13C2 isotopes and confirm no 34S presence).
Identification and Elucidation of Fractions 44 from Sample Number 25663 (Compound 6)
1H NMR (500 MHz, DMSO-d6): δ 9.20 (d, J = 5.6, 1H, H-21), 9.10 (dd, J = 4.5, 1.7, 1H, H-17), 9.00 (dd, J = 8.0, 1.1, 1H, H-6), 8.92 (d, J = 5.6, 1H, H-19), 8.63 (dd, J = 7.9, 1.7, 1H, H-15), 8.44 (d, J = 8.0, 1H, H-3), 8.09 (ddd, J = 8.0, 7.2, 1.1, 1H, H-2), 8.02 (ddd, J = 8.0, 7.2, 1.1, 1H, H-1), 7.80 (dd, J = 7.9, 4.5, 1H, H-16). HRMS (ESI): m/z 306.0635; the molecular formula C18H9ON3 gives an expected molecular [M + Na]+ ion at 306.0637 (err 0.8 ppm). This molecular formula gives a rdbe of 16, requiring many rings or double bonds. The NMR and mass data are in agreement with those in the literature for the known compound ascididemin [36,37,55].
Identification and Elucidation of Fractions 58 from Sample Number 25663 (Compound 7)
1H NMR (500 MHz, DMSO-d6): δ 9.25 (d, J = 5.3, 1H, H-21), 9.13 (d, J = 4.3, 1H, H-17), 8.98 (d, J = 8.8, 1H, H-6), 8.93 (m, 1H, H-19), 8.68 (s, 1H, H-15), 8.64 (d, J = 7.6, 1H, H-3), 8.19 (d, J = 8.8 1H, H-1), 7.81 (dd, J = 4.3, 7.6, 1H, H-16). HRMS (ESI): m/z 361.9925; the molecular formula C18H8BrN3O would give an expected [M + H]+ ion at 361.9923 (err 0.5 ppm). This molecular formula gives a rdbe of 16, requiring many rings or double bonds. The NMR and mass data agree with those in the literature for the known compound 2-bromoascididemin [36,37].
Identification and Elucidation of Purified Compounds from Sample Number 26104 (Compound 8)
1H NMR (500 MHz, DMSO-d6): δ 8.47 (br s, 1H, H-8), 8.46 (br s, 1H, H-7), 6.54 (d, J = 8.4, 1H, H-6), 6.44 (d, J = 3.2, 1H, H-3), 6.35 (dd, J =3.2, 8.4, 1H, H-5), 5.29 (m, 1H, H-25), 5.22 (t, J = 7.3, 1H, H-10), 4.19 (d, J = 11.4, 1H, H-18), 3.12 (d, J =7.3, 1H, H-9), 2.19 (m, 1H, H-21), 2.13 (m, 1H, H-16), 1.95 (t, J = 7.4, 1H, H-13), 1.91 (m, 1H, H-24), 1.8 (m, 1H, H-24′), 1.66 (d, J = 13.1, 1H, H-27), 1.62 (s, 3H, H-12), 1.6 (s, 3H, H-31), 1.42 (m, 1H, H-14), 1.41 (m, 1H, H-28), 1.37 (m, 1H, H-29), 1.34 (m, 1H, H-20), 1.21 (m, 1H, H-15), 1.13 (m, 1H, H-23), 1.12 (m, 1H, H-29′), 1.06 (m, 1H, H-20′), 1.03 (m, 1H, H-27′), 1.03 (m, 1H, H-15′), 0.84 (s, 3H, H-33), 0.83 (s, 3H, H-34), 0.78 (d, J= 6.9, 1H, H-26), 0.66 (s, 3H, H-32). LRMS (APCI): m/z 467.3, [M + H]+; HRMS (APCI): m/z 467.1, [M + H]+; the molecular of formula C30H17N2O4 calculated at 467.118, err 1.9 ppm. This results in rdbe calculation of 24 indicating a high level of unsaturation and rings. The NMR and mass data agree with those in the literature for the known compound halisulfate [55,56,57,58].
4. Conclusions
The emergence of antibiotic resistance highlights the need for novel, effective antibacterial agents which circumvent traditional resistance mechanisms. Thus, we assayed 1434 extracts from the AIMS Bioresources Library, Brisbane, Australia [28] against MRSA, finding five samples that have a promising combination of high antibacterial activity and low toxicity to mammalian cells. The NMR, high-resolution MS and tandem MS analysis was used to decipher structures. The proposed structures form samples 20608, 26051 and 19033 are all tertiary amines or quaternary amine salts: aniline 1 and benzylamine 2 (from Lendenfeldia sample number 20608), aliphatic amine 3 (from Dysidea herbacea sample number 19033), aliphatic tertiary amine 4 and quaternary amine salt 5 (from Ircinia sp. sample number 26,051). The proposed structures from samples 25663 and 26104 are acididemin 6 and 2-bromoascididemin 7 (from Flavobranchia sample number 25663), and halisulfate 8 (from Ircinia sample number 26104). The compounds discovered in this study add to the growing arsenal of antimicrobial agents from the sea [6,12], and offer interesting new avenues in the quest for new, effective agents to combat the growing scourge of multidrug resistant bacteria.
The structure activity relationship (SAR), close analogues studies have demonstrated active scaffolds of isolated natural products and synthetic derivatives compounds. These natural product analogues may be optimized further, inspiring further studies in the search for new lead compounds to fight against microbial pathogens. They will be helpful in broadening the understanding of the biological effects of the synthetic analogues of ascididemin and therefore may aid the development of novel anticancer and antimicrobial agents. Often the natural properties of natural compounds, such as molecular weight, a large number of chiral centres, and/or complex 3D structures can hamper the drug development process and make them unsuitable for synthesis or non-drug-like [59,60]. Interestingly, the simple molecules which have been found in this study may be suitable as potential drug leads.
Acknowledgments
We thank John Merlino (Concord Hospital, Sydney, Australia) for provision of the MRSA.This work was supported by the National Health and Medical Research Council (NHMRC) Project APP1084266, the NHMRC Centre of Research Excellence in Tuberculosis Control (APP1043225), and the University of Sydney, Sydney, Australia. M.D. was supported by an International Postgraduate Research Scholarship (IPRS) and Australian Postgraduate Award (APA) from the Australian Government.
Abbreviations
A549, human alveolar epithelial cells; AIMS, Australian Institute for Marine Science; DMEM, Dulbecco’s Modified Eagle’s medium; HEK293, human embryonic kidney cells 293; HPLC, high-performance liquid chromatography; HTS, high-throughput screening; MDCK, Madin-Darby canine kidney epithelial cells; MIC, minimum inhibitory concentration; MRSA, methicillin resistant Staphylococcus aureus; MS, mass spectrometry;MS/MS, tandem mass spectrometry; MTC, minimum toxic concentration; NMR, nuclear magnetic resonance;RPMI, Roswell Park Memorial Institute Medium; THP-1, human leukaemia cells; Hep-G2, human hepatocellular carcinoma cells; VRSA, vancomycin-resistant Staphylococcus aureus.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/10/3/337/s1. Supplementary Materials file 1.
Author Contributions
M.D. conceived and designed the experiments; M.D. and M.S. performed the experiments; analyzed the data; M.D. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing financial interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bassetti M., Baguneid M., Bouza E., Dryden M., Nathwani D., Wilcox M. European perspective and update on the management of complicated skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid. Clin. Microbiol. Infect. 2014;20:3–18. doi: 10.1111/1469-0691.12463. [DOI] [PubMed] [Google Scholar]
- 2.Richard R.W., Marisa H., Michael Z.D. Antimicrobial Resistance in Methicillin-Resistant Staphylococcus aureus to Newer Antimicrobial Agents. Antimicrob. Agents Chemother. 2019;63:1216–1219. doi: 10.1128/AAC.01216-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dookie N., Rambaran S., Padayatchi N., Mahomed S., Naidoo K. Evolution of drug resistance in Mycobacterium tuberculosis: A review on the molecular determinants of resistance and implications for personalized care. J. Antimicrob. Chemother. 2018;73:1138–1151. doi: 10.1093/jac/dkx506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pourakbari B., Mamishi S., Mohammadzadeh M., Mahmoudi S. First-Line Anti-Tubercular Drug Resistance of Mycobacterium tuberculosis in IRAN: A Systematic Review. Front. Microbiol. 2016;7:1139. doi: 10.3389/fmicb.2016.01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Avalos G., Gonzalez-Palomar G., Lopez-Rodriguez M., Vazquez-Chacon C.A., Mora-Aguilera G., Gonzalez-Barrios J.A., Villanueva-Arias J.C., Sandoval-Diaz M., Miranda-Hernández U., Alvarez-Maya I. Genetic diversity of Mycobacterium tuberculosis and transmission associated with first-line drug resistance: A first analysis in Jalisco, Mexico. J. Glob. Antimicrob. Resist. 2017;11:90–97. doi: 10.1016/j.jgar.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Indraningrat A., Smidt H., Sipkema D. Bioprospecting Sponge-Associated Microbes for Antimicrobial Compounds. Mar. Drugs. 2016;14:87. doi: 10.3390/md14050087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehbub M.F., Perkins M.V., Zhang W., Franco C.M.M. New marine natural products from sponges (Porifera) of the order Dictyoceratida (2001 to 2012); a promising source for drug discovery, exploration and future prospects. Biotechnol. Adv. 2016;34:473–491. doi: 10.1016/j.biotechadv.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 9.Pye C.R., Bertin M.J., Lokey R.S., Gerwick W.H., Linington R.G. Retrospective analysis of natural products provides insights for future discovery trends. Proc. Natl. Acad. Sci. USA. 2017;114:5601–5606. doi: 10.1073/pnas.1614680114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Demerdash A., Atanasov A.G., Horbanczuk O.K., Tammam M.A., Abdel-Mogib M., Hooper J.N.A., Sekeroglu N., Al-Mourabit A., Kijjoa A. Chemical Diversity and Biological Activities of Marine Sponges of the Genus Suberea: A Systematic Review. Mar. Drugs. 2019;17:115. doi: 10.3390/md17020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molinski T.F., Dalisay D.S., Lievens S.L., Saludes J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2008;8:69–85. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
- 12.Hughes C.C., Fenical W. Antibacterials from the Sea. Chem. A Eur. J. 2010;16:12512–12525. doi: 10.1002/chem.201001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroeder G., Bates S.S., La Barre S. Blue Biotechnology. Wiley; Hoboken, NJ, USA: 2018. Bioactive Marine Molecules and Derivatives with Biopharmaceutical Potential. [Google Scholar]
- 14.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2017;34:235–294. doi: 10.1039/C6NP00124F. [DOI] [PubMed] [Google Scholar]
- 15.Blunt J.W., Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2018;35:8–53. doi: 10.1039/C7NP00052A. [DOI] [PubMed] [Google Scholar]
- 16.Mayer A.M.S., Rodríguez A.D., Taglialatela-Scafati O., Fusetani N. Marine Pharmacology in 2012–2013: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs. 2017;15:273. doi: 10.3390/md15090273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGivern J.G. Ziconotide: A review of its pharmacology and use in the treatment of pain. Neuropsychiatr. Dis. Treat. 2007;3:69–85. doi: 10.2147/nedt.2007.3.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon E.M., Sankhala K.K., Chawla N., Chawla S.P. Trabectedin for Soft Tissue Sarcoma: Current Status and Future Perspectives. Adv. Ther. 2016;33:1055–1071. doi: 10.1007/s12325-016-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Damhougy K.A., El-Naggar H.A., Ibrahim H.A., Bashar M.A.E., Abou Senna F.M. Biological activities of some marine sponge extracts from Aqaba Gulf, Red Sea, Egypt. Int. J. Fish. Aquat. Stud. 2017:5. doi: 10.1186/s41936-017-0011-5. [DOI] [Google Scholar]
- 20.Matobole R.M., van Zyl L.J., Parker-Nance S., Davies-Coleman M.T., Trindade M. Antibacterial Activities of Bacteria Isolated from the Marine Sponges Isodictya compressa and Higginsia bidentifera Collected from Algoa Bay, South Africa. Mar. Drugs. 2017;15:47. doi: 10.3390/md15020047. [DOI] [Google Scholar]
- 21.Van Soest R.W.M., Boury-Esnault N., Vacelet J., Dohrmann M., Erpenbeck D., De Voogd N.J., Santodomingo N., Vanhoorne B., Kelly M., Hooper J.N.A. Global diversity of sponges (Porifera) PLoS ONE. 2012;7:e35105. doi: 10.1371/journal.pone.0035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohamed M., Radwan S.P.M., Samir A. Ross, Two New Sulfated Sterols from the Marine Sponge Lendenfeldia dendyi NPC. Nat. Prod. Commun. 2007;2:1934578X0700200905. [Google Scholar]
- 23.Liu Y., Liu R., Mao S.-C., Morgan J.B., Jekabsons M.B., Zhou Y.-D., Nagle D.G. Molecular-Targeted Antitumor Agents. 19. Furospongolide from a Marine Lendenfeldia sp. Sponge Inhibits Hypoxia-Inducible Factor-1 Activation in Breast Tumor Cells. J. Nat. Prod. 2008;71:1854–1860. doi: 10.1021/np800342s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sera Y., Adachi K., Shizuri Y. A New Epidioxy Sterol as an Antifouling Substance from a Palauan Marine Sponge, Lendenfeldia chondrodes. J. Nat. Prod. 1999;62:152–154. doi: 10.1021/np980263v. [DOI] [PubMed] [Google Scholar]
- 25.Thakur N.L., Anil A.C. Antibacterial Activity of the Sponge Ircinia Ramosa: Importance of its Surface-Associated Bacteria. J. Chem. Ecol. 2000;26:57–71. doi: 10.1023/A:1005485310488. [DOI] [Google Scholar]
- 26.Belma Konuklugġl B.G. Antimicrobial activity of marine samples collected from the different coasts of turkey. Turk. J. Pharm. Sci. 2015;12:337–344. [Google Scholar]
- 27.Mohamed N.M., Rao V., Hamann M.T., Kelly M., Hill R.T. Monitoring Bacterial Diversity of the Marine Sponge Ircinia strobilina upon Transfer into Aquaculture. Appl. Environ. Microbiol. 2008;74:4133. doi: 10.1128/AEM.00454-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans-Illidge E.A., Logan M., Doyle J., Fromont J., Battershill C.N., Ericson G., Wolff C.W., Muirhead A., Kearns P., Abdo D., et al. Phylogeny drives large scale patterns in Australian marine bioactivity and provides a new chemical ecology rationale for future biodiscovery. PLoS ONE. 2013;8:e73800. doi: 10.1371/journal.pone.0073800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinarvand M., Spain M.P., Vafaee F. Pharmacodynamic Functions of Synthetic Derivatives for Treatment of Methicillin-Resistant Staphylococcus aureus (MRSA) and Mycobacterium tuberculosis. Front. Microbiol. 2020;11:551189. doi: 10.3389/fmicb.2020.551189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simpson M., Poulsen S.-A. An Overview of Australia′s Compound Management Facility: The Queensland Compound Library. ACS Chem. Biol. 2014;9:28–33. doi: 10.1021/cb400912x. [DOI] [PubMed] [Google Scholar]
- 31.Compounds Australia. [(accessed on 20 September 2018)]; Available online: https://www.griffith.edu.au/griffith-sciences/compounds-australia.
- 32.Bouslimani A., Sanchez L.M., Garg N., Dorrestein P.C. Mass spectrometry of natural products: Current, emerging and future technologies. Nat. Prod. Rep. 2014;31:718–729. doi: 10.1039/c4np00044g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kind T., Fiehn O. Advances in structure elucidation of small molecules using mass spectrometry. Bioanal. Rev. 2010;2:23–60. doi: 10.1007/s12566-010-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herbert Júnior D., Nathalya Isabel de M., Antônio Eduardo Miller C. Tandem Mass Spectrometry—Applications and Principles. InTech; London, UK: 2012. Electrospray Ionization Tandem Mass Spectrometry as a Tool for the Structural Elucidation and Dereplication of Natural Products: An Overview. [Google Scholar]
- 35.McCracken S.T. Ph.D. Thesis. University of Auckland; Auckland, New Zealand: 2010. Synthetic Studies of Biologically Active Natural Products —Ascididemin and 6-Substituted 2-Pyranones. [Google Scholar]
- 36.Lindsay B.S. Ph.D. Thesis. University of Auckland; Auckland, New Zealand: 1998. Studies in Marine Natural Product Synthesis, lsolation and Ecology. [Google Scholar]
- 37.Da Silva Liberio M. Ph.D. Thesis. Griffith University; Brisbane, Australia: 2014. Chemical and Biological Investigations of Anticancer Compounds from Australian Ascidians. [Google Scholar]
- 38.Phuwapraisirisan P., Matsunaga S., van Soest R.W.M., Fusetani N. Shinsonefuran, a cytotoxic furanosesterterpene with a novel carbon skeleton, from the deep-sea sponge Stoeba extensa1See Ref. 1.1. Tetrahedron Lett. 2004;45:2125–2128. doi: 10.1016/j.tetlet.2004.01.041. [DOI] [Google Scholar]
- 39.Carr G. Ph.D. Thesis. The University of British Columbia; Vancouver, BC, Canada: 2010. Bioactive Marine Natural Products: Isolation, Structure Elucidation and Synthesis of Pharmacophore Analogues. [Google Scholar]
- 40.Veltri C.A. Ph.D. Thesis. The University of Utah; Salt Lake City, UT, USA: 2009. Identification of Cellular Targets of Marine Natural Products. [Google Scholar]
- 41.Zhu Q. A review of novel bacterial complex lipids: Implications for the pathogenesis of apical periodontitis. Iran. Endod. J. 2010;5:141–146. [PMC free article] [PubMed] [Google Scholar]
- 42.Bontemps N., Bry D., Lopez-Legentil S., Simon-Levert A., Long C., Banaigs B. Structures and antimicrobial activities of pyridoacridine alkaloids isolated from different chromotypes of the ascidian Cystodytes dellechiajei. J. Nat. Prod. 2010;73:1044–1048. doi: 10.1021/np900751k. [DOI] [PubMed] [Google Scholar]
- 43.Furuta A., Salam K.A., Hermawan I., Akimitsu N., Tanaka J., Tani H., Yamashita A., Moriishi K., Nakakoshi M., Tsubuki M., et al. Identifiction and biochemical characterization of halisulfate 3 and suvanine as novel inhibitors of hepatitis C virus NS3 helicase from a marine sponge. Mar. Drugs. 2014;12:462–476. doi: 10.3390/md12010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epand R.F., Savage P.B., Epand R.M. Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds (Ceragenins) Biochim. Biophys. Acta Biomembr. 2007;1768:2500–2509. doi: 10.1016/j.bbamem.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 45.Ibarguren M., López D.J., Escribá P.V. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim. Biophys. Acta Biomembr. 2014;1838:1518–1528. doi: 10.1016/j.bbamem.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 46.Gotoh A., Nara M., Sugiyama Y., Sakanaka M., Yachi H., Kitakata A., Nakagawa A., Minami H., Okuda S., Katoh T., et al. Use of Gifu Anaerobic Medium for culturing 32 dominant species of human gut microbes and its evaluation based on short-chain fatty acids fermentation profiles. Biosci. Biotechnol. Biochem. 2017;81:2009–2017. doi: 10.1080/09168451.2017.1359486. [DOI] [PubMed] [Google Scholar]
- 47.De Oliveira J.H.H.L., Seleghim M.H.R., Timm C., Grube A., Köck M., Nascimento G.G.F., Martins A.C.T., Silva E.G.O., de Souza A.O., Minarini P.R.R., et al. Antimicrobial and Antimycobacterial Activity of Cyclostellettamine Alkaloids from Sponge Pachychalina sp. Mar. Drugs. 2006;4:1–8. doi: 10.3390/md401001. [DOI] [Google Scholar]
- 48.Febles M., Montalvao S., Crespin G.D., Norte M., Padron J.M., Tammela P., Fernandez J.J., Daranas A.H. Synthesis and biological evaluation of crown ether acyl derivatives. Bioorganic Med. Chem. Lett. 2016;26:5591–5593. doi: 10.1016/j.bmcl.2016.09.066. [DOI] [PubMed] [Google Scholar]
- 49.Christopher J.M., Charles W.R., Thomas R. Synthesis of marine alkaloid ascididemin. Tetrahedron Lett. 1990;31:4375–4376. [Google Scholar]
- 50.Giard D.J., Aaronson S.A., Todaro G.J., Arnstein P., Kersey J.H., Dosik H., Parks W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 51.Gaush C.R., Hard W.L., Smith T.F. Characterization of an established line of canine kidney cells (MDCK). Proceedings of the Society for Experimental Biology and Medicine. Soc. Exp. Biol. Med. 1966;122:931–935. doi: 10.3181/00379727-122-31293. [DOI] [PubMed] [Google Scholar]
- 52.Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int. J. Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 53.Aden D.P., Fogel A., Plotkin S., Damjanov I., Knowles B.B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979;282:615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- 54.Graham F.L., Smiley J., Russell W.C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 55.Poigny S., Nouri S., Chiaroni A., Guyot M., Samadi M. Total Synthesis and Determination of the Absolute Configuration of Coscinosulfate. A New Selective Inhibitor of Cdc25 Protein Phosphatase. J. Org. Chem. 2001;66:7263–7269. doi: 10.1021/jo010154c. [DOI] [PubMed] [Google Scholar]
- 56.Bae J., Jeon J.-E., Lee Y.-J., Lee H.-S., Sim C.J., Oh K.-B., Shin J. Sesterterpenes from the Tropical Sponge Coscinoderma sp. J. Nat. Prod. 2011;74:1805–1811. doi: 10.1021/np200492k. [DOI] [PubMed] [Google Scholar]
- 57.Kernan M.R., Faulkner D.J. Sesterterpene sulfates from a sponge of the family Halichondriidae. J. Org. Chem. 1988;53:4574–4578. doi: 10.1021/jo00254a030. [DOI] [Google Scholar]
- 58.Loukaci A., Le Saout I., Samadi M., Leclerc S., Damiens E., Meijer L., Debitus C., Guyot M. Coscinosulfate, a CDC25 phosphatase inhibitor from the sponge Coscinoderma mathewsi. Bioorganic Med. Chem. 2001;9:3049–3054. doi: 10.1016/S0968-0896(01)00208-5. [DOI] [PubMed] [Google Scholar]
- 59.Harvey A.L., Edrada-Ebel R., Quinn R.J. The re-emergence of natural products for drug discovery in the genomics era. Nature reviews. Drug Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 60.Zidar N., Montalvão S., Hodnik Ž., Nawrot D., Žula A., Ilaš J., Kikelj D., Tammela P., Mašič L. Antimicrobial Activity of the Marine Alkaloids, Clathrodin and Oroidin, and Their Synthetic Analogues. Mar. Drugs. 2014;12:940. doi: 10.3390/md12020940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.