Abstract
Morus alba L. (M. alba) is a highly adaptable plant that is extensively incorporated in many traditional and Ayurveda medications. Various parts of the plant, such as leaves, fruits, and seeds, possess nutritional and medicinal value. M. alba has abundant phytochemicals, including phenolic acids, flavonoids, flavonols, anthocyanins, macronutrients, vitamins, minerals, and volatile aromatic compounds, indicating its excellent pharmacological abilities. M. alba also contains high nutraceutical values for protein, carbohydrates, fiber, organic acids, vitamins, and minerals, as well as a low lipid value. However, despite its excellent biological properties and nutritional value, M. alba has not been fully considered as a potential functional food ingredient. Therefore, this review reports on the nutrients and bioactive compounds available in M. alba leaves, fruit, and seeds; its nutraceutical properties, functional properties as an ingredient in foodstuffs, and a microencapsulation technique to enhance polyphenol stability. Finally, as scaling up to a bigger production plant is needed to accommodate industrial demand, the study and limitation on an M. alba upscaling process is reviewed.
Keywords: Morus alba, bioactive compounds, functional food ingredients
1. Introduction
Morus alba Linn (M. alba), also known as white mulberry, belongs to the Moraceae family [1]. It is a small deciduous tree cultivated in various tropical, subtropical, and temperate countries, including China, Japan, Korea, Thailand, Indonesia, India, Vietnam, Brazil, Africa, and others [2,3]. Many traditional medicines incorporate M. alba fruit, leaves, roots, branches, and bark in Ayurveda medication systems due to their health benefits and antioxidants [4].
M. alba contains abundant bioactive compounds, including phenolic acids, flavonoids, flavonols, anthocyanins, macronutrients, vitamins, minerals, and volatile aromatic compounds [5,6]. Its fruits and leaves contain significant amounts of quercetin, rutin, and apigenin; ferulic, chlorogenic, and protocatechuic acids are also the significant compounds in fruits. These natural bioactive compounds hold potent biological activities proven to exhibit excellent pharmacological effects against various diseases. These include antioxidative, diuretic, antiobesity, hypoglycemic, hypotensive, anticholesterol, antidiabetic, and antimicrobial properties [7,8]. Moreover, the high quantity of phenolic compounds also contributes to M. alba’s functional properties in food applications. For example, the flavonoids and caffeoylquinic acids in M. alba could benefit as colorants, flavorants, food fortificants, antioxidants, preservatives, and antimicrobial agents against bacteria and fungi, all of which are essential in the food industry. Simultaneously, their anthocyanins could act as natural antioxidative food colorants [9,10].
That aside, M. alba as a whole also has a high nutraceutical value due to its low lipid value and high levels of protein, carbohydrates, fiber, organic acids, vitamins, and minerals that are comparable to other berries [6,11]. To be precise, M. alba contains approximately 3.6 g/100 g DW crude fiber and 19.4% protein content, giving it great potential in contributing to the recommended dietary allowance (RDA) of proteins [12]. Moreover, macroelements such as Ca, N, K, and Mg were abundantly found in both leaves and fruit, with low Na values of 0.01 g/100 g DW, making them suitable for low-sodium diets [11,12].
As a result of public and industry interest in the importance of functional foods in disease prevention, a rise in active food-market exploitation has occurred. With their demonstrated nutrition and health benefits, M. alba leaves and fruit can be considered as suitable ingredients to contribute to a broader application of functional foods. M. alba products can widely be found in the market, including mulberry powder, dried fruit, juices, jellies, jams, marmalade, ice cream, desserts, candies, pastes, and wine. However, its potential is limited in producing food products, and its efficiency as a functional food product still needs to be discovered. Therefore, there are still many possibilities to be explored, as it is imperative to sustain and preserve M. alba health benefits in food. This review paper aims to discuss the nutritional and phytochemical properties of M. alba leaves, fruit, and seeds and their potential as food ingredients for developing novel and functional foods to enrich human nutrition.
2. Nutrients and Phytochemical Compositions of Morus alba
2.1. Nutrient Composition of Morus alba
People consume mulberry species in various countries due to their nutritiousness, deliciousness, nontoxicity, and abundant active benefits. The leaves of M. alba species are rich in protein, carbohydrates, fiber, and vitamins, especially ascorbic acid and β-carotene [13]. Studies have also found that the leaves contain a high amount of important minerals such as calcium (Ca), potassium (K), magnesium (Mg), zinc (Zn), and many others. Moreover, according to Sánchez-Salcedo et al. [12], M. alba leaves possessed high iron (Fe) values (119.3–241.8 mg/kg) and a low level of sodium (0.01 mg/100 g), making them a suitable diet material for sodium-restricted individuals. The leaves also contain a considerable amount of organic acids, including citric acid (0.26–3.85 mg/g FW), malic acid (7.37–12.49 mg/g FW), tartaric acid (0.085–0.212 mg/g FW), succinic acid (1.02–5.67 mg/g FW), lactic acid (0.29–0.83 mg/g FW), fumaric acid (0.058–0.39 mg/g FW), and acetic acid (0.029–0.1 mg/g FW), which contribute to the potential health benefits of M. alba leaves [14].
The same nourishing richness is in M. alba fruit, with a protein content higher (10.15–13.33%) than other mulberry species [6]. A study by Owon et al. [15] showed a higher protein value of M. alba fruit (12.98%) as compared to black mulberry (10.85%), golden berry (9.16%), and strawberry (7.65%). Their great protein amount has proven their capability in contributing to protein’s recommended dietary allowance (RDA), which is 0.8 g/kg of body weight [16]. A considerable percentage of minerals, including N, P, K, Mg, Mn, Ca, Zn, Cu, Fe, and Se, were observed in M. alba fruits, while a higher ascorbic acid value was obtained from M. alba fruit (22.4 mg/100 g) than the M. rubra species (19.4 mg/100 g) [1,11]. A summary of these macro- and microelements can be seen in Table 1 and Table 2.
Table 1.
Macronutrients and vitamins in M. alba.
| Nutrients | Carbohydrate | Protein | Fats | Fibre | Ascorbic Acid | β-Carotene | References | |
|---|---|---|---|---|---|---|---|---|
| Sample | ||||||||
| M. alba leaves | 28.8% | 27.1% | 1.9% | 26.5% | - | - | [17] | |
| 9.7–29.6% | 15.3–30.9% | 2.1–4.9% | 27.6–36.7% | 100–200 mg/100 g | 8.4–13.1 mg/100 g | [13] | ||
| 9.7–39.7% | 14.0–34.2% | 3.5–8.1% | 5.4–38.4% | - | - | [18] | ||
| M. alba fruit | - | - | 1.10% | - | 22.4 mg/100 mL | - | [1] | |
| - | 10.2– 13.3% | - | - | - | - | [6] | ||
| - | 12.98% | - | 8.32% | 351 mg/g | 13.7 mg/100 g | [15] | ||
| 71% | 13–15% | 2–3.5% | 12–14% | - | - | [19] | ||
| - | 1.55 g/100 g | 0.48 g/100 g | 1.47 g/100 g | 15.2 g/100 g | - | [20] | ||
Table 2.
Mineral contents in M. alba.
| Minerals | Ca | Zn | Fe | Mg | P | K | Mn | Na | References | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | ||||||||||
| M. alba leaves | 14.9 mg/g | 2.2 mg/100 g | 27.1 mg/100 g | 5.3 mg/g | 3.7 mg/g | 12.4 mg/g | - | 58.62 mg/100 g | [17] | |
| 1.0–1.6% | - | - | 0.4–0.7% | 0.2–0.3% | 1.3–1.9% | - | - | [21] | ||
| 11.2–27.6 mg/g | - | - | 2.2–3.4 mg/g | 3.8–7.02 mg/g | 16.1–18.6 mg/100 g | - | - | [22] | ||
| M. alba fruit | 1.52 mg/g | 2.8 mg/100 g | 4.2 mg/100 g | 1.06 mg/g | 2.47 mg/g | 16.68 mg/g | 3.8 mg/100 g | 60 mg/100 g | [1] | |
| 0.2–0.4 g/100 g | 14.9–19.6 mg/kg | 28.2–46.7 mg/kg | 0.1–0.2 g/100 g | 0.2–0.3 g/100 g | 1.6–2.1 g/100 g | 12.3–19.4 mg/kg | 0.01 g/100 g | [6] | ||
| 2.73 mg/g | 1.8 mg/100 g | 6.3 mg/100 g | 1.82 mg/g | 1.98 mg/g | 9.07 mg/g | 0.8 mg/100 g | 82.8 mg/100 g | [23] | ||
| 5.76 mg/g | 50.20 mg/100 g | 73 mg/100 g | 2.4 mg/g | - | 17.31 mg/g | - | 2.8 mg/g | [20] | ||
2.2. Phytochemicals of Morus alba Leaves
Flavonoids are essential bioactive compounds with excellent antioxidant properties that are found in M. alba leaves. Thabti et al. [24] reported the presence of three newly identified compounds (kaempferol-7-O-glucoside, quercetin-3-O-rhamnoside-7-O-glucoside, and quercetin 3-O-β-glucoside-7-O-α-rhamnoside), along with 10 other known compounds (1-caffeoylquinic acid, 5-caffeoylquinic acid, 4-caffeoylquinnic acid, caffeic acid, rutin, quercetin-3,7-d-O-β-d-glucopyranoside, quercetin-3-O-glucoside, quercetin-3-O-(6-malonyl)-β-d-glucopyranoside, kaempferol-3-O-glucopyranosyl-(1,6)-β-d-glucopyranoside, and kaempferol-3-O-(6-malonyl)glucoside). These findings were supported by Sánchez-Salcedo et al. [5] and Memon et al. [25]. They found chlorogenic, gallic, vanillic, p-hydroxybenzoic, syringic, p-coumaric, protocatechuic, ferulic, and m-coumaric acids as the leaves’ major phenolic acids.
Several new compounds, including flavan derivatives (moracinflavan A-G [26]) and 2-arylbenzofuran derivatives (moracinfurol A and B) were obtained from M. alba leaves [27]. The same significant phytochemical groups—alkaloids, phenolic, flavonoids, tannin, and terpenes—were isolated from leaves of two different maturities, with mature leaves containing higher 1-deoxynojirimycin (DNJ) and secondary metabolite values [28]. However, only simple terpenes were isolated via pyrolysis gas chromatography–mass spectrophotometry (Py/GC/MS), possibly due to terpenes’ lack of a polar group, which makes them more easily volatile compared to alkaloids and phenolics. Further analysis with different pyrolysis temperatures might isolate more compounds from M. alba leaves [29]. Ultimately, rutin, apigenin, and quercetin were the three highest bioactive constituents among the major phytochemical classes of M. alba leaves (Table 3) [30]. In addition, the presence of alkaloids, carbohydrates, fatty acids, phytosterols, glycosides, proteins, tannins, gums, and amino acids were also observed in M. alba leaves in five different solvents [31].
Table 3.
Major phytochemical classes in M. alba leaves.
| Phytochemical Class | Amount in Leaves | References |
|---|---|---|
| Flavonoids | 57.8% | [32] |
| 21.36–56.41 mg RE/g DW | [33] | |
| 20.4–187.23 mg QUE/g | [34] | |
| 3.66–6.11 mg/g DW | [5] | |
| Benzofurans | 17.9% | [32] |
| Phenolic acids | 10.7% | [32] |
| 0.84–1.07 mg/g DW | [25] | |
| 6.78–8.48 mg/g DW | [5] | |
| Alkaloids | 6.4% | [32] |
| 0.680–6.909 mg/g | [35] | |
| 1359 mg/kg | [36] | |
| Coumarins | 3.6% | [32] |
| Chalcones | 2.9% | [32] |
| Stilbenes | 0.7% | [32] |
| 188.57 mg/100 g DW | [37] |
2.3. Phytochemicals of Morus alba Fruit
M. alba fruit is not commonly integrated into traditional medicine, possibly due to its seasonal production and the limited dissemination of its health benefits. However, the increasing interest in analyzing, isolating, and quantifying M. alba fruit phytochemicals has led to reports of their richer phenolic- and volatile-compound content, as well as better antioxidant capacity than other berry species like blueberry, strawberry, blackberry, and raspberry [38]. Previously, Wang et al. [39] found a total of 17 phenolic compounds in the fruits, consisting of cinnamic acid derivatives (0.36–1.29 mg/g DW), flavonols (0.07–0.36 mg/g DW), anthocyanins (not quantified), and benzoic acid derivatives (0.81–2.33 mg/g DW); while caffeoylquinic acids (CQAs) lower than in the leaves were detected (0.16–3.62 mg/g DW and 6.78–8.48 mg/g DW, respectively) [5].
Calín-Sánchez et al. [40] found 35 volatile compounds and categorized them into aldehydes, esters, ketones, benzene terpenes, and oxygenated terpenes. Moreover, the presence of bioactive compounds in M. alba fruit, including rutin (293.5 μg/g), chlorogenic acid (226.9 μg/g), caffeic acid (17.2 μg/g), quercetin (15.2 μg/g), gallic acid (8.9 μg/g), kaempferol (5.8 μg/g), and apigenin (3.5 μg/g), has previously been detected [41]. The fruit’s major fatty acids were also reported, including linoleic acid (57.26%), palmitic acid (22.42%), oleic acid (10.5%), stearic acids (4.27%), and myristic acid (0.98%) [1]. Meanwhile, in a more recent study by Xu et al. [42], three new compounds were isolated: (2S,2″S)-2,3,8,9-tetrahydro-5-hydroxy-8-(1-methylethenyl)-2-(4-hydroxyphenyl)-4H-furo2,3-h]-1-benzopyran-4-one; (1″R,2″R)-4-(2-formyl-1H-pyrrol-1-yl)-1-(2-hydroxy-1-methylpropoxy)-butanoate; and (4′S,5′R)-1-[(Tetrahydro-4-hydroxyl-5-oxo-2-furanyl)methyl]-2,4(1H,3H)-pyrimidinedione.
Zhang et al. [43] suggested flavonols, flavonoids, anthocyanins, hydroxynamic acids, and benzoic acids as the significant polyphenol composition in M. alba fruit. The fruit’s polyphenol composition was significantly influenced by their maturity stages. The brackish fully ripe M. alba fruit extract contained higher total sugar and anthocyanins, while the ripe red fruit contained higher ß-carotene and ascorbic acid [44]. This is because sugar acts as a precursor in synthesizing anthocyanins, hence their higher value in mature fruit. A study by El-Baz et al. [45] found that types of solvent influenced the extraction of phytochemicals. Ethanol (EtOH) extract was the most efficient solvent, as 18 compounds were isolated, mostly esters (96.32%), followed by dichloromethane (DCM) and ethyl acetate (EtOAC) fractions with a total of 12 compounds. The major bioactive compound groups in M. alba fruit are shown in Table 4. The rich reports on M. alba fruit’s bioactive-compound content suggest its excellent potential for functional food-product development.
Table 4.
Major phytochemical classes of M. alba fruit.
| Phytochemical Class | Amount in Fruit | References |
|---|---|---|
| Phenolic acids | 0.013–0.57 mg CAE/g DW | [30] |
| 0.90–2.18 mg/g DW | [46] | |
| 1.17–3.62 mg/g DW | [6] | |
| 0.62–0.84 mg/g DW | [25] | |
| Flavonoids | 0.026–0.607 mg QE/g | [30] |
| 0.553–2.83 mg/g DW | [46] | |
| 3.66–6.11 mg/g DW | [6] | |
| Alkaloids | 660 mg/100 FW | [20] |
| 1047 mg/kg | [36] | |
| Anthocyanins | 0.95–28.61 mg/g DW | [47] |
| 6.52 mg/100 g DW | [24] | |
| Sterol | 28.07% | [45] |
| Stilbenes | 609.15 mg/100 g DW | [37] |
2.4. Phytochemicals of Morus alba Seeds
M. alba seeds alone are barely researched, as they usually are incorporated in the fruit analysis. The seeds are tiny in size and difficult to separate. However, the content of polyphenol in the seeds alone is currently becoming an interest to be explored. Lee et al. [48] have found and quantified 11 polyphenolic compounds in defatted M. alba seeds. These compounds are rutin (31.1–60.0 mg/100 g), 4-prenylmoracin (10.5–43.3 mg/100 g), quercitrin (7.2–34.2 mg/100 g), (+)-dihydroquercetin (13.2–33.1 mg/100 g), quercetin (15.8–19.5 mg/100 g), isoquercitrin (5.8–15.4 mg/100 g), chlorogenic acid (0.0–15.3 mg/100 g), moracin (4.7–7.2 mg/100 g), procatechuic acid (0.0–11.6 mg/100 g), and (+)-dihydrokaempferol and trans-resveratrol (<0.1 mg/100 g). Meanwhile, caffeic acid (1.66 mg/g), 3,4-dihydroxybenzoic acid (1.57 mg/g), rutin (1.54 mg/g), and cyaniding-3-rutinoside (1.30 mg/g) were the major compounds in M. alba seeds [49].
Studies have shown that M. alba fruit seeds were rich in carbohydrates, fatty acids, and protein [50,51,52]. Yılmaz and Durmaz [53] have successfully extracted oil amounting to 232–332 mg/g from M. alba seed, while lower contents of 220–280 mg/g and 213.3 mg/g were extracted by Yao et al. [54] and Argon et al. [50], respectively. Researchers have claimed that the seed oil contains high amounts of carbohydrates and protein, approximately 38–54.8% and 15–21.6%, respectively [50]. A high total tocopherol content of about 2031.20–2393.63 mg/kg, mostly comprising δ-tocopherol (1644.70–2012.27 mg/kg), γ-tocopherol (349.74–366.80 mg/kg oil), β-tocopherol (13.11–19.27 mg/kg oil), and α-tocopherol (6.60–12.35 mg/kg oil) were reported [50,53]. High antioxidative total phenolic content and total flavonoid content were also found in the seed oil. Moreover, the oil could be fractionized into mono-, di-, and triglycerides (1.47–2.05%, 3.25–4.10%, and 90.08–91.05%, respectively). The total lipids could be fractionized into the three major lipids groups: neutral lipids (92.97–93.10%), glycolipids (2.12–2.43%) and phosphor-lipids (1.85–1.92%) [55]. Interestingly, M. alba seed oil was reported to be rich in essential fatty acids, including linoleic acid (75.83–81.1%), palmitic acid (7.96–9.51%), oleic acid (5.64–10.38%), stearic acid (3.45–5.01%), linolenic acid (0.41–80.6%), and myristic acid (0.07%) [50,53]. Based on the capacity of M. alba seeds in oil form, it has a high potential, especially as a fat substitute in food and nonfood applications.
3. Nutraceutical Properties of Morus alba
3.1. Antioxidative Activity
M. alba is rich in bioactive compounds, and its flavonoids contain high antioxidative abilities, based on the analysis of different assays: 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonate) (ABTS), and ferric reducing antioxidant power (FRAP) (Table 5) [56]. The antioxidative property of flavonoids inhibited the oxidative modification of human lipoproteins [57]. Jha et al. [58] isolated 0.5–3 kDa of oligopeptides from M. alba leaves that possessed high scavenging activity in DPPH (322.67–876.92 μg/mL), ABTS (141.29–256.59 μg/mL), nitric oxide (5.11–176.38 μg/mL), and FRAP (24.32–258.99 AAE/g). The isolated peptides showed values of 169.55–328.57 μg/mL in ferrous ion chelation and 202.3–315.5 μg/mL in malondialdehyde (MDA) inhibition, which is important in hindering the production of highly reactive hydroxyl radicals.
Table 5.
Antioxidative properties of M. alba from various studies.
|
M. alba Materials |
Extraction Solvent |
Assay | Result | References |
|---|---|---|---|---|
| Leaves | Ethanol, Petroleum ether, Ethyl acetate, and n-butanol. |
DPPH | IC50 = 145–2070 mg/mL | [56] |
| ABTS | IC50 = 32.73–642.33 mg/mL | |||
| FRAP | EC50 = 868.67–2429.33 mg/mL | |||
| Leaves | Methanol | DPPH | 33.22–56.37 μmol TE/g DW | [33] |
| FRAP | 91.62–149.15 μmol AAE/g DW | |||
| ABTS | 51.28–70.84 μmol TE/g DW | |||
| Leaf oligopeptides |
Liquid nitrogen |
DPPH | 322.7–876.9 μg/mL | [58] |
| ABTS | 141.3–259.6 μg/mL | |||
| Nitric oxide scavenging |
5.11–176.4 μg/mL | |||
| Metal chelation | 169.6–328.6 μg/mL | |||
| Anti-lipid peroxidation |
202.3–315.5 μg/mL | |||
| Leaf Moracin N, Resveratrol, and Quercetin |
Ethanol | Oxygen radical absorption capacity |
1.55–10.86 μmol TE/μmol | [61] |
| DPPH | IC50 = 40.00–285.54 μM | |||
| Cellular antioxidant activity |
No PBS wash: 6.68–22.51 μmol QE/100 μmol |
|||
| Fruit | Ethanol | DPPH | IC50 = 0.518 mg/mL | [34] |
| FRAP | 0.522–0.685 | |||
| Ferrous ion chelation |
72.6% | |||
| Lipid peroxidation | 39–45.51% | |||
| Fruit | Ethanol, Hexane, Chloroform, Ethyl acetate and n-Butanol |
DPPH | EC50 = 71.12–623.86 mg/L | [39] |
| Superoxide anion radical-scavenging | EC50 = 82.37–921.83 mg/L | |||
| Fruit | Ethanol, N-hexane, Dichloromethane, and Ethyl acetate |
DPPH | 35.43–66.6% | [45] |
| Fruit polysaccharides | Ethanol | Oxygen radical absorption capacity |
1117.3–2159.8 μmol TE/g | [62] |
| Rapid peroxyl radical scavenging capacity |
75.23–461.32 μmol Vit C/g | |||
| Cellular antioxidant activity | No PBS wash: 19.64–66.41 μmol QE/100 g |
|||
| PBS wash: 3.13–7.66 μmol QE/10 g | ||||
| Seed polyphenols | Methanol | DPPH | IC50 = 20.2–48.2 μM | [48] |
M. alba fruit also has potent antioxidative activities. According to D’urso et al. [59], phenylpropanoids and flavonols were the two main compounds responsible for the fruit’s antioxidant activity. High total flavonoid content (187.23 mg/g QUE of DW) expressing strong DPPH (IC50 = 0.518 mg/mL) and FRAP (0.685 at 4.0 mg/mL) were obtained from M. alba fruit [34]. The flavonoids showed haemolytic and antihaemolytic ability in red-blood-cell haemolysis of H2O2 induced in mice. In addition, the inhibition of lipid peroxidation in the liver, microsome, and mitochondria were detected as 45.5%, 42.8%, and 39.4%, respectively. The total antioxidant activity of M. alba fruit was similar to that of strawberry (39.40 and 51.31 mg Trolox/g of fresh weight, respectively) [59].
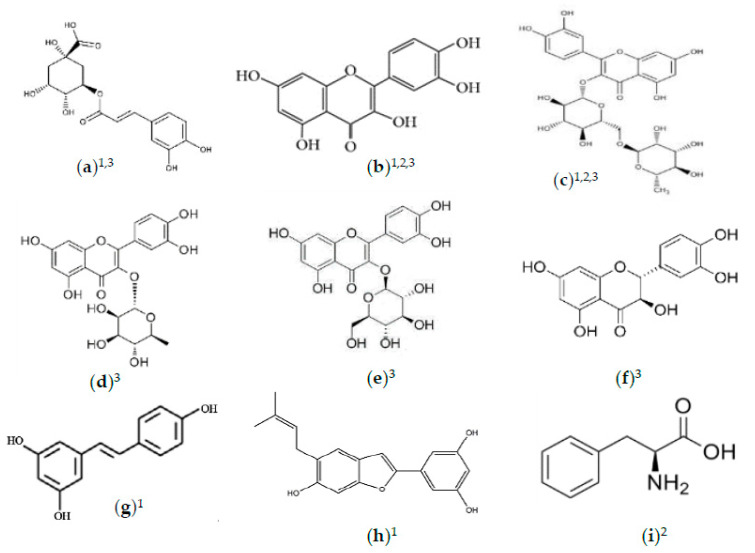
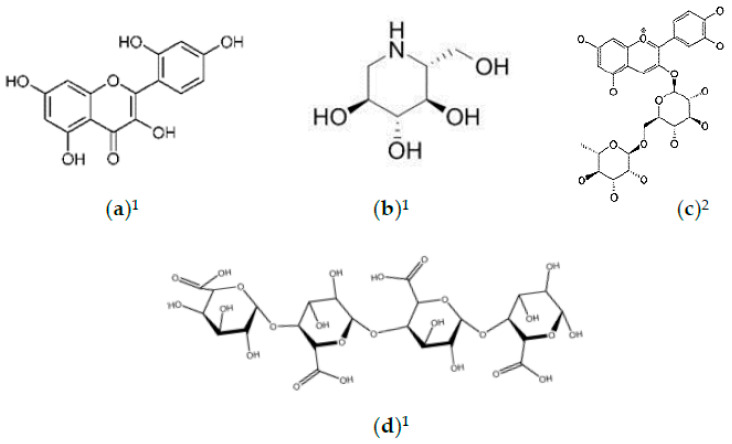
Meanwhile, in M. alba seeds, stronger DPPH radical activity than L-ascorbic acid (IC50 = 31.5 μM) and α-tocopherol (IC50 = 52.3 μM) were revealed by rutin (IC50 = 20.2 μM), isoquercitrin (IC50 = 22.5 μM), quercitrin (IC50 = 24.6 μM), quercetin (IC50 = 27.8 μM), (+)-dihydroquercetin (IC50 = 28.9 μM), and chlorogenic acid (IC50 = 30.6 μM) [48]. These results indicate that M. alba contains antioxidative phenolic compounds potentially useful as nutraceutical agents in functional foods (Figure 1). However, temperature influences the contents of phenolic acids and flavonoids during drying processes [60]. Therefore, studies using different drying methods, type of solvents, and extracting conditions that are more efficient and can effectively maintain yields, bioactive components, and biological activities should be pursued. Overall, the existing literature has evidently indicated the efficient antioxidative capacity of M. alba both in vivo and in vitro, making M. alba a promising ingredient for nutraceutical development.
Figure 1.
Natural compounds in M. alba with antioxidative abilities. (a) Chlorogenic acid; (b) quercetin; (c) rutin; (d) quercitrin; (e) isoquercitrin; (f) dihydroquercetin; (g) resveratrol; (h) moracin; (i) phenylpropanoids. 1 Refers to compounds in M. alba leaves; 2 refers to compounds in M. alba fruit; 3 refers to compounds in M. alba seeds.
3.2. Antidiabetic Property
Natural plant extracts can ameliorate insulin production and inhibit intestinal glucose absorption, thus becoming a significant catalyst in managing diabetes [63]. Ahn et al. [64] supplemented M. alba in diabetic mice for 14 days and found a significant reduction of plasma total cholesterol, hepatic T-C, and triglyceride concentrations. However, the M. alba leaf-supplemented group showed the most significant activities, including decreased plasma glucose and insulin, and elevated levels of protein S6 kinase (pS6K), phosphorylated Akt (pAKT), and phosphorylated (p)-AMP-activated protein kinase (pAMPK). These showed that M. alba leaves have better antidiabetic and antidyslipidemic properties compared to the fruits. Moreover, M. alba leaf extract and oligopeptides (0.5–3 kDa) were found to create potent inhibition of both α-glucosidase and α-amylase enzymes [58,65]. This allows a delay in the breakdown of sugars, thus reducing glucose’s absorption rate and controlling postprandial hyperglycemia. From this, it was discovered that M. alba leaves contain 30–170 mg/100 g DW of DNJ, which is a potent α-glucosidase inhibitor that effectively suppresses postprandial blood glucose elevation with just 6.5 mg of its administration [66]. Additionally, Jeon and Choi [67] have isolated eight compounds containing α-glucosidase inhibitory activity, with chalcomoracin (IC50 = 6.00 μM) and 4′-prenyloxyresveratrol (IC50 = 28.04 μM) holding the most significant inhibition ability.
That aside, a two-week supplementation of M. alba fruit extract could improve insulin sensitivity and reduce hepatic glucose production in type 2 diabetic mice [68]. The fruit-supplemented mice showed a significantly lower level of blood glucose and blood glycosylated haemoglobin (HbA1c) (9.083%) as compared to the diabetic-control and rosiglitazone mice groups (12.921% and 7.454%, respectively). The fruit extract also significantly enhanced the activation of pAMPK and p-Akt substrate of 160 kDa (pAS160). Consequently, it significantly increased the GLUT4 level in skeletal muscles and decreased the glucose 6-phosphatase and phosphoenolpyruvate carboxykinase levels in the liver, thus suppressing the hepatic gluconeogenesis process [68].
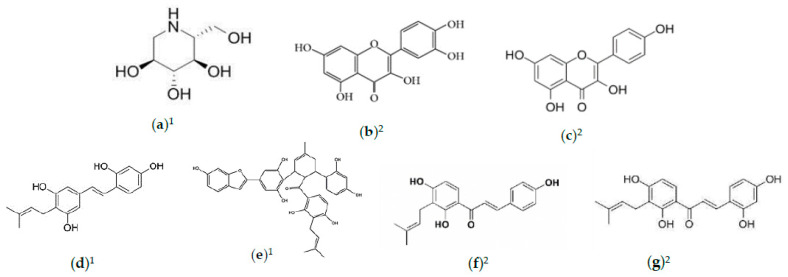
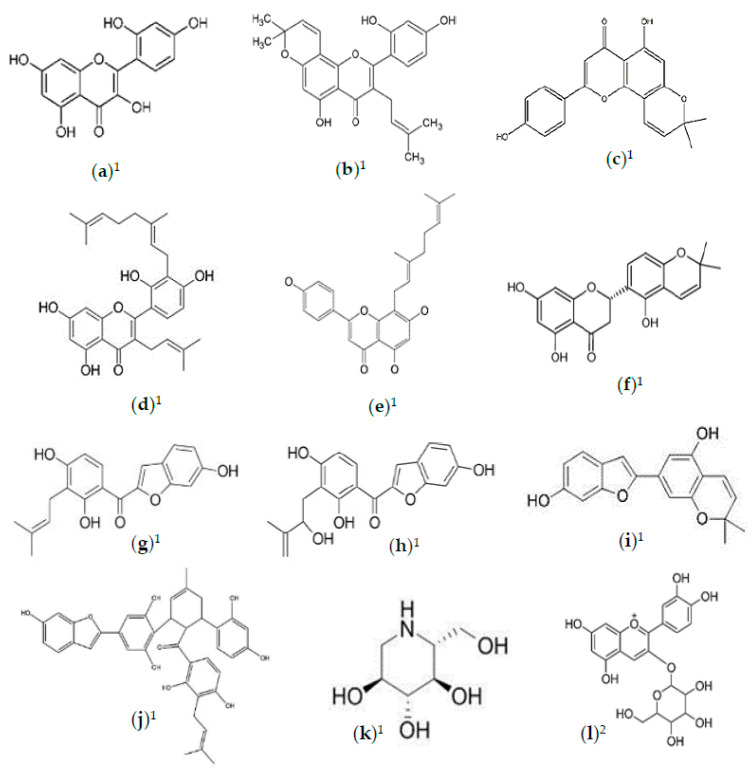
A recent study reported 16 flavonoids from M. alba fruit possessing α-glucosidase-inhibitory ability [42]. Among them, quercetin (IC50 = 16.00 μM), kaempferol (27.61 μM), morachalcone (49.07 μM), and isobavachalcone (51.84 μM) were predominant, while the rest presented higher IC50 values than acarbose (122.22 μM). The structures of these compounds are shown in Figure 2. The inhibitory ability of flavonoids is interchangeable based on any slight structural changes from the hydroxylation of the C-3 position of dihydroflavones, which significantly increased the α-glucosidase-inhibitory ability of (2R)-eriodictyol (IC50 = 168.72 μM) as compared to (2R, 3R)-dihydroquercetin (IC50 = 348.12 μM) [42]. Overall, M. alba leaves and fruit contain high α-glucosidase-inhibiting, antihyperglycemic, and antidyslipidemic properties, making them a positive source for diabetes treatment.
Figure 2.
Natural compounds with antidiabetic activities. (a) 1-Deoxynojirimycin (DNJ); (b) quercetin; (c) kaempferol; (d) 4′-prenyloxyresveratrol; (e) chalcomoracin; (f) isobavachalcone; (g) morachalcone. 1 Refers to compounds in M. alba leaves; 2 refers to compounds in M. alba fruit.
3.3. Antihyperlipidaemia and Antiobesity Activity
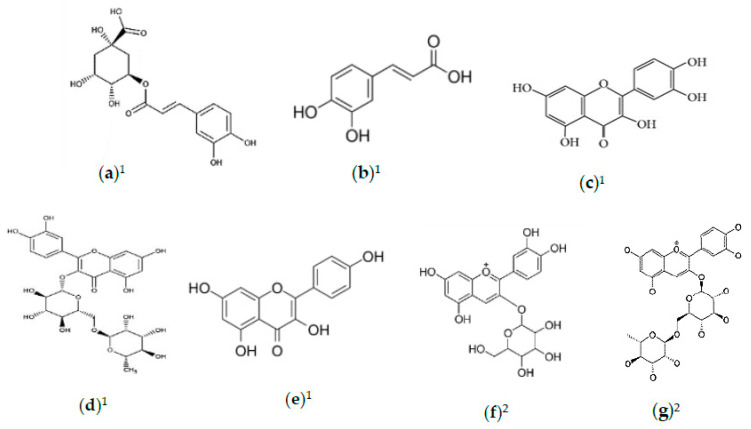
Studies have found that the M. alba leaves could significantly reduce the levels of cholesterol, triglyceride, and lipid peroxidation in blood plasma and post a mitochondrial fraction of cholesterol-induced mice, followed by decreases in body-weight gain, the atherogenic index, coronary artery indices (CRIs), Lee’s index, and waist circumference [69,70]. The downregulation of leptin (0.39-fold) and resistin (0.71-fold), along with the upregulation of adiponectin (1.53-fold), have aided M. alba leaves to work directly on visceral fat mass and attenuated their adiposity and derangements. The abilities of M. alba leaves in suppressing obesity, reducing visceral adiposity, and cardiometabolic alteration was accredited to their polyphenols, particularly chlorogenic acid, quercetin, caffeic acid, rutin, and kaempferol [70].
Ten compounds were found to have antiadipogenic activity, in which three were benzofuran derivatives, two were phenolic derivatives, two were flavonoids, one was an alkaloid, one was lignin, and one was coumarin. These compounds possessed 2.1–36.6% of antiadipogenic activity on 3T3-L1 adipocytes [71]. The efficacy of M. alba leaves to significantly reduce the expression of the key regulator of LDL receptor, the proprotein convertase subtilisin/kexin type 9 (PCSK9), was reported by Lupo et al. [72]. This report was followed with significant reduction of 3-hydroxy-3-methyl-3-glutaryl coenzyme A reductase (HMGCR) and fatty acid synthase (FAS) in mRNA levels of HepG2 cells by 51.1% and 37.2%, respectively. Whereas, at the protein level, expression of the LDL receptor was elevated by 1.8-fold. However, in hepatic cells, no significant effect on the PCSK9 promoter activity was found, despite its expression inhibition in both mRNA and protein levels.Both 5% and 10% M. alba fruit supplementation have significantly down-regulated serum and liver thiobarbituric-acid-related substances (TBARS) in mice [73]. In contrast, a significant increment of red blood cells, liver superoxide dismutase, and blood glutathione peroxidase levels only occurred in 10% fruit-supplemented mice. The fluctuated HDL-C and LDL-C in high-fat animals were claimed to be contributed by the total anthocyanin (0.0087%) and flavonoid (0.39%) contents in the fruit [73]. These findings showed the capacity of M. alba fruit to manage hyperlipidaemia. In another study, M. alba fruit modulated obesity-induced cardiac dysfunction by inhibiting lipogenesis and fibrosis, and enhancing lipolysis in high-fat diet-induced obesity [74]. Fruit extract also attenuated reactive oxygen species (ROS), vascular function, inflammatory markers, and lipid accumulation while reducing collagen in obese rats; these are considered factors for defense against and remediation of obesity-related cardiac dysfunction cardiac fibrosis. The chemical structures of compounds possessing antihyperlipidaemia can be seen in Figure 3. To sum up, M. alba leaf and fruit extracts contain significant obesity-repressing and cholesterol-reducing properties, making them an excellent defensive agent against obesity, atherosclerosis, and hyperlipidaemia-related disorder. Nevertheless, the active components’ mechanism to induce the uptake of LDLR and LDL is still unknown. Therefore, further study needs to explore a more profound understanding of M. alba’s antihyperlipidaemia mechanism.
Figure 3.
Natural compounds with antihyperlipidaemia activities. (a) Chlorogenic acid; (b) caffeic acid; (c) quercetin; (d) rutin; (e) kaempferol; (f) cyanidin-3-O-glucoside; (g) cyanidin-3-O-rutinoside. 1 Refers to compounds in M. alba leaves; 2 refers to compounds in M. alba fruit.
3.4. Neuroprotective Ability
According to Gupta et al. [75] and Rayam et al. [76], M. alba leaves could facilitate gamma-aminobutyric acid (GABA) transmission, thus exhibiting an anticonvulsant ability in rats [77]. A 25 mg/kg methanolic leaf extract actually postponed the onset of pentylenetetrazole (PTZ)-induced chronic seizure. In contrast, 50 and 100 mg/kg extracts decreased convulsion time length [75]. These concentrations also significantly reduced the maximal electroshock-induced tonic hindlimb extension [75]. Meanwhile, M. alba leaves’ significant antiepileptic activity at 200 and 400 mg/kg at 6.8 and 3.16 s, respectively, was observed by Rayam et al. [76].
In the context of Alzheimer’s disease, a 28 day administration of 100 mg/kg ethanolic M. alba leaves enhanced both learning and memory function in spatial-memory-impaired rats, whereas the 200 mg/kg and 400 mg/kg extracts inhibited memory impairment [78]. A high dose (160 mg/kg daily) administration of DNJ could improve cognitive impairment, alleviate Aβ deposition in the hippocampus through β-secretase 1 (BACE1) inhibition, and reduce the expression of brain inflammatory factors such as TNF-α, IL1β, and IL-6 in mice [79]. DNJ minimizes the decline of brain-derived neurotrophic factor/tyrosine kinase receptor (BDNF/TrkB) signal pathway in mouse hippocampus. This therefore implies its ability in improving the hippocampal neuron microenvironment [79]. Similar results for M. alba fruit extract, including improved spatial memory and learning aptitude and decreased neuron apoptosis and Aβ plaques in mice hippocampus and cortex tissues were reported [80]. M. alba fruit also increased anti-inflammatory cytokines (IL-4) and reduced astrocytes and proinflammatory cytokines (TNF-α, IL-1β, and IL-6) in both hippocampus and cortex. Thus, it alleviated neuroinflammation [80]. That aside, M. alba fruit extract has significantly reversed the AlCl3 neurotoxin-induced alteration of striatum neurotransmitters and decreased 50% of acetylcholinesterase (AChE) activity in the brain. A significant increment in norepinephrine, epinephrine, 5-hydroxytryptamine serotonin, and dopamine neurotransmitter were observed in the treated mice [81]. Based on these data, M. alba could attenuate Alzheimer’s disease via Aβ deposition alleviation, inflammatory-factor reduction, and BDNF/TrkB signaling pathway alleviation, thus proving their protective effects on memory and learning abilities.
Parkinson’s disease is a dysfunctional motor disorder caused by progressive degeneration of dopaminergic neurons and glyphosate linked to oxidative stress [82]. According to Rebai et al. [82], M. alba leaf extract’s protective ability could fight the neurotoxicity and harmful effects of glyphosate. At the same time, it attenuated the levels of lactate dehydrogenase, malondialdehyde, and protein carbonyls. Leaf extract also chelated free ions to scavenge H2O2, and increased calcium and superoxide dismutase activity in the brain. These neuroprotective effects of M. alba leaves are due to synergism or antagonism among the bioactive phenolic components in the leaf extract.
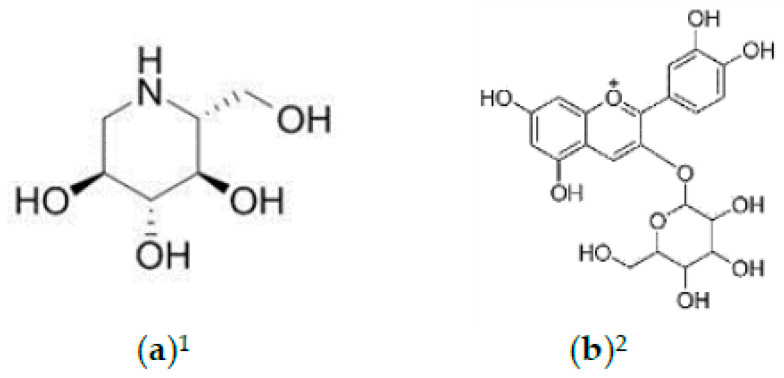
Moreover, M. alba fruit extract also significantly improved and delayed the progression of Parkinson’s-disease-like motor and nonmotor impairment symptoms in the 1-methyl-4-phenyl 1,2,3,6-tetrahydropyridine (MPTP)/probenecid-rat group [83]. M. alba fruit has inhibited olfactory dysfunction of rats based on shortened pellet retrieval time; amelioration of hypokinesia and bradykinesia, and inhibition of Gait dysfunction. Fruit extract also inhibited dopaminergic neuron degeneration and Lewy body formation through α-synuclein suppression, while amending the negative effects of MPTP/probenecid on ubiquitin expression in both the substantia nigra and striatum [83]. The cyanidin-3-glucoside (C3G) (Figure 4) from M. alba fruit has dose-dependently prevented membrane damage, and conserved mitochondrial function and mitochondrial membrane potential (MMP) of the primary cortical neurons in rats when exposed to oxygen–glucose deprivation for 3.5 h [84], suggesting the C3G neuroprotective effect. To sum up, results from various studies served as proof of the worthy neuroprotective effects of M. alba leaves and fruit. This makes them a promising nutraceutical ingredient to target neurodegenerative disorders, especially Alzheimer’s and Parkinson’s diseases.
Figure 4.
Structures of (a) 1-deoxynojirimycin (DNJ) and (b) cyanidin-3-O-glucoside. 1 Refers to compounds in M. alba leaves; 2 refers to compounds in M. alba fruit.
3.5. Antimicrobial and Antiviral Activity
Over the past decade, the interest in plant-derived antimicrobial drugs has increased dramatically. Due to the high production cost and side effects of antibiotics, as well as their non-targeted mechanism of actions, various medicinal plants have been tested and showed positive effects on bacteria and microorganisms. Therefore, they are being used as a means against bacteria-caused diseases. Accordingly, M. alba leaves were reported to inhibit the growth of Staphylococcus aureus, Bacillus cereus, and Pseudomonas fluorescence [85]. However, out of the chosen 13 cultivars, only two cultivars showed low to moderate inhibitory effects on Escherichia coli. The extracts with the highest total phenolic contents could inhibit the three bacterial strains, verifying the correlation between the leaf extract’s total phenolic content and their antibacterial ability [85]. Thabti et al. [86] found that the aqueous and methanolic M. alba leaf extract showed antibacterial activity against Salmonella ser. Typhimurium, Staphylococcus epidermis, and Staphylococcus aureus.
With the increasing antibiotic-resistant microbial strains, De Oliveira et al. [87] determined the effect of ethanolic M. alba leaves on medical-related bacteria and fungi. In their study, the strains Candida albicans LM-106, Candida albicans ATCC-76645, and Candida krusei LM-656 developed resistance to the positive controls. Nevertheless, M. alba leaves have exerted a vigorous antimicrobial activity on Candida albicans LM-106 with a minimal inhibitory concentration of 256 μg/mL. Moderate activity was exerted on other strains: Staphylococcus aureus (ATCC-13150 and M-177), Pseudomonas aeruginosa (ATCC-9027 and P-03), Candida albicans (ATCC-76645 and LM-106), Candida tropicalis (ATCC-13803 and LM-6), Candida krusei (LM-656 and LM-978), and Aspergillus flavus (LM-714). Staphylococcus aureus showed the highest sensitivity to the leaf extract, while no effect against Aspergillus niger was observed.
On the other hand, morin isolated from M. alba fruit exerted moderate growth-inhibiting activity against Streptococcus spp. [88]. Investigation of the antimicrobial abilities of pectin isolated from M. alba fruit (Figure 5) showed antibacterial activity against selected Gram-positive and Gram-negative bacteria: Bacillus cereus, Staphylococcus aureus, Streptococcus mutans, E. coli, Pseudomonas aeruginosa, and S. Typhimurium at concentrations of 500–1000 µg/mL [89]. In addition, methanolic and water extracts of M. alba leaves were reported to be active against S. Typhimurium and Staphylococcus aureus [90]. However, acetone extract expressed better inhibitory activity on Gram-positive bacteria, followed by ethanolic and methanolic extracts. In contrast, no inhibition was detected in any of the extracts against Gram-negative bacteria [91]. In another study, an attempt at biotransforming M. alba fruit extract with Lactobacillus brevis DF01 and Pediococcus acidilactici K10 yielded effective antibacterial activity against S. Typhimurium through the reduction of growth and bacterial-produced biofilm [92]. Moreover, in Jacob et al. [93], DNJ derived from M. alba fruit was shown to exert a positive effect against bovine viral diarrhea virus (BVDV), GB virus-B (GBV-B), woodchuck hepatitis virus (WHV), and hepatitis B virus (HBV).
Figure 5.
Natural compounds in M. alba possessing antimicrobial and antiviral activities. (a) Morin; (b) 1-deoxynojirimycin (DNJ); (c) cyanidin-3-O-rutinoside; (d) pectin.1 Refers to compounds in M. alba fruit; 2 refers to compounds in M. alba seeds.
Meanwhile, M. alba seeds exerted an antiviral effect against the foodborne viral surrogates feline calicivirus-F9 (FCV-F9) and murine norovirus1 (MNV-1) [49]. The addition of 1 mg/mL of seed extract during pretreatment significantly inhibited FCV-F9 and MNV-1 plaque formation by 65% and 47%, respectively. Among the polyphenols, cyanidin-3-rutinoside showed the best reduction of MNV-1 polymerase gene expression. The significant inhibition of viral infection by M. alba seeds in the pretreatment stage suggested their best effect was on the initial stage of viral replication.
Based on these studies, the antimicrobial and antibacterial activity of M. alba leaves and fruit have been demonstrated to be effective, especially against S. Typhimurium, which is a globally known foodborne enteric pathogen accountable for acute gastroenteritis associated with undercooked or contaminated foods. Moreover, a lack of antiviral drugs could allow M. alba to be utilized as an effective alternative to prevent and control virus-related diseases. Nonetheless, further studies focusing on specific high antioxidative compounds and biotransformation are still required to support the antimicrobial and antiviral ability of M. alba to better control foodborne bacterial and viral surrogates.
3.6. Cytotoxicity and Anticancer Activities
Mulberry species are well known as a source of compounds that inhibit the initiation and development of cancers. The primary anticancer protective ability is due to the number of antioxidants available in M. alba. Several studies on various human cancer cells, including cervical cancer, lung carcinoma, hepatocellular carcinoma, breast cancer, and colorectal cancer have been conducted to verify the anticancer ability of M. alba. A study on methanolic M. alba leaf extract used against P19 embryonal carcinoma showed the highest cytotoxic effect with IC50 of 273, 117, and 127 μg/mL at 48 h, 96 h, and 144 h of treatment, respectively, as compared to the other 3 plants extracts [94]. In Fathy et al. [95], leaf extract inhibited HepG2 proliferation by repressing nuclear factor kappa B (NF-κB) gene expression and modulating the biochemical markers alfa-fetoprotein (AFP), alkaline phosphatase (ALP), gammaglutamyl transpeptidase (γ-GT), and albumin (ALB). Moreover, the leaves induced morphological changes in HepG2 cells to a more mature hepatocyte form [95]. Morphological changes also occurred in HeLa cells with 50 mM of morin isolated from M. alba leaves, followed by the formation of flocculent apoptosomes at 150 mM and spherical suspensions of dead/apoptotic cells at 220 mM. This morin-induced apoptosis occurred via multiple pathways, including increment of death-receptor expression, mitochondrial pathway, the elevation of apoptosis-related gene expression, and ROS-induced apoptosis [96].
Interestingly, Fallah et al. [97] observed that flavonoids from M. alba leaves could induce a more potent dose-dependent cytotoxicity in colon cancer, compared to the established chemotherapy drug doxorubicin or their combination (flavonoids + doxorubicin). Dat et al. [2] found 10 flavonoid compounds exerting potent cytotoxicity toward HeLa cells (IC50 of 0.64–3.69 μM), MCF-7 (IC50 of 3.21–7.88 μM), and Hep-3B (IC50 of 3.09–9.21 μM). As such, the top three flavonoids with the highest cytotoxicity for each cancer cell were morusin, atalantoflavone, and 3′-Geranyl-3-prenyl-2′,4′,5,7-tetrahydroxyflavone in HeLa cells; 8-Geranylapigenin, sanggenon K, and 3′-Geranyl-3-prenyl-2′,4′,5,7-tetrahydroxyflavone in MCF-7 cells; and sanggenon K, 8-Geranylapigenin, and atalantoflavone in Hep-3B cells. Meanwhile, a more recent study suggested M. alba leaves had in vitro and in vivo cytotoxic activity on a number of compounds, including morusin, morachalcone B, morachalcone C, moracin D, moracin X, chalcomoracin, and DNJ [32]. The chemical structures of these compounds are shown in Figure 6.
Figure 6.
Natural compounds in M. alba with anticancer abilities. (a) Morin; (b) morusin; (c) atalantoflavone; (d) 3′-geranyl-3-prenyl-2′,4′,5,7-tetrahydroxyflavone; (e) 8-geranylapigenin; (f) sanggenon K; (g) morachalcone B; (h) morachalcone C; (i) moracin D; (j) chalcomoracin; (k) 1-deoxynojirimycin (DNJ); (l) cyanidin-3-glucoside. 1 Refers to compounds in M. alba leaves; 2 refers to compounds in M. alba fruit.
Furthermore, in the area of different extraction solvents, n-hexane fractions of M. alba fruit expressed the best cytotoxicity on HCT116 (IC50 = 32.3 µg/mL), while dichloromethane (DCM) fractions expressed best on MCF7 (IC50 = 43.9 µg/mL) [45]. Moreover, the DCM fruit fraction was safely recognized based on its zero effect on a normal cell line (Bj1), while EtOAc fraction afflicted 47.3% cytotoxicity on Bj1. Nonetheless, low to no effect was seen from the extracts on HepG2 (0–22.8%) and PC3 cancer cells (0–39.6%). This showed that a sample’s cytotoxicity varied with extraction yields based on the solvent’s varying polarity [98]. Cho et al. [99] showed that the cyanidin-3-glucoside (C3G) from M. alba fruit could inflict cytotoxicity and dose-dependently increased human breast cancer cell death. M. alba C3G’s active apoptosis occurred via elevation of cleaved caspase-3, reduction of Bcl-2, and DNA fragmentation. Moreover, 25 days of a C3G diet dose-dependently reduced the size of the tumor in tumor-transplanted mice. This proves their capability to inhibit the proliferation and growth of cancer cells in both in vitro and in vivo models [99].
A newly found indole acetic acid derivative from M. alba fruit revealed dose-dependent cytotoxicity on HeLa cells. The apoptosis mechanism was deduced via the death-receptor-mediated extrinsic pathway and mitochondria-mediated intrinsic path owing to the activation of caspase-8 and -9 [100]. Intriguingly, Ramis et al. [101] observed that the leaves exhibited only a slightly better cytotoxicity effect on colon cancer cells as compared to fruit, despite their higher total phenolics, total flavonoids, and antioxidants. However, a nearly similar cytotoxicity effect as the fruit was exerted on liver hepatocellular cells. Many studies have reported M. alba’s multidirectional mechanism of action, which consists of eliminating reactive oxygen and nitrogen species and reducing the number of negative mutations and inflammation, while promoting apoptosis and activating the immune system. However, further investigations on in vivo studies of M. alba cytotoxicity are suggested to be conducted on more aggressive and metastatic cancers or late-stage cancers, thus giving a better chance for advanced-stage cancer patients.
4. Toxicity Study of Morus alba
The toxicity of M. alba leaves was previously determined in 4 weeks of oral administration of low (1%) and high (5%) doses to both male and female rats [102]. Throughout the observation period, no disturbances to growth or organ weight, nor any effects on the biochemical, hematologic, or pathological examinations were reported, indicating the consumption of M. alba leaves was safe. In an acute toxicity study, an intraperitoneal administration of M. alba leaf extract showed a median lethal dose (LD50) of approximately 4 g/kg in mice and 5 g/kg in Winstar rats [103]. However, they found no significant toxicity from the same 5 g/kg leaf extract when orally administered to both subject groups. During the test, the only effect recorded was the depression of the central nervous and respiratory systems, which recovered within 15 to 30 min. In a subchronic toxicity study, 60 days of orally administered M. alba leaf extract (1, 2, and 3 g/kg daily) showed no significant effects on blood chemistry or hematologic values, as well as no significant histopathological abnormalities in the major organs of the mice. There were no deaths reported in any of the studies [103].
Throughout the 14 days of the trial, the behavioral signals and biomass of the mice were not affected by 300 and 2000 mg/kg of body weight of ethanolic M. alba leaves [87]. However, significant reduction of mean corpuscular volume (MCV) and mean corpuscular haemoglobin concentration (MCHC) occurred in the 2000 mg/kg-treated mice. The MCHC of the 300 mg/kg mice was also lower than the control group, indicating the activity of leaf compounds on erythrocytes, leading to lower cell production. Both mice groups showed a lower proportion of lymphocytes with an increase of segmented leukocytes. The 2000 mg/kg-treated mice showed significant alteration in alanine aminotransferase (ALT) and alkaline phosphatase enzymes. The liver cells’ instability signal of the Gamma-glutamyl transferase level (GGT) was the same in all groups, which indicated the nonhepatotoxic effect of M. alba leaves. Conversely, the 2000 mg/kg dose altered kidney and liver structure, whereas the 300 mg/kg only altered leukocyte proportion, with no toxicity or any irreversible cellular damages observed [87]. Nonetheless, compared to these results, the intraperitoneal injection of the same concentrations extracts was more damaging to the mice, as histological analysis showed significant alteration in the liver, kidney, and spleen [104]. Studies indicate that the ethanolic leaf extract at 2000 mg/k exerted low oral toxicity on mice. However, there were no deaths, so it is safe to use with caution. M. alba leaves could induce biochemical, haematological, and histopathological alterations.
An acute toxicity study on M. alba fruit showed a nonsignificant effect of 1000 mg/kg of polysaccharide intragastric administration on animal behavior. The results considered their respiratory distress, posture, emaciation, and mortality throughout the 1-week observational period [105]. A subchronic toxicity study of 90 days of M. alba fruit oral administration (0, 40, 200, and 1000 mg/kg) on Sprague Dawley rats reported no significant adverse reactions, with no influence on food and water intake, or on body mass gain [106]. No significant toxic impact was detected between the treated group and the control based on organ weight, biochemical values, and hematological and urine analysis [106]. No deaths were reported in any of the studies.
Furthermore, an in vivo neurobehavioral study of M. alba fruit (100, 300, 1000 mg/kg) showed no significant effect on the general health, metabolism, and growth of mice based on their alertness, body weight, daily food and water intake, and organ weight [107]. The low liver toxicity biomarkers, ALT and aspartate transaminase (AST), indicated a nondetrimental effect of M. alba fruit on liver. No indication of renal toxicity was observed, as the levels of renal-function biomarkers (blood urea nitrogen, creatinine, cholesterol, glucose, and albumin) were within normal ranges. The histological analysis confirmed that no morphological changes or internal injuries appeared, even at the highest oral dose of M. alba fruit (1000 mg/kg) throughout 28 days, indicating the safe consumption of M. alba fruit [107]. However, this study was not associated with neurochemical estimation. Therefore, to understand the precise mechanism of action, estimating the brain’s neurotransmitter levels is necessary, and could suggest the nontoxic effect of fruit extract on the normal growth of animals.
5. Functional Ingredients in Food Applications
M. alba leaves, fruit, and seeds are composed of different matrices, therefore varying their potential application in food industry. A substantial amount of nutrients was found in M. alba food products, including carbohydrates; protein; minerals like calcium, iron, and zinc; and vitamins. Yu et al. [33] found a high content of crude protein, total phenolic, and DNJ in M. alba leaves, indicating their health properties and potential as ingredients in the food industry. Recently, M. alba leaves have been used for herbal tea, especially in Asian countries [5]. The bitterness of this tea was found to be positively correlated with the content of TPC and TFC in the leaves and their radical-scavenging abilities [108].
The antioxidative properties of M. alba leaves also have demonstrated its food functionality in paratha [109]. Paratha with M. alba leaves added revealed an increase in the dough’s protein levels, fat, ash, polyphenol, DPPH, ABTS, and Fe3+-reducing and chelating capabilities in a dose-dependent manner. However, upon frying, a decrease in polyphenolic compounds (by 0.16–0.21%) and DPPH activity (by 22–34.6%) occurred due to polyphenol degradation caused by heating [110], while an increase in the activity of ABTS scavenging (by 1.4–6.6%), Fe3+ reduction (by 1.3–2.9%), and Fe3+ chelation (by 247.5–4906.3%) occurred due to Maillard-reaction products (MRPs) that allowed metal chelation to inhibit oxidation [111]. A higher cooking temperature with a shorter cooking time can have less impact on bound polyphenols and sugars than the contrary [112]. Further studies on commercial-range parathas, such as the frozen and the ready-to-cook forms, are more convenient alternatives, and the cooking method should be revised to limit the loss of mulberry’s functional activities.
Moreover, better pork quality was observed when 15% M. alba leaf powder (MLP) was added to the pig’s diet. The levels of backfat, shear force, lower cooking, and drip loss were decreased; and the content of inosinic acid, intramuscular fat value, pH, meat color value, and antioxidative capacity were increased [113,114]. In addition, 15% MLP-treated pigs showed higher mRNA levels of type I and IIa muscle fibers that were lower in shear, more sufficient in diameter, and more tender than the type IIb fiber [114]. However, the 15% MLP also caused adverse effects on the pig’s growth, as it reduced its average daily gain, feed efficiency, carcass weight, and dressing percentage.
On the contrary, the inclusion of 15% and 30% MLP in the diet of lambs maintained growth and carcass performance while increasing the total weight of their rumen and stomach. The 15% MLP supplementation showed to be the most promising concentration to maintain growth, intake, and carcass performance, and alleviate oxidative stress, improved blood metabolites, and improved quality of the longissimus lumborum muscle (redness) [115]. Despite the nonsignificant effect on the meat’s pH, the mRNA expressions of Cu/Zn SOD and GPx against lipid peroxidation were elevated, which implied MLP had an ability to enhance lamb meat’s redness, quality, and shelf life.
According to Zhang et al. [116], M. alba leaf extracts can prevent oxymyoglobin and metmyoglobin oxidation to maintain refrigerated beef’s color. In addition, leaf extract significantly reduced the values of peroxide and thiobarbituric acid reactive substances (TBARS), and increased the superoxide dismutase and glutathione peroxidase activities during beef storage, which suggested that mulberry leaves were able to reduce the lipid oxidation reaction. Nonetheless, M. alba leaves are a good supplement for ruminants and pigs, as well as a good natural antioxidant to preserve the color and increase the quality and shelf life of meat, which are important in food industries. Further research is needed to find a suitable MLP dietary level with a lower negative impact on pig growth, and a longer observation period is required to find the maximum capability of M. alba leaf extract on meat quality and preservation, to ensure the appropriate amount needed for the desired shelf life to prevent the food-processing industry from economic losses.
M. alba fruit, on the other hand, was found to be rich in volatile compounds with higher phenolic contents compared to other berries and mulberry variety [38,117]. The fruit is a good source of pectin (4.75–7%), which has a wide range of food-industry applications as an emulsifier, thickener, stabilizer, gelling agent, and a fat or sugar replacement in low-calorie foods [118]. Due to its remarkable gel-forming property, the authors utilized pectin to produce jellies, jams, and preservatives. They found that pectin promotes multiple biological activities, such as anti-inflammatory, antibacterial, antioxidant, and antitumour activities. Thus, pectin-containing M. alba fruit has a probable function as a food ingredient [89].
Currently, M. alba fruit is used in cooking, baking, and for dessert purposes due to its sweet taste and attractive bright color that is ideal for sherbets, jams, fruit tarts, pies, jellies, teas, wines, and cordials [19]. The utilization of fruit as a natural food colorant is derived from their cyanidin and delphinidin glycoside anthocyanins. They are reddish-purple to purple pigments that possess high FRAP and DPPH antioxidant activities [47,119]. Previously, Natić et al. [119] isolated 14 anthocyanins compounds with a total of 45.42–208.74 mg cyn-3-glu/100 g frozen weight, whereas a more recent study by Kim and Lee [47] obtained 16 types of anthocyanin with total value of 0.95–28.61 mg/g DW. Among them, cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside were the two significant anthocyanins in M. alba fruit, possessing antioxidant anti-inflammatory properties.
In jam processing, the color quality of M. alba jam was influenced by storage temperature. The 5 °C-stored jam possessed better color qualities (L* = lightness, a* = green to redness, b* = blue to yellowness) than the 25 °C-stored jam, but the difference was not statistically significant. In addition, during the four months of storage, no significant influence of light conditions on the quality and fluctuation of total anthocyanins and phenolic contents was observed [120]. This showed that mulberry jam was stable during storage. The high antioxidant and color-enhancing properties positively recommended M. alba and its anthocyanins as a natural, functional food colorant, since it is safer to consume than synthetics and is capable of delivering enhanced color quality with value-added properties to the product. However, a study on this colorant’s stability in other foods such as jellies, puddings, or yogurts should be explored to reinforce the coloring efficiency. To our knowledge, no one has conducted further analysis of the antioxidant capacity of an M. alba anthocyanin-containing finished product, or of other biological benefits, despite the proanthocyanidin-significant antimicrobial effects in the gastrointestinal tract and inhibition of sugar-, protein-, and fat-uptake-related enzymes [121], which are valuable in products targeting concerned about calories or their body weight. Therefore, such studies on M. alba anthocyanins and their stabilization methods will determine their benefits as functional food ingredients.
Furthermore, the investigation of M. alba fruit’s polyphenolic compounds (MFPs) in dried minced pork slices (DMPS) revealed a dose-dependent antioxidative activity during processing and storage [122]. The MFPs effectively inhibited oxidation of muscle protein and oxidation-induced texture deterioration, and reduced the DMPS hardness during heating and storage. MFP also significantly inhibited aerobic bacteria growth on DMPS and masked the odor and flavor of pork. Interestingly, MFP-added samples during the storage and post-heating process revealed higher redness (a*) values as compared to the preparation-stage samples and the control group. Meanwhile, an increment not surpassing the control group occurred for yellowness (b*) and lightness (L*) [122]. Similar results were found in MFP-added Cantonese sausages during 28 days of room-temperature storage in Xiang et al. [123]. A* and L* elevations were suggested to be caused by anthocyanin degradation during heating and storage. Simultaneously, the rise of b* value was attributed to the oxidation of ferrous heme iron induced by lipid oxidized products. The samples’ hardness showed a good increment, owing to the polyphenols’ physicochemical modifications during storage. Moreover, MFPs at 1.0 g/kg provided protection against lipid- and protein oxidation-induced damage, based on the lower TBARS and carbonyl contents, yet at a higher level of sulfhydryl than sausages without MFPs. Aside from the effective control of volatile base nitrogen (TVB-N) and microbial stability, MFPs also significantly reduced residual nitrite, which indirectly reduced the production of carcinogenic nitrosamine, causing MFPs to be trusted in biosafety [123]. In summary, MFPs are a promising natural antioxidant with effective protection against TVB-N increment, lipids, and protein oxidation, which could also effectively regulate Cantonese sausages’ biosafety. While this study can be used as a preliminary reference for research on other meat-based products, due to its slight unfavorable color defect, further research using the antioxidant function for better sensory and quality properties should be prioritized.
A recent study added M. alba leaves and fruit respectively to liquefied and creamed rape honey to assess their effects on the enriched honey’s phenolic profile, antioxidant, and glycoside hydrolysis activities [124]. Rape honey is naturally deficient in polyphenols, but its antioxidant activity was enriched and diversified by 70- and 7-fold after the addition of M. alba leaf and fruit extracts, respectively. A similar increment also occurred in M. alba-enriched creamed rape honey. Furthermore, dose-dependent elevations of α-glucosidase and β-galactosidase were observed in leaf-enriched creamed honey. Meanwhile, in M. alba-fruit-enriched honey, a slight decrease in α-glucosidase and a non-dose-dependent β-galactosidase increase occurred. The addition of M. alba, especially the leaves, reduced about 50% of diastase, suggesting their inhibitory effect against carbohydrate-hydrolyzing enzymes [124]. This is advantageous, as it slows down the loss of the diastase enzyme in honey, which allows longer storage while still containing a major amount of beneficial enzymes.
The same compound enrichment effect was observed in M. alba (leaf extract + fruit) added to baked bread (MAB) studied by Kobus-Cisowska et al. [125]. They reported significantly high total phenolic acids, flavonols, and antiradical activities in bread after baking and after 30 days of frozen storage. The MAB also revealed substantially high isoquercetin, chlorogenic acids, and protocatechuic acid. Respective positive correlations between the sum of phenolic acid and the sum of flavonols with DPPH inactivation, ABTS, and reducing the power activities were reported. These high values show significant M. alba functionality in enhancing health benefits in foods for consumption. MAB also revealed higher sweet and insipid scent intensity, despite its grassier and bittersweet taste compared to the control bread’s sour and salty taste. MAB bitterness is due to M. alba’s polyphenols, such as vanillic acids and ferulic acids, that contribute to a bitter, bean-like taste. Meanwhile, the flavonol glycosides affect bitter and sour taste. Despite the differences, MAB received a similar level of scent desirability with higher taste desirability, expressed via sensory profiling compared to bread without M. alba enrichment. DPPH and ABTS activity, which expressed the continuous activation of the responsible antiradical compounds, was stable throughout 30 days of frozen storage in another study on MAB [125]. However, changes in the intensity of frozen MAB characteristics did occur; its sweet scent and grassy taste declined, yet its grassy scent and sweet taste intensified. From these data, M. alba leaf and fruit extracts could be used as natural food fortifiers in bread, as they enhanced the nutrition level, bioactive compounds, and antiradical activity without negatively influencing the bread’s sensory and microbiological qualities. Nevertheless, they should have conducted a prior analysis on the dough’s bioactive compounds and antiradical levels in this study in order to know the percentage of compound and antioxidant-activity loss after baking. Despite the positive results from various studies, these data are still far from enough to solidify a food-industry function. A broader food-variety study, concise shelf-life of products, and analysis of M. alba extracts’ effects, abilities, and best preservation of shelf-life are some aspects for further exploration.
Essential oils (EOs) are plant-based aromatic oily liquids usable in several industries, including the food industry, as functional flavoring substances due to their health benefits. They are also integrated into food packaging to increase food shelf-life due to their antimicrobial and antioxidative activities [126]. Despite EOs’ increasing popularity, there have only been a couple of studies conducted on M. alba EO compound constitution. Zhi-ming et al. [127] and Radulović et al. [128] found some volatile compounds in M. alba leaf EOs with noteworthy biological effects. These compounds mainly consist of terpenoids, phytol, heptacosane, hexahydrofamesyl acetone, hexadeconic acids, β-bisabolene, carotenoid derivatives, and geranyl acetone. These compounds are used as food additives and flavor enhancers. For example, phytol contains antioxidant, anti-inflammatory, and antinociceptive activities [121,129]. Hexadecanoic acid is famous for its anti-inflammatory [130] and cytotoxicity potentials [131], whereas β-bisabolene possesses a strong cancer cytotoxicity effect [132] and has a balsamic odor that led to its approval as a food additive. Heptacosane has been used as a nutritional supplement and sweetener to mend aftertaste [133]. Meanwhile, terpenoids are used for their medicinal, flavor-enhancing, and fragrance properties in various industries [134]. The same wide application occurred for carotenoid derivatives, which are also extensively utilized as a food colorant and provitamin, and as a fortifier to introduce its health benefits into food [135]. Nonetheless, no specific analysis of M. alba EOs or their compound potentials have been conducted before. Therefore, further studies of their biological properties and applications as a functional food additive are needed.
Leaf EOs aside, M. alba-fruit-derived oil revealed rich fatty-acid contents, such as linoleic acids (58.89% DW), palmitic acids (12.46% DW), oleic acids (11.87% DW), and stearic acids (5.67% DW) [136]. Moreover, M. alba seed oil revealed its high content of total phenolics, total flavonoids, tocopherols (1644.70–2012.27 mg/kg oil), and fatty acids such as linoleic acid (75.83–81.1%), palmitic acid (8.80–9.51%), oleic acid (5.64–10.38%), stearic acid (3.96–5.01%), linolenic acid (0.41–0.45%), and myristic acid (0.07%) [53,54,55]. The high amount of bioactive compounds in the seeds contributed to its high DPPH (62–72%), ABTS (0.026–0.072 mmol/g), and FRAP (0.15–0.68 mmol Fe2+/g) abilities, which were proven by the strong correlation between total phenolic content with DPPH and FRAP (r = 0.965 and r = 0.951, respectively) [54]. Moreover, the high flavor and volatile compounds in the leaf EOs could offer antioxidant [137], antifungal [138] and antibacterial [139] effects in medicine when utilized as a flavorer, fragrance enhancer, antimicrobial agent, and food preservative for pulses, grains, cereals, fruits, and vegetables in the food industry [140]. Meanwhile, M. alba seed oil could be a good source of tocopherol and essential fatty acids, especially linoleic acid, an omega-6 PUFA usable as a blood-vessel cleaner to decrease serum cholesterol and inhibit arterial thrombosis formation [141]. Therefore, the oil derived from M. alba leaves, fruit, and seeds contains many beneficial compounds and is potentially applicable in the food industry as a functional food ingredient. Nonetheless, one of the main complications in utilizing an oil is the quantitative or qualitative diversity of its compound content, which could cause variable biological efficacy [142]. EOs and oil carry a strong aroma, which might restrict their application due to the complex matrices and different interconnecting microenvironments of foods. It also requires a level of natural flavor complexes for adequate effectiveness that might exceed the organoleptic acceptable range, affecting the natural taste of food and human health [126,143]. The limited number of studies and data on M. alba-derived EOs and oils are far from enough to solidify M. alba’s potentiality in any industry. Therefore, further studies that isolate more of their compounds, and analyses of M. alba-derived EOs and oils for their nutrition, biological activities, potential applications, effectiveness, toxicity, and stability in the food system are implored to solidify their place as functional food ingredients.
Microencapsulation of Morus alba
M. alba, with its biologically beneficial polyphenolic compounds, is a suitable functional food ingredient. Unfortunately, the poor stability and heat and light sensitivity of phenolic compounds are a widely known weakness [144]. There is evidence that the stability of M. alba polyphenols can be destroyed by environmental factors, including pH, temperature, and oxygen [145]. These downsides have limited their commercial applications, especially for hot-processed foods, as reported in Cheng et al. [146] and their previous studies. Many studies have investigated methods to stabilize and protect polyphenols from degradation. Microencapsulation is an effective technique to ameliorate the unstable physiochemical properties of a substance or compound, including their solubility and dispensability [147]. Xu et al. [145] stated that M. alba polyphenol (MP) stability in dried minced pork slices was significantly enhanced after gum Arabic microencapsulation with a core/wall ratio of 1:90. After 20 days of storage, the microencapsulated polyphenol (MMP) pork slices showed lower loss of total phenolic, flavonoid, and anthocyanin contents (15.10%, 16.87%, and 19.88%, respectively), with higher recovery (by 1.35-fold, 1.26-fold, and 1.16-fold, respectively). Moreover, both MP and MMP exhibited a synergistic inhibitory effect on protein and lipid oxidation, with MMP showing better oxidation and color stability. In addition, Cheng et al. [146] found 1% β-cyclodextrin MMP significantly improved the dried minced pork slices’ recovery of total phenolic, flavonoid, and anthocyanin contents as compared to the nonencapsulated product by 5.6%, 5.8%, and 18.6%, respectively. Furthermore, as compared to the control, the MP-added pork slices showed 1.03-fold higher FRAP and 1.18-fold higher ABTS▪+ scavenging ability, whereas the β-cyclodextrin MMP showed 1.2-fold and 3.58-fold increases, respectively. These positive effects were attributed to β-cyclodextrin’s protective ability on phenolic compounds. However, the degradation of certain compounds such as quercetin, cryptochlorogenic acid, and caffeic acid, were not effectively avoided, presumably due to the difference in structures, thermochemical stabilities, and reaction modes between the compounds and β-cyclodextrin.
The promising results for phenolic-compound recovery was strongly supported by the β-cyclodextrin microcapsule of MP, which showed optimal encapsulation efficiency at a core/wall ratio of 1:6 with ultrasound treatment at 450 W, 25 °C for 90 min. Under the optimized parameters, the processing stability of MMP, including the thermal, light, and storage stabilities, were significantly enhanced. Simultaneously, the encapsulation efficiencies of MMP for total polyphenol, flavonoid, and anthocyanin contents were higher than 97%, thus verifying the success of MP encapsulation in ameliorating the unstable properties of M. alba’s polyphenols [144]. Gum Arabic and β-cyclodextrin are some credible encapsulating agents for M. alba polyphenols to add higher nutritional value to food. The results created a solid base for further MMP studies on their natural-pigment and biopreservative abilities in other thermal-processed products when substituted for synthetics. However, detailed laboratory measurements with evaluations of color and sensory characteristics should be the focus of future studies. Moreover, the bioavailability of MMP and its synergistic inhibitory effect on protein and lipid oxidation in functional meat products must be further studied in larger-scale production to accommodate MMP integration at the commercial scale.
6. Industrial Scale-Up
The assimilation into industrial and commercial production involves a larger production size to accommodate market demand, thus the need for upscaling, which proceeds from the bench or laboratory scale to the pilot scale, and finally to the demonstration scale for commercial demand [148]. The bench or laboratory plant is an early-stage study that provides a sample’s successful assessment, clinical trials, and system parameters. This stage provides initial upscaling data and factors before following up with efficient pilot-plant production. However, the procedures and materials operated at a laboratory scale are usually not practical on a larger scale. The unsuitable scale-up factor, policies, and parameters often critically influence products’ values, properties, and volumes, leading to energy and economic losses [148]. The upscaling process requires deliberate time, efforts, experiences, in-depth research, and high capital before its successful implementation. These difficulties are the reasons for lower upscaling information than the same sample’s laboratory-scale study.
So far, only a handful of the attempted pilot-scale studies were successful, while most of them require more modification and trials. In Flaczyk et al. [149], the authors obtained about a 4% lower M. alba leaf-extraction yield than at the laboratory scale. The study also reported lower protein (by 13.61%), glucose (by 38.78%), phenolics (by 44.94%), phenolic acids (by 53.03%), and flavonols (by 11.36%), as well lower DPPH (by 35.72%) and ABTS (by 20.1%) activities. Nonetheless, at the pilot scale, they found higher concentrations of saccharose and galactose by 23.77% and 23.68%, respectively. These values were due to intermittent loss during pilot-scale processing, as well as the different extraction and concentration conditions between the two scales. Therefore, further analysis and determination of qualitative indicators are necessary for the optimization process.
Gang et al. [150] obtained a polysaccharides’ optimal extraction preceding data focusing on M. alba leaves. This study concluded the optimized temperature, sample-to-solvent ratio, and extraction time via a Box–Behnken response surface design (BBD) coupled with response surface methodology (RSM). As such, extraction at 76.7 °C with a 1: 20.6 sample-to-solvent ratio, extracted for 1.87 h, was reported to produce the highest polysaccharides (105.57 mg/g), consistent with their predicted value (106.64 mg/g). A recent pilot-plant study reported the optimal extraction condition for DNJ from M. alba leaves [151]. The RSM exploration showed the best DNJ extraction value (31.62 mg/g) at 79.2 °C with a 1:9.82 sample-to-solvent ratio for 1.46 h. The value was higher than for the small-scale extraction process. These studies showed that extraction-parameter optimization is crucial in obtaining the best outcome value, and that RSM or BBD coupled with RSM were reliable methods for optimization of upscaled extraction. The obtained data on optimized extraction of M. alba leaf polysaccharides and DNJ can be used as a foundation for industrial applications of M. alba.
Sample-processing parameters aside, the equipment and types of machinery used at the pilot scale also affect the process and outcome. Komaikul et al. [152] found that both stilbenoid and mulberroside A from an M. alba cell culture were affected by the usage of different types of bioreactors. The study revealed that round-bottom bioreactors could produce three times higher biomass than the flat-bottom bioreactor, though no significant difference in mulberroside A production was found. However, significantly higher mulberroside A was obtained from shake-flask cultured cells without an air-driven system compared to air-driven bioreactors. Meanwhile, the stilbenoid production preferred smaller air-driven bioreactors (1 L) with low aeration rates. In sum, the production of mulberroside A and stilbenoid in M. alba cell cultures could be affected by factors such as biomass circulation, aeration, and endogenous enzymatic hydrolysis. Therefore, a study focusing on target-compound stabilization and reduction of aeration-induced negative effects would be a valuable approach for M. alba at the pilot-plant scale. More studies and optimization of M. alba pilot-plant processing should include factors of formulae standardization, chemical equilibrium, product-quality maintenance, thermodynamics, processing equipment, energy consumption, etc., to ensure M. alba’s successful application at the industrial level.
7. Conclusions and Future Perspectives
Taken together, M. alba leaves and fruit have a high content of bioactive compounds such as phenolic acids, flavonoids, flavonols, anthocyanins, macronutrients, vitamins, and volatile aromatic compounds. These compounds contribute significantly to the properties of M. alba in preventing and treating illnesses such as oxidative stress, diabetes, hyperlipidaemia, neurological disorders, microbial infections, and cancer. Numerous studies have been conducted on M. alba fruits and leaves, especially to determine their bioactive compounds, pharmaceutical potentials, and toxicity effects. However, limited studies have been done on M. alba seeds. Therefore, it is essential to include knowledge about the seeds so that the full potential of M. alba can be achieved.
There are significant knowledge gaps between M. alba leaves and fruit regarding their working mechanisms, bioavailability, and biochemistry in the human body. Moreover, the multiple phytochemicals and bioactive compounds contained in different M. alba cultivars, maturity stages, extraction conditions, and methodologies have produced heterogeneous data. This has led to difficulty in evaluating and standardizing their activities. For that reason, more suggestive scale trials with well-designed and uniform parameters will provide a substantial input in obtaining homogenous data, which would benefit further comprehension of the in vivo effect on human health of M. alba leaves and fruit, including their bioavailability and biological effect against enzymes, pH, and especially gut microbiota.
There is limited information available on the application of M. alba in the food industry as a functional food ingredient, despite its current presence in commercial products. Studies have found that M. alba has promising prospects for functional food applications. Its flavonoids and phenolic acids can act as flavorants, food fortificants, antioxidants, preservatives, and antimicrobial agents against bacteria and fungi. In contrast, its anthocyanins could be used as natural antioxidative food colorants to substitute for synthetic colorant usage. With the wide variations of food, more studies on a broader range of foods such as frozen, ready-to-eat, or processed foods are necessary to understand the reactions and impact of M. alba on other food ingredients, as the food system itself is very complex. In the vast food-application potential, research emphasizing the effect of processing and storing on the content and stability of bioactive compounds in M. alba-based foodstuffs is needed for a better understanding of the bioactive compounds’ role and interaction in the food matrix to enhance food quality and properties.
Additionally, in regard to the degradation of heat-sensitive bioactive compounds, microencapsulation techniques have been proven to naturally protect and significantly enhance M. alba polyphenols’ stability and properties. This result could establish a factual basis for further study of their utilization in heat-processed products and better industrial-processing performance, thus expanding the application of M. alba in various food products. However, integration into the industry requires scaling up from the laboratory scale to the pilot plant, and subsequently to the industrial plant, to produce a larger production scale to ensure demand can be met. Regrettably, studies on the pilot scale-up process for M. alba leaves and fruit are still very lacking. This is possibly due to the difficulty of scale-up implementation of bioprocessing. It requires deliberate time, efforts, experiences, in-depth research, and high capital. Unsuitable scale-up factors, procedures, and parameters would critically influence product values, properties, and volumes, leading to undesirable energy and economic losses to a company. In conclusion, M. alba indeed possesses beneficial biological properties and is suitable to be included as a functional food ingredient. Nevertheless, the exploitation of M. alba’s functionality and properties within the industrial field are still scarcely explored.
Acknowledgments
The authors gratefully acknowledge the financial support from F.H.S., with grant number SDK0054-2018 and University Malaysia Sabah, Malaysia. Also, special thanks to Gao Peiru for the guidance and contribution to completing this work.
Author Contributions
Conceptualization, N.Q.I.M.N.; writing—original draft preparation, C.C.; writing—review and editing, N.Q.I.M.N.; visualization, U.H.M.R.; supervision, N.Q.I.M.N.; project administration, A.M.; funding acquisition, F.H.S. All authors have read and agreed to the published version of the manuscript.
Funding
F.H.S.: partly funded the research, grant number SDK0054-2018; and the APC was funded by University Malaysia Sabah.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study’s design; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ercisli S., Orhan E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007;103:1380–1384. doi: 10.1016/j.foodchem.2006.10.054. [DOI] [Google Scholar]
- 2.Dat N.T., Binh P.T.X., Van Minh C., Huong H.T., Lee J.J. Cytotoxic prenylated flavonoids from Morus alba. Fitoterapia. 2010;81:1224–1227. doi: 10.1016/j.fitote.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Sánchez M.D., editor. Proceedings of FAO Electronic Conference Mulberry Animal Production (Morus1-L) Food and Agriculture Organization; Rome, Italy: 2000. World distribution and utilization of mulberry, potential for animal feeding. [Google Scholar]
- 4.Xie H.-H., Wei J.-G., Liu F., Pan X.-H., Yang X.-B. First report of mulberry root rot caused by Lasiodiplodia theobromae in China. Plant Dis. 2014;98:1581. doi: 10.1094/PDIS-03-14-0261-PDN. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez-Salcedo E.M., Mena P., García-Viguera C., Hernández F., Martínez J.J. (Poly) phenolic compounds and antioxidant activity of white (Morus alba) and black (Morus nigra) mulberry leaves: Their potential for new products rich in phytochemicals. J. Funct. Foods. 2015;18:1039–1046. doi: 10.1016/j.jff.2015.03.053. [DOI] [Google Scholar]
- 6.Sánchez-Salcedo E.M., Mena P., García-Viguera C., Martínez J.J., Hernández F. Phytochemical evaluation of white (Morus alba L.) and black (Morus nigra L.) mulberry fruits, a starting point for the assessment of their beneficial properties. J. Funct. Foods. 2015;12:399–408. doi: 10.1016/j.jff.2014.12.010. [DOI] [Google Scholar]
- 7.Hussain F., Rana Z., Shafique H., Malik A., Hussain Z. Phytopharmacological potential of different species of Morus alba and their bioactive phytochemicals: A review. Asian Pac. J. Trop. Biomed. 2017;7:950–956. doi: 10.1016/j.apjtb.2017.09.015. [DOI] [Google Scholar]
- 8.Wen P., Hu T.-G., Linhardt R.J., Liao S.-T., Wu H., Zou Y.-X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019;83:138–158. doi: 10.1016/j.tifs.2018.11.017. [DOI] [Google Scholar]
- 9.Ludwig I.A., Mena P., Calani L., Cid C., Del Rio D., Lean M.E., Crozier A. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking? Food Funct. 2014;5:1718–1726. doi: 10.1039/C4FO00290C. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Cruz S., Chaparro-Hernández S., Hernández-Ruiz K.L., Cira-Chávez L.A., Estrada-Alvarado M.I., Gassos Ortega L., Ornelas-Paz J.d.J., Lopez Mata M. Flavonoids: Important biocompounds in food. In: Justino G., editor. Flavonoids: From Biosynthesis to Human Health. Intechopen; London, UK: 2017. pp. 353–370. [DOI] [Google Scholar]
- 11.Jiang Y., Nie W.-J. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 2015;174:460–466. doi: 10.1016/j.foodchem.2014.11.083. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Salcedo E.M., Amorós A., Hernández F., Martínez J.J. Physicochemical properties of white (Morus alba) and black (Morus nigra) mulberry leaves, a new food supplement. J. Food Nutr. Res. 2017;5:253–261. doi: 10.12691/jfnr-5-4-7. [DOI] [Google Scholar]
- 13.Srivastava S., Kapoor R., Thathola A., Srivastava R.P. Nutritional quality of leaves of some genotypes of mulberry (Morus alba) Int. J. Food Sci. Nutr. 2006;57:305–313. doi: 10.1080/09637480600801837. [DOI] [PubMed] [Google Scholar]
- 14.Bozhüyük M.R., Pehluvan M., Tuncay K., Doğru B. Organic acid composition of selected mulberry genotypes from Aras Valley. Atatürk Üniversitesi Ziraat Fakültesi Dergisi. 2015;46:69–74. [Google Scholar]
- 15.Owon M.A., Gafar A., Saleh S.M., Shaheen M. Identification of bioactive compounds from egyptian mulberry fruits and their uses in improvement the quality of some foods. J. Agric. Res. Kafr El-Sheikh Univ. 2016;42:33–52. [Google Scholar]
- 16.Lonnie M., Hooker E., Brunstrom J.M., Corfe B.M., Green M.A., Watson A.W., Williams E.A., Stevenson E.J., Penson S., Johnstone A.M. Protein for life: Review of optimal protein intake, sustainable dietary sources and the effect on appetite in ageing adults. Nutrients. 2018;10:360. doi: 10.3390/nu10030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamiaa M. Nutritive value and Hypolipidrmic effects of mulberry leaves powder. Indian J. Appl. Res. 2014;4:7–12. [Google Scholar]
- 18.Hassan F.-U., Arshad M.A., Li M., Rehman M.S.-U., Loor J.J., Huang J. Potential of Mulberry Leaf Biomass and Its Flavonoids to Improve Production and Health in Ruminants: Mechanistic Insights and Prospects. Animals. 2020;10:2076. doi: 10.3390/ani10112076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munir A., Khera R.A., Rehman R., Nisar S. Multipurpose White mulberry: A Review. Int. J. Biol. Chem. Sci. 2018;13:31–35. [Google Scholar]
- 20.Imran M., Khan H., Shah M., Khan R., Khan F. Chemical composition and antioxidant activity of certain Morus species. J. Zhejiang Univ. Sci. B. 2010;11:973–980. doi: 10.1631/jzus.B1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nooruldin S., Kamili S., Mir M., Wani A., Malik G., Raja A., Bilal S. Seasonal variation in macro nutrient contents of mulberry (Morus alba) leaves under temperate climatic conditions of Kashmir. Int. J. Agric. Innov. Res. 2015;4:147–152. [Google Scholar]
- 22.Levickienė D., Vaitkevičienė N., Jarienė E., Mažeika R. The content of macroelements in white mulberry (Morus alba L.) leaves. Žemės Ūkio Mokslai. 2018;25:177–183. doi: 10.6001/zemesukiomokslai.v25i4.3867. [DOI] [Google Scholar]
- 23.Liang L., Wu X., Zhu M., Zhao W., Li F., Zou Y., Yang L. Chemical composition, nutritional value, and antioxidant activities of eight mulberry cultivars from China. Pharmacogn. Mag. 2012;8:215. doi: 10.4103/0973-1296.99287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thabti I., Elfalleh W., Hannachi H., Ferchichi A., Campos M.D.G. Identification and quantification of phenolic acids and flavonol glycosides in Tunisian Morus species by HPLC-DAD and HPLC–MS. J. Funct. Foods. 2012;4:367–374. doi: 10.1016/j.jff.2012.01.006. [DOI] [Google Scholar]
- 25.Memon A.A., Memon N., Luthria D.L., Bhanger M.I., Pitafi A.A. Phenolic acids profiling and antioxidant potential of mulberry (Morus laevigata W., Morus nigra L., Morus alba L.) leaves and fruits grown in Pakistan. Pol. J. Food Nutr. Sci. 2010;60:25–32. [Google Scholar]
- 26.Zhang H.-R., Li M., Wang M.-M., Wang X.-N., Shen T., Wang S.-Q., Ren D.-M. Antioxidant flavan derivatives from the leaves of Morus alba. Phytochem. Lett. 2019;29:84–90. doi: 10.1016/j.phytol.2018.11.002. [DOI] [Google Scholar]
- 27.Wu X., Li M., Wang X., Shen T., Wang S., Ren D. Two new 2-arylbenzofurnan derivatives from the leaves of Morus alba. Nat. Prod. Res. 2019;33:204–211. doi: 10.1080/14786419.2018.1443095. [DOI] [PubMed] [Google Scholar]
- 28.Wulandari Y.R.E., Prasasty V.D., Rio A., Geniola C. Determination of 1-Deoxynojirimycin Content and Phytochemical Profiles from Young and Mature Mulberry Leaves of Morus Spp. OnLine J. Biol. Sci. 2019;19:124–131. doi: 10.3844/ojbsci.2019.124.131. [DOI] [Google Scholar]
- 29.Jia C., Li Y., Zhang S., Fei T., Pang S. Thermogravimetric analysis, kinetic study, and pyrolysis–GC/MS analysis of 1, 1′-azobis-1, 2, 3-triazole and 4, 4′-azobis-1, 2, 4-triazole. Chem. Cent. J. 2018;12:22. doi: 10.1186/s13065-018-0381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polumackanycz M., Sledzinski T., Goyke E., Wesolowski M., Viapiana A. A Comparative Study on the Phenolic Composition and Biological Activities of Morus alba L. Commercial Samples. Molecules. 2019;24:3082. doi: 10.3390/molecules24173082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali M., Rathaur B., Nishad U. Phytochemical Screening, Physicochemical and Antioxidant Activity, TLC & Finger Print of HPTLC, Morus Alba Ethanol Extraction. Int. J. Innov. Sci. Res. Technol. 2020;5:1–14. [Google Scholar]
- 32.Chan E.W.C., Wong S.K., Tangah J., Inoue T., Chan H.T. Phenolic constituents and anticancer properties of Morus alba (white mulberry) leaves. J. Integr. Med. 2020;18:189–195. doi: 10.1016/j.joim.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Yu Y., Li H., Zhang B., Wang J., Shi X., Huang J., Yang J., Zhang Y., Deng Z. Nutritional and functional components of mulberry leaves from different varieties: Evaluation of their potential as food materials. Int. J. Food Prop. 2018;21:1495–1507. doi: 10.1080/10942912.2018.1489833. [DOI] [Google Scholar]
- 34.Raman S.T., Ganeshan A.K.P.G., Chen C., Jin C., Li S.-H., Chen H.-J., Gui Z. In vitro and in vivo antioxidant activity of flavonoid extracted from mulberry fruit (Morus alba L.) Pharmacogn. Mag. 2016;12:128. doi: 10.4103/0973-1296.177910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao J.-Y., Wan Y., Yao X.-H., Zhao W.-G., Hu R.-Z., Chen C., Li L., Zhang D.-Y., Wu G.-H. Effect of different planting areas on the chemical compositions and hypoglycemic and antioxidant activities of mulberry leaf extracts in Southern China. PLoS ONE. 2018;13:e0198072. doi: 10.1371/journal.pone.0198072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asano N., Yamashita T., Yasuda K., Ikeda K., Kizu H., Kameda Y., Kato A., Nash R.J., Lee H.S., Ryu K.S. Polyhydroxylated alkaloids isolated from mulberry trees (Morus alba L.) and silkworms (Bombyx mori L.) J. Agric. Food Chem. 2001;49:4208–4213. doi: 10.1021/jf010567e. [DOI] [PubMed] [Google Scholar]
- 37.Kim J.-S., Ha T.-Y., Ahn J.-Y., Kim H.-K., Kim S.-A. Composition and quantitative analysis of stilbenoids in mulberry (Morus alba L.) leaves and fruits with DAD/UV HPLC. J. Korean Soc. Food Sci. Nutr. 2008;37:124–128. doi: 10.3746/jkfn.2008.37.1.124. [DOI] [Google Scholar]
- 38.Chen W., Li Y., Bao T., Gowd V. Mulberry fruit extract affords protection against ethyl carbamate-induced cytotoxicity and oxidative stress. Oxidative. Med. Cell. Longev. 2017;2017:1594963. doi: 10.1155/2017/1594963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Xiang L., Wang C., Tang C., He X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS ONE. 2013;8:e71144. doi: 10.1371/journal.pone.0071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calín-Sánchez Á., Martínez-Nicolás J.J., Munera-Picazo S., Carbonell-Barrachina Á.A., Legua P., Hernández F. Bioactive compounds and sensory quality of black and white mulberries grown in Spain. Plant Foods Hum. Nutr. 2013;68:370–377. doi: 10.1007/s11130-013-0382-9. [DOI] [PubMed] [Google Scholar]
- 41.Chu Q., Lin M., Tian X., Ye J. Study on capillary electrophoresis–amperometric detection profiles of different parts of Morus alba L. J. Chromatogr. A. 2006;1116:286–290. doi: 10.1016/j.chroma.2006.03.118. [DOI] [PubMed] [Google Scholar]
- 42.Xu X., Huang Y., Xu J., He X., Wang Y. Anti-neuroinflammatory and antioxidant phenols from mulberry fruit (Morus alba L.) J. Funct. Foods. 2020;68:103914. doi: 10.1016/j.jff.2020.103914. [DOI] [Google Scholar]
- 43.Zhang H., Ma Z.F., Luo X., Li X. Effects of mulberry fruit (Morus alba L.) consumption on health outcomes: A mini-review. Antioxidants. 2018;7:69. doi: 10.3390/antiox7050069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aramwit P., Bang N., Srichana T. The properties and stability of anthocyanins in mulberry fruits. Food Res. Int. 2010;43:1093–1097. doi: 10.1016/j.foodres.2010.01.022. [DOI] [Google Scholar]
- 45.El-Baz F.K., Hassan A.Z., Abd-Alla H.I., Aly H.F., Mahmoud K. Phytochemical analysis, assessment of antiproliferative and free radical scavenging activity of Morus alba and Morus rubra fruits. Asian J. Pharm. Clin. Res. 2017;10:189. doi: 10.22159/ajpcr.2017.v10i6.18029. [DOI] [Google Scholar]
- 46.Kobus-Cisowska J., Szczepaniak O., Szymanowska-Powałowska D., Piechocka J., Szulc P., Dziedziński M. Antioxidant potential of various solvent extract from Morus alba fruits and its major polyphenols composition. Ciênc. Rural. 2020;50:50. doi: 10.1590/0103-8478cr20190371. [DOI] [Google Scholar]
- 47.Kim I., Lee J. Variations in Anthocyanin Profiles and Antioxidant Activity of 12 Genotypes of Mulberry (Morus spp.) Fruits and Their Changes during Processing. Antioxidants. 2020;9:242. doi: 10.3390/antiox9030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee Y.-J., Kim E.-O., Choi S.-W. Isolation and identification of antioxidant polyphenolic compounds in mulberry (Morus alba L.) seeds. J. Korean Soc. Food Sci. Nutr. 2011;40:517–524. doi: 10.3746/jkfn.2011.40.4.517. [DOI] [Google Scholar]
- 49.Oh M., Bae S.Y., Chung M.S. Mulberry (Morus alba) seed extract and its polyphenol compounds for control of foodborne viral surrogates. J. Korean Soc. Appl. Biol. Chem. 2013;56:655–660. doi: 10.1007/s13765-013-3266-7. [DOI] [Google Scholar]
- 50.Argon Z.Ü., İlhan N., Gökyer A., Öztürk S.B., Koparal B. Phytochemical Evaluation of Morus alba Seeds and Cold Pressed Oil. J. Turk. Chem. Soc. Sect. A Chem. 2019;6:41–50. doi: 10.18596/jotcsa.470279. [DOI] [Google Scholar]
- 51.Sbihi H.M., Nehdi I.A., Mokbli S., Romdhani-Younes M., Al-Resayes S.I. Hexane and ethanol extracted seed oils and leaf essential compositions from two castor plant (Ricinus communis L.) varieties. Ind. Crops Prod. 2018;122:174–181. doi: 10.1016/j.indcrop.2018.05.072. [DOI] [Google Scholar]
- 52.Trigui I., Zarai Z., Chevance S., Cheikh-Rouhou S., Attia H., Ayadi M. Physicochemical properties, antioxidant activity and in vitro gastrointestinal digestion of purified proteins from black cumin seeds. Int. J. Biol. Macromol. 2019;126:454–465. doi: 10.1016/j.ijbiomac.2018.12.198. [DOI] [PubMed] [Google Scholar]
- 53.Yılmaz M.A., Durmaz G. Mulberry seed oil: A rich source of δ-tocopherol. J. Am. Oil Chem. Soc. 2015;92:553–559. doi: 10.1007/s11746-015-2627-2. [DOI] [Google Scholar]
- 54.Yao X.-H., Shen Y.-S., Hu R.-Z., Xu M., Huang J.-X., He C.-X., Cao F.-L., Fu Y.-J., Zhang D.-Y., Zhao W.-G. The antioxidant activity and composition of the seed oil of mulberry cultivars. Food Biosci. 2020;37:100709. doi: 10.1016/j.fbio.2020.100709. [DOI] [Google Scholar]
- 55.Rahman M.M., Akther A., Moinuddin M., Yeasmin M.S., Rahman M.M., Rahman M.S., Ferdousi S.A., Sayeed M.A. Investigation some physicochemical properties, lipids, glycerides and fatty acid composition of mulberry (Morus alba L.) seed oil of three different regions of Bangladesh. Am. J. Appl. Chem. 2014;2:38–41. doi: 10.11648/j.ajac.20140203.11. [DOI] [Google Scholar]
- 56.Cui H., Lu T., Wang M., Zou X., Zhang Y., Yang X., Dong Y., Zhou H. Flavonoids from Morus alba L. leaves: Optimization of extraction by response surface methodology and comprehensive evaluation of their antioxidant, antimicrobial, and inhibition of α-amylase activities through analytical hierarchy process. Molecules. 2019;24:2398. doi: 10.3390/molecules24132398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swapana N., Jotinkumar T., Devi C.B., Singh M.S., Singh S.B., Singh C. Total Phenolic, Total Flavonoid Contents And Antioxidant Activity Of A Fe w Indigenous Fruits Grown In Manipur. Bioscan. 2012;7:73–76. [Google Scholar]
- 58.Jha S., Gupta S.K., Bhattacharyya P., Ghosh A., Mandal P. In vitro antioxidant and antidiabetic activity of oligopeptides derived from different mulberry (Morus alba L.) cultivars. Pharmacogn. Res. 2018;10:361. doi: 10.4103/pr.pr_70_18. [DOI] [Google Scholar]
- 59.D’urso G., Mes J.J., Montoro P., Hall R.D., de Vos R.C. Identification of bioactive phytochemicals in mulberries. Metabolites. 2020;10:7. doi: 10.3390/metabo10010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Przeor M., Flaczyk E., Beszterda M., Szymandera-Buszka K.E., Piechocka J., Kmiecik D., Szczepaniak O., Kobus-Cisowska J., Jarzębski M., Tylewicz U. Air-drying temperature changes the content of the phenolic acids and flavonols in white mulberry (Morus alba L.) leaves. Ciênc. Rural. 2019;49:1–4. doi: 10.1590/0103-8478cr20190489. [DOI] [Google Scholar]
- 61.Tu J., Shi D., Wen L., Jiang Y., Zhao Y., Yang J., Liu H., Liu G., Yang B. Identification of moracin N in mulberry leaf and evaluation of antioxidant activity. Food Chem. Toxicol. 2019;132:110730. doi: 10.1016/j.fct.2019.110730. [DOI] [PubMed] [Google Scholar]
- 62.Khan M.S., Chen C., Fu X. The effect of geographic variation on chemical composition, antioxidant and hypoglycemic activities of Morus alba L. polysaccharides. J. Food Process. Preserv. 2019;43:e14206. doi: 10.1111/jfpp.14206. [DOI] [Google Scholar]
- 63.Malviya N., Jain S., Malviya S. Antidiabetic potential of medicinal plants. Acta Pol. Pharm. 2010;67:113–118. [PubMed] [Google Scholar]
- 64.Ahn E., Choi S.-W., Kim E. Anti-diabetic effect of sericultural product in high fat diet-fed mice. J. Korean Soc. Food Sci. Nutr. 2017;46:289–297. doi: 10.3746/jkfn.2017.46.3.289. [DOI] [Google Scholar]
- 65.Eruygur N., Dural E. Determination of 1-deoxynojirimycin by a developed and validated HPLC-FLD method and assessment of in-vitro antioxidant, α-amylase and α-glucosidase inhibitory activity in mulberry varieties from Turkey. Phytomedicine. 2019;53:234–242. doi: 10.1016/j.phymed.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 66.Vichasilp C., Nakagawa K., Sookwong P., Higuchi O., Luemunkong S., Miyazawa T. Development of high 1-deoxynojirimycin (DNJ) content mulberry tea and use of response surface methodology to optimize tea-making conditions for highest DNJ extraction. LWT Food Sci. Technol. 2012;45:226–232. doi: 10.1016/j.lwt.2011.09.008. [DOI] [Google Scholar]
- 67.Jeon Y.-H., Choi S.-W. Isolation, Identification, and Quantification of Tyrosinase and α-Glucosidase Inhibitors from UVC-Irradiated Mulberry (Morus alba L.) Leaves. Prev. Nutr. Food Sci. 2019;24:84. doi: 10.3746/pnf.2019.24.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi K.H., Lee H.A., Park M.H., Han J.-S. Mulberry (Morus alba L.) fruit extract containing anthocyanins improves glycemic control and insulin sensitivity via activation of AMP-activated protein kinase in diabetic C57BL/Ksj-db/db mice. J. Med. Food. 2016;19:737–745. doi: 10.1089/jmf.2016.3665. [DOI] [PubMed] [Google Scholar]
- 69.Khyade V.B. Utilization of mulberry methanolic leaf extractive (MMLE) for treating hypercholesterolemia in rat, rattus norvegicus (l) Int. J. Acad. Res. 2019;1:404–418. [Google Scholar]
- 70.Metwally F.M., Rashad H., Mahmoud A.A. Morus alba L. Diminishes visceral adiposity, insulin resistance, behavioral alterations via regulation of gene expression of leptin, resistin and adiponectin in rats fed a high-cholesterol diet. Physiol. Behav. 2019;201:1–11. doi: 10.1016/j.physbeh.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 71.Li H.X., Jo E., Myung C.-S., Kim Y.H., Yang S.Y. Lipolytic effect of compounds isolated from leaves of mulberry (Morus alba L.) in 3T3-L1 adipocytes. Nat. Prod. Res. 2018;32:1963–1966. doi: 10.1080/14786419.2017.1354190. [DOI] [PubMed] [Google Scholar]
- 72.Lupo M.G., Macchi C., Marchianò S., Cristofani R., Greco M.F., Dall’Acqua S., Chen H., Sirtori C.R., Corsini A., Ruscica M. Differential effects of red yeast rice, Berberis aristata and Morus alba extracts on PCSK9 and LDL uptake. Nutr. Metab. Cardiovasc. Dis. 2019;29:1245–1253. doi: 10.1016/j.numecd.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Yang X., Yang L., Zheng H. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem. Toxicol. 2010;48:2374–2379. doi: 10.1016/j.fct.2010.05.074. [DOI] [PubMed] [Google Scholar]
- 74.El-Baz F.K., Aly H.F., Abd-Alla H.I., Fayed D.B. Therapeutic impact of berries (Morus alba and Morus rubra) fruit extract in the regression of high-fat diet-induced cardiac dysfunction in rats. Asian J. Pharm. Clin. Res. 2018;11:314–320. doi: 10.22159/ajpcr.2018.v11i7.25859. [DOI] [Google Scholar]
- 75.Gupta G., Afzal M., David S.R., Verma R., Candaswamy M., Anwar F. Anticonvulsant activity of Morus alba and its effect on brain gamma-aminobutyric acid level in rats. Pharmacogn. Res. 2014;6:188. doi: 10.4103/0974-8490.129046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rayam S., Kudagi B., Sufyan U., Buchineni M., Pathapati R.M. Assessment of Morus alba (Mulberry) leaves extract for anti convulsant property in rats. Int. J. Basic Clin. Pharmacol. 2019;8:520–523. doi: 10.18203/2319-2003.ijbcp20190658. [DOI] [Google Scholar]
- 77.Diniz T.C., Silva J.C., Lima-Saraiva S.R., Ribeiro F.P., Pacheco A.G., de Freitas R.M., Quintans-Júnior L.J., Quintans J.D., Mendes R.L., Almeida J.R. The role of flavonoids on oxidative stress in epilepsy. Oxid. Med. Cell. Longev. 2015;2015:1–9. doi: 10.1155/2015/171756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tamtaji O.R., Mohammadifar M., Behnam M., Taghizadeh M., Talaei S.A. Investigation of the effect of alcoholic Morus alba leave extract on scopolamine-induced spatial memory impairment in rats. Sci. J. Kurd. Univ. Med. Sci. 2016;21:30–39. [Google Scholar]
- 79.Chen W., Liang T., Zuo W., Wu X., Shen Z., Wang F., Li C., Zheng Y., Peng G. Neuroprotective effect of 1-Deoxynojirimycin on cognitive impairment, β-amyloid deposition, and neuroinflammation in the SAMP8 mice. Biomed. Pharmacother. 2018;106:92–97. doi: 10.1016/j.biopha.2018.06.106. [DOI] [PubMed] [Google Scholar]
- 80.Liu D., Du D. Mulberry Fruit Extract Alleviates Cognitive Impairment by Promoting the Clearance of Amyloid-β and Inhibiting Neuroinflammation in Alzheimer’s Disease Mice. Neurochem. Res. 2020;45:2009–2019. doi: 10.1007/s11064-020-03062-7. [DOI] [PubMed] [Google Scholar]
- 81.El-Baz F.K., Aly H.F., Abd-Alla H.I., Ali S.A. Neurorestorative mulberries potential of alzheimer’s disease in animal model. Asian J. Pharm. Clin. Res. 2018;11:318–324. doi: 10.22159/ajpcr.2018.v11i10.27155. [DOI] [Google Scholar]
- 82.Rebai O., Belkhir M., Boujelben A., Fattouch S., Amri M. Morus alba leaf extract mediates neuroprotection against glyphosate-induced toxicity and biochemical alterations in the brain. Environ. Sci. Pollut. Res. 2017;24:9605–9613. doi: 10.1007/s11356-017-8584-6. [DOI] [PubMed] [Google Scholar]
- 83.Gu P.S., Moon M., Choi J.G., Oh M.S. Mulberry fruit ameliorates Parkinson’s-disease-related pathology by reducing α-synuclein and ubiquitin levels in a 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine/probenecid model. J. Nutr. Biochem. 2017;39:15–21. doi: 10.1016/j.jnutbio.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 84.Bhuiyan M.I.H., Kim H.-B., Kim S.Y., Cho K.-O. The neuroprotective potential of cyanidin-3-glucoside fraction extracted from mulberry following oxygen-glucose deprivation. Korean J. Physiol. Pharmacol. 2011;15:353. doi: 10.4196/kjpp.2011.15.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suwansri S., Khaoprasert S., Ratanatriwong P., Promboon A. Natural preservative from Thai mulberry: The antioxidant and antibacterial properties of leaf extracts from different cultivars. Acta Hortic. 2008;786:115–124. doi: 10.17660/ActaHortic.2008.786.12. [DOI] [Google Scholar]
- 86.Thabti I., Elfalleh W., Tlili N., Ziadi M., Campos M.G., Ferchichi A. Phenols, flavonoids, and antioxidant and antibacterial activity of leaves and stem bark of Morus species. Int. J. Food Prop. 2014;17:842–854. doi: 10.1080/10942912.2012.660722. [DOI] [Google Scholar]
- 87.De Oliveira A.M., Mesquita M.d.S., da Silva G.C., de Oliveira Lima E., de Medeiros P.L., Paiva P.M.G., Souza I.A.d., Napoleão T.H. Evaluation of toxicity and antimicrobial activity of an ethanolic extract from leaves of Morus alba L. (Moraceae) Evid. Based Complementary Altern. Med. 2015;2015:7. doi: 10.1155/2015/513978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang J.-Y., Lee H.-S. Evaluation of antioxidant and antibacterial activities of morin isolated from mulberry fruits (Morus alba L.) J. Korean Soc. Appl. Biol. Chem. 2012;55:485–489. doi: 10.1007/s13765-012-2110-9. [DOI] [Google Scholar]
- 89.Kumar R.V., Srivastava D., Singh V., Kumar U., Vishvakarma V.K., Singh P., Kumar D., Kumar R. Characterization, biological evaluation and molecular docking of mulberry fruit pectin. Sci. Rep. 2020;10:1–16. doi: 10.1038/s41598-020-78086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kostić D.S.D.D.A., Mitić G.S.S.S.-S., Đorđević M.N.M.A.S. Phenolic composition, antioxidant activity, mineral content and antimicrobial activity of fresh fruit extracts of Morus alba L. J. Food Nutr. Res. 2014;53:22–30. [Google Scholar]
- 91.Genovese C., Scarpaci K., D’angeli F., Caserta G., Nicolosi D. Antibacterial activity of Morus alba L. leaf extracts. Ann. Clin. Case Stud. 2020;2:1017. [Google Scholar]
- 92.Kim B.S., Kim H., Kang S.-S. In vitro anti-bacterial and anti-inflammatory activities of lactic acid bacteria-biotransformed mulberry (Morus alba Linnaeus) fruit extract against Salmonella Typhimurium. Food Control. 2019;106:106758. doi: 10.1016/j.foodcont.2019.106758. [DOI] [Google Scholar]
- 93.Jacob J.R., Mansfield K., You J.-E., Tennant B.C., Kim Y.-H. Natural iminosugar derivatives of 1-deoxynojirimycin inhibit glycosylation of hepatitis viral envelope proteins. J. Microbiol. 2007;45:431–440. [PubMed] [Google Scholar]
- 94.Soltanian S., Sheikhbahaei M., Mohamadi N. Cytotoxicity evaluation of methanol extracts of some medicinal plants on P19 embryonal carcinoma cells. J. Appl. Pharm. Sci. 2017;7:142–149. [Google Scholar]
- 95.Fathy S.A., Singab A.N.B., Agwa S.A., El Hamid D.M.A., Zahra F.A., El Moneim S.M.A. The antiproliferative effect of mulberry (Morus alba L.) plant on hepatocarcinoma cell line HepG2. Egypt. J. Med. Hum. Genet. 2013;14:375–382. doi: 10.1016/j.ejmhg.2013.07.001. [DOI] [Google Scholar]
- 96.Zhang Q., Zhang F., Thakur K., Wang J., Wang H., Hu F., Zhang J.-G., Wei Z.-J. Molecular mechanism of anti-cancerous potential of Morin extracted from mulberry in Hela cells. Food Chem. Toxicol. 2018;112:466–475. doi: 10.1016/j.fct.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Fallah S., Karimi A., Panahi G., Nejad S.G., Fadaei R., Seifi M. Human colon cancer HT-29 cell death responses to doxorubicin and Morus alba leaves flavonoid extract. Cell. Mol. Biol. 2016;62:72–77. [PubMed] [Google Scholar]
- 98.Jyoti A., Anand K., Sunanda P. Synergistic action of phytochemicals augments their antioxidative efficacy: An in vitro comparative study. Asian J. Pharm. Clin. Res. 2013;6:121–126. [Google Scholar]
- 99.Cho E., Y Chung E., Jang H.-Y., Hong O.-Y., S Chae H., Jeong Y.-J., Kim S.-Y., Kim B.-S., J Yoo D., Kim J.-S. Anti-cancer effect of cyanidin-3-glucoside from mulberry via caspase-3 cleavage and DNA fragmentation in vitro and in vivo. Anticancer Agents Med. Chem. 2017;17:1519–1525. doi: 10.2174/1871520617666170327152026. [DOI] [PubMed] [Google Scholar]
- 100.Yu J.S., Lee D., Lee S.R., Lee J.W., Choi C.-I., Jang T.S., Kang K.S., Kim K.H. Chemical characterization of cytotoxic indole acetic acid derivative from mulberry fruit (Morus alba L.) against human cervical cancer. Bioorg. Chem. 2018;76:28–36. doi: 10.1016/j.bioorg.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 101.Ramis E.S., Hammoud G.M., ElSawy K.M. In vitro and In vivo Studies on Mulberry Extracts: Evaluation of Chemical and Anticancer Activities and Attenuation of Lead Toxicity. Asian J. Res. Biochem. 2018:1–14. doi: 10.9734/ajrb/2018/v2i3525. [DOI] [Google Scholar]
- 102.Mitsuya M. Four-week oral toxicity studies of the leaf powder of mulberry (Morus alba L.) in rats. Pharmacometrics. 2001;61:169–179. [Google Scholar]
- 103.Phornchirasilp S., Luanratana O., Sirikulchayanonta V., Tiengda C. A Toxicity Study of Morus alba L. Leaf Extract. Thai J. Pharm. Sci. 2004;26:70. [Google Scholar]
- 104.de Oliveira A.M., do Nascimento M.F., Ferreira M.R.A., de Moura D.F., dos Santos Souza T.G., da Silva G.C., da Silva Ramos E.H., Paiva P.M.G., de Medeiros P.L., da Silva T.G. Evaluation of acute toxicity, genotoxicity and inhibitory effect on acute inflammation of an ethanol extract of Morus alba L. (Moraceae) in mice. J. Ethnopharmacol. 2016;194:162–168. doi: 10.1016/j.jep.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 105.Jiao Y., Wang X., Jiang X., Kong F., Wang S., Yan C. Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet-and streptozotocin-induced type 2 diabetes in rats. J. Ethnopharmacol. 2017;199:119–127. doi: 10.1016/j.jep.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 106.Chang B.Y., Kim S.B., Lee M.K., Park H., Kim S.Y. Nonclinical Safety Assessment of Morus alba L. Fruits: Study of 90-D Toxicity in Sprague Dawley Rats and Genotoxicity in Salmonella. J. Food Sci. 2016;81:T1328–T1335. doi: 10.1111/1750-3841.13285. [DOI] [PubMed] [Google Scholar]
- 107.Paul A., Shakya A., Zaman M.K. Assessment of acute and sub-chronic neurotoxicity of Morus alba L. fruits in rodents. Future J. Pharm. Sci. 2020;6:1–11. doi: 10.1186/s43094-020-00110-5. [DOI] [Google Scholar]
- 108.Ruengdech A., Siripatrawan U., Sangnark A., Benedetti S., Buratti S. Rapid evaluation of phenolic compounds and antioxidant activity of mulberry leaf tea during storage using electronic tongue coupled with chemometrics. J. Berry Res. 2019;9:563–574. doi: 10.3233/JBR-190395. [DOI] [Google Scholar]
- 109.Bhosale S.C., Khyade V.B., Patil V.S. Preparation of functional and healthy food: The paratha (cooked dough) through the use of leaves of mulberry, Morus alba (L) from the moriculture unit of agricultural development Trust, Baramati (India) Int. J. Recent Acad. Res. 2019;1:506–518. [Google Scholar]
- 110.Lim H.S., Park S.H., Ghafoor K., Hwang S.Y., Park J. Quality and antioxidant properties of bread containing turmeric (Curcuma longa L.) cultivated in South Korea. Food Chem. 2011;124:1577–1582. doi: 10.1016/j.foodchem.2010.08.016. [DOI] [Google Scholar]
- 111.Jomova K., Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 112.Alfeo V., Bravi E., Ceccaroni D., Perretti G., Marconi O., Sileoni V. Effect of Baking Time and Temperature on Nutrients and Phenolic Compounds Content of Fresh Sprouts Breadlike Product. Foods. 2020;9:1447. doi: 10.3390/foods9101447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang J., Li T., Cao H., Bu H., Hou X., Lu C. Effects of dietary forage mulberry on the growth performance and meat quality of finishing pigs. Chin. J. Anim. Sci. 2014;50:52–56. doi: 10.1016/S2095-3119(18)62072-6. [DOI] [Google Scholar]
- 114.Zhu Z., Jiang J.-J., Jie Y., Mao X.-B., Bing Y., Chen D.-W. Effect of dietary supplementation with mulberry (Morus alba L.) leaves on the growth performance, meat quality and antioxidative capacity of finishing pigs. J. Integr. Agric. 2019;18:143–151. [Google Scholar]
- 115.Ouyang J., Hou Q., Wang M., Zhao W., Feng D., Pi Y., Sun X.-Z. Effects of dietary mulberry leaf powder on growth performance, blood metabolites, meat quality and antioxidant enzyme related gene expression of fattening Hu lambs. Can. J. Anim. Sci. 2020;100:510–521. doi: 10.1139/cjas-2019-0119. [DOI] [Google Scholar]
- 116.Zhang X., Li D., Meng Q., He C., Ren L. Effect of mulberry leaf extracts on color, lipid oxidation, antioxidant enzyme activities and oxidative breakdown products of raw ground beef during refrigerated storage. J. Food Qual. 2016;39:159–170. doi: 10.1111/jfq.12187. [DOI] [Google Scholar]
- 117.Butkhup L., Samappito W., Samappito S. Phenolic composition and antioxidant activity of white mulberry (Morus alba L.) fruits. Int. J. Food Sci. Technol. 2013;48:934–940. doi: 10.1111/ijfs.12044. [DOI] [Google Scholar]
- 118.Vanitha T., Khan M. Pectins-Extraction, Purification, Characterization and Applications. Books on Demand; Norderstedt, Germany: 2019. Role of pectin in food processing and food packaging. [Google Scholar]
- 119.Natić M.M., Dabić D.Č., Papetti A., Akšić M.M.F., Ognjanov V., Ljubojević M., Tešić Ž.L. Analysis and characterisation of phytochemicals in mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chem. 2015;171:128–136. doi: 10.1016/j.foodchem.2014.08.101. [DOI] [PubMed] [Google Scholar]
- 120.ve Depolama A.K.Ü.Ç. Phenolic Composition, Colour and Anthocyanin Stability of Black Mulberry and White Mulberry Jam as Affected by Cultivar and Storage Temperature. J. Biol. Chem. 2016;44:395–399. doi: 10.15671/HJBC.2016.119. [DOI] [Google Scholar]
- 121.Olofsson P., Hultqvist M., Hellgren L.I., Holmdahl R. Phytol: A chlorophyll component with anti-inflammatory and metabolic properties. In: Claus J., Gilbert K., Alan S., Paul G.W., Torsten B., editors. Recent Advances in Redox Active Plant and Microbial Products. Springer; Dordrecht, Netherland: 2014. pp. 345–359. [Google Scholar]
- 122.Xu L., Zhu M.-J., Liu X.-M., Cheng J.-R. Inhibitory effect of mulberry (Morus alba) polyphenol on the lipid and protein oxidation of dried minced pork slices during heat processing and storage. LWT. 2018;91:222–228. doi: 10.1016/j.lwt.2018.01.040. [DOI] [Google Scholar]
- 123.Xiang R., Cheng J., Zhu M., Liu X. Effect of mulberry (Morus alba) polyphenols as antioxidant on physiochemical properties, oxidation and bio-safety in Cantonese sausages. LWT. 2019;116:108504. doi: 10.1016/j.lwt.2019.108504. [DOI] [Google Scholar]
- 124.Tomczyk M., Miłek M., Sidor E., Kapusta I., Litwińczuk W., Puchalski C., Dżugan M. The Effect of Adding the Leaves and Fruits of Morus alba to Rape Honey on Its Antioxidant Properties, Polyphenolic Profile, and Amylase Activity. Molecules. 2020;25:84. doi: 10.3390/molecules25010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kobus-Cisowska J., Dziedziński M., Szymanowska D., Szczepaniak O., Byczkiewicz S., Telichowska A., Szulc P. The Effects of Morus alba L. Fortification on the Quality, Functional Properties and Sensory Attributes of Bread Stored under Refrigerated Conditions. Sustainability. 2020;12:6691. doi: 10.3390/su12166691. [DOI] [Google Scholar]
- 126.Ribeiro-Santos R., Andrade M., de Melo N.R., Sanches-Silva A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017;61:132–140. doi: 10.1016/j.tifs.2016.11.021. [DOI] [Google Scholar]
- 127.Zhi-ming L., Hai-ying W., Shan-shan L., Nai-xiang J. Volatile Components of Essential Oil from Mulberry Variety “Longsang 1” Leaves. Nat. Prod. Res. 2011;23:1069–1084. [Google Scholar]
- 128.Radulović N.S., Miljković V.M., Mladenović M.Z., Nikolić G.S. Essential oils of Morus alba and M. nigra leaves: Effect of drying on the chemical composition. Nat. Prod. Commun. 2017;12:1934578X1701200133. doi: 10.1177/1934578X1701200133. [DOI] [PubMed] [Google Scholar]
- 129.Santos C.C.D.M.P., Salvadori M.S., Mota V.G., Costa L.M., de Almeida A.A.C., de Oliveira G.A.L., Costa J.P., de Sousa D.P., de Freitas R.M., de Almeida R.N. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci. J. 2013;2013:94945. doi: 10.1155/2013/949452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aparna V., Dileep K.V., Mandal P.K., Karthe P., Sadasivan C., Haridas M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012;80:434–439. doi: 10.1111/j.1747-0285.2012.01418.x. [DOI] [PubMed] [Google Scholar]
- 131.Ravi L., Krishnan K. Research Article Cytotoxic Potential of N-hexadecanoic Acid Extracted from Kigelia pinnata Leaves. Asian J. Cell Biol. 2017;12:20–27. doi: 10.3923/ajcb.2017.20.27. [DOI] [Google Scholar]
- 132.Yeo S.K., Ali A.Y., Hayward O.A., Turnham D., Jackson T., Bowen I.D., Clarkson R. β-Bisabolene, a sesquiterpene from the essential oil extract of opoponax (Commiphora guidottii), exhibits cytotoxicity in breast cancer cell lines. Phytother. Res. 2016;30:418–425. doi: 10.1002/ptr.5543. [DOI] [PubMed] [Google Scholar]
- 133.Dong X., Gao X., Liu L., Chen L., Liu Q., Zhang D. Function-specific volatiles and volatilization characteristics of Dendrobium officinale. J. King Saud Univ. Sci. 2020;32:2020–2028. doi: 10.1016/j.jksus.2020.02.001. [DOI] [Google Scholar]
- 134.Caputi L., Aprea E. Use of terpenoids as natural flavouring compounds in food industry. Recent Pat. Food Nutr. Agric. 2011;3:9–16. doi: 10.2174/2212798411103010009. [DOI] [PubMed] [Google Scholar]
- 135.Mezzomo N., Ferreira S.R. Carotenoids functionality, sources, and processing by supercritical technology: A review. J. Chem. 2016;2016:3164312. doi: 10.1155/2016/3164312. [DOI] [Google Scholar]
- 136.Özcan M.M., Lemiasheuski V., Özcan M.M. Fatty acid compositions of white and black mulberry fruit oils. J. Agroaliment. Process. Technol. 2019;25:179–181. [Google Scholar]
- 137.Mimica-Dukić N., Božin B., Soković M., Mihajlović B., Matavulj M. Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Med. 2003;69:413–419. doi: 10.1055/s-2003-39704. [DOI] [PubMed] [Google Scholar]
- 138.Fitzgerald D.J., Stratford M., Narbad A. Analysis of the inhibition of food spoilage yeasts by vanillin. Int. J. Food Microbiol. 2003;86:113–122. doi: 10.1016/S0168-1605(03)00059-X. [DOI] [PubMed] [Google Scholar]
- 139.Oussalah M., Caillet S., Saucier L., Lacroix M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157: H7, Salmonella typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control. 2007;18:414–420. doi: 10.1016/j.foodcont.2005.11.009. [DOI] [Google Scholar]
- 140.Pandey A.K., Kumar P., Singh P., Tripathi N.N., Bajpai V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017;7:2161. doi: 10.3389/fmicb.2016.02161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chang J., Kang X., Yuan J.-L. Enhancing emulsification and antioxidant ability of egg albumin by moderately acid hydrolysis: Modulating an emulsion-based system for mulberry seed oil. Food Res. Int. 2018;109:334–342. doi: 10.1016/j.foodres.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 142.Li Y.-Q., Kong D.-X., Huang R.-S., Liang H.-L., Xu C.-G., Wu H. Variations in essential oil yields and compositions of Cinnamomum cassia leaves at different developmental stages. Ind. Crops Prod. 2013;47:92–101. doi: 10.1016/j.indcrop.2013.02.031. [DOI] [Google Scholar]
- 143.Negi P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012;156:7–17. doi: 10.1016/j.ijfoodmicro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 144.Li D., Zhu M., Liu X., Wang Y., Cheng J. Insight into the effect of microcapsule technology on the processing stability of mulberry polyphenols. LWT. 2020;126:109144. doi: 10.1016/j.lwt.2020.109144. [DOI] [Google Scholar]
- 145.Xu L., Cheng J.-R., Liu X.-M., Zhu M.-J. Effect of microencapsulated process on stability of mulberry polyphenol and oxidation property of dried minced pork slices during heat processing and storage. LWT. 2019;100:62–68. doi: 10.1016/j.lwt.2018.10.025. [DOI] [Google Scholar]
- 146.Cheng J.-R., Xiang R., Liu X.-M., Zhu M.-J. The effects of thermal processing and β-cyclodextrin on extractable polyphenols in mulberry juice-enriched dried minced pork slices. LWT. 2019;116:108503. doi: 10.1016/j.lwt.2019.108503. [DOI] [Google Scholar]
- 147.Butstraen C., Salaün F. Preparation of microcapsules by complex coacervation of gum Arabic and chitosan. Carbohydr. Polym. 2014;99:608–616. doi: 10.1016/j.carbpol.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 148.Efara É., Marquis F., Tremblay A. Scaling Up Biotechnological Chemical Processes: A Better Alternative to the Traditional Develop-Then-Scale Model. Ind. Biotechnol. 2019;15:157–161. doi: 10.1089/ind.2019.29173.eef. [DOI] [Google Scholar]
- 149.Flaczyk E., Kobus-Cisowska J., Przeor M., Korczak J., Remiszewski M., Korbas E., Buchowski M. Chemical characterization and antioxidative properties of Polish variety of Morus alba L. leaf aqueous extracts from the laboratory and pilot-scale processes. J. Agric. Sci. 2013;4:141. doi: 10.4236/as.2013.45B026. [DOI] [Google Scholar]
- 150.Gang T., Xin-Fu Y., Xiao-Ming P., Cui-Qing L., Teng W., Qi Y. Response Surface Methodology-Optimized Pilot-Plant Extraction of Polysaccharides from Mulberry Leaves; Proceedings of the Joint International Conference on Social Science and Environment Science (SSES) and International Conference on Food Science and Engneering (ICFSE); Guangzhou, China. 15–16 October 2016; Lancaster, PA, USA: DEStech; 2016. pp. 1–13. [Google Scholar]
- 151.Tian G., Yin X., Peng X., Li C., Wang T., Feng J., Ju R. Response Surface Methodology Optimized Pilot Plant Extraction Process of 1-Deoxynojirimycin from Mulberry Leaves. J. Biobased Mater. Bioenergy. 2019;13:207–213. doi: 10.1166/jbmb.2019.1839. [DOI] [Google Scholar]
- 152.Komaikul J., Kitisripanya T., Inyai C., Likhitwitayawuid K., Sritularak B., Tanaka H., Putalun W. Phytostilbenoid production in white mulberry (Morus alba L.) cell culture using bioreactors and simple deglycosylation by endogenous enzymatic hydrolysis. In Vitro Cell. Dev. Biol. Plant. 2019;55:199–208. doi: 10.1007/s11627-018-09953-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.