Abstract
Individual differences in working memory relate to performance differences in general cognitive ability. The neural bases of such individual differences, however, remain poorly understood. Here, using a data-driven technique known as connectome-based predictive modeling, we built models to predict individual working memory performance from whole-brain functional connectivity patterns. Using n-back or rest data from the Human Connectome Project, connectome-based predictive models significantly predicted a novel individual’s 2-back accuracy. Model predictions also correlated with measures of fluid intelligence and, with less strength, sustained attention. Separate fluid intelligence models predicted working memory score, as did sustained attention models, again with less strength. Anatomical feature analysis revealed significant overlap between working memory and fluid intelligence models, particularly in utilization of prefrontal and parietal regions, and less overlap in predictive features between working memory and sustained attention models. Furthermore, showing the generality of these models, the working memory model developed from Human Connectome Project data generalized to predict memory deterioration in an independent dataset of 157 older adults (mean age 69; 48 healthy, 54 amnestic mild cognitive impairment, 55 Alzheimer’s disease). The present results demonstrate that distributed functional connectivity patterns predict individual variation in working memory capability across the adult lifespan, correlating with constructs including fluid intelligence and sustained attention.
Keywords: functional connectivity, predictive model, working memory, fluid intelligence, attention
Introduction
Working memory (WM) plays a critical role in our daily lives. From reading a news article to remembering the items we need at the grocery store, our capacity to temporarily store and manipulate information facilitates our interactions with the world around us and guides our behavior. In this role, WM has been deemed the “workbench of cognition” (Klatzky, 1975), and, as such, WM capability, its relation to other cognitive skills, and its variation between individuals have been of particular interest (Baddeley, 2003; D’Esposito & Postle, 2015; Luck & Vogel, 2013).
Individual differences in WM relate to performance differences in general cognitive ability (Cowan et al., 2005; Kyllonen & Christal, 1990; Kane & Engle, 2002). WM capacity, for example, accounts for 40% of variance in global fluid intelligence (gF; Fukuda et al., 2010). This strong relationship is thought to reflect shared neural systems, particularly involving prefrontal and parietal regions, which allow us to keep a representation active in the face of interference (Engle et al., 1999; Prabhakaran et al., 1997). Relationships between WM and controlled attention have also been of particular interest (Chun et al., 2011), and the ability to control attention has been proposed to play a major role in complex WM tasks (Kane et al., 2001; Barrett, Tugade, & Engle, 2004). For example, the efficiency of information flow between attentional and perceptual neural systems may impact WM precision performance, though not WM capacity (Galeano Weber et al., 2017).
Deficits in WM affect a wide range of cognitive abilities and are common to a range of neurological disorders including schizophrenia, attention deficit disorder, and reading disabilities (Luck & Vogel, 2013). The question of what drives such individual differences in WM, however, requires more study. WM has classically been described as a limited capacity workspace (Baddeley & Hitch, 1974), wherein individual differences in storage capacity and the ability to use this capacity efficiently account for individual variation in WM capability (Just & Carpenter, 1992; Luck & Vogel, 1997). More recent work has expanded on this foundation, with leading cognitive psychological theories positing that capacity arises from a fixed number of memory slots (Luck & Vogel, 2013) or a fixed amount of attentional resources (Ma, Husain, & Bays, 2014).
Recent functional magnetic resonance imaging (fMRI) studies have characterized the neural representations underlying models of WM, with multivariate pattern analysis techniques showing that WM representations are sustained in regions including prefrontal, parietal, and sensory cortices (Christophel, Hebart, & Haynes, 2012; Han et al., 2013; Sreenivasan, Vytlacil, & D’Esposito, 2014). The posterior intraparietal sulcus (IPS) in particular has been strongly linked to variation in WM capacity: IPS activity scales with WM load until capacity is reached (Todd & Marois, 2004; Xu & Chun, 2006), and functional connectivity analyses have implicated the IPS as a central hub in networks of WM-associated regions (Palva et al., 2010). More generally, work has emphasized the importance of functional connections between distributed brain regions including frontal, parietal, premotor, and occipitotemporal regions in mediating WM (Gazzaley, Rissman, & D’Esposito, 2004; Fiebach, Rissman, & D’Esposito, 2006; Liebe et al., 2012; Liu et al., 2018; Yamashita et al., 2018), with recent work implicating large-scale, distributed networks in WM capacity (Miller & Buschman, 2015; Soreq, Leech, & Hampshire, 2019; Luck & Vogel, 2013; Cohen & D’Esposito, 2016). In this vein, the functional networks and anatomical features giving rise to WM processes remains a topic of interest, including their relationship to WM deterioration in older age.
In addition to the neuroimaging studies that have focused on neural correlates of WM, related work has investigated individual differences in this cognitive ability (Todd & Marois, 2005). Such differences have been traditionally measured by performance on cognitive tasks, such as verbal repetition span tasks and continuous recognition tasks (Redick et al., 2012; Kane et al., 2007), with more recent work relating performance on such tasks to individual differences in brain function (Bertolero et al., 2019; Luck & Vogel, 2013). Here, we propose a novel, brain-based measure of individual WM performance, which goes beyond former work in being applicable to novel participants and directly comparable to models of other cognitive and attentional abilities. Employing a data-driven technique known as connectome-based predictive modeling (CPM: Finn et al., 2015; Rosenberg et al., 2016a; Shen et al., 2017), we built models predicting individual WM performance from whole-brain functional connectivity patterns observed in participants of the Human Connectome Project (HCP) as they completed a WM (n-back) task or rested. This approach complements a growing body of work predicting outcomes and behavior from large-scale functional connectivity patterns (e.g., Smith et al., 2015; Gong et al., 2019; see Woo et al., 2017 and Dubois & Adolphs 2016 for recent reviews).
Using 10-fold cross validation in the HCP dataset, CPMs built from task-based or resting-state fMRI data successfully predicted previously unseen individuals’ 2-back accuracy. Distinct models predicted measures of attention, and, replicating previous work, gF (Finn et al., 2015), and comparisons of these models revealed relationships between WM, gF, and, to a lesser degree, sustained attention. Furthermore, the same CPMs trained to predict 2-back accuracy the HCP sample generalized to predict individual differences in memory deterioration in an independent sample of older adults, including patients with amnestic mild cognitive impairment (aMCI) and with Alzheimer’s disease (AD), recruited from the Samsung Medical Center (SMC) in Seoul, Korea. Thus, we provide a novel demonstration of the use of distributed patterns of functional connectivity to measure individual differences in WM, correlating with constructs such as gF and sustained attention. We also characterize commonalities and differences in the functional anatomy of brain networks that underlie WM, gF, and sustained attention.
Methods
The present study used two independent fMRI datasets.
Dataset 1: Human Connectome Project S900 release
Data were obtained from the Human Connectome Project (HCP; Van Essen et al., 2013) 900-subject release of December 2015. From the available data from multiple neuroimaging modalities, we utilized functional MRI data and performance on behavioral cognitive tasks. Scan parameter details can be found in Ugurbil et al. (2013). Of the 900 participants (aged 22-35 years), we identified the 515 who completed all resting and task fMRI runs with average frame-to-frame motion <0.10mm and maximum frame-to-frame motion <0.15mm (Greene et al., 2018). Mean frame-to-frame motion for each condition (e.g., n-back task) was calculated by averaging the mean motion for corresponding left/right and right/left phase-encoding runs. Of these 515 participants, we analyzed the 502 (mean age 28 ± 3.6 years; 274 females) who did not have any missing functional atlas nodes (see Connectome-based predictive modeling section) or time points. Each participant provided written consent in accordance with the Institutional Review Boards of Washington University in Saint Louis, University of Minnesota, Oxford University, Saint Louis University, Indiana University, University d’Annunzio, Ernst Strungmann Institute, Warwick University, Radboud University Nijmegen, and Duke University.
Connectivity matrices were created as described in Finn et al. (2015). From minimally processed HCP data (Glasser et al., 2013), further preprocessing was completed using BioImage Suite and included the regression of 12 motion parameters and global, white matter, and cerebral spinal fluid signal, as well as removal of linear trend, and low-pass filtering (Finn et al., 2015). Task-evoked activity was not regressed. After preprocessing, the whole brain was parcellated by applying a 268-node whole-brain gray matter atlas spanning cortical, subcortical, and cerebellar regions to define functional network nodes (Shen et al., 2013). The fMRI signal time course in each node was averaged, and the Pearson correlation between the time courses of each pair of nodes was then calculated. Pearson r-values were converted to z-values using the Fisher transform to produce a 268x268 matrix of functional connection (edge) strengths for each participant.
Functional connectivity matrices were derived from resting state fMRI data (4 runs of 15 minutes each) and WM task data (n-back; 2 runs of 5 minutes each). Full details of task and rest scan procedures can be found in Barch et al. (2013) and Smith et al. (2013), respectively. Although all participants completed a total of 7 different task fMRI scans targeting specific cognitive functions, we selected the WM task fMRI scan for our analyses: In addition to directly targeting our construct of focus, this 10-minute n-back (0-back and 2-back) task also provided more data than any of the other 6 tasks, which ranged from 2.5 to 4 minutes in length. We also chose to include resting-state fMRI data because previous work has indicated that CPM is successful even when applied to data collected in the absence of an explicit task challenge (Finn et al., 2015; Rosenberg et al., 2016a, 2016b). Connectome-based models were trained to predict participants’ WM performance, operationalized as 2-back task accuracy, from n-back and resting-state functional connectivity patterns.
In consideration of the known mechanistic and behavioral relationships between WM and cognitive abilities such as gF and controlled attention (Kane & Engle, 2002; Barrett, Tugade, & Engle, 2004), we also assessed the degree to which our models of WM, gF, and attention reflected this overlap. To test whether models built to predict performance on the 2-back task generalized to predict other measures of cognitive task performance, we correlated model predictions from task-based and resting-state data with measures of gF and sustained attention. gF was quantified by number of correct responses on the Penn’s Progressive Matrices (PMAT), an increasingly difficult pattern-matching task during which subjects were instructed to select the missing piece of a puzzle containing visual patterns (Moore et al., 2015). Although gF and WM are related (Fukuda et al., 2010; Colom, Flores-Mendoza, & Rebollo, 2002), the PMAT does not explicitly recruit or assess WM capability. Sustained attention was quantified with d’ (sensitivity) on the Short Penn Continuous Performance task (SCPT), during which participants responded to displays of 7 line segments whenever they formed a number or a letter (Kurtz et al., 2001). Although attention is also required for n-back and PMAT task completion, as it is for nearly every task in daily life (Rosenberg et al., 2016a), we utilized 2-back accuracy, PMAT score, and SCPT d’ as commonly used indications of a participant’s WM, gF, or attentional ability, respectively. We report the correlations between these observed behavioral scores of gF, attention, and WM in Supplementary Table S3.
Dataset 2: Samsung Medical Center
Connectome-based models of WM defined in the full HCP sample were externally validated with an independent dataset collected at the Samsung Medical Center (SMC) in Seoul, Korea. Models applied to the SMC dataset were trained using data from the full HCP sample to maximize the number of training observations. Between March 2008 and February 2009, fMRI data and neuropsychological assessments (Seoul Neuropsychological Screening Battery) were collected from 278 individuals classified into three levels of memory function: Alzheimer’s disease patients (AD; n=112), amnestic mild cognitive impairment patients (aMCI; n=87), and healthy controls (n=79). Full information regarding patients’ medical histories and diagnostic criteria as well as neuropsychological assessment and fMRI scan procedures are detailed in Ahn et al. (2010) and Kim et al. (2015). Of the 278 patients, aged 44-90 years, we identified the 157 (mean age 68.7 ± 9.6 years; 105 females; 55 AD, 54 aMCI, 48 healthy) who completed all neuropsychological assessments and fMRI runs with average frame-to-frame motion <0.2mm, maximum translation <2mm, and maximum rotational motion <3°. These motion thresholds were less stringent than those applied to our HCP sample in consideration of the fact that older adults, on average, tend to exhibit higher motion levels during fMRI (D’Esposito et al., 1999; Geerligs et al., 2017; Van Dijk, Sabuncu, & Buckner, 2012; Fountain-Zaragoza et al., 2019). Recent work utilizing older adult fMRI data has thus tended to use motion thresholds that are less stringent than those typically applied to young adult samples (Lin et al., 2018; Guo et al., 2012; Ystad et al., 2010).
Resting-state fMRI preprocessing, including motion-correction and regression of nuisance covariates, was performed using SPM12 and custom scripts (MATLAB R2016b). Nuisance covariates included 24 motion-related parameters (6 translational and rotational motions, 6 derivatives of these motions, and 12 squares of these motions), 3 mean signals (global, white matter, and cerebrospinal fluid), and linear and quadratic trends. Functional connectivity matrices were created by calculating the Pearson correlation between each of 268 nodes of the whole brain (Shen et al., 2013 whole-brain gray matter atlas). Pearson r-values were converted to z-values using the Fisher transform to produce a 268x268 connectivity matrix of edge strengths for each participant. Each participant provided written informed consent in accordance with the Institutional Review Board of the SMC.
Connectome-based predictive modeling
CPM was used to construct models of WM following the procedures of Finn et al. (2015) and Rosenberg et al. (2016), with a detailed protocol and scripts available in Shen et al. (2017).
First, using the HCP dataset, subjects’ functional connectivity and behavioral data were split into 10 groups. Throughout 10-fold cross validation, nine of these groups comprised a training dataset, while the remaining group was designated as a testing dataset. As some of the 502 subjects in our HCP sample were related (194 sharing the same mother, 184 sharing the same father, 123 twins), group assignments were made with regard to familial relatedness between subjects such that related subjects were always placed in the same groups (that is, models were never trained on data from one family member and tested on data from another, which could compromise the independence of the training and test sets).
Using data from the nine groups of the training set, a given edge (i.e., functional connection) in the n-back or resting-state functional connectivity matrices across all training set participants was Pearson correlated with 2-back accuracy scores for those participants, and this process was repeated for every edge to identify the functional connections most strongly related to accuracy. A significance threshold of p<0.01 was applied to select edges that were positively and negatively correlated with behavior across individuals. A relationship was found between individual motion and behavioral task scores (see Supplementary Table S1), and we thus controlled for motion at this edge-selection step using partial Pearson correlation. Following selection of significant edges, the strength of these retained edges was then averaged for each participant to generate two summary statistics per individual: positive network strength (the mean edge value in the set of edges positively correlated with behavior) and negative network strength (the mean edge value in the set of edges negatively correlated with behavior). Linear models were then constructed to relate 2-back accuracy scores to network strength in the training sample, and these models were then applied to left-out individuals’ functional connectivity data to predict their task performance. This process was repeated 1000 times with random assignment of subjects into folds so as to provide a reliable statistic of average model performance. Model performance was assessed at each iteration by the strength of Pearson correlation between predicted and observed scores for each novel individual. The average of the resulting 1000 correlation coefficients was taken to represent average model performance
To assess the significance of the observed correlation between predicted and observed scores, we performed 1000 additional iterations of 10-fold cross validation using shuffled behavioral scores. This permutation analysis using mismatched brain and behavioral data allowed us to create a null model performance distribution. The significance of true model performance was assessed by comparing the distributions of correlation coefficients generated by the true and null models using the equation p(r) = (1 + number of null r-values >= observed r-value)/(1 + 1000). The effect size of the difference between null and true distribution means was also measured with Cohen’s D to further characterize model performance.
To compare the features and performance of WM models with those of other related cognitive abilities, we repeated the CPM procedure described above twice more, once to predict PMAT scores and once to predict SCPT performance. The predictions of gF and sustained attention CPMs from both task and resting-state data were correlated with observed 2-back accuracy values to assess their ability to predict WM task performance.
Cross-predictive power was expected and indeed observed between WM and gF models. As such overlap is thought to reflect similar, but not identical, neural underpinnings of WM and gF (Kane & Engle, 2002), we sought to isolate these cognitive abilities and their respective networks from each other to assess the degree to which they may be distinct. To this end, we applied CPM to predict 2-back task accuracy from functional connectivity matrices while controlling for gF at the edge selection step. We constructed predictive networks as described above, but selected only the edges that were correlated with 2-back accuracy values and were not correlated with PMAT scores (threshold p<0.01). The features and predictive power of these models were compared to those of the original WM models described above. We did not repeat this procedure to remove edges correlated with attention (i.e., SCPT task performance) because the observed correlation between WM and sustained attention performance was weaker (see Table 1).
Table 1. Summary of internal and external performance of WM, gF, and attention CPMs.
WM CPMs strongly predict HCP 2-back accuracy, HCP PMAT, and memory performance in the SMC dataset. WM CPMs built while controlling for gF at edge selection continue to strongly predict HCP 2-back accuracy. GF CPMs strongly predict HCP PMAT score, HCP 2-back accuracy, and SMC memory performance. When trained on n-back task data, but not rest data, attention CPMs predict HCP SCPT d’. P-values are derived from 1000 permutations of true and null models. Task data is HCP n-back task fMRI data.
| Analysis # | Training behavior, training data | Controlled covariate | Test behavior, test data | Model performance | Validation Type | Data Type | |
|---|---|---|---|---|---|---|---|
| 1 | 2-back accuracy, Task | 2-back accuracy, Task | r = 0.36, P = 1/1001 | * | 10-fold cross-validation | Internal data | |
| 2 | SMC composite memory, Rest | r = 0.37, P = 1/1001 | * | External validation | External data, different behavior | ||
| 3 | PMAT score, Task | r = 0.28, P = 1/1001 | * | 10-fold cross-validation | Internal data, different behavior | ||
| 4 | SCPT d’, Task | r = 0.09, P = 7.19E-2 | 10-fold cross-validation | Internal data, different behavior | |||
| 5 | 2-back accuracy, Task | gF** | 2-back accuracy, Task | r = 0.33, P = 1/1001 | * | 10-fold cross-validation | Internal data |
| 6 | 2-back accuracy, Rest | 2-back accuracy, Rest | r = 0.20, P = 1/1001 | * | 10-fold cross-validation | Internal data | |
| 7 | SMC composite memory, Rest | r = 0.30, P = 1/1001 | External validation | External data, different behavior | |||
| 8 | PMAT score, Rest | r = 0.13, P = 2.00E-3 | 10-fold cross-validation | Internal data, different behavior | |||
| 9 | SCPT d’, Rest | r = 0.06, P = 6.29E-2 | 10-fold cross-validation | Internal data, different behavior | |||
| 10 | 2-back accuracy, Rest | gF** | 2-back accuracy, Rest | r = 0.17, P = 2.00E-3 | * | 10-fold cross-validation | Internal data |
| 11 | PMAT score, Task | PMAT score, Task | r = 0.33, P = 1/1001 | * | 10-fold cross-validation | Internal data | |
| 12 | SMC composite memory, Rest | r = 0.30, P = 1/1001 | * | External validation | External data, different behavior | ||
| 13 | 2-back accuracy, Task | r = 0.34, P = 2.00E-3 | * | 10-fold cross-validation | Internal data, different behavior | ||
| 14 | PMAT score, Rest | PMAT score, Rest | r = 0.12, P = 1.70E-2 | 10-fold cross-validation | Internal data | ||
| 15 | SMC composite memory, Rest | r = 0.36, P = 1/1001 | * | External validation | External data, different behavior | ||
| 16 | 2-back accuracy, Rest | r = 0.13, P = 2.70E-2 | 10-fold cross-validation | Internal data, different behavior | |||
| 17 | SCPT d’, Task | SCPT d’, Task | r = 0.14, P = 5.00E-3 | * | 10-fold cross-validation | Internal data | |
| 18 | 2-back accuracy, Task | r = 0.16, P = 6.39E-2 | 10-fold cross-validation | Internal data, different behavior | |||
| 19 | SCPT d’, Rest | SCPT d’, Rest | r = −5.7E-3, P = 0.533 | 10-fold cross-validation | Internal data | ||
| 20 | 2-back accuracy, Rest | r = 0.11, P = 5.59E-2 | 10-fold cross-validation | Internal data, different behavior |
Correction for multiple comparison: Analyses 1-4, 6-9: alpha= 0.0125; Analyses 5 and 10: alpha=0.05; Analyses 11-13, 14-16: alpha= .016; Analyses 17-18, 19-20: alpha= .025
Significant with P < 0.05, Bonferroni corrected for multiple comparisons within models defined by training behavior and data (Analyses 1-4, 6-9, 11-13, 14-16, 17-18, 19-20).
Additional controlling for gF at edge selection in model construction.
External validation in an independent, older age sample with memory impairment
To test whether WM CPMs defined in the HCP dataset were robust to participant population, scan site and parameters, and memory measure, we applied them to resting-state functional connectivity matrices from the SMC sample. We began this analysis by defining a single set of WM CPMs (i.e., network masks and linear models relating network strength to 2-back accuracy) using data from the full HCP dataset rather than utilizing 10-fold cross validation. The performance of these CPMs in the external dataset was assessed by correlating predicted 2-back accuracy scores with a composite score of visual and verbal memory performance in the SMC sample. Visual memory was quantified by performance on the Rey Complex Figure Test, during which subjects visually reproduced a complex line drawing by both copying and drawing from memory (Meyers & Meyers, 1995). Verbal memory was quantified by verbal recall performance on the Seoul Verbal Learning Task, during which subjects verbally recalled items from a list of 12 words before and after a 20-minute delay (Ahn et al., 2010). The composite score was calculated as the sum of visual and verbal memory task performance. Motion was not significantly correlated with memory score in our group of 157 subjects (mean frame-to-frame displacement: r = 0.12, P = 0.12; max translation: r = 0.06, P = 0.49; max rotation: r = 0.05, P = 0.54).
Network overlap
We characterized the overlap of the networks predicting WM, gF, and sustained attention by first defining networks that positively and negatively predicted each measure in each of 1000 rounds of 10-fold cross-validation in the HCP sample. (Note that these networks, visualized in Figures 2, 5, and 6, represent a subset of edges included in the model trained on the full HCP sample and tested on the SMC sample. We elected to visualize these subset networks because they include the edges most consistently related to behavior.) We next counted the number of edges in common between every pair of networks and assessed the statistical significance of this overlap with the hypergeometric cumulative distribution function (Rosenberg et al., 2018). We also report the anatomical location of overlapping edges.
Figure 2. Functional anatomy of positive and negative networks of WM task-trained (top) and rest-trained (bottom) WM CPMs.
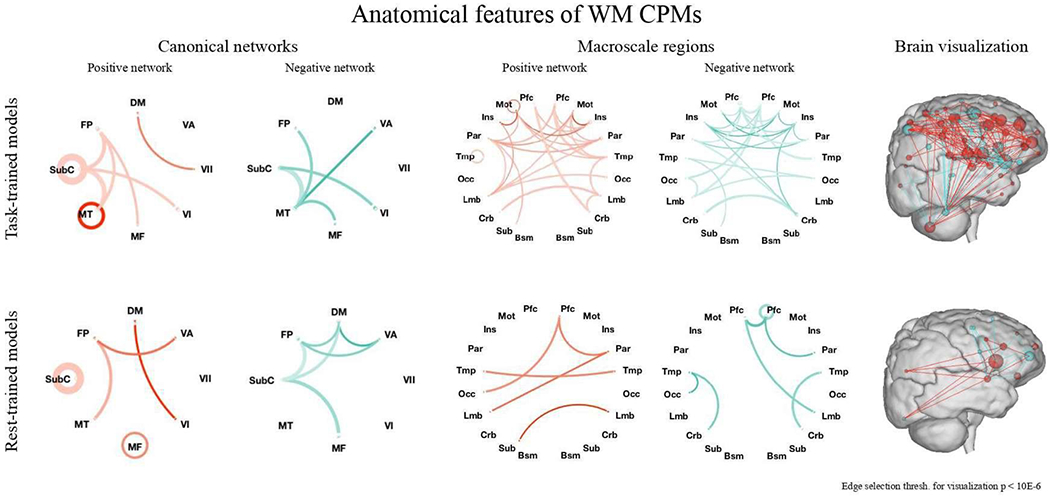
Left and center: In both positive (red) and negative (blue) networks of task- and rest-trained models, a broad range of connections was utilized. Line saturation indicates the proportion of edges of the particular network pair involved in prediction, with darker lines indicating a higher proportion of edges utilized. Line width indicates the total number of edges possible in the pair, with thicker lines indicating more possible edges. Canonical networks include default mode (DM), subcortical-cerebellum (SubC), frontoparietal (FP), motor (MT), medial frontal (MF), visual association (VA), VI, and VII. Edge selection threshold for visualization is P < 1*10−6 for clarity. Right: Positive (red) and negative (blue) edges of the task-trained (left) and rest-trained (right) models of WM described in the manuscript. Spheres represent nodes and lines represent functional connections between them. Nodes are sized according to the total number of connections they have in both the positive and negative networks and are colored according to the network in which they have more connections. The right surface representation images were created using the publicly available BioImage Suite Connectivity Viewer Tool.
Figure 5.
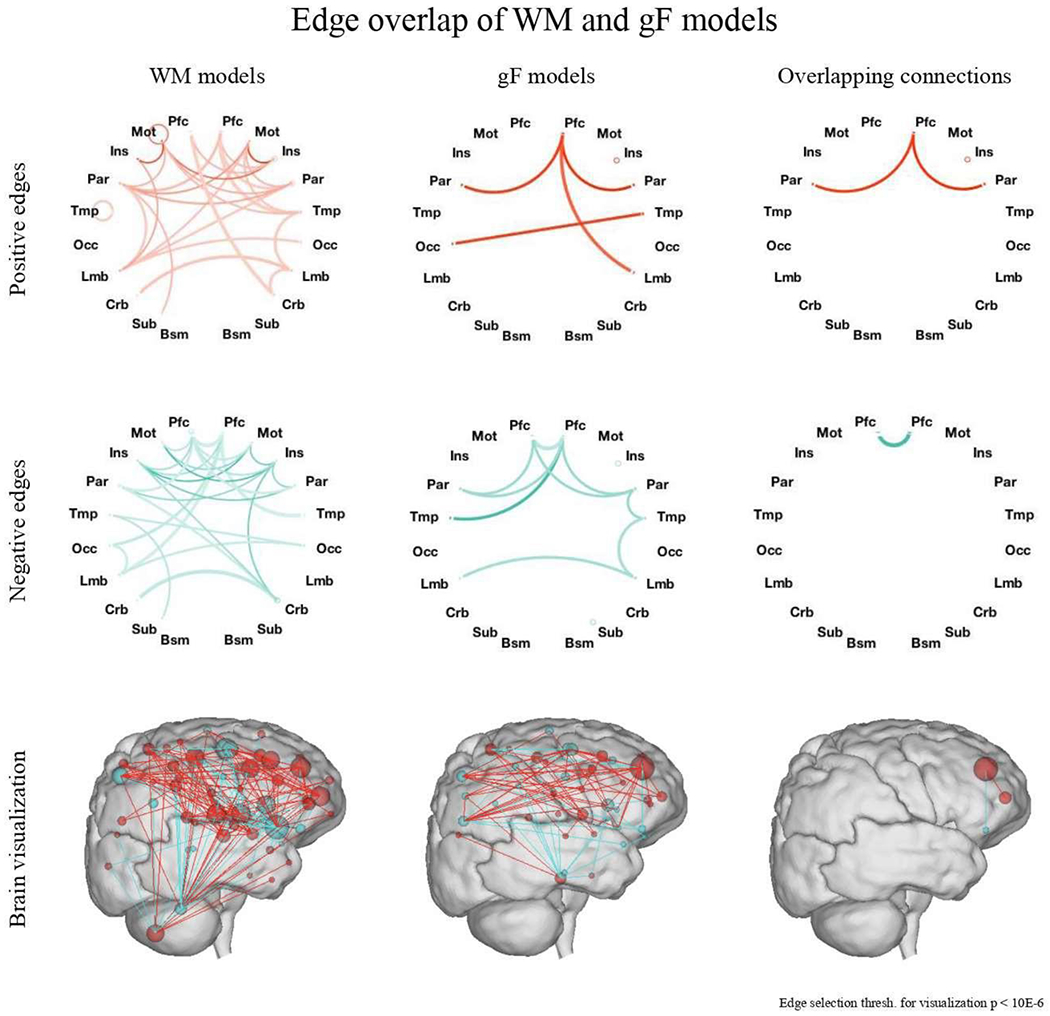
Edge overlap between WM and gF models. WM models utilize a wide range of connections throughout the brain, including subcortical and cerebellar regions, while gF models largely utilize connections between prefrontal and parietal regions. Overlap is predominantly observed in connections between parietal, prefrontal, and motor regions. Top and middle: Line saturation indicates the proportion of edges of the particular network pair involved in prediction, with darker lines indicating a higher proportion of edges utilized. Line width indicates the total number of edges possible in the pair, with thicker lines indicating more possible edges. Circles represent within-region edges. Macroscale regions include prefrontal cortex (PFC), motor cortex (Mot), insula (Ins), parietal (Par), temporal (Tem), occipital (Occ), limbic (including the cingulate cortex, amygdala and hippocampus; Lim), cerebellum (Cer), subcortical (thalamus and striatum; Sub) and brainstem (Bsm). Bottom: Positive (red) and negative (blue) edges of the task-trained WM (left) and gF (middle) models, as well as the overlap between these two models (right). Spheres represent nodes and lines represent functional connections between them. Nodes are sized according to the total number of connections they have in both the positive and negative networks and are colored according to the network in which they have more connections. The bottom surface representation images were created using the publicly available BioImage Suite Connectivity Viewer Tool.
Figure 6. Overlap between positive and negative networks of task-trained WM, gF, and attention models.
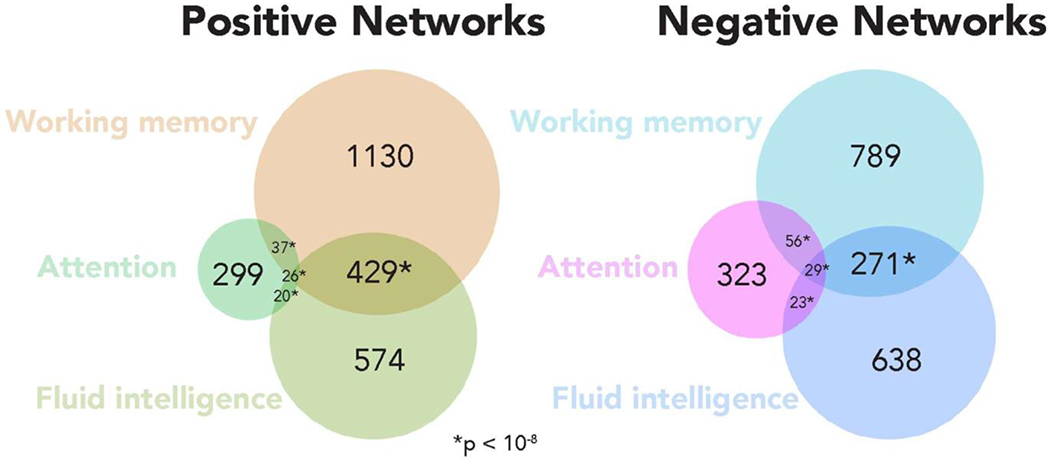
Positive network overlap between WM and gF CPMs was significant (P = 1.4*10−232), totaling 455 edges, as was negative network overlap between these models (P = 9.9*10−207), totaling 300 edges. Overlap between WM and attention CPMs was also significant but numerically less, totaling 63 positive network edges (P = 6.6*10−19) and 85 negative network edges (P = 3.0*10−41). Forty-six edges were common to the gF and attention positive networks (P = 3.3*10−16), and 52 to their negative networks (P = 1.2*10−19). Overall, 26 positive network edges (significant, P = 7.5*10−5) and 29 negative network edges (significant, P = 2.8*10−6) were shared by all 3 CPMs.
Results
Internal validation: prediction of working memory from task-based and resting-state connectivity
Connectome-based predictive models defined using both task-based and resting-state data demonstrated significant correlation between observed and predicted 2-back accuracy scores of previously unseen individuals from the HCP sample (Table 1, analyses 1 & 6; Figure 1).
Figure 1. Summary of WM CPM performance.
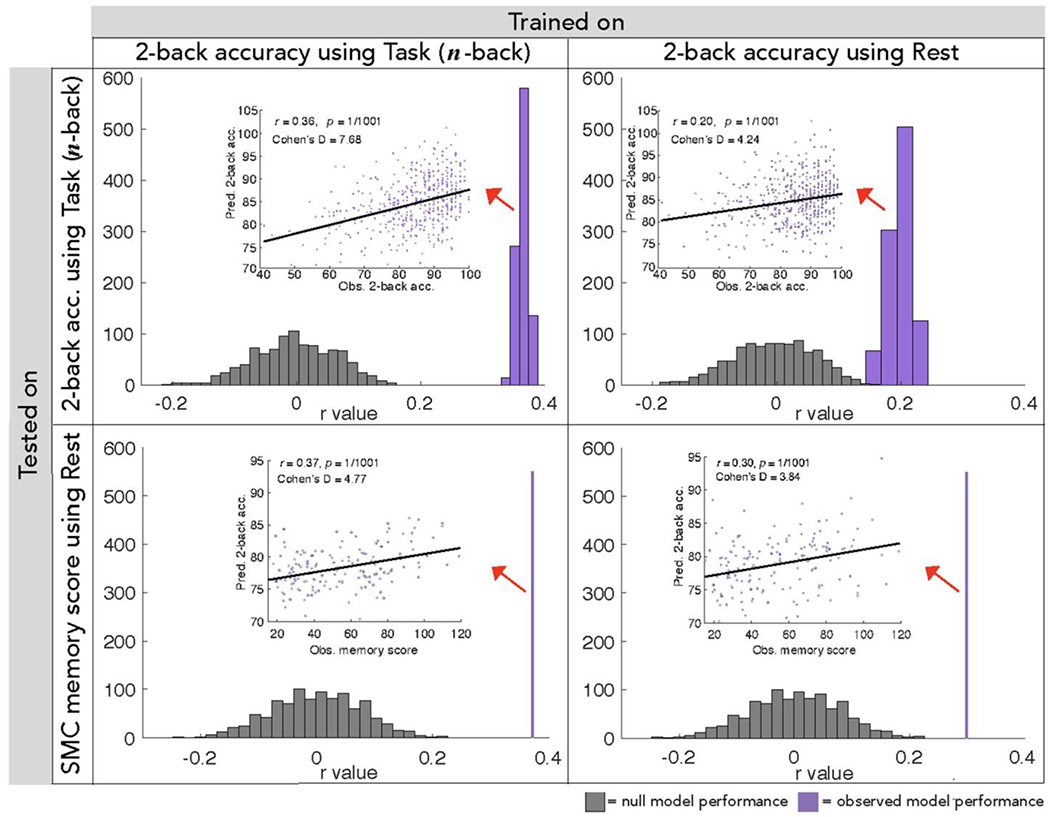
Correlations between predicted 2-back accuracy and observed 2-back accuracy in the HCP sample (top) or observed composite memory score in the external SMC sample (bottom) over 1000 true model iterations (purple) or 1000 null model permutations (gray). Scatter plots depict the correlation between each individual’s predicted and observed behavioral score in one model iteration whose r- and P- values match that of the average values reported in these plots. P-values are Bonferroni-corrected for multiple comparisons. Models were trained on HCP participants’ n-back task (left) or resting-state (right) functional connectivity data, and tested on functional connectivity data from previously unseen individuals. Whether built from n-back task or resting-state fMRI data, our WM models significantly predict both 2-back accuracy in the HCP dataset and memory performance in the independent SMC dataset, including memory-impaired individuals.
External validation: prediction of working memory performance in a memory-impaired population
To assess the robustness and generalizability of WM CPMs, we applied them to a completely independent sample of 157 older adults of the SMC dataset, 109 of whom were memory-impaired (AD or aMCI). Applying the n-back task-trained network models to resting-state functional connectivity from these individuals, we found a strong correlation between predicted 2-back accuracy values and observed composite memory score of visual and verbal memory task performance (Table 1, analysis 2; Figure 1). Strong prediction of these three measures was also achieved when rest-trained models were applied (Table 1, analysis 7; Figure 1). Model performance remained significant in the subset of 28 individuals from the SMC dataset whose motion values met the stricter motion threshold that was appropriate for young adult HCP participants (average frame-to-frame motion < 0.10mm: task-trained r = 0.46, P = 7.99*10−3; rest-trained r = 0.40, P = 2.60*10−2; Supplementary Figure S2).
Functional anatomy of WM networks
To gain a better understanding of the brain regions and systems underlying the strong performance of our WM models (defined using the full HCP sample), we examined the connections utilized between 10 macroscale regions and 8 canonical brain networks. Utilization is here operationalized as the percent of possible network-network edges included in the full set of features that were selected on every iteration. Macroscale regions were anatomically defined and covered a range of cortical and subcortical areas. Canonical networks were functionally defined using the data used for parcellation as described in Finn et al., 2015. Grouping our 268 nodes into these regions and networks, we found that a broad range of connections was utilized in the positive and negative networks of both our task- and rest-trained WM models, spanning both cortical and subcortical regions (Figure 2; anatomical, network, and surface representations). Frontoparietal, subcortical-cerebellar, and motor networks and regions were particularly heavily utilized.
Default mode network (DMN) connectivity was also utilized in both task- and rest-trained WM models. In task-trained models, the DMN and DMN-associated regions (including limbic, prefrontal, parietal, and temporal cortices) were utilized more heavily in networks that positively predicted WM performance than in negatively predictive networks. Both positive and negative task-trained networks also included connections in insular regions.
Modeling fluid intelligence and attention in the HCP dataset
To compare the features and performance of WM CPMs to independent CPMs computed for gF and for sustained attention, we repeated the CPM pipeline to construct models predicting PMAT scores, as a measure of gF, and SCPT d’ values, as a measure of sustained attention, using 10-fold cross-validation in the same dataset of 502 HCP subjects. Replicating previous work (Finn et al., 2015; Finn et al., 2017), gF models generated predicted PMAT scores that strongly correlated with observed PMAT scores when trained on task fMRI data (Figure 3; Table 1, analyses 11 & 14). Sustained attention models also generated predicted SCPT d’ scores that significantly correlated with observed SCPT d’ scores, though with less internal prediction strength than that of gF models (Steiger’s Z = 3.0, P = 2.4*10−3; Figure 3; Table 1, analyses 17 & 19).
Figure 3. Summary of gF and attention CPM performance.
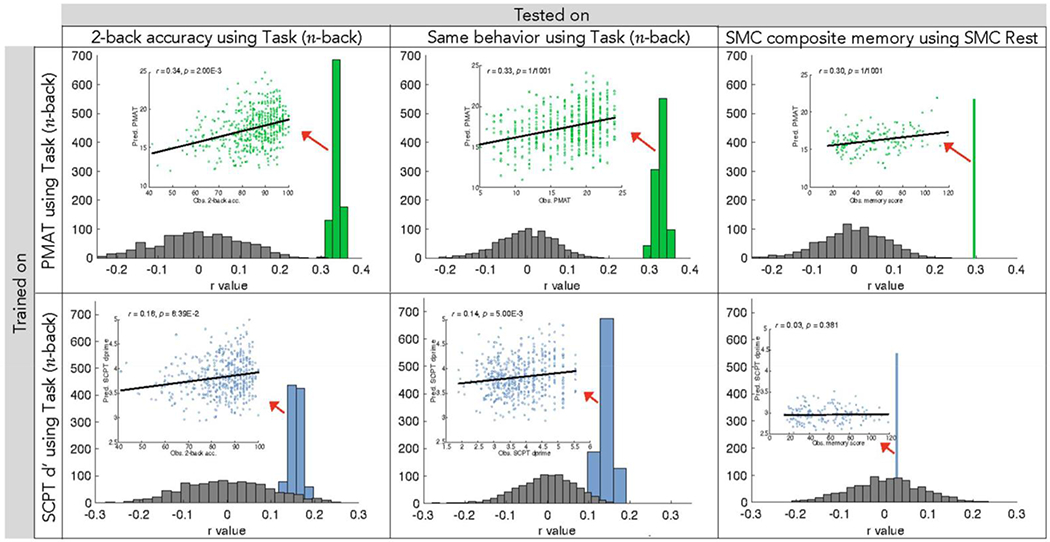
Correlations between predicted and observed behavioral scores over 1000 model iterations (green and blue) or 1000 null model permutations (gray) utilizing gF (top) and attention (bottom) models. Scatter plots depict the correlation between each individual’s predicted and observed behavioral score in one model iteration with average correlation coefficient. P-values are corrected for multiple comparisons as shown in Table 1. Behavioral measures include 2-back accuracy (HCP data, left), PMAT (HCP data, middle top) or SCPT d’ (HCP data, middle bottom), and SMC composite memory score (external data, right). Models were trained on HCP participants’ n-back task functional connectivity data and were tested on functional connectivity data from previously unseen individuals. GF models predict scores of HCP subject 2-back accuracy (r = 0.34) and PMAT (r = 0.33), as well as external SMC subject memory scores (r = 0.30). Attention models perform with significantly less strength (Steiger’s Z = 3.0, P = 2.4*10−3), more weakly predicting HCP 2-back accuracy (r = 0.16) and SCPT d’ (r = 0.14) scores. Attention models do not predict external SMC memory scores (r = 0.03). The difference in correlation between predicted and observed scores of 2-back accuracy and scores of the trained behavioral measure (PMAT or SCPT d’) is not significant for gF (Steiger’s Z = 0.18, P = 0.86) or attention (Steiger’s Z = 0.32, P = 0.75) models.
To further characterize relationships between WM, gF, and sustained attention and their respective network models, we assessed the ability of PMAT and SCPT d’ models to predict n-back task performance and vice versa. Throughout 1000 rounds of 10-fold cross validation, these models were trained on the data of 90% of HCP subjects in the specified training behavior (e.g., PMAT score) and applied to the left out 10% to predict the other behavioral measure of interest (e.g., SCPT d’). Utilizing gF networks, we found a strong correlation between observed 2-back accuracy and predicted gF performance (Figure 3; Table 1, analyses 13 & 16; Supplementary Table S3). We observed similarly strong performance when using WM networks to predict PMAT scores (Table 1, analyses 3 & 8; Supplementary Table S3). In comparison to that of gF and WM, the relationship between predicted attention and WM performance was significantly less strong (Steiger’s Z = 3.36, P = 7.9*10−4): attention network predictions correlated more weakly with observed scores of 2-back accuracy (Figure 3; Table 1, analyses 18 & 20; Supplementary Table S3), as did predicted 2-back accuracy and observed SCPT d’ scores (Table 1, analyses 4 & 9; Supplementary Table S3).
Working memory models and fluid intelligence models predict memory score in an external (SMC) dataset
To directly compare the predictive power of WM and gF models, we also applied our gF CPMs to the external SMC dataset including memory-impaired individuals. Predicted gF was correlated with observed composite memory scores (Figure 3; Table 1, analyses 12 & 15). This correlation was of similar strength to that achieved when WM model predictions were correlated with observed composite memory scores (Table 1, analyses 2 & 12).
The current results do not speak to why we observe some cases of improved model performance when testing on untrained behavior or even an independent fMRI dataset. Several possibilities include differences in participant setting during behavioral testing (in scanner or out of scanner), differences in reliability among behavioral tests used, and differences in the distribution of behavior or age ranges of HCP and SMC subjects, as individual differences in memory are thought to be magnified in aging (Nagel et al., 2008).
WM CPMs retain predictive power when controlling for gF at edge selection
To further assess the relationship between models of WM and gF, we repeated CPM and 10-fold cross validation procedures to create a WM model in which we selected edges correlated with 2-back accuracy while controlling for gF (PMAT score) in each fold of 10-fold cross validation. The resulting model, consisting of edges that appeared in every round of 10-fold cross validation, contained 1036 edges in the positive network (previously 1674) and 755 edges in the negative network (previously 1075). Despite this substantial decrease in numbers of network edges, this model retained predictive power (Figure 4 Table 1, analyses 5 & 10), which was not significantly different (task-trained models: Steiger’s Z = 0.54, P = 0.59; rest-trained models: Z = 0.49, P = 0.62) from that of our original WM model created without controlling for gF during edge selection (Figure 4; Table 1, analyses 1 & 6).
Figure 4. Correlations between observed 2-back accuracy scores and scores predicted by n-back task-trained combined networks.
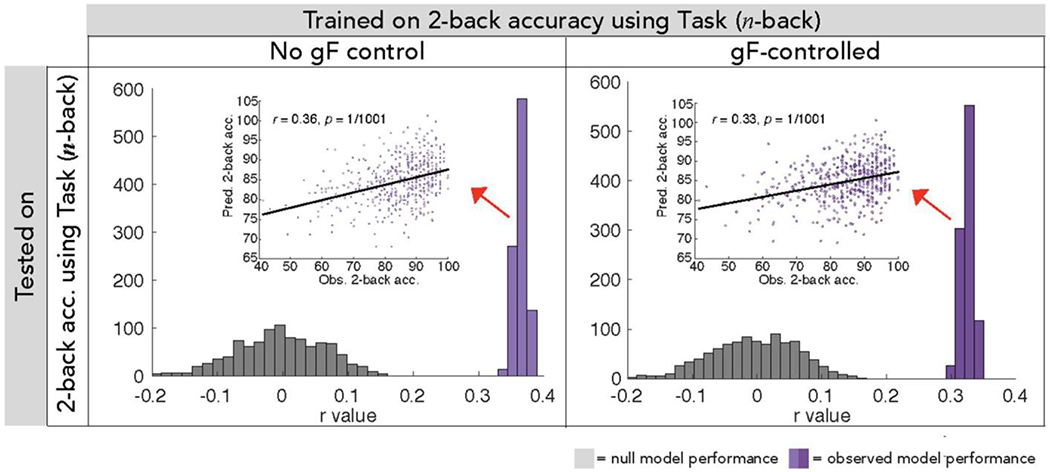
Though exclusion of gF-correlated edges (right) resulted in models that contained 38% fewer edges in the positive network and 30% fewer edges in the negative network, these models continued to predict WM strongly, performing comparably to models created with no gF-related edge exclusion criteria (left).
Anatomical features of WM and gF networks
In a final assessment of the relationship between our models of WM and gF, we then compared the functional anatomy of these networks. To do so, we first followed the procedures of Finn et al., 2015 to group our 268 nodes into ten anatomically defined brain regions and eight functionally defined canonical networks. We then considered the relative numbers of connections between the nodes comprising each pair of regions. As depicted in Figure 5, we found that overlap between our WM and gF networks was largely limited to connections between prefrontal, parietal, and motor regions. In general, WM networks utilized a wider range of connections throughout the brain, including subcortical and cerebellar regions, while gF networks utilized connections primarily between prefrontal and parietal regions. These gF network connections align with previous analyses of gF model anatomical features (Finn et al., 2017).
Given that WM models generalized to predict gF and vice versa, we assessed the degree of overlap between networks. With regard to network overlap models trained on n-back task data, of 1674 total edges in the positively predictive WM network, 455 were shared with the positively predictive gF network in every round of 10-fold cross validation. This overlap was determined to be significant (P = 1.4*10−232) using the hypergeometric cumulative distribution function (Rosenberg et al., 2018). Of 1203 total edges in the negative predictive WM network, 300 (significant, P = 9.9*10−207) were shared with the negatively predictive gF network. There was no overlap between positive WM networks and negative gF networks or vice versa, nor was there any overlap between any positive and any negative network. Thus, of 2877 total edges in the positive and negative networks added together, 755 overlapped in the expected direction between positive and negative WM and gF networks (significant, P = 4.4*10−321). Overlap was also significant but numerically less extensive between WM and sustained attention models, totaling 63 positive network edges (P = 6.6*10−19) and 85 negative network edges (P = 3.0*10−41).
Discussion
In the present study, we provide a novel demonstration that distributed patterns of functional connectivity are a broadly applicable predictor of individual differences in WM. Using connectome-based predictive modeling, we defined predictive models of 2-back task performance in 502 individuals of the Human Connectome Project dataset. Indicating that our models characterize individual differences in working memory-related processes in general rather than 2-back accuracy in particular, these WM CPMs generalized to predict memory deterioration in the independent Samsung Medical Center dataset of 157 older adults. Complementing previous work that has identified neural correlates of WM (Magnuson et al., 2015; McNab and Klingberg, 2008), explored individual differences (Todd and Marois, 2005), and predicted memory impairments related to psychiatric disease (Yamashita et al., 2018), our present work predicts WM function in completely novel individuals, including older adults, with relatively high accuracy (Hsu et al., 2018; Lin et al., 2018; Greene et al., 2018). Furthermore, we characterize overlap and differences in the functional brain networks underlying WM, fluid intelligence, and sustained attention.
The present results align with previous demonstrations of the strong link between WM and gF: gF models strongly predicted WM score in novel individuals, and vice versa. Though not complete, overlap between gF and WM networks was substantial (755 of 2877 total WM CPM edges, 26.2%), and this overlap was predominantly observed in connections between parietal, prefrontal, and motor regions. This supports the notion that WM and gF rely on shared neural systems, particularly those involving parietal and prefrontal regions, which allow us to keep a representation active in the face of interference (Engle et al., 1999; Prabhakaran et al., 1997).
While our results support the strong link between WM and gF, we also find that a set of edges uniquely predicts memory task performance. WM models built while controlling for gF-correlation at edge selection showed similar model performance to original WM models built without gF control.
This suggests that predictive network models of WM do not rely on functional connections that support gF. Additionally, as SMC participants were older adults, the successful external validation of WM models indicates some degree of persistence in the neural systems supporting WM into older age, even in individuals with memory impairment. Anatomically, WM models also utilized connections between a wider range of regions than did gF networks, including more subcortical and cerebellar regions. Though connections between prefrontal and parietal regions have classically been implicated in WM performance and training (Engle et al., 1999; Constantinidis & Klingberg, 2016) and are included in our networks, our findings of additional, broad WM connectivity suggest that WM processes are facilitated by coordinated activity across a wide range of cortical and subcortical regions. This notion aligns with recent propositions that communication between large-scale networks underlies WM performance (Cohen & D’Esposito, 2016; Gazzaley, Rissman, & D’Esposito, 2004).
The present results also align with previous indications of a link between WM and attention. An individual’s ability to control attention is related to performance on complex WM tasks (Kane et al., 2001; Barrett, Tugade, & Engle, 2004), and our work indeed supports this notion by demonstrating some network components are shared between WM and attention models (148 of 2877 total WM CPM edges overlapping, 5.1%). This degree of overlap, however, was less extensive than that of WM and gF models. Furthermore, models of WM did not correlate with sustained attention performance, and models of attention did not correlate with WM performance. The fact that that our models of WM capacity and attention lack cross applicability and do not predominantly utilize the same functional connections aligns with recent propositions that WM precision, rather than WM capacity, is what is most influenced by attentional processes and information flow between attentional and perceptual neural systems (Galeano Weber et al., 2017). Furthermore, the weak performance of attention models indicates that our modeling approach reflects more than an ability to predict general cognitive performance from functional connectivity: the strong performance we observe in models of WM is specific and cannot be replicated by just any selected measure of cognitive performance. In general, our use of predictive modeling techniques to compare different cognitive abilities is novel, providing a new approach in the study of the relationships between cognitive and attentional abilities.
Additionally, the anatomical features of our task-trained and rest-trained WM models suggest that connectivity within and between regions of the default mode network (DMN) is highly utilized in predicting WM performance, supporting hypotheses of a role for task-persistent DMN connectivity (Hampson et al., 2006; Hampson et al., 2010). Particularly in task-trained models, DMN connectivity was utilized in positive but not negative networks, and the notion that more extensive DMN connectivity may be indicative of higher WM performance is a novel finding. This aligns with previous indications that DMN connectivity is degraded in Alzheimer’s disease patients, though reduction in the integrity of several other brain networks has also been reported in these patients (Brier et al., 2012; Greicius et al., 2004). Furthermore, the insula was bilaterally utilized in both positive and negative task-trained networks, providing support for propositions that the insula plays a role in switching between central executive and default mode networks (Sridharan, Levitin, & Menon, 2008).
In all, our present work demonstrates, for the first time, that distributed sets of task-based and resting-state functional connections predict WM performance and can help characterize relationships between memory and other cognitive abilities, including gF and sustained attention. Though not intended to be used as a diagnostic tool, our results are theoretically informative, linking brain variables to behavioral ones to enhance our understanding of the neural infrastructure that gives rise to WM processes. Further applications of modeling and analysis techniques will be of use in relating our WM models to other memory types, including long-term memory, and other cognitive abilities and clinical symptoms.
Supplementary Material
Acknowledgments
This work was supported by the Yale Faculty of Arts and Sciences MRI Program funded by the Office of the Provost and the Department of Psychology, National Science Foundation Grant BCS1558497 to M.M.C., and National Institutes of Health Grant MH108591 to M.M.C.
Footnotes
Declaration of Interests
The authors declare no competing interests.
References
- Ahn H-J, Chin J, Park A, Lee BH, Suh MK, Seo SW, & Na DL (2010). Seoul Neuropsychological Screening Battery-Dementia Version (SNSB-D): A Useful Tool for Assessing and Monitoring Cognitive Impairments in Dementia Patients. Journal of Korean Medical Science, 25(7), 1071–1076. 10.3346/jkms.2010.25.7.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A (2003). Working memory: looking back and looking forward. Nature Reviews Neuroscience, 4(10), 829–839. 10.1038/nrn1201 [DOI] [PubMed] [Google Scholar]
- Baddeley AD, & Hitch G (1974). Working Memory. In Bower GH (Ed.), Psychology of Learning and Motivation (Vol. 8, pp. 47–89). Academic Press. 10.1016/S0079-7421(08)60452-1 [DOI] [Google Scholar]
- Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, … Van Essen DC (2013). Function in the human connectome: Task-fMRI and individual differences in behavior. NeuroImage, 80, 169–189. 10.1016/j.neuroimage.2013.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Tugade MM, & Engle RW (2004). Individual Differences in Working Memory Capacity and Dual-Process Theories of the Mind. Psychological Bulletin, 130(4), 553–573. 10.1037/0033-2909.130.4.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolero MA, Yeo BTT, Bassett DS, D’Esposito M (2018) A mechanistic model of connector hubs, modularity and cognition. Nat Hum Behav 2:765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, Raichle ME, … Ances BM (2012). Loss of Intranetwork and Internetwork Resting State Functional Connections with Alzheimer’s Disease Progression. Journal of Neuroscience, 32(26), 8890–8899. 10.1523/JNEUROSCI.5698-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophel TB, Hebart MN, & Haynes J-D (2012). Decoding the Contents of Visual Short-Term Memory from Human Visual and Parietal Cortex. Journal of Neuroscience, 32(38), 12983–12989. 10.1523/JNEUROSCI.0184-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Golomb JD, & Turk-Browne NB (2011). A Taxonomy of External and Internal Attention. Annual Review of Psychology, 62(1), 73–101. 10.1146/annurev.psych.093008.100427 [DOI] [PubMed] [Google Scholar]
- Cohen JR, & D’Esposito M (2016). The Segregation and Integration of Distinct Brain Networks and Their Relationship to Cognition. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 36(48), 12083–12094. 10.1523/JNEUROSCI.2965-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom R, Flores-Mendoza C, & Rebollo I (2002). Working memory and intelligence. Personality and Individual Differences, 34(1), 33–39. [Google Scholar]
- Constantinidis C, & Klingberg T (2016). The neuroscience of working memory capacity and training. Nature Reviews. Neuroscience, 17(7), 438–449. 10.1038/nrn.2016.43 [DOI] [PubMed] [Google Scholar]
- Cowan N, Elliott EM, Scott Saults J, Morey CC, Mattox S, Hismjatullina A, & Conway ARA (2005). On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology, 51(1), 42–100. 10.1016/j.cogpsych.2004.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, & Postle BR (2015). The Cognitive Neuroscience of Working Memory. Annual Review of Psychology, 66(1), 115–142. 10.1146/annurev-psych-010814-015031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Zarahn E, Aguirre GK, Rypma B (1999) The Effect of Normal Aging on the Coupling of Neural Activity to the Bold Hemodynamic Response. NeuroImage 10:6–14. [DOI] [PubMed] [Google Scholar]
- Dubois J, Adolphs R (2016) Building a Science of Individual Differences from fMRI. Trends in Cognitive Sciences 20:425–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, & Conway AR (1999). Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. Journal of Experimental Psychology: General, 128(3), 309. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Rissman J, & D’Esposito M (2006). Modulation of Inferotemporal Cortex Activation during Verbal Working Memory Maintenance. Neuron, 51(2), 251–261. 10.1016/j.neuron.2006.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES, Scheinost D, Finn DM, Shen X, Papademetris X, & Constable RT (2017). Can brain state be manipulated to emphasize individual differences in functional connectivity? NeuroImage, 160, 140–151. 10.1016/j.neuroimage.2017.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, … Constable RT (2015). Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nature Neuroscience, 18(11), 1664–1671. 10.1038/nn.4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain-Zaragoza S, Samimy S, Rosenberg MD, Prakash RS (2019) Connectome-based models predict attentional control in aging adults. NeuroImage 186:1–13. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Vogel E, Mayr U, & Awh E (2010). Quantity, not quality: the relationship between fluid intelligence and working memory capacity. Psychonomic Bulletin & Review, 17(5), 673–679. 10.3758/17.5.673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeano Weber EM, Hahn T, Hilger K, & Fiebach CJ (2017). Distributed patterns of occipito-parietal functional connectivity predict the precision of visual working memory. NeuroImage, 146, 404–418. 10.1016/j.neuroimage.2016.10.006 [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, & D’esposito M (2004). Functional connectivity during working memory maintenance. Cognitive, Affective, & Behavioral Neuroscience, 4(4), 580–599. 10.3758/CABN.4.4.580 [DOI] [PubMed] [Google Scholar]
- Geerligs L, Tsvetanov KA, Cam-CAN, Henson RN (2017) Challenges in measuring individual differences in functional connectivity using fMRI: The case of healthy aging: Measuring Individual Differences Using fMRI. Human Brain Mapping 38:4125–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, … Jenkinson M (2013). The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage, 80, 105–124. 10.1016/j.neuroimage.2013.04.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Cheng F, Rolls ET, Lo C-YZ, Huang C-C, Tsai S-J, Yang AC, Lin C-P, Feng J (2019) A powerful and efficient multivariate approach for voxel-level connectome-wide association studies. NeuroImage 188:628–641. [DOI] [PubMed] [Google Scholar]
- Greene AS, Gao S, Scheinost D, & Constable RT (2018). Task-induced brain state manipulation improves prediction of individual traits. Nature Communications, 9(1). 10.1038/s41467-018-04920-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, & Menon V (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences, 101(13), 4637–4642. 10.1073/pnas.0308627101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CC, Kurth F, Zhou J, Mayer EA, Eickhoff SB, Kramer JH, Seeley WW (2012) One-year test-retest reliability of intrinsic connectivity network fMRI in older adults. Neuroimage 61:1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, & Constable RT (2006). Brain Connectivity Related to Working Memory Performance. Journal of Neuroscience, 26(51), 13338–13343. 10.1523/JNEUROSCI.3408-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson Michelle, Driesen N, Roth JK, Gore JC, & Constable RT (2010). Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magnetic Resonance Imaging, 28(8), 1051–1057. 10.1016/j.mri.2010.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Berg AC, Oh H, Samaras D, & Leung H-C (2013). Multi-voxel pattern analysis of selective representation of visual working memory in ventral temporal and occipital regions. NeuroImage, 73, 8–15. 10.1016/j.neuroimage.2013.01.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W-T, Rosenberg MD, Scheinost D, Constable RT, Chun MM (2018) Resting-state functional connectivity predicts neuroticism and extraversion in novel individuals. Soc Cogn Affect Neurosci 13:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, & Carpenter PA (1992). A capacity theory of comprehension: Individual differences in working memory. Psychological Review, 99(1), 122–149. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Bleckley MK, Conway ARA, & Engle RW (2001). A controlled-attention view of working-memory capacity. Journal of Experimental Psychology: General, 130(2), 169–183. 10.1037//0096-3445.130.2.169 [DOI] [PubMed] [Google Scholar]
- Kane MJ, Conway ARA, Miura TK, & Colflesh GJH (2007). Working memory, attention control, and the n-back task: A question of construct validity. Journal of Experimental Psychology: Learning, Memory, and Cognition, 33(3), 615–622. 10.1037/0278-7393.33.3.615 [DOI] [PubMed] [Google Scholar]
- Kane MJ, & Engle RW (2002). The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review, 9(4), 637–671. 10.3758/BF03196323 [DOI] [PubMed] [Google Scholar]
- Kim H, Yoo K, Na DL, Seo SW, Jeong J, & Jeong Y (2015). Non-monotonic reorganization of brain networks with Alzheimer’s disease progression. Frontiers in Aging Neuroscience, 7. 10.3389/fnagi.2015.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzky RL (1975). Human memory: Structures and processes. Oxford, England: W. H. Freeman. [Google Scholar]
- Kurtz MM, Ragland JD, Bilker W, Gur RC, & Gur RE (2001). Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophrenia Research, 48(2-3), 307–316. 10.1016/S0920-9964(00)00060-8 [DOI] [PubMed] [Google Scholar]
- Kyllonen PC, & Christal RE (1990). Reasoning ability is (little more than) working-memory capacity?! Intelligence, 14(4), 389–433. 10.1016/S0160-2896(05)80012-1 [DOI] [Google Scholar]
- Liebe S, Hoerzer GM, Logothetis NK, & Rainer G (2012). Theta coupling between V4 and prefrontal cortex predicts visual short-term memory performance. Nature Neuroscience, 15(3), 456–462. 10.1038/nn.3038 [DOI] [PubMed] [Google Scholar]
- Lin Q, Rosenberg MD, Yoo K, Hsu TW, O’Connell TP, Chun MM, Chun MM, Chun MM (2018) Resting-State Functional Connectivity Predicts Cognitive Impairment Related to Alzheimer’s Disease. Frontiers in Aging Neuroscience 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Poh J-H, Koh HL, Ng KK, Loke YM, Lim JKW, … Zhou J (2018). Carrying the past to the future: Distinct brain networks underlie individual differences in human spatial working memory capacity. NeuroImage, 176, 1–10. 10.1016/j.neuroimage.2018.04.014 [DOI] [PubMed] [Google Scholar]
- Luck SJ, & Vogel EK (1997). The capacity of visual working memory for features and conjunctions. Nature, 390(6657), 279–281. 10.1038/36846 [DOI] [PubMed] [Google Scholar]
- Luck SJ, & Vogel EK (2013). Visual working memory capacity: from psychophysics and neurobiology to individual differences. Trends in Cognitive Sciences, 17(8), 391–400. 10.1016/j.tics.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WJ, Husain M, & Bays PM (2014). Changing concepts of working memory. Nature Neuroscience, 17(3), 347–356. 10.1038/nn.3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson ME, Thompson GJ, Schwarb H, Pan W-J, McKinley A, Schumacher EH, & Keilholz SD (2015). Errors on interrupter tasks presented during spatial and verbal working memory performance are linearly linked to large-scale functional network connectivity in high temporal resolution resting state fMRI. Brain Imaging and Behavior, 9(4), 854–867. 10.1007/s11682-014-9347-3 [DOI] [PubMed] [Google Scholar]
- McNab F, & Klingberg T (2008). Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience, 11(1), 103–107. 10.1038/nn2024 [DOI] [PubMed] [Google Scholar]
- Meyers JE, & Meyers KR (1995). Rey complex figure test under four different administration procedures. The Clinical Neuropsychologist, 9(1), 63–67. 10.1080/13854049508402059 [DOI] [Google Scholar]
- Miller EK, Buschman TJ (2015) Working Memory Capacity: Limits on the Bandwidth of Cognition. Daedalus 144:112–122. [Google Scholar]
- Moore TM, Reise SP, Gur RE, Hakonarson H, & Gur RC (2015). Psychometric Properties of the Penn Computerized Neurocognitive Battery. Neuropsychology, 29(2), 235–246. 10.1037/neu0000093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li S-C, Von Oertzen T, Sander T, Villringer A, … Lindenberger U (2008). Human aging magnifies genetic effects on executive functioning and working memory. Frontiers in Human Neuroscience, 2. 10.3389/neuro.09.001.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva JM, Monto S, Kulashekhar S, & Palva S (2010). Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proceedings of the National Academy of Sciences, 107(16), 7580–7585. 10.1073/pnas.0913113107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran V, Smith JAL, Desmond JE, Glover GH, & Gabrieli JDE (1997). Neural Substrates of Fluid Reasoning: An fMRI Study of Neocortical Activation during Performance of the Raven’s Progressive Matrices Test. Cognitive Psychology, 33(1), 43–63. 10.1006/cogp.1997.0659 [DOI] [PubMed] [Google Scholar]
- Redick TS, Broadway JM, Meier ME, Kuriakose PS, Unsworth N, Kane MJ, & Engle RW (2012). Measuring Working Memory Capacity With Automated Complex Span Tasks. European Journal of Psychological Assessment, 28(3), 164–171. 10.1027/1015-5759/a000123 [DOI] [Google Scholar]
- Rosenberg MD, Zhang S, Hsu W-T, Scheinost D, Finn ES, Shen X, … Chun MM (2016). Methylphenidate Modulates Functional Network Connectivity to Enhance Attention. Journal of Neuroscience, 36(37), 9547–9557. 10.1523/JNEUROSCI.1746-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg Monica D., Finn ES, Scheinost D, Papademetris X, Shen X, Constable RT, & Chun MM (2016). A neuromarker of sustained attention from whole-brain functional connectivity. Nature Neuroscience, 19(1), 165–171. 10.1038/nn.4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg Monica D., Hsu W-T, Scheinost D, Todd Constable R, & Chun MM (2018). Connectome-based Models Predict Separable Components of Attention in Novel Individuals. Journal of Cognitive Neuroscience, 30(2), 160–173. 10.1162/jocn_a_01197 [DOI] [PubMed] [Google Scholar]
- Shen X, Tokoglu F, Papademetris X, & Constable RT (2013). Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. NeuroImage, 82, 403–415. 10.1016/j.neuroimage.2013.05.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Xilin, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X, & Constable RT (2017). Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nature Protocols, 12(3), 506–518. 10.1038/nprot.2016.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, … Glasser MF (2013). Resting-state fMRI in the Human Connectome Project. NeuroImage, 80, 144–168. 10.1016/j.neuroimage.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Nichols T, Vidaurre D, Winkler A, Behrens T, Glasser M, Ugurbil K, Barch D, Van Essen D, Miller K (2015) A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci 18:1565–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq E, Leech R, Hampshire A (2019) Dynamic network coding of working-memory domains and working-memory processes. Nature Communications 10:936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan KK, Vytlacil J, & D’Esposito M (2014). Distributed and Dynamic Storage of Working Memory Stimulus Information in Extrastriate Cortex. Journal of Cognitive Neuroscience, 26(5), 1141–1153. 10.1162/jocn_a_00556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, & Menon V (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences, 105(34), 12569–12574. 10.1073/pnas.0800005105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JJ, & Marois R (2004). Capacity limit of visual short-term memory in human posterior parietal cortex. Nature, 428(6984), 751–754. 10.1038/nature02466 [DOI] [PubMed] [Google Scholar]
- Todd JJ, & Marois R (2005). Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cognitive, Affective, & Behavioral Neuroscience, 5(2), 144–155. 10.3758/CABN.5.2.144 [DOI] [PubMed] [Google Scholar]
- Uğurbil K, Xu J, Auerbach EJ, Moeller S, Vu AT, Duarte-Carvajalino JM, … Yacoub E (2013). Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project. NeuroImage, 80, 80–104. 10.1016/j.neuroimage.2013.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL (2012) The influence of head motion on intrinsic functional connectivity MRI. NeuroImage 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, & Ugurbil K (2013). The WU-Minn Human Connectome Project: An overview. NeuroImage, 80, 62–79. 10.1016/j.neuroimage.2013.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C-W, Chang LJ, Lindquist MA, Wager TD (2017) Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci 20:365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, & Chun MM (2006). Dissociable neural mechanisms supporting visual short-term memory for objects. Nature, 440(7080), 91–95. 10.1038/nature04262 [DOI] [PubMed] [Google Scholar]
- Yamashita M, Yoshihara Y, Hashimoto R, Yahata N, Ichikawa N, Sakai Y, … Imamizu H (2018). A prediction model of working memory across health and psychiatric disease using whole-brain functional connectivity. ELife, 7, e38844. 10.7554/eLife.38844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad M, Eichele T, Lundervold AJ, Lundervold A (2010) Subcortical functional connectivity and verbal episodic memory in healthy elderly—A resting state fMRI study. NeuroImage 52:379–388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


