Abstract
Lipopolysaccharide (LPS), the major component of the outer membrane of Gram-negative bacteria, is important for bacterial viability in general and host–pathogen interactions in particular. Negative charges at its core oligosaccharide (core-OS) contribute to membrane integrity through bridging interactions with divalent cations. The molecular structure and synthesis of the core-OS have been resolved in various bacteria including the mammalian pathogen Pseudomonas aeruginosa. A few core-OS structures of plant-associated Pseudomonas strains have been solved to date, but the genetic components of the underlying biosynthesis remained unclear. We conducted a comparative genome analysis of the core-OS gene cluster in Pseudomonas syringae pv. tomato (Pst) DC3000, a widely used model pathogen in plant–microbe interactions, within the P. syringae species complex and to other plant-associated Pseudomonas strains. Our results suggest a genetic and structural conservation of the inner core-OS but variation in outer core-OS composition within the P. syringae species complex. Structural analysis of the core-OS of Pst DC3000 shows an uncommonly high phosphorylation and presence of an O-acetylated sugar. Finally, we combined the results of our genomic survey with available structure information to estimate the core-OS composition of other Pseudomonas species.
Keywords: lipopolysaccharide, core oligosaccharide, Pseudomonas syringae, NMR spectroscopy, mass spectrometry, structural characterization
1. Introduction
The Pseudomonas syringae species complex comprises numerous highly adapted pathovars and is considered an indispensable model for studying plant–bacteria interactions. The genetic diversity of the P. syringae complex is reflected by the subdivision into 13 distinct phylogenetic groups [1]. Among them are economically relevant pathogens, which cause substantial yield losses each year [2]. Research aiming to enlighten the mechanism of P. syringae pathogenesis contributes to the development of agronomical solutions to prevent and control bacterial disease outbreaks in the field.
P. syringae first colonizes the phylloplane but switches to an endophytic lifestyle to establish an infection. While the prevalence of epiphytic or endophytic growth is strain specific, disease symptoms only emerge when P. syringae colonizes the apoplast [1]. During transition between these two lifestyles, bacteria are exposed to profound environmental changes and rely on specific cellular properties to withstand these stresses [3]. The cell wall protects the bacteria from harsh chemical conditions, shields off antimicrobial substances, and contributes to immune evasion processes while simultaneously maintaining cell integrity, nutrient uptake, and material exchange with the environment. In most Gram-negative bacteria, these cell wall characteristics are largely mediated by lipopolysaccharide (LPS), the main component of the outer leaflet of the outer membrane (OM) [4]. The general molecular architecture of LPS is conserved between bacterial species and can be divided into three subdomains: lipid A (LA), core oligosaccharide (core-OS), and O-polysaccharide (OPS) [3].
The structural composition of OPS, the most distal LPS domain, is very diverse but often strain-specific and determines the serotypic specificity [5]. Previously analyzed OPS structures of P. syringae show a prevalence of l-rhamnose (Rha) residues [6]. Similar to OPS, the outer core-OS is structurally quite variable, while the saccharide composition of the inner core-OS is often conserved and typically contains l-glycero-d-manno-heptose and the LPS specific sugar 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) [7]. The LA usually has a di-phosphorylated di-glucosamine backbone which is commonly acylated with up to four primary and from one to three secondary fatty acids. The acylation pattern and length of the acyl chains vary between bacterial families and influence the endotoxic potential of LPS in mammals and humans [8]. For example, the prototypical enterobacterial LA is asymmetrically hexa-acylated with long acyl chains (C12/C14), whereas Pseudomonas LA is often penta-acylated with shorter acyl chains (C10/C12) [9,10]. E. coli LA is a strong agonist of the human Toll-like receptor 4 (TLR4), while recognition of P. aeruginosa LA is weaker due to its shorter acyl chains and is further reduced when penta-acylated [11].
In the OM, ionic interactions between negatively charged residues in the LA:core-OS unit and divalent cations result in tight packing of LPS molecules and influence permeability and stability of the OM [12]. They are often targeted by host defenses, e.g., cationic antimicrobial peptides (CAMPs), which disrupt these ionic interactions and destabilize the OM [13]. The core-OS can also be sensed by pattern recognition proteins of the host immune system. Examples in mammals include the membrane proteins brain angiogenesis 51 inhibitor 1 (BAI1), which facilitates phagocytosis of Enterobacteria by macrophages [14], and cystic fibrosis transmembrane conductance regulator (CFTR), which binds the outer core-OS of P. aeruginosa LPS [15]. The core-OS of Xanthomonas campestris pv. campestris has been reported to elicit immune responses in Arabidopsis thaliana [16] and Nicotiana tabacum [17], but no corresponding sensory components have yet been identified in plants.
The basic core-OS structure can be altered with stoichiometric and nonstoichiometric substitutions to counteract recognition and targeting by host immune components. Such modifications include the addition of phosphoethanolamine to mask negative charges of the phosphates and increase CAMP resistance [18]. The core-OS of P. aeruginosa also displays a high degree of nonstoichiometric O-acetylation which is considered characteristic for respiratory and mucosal pathogens [19,20]. O-acetylation might influence cell-surface hydrophobicity and possibly increase resistance to opsonophagocytosis [19,21]. Similarly, the potential influence of O-carbamylation or alanine (Ala) substitutions on immune recognition remains unresolved [10].
Biosynthesis of the core-OS takes place at the cytoplasmic face of the inner membrane (IM). The first sugars of the inner core-OS, Kdo, are transferred to the tetra-acylated lipid IVA precursor during the LA biosynthesis. The subsequent addition of further saccharides to the nascent core-OS is mediated by several different membrane-associated glycosyltransferases [8]. In E. coli, all enzymes involved in the synthesis of the five known core-OS variations were identified [22]. Similarly, core-OS structure and underlying synthesis genes of P. aeruginosa are characterized. Most of the respective genes are located in a proposed gene cluster (genes PA4996-PA5012) and could be associated with a specific function [9,23]. Structural data suggest that two l-glycero-d-manno-heptose residues (HepI and HepII) of the inner core-OS are conserved in all Pseudomonas species analyzed to date, with Pseudomonas cichorii possibly being the only known exception [24]. HepI and HepII are usually highly phosphorylated and substituted with a carbamoyl group which is transferred by the putative carbamoyltransferase wapO (PA5005) in P. aeruginosa [10,20]. The core phosphates are considered essential for P. aeruginosa viability and might contribute to its intrinsic drug resistance [20,25]. The outer core-OS structures vary in their composition between the different Pseudomonas species analyzed to date. In P. syringae, only the core-OS structure of the pathovar phaseolicola was completely analyzed. It contains β-N-acetyl-d-glucosamine (GlcNAcIII)-(1→2)-α-d-glucose (GlcI)-(1→3) and α-l-rhamnose (l-Rha)-(1→6)-β-GlcII-(1→4) or α-KdoIII-(2→6)-β-GlcII-(1→4) chains in the outer core-OS which are linked to the inner core-OS via a →3,4)-α-d-galactosamine (GalN)-(1→3) residue substituted with l-Ala-2) [26]. The relative core-OS sugar content in the P. syringae pathovars maculicola and atrofaciens suggest that the P. syringae outer core-OS might be generally defined by the presence of GlcN and Rha residues [26,27,28]. P. syringae pv. tomato DC3000 (Pst DC3000) is a widely used model pathogen for studying molecular microbe–host interactions with Arabidopsis thaliana, but yet its core-OS composition and the respective synthesis genes are unknown.
Herein, we elucidate the genetic background of the core-OS synthesis in bacteria from the P. syringae species complex and other plant-associated bacteria by comparative analysis of publicly available genomes and predicted proteomes. We identified the core-OS gene cluster in Pst DC3000 and could associate most genes with a proposed function. The comparative genome analysis revealed that the gene cluster is highly conserved in P. syringae pathovars and predicts a general conservation of the core-OS composition. Supporting this, structural analysis of the core-OS of an OPS-deficient Pst DC3000 ΔwbpL mutant showed a basically similar composition to the core-OS of P. syringae pv. phaseolicola. However, in Pst DC3000 LPS, we observed a higher degree of core-OS phosphorylation and an O-acetylated sugar.
2. Results
2.1. Pst DC3000 Core-OS Gene Cluster Contains an Insertion Sequence Element
In P. aeruginosa, the core-OS biosynthetic genes localize in a cluster [20]. Most of the proteins encoded in this gene cluster could be associated with a specific function in core-OS biosynthesis [9]. The position of a putative core-OS gene cluster in the Pst DC3000 genome was identified by synteny analysis followed by multiple BLAST searches and pairwise alignments with the corresponding P. aeruginosa PAO1 gene products as reference. In total, 15 out of 17 genes in the P. aeruginosa core-OS cluster could be matched to sequences between PSPTO_4983 and PSPTO_5003 with predicted protein sequence identities ranging from 86.9% to 55.6% (Table 1). Pairwise alignment of the two unmatched P. aeruginosa protein sequences with the sequences of the syntenic Pst DC3000 genes PSPTO_4986 (PA4999, waaL) or PSPTO_4987 (PA5000, wapR) resulted in identities of 19.4% and 7.9%, respectively (Table 1).
Table 1.
Genes of the core oligosaccharide (core-OS) cluster and putative function of the encoded enzymes in Pseudomonas syringae pv. tomato DC3000 in comparison to P. aeruginosa PAO1 based on publicly available data.
| Identifier | Annotation 1 | Putative function in Pst DC3000 | PAO1 Equivalent | Putative/Proven Function in PAO1 | Identity % |
|---|---|---|---|---|---|
| Genes within core-OS cluster | |||||
| PSPTO_4983 | Lipopolysaccharide biosynthesis protein RfaE | Heptose biosynthesis | hldE (PA4996) | Heptose biosynthesis | 86.9 |
| PSPTO_4984 | Lipid A ABC transporter, ATP-binding/permease protein | Lipid-A:core-OS transport | msbA (PA4997) | Lipid-A:core-OS transport | 83.8 |
| PSPTO_4985 | Toluene tolerance protein | Unknown | PA4998 | Kinase | 55.6 |
| PSPTO_4986 | Membrane protein | Putative OPS ligase | waaL (PA4999) 2 | OPS ligase | No hit/19.4 3 |
| PSPTO_4987 | Hypothetical protein | WbcX-like glycosyltransferase | wapR (PA5000) 2 | Glycosyltransferase (Rha) | No hit/7.9 3 |
| PSPTO_4988 | Hypothetical protein | RfaB family glycosyltransferase | PA5001 | Glycosyltransferase | 73.6 |
| PSPTO_4989 | Hypothetical protein | PIG-L family deacetylase | PA5002 | Unknown | 65.5 |
| PSPTO_4990 | Hypothetical protein | GNAT family N-acetyltransferase | PA5003 | Unknown | 69.1 |
| PSPTO_4991 | Glycoside hydrolase family protein | Glycosyltransferase (GlcII) | wapH (PA5004) | Glycosyltransferase (GlcII) | 71.2 |
| PSPTO_4992 | Carbamoyltransferase family protein | Cm-(→7) carbamoyltransferase | wapO (PA5005) | Carbamoyltransferase | 86.5 |
| PSPTO_4993 | Hypothetical protein | Type III effector HopAC1 (segment) | No hit | ||
| PSPTO_4994 | ISPsy5, transposase | ISPsy5, transposase | No hit | ||
| PSPTO_4995 | ISPsy5, Orf1 | ISPsy5, Orf1 | No hit | ||
| PSPTO_4996 | Hypothetical protein | Type III effector HopAC1 (segment) | No hit | ||
| PSPTO_4997 | Hypothetical protein | Unknown | PA5006 | Kinase | 67.7 |
| PSPTO_4998 | Lipopolysaccharide biosynthesis protein | Heptose kinase | wapQ (PA5007) | Heptose kinase | 57.5 |
| PSPTO_4999 | Lipopolysaccharide core biosynthesis protein | Heptose kinase | wapP (PA5008) | Heptose kinase | 76.2 |
| PSPTO_5000 | Lipopolysaccharide core biosynthesis protein WaaP | Heptose kinase | waaP (PA5009) | Heptose kinase | 79.5 |
| PSPTO_5001 | Lipopolysaccharide core biosynthesis protein WaaG | α-GalN-(1→3) Glycosyltransferase | wapG (PA5010) | Glycosyltransferase (GalN) | 78.6 |
| PSPTO_5002 | Lipopolysaccharide heptosyltransferase | α-HepI-(1→5) Glycosyltransferase | waaC (PA5011) | Glycosyltransferase (HepI) | 74.9 |
| PSPTO_5003 | ADP-heptose--LPS heptosyltransferase II | α-HepII-(1→3) Glycosyltransferase | waaF (PA5012) | Glycosyltransferase (HepII) | 83.5 |
| Genes outside of the cluster | |||||
| PSPTO_1330 | Glycosyltransferase family protein | Glycosyltransferase α-l-Rha-(1→6) | migA (PA0705) | Glycosyltransferase (Rha1→6) | 63.9 |
| PSPTO_2767 | Lipopolysaccharide core biosynthesis domain protein | No hit | |||
1 according to www.pseudomonas.com accessed on 11.01.2021; 2 located outside of the cluster; 3 BLAST yielded no hit, identity result from pairwise alignment of syntenic gene sequences.
Notably, the putative core-OS cluster of Pst DC3000 (PSPTO_4983-PSPTO_5003) includes four additional open reading frames (ORFs, PSPTO_4993-PSPTO_4996). A comparison of the sequences and the gene ontology terms indicates that these might constitute a putative type III effector HopAC1 encoding gene (first segment: PSPTO_4993, second segment PSPTO_4996) which is disrupted by an insertion sequence element (IS-element, ISPsy5 transposase: PSPTO_4994, ISPsy5 ORF: PSPTO_4995). Finally, each gene of the putative core-OS cluster of Pst DC3000 (PSPTO_4983-PSPTO_5003) was associated with a putative function by taking gene annotation, gene ontology, and the corresponding function of the P. aeruginosa PAO1 orthologs into account (Table 1).
2.2. Genes Involved in Synthesis of the Inner Core-OS Are Conserved in Pseudomonas
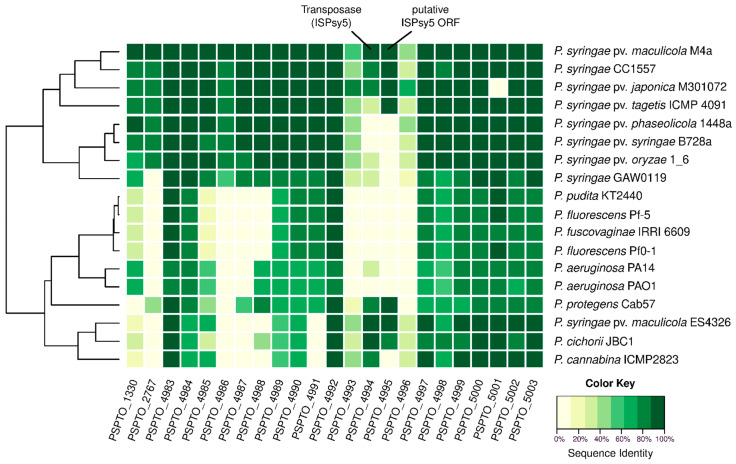
The results of the analysis of the Pst DC3000 core-OS gene cluster (PSPTO_4983-PSPTO_5003; Table 1) were used to elucidate core-OS synthesis from bacteria of the P. syringae species complex and other representative Pseudomonas species. Comparative analysis with predicted proteomes (Table S1) was performed to identify orthologs of known core-OS biosynthesis components and to reveal possible differences. The results suggest a strong conservation of genes associated with inner core-OS compared to outer core-OS synthesis among Pseudomonas (Figure 1).
Figure 1.
Comparison of core-OS synthesis enzymes. Heatmap of results from an NCBI BLASTP search for homologous core-OS biosynthetic enzymes in different predicted proteomes (Table S1). Pst DC3000 sequences were used as reference, e-value cutoff = 10−9. Sequence identity values of the BLASTP results are provided in Supplementary Data File 1. Dendrogram according to Euclidean distances calculated from the BLASTP results.
The respective sequence identities of P. syringae pathovars ranged from 100% to 74.5%, whereas the comparison with P. aeruginosa PAO1 genes showed the lowest sequence identities (83.5–57.5%) of the analyzed Pseudomonas species. Notably, BLASTP search for a PSPTO_5001 protein ortholog in P. syringae pv. japonica M301072 yielded no hit, while sequence identities for other core-OS synthesis components ranged from 97.4% to 81.0%. Closer inspection of the respective gene sequence shows a potential frame shift resulting in a premature stop codon. Comparison of further core-OS synthesis elements in the Pst DC3000 cluster (PSPTO_4983-PSPTO_4992) indicated a conservation of the respective proteins in all analyzed P. syringae pathovars (sequence identities: 100.0–60.0%) except P. syringae pv. maculicola ES4326 (Figure 1). While some of these proteins, e.g., carbamoyltransferase PSPTO_4992 and LA:core-OS transporter PSPTO_4984, seem to be conserved in other Pseudomonas species, only single orthologs of the putative glycosyltransferases PSPTO_4991, PSPTO_4988, or PSPTO_4997 could be identified in some predicted Pseudomonas proteomes (Figure 1).
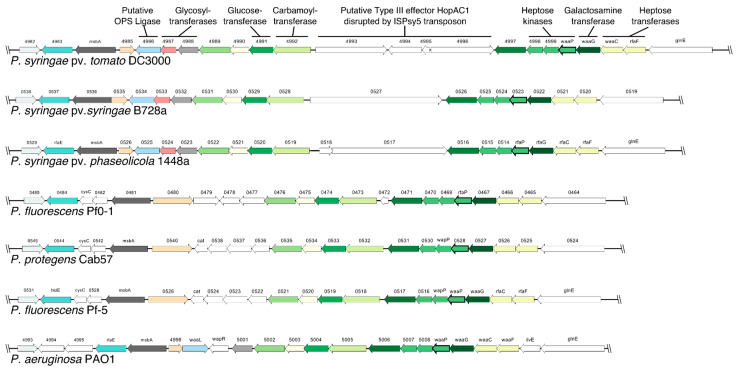
Three P. syringae pathovars and four Pseudomonas species were analyzed for synteny within the core-OS gene cluster to identify possible differences in a gene context (Figure 2). The IS-element disrupting the putative type III effector gene hopAC1 in Pst DC3000 is not present in other genomes, but hopAC1 or homologous sequences were identified in P. syringae pv. syringae B728a (Psyr_0527) and P. syringae pv. phaseolicola 1448a (PSPPH_0517/PSPPH_0518). Otherwise, gene synteny is highly conserved among P. syringae strains and mainly differs in the sequence and orientation of putative glycosyltransferase and OPS ligase genes upstream of PSPTO_4889.
Figure 2.
Synteny analysis of the core-OS cluster in Pseudomonas strains. Gene structure of the Pst DC3000 core-OS cluster and hits from synteny analysis of corresponding genes in selected Pseudomonas strains. Matching colors indicate corresponding gene hits.
In summary, the sequence analysis revealed conserved core-OS gene clusters in P. syringae strains and suggests these strains share a common core-OS structure. Available data on the core-OS structure of the P. syringae pv. phaseolicola and the relative sugar content of pathovar maculicola and atrofaciens indicate a similar sugar composition [26,27,28]. Next, we analyzed the core-OS structure of the model plant pathogen Pst DC3000 in detail.
2.3. Structural Analysis of the Pst DC3000 LPS Core-Oligosaccharide
Since we focused on the analysis of the core-OS structure, we isolated LPS of the previously established OPS-deficient Pst DC3000 ΔwbpL mutant [29], as this yields higher amounts of core-OS. We analyzed the O- and N-deacylated core-LA carbohydrate backbone generated by hydrazinolysis and alkaline hydrolysis (HyKOH-treatment) as well as the core sugar after mild acidic hydrolysis to check for loss of substituents during HyKOH-treatment.
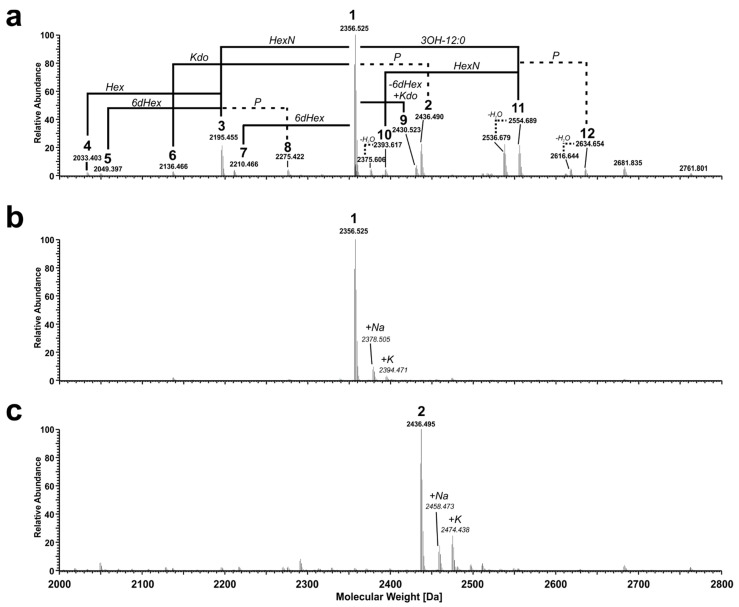
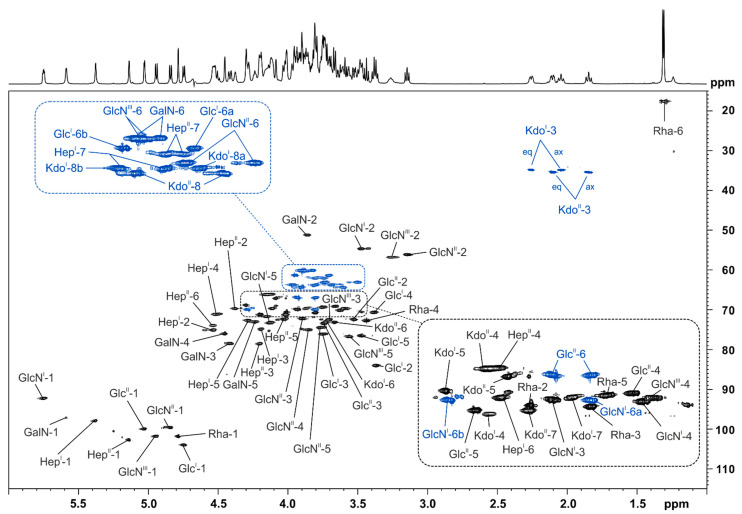
LPS from Pst DC3000 ΔwbpL was O-deacylated by mild hydrazinolysis and then N-deacylated under strong alkaline conditions. After desalting, the resultant mixture of oligosaccharides (OS-HyKOH; MS spectrum shown in Figure 3a) was further fractionated by HPAEC. A representative analytical HPAEC run of this mixture is depicted in Figure S1. One major (1), two minor (2 and 3, respectively), and some very minor molecules (4–9) have been observed; 2 contains one phosphate more than 1, while 3 lacks one HexN compared to 1. In addition, not completely deacylated variants of 1, 2, and 3 have been found (10, 10anh, 11, 11anh, 12, and 12anh). All detected species are summarized in Table 2. The MS spectrum of the HPAEC-purified and desalted major observed molecule 1 (pool 2 in HPAEC, Figure S1) is depicted in Figure 3b. Notably, 1 has an exact mass of 2356.525 Da, equivalent to the composition Kdo2Hep2Hex26dHex1HexN4P5, which is identical to the mass and composition observed for the major core-backbone oligosaccharide in Pseudomonas syringae pv. phaseolicola [26]. This was further corroborated by one- and two-dimensional NMR experiments on compound 1. The corresponding 1H, 13C-HSQC NMR spectrum is shown in Figure 4, and the respective NMR chemical shift data are summarized in Table 3, Table 4 and Table 5. By this, the identical structure of 1 and the major core-backbone oligosaccharide identified in P. syringae pv. phaseolicola [26] were proven.
Figure 3.
Mass spectrometric analysis of the O- and N-deacylated core-LA carbohydrate backbone of Pst DC3000 ΔwbpL. (a) Molecular species distribution in the mixture of oligosaccharides obtained after hydrazinolysis and alkaline hydrolysis (OS-HyKOH). The charge-deconvoluted spectrum of the MS-analysis performed in negative ion mode is depicted; the observed molecular species are listed in Table 2. MS-spectra of selected pools from the further fractionation by HPAEC containing molecules 1 (b) and 2 (c), respectively. Molecular masses given in italic style represent sodium (Δm = 21.98 Da) or potassium (Δm = 37.95 Da) adduct ions of the respective base peak.
Table 2.
Mass spectrometric analysis of the O- and N-deacylated core-LA carbohydrate backbone of Pst DC3000 ΔwbpL. Summary of calculated monoisotopic neutral masses and observed molecular masses [Da] in the OS-HyKOH preparation, the corresponding MS spectrum is depicted in Figure 3a. Accuracy of the measurement is stated as Δppm; anh = anhydro.
| Molecule | Composition | Calculated Mass [Da] | Observed Mass [Da] | Error [ppm] | HPAEC * |
|---|---|---|---|---|---|
| 4 | Kdo2Hep2Hex16dHex1HexN3P5 | 2033.403 | 2033.403 | 0.0 | pool 4 |
| 5 | Kdo2Hep2Hex2HexN3P5 | 2049.398 | 2049.397 | −0.5 | pool 3 |
| 6 | Kdo1Hep2Hex26dHex1HexN4P5 | 2136.466 | 2136.466 | 0.0 | pool 1 |
| 3 | Kdo2Hep2Hex26dHex1HexN3P5 | 2195.456 | 2195.455 | −0.5 | pool 4 |
| 7 | Kdo2Hep2Hex2HexN4P5 | 2210.467 | 2210.466 | −0.5 | pool 3 |
| 8 | Kdo2Hep2Hex26dHex1HexN3P6 | 2275.422 | 2275.422 | 0.0 | pool 6 |
| 1 | Kdo2Hep2Hex26dHex1HexN4P5 | 2356.524 | 2356.525 | 0.4 | pool 2/3 |
| 10anh | Kdo2Hep2Hex26dHex1HexN3P5[12:0(3-OH)]–H2O | 2375.607 | 2375.606 | −0.4 | ** |
| 10 | Kdo2Hep2Hex26dHex1HexN3P5[12:0(3-OH)] | 2393.618 | 2393.617 | −0.4 | ** |
| 9 | Kdo3Hep2Hex2HexN4P5 | 2430.525 | 2430.523 | −0.8 | pool 4 |
| 2 | Kdo2Hep2Hex26dHex1HexN4P6 | 2436.491 | 2436.490 | −0.4 | pool 5 |
| 11anh | Kdo2Hep2Hex26dHex1HexN4P5[12:0(3-OH)]–H2O | 2536.676 | 2536.679 | 1.2 | ** |
| 11 | Kdo2Hep2Hex26dHex1HexN4P5[12:0(3-OH)] | 2554.686 | 2554.689 | 1.2 | ** |
| 12anh | Kdo2Hep2Hex26dHex1HexN4P6[12:0(3-OH)]–H2O | 2616.642 | 2616.644 | 0.8 | ** |
| 12 | Kdo2Hep2Hex26dHex1HexN4P6[12:0(3-OH)] | 2634.653 | 2634.654 | 0.4 | ** |
* see Figure S1, ** these monoacylated molecules elute at later retention times, and pools were of minor yield.
Figure 4.
NMR analysis of the major O- and N-deacylated core-LA carbohydrate backbone molecule (1) of Pst DC3000 ΔwbpL after HPAEC-fractionation. Shown above is a section (δH 6.0–1.0; δC 115–15) of the 1H,13C-HSQC NMR spectrum, recorded at 310 K in D2O as dept-version (blue: CH2-groups, black: CH/CH3-groups), including assignment of signals. The corresponding NMR chemical shift data are listed in Table 3, Table 4 and Table 5.
Table 3.
1H NMR chemical shift data of 1 recorded in D2O at 310 K.
| Residue | H1 | H2 | H3 | H4 | H5 | H6a | H6b | H7a | H7b |
| →6)-α-GlcNI-(1→P | 5.77–5.73 | 3.50–3.45 | 3.96–3.91 | 3.65–3.60 | 4.15–4.10 | 3.83–3.78 | 4.31–4.27 | ||
| →6)-β-GlcNII4P-(1→ | 4.84 [d, 8.5 Hz] | 3.17–3.12 | 3.92–3.87 | 3.88–3.83 | 3.79–3.73 | 3.52–3.48 | 3.76-3.71 | ||
| →3)-α-HepI2P4P-(1→ | 5.39–5.36 | 4.54–4.51 | 4.22–4.17 | 4.54–4.48 | 4.30–4.26 | 4.14–4.09 | 3.83–3.79 | 3.99–3.94 | |
| →3)-α-HepII6P-(1→ | 5.15–5.12 | 4.39–4.36 | 4.21–4.18 | 4.13–4.09 | 4.05–4.00 | 4.56–4.50 | 3.77–3.71 | 3.82–3.77 | |
| →3,4)-α-GalN-(1→ | 5.60–5.57 | 3.88–3.83 | 4.43–4.39 | 4.46–4.44 | 4.24–4.21 | 3.84–3.80 | 3.93–3.88 | ||
| →2)-β-GlcI-(1→ | 4.75 [d, 7.9 Hz] | 3.38–3.34 | 3.77–3.73 | 3.40–3.36 | 3.50–3.45 | 3.73–3.69 | 3.97–3.93 | ||
| →6)-α-GlcII-(1→ | 5.03 [d, 7.9 Hz] | 3.55–3.50 | 3.76–3.72 | 3.68–3.63 | 4.22–4.18 | 3.81–3.78 | 3.95–3.92 | ||
| β-GlcNIII-(1→ | 4.94 [d, 8.4 Hz] | 3.29–3.22 | 3.74–3.68 | 3.61–3.55 | 3.58–3.53 | 3.95–3.85 | 3.95–3.85 | ||
| α-l-Rha-(1→ | 4.80–4.77 | 4.03–4.00 | 3.81–3.79 | 3.46–3.42 | 3.77–3.72 | 1.31 [d, 6.1 Hz] | |||
| Residue | H3eq | H3ax | H4 | H5 | H6 | H7 | H8a | H8b | |
| →4,5)-α-KdoI-(2→ | 2.30–2.23 | 2.04 [dd, 12.3, 12.0 Hz] | 4.17–4.12 | 4.31–4.27 | 3.74–3.71 | 3.89–3.85 | 3.63–3.58 | 3.93–3.88 | |
| α-KdoII-(2→ | 2.11 [dd, 12.9, 4.3 Hz] | 1.85 [dd, 12.9, 12.6 Hz] | 4.18–4.13 | 4.09–4.07 | 3.68–3.65 | 4.04–3.99 | 3.71–3.66 | 3.99–3.94 |
Table 4.
13C NMR chemical shift data of 1 recorded in D2O at 310 K.
| Residue | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 |
|---|---|---|---|---|---|---|---|---|
| →6)-α-GlcNI-(1→P | 92.4–92.2 | 54.6 | 69.9 | 70.1 | 73.3 | 69.9 | ||
| →6)-β-GlcNII4P-(1→ | 99.6 | 56.1 | 72.1 | 75.0 | 74.4 | 63.1 | ||
| →4,5)-α-KdoI-(2→ | n.d. | n.d. | 34.8 | 71.6 | 68.8 | 72.9 | 69.7 | 64.3 |
| α-KdoII-(2→ | n.d. | n.d. | 35.5 | 66.1 | 67.1 | 73.1 | 71.2 | 63.7 |
| →3)-α-HepI2P4P-(1→ | 98.0 | 75.0 | 74.8 | 71.1 | 72.6 | 69.7 | 63.7 | |
| →3)-α-HepII6P-(1→ | 102.8 | 69.7 | 78.3 | 66.1 | 72.3 | 74.0 | 62.0 | |
| →3,4)-α-GalN-(1→ | 97.2 | 51.1 | 78.4 | 75.9 | 73.0 | 60.1 | ||
| →2)-β-GlcI-(1→ | 104.0 | 84.0 | 76.0 | 70.6 | 76.4 | 61.3 | ||
| →6)-α-GlcII-(1→ | 100.0 | 72.3 | 73.3 | 69.1 | 71.1 | 66.9 | ||
| β-GlcNIII-(1→ | 101.8 | 56.7 | 72.4 | 69.6 | 76.6 | 60.2 | ||
| α-l-Rha-(1→ | 101.9 | 70.6 | 70.8 | 72.7 | 69.4 | 17.7 |
Table 5.
31P NMR chemical shift data of 1 recorded in D2O at 310 K.
| Residue | 31P Chemical Shift [ppm] |
|---|---|
| →6)-α-GlcNI-(1→P | -1.44 |
| →6)-β-GlcNII4P-(1→ | 0.48 |
| →3)-α-HepI2P4P-(1→ | 0.28 |
| →3)-α-HepI2P4P-(1→ | 1.57 |
| →3)-α-HepII6P-(1→ | 1.70 |
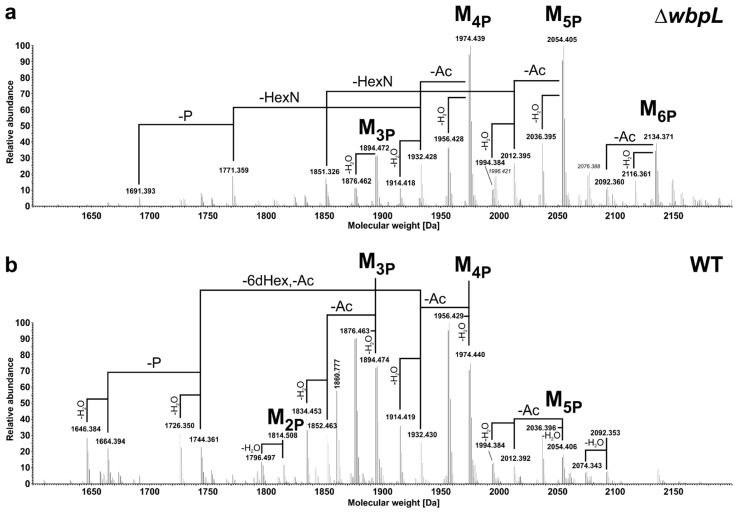
A fraction of molecules with an additional phosphate (2; exact mass of 2436.495 Da, Figure 3c) was also isolated by HPAEC (pool 5, Figure S1). Although this pool was represented by multiple peaks in the HPAEC, it was almost homogeneous in the molecular mass. However, the NMR analysis indicated that this pool contains multiple molecules, therefore the positions of the additional phosphate group could not be determined unequivocally, but all of them were monophosphate groups (data not shown). To check for further substituents that are known to be cleaved off during HyKOH-treatment, LPS from Pst DC3000 ΔwbpL was subjected to hydrolysis with 1% acetic acid. This treatment cleaves the linkage between LA and core-OS under elimination of one Kdo (KdoII) but leaves N-alanyl-, N-/O-acetyl-, and O-carbamoyl-residues as well as diphosphate bonds intact [30]. The MS spectrum of the desalted core-OS preparation (OSHOAc) is depicted in Figure 5a. Compared to the core-OS molecules observed in P. syringae pv. phaseolicola [26], two major differences are obvious: the core-OS of Pst DC3000 ΔwbpL bears more phosphate moieties (up to six instead of up to four in P. syringae pv. phaseolicola) and contains additional acetyl moieties.
Figure 5.
MS analysis of core-OS preparations obtained by hydrolysis with 1% acetic acid from lipopolysaccharide (LPS) of ΔwbpL and wild-type Pst DC3000. Molecular species distribution in the mixture of core-OS preparations (OSHOAc) obtained after treatment of LPS isolated from Pst DC3000 ΔwbpL (a) and wild-type (b) with 1% acetic acid. Charge-deconvoluted spectra of the MS-analysis performed in negative ion mode are depicted. Molecular masses given in italic style represent sodium (Δm = 21.98 Da) adduct ions of the respective base peak.
The structure of the basic core-OS molecule (M) has the following composition as judged by calculated masses: Kdo1Hep1HepCm1Hex26dHex1HexN2Ala1Ac3 with varying numbers of phosphate residues (three to six; M3P to M6P, respectively). Calculated and observed masses for core-OS molecules present in this preparation are summarized in Table 6. To prove that the additional two predominant acetylations are not an effect of the wbpL-knockout and a resulting loss of OPS, the same treatment and analysis was performed with LPS isolated from wild-type Pst DC3000. The MS spectrum of this desalted core-OS preparation is depicted in Figure 5b and major core-OS molecules present are summarized in Table 6. Molecules with a mass difference of −18 Da are the result of the known release of water from the reducing Kdo under such chemical treatment conditions.
Table 6.
Mass spectrometric analysis of core-OS of Pst DC3000 ΔwbpL and wild type. Summary of calculated and observed monoisotopic neutral masses [Da] is given. Accuracy of the measurement is stated as Δppm; n.d. = not detected; * detected, but only with <5% of relative intensity to the major base peak.
| Molecule | Pst DC3000 ΔwbpL | Pst DC3000 WT | |||
|---|---|---|---|---|---|
| Calculated Mass [Da] | Observed Mass [Da] | Error [Δppm] | Observed Mass [Da] | Error [Δppm] | |
| Kdo1Hep1HepCm1Hex2HexN2Ala1Ac1P3–H2O | 1646.381 | 1646.383 * | 1.2 | 1646.384 | 1.8 |
| Kdo1Hep1HepCm1Hex2HexN2Ala1Ac1P3 | 1664.390 | 1664.394 * | 2.4 | 1664.394 | 2.4 |
| Kdo1Hep1HepCm1Hex26dHex1HexN1Ala1Ac2P3–H2O | 1673.380 | 1673.383 * | 1.8 | 1673.384 | 2.4 |
| Kdo1Hep1HepCm1Hex26dHex1HexN1Ala1Ac2P3 | 1691.391 | 1691.393 | 1.2 | 1691.395 | 2.4 |
| Kdo1Hep1HepCm1Hex2HexN2Ala1Ac1P4–H2O | 1726.347 | 1726.349 | 1.2 | 1726.350 | 1.7 |
| Kdo1Hep1HepCm1Hex26dHex1HexN1Ala1Ac1P4 | 1729.347 | 1729.348 | 0.6 | n.d. | - |
| Kdo1Hep1HepCm1Hex2HexN2Ala1Ac1P4 | 1744.358 | 1744.360 | 1.1 | 1744.361 | 1.7 |
| Kdo1Hep1HepCm1Hex26dHex1HexN1Ala1Ac2P4 | 1771.357 | 1771.359 | 1.1 | 1771.361 | 2.3 |
| Kdo1Hep1HepCm1Hex26dHex1HexN2Ala1Ac3P2–H2O | 1796.494 | n.d. | - | 1796.497 | 1.7 |
| Kdo1Hep1HepCm1Hex26dHex1HexN2Ala1Ac3P2 (M2P) | 1814.504 | 1814.509 * | 2.8 | 1814.508 | 2.2 |
| Kdo1Hep1HepCm1Hex26dHex1 HexN2Ala1Ac2P3–H2O | 1834.449 | 1834.452 * | 1.6 | 1834.453 | 2.2 |
| Kdo1Hep1HepCm1Hex26dHex1HexN1Ala1Ac2P5 | 1851.324 | 1851.326 | 1.1 | n.d. | - |
| Kdo1Hep1HepCm1Hex26dHex1HexN2Ala1Ac2P3 | 1852.460 | 1852.462 | 1.1 | 1852.463 | 1.6 |
| Kdo1Hep1HepCm1Hex26dHex1 HexN2Ala1Ac3P3–H2O | 1876.460 | 1876.462 | 1.1 | 1876.463 | 1.6 |
| Kdo1Hep1HepCm1Hex26dHex1HexN2Ala1Ac3P3 (M3P) | 1894.470 | 1894.472 | 1.1 | 1894.474 | 2.1 |
| Kdo1Hep1HepCm1Hex26dHex1 HexN2Ala1Ac2P4–H2O | 1914.416 | 1914.418 | 1.0 | 1914.419 | 1.6 |
| Kdo1Hep1HepCm1Hex26dHex1HexN2Ala1Ac2P4 | 1932.426 | 1932.428 | 1.0 | 1932.430 | 2.1 |
| Kdo1Hep1HepCm1Hex26dHex1 HexN2Ala1Ac3P4–H2O | 1956.426 | 1956.428 | 1.0 | 1956.429 | 1.5 |
| Kdo1Hep1HepCm1Hex26dHex1HexN2Ala1Ac3P4 (M4P) | 1974.437 | 1974.439 | 1.0 | 1974.440 | 1.5 |
| Kdo1Hep1HepCm1Hex26dHex1 HexN2Ala1Ac2P5–H2O | 1994.382 | 1994.384 | 1.0 | 1994.384 | 1.0 |
| Kdo1Hep1HepCm1Hex26dHex1HexN2Ala1Ac2P5 | 2012.393 | 2012.395 | 1.0 | 2012.392 | −0.5 |
| Kdo1Hep1HepCm1Hex26dHex1 HexN2Ala1Ac3P5–H2O | 2036.393 | 2036.395 | 1.0 | 2036.396 | 1.5 |
| Kdo1Hep1HepCm1Hex26dHex1HexN2Ala1Ac3P5 (M5P) | 2054.403 | 2054.405 | 1.0 | 2054.406 | 1.5 |
| Kdo1Hep1HepCm1Hex26dHex1HexN2Ala1Ac2P6–H2O | 2074.348 | n.d. | - | 2074.343 | −2.4 |
| Kdo1Hep1HepCm1Hex26dHex1HexN2Ala1Ac2P6 | 2092.359 | 2092.360 | 0.5 | 2092.353 | −3.3 |
| Kdo1Hep1HepCm1Hex26dHex1 HexN2Ala1Ac3P6–H2O | 2116.359 | 2116.361 | 0.9 | n.d. | - |
| Kdo1Hep1HepCm1Hex26dHex1HexN2Ala1Ac3P6 (M6P) | 2134.369 | 2134.371 | 0.9 | n.d. | - |
This analysis verified that these acetylations also occur in core-OS of WT Pst DC3000. The majority of observed molecules was similar in preparations of both strains (Table 6), albeit molecular species were observed in significantly different relative abundances. One major difference was the higher content of phosphorylation in OSHOAc obtained from Pst DC3000 ΔwbpL LPS. In the OSHOAc of this mutant, the major molecules were M4P and M5P, as well as their variants with one acetyl group less (-Ac) and respective anhydro-compounds (-H2O). This is shifted in WT to molecules of M3P and M4P type, respectively. Furthermore, only in the preparation of the ΔwbpL mutant molecular species lacking one hexosamine (-HexN) are significantly present (1851.326 Da, 1771.359 Da; Figure 5a). By contrast, OSHOAc obtained from Pst DC3000 WT LPS contained molecular species to a significant degree, in which the 6-deoxyhexose together with one acetyl moiety is lacking (-6dHex, -Ac; 1744.361 Da, 1664.394 Da, and their respective anhydro-variants; Figure 5b). Notably, in these 6dHex (Rha)-lacking molecules only one acetyl moiety is present, pointing to the potential presence of these modifications at this terminal residue.
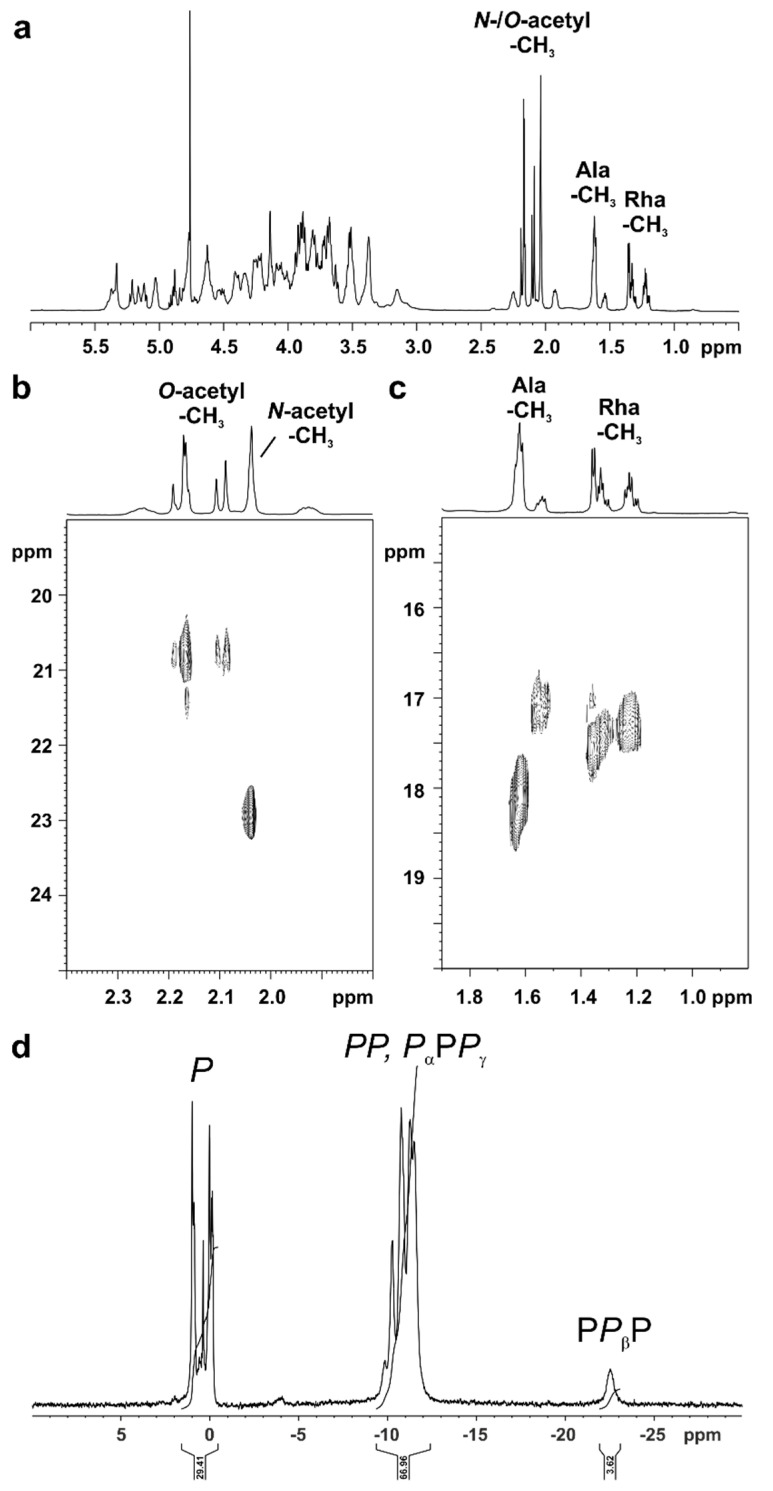
Despite the known complexity of such core-OS preparations due to the high degree of structural heterogeneity (e.g., caused by two outer core glycoforms (w/wo HexNAc), varying degree of phosphorylation, and anhydro versions of all molecules) we analyzed the OSHOAc preparation derived from Pst DC3000 ΔwbpL by NMR, especially aiming to identify the position of the additional acetyl substituents. The full 1H NMR spectrum is shown in Figure 6a, and the region for CH3 groups of the 1H, 13C-HSQC NMR is displayed in Figure 6b,c. Besides the presence of an N-acetyl group (δH 2.04; δC 23.0) multiple O-acetyl groups (δH 2.20–2.08; δC 20.9/20.8) can be detected (see Figure 6b). The major portion of the N-alanyl (Ala) CH3 group is represented by two overlapping doublets at δH 1.63/1.62 with δC 18.2 (most likely in the major occurring glycoform including the terminal HexNAc). A minor portion can be detected again as two overlapping doublets at δH 1.55/1.54 with δC 17.2 (most likely derived from the minor glycoform lacking the terminal HexNAc).
Figure 6.
NMR analysis of the OSHOAc preparation of Pst DC3000 ΔwbpL LPS. (a) Shown above is the full 1H NMR spectrum (δH 6.0–0.5; recorded in D2O at 300 K); regions for signals resulting from N-/O-acetyl groups, Ala-CH3, and Rha-CH3, respectively, are indicated. (b,c) Sections of the 1H,13C-HSQC NMR spectrum showing regions with 1H,13C-cross correlations for N-/O-acetyl groups (δH 2.40–1.80; δC 25–19; (b)) as well as Ala-CH3 and Rha-CH3 moieties (δH 1.90–0.80; δC 20–15; (c)) are shown. The signal assignment is discussed in the text. (d) Shown above is the 31P NMR spectrum (δP 10-(-30)) recorded in D2O at 300 K. Monophosphates (P) are represented by the group of signals between δP 2 and −1 ppm, diphosphates (PP) as well as Pα and Pγ of triphosphates appear between δP −9 and −12 ppm, and Pβ of triphosphates is represented by the broad signal between δP −22 and −23 ppm.
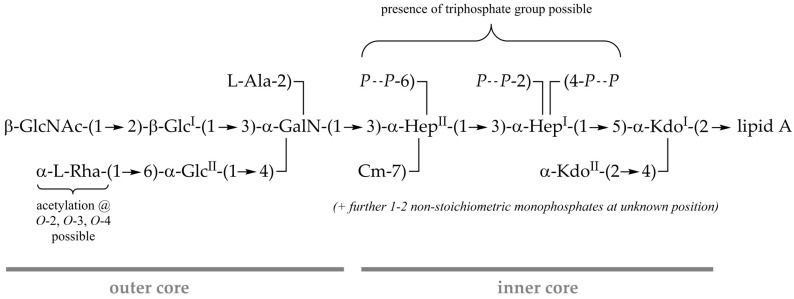
Interestingly, multiple doublets between δH 1.38–1.18, all with corresponding carbons at δC 17.6–17.2, point to the presence of various versions of the terminal Rha residue (Figure 6c). Furthermore, the presence of an O-carbamoyl group was indicated in the 13C-NMR spectrum of this OSHOAc preparation (not shown) at δC 158.9 (compared to δC 159.4 in the core of Pseudomonas syringae pv. phaseolicola [26]). Its analysis by 31P NMR revealed a significant proportion of di- and to a minor extent even triphosphates (appr. 1:1:0.1 as judged by sum-integration of signals). Diphosphates and Pα and Pγ of triphosphates are represented by the group of signals between δP −9 and −12 ppm, Pβ of triphosphates by the broad signal between δP −22 and −23 ppm (Figure 6d). Unfortunately, neither attempt aiming for the isolation of homogeneous core-OS molecules by HPAEC directly from this preparation nor after dephosphorylation by HF treatment (which also partially cleaves off O-acetyl residues) has been successful (data not shown). Therefore, the final assignment of the positions of these diphosphates and the combinations of O-acetylation of the Rha moiety remains partially elusive. The chemical structure of the core-OS of Pst DC3000 LPS as revealed here is summarized in Figure 7.
Figure 7.
Scheme of the core-OS of Pst DC3000 LPS. It has the same basic structure as the core-OS identified in P. syringae pv. phaseolicola (glycoform 1) [26], but for Pst DC3000 core-OS the observed degree of phosphorylation is significantly higher. Position 2 and 4 of HepI or position 6 of HepII are occupied by a diphosphate group in a significant proportion (dashed lines indicate nonstoichiometric substitution) and further nonstoichiometric monophosphates at so far unknown positions can be present. To a lesser extent, triphosphate groups are present as well. The terminal Rha moiety is O-acetylated with up to two acetyl groups.
3. Discussion
In this study, we identified the core-OS gene cluster in Pst DC3000 and analyzed publicly available sequence data to compare the genetic background of core-OS synthesis in the P. syringae species complex and other plant-associated Pseudomonas bacteria. The core-OS cluster is generally conserved within Pseudomonas. While the gene content is very similar in most P. syringae pathovars, variations in genes responsible for outer core-OS synthesis are apparent in other bacteria of the species complex. Previous core-OS structural analyses of P. syringae suggest that GalN and Rha residues are characteristic for the outer core region [26,27,28]. According to our analysis, GalN might be present in all Pseudomonas core-OS. The putative core-OS Rha transferase of Pst DC3000 (PSPTO_1330) is only conserved among P. syringae pathovars but not in the P. syringae species complex [26,27,28]. While other Rha transferases might be involved in core-OS synthesis in these strains, it is likely that they lack a Rha residue as observed in other Pseudomonas species, e.g., P. tolaasii [31].
The presence of two putative Hep transferases (PSPTO_5002, PSPTO_5003), three Hep kinases (PSPTO_4998-PSPTO_5000), a putative GalN transferase (PSPTO_5001), and a putative carbamoyltransferase in all Pseudomonas genomes suggests that they possess a common proximal core-OS structure with →3)-α-GalN-(1→3)-α-HepII-(1→3)-α-HepI-(1. Both Hep residues are phosphorylated and a carbamoyl residue is linked to HepII. The inner core-OS phosphates are essential for the viability and drug resistance of P. aeruginosa [25,32]. The conservation of the high degree of phosphorylations might be one of the factors responsible for the intrinsic resistance against harsh environmental conditions and the resulting versatility of bacteria belonging to the genus Pseudomonas [33]. Notably, although structural analysis of the P. cichorii 5707 core-OS indicated that it lacks Hep residues and phosphates [24], the corresponding biosynthetic genes are conserved in the P. cichorii JBC1 genome and are presumably functional. Possibly, this is due to genetic differences between P. cichorii strains. Alternatively, since the core-OS analysis of P. cichorii 5707 was conducted with bacteria cultivated in minimal media [34], the adaptation of bacteria to such conditions could result in a loss of Hep residues in the core-OS.
In accordance with the results from the genomic survey, analysis of the Pst DC3000 core-OS structure revealed that it is mostly identical to the P. syringae pv. phaseolicola core-OS analyzed by Zdorovenko et al. [26]. The major observed molecule after HyKOH-treatment was of same composition as the glycoform 1 observed in this P. syringae pathovar. Glycoform 2 observed in the P. syringae pv. phaseolicola rough-type strain (GSPB 711) used in that study, in which the terminal Rha is exchanged to a Kdo moiety, was only marginally observed in Pst DC3000 (molecule 9). Moreover, a part of molecules (molecules 3 and 8; and to a much lesser extent, molecules 4 and 5) lacked the terminal β-GlcNAc residue. However, this might be caused by the wbpL-knockout, which impairs OPS addition [29], since comparable variants of the core-OS after mild acidic hydrolysis are only observable for the OSHOAc of the wbpL-knockout strain. In the analogous preparation from LPS of the isogenic wild-type strain, this substituent is stoichiometrically present. Here, in turn, to some extent the terminal, O-acetylated Rha residue is lacking. The core-OS of some P. aeruginosa strains [20,23] and of P. fluorescens ATCC 49271 [35] have been described to display a high degree of nonstoichiometric O-acetylation. The specific positions of these O-acetylations in P. aeruginosa strains could not be completely assigned, but at least one was located at O-2 of a terminal Rha residue [30], another at O-6 of the GlcII [20]. A random distribution of O-acetylation at O-2, O-3, and O-4 of the terminal Rha has been observed in other studies of P. aeruginosa core-OS structures [36,37]. For the core-OS of the Pst DC3000 LPS, the assignment of O-acetyl groups to specific positions was also not completely possible. However, our data clearly suggest the presence of O-acetyl groups at the terminal Rha and a mixture of mono- and di-O-acetylated (2,3-di-O-acetyl, 2,4-di-O-acetyl, 3,4-di-O-acetyl) molecules. O-acetylation is associated with specialized respiratory and mucosal pathogens and might influence cell surface hydrophobicity and possibly increase resistance to opsonophagocytosis [19,21,30]. However, the investigation of its influence on plant-host colonization requires identification of the respective O-acetyl transferases, their specific knockout, and a subsequent comparative analysis of mutant and wild-type bacteria in in vivo assays. Moreover, P. aeruginosa is known to contain a high phosphorus content in its LPS core-OS, especially the inner core-OS, present as mono-, di-, and even tri-phosphates at multiple positions [30,38]. By contrast, the core-OS of P. syringae pv. phaseolicola contains only three stoichiometrically defined phosphates (position 2 and 4 of HepI, position 6 of HepII), whereas the phosphate at position 2 of HepI can be in part substituted with a phosphoethanolamine as well. Our analysis of the Pst DC3000 core-OS showed that here the phosphorylation pattern is more similar to that of P. aeruginosa. A high degree of diphosphate groups and the presence of triphosphates was observed. However, attempts to determine the exact sites of attachment of these groups by NMR analysis failed due to the high degree of heterogeneity of core-OS molecules, which is caused by the occurrence of Kdo in multiple forms and, most likely, by nonstoichiometric phosphorylation resulting in the splitting of the signals. In light of the same basic structure of the core-OS for Pst DC3000 as elucidated in this work in comparison with the core-OS found in P. syringae pv. phaseolicola, the disruption of the putative hopAC1 homolog by an IS-element, which was also described in previous reports of type III effector proteins in Pst DC3000 [39], is unlikely to have a major influence on core-OS biosynthesis. Given the critical role of the core-OS for bacterial viability and potentially virulence in interactions with host plants it will be interesting to see in future studies whether the structural features observed here for the Pst DC3000 LPS core-OS are common to other plant-adapted Pseudomonas species.
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
Pst DC3000 WT or ΔwbpL were grown at 26 °C in King’s B (KB) media liquid culture with shaking (230 rpm) or on KB agar plates with 2% (w/v) agar.
4.2. Hot Phenol-Water and Phenol-Chloroform-Petroleum Ether Extraction of LPS
Preparation of LPS from Pst DC3000 WT and ΔwbpL is described in or was performed according to reference [29].
4.3. Alkaline Degradation of the Lipopolysaccharide
LPS (151.8 mg) of Pst DC3000 ΔwbpL was treated with anhydrous hydrazine (1.5 mL) in a 10 mL rim rolled bottle (Macherey-Nagel, Düren, Germany) for 2 h at 37 °C. Afterwards, approximately 5 mL ice-cold acetone were added dropwise and precipitation was allowed overnight at −50 °C. The complete material was transferred into a 30 mL Nalgene™ Oak Ridge high-speed centrifuge tube (FEP; Thermo Scientific Nalgene Products, Rochester, NY, United States), filled with ice-cold acetone, and centrifuged (47,000× g) for 30 min at 4 °C. The precipitate was washed four times with ice-cold acetone, all supernatants were discarded. The sediment was dried under a nitrogen stream, transferred with Millipore-grade water (MP-water) into a 10-mL rim-rolled bottle and lyophilized (yield: 114.4 mg). Next, 2.28 mL 4 M KOH were added, and the sediment suspended using ultrasonics. The solution was purged for 15 min with a slight stream of nitrogen at RT and then heated for 18 h at 120 °C. After cooling to RT, the solution was transferred into a high strength centrifuge tube (chemically strengthened borosilicate glass, Type 1, Class B, Kimble-Chase, Rockwood, TN, United States) and centrifuged (2500× g) for 15 min at 4 °C. The pH of the resulting supernatant was adjusted with 4 M HCl to approximately 6.0 and afterwards extracted three times with 5 mL dichloromethane each (centrifugation: (1) 2500 g, for 15 min at 4 °C; (2) 2500 g, for 30 min at 4 °C; and (3) 5000 g, for 30 min at 4 °C). The aqueous phase was evaporated under reduced pressure and lyophilized. Further purification was achieved by gel-permeation chromatography on a Sephadex G-50 column (2.5 × 50 cm; GE Healthcare Bio-Sciences, Uppsala, Sweden) using pyridine–acetic acid–water (8:20:2000 (vol/vol), pH~4.7) as eluent. Oligosaccharides were monitored by a Knauer differential refractometer. For application to the column, the material was resuspended in 2 mL MP-water and centrifuged (371× g) for 5 min at 20 °C. Fractions containing the O- and N-deacylated core-LA carbohydrate backbone molecules (OS-HyKOH) were collected and lyophilized (yield: 57 mg).
A portion of the isolated OS-HyKOH mixture (dissolved 20 mg/mL in MP-water; approximately 10 mg per run) was fractionated by high-performance anion-exchange chromatography (HPAEC) on a semipreparative CarboPac PA1 column (250 × 9 mm; Dionex, Sunnyvale, CA, USA) using the following gradient (mobile phase A: MP-water; mobile phase B: 1 M NaOAc pH 6.0): 1% B for 5 min, linear gradient raising from 1 to 15% B (5–20 min), maintaining at 15% B for 10 min, linear gradient raising from 15 to 38% B (30–50 min), followed by a linear gradient raising from 38 to 100% B (50–90 min), and held at 100% B for further 30 min. Afterwards, the column was run for 10 min at the initial condition (1% B) to prepare for next injection. The flow rate was 2 mL/min and 2 mL fractions were collected. Selected fractions were analyzed by HPAEC using pulsed amperometric detection with postcolumn addition of 0.5 M NaOH (Dionex) on an analytical CarboPac PA1 column (250 × 4.0 mm) using the same eluents with a flow rate of 1 mL/min with the following gradient: 1% B for 5 min, linear gradient raising from 1 to 15% B (5–20 min), maintaining at 15% B for 15 min, linear gradient raising from 15 to 38% B (35–55 min), followed by a linear gradient raising from 38 to 100% B (55–70 min), and held at 100% B for 8 min more. Afterwards, the column was run for 8 min at initial condition (1% B) to prepare for the next injection. Appropriate fractions were combined and lyophilized. Desalting was performed on a Sephadex G-50 column as described above. Yields resulting from separation of 27.8 mg OS-HyKOH mixture in three runs were as follows: pool 1, 0.78 mg; pool 2, 8.81 mg; pool 3, 1.28 mg; pool 4, 2.62 mg; pool 5, 3.23 mg; and pool 6, 1.27 mg. For assignment of molecules to HPAEC pools, see Table 2 and Figure S1.
4.4. Mild-Acid Degradation of the Lipopolysaccharide
LPS (26 mg for Pst DC3000 WT; 2 × 55 mg for Pst DC3000 ΔwbpL) was dissolved in aqueous 1% HOAc (3 mg/mL) and heated for 1.5 h at 100 °C. The further procedure is described for core-OS from Pst DC3000 ΔwbpL: to enable parallel extraction of LA (not further discussed here, for composition see reference [40]), samples were equally portioned in four 30 mL Nalgene™ Oak Ridge high-speed centrifuge tubes (FEP; Thermo Scientific Nalgene Products, Rochester, NY, United States), and chloroform/methanol 8:2 (v/v) was added until the tubes were completely filled and thoroughly mixed. After centrifugation (6000× g) for 10 min at 4 °C, the lower organic phase was collected. The tubes were refilled with chloroform, thoroughly mixed, and centrifuged again. This procedure was repeated four times in total. The organic phase from the initial chloroform-/methanol-extraction and the first three chloroform extractions were sequentially combined in a pear-shaped flask and reduced to residual water by evaporation under reduced pressure. The chloroform of the last extraction was used to solubilize the material in the pear-shaped flask again (with ultrasonic) and equally portioned into four 30 mL Nalgene™ tubes. Remaining material in the pear-shaped flask was transferred with 4 mL chloroform/methanol 8:2 (v/v) in total into these tubes as well, using ultrasonic for solubilization. These combined organic phases (containing LA) were washed three times with water and finally evaporated under reduced pressure. All aqueous phases (containing core OS) were combined, neutralized with 1 M NaOH (in ΔwbpL core-OS preparation), evaporated under reduced pressure to remove residual organic solvents, and finally lyophilized. The core-OS preparation was further purified by gel permeation chromatography (GPC) on Sephadex G-50 (GE Healthcare Bio-Sciences, Uppsala, Sweden) on a column (2.5 × 50 cm) as described [41]. This yielded 4.5 mg core-OS of Pst DC3000 WT and 45.8 mg of Pst DC3000 ΔwbpL, respectively.
4.5. NMR Spectroscopy
Deuterated solvents were purchased from Deutero GmbH (Kastellaun, Germany). NMR spectroscopic measurements were performed in D2O at stated temperatures on a Bruker AvanceIII 700 MHz (equipped with an inverse 5 mm quadruple-resonance Z-grad cryoprobe). Acetone was used as an external standard for calibration of 1H (δH = 2.225) and 13C (δC = 30.89) NMR spectra [42], and 85% of phosphoric acid was used as an external standard for calibration of 31P NMR spectra (δP = 0.00). All data were acquired and processed by using Bruker TOPSPIN V 3.1 or higher (Bruker BioSpin Corporation, Billerica, MA, USA). 1H NMR assignments were confirmed by 2D 1H,1H-COSY, and total correlation spectroscopy (TOCSY) experiments. 13C NMR assignments were indicated by 2D 1H,13C-HSQC, based on the 1H NMR assignments. Inter-residue connectivity and further evidence for 13C assignment were obtained from 2D 1H,13C-heteronuclear multiple bond correlation and 1H,13C-HSQC-TOCSY. Connectivity of phosphate groups were assigned by 2D 1H,31P-HMQC and 1H,31P-HMQC-TOCSY.
4.6. Mass Spectrometry
All samples were measured on a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) using a Triversa Nanomate (Advion, Ithaca, NY, USA) as ion source. All measurements were performed in negative-ion mode using a spray voltage of −1.1 kV. Samples were dissolved in a water/propan-2-ol/trimethylamine/acetic acid mixture (50:50:0.06:0.02, v/v/v/v) in a final concentration of approximately 0.07 mg/mL (mixtures) or 0.03 mg/mL (HPAEC pools). The mass spectrometer was externally calibrated with glycolipids of known structure. All mass spectra were charge deconvoluted and given mass values that refer to the monoisotopic mass of the neutral molecules. Deconvoluted spectra were computed using Xtract module of Xcalibur 3.1. software (Thermo Fisher Scientific, Bremen, Germany).
4.7. Sequence Analysis
Reciprocal BLAST experiments of protein-coding regions in the Pst DC3000 core-OS cluster (UniProt proteome ID: UP000002515) were conducted against the predicted proteomes listed in Table S1. A Python script was used to identify homologs of the query sequences (NCBI BLASTP) in each proteome which were set up as individual local databases. The sequence of the first hit was retrieved by the algorithm and the sequence identity was calculated from the quotient of hit sequence length and corresponding identities. Heatmaps were generated from the sequence identity values of the BLASTP results (Supplementary Data File 1), and dendrograms were calculated from the corresponding Euclidean distances. All scripts are available online (https://gitlab.com/alexander.kutschera/quickblast, accessed on 11 January 2021).
Gene synteny was analyzed using SyntTax with standard settings [43]. The graphical output of multiple analyses was merged and modified with Inkscape 0.92.
Acknowledgments
We gratefully acknowledge H. Käßner and B. Kunz (both RCB) for excellent technical assistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/22/6/3250/s1.
Author Contributions
Conceptualization, A.K., S.R., and N.G.; methodology, A.K., U.S., S.R., and N.G.; validation, A.K., U.S., S.R., and N.G.; formal analysis, A.K. and N.G.; investigation, A.K., U.S., and N.G.; resources, D.S., S.R., and N.G.; data curation, A.K. and N.G.; writing—original draft preparation, A.K., S.R., and N.G.; writing—review and editing, A.K., U.S., D.S., S.R., and N.G.; visualization, A.K. and N.G.; supervision, S.R., D.S., and N.G.; project administration, S.R. and N.G.; funding acquisition, S.R. All authors have read and agreed to the published version of the manuscript.
Funding
Work in the Ranf lab was funded by Deutsche Forschungsgemeinschaft, Emmy Noether programme RA2541/1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting the findings of this study are provided in the manuscript and its Supplementary Files. Additional data supporting the findings of this study are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xin X.-F., Kvitko B., He S.Y. Pseudomonas syringae: What it takes to be a pathogen. Nat. Rev. Microbiol. 2018;16:316–328. doi: 10.1038/nrmicro.2018.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baltrus D.A., McCann H.C., Guttman D.S. Evolution, genomics and epidemiology of Pseudomonas syringae: Challenges in bacterial molecular plant pathology. Mol. Plant Pathol. 2017;18:152–168. doi: 10.1111/mpp.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raetz C.R.H., Whitfield C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander C., Rietschel E.T. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001;7:167–202. doi: 10.1179/096805101101532675. [DOI] [PubMed] [Google Scholar]
- 5.Lerouge I., Vanderleyden J. O-antigen structural variation: Mechanisms and possible roles in animal/plant-microbe interactions. FEMS Microbiol. Rev. 2002;26:17–47. doi: 10.1111/j.1574-6976.2002.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 6.Molinaro A., Newman M.-A., Lanzetta R., Parrilli M. The structures of lipopolysaccharides from plant-associated Gram-negative bacteria. Eur. J. Org. Chem. 2009;2009:5887–5896. doi: 10.1002/ejoc.200900682. [DOI] [Google Scholar]
- 7.Trent M.S., Stead C.M., Tran A.X., Hankins J.V. Diversity of endotoxin and its impact on pathogenesis. J. Endotoxin Res. 2006;12:205–223. doi: 10.1179/096805106X118825. [DOI] [PubMed] [Google Scholar]
- 8.Whitfield C., Trent M.S. Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 2014;83:99–128. doi: 10.1146/annurev-biochem-060713-035600. [DOI] [PubMed] [Google Scholar]
- 9.Lam J.S., Taylor V.L., Islam S.T., Hao Y., Kocíncová D. Genetic and functional diversity of Pseudomonas aeruginosa lipopolysaccharide. Front. Microbiol. 2011;2:118. doi: 10.3389/fmicb.2011.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knirel Y.A., Bystrova O.V., Kocharova N.A., Zähringer U., Pier G.B. Conserved and variable structural features in the lipopolysaccharide of Pseudomonas aeruginosa. J. Endotoxin Res. 2006;12:324–336. doi: 10.1179/096805106X118906. [DOI] [PubMed] [Google Scholar]
- 11.Ernst R.K., Hajjar A.M., Tsai J.H., Moskowitz S.M., Wilson C.B., Miller S.I. Pseudomonas aeruginosa lipid A diversity and its recognition by Toll-like receptor 4. J. Endotoxin Res. 2003;9:395–400. doi: 10.1177/09680519030090060201. [DOI] [PubMed] [Google Scholar]
- 12.Frirdich E., Whitfield C. Lipopolysaccharide inner core oligosaccharide structure and outer membrane stability in human pathogens belonging to the Enterobacteriaceae. J. Endotoxin Res. 2005;11:133–144. doi: 10.1179/096805105X46592. [DOI] [PubMed] [Google Scholar]
- 13.Kutschera A., Ranf S. The multifaceted functions of lipopolysaccharide in plant-bacteria interactions. Biochimie. 2019;159:93–98. doi: 10.1016/j.biochi.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Das S., Owen K.A., Ly K.T., Park D., Black S.G., Wilson J.M., Sifri C.D., Ravichandran K.S., Ernst P.B., Casanova J.E. Brain angiogenesis inhibitor 1 (BAI1) is a pattern recognition receptor that mediates macrophage binding and engulfment of Gram-negative bacteria. Proc. Natl. Acad. Sci. USA. 2011;108:2136–2141. doi: 10.1073/pnas.1014775108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder T.H., Lee M.M., Yacono P.W., Cannon C.L., Gerçeker A.A., Golan D.E., Pier G.B. CFTR is a pattern recognition molecule that extracts Pseudomonas aeruginosa LPS from the outer membrane into epithelial cells and activates NF-kB translocation. Proc. Natl. Acad. Sci. USA. 2002;99:6907–6912. doi: 10.1073/pnas.092160899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silipo A., Molinaro A., Sturiale L., Dow J.M., Erbs G., Lanzetta R., Newman M.-A., Parrilli M. The elicitation of plant innate immunity by lipooligosaccharide of Xanthomonas campestris. J. Biol. Chem. 2005;280:33660–33668. doi: 10.1074/jbc.M506254200. [DOI] [PubMed] [Google Scholar]
- 17.Braun S.G., Meyer A., Holst O., Pühler A., Niehaus K. Characterization of the Xanthomonas campestris pv. campestris lipopolysaccharide substructures essential for elicitation of an oxidative burst in tobacco cells. Mol. Plant Microbe Interact. 2005;18:674–681. doi: 10.1094/MPMI-18-0674. [DOI] [PubMed] [Google Scholar]
- 18.Needham B.D., Trent M.S. Fortifying the barrier: The impact of lipid a remodelling on bacterial pathogenesis. Nat. Rev. Microbiol. 2013;11:467–481. doi: 10.1038/nrmicro3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kooistra O., Lüneberg E., Lindner B., Knirel Y.A., Frosch M., Zähringer U. Complex O-acetylation in Legionella pneumophila serogroup 1 lipopolysaccharide. Evidence for two genes involved in 8-O-acetylation of legionaminic acid. Biochemistry. 2001;40:7630–7640. doi: 10.1021/bi002946r. [DOI] [PubMed] [Google Scholar]
- 20.Kocincova D., Lam J.S. Structural diversity of the core oligosaccharide domain of Pseudomonas aeruginosa lipopolysaccharide. Biochem. Mosc. 2011;76:755–760. doi: 10.1134/S0006297911070054. [DOI] [PubMed] [Google Scholar]
- 21.Bhasin N., Albus A., Michon F., Livolsi P.J., Park J.S., Lee J.C. Identification of a gene essential for O-acetylation of the Staphylococcus aureus type 5 capsular polysaccharide. Mol. Microbiol. 1998;27:9–21. doi: 10.1046/j.1365-2958.1998.00646.x. [DOI] [PubMed] [Google Scholar]
- 22.Amor K., Heinrichs D.E., Frirdich E., Ziebell K., Johnson R.P., Whitfield C. Distribution of core oligosaccharide types in lipopolysaccharides from Escherichia coli. Infect. Immun. 2000;68:1116–1124. doi: 10.1128/IAI.68.3.1116-1124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King J.D., Kocíncová D., Westman E.L., Lam J.S. Review: Lipopolysaccharide biosynthesis in Pseudomonas aeruginosa. Innate Immun. 2009;15:261–312. doi: 10.1177/1753425909106436. [DOI] [PubMed] [Google Scholar]
- 24.De Castro C., Molinaro A., Nunziata R., Lanzetta R., Parrilli M., Holst O. A novel core region, lacking heptose and phosphate, of the lipopolysaccharide from the Gram-negative bacterium Pseudomonas cichorii (Pseudomonadaceae RNA group 1) Eur. J. Org. Chem. 2004;2004:2427–2435. doi: 10.1002/ejoc.200300799. [DOI] [Google Scholar]
- 25.Walsh A.G., Matewish M.J., Burrows L.L., Monteiro M.A., Perry M.B., Lam J.S. Lipopolysaccharide core phosphates are required for viability and intrinsic drug resistance in Pseudomonas aeruginosa. Mol. Microbiol. 2000;35:718–727. doi: 10.1046/j.1365-2958.2000.01741.x. [DOI] [PubMed] [Google Scholar]
- 26.Zdorovenko E.L., Vinogradov E., Zdorovenko G.M., Lindner B., Bystrova O.V., Shashkov A.S., Rudolph K., Zähringer U., Knirel Y.A. Structure of the core oligosaccharide of a rough-type lipopolysaccharide of Pseudomonas syringae pv. phaseolicola. Eur. J. Biochem. FEBS. 2004;271:4968–4977. doi: 10.1111/j.1432-1033.2004.04467.x. [DOI] [PubMed] [Google Scholar]
- 27.Zdorovenko G.M., Varbanets L.D., Zdorovenko E.L., Vinarskaya N.V., Yakovleva L.M. Chemical and biological characterization of lipopolysaccharides from the Pseudomonas syringae pv. maculicola IMV 381 collection culture and its dissociants. Microbiology. 2004;73:678–688. doi: 10.1007/s11021-005-0009-8. [DOI] [PubMed] [Google Scholar]
- 28.Zdorovenko G.M., Zdorovenko E.L., Varbanets L.D. Composition, structure, and biological properties of lipopolysaccharides from different strains of Pseudomonas syringae pv. atrofaciens. Microbiology. 2007;76:683–697. doi: 10.1134/S0026261707060069. [DOI] [PubMed] [Google Scholar]
- 29.Kutschera A., Schombel U., Wröbel M., Gisch N., Ranf S. Loss of wbpL disrupts O-polysaccharide synthesis and impairs virulence of plant-associated Pseudomonas strains. Mol. Plant Pathol. 2019;20:1535–1549. doi: 10.1111/mpp.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knirel Y.A., Bystrova O.V., Shashkov A.S., Lindner B., Kocharova N.A., Senchenkova S.N., Moll H., Zähringer U., Hatano K., Pier G.B. Structural analysis of the lipopolysaccharide core of a rough, cystic fibrosis isolate of Pseudomonas aeruginosa. Eur. J. Biochem. FEBS. 2001;268:4708–4719. doi: 10.1046/j.1432-1327.2001.02396.x. [DOI] [PubMed] [Google Scholar]
- 31.Silipo A., Leone S., Molinaro A., Lanzetta R., Parrilli M. The structure of the phosphorylated carbohydrate backbone of the lipopolysaccharide of the phytopathogen bacterium Pseudomonas tolaasii. Carbohydr. Res. 2004;339:2241–2248. doi: 10.1016/j.carres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 32.DeLucia A.M., Six D.A., Caughlan R.E., Gee P., Hunt I., Lam J.S., Dean C.R. Lipopolysaccharide (LPS) inner-core phosphates are required for complete LPS synthesis and transport to the outer membrane in Pseudomonas aeruginosa PAO1. mBio. 2011;2 doi: 10.1128/mBio.00142-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silby M.W., Winstanley C., Godfrey S.A., Levy S.B., Jackson R.W. Pseudomonas genomes: Diverse and adaptable. FEMS Microbiol. Rev. 2011;35:652–680. doi: 10.1111/j.1574-6976.2011.00269.x. [DOI] [PubMed] [Google Scholar]
- 34.Jimenez-Barbero J., de Castro C., Evidente A., Molinaro A., Parrilli M., Surico G. Structural determination of the O-specific chain of the lipopolysaccharide from Pseudomonas cichorii. Eur. J. Org. Chem. 2002;2002:1770–1775. doi: 10.1002/1099-0690(200206)2002:11<1770::AID-EJOC1770>3.0.CO;2-5. [DOI] [Google Scholar]
- 35.Knirel Y.A., Helbig J.H., Zähringer U. Structure of a decasaccharide isolated by mild acid degradation and dephosphorylation of the lipopolysaccharide of Pseudomonas fluorescens strain ATCC 49271. Carbohydr. Res. 1996;283:129–139. doi: 10.1016/0008-6215(95)00401-7. [DOI] [PubMed] [Google Scholar]
- 36.Bystrova O.V., Lindner B., Moll H., Kocharova N.A., Knirel Y.A., Zähringer U., Pier G.B. Structure of the lipopolysaccharide of Pseudomonas aeruginosa O-12 with a randomly O-acetylated core region. Carbohydr. Res. 2003;338:1895–1905. doi: 10.1016/S0008-6215(03)00290-8. [DOI] [PubMed] [Google Scholar]
- 37.Bystrova O.V., Knirel Y.A., Lindner B., Kocharova N.A., Kondakova A.N., Zähringer U., Pier G.B. Structures of the core oligosaccharide and O-units in the R- and SR-type lipopolysaccharides of reference strains of Pseudomonas aeruginosa O-serogroups. FEMS Immunol. Med. Microbiol. 2006;46:85–99. doi: 10.1111/j.1574-695X.2005.00004.x. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson S.G. 31P N.m.r. evidence for the presence of triphosphate residues in lipopolysaccharides from Pseudomonas aeruginosa. Biochem. J. 1981;199:833–835. doi: 10.1042/bj1990833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schechter L.M., Vencato M., Jordan K.L., Schneider S.E., Schneider D.J., Collmer A. Multiple approaches to a complete inventory of Pseudomonas syringae pv. tomato DC3000 type III secretion system effector proteins. Mol. Plant Microbe Interact. 2006;19:1180–1192. doi: 10.1094/MPMI-19-1180. [DOI] [PubMed] [Google Scholar]
- 40.Kutschera A., Dawid C., Gisch N., Schmid C., Raasch L., Gerster T., Schäffer M., Smakowska-Luzan E., Belkhadir Y., Vlot A.C., et al. Bacterial medium-chain 3-hydroxy fatty acid metabolites trigger immunity in Arabidopsis plants. Science. 2019;364:178–181. doi: 10.1126/science.aau1279. [DOI] [PubMed] [Google Scholar]
- 41.Ranf S., Gisch N., Schäffer M., Illig T., Westphal L., Knirel Y.A., Sánchez-Carballo P.M., Zähringer U., Hückelhoven R., Lee J., et al. A lectin S-domain receptor kinase mediates lipopolysaccharide sensing in Arabidopsis thaliana. Nat. Immunol. 2015;16:426–433. doi: 10.1038/ni.3124. [DOI] [PubMed] [Google Scholar]
- 42.Gottlieb H.E., Kotlyar V., Nudelman A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997;62:7512–7515. doi: 10.1021/jo971176v. [DOI] [PubMed] [Google Scholar]
- 43.Oberto J. SyntTax: A web server linking synteny to prokaryotic taxonomy. BMC Bioinform. 2013;14:4. doi: 10.1186/1471-2105-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are provided in the manuscript and its Supplementary Files. Additional data supporting the findings of this study are available from the corresponding authors upon request.