Abstract
Industrial hemp is characterized by a huge amount of by-products, such as inflorescences, that may represent high-quality sources of biomolecules with pharmaceutical interest. In the present study, we have evaluated the phytochemical profile, including terpene and terpenophenolic compounds, of the essential oils (EOs) of Futura 75, Carmagnola selezionata and Eletta campana hemp varieties. The EOs were also tested for antifungal properties toward Trichophyton mentagrophytes, Trichophyton rubrum, Arthroderma crocatum, Arthroderma quadrifidum, Arthroderma gypseum, Arthroderma curreyi, and Arthroderma insingulare. In parallel, we investigated the inhibitory effects of the EOs against tyrosinase, and the production of prostaglandin E2 in isolated mouse skin exposed to hydrogen peroxide. In human H1299 lung adenocarcinoma cells, we also evaluated the influence of the EOs on the gene expression of angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2), which are involved in SARS-CoV-2 entry in human host. E-caryophyllene and α-pinene were the prominent terpenes in the EOs, whereas the cannabidiolic acid was the terpenophenol present at higher concentration. The EOs inhibited the growth of all tested dermatophytes species. In isolated skin specimens, EOs prevented the hydrogen-peroxide-induced synthesis of prostaglandin E2, consistent with the intrinsic antityrosinase activity. Finally, in H1299 cells, all tested EOs reduced the gene expression of ACE-2 and TMPRSS2, as well. Therefore, the present findings highlight the rationale for the use of the present EOs against infectious diseases.
Keywords: industrial hemp, essential oil, inflammation, dermatophytes, gene expression, bioinformatics
1. Introduction
Industrial hemp (Cannabis sativa L.) is a traditional multiuse crop that has been extensively cultivated throughout the history as a valuable source of fibers and nutrients, rather than for the extraction of the Δ9-tetrahydrocannabinol (THC) [1,2]. THC represents the sole recognized psychotropic phytocompound present in the industrial hemp cultivars, which are bred to produce THC in traces (≤0.3 % w/w). However, recent findings suggest the presence of other terpenophenolics potentially endowed with THC-like effects [3]. Currently, only hemp varieties that are certified for the THC content <0.2% are permitted to be cultivated in the European Union (EU Regulation N°1124/2008-12 November 2008; Italian Regulation N°172/2017). The Italian Government also promoted the cultivation of industrial hemp under an agricultural policy aimed to implement environmentally friendly crops and contrast the loss of agricultural lands. The application of this strategy is particularly evident in the middle Italy regions, including Abruzzo, where industrial hemp is the object of a renewed interest, as indicated by the increased surface dedicated to the cultivation. The national and supranational interests toward this crop have led to the development of a huge number of new varieties that, besides being certified for the low THC content, are sources of many hemp-seed-deriving foods, characterized by the valuable content in vitamins, minerals, proteins, carbohydrates, and lipids. In this regard, it is sensitive to highlight the seed content in fatty acids, namely linoleic (ω-6) and linolenic (ω-3), often in the 3:1 ratio, which is ideal for the prevention of chronic inflammatory diseases, in humans [4]. However, the industrial hemp chain is also characterized by a huge number of by-products, such as inflorescences, which may represent high-quality sources for extracting biomolecules of pharmaceutical interest, thus potentially contributing to the improvement of the whole botanical chain production [5]. Although the inflorescences have long been considered as waste material to be sold in the dried form for the sole technical use [6], their inclusion as tonics in beverages or flavorings and nutraceuticals in certified foods has been suggested [7]. The valorization of hemp inflorescences is also supported by recent studies, pointing out the use of inflorescences as sources of bioactive extracts and biomolecules, including terpenes, terpenophenolics, and phenolic compounds [8,9,10,11,12,13]. In this regard, inflorescence essential oils (EOs) have been described as effective insecticide, antibacterial, and antiproliferative agents [8,14], whereas the water extracts displayed anti-inflammatory and antityrosinase properties together with intriguing inhibitory effects on the growth of multiple fungi strains, including Candida and dermatophytes species [9,10,11]. Very recently, industrial hemp extracts characterized by different polarities have also been explored as anti-COVID-19 agents, with particular regards to the inhibitory effects on the gene expression of angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) [12,13], which are reputed to play master roles in mediating SARS-CoV-2 virus entry in the human host [15,16]. Aiming to enlarge our comprehension about antimicrobial properties of industrial hemp by-products, in the present multidirectional study, we have tested the antimycotic properties of three industrial hemp varieties, namely, Futura 75, Carmagnola selezionata, and Eletta campana, toward several dermatophytes species (Trichophyton mentagrophytes, Trichophyton rubrum, Arthroderma crocatum, Arthroderma quadrifidum, Arthroderma gypseum, Arthroderma curreyi, and Arthroderma insingulare) which are reputed to be involved in skin inflammatory disorders, including hyperpigmentation [17,18,19]. In parallel, we have evaluated the inhibitory effects of the EOs against the activity of tyrosinase, an enzyme playing a master role in hyperpigmentation [20], and the production of prostaglandin E2 in an ex vivo toxicity model constituted by isolated mouse skin specimens challenged with hydrogen peroxide [21]. Also considering the recent interest of scientific research toward hemp-deriving extracts as putative anti-COVID-19 agents [12,13], we investigated the influence of the aforementioned EOs on the gene expression of ACE-2 and TMPRSS2 in human H1299 lung adenocarcinoma cell line. In parallel, we studied the effects of the EOs on cell viability and the gene expression of apoptosis regulators, namely transforming growth factor (TGF)-β and B-cell lymphoma 2 (Bcl-2) [22,23], which could be targeted by hemp phytocompounds [24]. Finally, detailed phytochemical analyses were conducted for unravelling the composition of the EOs in terms of terpene and terpenophenolic compounds.
2. Results and Discussion
In the present study, a multidirectional approach was followed for exploring phytochemical composition and pharmacological applications of the EOs of the industrial hemp varieties Futura 75, Eletta campana, and Carmagnola selezionata. Recently, we characterized the terpene profile of the aforementioned EOs [25], and the chromatographic analyses indicated the E-caryophyllene and α-pinene as the most representative terpenes, in all tested EOs. These results agreed with previous literature data [8,26], as well. The gas chromatography coupled to mass spectrometry detector (GC–MS) profile of the three EOs is also available in Table 1, Table 2 and Table 3, whereas the high-performance liquid chromatography coupled to ultraviolet and mass spectrometry detectors (HPLC–UV-MS) chromatograms show the cannabidiolic acid as the prominent terpenophenol (range 57.4–70.6%), in all tested samples (Figure 1A–C). This is consistent, albeit partially, with literature data highlighting the presence of terpenophenols in the EO of industrial hemp [27]. However, the study conducted by Nagy et al. [27] showed the cannabidiol as the main terpenophenol in the EO (24.9%) of the Vavilov variety, whereas in the present cultivars, the cannabidiol was present at much lower percentages (1.79–3.58%). By contrast, in the Vavilov variety, cannabidiolic acid was identified in the ethyl acetate extract but not in the EO. We cannot exclude that these discrepancies could depend upon either variability in the plant’s secondary metabolism or harvesting/extractive conditions. The diverse pattern in cannabinoid content is also mirrored by differences in the E-caryophyllene yield that was higher (28.9%) in the EO of Vavilov variety, compared to the present EOs (13.5–19.3%). Given our recent findings about the pharmacological properties of the industrial hemp (Futura 75 variety) water extract as antioxidant/anti-inflammatory, antityrosinase, and antidermatophytes agent against T. rubrum, T. interdigitale, and Microsporum gypseum [10,11], in the present study, a deeper investigation about the antimycotic properties of the EOs was conducted on a broader spectrum of pathogen strains, namely T. mentagrophytes, Trichophyton rubrum, Arthroderma crocatum, Arthroderma quadrifidum, Arthroderma gypseum, Arthroderma curreyi, and Arthroderma insingulare. As depicted in Table 4, the EOs were effective in inhibiting the growth of all tested dermatophytes species, with IC50 values in the range 0.312–10 µg/mL. Overall, Futura 75 variety displayed the higher mycostatic activity, compared to the other two cultivars, although all EOs were generally more potent than the reference antimycotic drug griseofulvin. The mycostatic effects induced by the EOs also agree with the efficacy of caryophyllene oxide as antimycotic, in an in vitro model of onichomycosis [28].
Table 1.
Gas chromatography coupled to mass spectrometry detector (GC–MS) analysis of Eletta campana EO [25].
| Compound | Area % | RI a | RIL b |
|---|---|---|---|
| α-pinene | 11.9 | 934 | 933 |
| camphene | 0.3 | 949 | 953 |
| β-pinene | 3.6 | 977 | 978 |
| myrcene | 6.8 | 994 | 991 |
| α-phellandrene | 0.1 | 1006 | 1007 |
| δ-3-carene | 0.5 | 1011 | 1009 |
| α-terpinene | 0.1 | 1019 | 1018 |
| para-cymene | 0.2 | 1028 | 1024 |
| limonene | 2.2 | 1031 | 1030 |
| eucalyptol | 0.4 | 1040 | 1032 |
| Z-β-ocimene | 0.5 | 1043 | 1035 |
| E-β-ocimene | 1.9 | 1053 | 1046 |
| γ-terpinene | 0.2 | 1062 | 1058 |
| terpinolene | 2.3 | 1091 | 1086 |
| linalyl anthranilate | 0.2 | 1110 | 1104 |
| fenchyl alcohol | 0.1 | 1123 | 1123 |
| terpinen-4-ol | 0.2 | 1187 | 1184 |
| α-ylangene | 0.2 | 1378 | 1371 |
| Z-caryophyllene | 0.3 | 1414 | 1413 |
| E-caryophyllene | 13.5 | 1428 | 1424 |
| α-trans-bergamotene | 0.6 | 1442 | 1432 |
| α-humulene | 5.3 | 1462 | 1454 |
| 9-epi-E-caryophyllene | 0.7 | 1470 | 1464 |
| γ-muurolene | 0.5 | 1485 | 1478 |
| α-amorphene | 0.2 | 1488 | 1482 |
| β-selinene | 1.5 | 1495 | 1492 |
| α-selinene | 1.7 | 1504 | 1501 |
| Z-γ-bisabolene | 1.4 | 1516 | 1511 |
| γ-cadinene | 0.4 | 1523 | 1512 |
| selina-4(15),7(11)-diene | 1.3 | 1545 | 1540 |
| selina-3,7(11)-diene | 2.5 | 1552 | 1546 |
| B-germacrene | 0.3 | 1569 | 1557 |
| caryophyllene oxide | 2.2 | 1599 | 1587 |
| humulene epoxide | 0.7 | 1626 | 1613 |
| α-bisabolol | 0.5 | 1697 | 1688 |
| tetracosane | 6.0 | 2406 | 2400 |
| heptacosane | 23.9 | 2690 | 2700 |
| 12 unknown compounds | 4.8 |
a calculated retention index. b retention index reported in literature or commercial database.
Table 2.
GC–MS analysis of Futura 75 EO [25].
| Compound | Area % | RI a | RIL b |
|---|---|---|---|
| α-pinene | 14.9 | 934 | 933 |
| camphene | 0.3 | 949 | 953 |
| β-pinene | 3.8 | 977 | 978 |
| myrcene | 11.8 | 994 | 991 |
| α-phellandrene | 0.2 | 1006 | 1007 |
| δ-3-carene | 0.5 | 1011 | 1009 |
| α-terpinene | 0.2 | 1018 | 1018 |
| para-cymene | 0.1 | 1028 | 1025 |
| limonene | 1.8 | 1031 | 1030 |
| eucalyptol | 0.2 | 1041 | 1032 |
| E-β-ocimene | 2.9 | 1053 | 1046 |
| γ-terpinene | 0.2 | 1062 | 1058 |
| terpinolene | 5.1 | 1091 | 1086 |
| Z-caryophyllene | 0.5 | 1414 | 1413 |
| α-cis-bergamotene | 0.3 | 1422 | 1416 |
| E-caryophyllene | 19.3 | 1428 | 1424 |
| α-trans-bergamotene | 1.9 | 1442 | 1432 |
| α-humulene | 8.3 | 1462 | 1454 |
| 9-epi-caryophyllene | 1.1 | 1470 | 1464 |
| β-selinene | 1.7 | 1495 | 1492 |
| α-selinene | 1.3 | 1504 | 1501 |
| selina-4(15),7(11)-diene | 0.9 | 1545 | 1540 |
| selina-3,7(11)-diene | 1.5 | 1552 | 1546 |
| caryophyllene oxide | 4.3 | 1599 | 1587 |
| humulene epoxide | 1.1 | 1626 | 1613 |
| allo-aromadendrene epoxide | 0.4 | 1650 | 1644 |
| tetracosane | 8.8 | 2407 | 2400 |
| 13 unknown compounds | 6.6 |
a calculated retention index. b retention index reported in literature or commercial database.
Table 3.
GC–MS analysis of Carmagnola selezionata EO [25].
| Compound | Area % | RI a | RIL b |
|---|---|---|---|
| α-pinene | 12.6 | 934 | 933 |
| camphene | 0.3 | 949 | 953 |
| β-pinene | 4.1 | 977 | 978 |
| myrcene | 26.4 | 995 | 991 |
| α-phellandrene | 0.3 | 1006 | 1007 |
| δ-3-carene | 0.3 | 1012 | 1009 |
| α-terpinene | 0.3 | 1019 | 1018 |
| para-cymene | 0.2 | 1028 | 1025 |
| limonene | 4.7 | 1031 | 1030 |
| eucalyptol | 0.5 | 1041 | 1032 |
| Z-β-ocimene | 0.4 | 1043 | 1035 |
| E-β-ocimene | 2.5 | 1053 | 1046 |
| γ-terpinene | 0.3 | 1062 | 1058 |
| terpinolene | 7.0 | 1091 | 1086 |
| linalyl anthranilate | 0.3 | 1110 | 1104 |
| fenchyl-alcohol | 0.2 | 1123 | 1123 |
| Z-caryophyllene | 0.4 | 1414 | 1413 |
| E-caryophyllene | 19.1 | 1428 | 1424 |
| α-trans-bergamotene | 0.2 | 1442 | 1432 |
| α-humulene | 7.2 | 1462 | 1454 |
| 9-epi-E-caryophyllene | 0.6 | 1470 | 1464 |
| β-selinene | 1.3 | 1495 | 1492 |
| α-selinene | 1.1 | 1504 | 1501 |
| Z-γ-bisabolene | 1.1 | 1514 | 1511 |
| selina-4(15),7(11)-diene | 0.3 | 1545 | 1540 |
| selina-3,7(11)-diene | 0.5 | 1552 | 1546 |
| caryophyllene oxide | 3.2 | 1599 | 1587 |
| humulene epoxide | 1.0 | 1626 | 1613 |
| 10 unknown compounds | 3.6 |
a calculated retention index. b retention index reported in literature or commercial database.
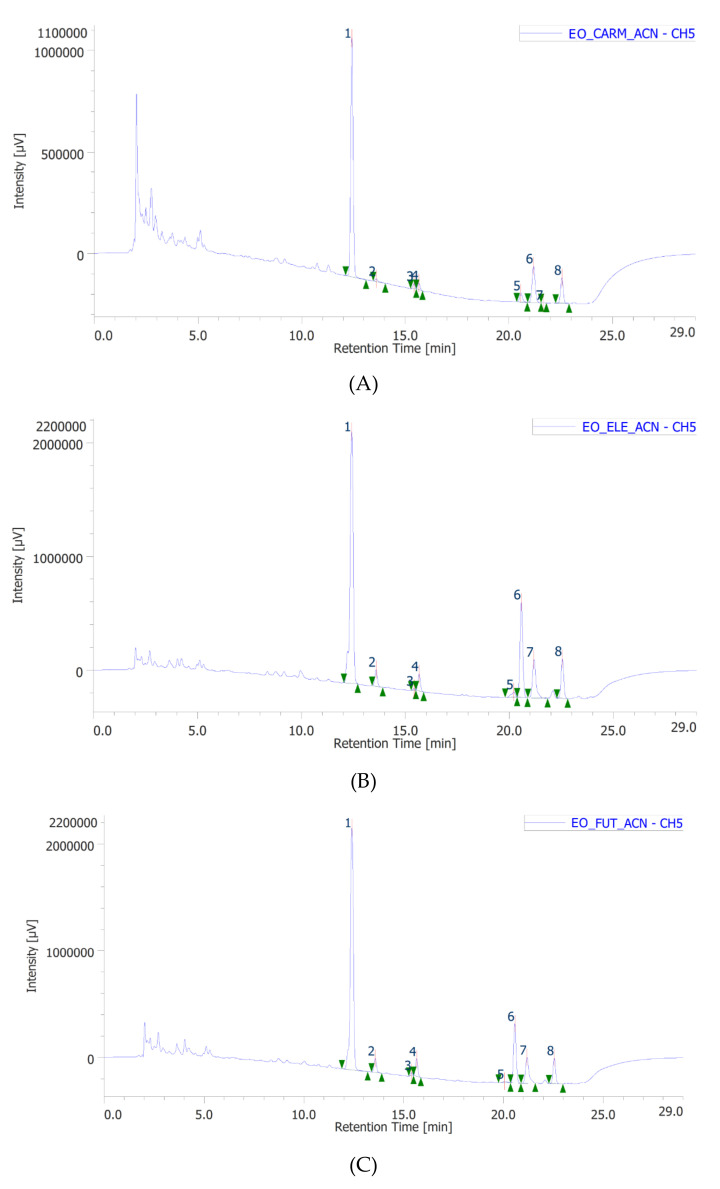
Figure 1.
High-performance liquid chromatography coupled to ultraviolet and mass spectrometry detectors (HPLC–UV-MS) analysis of Carmagnola selezionata (A), Eletta campana, (B) and Futura 75 (C) terpenophenols. In all tested essential oils, cannabidiolic acid (1) was the prominent phytocompound compared to the other identified terpenophenols. Specifically, the cannabidiolic acid was present in the range 57.4–70.6%, corresponding to 24.6–62.9 µg/mg EO.
Table 4.
Minimum inhibitory concentration (MIC) of industrial hemp essential oils toward selected dermatophyte strains.
| Dermatophytes Species | MIC * (µg/mL) | |||
|---|---|---|---|---|
| Carmagnola Selezionata | Eletta Campana | Futura 75 | Griseofulvin | |
| T. mentagrophytes (CCF 4823) | 0.79(0.625–1.25) | < 0.312 | 0.79(0.625–1.25) | 2.52 (2–4) |
| T. rubrum (CCF 4879) | 3.15(2.5–5) | 6.3(5–10) | 6.3(5–10) | 3.175(2–4) |
| T. rubrum (CCF 4933) | 0.99(0.625–1.25) | 1.57(1.25–2.5) | 0.99(0.625–1.25) | 1.26(1–2) |
| A. crocatum (CCF 5300) | 1.57(1.25–2.5) | 1.57(1.25–2.5) | 0.39(0.625–0.312) | >8 |
| A. quadrifidum (CCF 5792) | 3.15(2.5–5) | 3.15(2.5–5) | 0.39(0.625–0.312) | >8 |
| A. gypseum (CCF 6261) | 1.57(1.25–2.5) | 3.15(2.5–5) | 0.49(0.312–0.625) | 3.174 (2–4) |
| A. curreyi (CCF 5207) | 1.98(1.25–2.5) | 6.3(5–10) | 1.57(1.25–2.5) | >8 |
| A. insingulare (CCF 5417) | 6.3(5–10) | 0.99(0.625–1.25) | 0.79(0.625–1.25) | >8 |
* MIC values are reported as geometric means of three independent replicates (n=3); MIC range concentrations are reported within brackets.
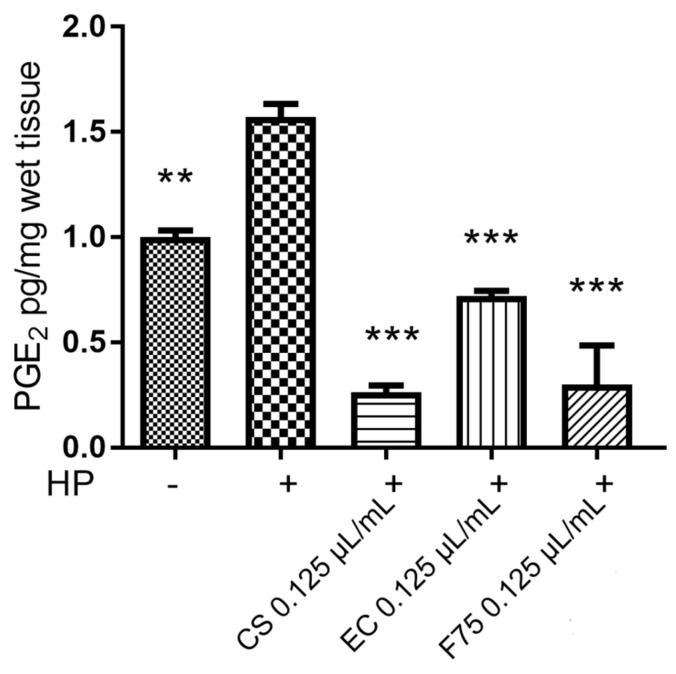
In view of potential applications as skin protective agents, the EOs were assayed for measuring their intrinsic antityrosinase activity. Tyrosinase is a key enzyme involved in the melanogenesis process; therefore, the enzyme inhibition could be crucial to counteract inflammatory-induced skin hyperpigmentation, especially during aging [28]. Eletta campana was the most active as enzyme inhibitory agent (Table 5), and this could be related, albeit partially, to its higher scavenging/reducing activity (Table 6) [25]. Plant secondary metabolites have been reported to exert antioxidant and enzyme inhibitory effects, and high correlation also exists between radical scavenging and enzyme inhibition [29]. Overall, the antidermatophytes and antityrosinase effects induced by hemp EOs suggest protective effects against skin inflammatory disorders that have been partly explored through an ex vivo approach. In this regard, isolated mouse skin specimens were exposed to hydrogen peroxide 1 mM and treated with 0.125 µL/mL (about 100 µg/mL) of EOs. In isolated skin tissue, the hydrogen peroxide stimulus is able to induce both tyrosinase and prostaglandin E2 (PGE2) synthesis [21]. In this context, the blunting effect induced by all tested EOs on hydrogen-peroxide-induced PGE2 level (Figure 2) further supports the use of hemp EOs against infectious and inflammatory skin diseases.
Table 5.
Antityrosinase activity.
| EO | Tyrosinase inhibition (mg KAE/g oil) |
|---|---|
| EC | 31.73 ± 0.63 a |
| F75 | 29.41 ± 0.61 b |
| CS | 21.31 ± 1.37 c |
Values are reported mean ± SD of three parallel experiments. Different superscripts indicate significant differences in the samples (p < 0.05) EO: essential oil; EC: Eletta campana; CS: Carmagnola selezionata; and F75: Futura 75. a,b,c.
Table 6.
Scavenging/reducing and metal-chelating properties of the tested hemp essential oils [25].
| EO | DPPH (mg TE/g EO) |
ABTS (mg TE/g EO) |
CUPRAC (mg TE/g EO) |
FRAP (mg TE/g EO) |
Metal Chelating (mg EDTAE/g EO) |
Phosphomolybdenum (mmol TE/g EO) |
|---|---|---|---|---|---|---|
| EC | 2.53±0.23 a* | 32.44 ± 0.03 a | 45.51 ± 0.75 a | 19.29 ± 0.34 a | 11.55 ± 0.84 a | 17.95 ± 0.34 b |
| CS | 1.18 ± 0.09 c | 32.15 ± 0.08 b | 29.91 ± 0.95 c | 13.55 ± 0.46 c | 7.19 ± 0.42 b | 17.52 ± 1.12 b |
| F75 | 2.11 ± 0.15 b | 32.47 ± 0.04 a | 35.05 ± 0.85 b | 16.16 ± 0.47 b | 10.84 ± 0.46 a | 18.80 ± 0.47 a |
Values are reported as mean ± SD of three parallel experiments. TE: trolox equivalent; EDTAE: EDTA equivalent. Different superscripts indicate significant differences in the samples (p < 0.05). EO: essential oil; EC: Eletta campana; CS: Carmagnola selezionata; and F75: Futura 75. a,b,c. * Values are reported as mean ± SD of three parallel experiments.
Figure 2.
Inhibitory effect induced by the essential oils (EOs: 0.125 µL/mL) of Carmagnola selezionata (CS), Eletta campana (EC), and Futura 75 (F75) cultivars on hydrogen-peroxide (HP)-induced prostaglandin E2 (PGE2) level (pg/mg wet tissue), in isolated mouse skin specimens. ANOVA, p < 0.0001; ** p < 0.01, *** p < 0.001 vs. hydrogen peroxide (HP) group.
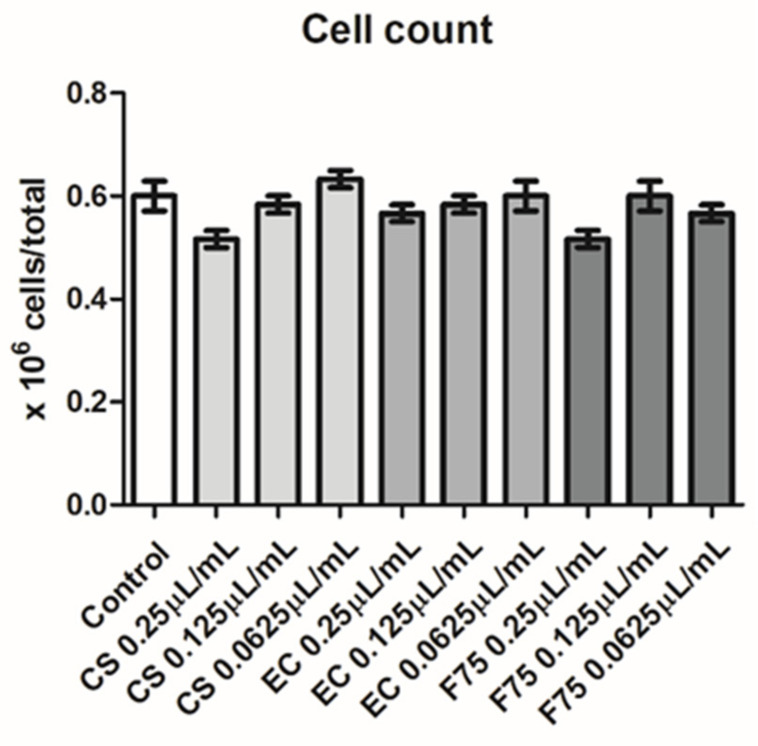
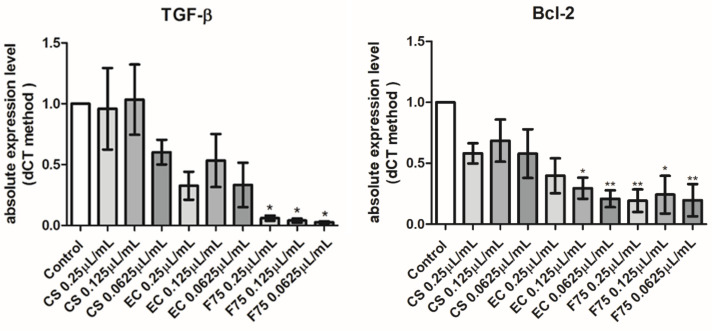
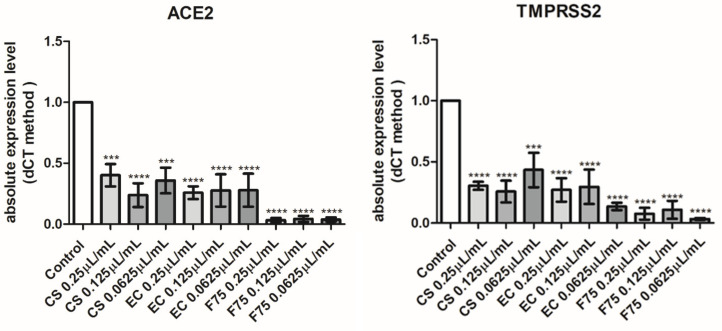
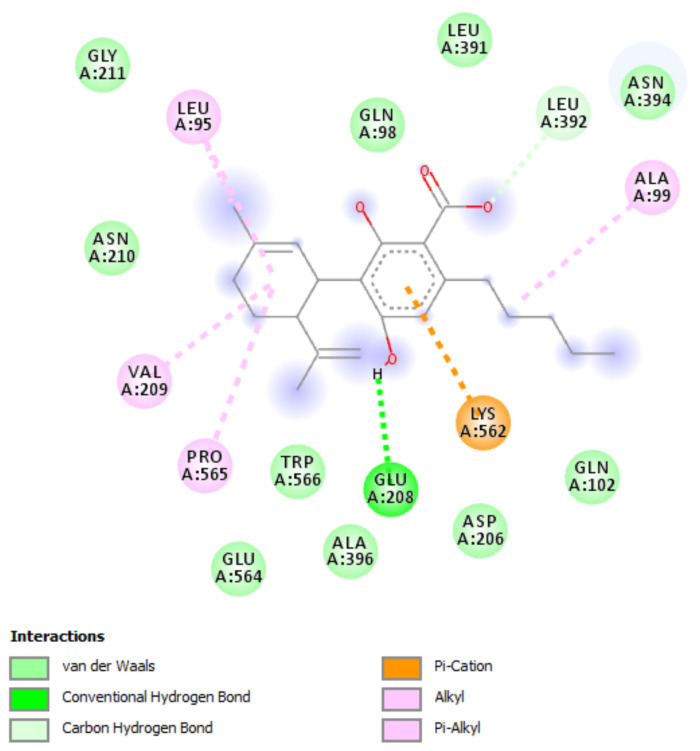
Hemp EOs were also investigated in an in vitro model constituted by human H1299 lung adenocarcinoma cells. As depicted in Figure 3, the EOs (0.0625–0.25 µL/mL) did not alter cell number following pharmacological treatment, thus excluding any significant effect on cell survival, in basal conditions. On the other hand, the literature’s data suggest antiproliferative effects exerted by hemp EOs tested in a similar concentration range [8]. However, the capability of the EO to reduce the cell viability under the limit of biocompatibility (70% viability) could be related to the tumor-cell-line sensitivity [8]. In the present study, the null effect on cell viability is also mirrored by the EOs-induced simultaneous inhibition of the gene expression of both Bcl-2 and TGFβ (Figure 4), which are known to regulate apoptosis in opposite ways [30,31,32]. Regarding the Futura 75 variety, the inhibition of Bcl-2 and TGFβ was statistically significant at all tested concentrations, whereas the EOs of the other two cultivars did not significantly alter Bcl-2 and TGFβ gene expression. Recent studies explored the potential of industrial hemp polar extracts as anti-COVID-19 agents, with particular regards to the gene expression inhibition of ACE2 and TMPRSS2 [12,13], which, besides being co-expressed [33], are reputed to play master roles in mediating SARS-CoV-2 virus entry in the human host [15,16]. This is as also summarized by the pathway map hsa05171 deposited on the KEGG PATHWAY Database (https://www.genome.jp/kegg/pathway.html). Considering the expression of both ACE2 and TMPRSS2 in H1299 cells [22], hemp EOs were assayed for evaluating their influence on ACE2 and TMPRSS2 gene expression, as well. As showed in Figure 5, all investigated EOs were able to significantly inhibit the gene expression of ACE2 and TMPRSS2 in H1299 cells exposed to the treatment, in basal conditions. Considering the results of the quantitative phytochemical analysis, a docking approach was also conducted on the sole ACE2 (PDBID: 1R4L), with the aim of exploring putative direct interactions between ACE2 and the main EO terpenophenol, namely cannabidiolic acid. Indeed, the molecular structure of TMPRSS2 is currently unknown, and the bioinformatics data and predictions available in literature [34] and database (STRINGH, SWISSMODEL) are based on the use of hepsin (PDB ID: 1Z8G), which is currently chosen for its homology with TMPRSS2. The result of the present docking runs yielded putative affinity (Ki) in the micromolar range (Figure 6), thus suggesting binding interactions of cannabidiolic acid with ACE2, which could partly explain the pattern of gene expression observed following treatment. Overall, these findings suggest the hemp EOs as promising agents to be further investigated with the final goal of optimizing their use in protective devices for counteracting the SARS-CoV-2 virus entry in the human host.
Figure 3.
Null effects of the industrial hemp essential oils (CS), (EC), and (F75) (0.0625–0.25 µL/mL) on the viability of the human H1299 lung adenocarcinoma cell line.
Figure 4.
Real-time PCR analyses from three independent experiments (mean ± SEM) of Bcl-2 and TGF-β, in cells treated with vehicle (Control) or 0.25, 0.125, and 0.0625 μL/mL of essential oils (CS), (EC), and (F75). Differences between groups were evaluated using one-way ANOVA test. Differences were considered statistically significant according to the following criteria: * p < 0.05; ** p < 0.01.
Figure 5.
Real-time PCR analyses from five independent experiments (mean ± SEM) of ACE2 and TMPRSS2, in cells treated with vehicle (Control) or 0.25, 0.125, 0.0625 μL/mL of essential oils (CS), (EC), and (F75). Differences between groups were evaluated using one-way ANOVA test. Differences were considered statistically significant according to the following criteria: *** p < 0.001, **** p < 0.0001.
Figure 6.
Putative interactions between cannabidiolic acid and angiotensin-converting enzyme 2 (ACE2; PDBID: 1R4L). Free energy of binding (ΔG) and affinity (Ki) are −7.3 kcal/mol and 4.5 µM, respectively.
3. Materials and Methods
3.1. Pharmacognostic Analysis
Three selected cultivars of industrial hemp, namely Cannabis sativa L. cultivar “Futura 75” (F75), C. sativa L. cultivar “Eletta campana”, and C. sativa L. cultivar “Carmagnola selezionata” were cultivated in experimental crops, in Abruzzo region, and more specifically in the surrounding of the Pescara river valley, Italy (Table 7). This valley is a typical piedmont-hilly zone of the central-eastern Apennines, which originates from the ecotone between the Gran Sasso and Maiella-Morrone massifs, in the northern and southern part, respectively, and declines within 45 km in an extended drainage basin to the Adriatic coast. The basin area expresses the Mediterranean bi-climate characters typical of the Adriatic coast with dry summer characterized by law rainfall. The total extension of the experimental crops were 24,000 square meters on level ground, with a mean slope lower than 5% and altitude range 70–100 m asl. The planting scheme was in lines with a central 2 m irrigation strip widths.
Table 7.
Characteristics of the experimental fields.
| EO | GPS Coordinates | Extension (sqm) | Previous Crops (2 Years) |
Sowing Scheme (cm Inter/Intra Lines) |
Sowing Density |
|---|---|---|---|---|---|
| F75 | 42.363059, 14.093390 | 4000 | Wheat/alfalfa | 20 cm/50 cm | 15 Kg/ha |
| CS | 42.359222, 14.108528 | 14,000 | Wheat/ Wheat | 40 cm/50 cm | 20 Kg/ha |
| EC | 42.343824, 14.102808 | 6000 | Not cultivated | 20 cm/40 cm | 20 Kg/ha |
EO: essential oil; EC: Eletta campana; CS: Carmagnola selezionata; and F75: Futura 75.
In order to improve the development of plants according to the high temperature and long daylight, the sowings were performed between mid and late March 2019, with a prevision to reach a scalar blooming stage, ready for harvesting, in late August/September. The cultivar Eletta campana is a dioecious Italian selection deriving from original crops of the early 1900 s, largely cultivated in the south of Italy for fiber production. It is in the European community list of varieties admitted for cultivation and its agronomic needs fit with the Mediterranean environment. It is considered to be a late-flowering cultivar that produces a high amount of biomass, due to the large leaf surface and compact smelling inflorescences, endowed with a rich presence of terpenes. The cultivar name refers to Campania region, and the selected cultivar is largely cultivated throughout central and south Italy. Similarly, Carmagnola selezionata is a dioecious cultivar originally selected for the production of fiber. The mature plants can grow over 6 meters but are also adapted for seeds and flowers production. Futura 75 is a French selection of monoecious variety for seeds and biomass production, characterized by high adaptability for multipurpose cultivation. Previous experiences of cultivation confirmed the suitability of this cultivar for local environmental conditions, also in central Italy [8]. All selected seeds are present in the plant variety database of the European Commission in the agricultural species list. The genus Cannabis and specifically the selected cultivars are characterized by high adaptability, and based on previous experiences, no specific agronomical practices were planned but just a superficial soil preparation (ploughing, disc harrowing, and milling), automated sowing for optimized seed density, no fertilization, and only three programmed automated irrigation cycles. During the phase of flowering, an accurate manual collection of only mature flowers was done. The collection was repeated daily to obtain only high-quality mature flowers. Fresh plant material was immediately transferred to the laboratory and extracted within 6 hours. Random samples from collections were selected and botanical identity was confirmed by classical taxonomical approach (done by Prof. L. Menghini, Full Professor in Pharmaceutical Botany at the Department of Pharmacy, “G. d’Annunzio” University, Chieti, Italy). Fresh inflorescences were distributed to obtain a homogeneous dispersion in the distillation chamber in a stainless-steel distillatory Clevenger-type apparatus (Albrigi Luigi S.r.l., Stallavena, Italy). The volumetric EO yields (%, v/w) were determined and expressed for phytochemical (mL in plant fresh weight) or agronomical (mL from hectare) interpretations. Samples of EOs to be used for analytical characterization and biological tests were dehydrated through a passage in anhydrous sodium sulfate and stored in a dark glass bottles with a PTFE septa cap at 4 °C until used.
3.2. HPLC–UV-MS Determination of Terpenophenolic Compounds
Industrial hemp EOs were analyzed for terpenophenol quantitative determination using a reversed-phase HPLC–UV-MS in gradient elution mode. HPLC: the separation was conducted within 30 minutes, starting from the following conditions: 0.007% formic acid, 7% water, and 93% acetonitrile. The details about gradient are listed in Table 8. The separation was performed on an Infinity lab Poroshell 120 reverse phase column (C18, 150 mm × 4.6 mm i.d., 2.7 µm) (Agilent Santa Clara, CA, USA). Column temperature was set at 30 °C. The EOs were qualitatively analyzed with MS detector in positive ion mode. MS signal identification was realized through comparison with standard solutions and MS spectra present in the MassBank Europe database (https://massbank.eu/MassBank/). Specifically, the mass-to-charge ratio (m/z), the wavelength, and retention times considered for compound identification are listed in Table 9.
Table 8.
Gradient elution of HPLC–UV-MS.
| Time (min) | Flow (mL/min) | %A | %B |
|---|---|---|---|
| 0 | 0.750 | 32.5 | 67.5 |
| 0.5 | 0.750 | 32.5 | 67.5 |
| 14 | 0.750 | 7 | 93 |
| 22 | 0.750 | 7 | 93 |
| 22.1 | 1.05 | 32.5 | 67.5 |
| 28 | 1.05 | 32.5 | 67.5 |
| 28.1 | 0.750 | 32.5 | 67.5 |
| 30 | 0.750 | 32.5 | 67.5 |
Table 9.
Mass-to-charge ratios (m/z) and retention times related to the investigated terpenophenolic compounds.
| Standard | m/z | Wavelength (nm) | Retention Time (min) | |
|---|---|---|---|---|
| 1 | CBDA | 357.3 | 230 | 12.4 |
| 2 | CBGA | 343.3–260.1 | 230 | 13.6 |
| 3 | CBG | 317.3–234.1–193.1 | 230 | 15.4 |
| 4 | CBD | 315.2 | 230 | 15.6 |
| 5 | CBN | 311.3–293.25 | 230 | 20.0 |
| 6 | THC-d3 | 318.9; [315.2: EO pool of THC] | 230 | 20.6 |
| 7 | CBC | 315.3–259.13–193.13 | 230 | 21.2 |
| 8 | THCA | 341.3 | 230 | 22.5 |
3.3. Antityrosinase Activity
The enzyme inhibition toward tyrosinase was conducted with colorimetric assay, as well, and expressed as kojic acid equivalents. The experimental procedures for all these assays were comprehensively described in our previous study [35].
3.4. Antimycotic Activities
The industrial hemp EOs were assayed for antimycotic effects against different dermatophytes species: Trichophyton mentagrophytes (CCF 4823), Trichophyton rubrum (CCF 4879), Trichophyton rubrum (CCF 4933), Arthroderma crocatum (CCF 5300), Arthroderma quadrifidum (CCF 5792), Arthroderma gypseum (CCF 6261), Arthroderma curreyi (CCF 5207), and Arthroderma insingulare (CCF 5417). The detailed protocol of the present antimycotic evaluations is fully described in recent published papers of ours [36]. The antimycotic effects were compared to the reference drug griseofulvin.
3.5. Ex Vivo Model of Hydrogen-Peroxide-Induced Toxicity in Isolated Mouse Skin Tissue
Twenty male adult C57BL6 mice (20–25 g) were sacrificed by CO2 inhalation (100% CO2 at a flow rate of 20% of the chamber volume per min), and skin specimens were immediately collected from the back of the animals and maintained in a humidified incubator with 5% CO2 at 37 °C for 4 h, in DMEM buffer with the addition of hydrogen peroxide (1 mM). The experimental procedure was approved by the Italian Ministry of Health (Authorization Number: F4738.N.5QP). During the incubation period, skin specimens were stimulated with different industrial hemp EOs (0.125 μL/mL). Tissue supernatants were collected, and the PGE2 level (ng/mg wet tissue) was measured by radioimmunoassay (RIA), as previously reported [37].
3.6. Cell Culture
The human H1299 lung adenocarcinoma cell line was cultured with RMPI-16140 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated at 37 °C with 5% CO2. The H1299 cell line was purchased from ATCC (Manassas, VA, United States). In the experiments, cells were seeded into 6 well culture plates, kept ad concentration of 2 × 105 cells/mL, and after 24 h, were treated with different concentrations of essential oils for 24 h at the final concentrations reported in the figures.
3.7. Real-Time Reverse Transcription Polymerase Chain Reaction (Real-Time RT PCR)
Total RNA was extracted from cells by RNeasy Plus Micro Kit (Qiagen), and generation of cDNA was performed in triplicate using a QuantiTect reverse transcription kit (Qiagen). All reactions were performed using an ABI-7300 real-time Cycler, and amplification was performed using TaqMan Assay (Hs01085333 for ACE2, Hs01122322 for TMPRSS, HS 00608023 for Bcl-2, HS 00998133 for TGFβ, and eukaryotic 18S rRNA as an endogenous control).
3.8. Bioinformatics
Docking calculations were conducted through the Autodock Vina of PyRx 0.8 software, as recently described [38,39]. Crystal structures of target proteins were derived from the Protein Data Bank (PDB) with PDB ID as follows: 1R4L (inhibitor-bound human angiotensin-converting enzyme-related carboxypeptidase (ACE2)). Discovery studio 2020 visualizer was employed to investigate the protein–ligand nonbonding interactions.
3.9. Statistical Analysis
The statistical significance of difference between controls and experimental groups was evaluated using one-way analysis of variance (ANOVA). Results were expressed as means ± SD (standard deviation). Statistical analysis was performed using GraphPad Prism™ (Version 6.01) software (GraphPad Software, San Diego, CA, USA). A p-value < 0.05 was considered as statistically significant.
4. Conclusions
In conclusion, the present findings highlight the rationale for improving the whole botanical chain of the industrial hemp, considering the inflorescence by-product as a valuable source of biomolecules with pharmacological applications. Specifically, the present study aims to be a starting point for the development of innovative topical pharmaceutical preparations and devices. Indeed, the industrial hemp EOs could be included in dermatological formulations for counteracting the inflammatory burden of oxidative stress and inflammation occurring during dermatophytes infections. Additionally, considering the inhibitory effects on ACE2 and TMPRSS2 gene expression, we hypothesize the inclusion of the present EOs in protection devices, such as chirurgical masks, functioning as physical barriers against COVID-19.
Acknowledgments
The authors would like to express their gratitude to Stefania Cesa and her research group at the Department of Drug Chemistry and Technology, Sapienza University of Rome, for the very kind assistance in the GC–MS analysis. The present study is also part of the third mission activities of the Botanic Garden “Giardino dei Semplici” planned for the 20th anniversary of the establishment.
Author Contributions
Conceptualization, G.O., C.F., L.M., D.D., and L.B.; methodology, C.F. and M.T.; software, L.M. and C.F.; validation, C.F., L.M., G.O., and M.T.; formal analysis, C.F. and G.Z.; investigation, S.A., A.C., S.C.D.S., L.R., S.L., A.A.; M.A., M.D.A, P.A., G.A.F., and R.V.; resources, G.O., L.M., and C.F.; data curation, C.F., G.O., and L.M.; writing—original draft preparation, C.F.; writing—review and editing, C.F., G.O., L.M., and M.T.; visualization, L.B.; G.Z., D.D., P.A., and R.V.; supervision, L.B.; project administration, L.M., G.O., and C.F.; funding acquisition, G.O., C.F., and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
The study was conducted within the joint project between Department of Pharmacy of “G. d’Annunzio” University and Veridia S.r.l. (Research Program 2020-2023). Study coordinators were Giustino Orlando, Luigi Menghini, and Claudio Ferrante.
Institutional Review Board Statement
The experiments were approved by the Local Ethical Committee (University “G. d’Annunzio” of Chieti-Pescara) and the Italian Health Ministry (authorization number: F4738.N.5QP).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Montserrat-de la Paz S., Marín-Aguilar F., García-Gimenez M.D., Fernández-Arche M. Hemp (Cannabis sativa L.) seed oil: Analytical and phytochemical characterization of the unsaponifiable fraction. J. Agric. Food Chem. 2014;62:1105–1110. doi: 10.1021/jf404278q. [DOI] [PubMed] [Google Scholar]
- 2.Vonapartis E., Aubin M.-P., Seguin P., Mustafa A.F., Charron J.-B. Seed composition of ten industrial hemp cultivars approved for production in Canada. J. Food Compos. Anal. 2015;39:8–12. doi: 10.1016/j.jfca.2014.11.004. [DOI] [Google Scholar]
- 3.Citti C., Linciano P., Russo F., Luongo L., Iannotta M., Maione S., Laganà A., Capriotti A.L., Forni F., Vandelli M.A., et al. A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci. Rep. 2019;9:20335. doi: 10.1038/s41598-019-56785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiralan M., Gül V., Metin Kara S. Fatty acid composition of hemp seed oils from different locations in Turkey. Span. J. Agric. Res. 2010;8:385–390. doi: 10.5424/sjar/2010082-1220. [DOI] [Google Scholar]
- 5.Amaducci S., Scordia D., Liu F., Zhang Q., Guo H., Testa G., Cosentino S. Key cultivation techniques for hemp in Europe and China. Ind. Crop. Prod. 2015;68:2–16. doi: 10.1016/j.indcrop.2014.06.041. [DOI] [Google Scholar]
- 6.Bertoli A., Tozzi S., Pistelli L., Angelini L.G. Fibre hemp inflorescences: From crop-residues to essential oil production. Ind. Crop. Prod. 2010;32:329–337. doi: 10.1016/j.indcrop.2010.05.012. [DOI] [Google Scholar]
- 7.Fernández-Ruiz J., Sagredo O., Pazos M.R., García C., Pertwee R., Mechoulam R., Martínez-Orgado J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013;75:323–333. doi: 10.1111/j.1365-2125.2012.04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zengin G., Menghini L., Di Sotto A., Mancinelli R., Sisto F., Carradori S., Cesa S., Fraschetti C., Filippi A., Angiolella L., et al. Chromatographic Analyses, In Vitro Biological Activities, and Cytotoxicity of Cannabis sativa L. Essential Oil: A Multidisciplinary Study. Molecules. 2018;23:3266. doi: 10.3390/molecules23123266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrante C., Recinella L., Ronci M., Menghini L., Brunetti L., Chiavaroli A., Leone S., Di Iorio L., Carradori S., Tirillini B., et al. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019;125:452–461. doi: 10.1016/j.fct.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Orlando G., Recinella L., Chiavaroli A., Brunetti L., Leone S., Carradori S., Di Simone S., Ciferri M.C., Zengin G., Ak G., et al. Water Extract from Inflorescences of Industrial Hemp Futura 75 Variety as a Source of Anti-Inflammatory, Anti-Proliferative and Antimycotic Agents: Results from In Silico, In Vitro and Ex Vivo Studies. Antioxidants. 2020;9 doi: 10.3390/antiox9050437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Giacomo V., Recinella L., Chiavaroli A., Orlando G., Cataldi A., Rapino M., Di Valerio V., Politi M., Antolini M.D., Acquaviva A., et al. Metabolomic Profile and Antioxidant/Anti-Inflammatory Effects of Industrial Hemp Water Extract in Fibroblasts, Keratinocytes and Isolated Mouse Skin Specimens. Antioxidants. 2021;10:44. doi: 10.3390/antiox10010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B., Kovalchuk A., Li D., Rodriguez-Juarez R., Ilnytskyy Y., Kovalchuk I., Kovalchuk O. In search of preventive strategies: Novel high-CBD Cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. Aging. 2020;12:22425–22444. doi: 10.18632/aging.202225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anil S.M., Shalev N., Vinayaka A.C., Nadarajan S., Namdar D., Belausov E., Shoval I., Mani K.A., Mechrez G., Koltai H. Cannabis compounds exhibit anti-inflammatory activity in vitro in COVID-19-related inflammation in lung epithelial cells and pro-inflammatory activity in macrophages. Sci Rep. 2021;11:1462. doi: 10.1038/s41598-021-81049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regnault-Roger C., Vincent C., Arnason J.T. Essential oils in insect control: Low-risk products in ahigh-stakes world. Annu. Rev. Entomol. 2012;57:405–424. doi: 10.1146/annurev-ento-120710-100554. [DOI] [PubMed] [Google Scholar]
- 15.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: From vasopeptidase to SARS virus receptor. Trends Pharm. Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., et al. HCA Lung Biological Network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isa-Isa R., Arenas R., Isa M. Inflammatory tinea capitis: Kerion, dermatophytic granuloma, and mycetoma. Clin. Dermatol. 2010;28:133–136. doi: 10.1016/j.clindermatol.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M., Jiang L., Li F., Xu Y., Lv S., Wang B. Simultaneous dermatophytosis and keratomycosis caused by Trichophyton interdigitale infection: A case report and literature review. BMC Infect. Dis. 2019;19:1–8. doi: 10.1186/s12879-019-4612-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Zawawy N.A., Ali S.S. Pyocyanin as anti-tyrosinase and anti tinea corporis: A novel treatment study. Microb. Pathog. 2016;100:213–220. doi: 10.1016/j.micpath.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Kolbe L., Mann T., Gerwat W., Batzer J., Ahlheit S., Scherner C., Wenck H., Stäb F. 4-n-butylresorcinol, a highly effective tyrosinase inhibitor for the topical treatment of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol. 2013;27:19–23. doi: 10.1111/jdv.12051. [DOI] [PubMed] [Google Scholar]
- 21.Chiavaroli A., Di Simone S.C., Sinan K.I., Ciferri M.C., Angeles Flores G., Zengin G., Etienne O.K., Ak G., Fawzi Mahomoodally M., Jugreet S., et al. Pharmacological Properties and Chemical Profiles of Passiflora foetida L. Extracts: Novel Insights for Pharmaceuticals and Nutraceuticals. Processes. 2020;8:1034. doi: 10.3390/pr8091034. [DOI] [Google Scholar]
- 22.Dong Z., Zhang L., Xu W., Zhang G. EGFR may participate in immune evasion through regulation of B7-H5 expression in non-small cell lung carcinoma. Mol. Med. Rep. 2018;18:3769–3779. doi: 10.3892/mmr.2018.9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S., Ren J., Ten Dijke P. Targeting TGFβ signal transduction for cancer therapy. Transduct. Target. Ther. 2021;6:8. doi: 10.1038/s41392-020-00436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atalay S., Gęgotek A., Wroński A., Domigues P., Skrzydlewska E. Therapeutic application of cannabidiol on UVA and UVB irradiated rat skin. A Proteomic Study. J. Pharm. Biomed. Anal. 2021;192:113656. doi: 10.1016/j.jpba.2020.113656. [DOI] [PubMed] [Google Scholar]
- 25.Menghini L., Ferrante C., Carradori S., D’Antonio M., Orlando G., Cairone F., Cesa S., Filippi A., Fraschetti C., Zengin G., et al. Chemical and Bioinformatics Analyses of the Anti-Leishmanial and Anti-Oxidant Activities of Hemp Essential Oil. Biomolecules. 2021;11:272. doi: 10.3390/biom11020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheljazkov V.D., Sikora V., Semerdjieva I.B., Kačániová M., Astatkie T., Dincheva I. Grinding and Fractionation during Distillation Alter Hemp Essential Oil Profile and Its Antimicrobial Activity. Molecules. 2020;25:3943. doi: 10.3390/molecules25173943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagy D.U., Cianfaglione K., Maggi F., Sut S., Dall’Acqua S. Chemical Characterization of Leaves, Male and Female Flowers from Spontaneous Cannabis (Cannabis sativa L.) Growing in Hungary. Chem. Biodivers. 2019;16:e1800562. doi: 10.1002/cbdv.201800562. [DOI] [PubMed] [Google Scholar]
- 28.Yang D., Michel L., Chaumont J.P., Millet-Clerc J. Use of caryophyllene oxide as an antifungal agent in an in vitro experimental model of onychomycosis. Mycopathologia. 1999;148:79–82. doi: 10.1023/A:1007178924408. [DOI] [PubMed] [Google Scholar]
- 29.Chatatikun M., Supjaroen P., Promlat P., Chantarangkul C., Waranuntakul S., Nawarat J., Tangpong J., Chiabchalard A. Antioxidant and tyrosinase inhibitor properties of an aqueous extract og Garcinia atrovirdis Griff. Ex. T. Anderson fruit pericarps. Pharm. J. 2020;12:71–78. [Google Scholar]
- 30.Pillaiyar T., Manickam M., Namasivayam V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017;32:403–425. doi: 10.1080/14756366.2016.1256882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuster N., Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res. 2002;307:1–14. doi: 10.1007/s00441-001-0479-6. [DOI] [PubMed] [Google Scholar]
- 32.Senichkin V.V., Pervushin N.V., Zuev A.P., Zhivotovsky B., Kopeina G.S. Target. Bcl-2 Fam. Proteins: WhatWhereWhen? Biochemistry. 2020;85:1210–1226. doi: 10.1134/S0006297920100090. [DOI] [PubMed] [Google Scholar]
- 33.Zarubin A., Stepanov V., Markov A., Kolesnikov N., Marusin A., Khitrinskaya I., Swarovskaya M., Litvinov S., Ekomasova N., Dzhaubermezov M., et al. Structural Variability, Expression Profile, and Pharmacogenetic Properties of TMPRSS2 Gene as a Potential Target for COVID-19 Therapy. Genes. 2020;12:E19. doi: 10.3390/genes12010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussain M., Jabeen N., Amanullah A., Baig A.A., Aziz B., Shabbir S., Raza F., Uddin N. Molecular docking between human TMPRSS2 and SARS-CoV-2 spike protein: Conformation and intermolecular interactions. Aims Microbiol. 2020;6:350–360. doi: 10.3934/microbiol.2020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiavaroli A., Recinella L., Ferrante C., Locatelli M., Macchione N., Zengin G., Leporini L., Leone S., Martinotti S., Brunetti L., et al. Crocus sativus, Serenoa repens and Pinus massoniana extracts modulate inflammatory response in isolated rat prostate challenged with LPS. J. Biol. Regul. Homeost. Agents. 2017;31:531–541.15. [PubMed] [Google Scholar]
- 36.Ferrante C., Angelini P., Venanzoni R., Angeles Flores G., Tirillini B., Recinella L., Chiavaroli. A., Brunetti L., Leone S., Di Simone S.C., et al. Antimicrobial, Antioxidant, and Antiproliferative Effects of Coronilla minima: An Unexplored Botanical Species. Antibiotics. 2020;9 doi: 10.3390/antibiotics9090611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menghini L., Ferrante C., Leporini L., Recinella L., Chiavaroli A., Leone S., Pintore G., Vacca M., Orlando G., Brunetti L. An Hydroalcoholic Chamomile Extract Modulates Inflammatory and Immune Response in HT29 Cells and Isolated Rat Colon. Phytother. Res. 2016;30:1513–1518. doi: 10.1002/ptr.5655. [DOI] [PubMed] [Google Scholar]
- 38.Angelini P., Venanzoni R., Angeles Flores G., Tirillini B., Orlando G., Recinella L., Chiavaroli A., Brunetti L., Leone S., Di Simone S.C., et al. Evaluation of Antioxidant, Antimicrobial and Tyrosinase Inhibitory Activities of Extracts from Tricholosporum goniospermum, an Edible Wild Mushroom. Antibiotics. 2020;9 doi: 10.3390/antibiotics9080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrante C., Chiavaroli A., Angelini P., Venanzoni R., Angeles Flores G., Brunetti L., Petrucci M., Politi M., Menghini L., Leone S., et al. Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts. Antibiotics. 2020;9:783. doi: 10.3390/antibiotics9110783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.