Abstract
The practice of transplanting hepatitis C (HCV)-infected livers into HCV-uninfected recipients has not previously been recommended in transplant guidelines, in part because of concerns over uncontrolled HCV infection of the allograft. Direct-acting antivirals (DAAs) provide an opportunity to treat donor derived HCV-infection, and should be administered early in the post-transplant period. However, evidence on the safety and efficacy of an immediate DAA treatment approach, including how to manage logistical barriers surrounding timely DAA procurement, are required prior to broader use of HCV-positive donor organs. We report the results of a trial in which fourteen HCV-negative patients underwent successful liver transplantation from HCV-positive donors. Nine patients received viremic (nucleic acid testing (NAT)-positive) livers, and started a 12-week course of oral glecaprevir-pibrentasvir (GP) within 5 days of transplant. Five patients received livers from HCV antibody-positive non-viremic donors and were followed using a reactive approach. Survival in NAT-positive recipients is 100% at a median follow-up of 46 weeks. An immediate treatment approach for HCV NAT-positive liver transplantation into uninfected recipients is safe and efficacious. Securing payer approval for DAAs early in the post-transplant course could enable need-based allocation of HCV-positive donor organs irrespective of candidate HCV status, while averting chronic HCV allograft infection.
1. INTRODUCTION
Management of end-stage organ disease has increased in sophistication during the past decade, leading to improved survival rates and a larger pool of transplant candidates. However, there remains a serious deficit in the availability of organs deemed suitable for transplantation. These factors have resulted in progressively longer transplant waiting times (1). Maximizing utility of available organs, while optimizing post-transplantation outcomes, is therefore a pressing concern within the transplant community.
The ongoing opioid epidemic has resulted in a surge of overdose fatalities, and persons who inject drugs are currently the fastest growing category of organ donor (2,3). A parallel increase in intravenous drug use-related hepatitis C virus (HCV) infection has been demonstrated in this population. HCV-infected organs have traditionally been restricted for transplantation into HCV-positive recipients, with studies showing patient and graft survival rates comparable to those observed after receipt of HCV-negative livers (4–9). Prior practice guidelines have recommended against transplanting livers from HCV-positive donors into HCV-negative recipients, due to concern for HCV transmission and potential complications including accelerated allograft hepatitis, premature graft failure or death (10,11). These guidelines were conceived during the era of traditional pegylated interferon-based HCV regimens when efficacy was suboptimal, and undesirable side effects triggered high rates of treatment discontinuation. Direct-acting antivirals (DAAs) are highly effective and well-tolerated, with sustained virologic response (SVR) rates much higher (>95%) than previously observed with interferon-based regimens (20–30%) (12). In the transplant population, DAAs have been shown to effectively induce SVR in HCV-negative recipients transplanted with viremic organs, when administered as part of a reactive treatment approach, after detection of viremia (13–21). Preemptive or early treatment with DAAs, prior to development of viremia, reduces recipient risk and has been recently evaluated in a small number of studies involving kidney, heart and lung transplantation (22–24). However, the challenge of obtaining insurance coverage for preemptive or early treatment has impeded efforts to implement this approach outside of industry-sponsored trials. A similar early treatment strategy has not previously been evaluated in liver recipients.
We characterize 14 HCV-negative patients at a single center who successfully underwent liver transplantation with HCV-positive donor organ(s). This is the largest study of HCV donor-positive to recipient-negative liver transplantation that includes viremic donors, utilizes an immediate DAA treatment strategy, and evaluates the efficacy of glecaprevir-pibrentasvir (GP). We discuss the logistical barriers (insurance approval of preemptive or immediate DAA therapy) that currently preclude this strategy from being safely implemented, and call for a shift in payer approach to allow this to become standard of care.
2. MATERIALS AND METHODS
Trial Design and Oversight
This non-industry funded trial was conceived and undertaken via the collective efforts of members of the Massachusetts General Hospital Transplant Center, including Hepatology, Renal, Infectious Diseases, and Transplant Surgery Divisions, together with Inpatient and Outpatient Pharmacy Departments and Core Laboratory Support Services, with a view to optimize organ allocation strategies and improve care amongst recipients of HCV-positive donor organs.
Our trial (CT.gov NCT03208127) was an open-label, unblinded single-center trial commencing July 2018; we now present the results of an interim analysis. The trial was approved by the Massachusetts General Hospital and Partners Human Research Committee Institutional Review Board and conformed to Good Clinical Practice guidelines and the Declaration of Helsinki. Institutional Review Board protocol number is 2017P000653. Written informed consent was obtained from each patient. No donor organs were obtained from executed prisoners or other institutionalized persons. All authors vouch for the integrity, completeness, and accuracy of the data and analyses and assume responsibility for the fidelity of the trial to the protocol and statistical analysis plan (see data supplement). This trial was conducted in collaboration with New England Donor Services, our regional arm of the United Network for Organ Sharing.
Patient Population and Treatment
Commencing in July 2018, all patients at our center who were undergoing evaluation for liver transplant or registered on the transplant waitlist were screened for trial participation. Patients in need of dual heart-liver transplant were excluded. Patients requiring dual liver-kidney transplant were eligible for inclusion following comprehensive review by the study team’s transplant nephrologist and hepatologist. Patients awaiting retransplantation were also considered for enrollment. Following trial recruitment, United Network for Organ Sharing system entries were updated to indicate each enrolled patient’s status as being open to receipt of an HCV-positive organ. Donor inclusion criteria required a positive test for HCV antibody with or without positive NAT. All accepted donor organs had to otherwise meet standard donor selection criteria, and the management of their procurement was identical to that for HCV-negative donor organs at our center.
If an enrolled patient received an organ offer from an HCV-negative donor, they proceeded with liver transplantation according to the standard center protocol. Recipients of livers from NAT-positive (viremic) donors were administered GP under close observation once adequate graft function was demonstrated in the form of an improving international normalized ratio less than 1.4 and a total bilirubin less than 10 (or less than 50% pre-transplant value within 12 to 24 hours post-transplantation). Each patient then completed a 12-week course of GP (Figure 1a). HCV RNA assays were regularly obtained throughout the course of GP treatment and following treatment completion, to confirm adequate viral suppression and attainment of SVR. The primary endpoint was SVR12 (negative HCV RNA 12 weeks after completion of antiviral therapy). If an enrolled patient received an offer for an HCV antibody-positive donor without detectable circulating virus by NAT (non-viremic), they underwent transplantation and were then followed with a reactive treatment approach and started on GP therapy only if they developed viremia during serial surveillance at days 0, 3, 7, 14, and 28 after transplantation and every 4 weeks thereafter, until week 52 (Figure 1b).
Figure 1. a: Immediate treatment group.
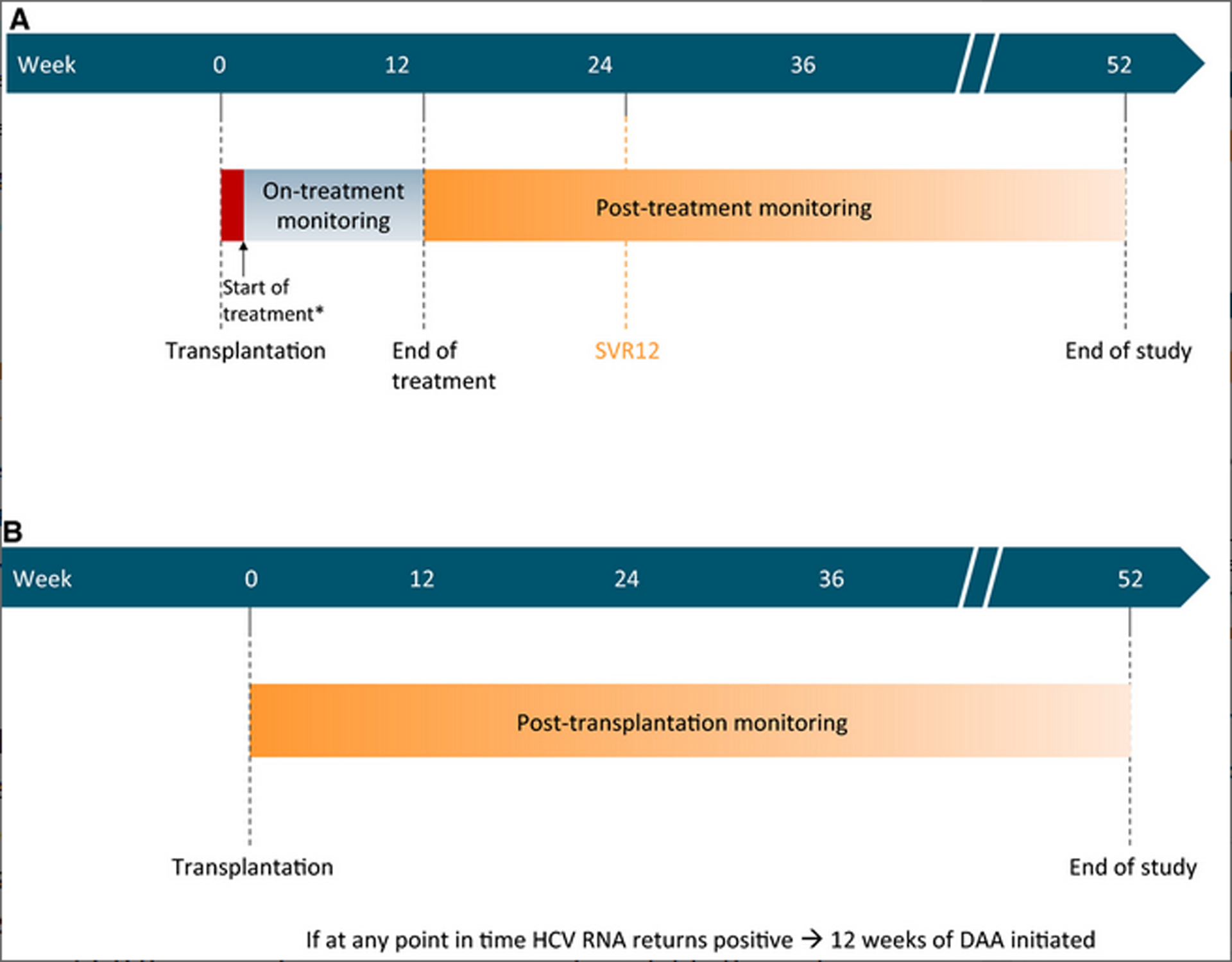
SVR = sustained virologic response 12 weeks after completion of treatment. DAA = direct-acting antiviral. *Treatment was commenced as soon as feasible after transplantation (1–5 post-operative days)
b: Reactive treatment group
SVR = sustained virologic response 12 weeks after completion of treatment. DAA = direct-acting antiviral.
DAA therapy was administered orally as co-formulated glecaprevir (300mg)/pibrentasvir (120mg) for 12 weeks post-transplant. No dose adjustments were required for renal dysfunction. Risks for drug-drug interactions were reviewed on an individual basis. Where potential for interaction was identified, vigilant monitoring formed the mainstay of management, together with dose and/or timing modifications as required. This routinely included evaluation of proton pump inhibitors and certain HMG-CoA reductase inhibitors (statins), as outlined in the GP standard package insert recommendations. For patients unable to take medications orally within 24 hours of transplantation, GP was crushed and placed into suspension to facilitate administration via nasogastric or orogastric tube.
Immunosuppression.
Patients were treated with a combination of mycophenolate mofetil, tacrolimus, and glucocorticoids, per our center’s liver transplant protocol. Doses of immunosuppressants were individually tailored based on serum drug levels per routine clinical practice.
DAA Therapy Procurement.
To obtain coverage of DAA therapy, a team of study staff (including MDs and PharmDs) applied to payers on behalf of transplant recipients and completed an appeals procedure if the initial application was denied. The hospital and transplant center administration were directly involved in protocol development and provided key financial support, including agreement to: 1) purchase GP and register it on the inpatient formulary exclusively for inpatient study use, and 2) provide a safety-net backstop for study patients, with an agreement to cover the cost of a course of GP in the event coverage was denied by the insurance provider.
Analysis, Endpoints, and Assessments
The primary endpoint of the study was achievement of SVR12. Patients underwent serial viral load measurements to gauge response to antiviral therapy. An initial HCV viral load was obtained at the time of transplantation, with subsequent measurements on post-operative days 1, 3, 7, 14, 21, and 28, followed by 4-week intervals until attainment of SVR12, and subsequently at 8 to 12-week intervals until week 52. Key secondary endpoints included allograft function, as assessed by liver function tests, and overall patient survival. Additional secondary endpoints included rates of HCV or DAA-related adverse events and GP discontinuation.
Recruitment of patients to our trial began in April 2018. Enrolled patients remain on study protocol until 52 weeks post-transplant. Enrollment of new trial patients is ongoing. Fourteen protocol patients have undergone transplant with an HCV-positive donor. A decision was made to undertake interim analysis in September 2019, when all 9 NAT-positive recipients had achieved the SVR12 primary endpoint. Data presented reflects follow-up through September 20, 2019.
Statistical analysis
Data collection and analysis were performed after obtaining informed patient consent and approval from the institutional review board. Data collected includes: (i) pre-transplant liver function and HCV serology status (ii) details of donor and recipient HCV testing and treatment (iii) details of recipient post-transplant course, including immunosuppression, rejection, and graft function. Descriptive analysis was performed for various patient characteristics. Summary statistics for continuous variable are reported as mean (standard deviation) or median (interquartile range). Statistical analyses were performed using STATA version 15 (College Station, TX). Data presented reflect follow up through September 20, 2019.
Role of funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
3. RESULTS
From July 2018, 14 patients underwent liver transplantation with HCV-positive donor livers, 4 of whom underwent simultaneous liver-kidney transplant (Table 1). Five livers were procured from HCV antibody-positive/NAT-negative donors, and the remainder were from NAT-positive (viremic) donors. Ten patients received GP for the treatment of donor derived HCV infection: nine NAT-positive recipients and one NAT-negative recipient who developed viremia post-transplant. Transplantation using NAT-positive livers followed by immediate treatment with GP was associated with recipient peak viral loads ranging from unquantifiable levels to 70,500,000 IU/mL. Average time to GP initiation was 1.7 days after transplant, and the longest delay in GP initiation was 5 days post-transplant. Total bilirubin and international normalized ratio values at the time of GP initiation ranged from 0.5 to 10.3 mg/dL and 1.1 to 1.8, respectively. Total bilirubin values continued to decline notwithstanding GP initiation (Figure 2). Median time to undetectable or unquantifiable viral load in NAT-positive liver recipients was 15 days (interquartile range 7 to 26 days). All nine NAT-positive recipients are ≥12 weeks post treatment completion and have achieved the primary endpoint of SVR12 (Table 2).
Table 1:
Baseline Characteristics
| Characteristic | HCV-positive liver transplant recipients, N=14 |
|---|---|
| Age, years | 59 (29–70) |
| Sex | |
| Male | 8 (57%) |
| Female | 6 (43%) |
| Height, m | 1.72 (1.55–1.88) |
| Weight, kg | 95.7 (51.3–121.1) |
| Blood Group | |
| O | 6 (43%) |
| A | 6 (43%) |
| B | 1 (7%) |
| AB | 1 (7%) |
| Overall waitlist time, days | 260 (4–4181) |
| Waitlist time after consent, days | 69.5 (1–448) |
Values are median (IQR) or n (%). Non-study enrolled patients who received an HCV-negative liver at our institution during 2018, and between Jan 1 and June 30, 2019, had an overall waitlist time (days) of 259 (1–5330)
Figure 2: Trends in liver function in HCV-negative recipients of an HCV-positive liver: Pre- and post-transplantation.
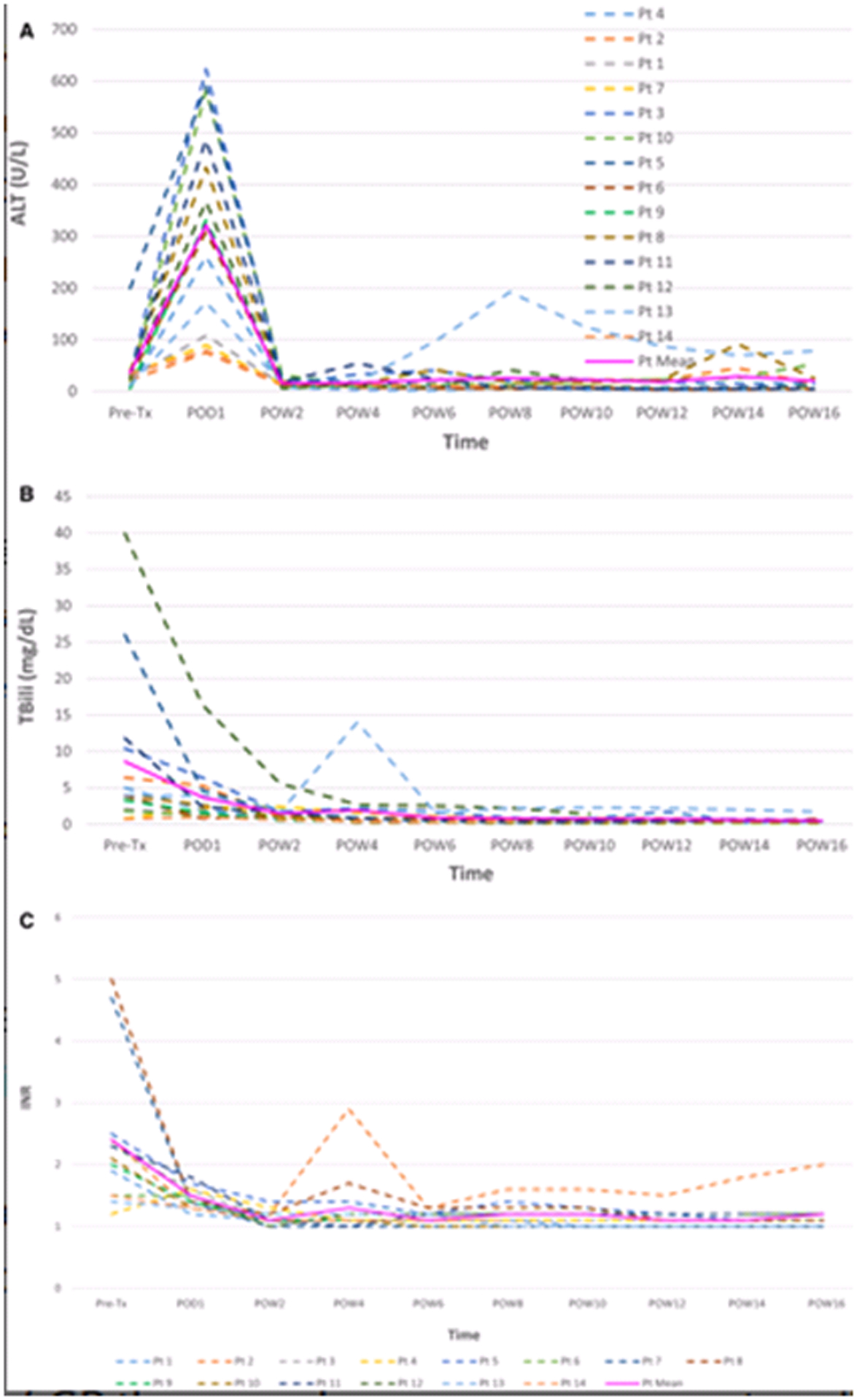
ALT = alanine aminotransferase. Tx = transplant. POD = post-operative day. POW = post-operative week. Tbili = total bilirubin. INR = international normalized ratio. Pt = patient. The elevated ALT readings in Pt 13 are attributable to an episode of acute cellular rejection which was successfully managed with increased immunosuppression. The elevated INR reading during POW4 in Pt 2 is a consequence of restarting Coumadin treatment.
Table 2:
Characteristics of HCV-positive organ recipients, HCV-positive donor organs, and recipient treatment responses
| Case | Indication for transplantation | Natural MELD Score | MELD Exception | eGFR (mL/min/1.73m2) | Time from HCV+ consent to transplantation (days) | Donor NAT | Donor GT | Donor VL (IU/mL) | Time to GP initiation post-transplantation (days) | Peak recipient VL (IU/mL) | Time to UQ/UD VL (days) | EOT Achieved | SVR12† | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At transplantation listing | At study enrollment | At transplantation | |||||||||||||
| 1 | NASH | 19 | 30 | 30 | NA | <10* | 24 | positive | 1a | 840,000 | 2 | UQ | 0 | Yes | Yes |
| 2 | ARLD | 20 | 24 | 40 | NA | <10* | 127 | positive | 1a | 870 | 0 | 1,330,000 | 16 | Yes | Yes |
| 3 | NASH | 29 | 31 | 34 | NA | <10* | 14 | positive | 3 | 6,100,000 | 1 | 1820 | 19 | Yes | Yes |
| 4 | ARLD/NASH | 21 | 29 | 35 | NA | <10* | 174 | negative | NA | UD (NAT-) | NA | UD (NAT-) | NA | NA | NA |
| 5 | ALF (secondary to drug effect) | - | - | >40 | Status 1A | <10* | 0 | positive | 1a | 22,320,000 | 1 | 31,090,000 | 35 | Yes | Yes |
| 6 | HCV | 20 | 34 | 34 | NA | 26 | 12 | positive | 1a | 42,000 | 2 | 785,000 | 14 | Yes | Yes |
| 7 | NASH | 24 | 24 | 22 | NA | <10* | 215 | positive | 1a | 512,000 | 2 | 70,500,000 | 47 | Yes | Yes |
| 8 | ARLD | 40 | 29 | 30 | NA | 37 | 26 | positive | 3 | 660,000 | 3 | 899,000 | 28 | Yes | Yes |
| 9 | Chronic Budd-Chiari syndrome complicated by HCC | 11 | 28 | 28 | NA | 105 | 68 | negative | NA | UD (NAT-) | NA | UD (NAT-) | NA | NA | NA |
| 10 | NASH | 12 | 13 | 13 | NA | 44 | 155 | positive | 1a | 720,000 | 0 | 17,700 | 9 | Yes | Yes |
| 11 | PBC | 22 | 25 | 32 | NA | 128 | 71 | negative | NA | UD (NAT-) | NA | UD (NAT-) | NA | NA | NA |
| 12 | ARLD | 40 | 40 | 40 | NA | <10* | 2 | positive | 1a | 6,700 | 5 | 174 | 5 | Yes | Yes |
| 13 | NASH | 16 | 21 | 21 | NA | 37 | 272 | positive | 1a | 48,000 | 1 | 106,000 | 6 | Yes | Yes |
| 14 | PLD | 7 | 9 | 7 | 30 | 49 | 448 | negative | 2§ | UD (NAT-) | 29∥ | 28,098,307 (NAT-) | - | On treatment | On treatment |
ARLD=alcohol-related liver disease. NASH=non-alcoholic steatohepatitis. ALF=acute liver failure. HCV=hepatitis C virus. HCC=hepatocellular carcinoma. PBC=primary biliary cirrhosis. PLD=polycystic liver disease. MELD=model for end-stage liver disease. NA=not applicable. eGFR=estimated glomerular filtration rate. NAT=nucleic acid testing. GT=genotype. VL=viral load. UD=undetectable. GP=glecaprevir-pibrentasvir. UQ=unquantifiable. EOT=end of treatment. SVR=sustained virologic response.
On renal replacement therapy.
For all study patients, all viral load checks after initial suppression have returned undetectable, including checks that have occurred after SVR12.
Recipient developed viremia within 24 hours of transplantation, despite donor serum testing NAT-negative.
Recipient followed the reactive protocol, since donor serum tested NAT-negative. GP was initiated in response to detectable VL.
Insurance approval for GP coverage was successfully secured in seven of the nine NAT-positive recipients, and in the single NAT-negative recipient who developed viremia; however, obtaining payer approval early in the post-transplant course was a major challenge. Considerable effort was required for each patient determining the need to submit for urgent review, draft appeal letters, request peer-to-peer consultation, and work to overturn initial payer denials. A patient assistance grant and hospital financial support provided the funds necessary to cover the course of GP therapy when coverage was not available through insurance.
No GP drug reactions or interactions necessitated a lapse or cessation of GP or immunosuppressive therapy. No treatment-related or HCV-attributable adverse events have occurred to date. One NAT-positive liver recipient developed acute cellular rejection, which was substantiated by biopsy findings on post-operative day 50, and successfully managed with increased baseline immunosuppression. All study participants continue to exhibit broadly preserved allograft function, as assessed by serial liver function test and international normalized ratio readings (Table 3). The survival rate for the cohort stands at 100%, with a median of 46 weeks (range 20 to 76 weeks) follow up (Supplementary Figure 1).
Table 3:
Allograft Function
| Laboratory Parameter | POD0 | POD30 | POD90 |
|---|---|---|---|
| ALT | 351 (89–869) | 12 (4–54) | 15 (3–91) |
| AST | 850 (334–1518) | 12 (7–64) | 19 (6–93) |
| ALP | 58 (40–117) | 120 (64–387) | 96.5 (48–502) |
| Bilirubin (Total) | 3.1 (1.3–22.7) | 0.8 (0.3–2.5) | 0.45 (0.3–1.1) |
| Bilirubin (Direct) | 1.7 (0.6–19.6) | 0.4 (0.1–1.5) | 0.2 (0–1.5) |
| Albumin | 2.8 (1.8–4) | 3.3 (2.4–4.3) | 3.95 (2.5–4.7) |
| INR | 2.2 (1.5–5.1) | 1.1 (1–1.8) | 1.05 (1–1.7) |
Values are median (IQR). ALT=alanine aminotransferase. AST=aspartate aminotransferase. ALP=alkaline phosphatase. INR=international normalized ratio. POD=post-operative day.
4. DISCUSSION
We report the results of an open-label, unblinded prospective single-center trial: 14 HCV-negative candidates were transplanted with livers from HCV-seropositive donors, of which 9 were viremic (NAT-positive) and 5 non-viremic (NAT-negative). In the case of NAT-positive donation, immediate administration of DAA therapy was initiated within 5 days of transplantation. This treatment approach demonstrated excellent efficacy and allowed achievement of early viral suppression and successful eradication. The regimen was well-tolerated with no major GP-related adverse events or premature treatment discontinuation. Despite the combination of HCV infection and intensive immunosuppression, with early GP treatment initiation we did not experience any cases of HCV related complications such as fibrosing cholestatic hepatitis.
Glecaprevir’s excretion via the hepatic route, and the consequent potential for hepatic accumulation of intermediate compounds, have led to recommendations against use of GP in hyperbilirubinemia or decompensated cirrhosis in an effort to prevent hepatotoxicity. However, at present, no formal guidance has been issued with regards to a ‘safe threshold’ of bilirubin for administering GP in liver disease patients. In this regard, it is of interest that we observed no instances of hepatotoxicity arising from early use of GP in our protocol. GP was safely initiated at total bilirubin levels up to 10.3 mg/dL, shortly after transplantation in the setting of improving allograft function. At the time of trial initiation, the only available alternative pangenotypic DAA regimen was sofosbuvir-based and had not yet been approved for use in patients with limited renal function. The safety of sofosbuvir-based DAA regimens in patients with renal compromise has now been demonstrated (25), and given that these regimens have not been found to cause hepatotoxicity, we acknowledge that alongside GP, consideration should be given to use of sofosbuvir-based regimens in patients experiencing delayed recovery of liver graft function.
Our protocol utilized a 12-week course of DAA therapy. There are data to suggest that shorter (4–8 week) courses may be sufficient to achieve SVR in recipients of non-reservoir organs, such as heart, lung or kidney. Furthermore, Gupta et al successfully utilized an ultra-short, four-dose regimen to prevent HCV transmission in the setting of viremic kidney transplantation, with the initial dose administered during the 24 hours preceding transplant (26). Similarly, Feld et al showed that initiating combined GP and ezetimibe (an HCV entry blocker) prior to lung, heart, kidney or kidney-pancreas transplant, and continuing treatment for seven post-transplant days, led to prevention of quantifiable viremia in the majority of recipients, alongside rapid viral clearance in the remainder (27). However, no prior studies have looked specifically at shorter durations of therapy after liver transplantation. The 12 weeks of therapy used here proved adequate to induce viral clearance in all patients notwithstanding their concomitant use of immunosuppressive medications. The safety of limiting DAA duration to under 12 weeks requires further investigation, as we do not have the ability to extrapolate from other solid organ experiences given the nature of the liver as the reservoir organ. Additionally, commencing DAA therapy before transplanting a non-reservoir organ represents a ‘true preemptive’ approach, since HCV is transmitted via infected blood and not via the organ per se. It is unclear whether initiating DAA therapy prior to liver transplant, instead of in the early post-transplant course, will entail lower rates of viremia, more rapid viral clearance or improved clinical outcomes since the viremic liver serves as the site of viral replication.
Despite the lack of consensus regarding whether higher on-treatment viral loads predict poorer treatment responses (28,29), we observed uniform viral suppression with no post-treatment relapses.
Our findings build on a growing body of evidence that supports the efficacy and safety of utilizing DAAs as part of an immediate strategy in organ transplantation (22–24). This strategy involves administering treatment prior to, or within hours to days of transplantation, before HCV specific genotype and viral load information are available. Given that transplantation of livers from viremic donors entails a universal risk of HCV transmission, we believe early DAA initiation, in light of demonstrated safety, warrants serious consideration. Delaying the treatment of HCV-negative recipients transplanted with NAT-positive organs increases the risk for adverse outcomes. Kapila et al. reported the development of fibrosing cholestatic hepatitis in two HCV-negative patients transplanted with kidneys from viremic donors (30, 31). Those patients presented on post-operative day 3 and week 24, respectively, with deranged liver function tests and HCV viral loads >100,000,000 IU/mL. This prompted urgent initiation of DAA therapy. In addition, Wadei et al. reported the case of an HCV-negative patient transplanted with a liver from a viremic donor who was initially denied insurance coverage, delaying DAA administration until post-operative day 24 (32). The patient developed HCV‐induced membranous nephropathy on post-operative day 18, which necessitated dialysis. Renal function recovered once the HCV viral load became undetectable on DAA treatment, although significant proteinuria persisted. Hence, we believe it is imperative that HCV-negative recipients transplanted with NAT-positive organs receive immediate treatment with DAAs. In recipients of organs from HCV antibody-positive, NAT-negative donors, HCV transmission is not assured, and it is appropriate to employ a reactive treatment strategy, with plans to initiate prompt DAA therapy in the event that viremia occurs.
We report one biopsy-confirmed instance of acute cellular rejection, out of nine NAT-positive liver recipients who underwent immediate treatment with DAAs. During the same period, five HCV-infected patients at our institution underwent transplantation with livers from viremic donors, and two of these five patients experienced at least one episode of biopsy confirmed acute cellular rejection. Further data are needed to evaluate if altered rates of rejection are seen following transplant with an HCV-infected donor organ, and if early DAA administration impacts the risk of rejection among HCV-negative recipients of HCV-positive organs.
Significant logistical barriers currently impede early access to DAAs, and there is a lack of uniformity regarding the willingness of payers to support immediate therapy, despite the sound scientific rationale. DAA regimens remain expensive, and even when coverage is granted, lengthy approval processes may incur unacceptable delays in treatment commencement (33). Furthermore, we are seeing an increase in denials, with many payers beginning to cite documentation of disease chronicity (>6 months viremia) as a prerequisite to drug approval, arguing that a percentage of patients with acute infection may spontaneously clear the virus and negate the need for DAA therapy. In the highly immunosuppressed post-transplant setting, the likelihood of spontaneous clearance is exceedingly small, and the risk of waiting significant (34). This provides an example of broad policies put in place by payers that interfere with optimization of care for the individual.
Since our trial was not industry-sponsored, we were required to navigate the real-world challenges of obtaining DAA insurance coverage post-transplant. Timely insurance approval was made possible through the efforts of a dedicated team of study staff and required institutional support with an agreement to cover the cost of a bridge supply of GP as our team worked through appeals and overturned initial denials. Time to approval ranged from 24 hours to 10 days. A similar degree of time and resource expenditure may not be feasible in the setting of routine clinical practice. However, this treatment approach should not be abandoned on the basis of anticipated barriers to procurement of payer approval for DAA therapy in the immediate post-transplant setting. We instead advocate a system in which payers pre-approve DAA coverage for transplant candidates specifically in the event they receive an HCV NAT-positive organ. Under these conditions, timely access to post-transplant DAA therapy would be assured. Such an approach is a prerequisite to the broad and safe implementation of this practice as standard of care. The costs of life-saving therapies cannot be routinely absorbed by patients or by hospitals.
Given the relative dearth of prospectively-collected clinical and cost-effectiveness data, modelling studies have been conducted to demonstrate that the additional expenses incurred in supplying preemptive DAA therapy are offset when compared with the high costs of maintaining patients on transplant waitlists. In a recent study, readiness to accept an HCV-positive or HCV-negative liver versus only an HCV-negative liver entailed incremental cost-effectiveness ratios ranging from $56,100 to $91,700 per quality-adjusted life-year in patients with MELD score ≥ 22, indicating cost-effectiveness at the standard willingness-to-pay threshold of $100,000 per quality-adjusted life-year. This suggests that the curative potential of an inclusive treatment approach, which extends to coverage of donor-derived HCV infection, will outweigh higher upfront costs (35). These findings should inform updates to policies regarding reimbursement for treatment of donor-derived HCV infection.
Our study has several limitations. Despite considerable promise shown by our interim results, the long-term outcomes of transplanting viremic livers into HCV-negative recipients, including impact on the incidence of acute rejection, chronic rejection, and 1-year graft and patient survival rates, have yet to be established. However, based on the success with other solid organ HCV donor-positive to recipient-negative transplants, the expectation is that graft outcomes will be excellent in the setting of SVR12, supporting the generalizability of this approach (24,36). Continued patient monitoring will enable us to investigate these long-term outcomes.
CONCLUSION
We report the results of a non-industry sponsored trial, in which livers from HCV-seropositive (either NAT-positive or NAT-negative) donors were transplanted into HCV-negative recipients, with immediate administration of GP therapy. Short-term outcomes have been highly favorable. At present, insurance companies generally do not issue advanced approval for DAA coverage prior to receipt of an infected organ. Therefore, without supplementary funding for this trial, we would have been unable to guarantee patients prompt access to DAAs post-transplant. We believe that preapproval of DAA therapy for transplantation of NAT-positive livers into uninfected recipients represents a justifiable and crucial step for implementation of this practice as standard of care, and in turn deriving maximum utility from the pool of available donor livers.
Supplementary Material
Acknowledgments
This study was supported in part by the American Association for the Study of Liver Diseases (AASLD) Transplant Hepatology Career Development Award (EB), the AASLD Clinical and Translational Research Fellowship in Liver Disease (AA), National Institutes of Health (NIH) K24 DK078772 (RTC), NIH K23 DK117014 (MS) and the Massachusetts General Hospital Research Scholars Program (RTC).
Abbreviations
- GP
Glecaprevir-pibrentasvir
- NAT
Nucleic acid testing
- SVR
Sustained virologic response
- DAAs
Direct-acting antivirals
- HCV
Hepatitis C virus
Footnotes
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr. Chung received grant support to his institution from Gilead, AbbVie, Merck, Janssen, Boehringer, and Bristol Myers Squibb. Dr Sise received grant support from Gilead, AbbVie and Merck. The other authors have no conflicts of interest to disclose.
REFERENCES
- 1.OPTN Data accessed July 10, 2019. Available at https://optn.transplant.hrsa.gov/data/view-datareports/national-data/.
- 2.Levitsky J, Formica RN, Bloom RD et al. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. Am J Transplant 2017;17:2790–2802. [DOI] [PubMed] [Google Scholar]
- 3.Durand CM, Bowring MG, Thomas AG et al. The Drug Overdose Epidemic and Deceased-Donor Transplantation in the United States: A National Registry Study. Ann Intern Med 2018;168:702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vargas HE, Laskus T, Wang LF et al. Outcome of liver transplantation in hepatitis C virus-infected patients who received hepatitis C virus-infected grafts. Gastroenterology 1999;117:149–153. [DOI] [PubMed] [Google Scholar]
- 5.Testa G, Goldstein RM, Netto et al. Long-term outcome of patients transplanted with livers from hepatitis C-positive donors. Transplantation 1998;65:925–929. [DOI] [PubMed] [Google Scholar]
- 6.Marroquin CE, Marino G, Kuo PC et al. Transplantation of hepatitis C-positive livers in hepatitis C-positive patients is equivalent to transplanting hepatitis C-negative livers. Liver Transpl 2001;7:762–768. [DOI] [PubMed] [Google Scholar]
- 7.Saab S, Ghobrial RM, Ibrahim AB et al. Hepatitis C positive grafts may be used in orthotopic liver transplantation: A matched analysis. Am J Transplant 2003;3:1167–1172. [DOI] [PubMed] [Google Scholar]
- 8.Ballarin R, Cucchetti A, Spaggiari M et al. Long-term follow-up and outcome of liver transplantation from anti-hepatitis C virus- positive donors: A European multicentric case-control study. Transplantation 2011;91:1265–1272. [DOI] [PubMed] [Google Scholar]
- 9.Velidedeoglu E,Desai NM,Campos L et al. The outcome of liver grafts procured from hepatitis C-positive donors. Transplantation 2002;73:582–587. [DOI] [PubMed] [Google Scholar]
- 10.Berenguer M Treatment of hepatitis C after liver transplantation. Clin Liver Dis 2005;9:579–600. [DOI] [PubMed] [Google Scholar]
- 11.Berenguer M, Palau A, Aguilera V et al. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant 2008;8:679–687. [DOI] [PubMed] [Google Scholar]
- 12.Foster GSA. Treatment of HCV infection with pegylated interferons. Current Hepatitis Reports [serial online];2005;(2):49. Available from: Academic OneFile, Ipswich, MA. Accessed July, 2019. [Google Scholar]
- 13.Shah AP, Cameron A, Singh P et al. Successful treatment of donor-derived hepatitis C viral infection in three transplant recipients from a donor at increased risk for bloodborne pathogens. Transpl Infect Dis 2017;19. [DOI] [PubMed] [Google Scholar]
- 14.Khan B, Singer LG, Lilly LB et al. Successful Lung Transplantation From Hepatitis C Positive Donor to Seronegative Recipient. Am J Transplant 2017;17:1129–1131. [DOI] [PubMed] [Google Scholar]
- 15.Saberi B, Hamilton JP, Durand CM et al. Utilization of hepatitis C virus RNA–positive donor liver for transplant to hepatitis C virus RNA–negative recipient. Liver Transpl 2018;24:140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg DS, Abt PL, Blumberg EA et al. Trial of Transplantation of HCV-Infected Kidneys into Uninfected Recipients. N Engl J Med 2017;376:2394–2395. [DOI] [PubMed] [Google Scholar]
- 17.Schlendorf KH, Zalawadiya S, Shah AS et al. Early outcomes using hepatitis C-positive donors for cardiac transplantation in the era of effective direct-acting anti-viral therapies. J Heart Lung Transplant 2018;37:763–769. [DOI] [PubMed] [Google Scholar]
- 18.McLean RC, Reese PP, Acker M et al. Transplanting hepatitis C virus-infected hearts into uninfected recipients: A single-arm trial. Am J Transplant 2019;19:2533–2542. [DOI] [PubMed] [Google Scholar]
- 19.Cholankeril G,Li AA,Dennis BB et al. Increasing Trends in Transplantation of HCV-Positive Livers Into Uninfected Recipients. Clin Gastroenterol Hepatol.2019;17:1634–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwong AJ,Wall A,Melcher M et al. Liver transplantation for hepatitis C virus (HCV) non-viremic recipients with HCV viremic donors. Am J Transplant 2019;19:1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cotter TG, Paul S, Sandıkçı B et al. Increasing Utilization and Excellent Initial Outcomes Following Liver Transplant of Hepatitis C Virus (HCV)-Viremic Donors Into HCV-Negative Recipients: Outcomes Following Liver Transplant of HCV-Viremic Donors. Hepatology 2019;69:2381–2395. [DOI] [PubMed] [Google Scholar]
- 22.Durand CM, Bowring MG, Brown DM et al. Direct-Acting Antiviral Prophylaxis in Kidney Transplantation From Hepatitis C Virus-Infected Donors to Noninfected Recipients: An Open-Label Nonrandomized Trial. Ann Intern Med 2018;168:533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolley AE, Singh SK, Goldberg HJ et al. Heart and Lung Transplants from HCV-Infected Donors to Uninfected Recipients. N Engl J Med 2019;380:1606–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bethea ED, Gaj K, Gustafson J et al. Pre-emptive pangenotypic direct acting antiviral therapy in donor HCV-positive to recipient HCV-negative heart transplantation: an open-label study. Lancet Gastroenterol Hepatol 2019;4:771–780. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Chen J, Fang Z et al. Sofosbuvir-based regimen is safe and effective for hepatitis C infected patients with stage 4–5 chronic kidney disease: a systematic review and meta-analysis. Virol J 2019;16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta G, Yakubu I, Bhati CS et al. Ultra-short duration direct acting antiviral prophylaxis to prevent virus transmission from hepatitis C viremic donors to hepatitis C negative kidney transplant recipients. Am J Transplant 2019. [DOI] [PubMed] [Google Scholar]
- 27.Feld JJ, Cypel M, Kumar D et al. Transplantation from HCV-infected donors to HCV-uninfected recipients: Short course therapy to prevent transmission [abstract]. Hepatology. 2019;40(Suppl 1):38. [Google Scholar]
- 28.Maasoumy B, Vermehren J, Welker MW et al. Clinical value of on-treatment HCV RNA levels during different sofosbuvir-based antiviral regimens. J Hepatol 2016;65:473–482. [DOI] [PubMed] [Google Scholar]
- 29.Childs K, Merritt E, Considine A et al. Immunological Predictors of Nonresponse to Directly Acting Antiviral Therapy in Patients With Chronic Hepatitis C and Decompensated Cirrhosis. Open Forum Infect Dis 2017;4:ofx067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapila N, Al-Khalloufi K, Bejarano PA et al. Fibrosing cholestatic hepatitis after kidney transplantation from HCV viremic donors to HCV negative recipients: A unique complication in the DAA era. Am J Transplant 2019. [DOI] [PubMed] [Google Scholar]
- 31.Kapila N, Narayanan Menon KV, Al-Khalloufi K et al. HCV NAT positive solid organ allografts transplanted into HCV negative recipients: A real-world experience. Hepatology 2019. [DOI] [PubMed] [Google Scholar]
- 32.Wadei HM, Pungpapong S, Cortese C et al. Transplantation of HCV-infected organs into uninfected recipients: Advance with caution. Am J Transplant 2019;19:960–961. [DOI] [PubMed] [Google Scholar]
- 33.Cost and Access to Direct-Acting Antiviral Agents. Accessed July 10, 2019. Available at https://www.hepatitisc.uw.edu/go/evaluation-treatment/cost-access-medications.
- 34.Haque M, Hashim A, Greanya ED et al. Spontaneous clearance of hepatitis C infection post-liver transplant: A rare but real phenomenon? A case report and review of the literature. Ann Hepatol 2010;9:202–206. [PubMed] [Google Scholar]
- 35.Bethea ED, Samur S, Kanwal F et al. Cost Effectiveness of Transplanting HCV-Infected Livers Into Uninfected Recipients With Preemptive Antiviral Therapy. Clin Gastroenterol Hepatol 2019;17:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reese PP, Abt PL, Blumberg EA et al. Twelve-Month Outcomes After Transplant of Hepatitis C-Infected Kidneys Into Uninfected Recipients: A Single-Group Trial. Ann Intern Med 2018;169:273–281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


