Abstract
Bacterial endospores (spores) are among the most resistant living forms on earth. Spores of Bacillus subtilis A163 show extremely high resistance to wet heat compared to spores of laboratory strains. In this study, we found that spores of B. subtilis A163 were indeed very wet heat resistant and released dipicolinic acid (DPA) very slowly during heat treatment. We also determined the proteome of vegetative cells and spores of B. subtilis A163 and the differences in these proteomes from those of the laboratory strain PY79, spores of which are much less heat resistant. This proteomic characterization identified 2011 proteins in spores and 1901 proteins in vegetative cells of B. subtilis A163. Surprisingly, spore morphogenic protein SpoVM had no homologs in B. subtilis A163. Comparing protein expression between these two strains uncovered 108 proteins that were differentially present in spores and 93 proteins differentially present in cells. In addition, five of the seven proteins on an operon in strain A163, which is thought to be primarily responsible for this strain’s spores high heat resistance, were also identified. These findings reveal proteomic differences of the two strains exhibiting different resistance to heat and form a basis for further mechanistic analysis of the high heat resistance of B. subtilis A163 spores.
Keywords: Bacillus subtilis A163, proteome, high heat resistance
1. Introduction
Strains from Bacillus subtilis are causative agents of food spoilage, which can cause problems in the food industry [1,2,3]. This is largely due to the high stress resistance of the spores produced by this species. In response to nutritional and environmental stresses, vegetative cells of B. subtilis can form metabolically dormant spores which exhibit extreme resistance properties compared to their corresponding vegetative cells [4]. Surviving spores can, depending on the environmental conditions, quickly germinate, resume vegetative growth and subsequently cause food spoilage [5]. Compared to vegetative cells, spores are protected by multiple proteinaceous coat layers [6]. In particular, the water content within the spores’ core is low, and DNA in the core is surrounded by dipicolinic acid (DPA) and saturated with protective α/β-type small acid-soluble proteins (SASPs). Many factors can affect spores’ wet heat resistance. For example, spores prepared on solid media have higher wet heat resistance than those prepared in liquid [7]. Higher sporulation temperatures can also result in higher heat resistance of spores [8]. In addition, the protein composition and DPA levels of spores are affected by sporulation conditions and temperatures [8,9,10]. With respect to heat resistance of spores of different Bacillus strains, two distinct groups have been identified [11]. One group, which includes B. subtilis strains 168 and PY79, commonly used strains in laboratory research, has spores with low heat resistance, while the other group, which includes the foodborne isolate B. subtilis strain A163, has spores with high heat resistance. A mobile genetic element, Tn1546, is commonly present in the high resistance strains and this transposon contains the spoVA2 mob operon which can profoundly heighten resistance of spores to heat and pressure, encoding four genes of unknown function and a three gene spoVA operon, which encodes proteins involved in DPA uptake into developing spores, and its release in spore germination [12,13,14,15]. Regrettably, the proteome of strain B. subtilis A163 has not yet been studied extensively using high resolution mass spectrometry-based proteomics so the protein expression profile has not been compared with that of low resistance spores.
First, we confirmed the higher thermal resistance of B. subtilis A163 spores compared to spores of B. subtilis PY79, and that rates of DPA release at elevated temperatures were much slower from A163 spores than from PY79 spores. We also determined the proteomes of spores and cells of B. subtilis A163. This work found 2011 spore and 1901 cell proteins, while 2045 cell and 2170 spore proteins were identified in B. subtilis strain PY79, a prototrophic derivative of strain 168 [16]. Among the proteome of spores of B. subtilis A163 were five of the seven proteins of the spoVA2mob operon found, and homologs of SpoVM, which are important in spore coat assembly in PY79 [17] were not found. Previous studies show that strain A163 and strain 168 are very similar at the genomic level [18]. By searching every protein sequence of B. subtilis PY79 against all protein sequences of B. subtilis A163, 4030 protein sequences of two strains showed more than 50% identity. Among them, 1312 and 1276 proteins were quantified between the two strains in spores and cells, respectively. Both high-abundance and low-abundance proteins were revealed in spores and cells. In spores of B. subtilis A163, the high-abundance proteins are enriched in the Uniprot categories glycosyltransferases and proteases, while low-abundance proteins are mainly enriched in membrane and sporulation proteins. In cells of B. subtilis A163, high-abundance proteins are enriched in the transport of proteins and peptides, as well as competence. Proteins involved in biosynthesis and metabolism of fatty acids and lipids, as well as oxidoreductase, are enriched in the low-abundant cellular proteins.
2. Materials and Methods
2.1. Strains and Sporulation
Low spore wet heat resistant B. subtilis strain PY79 and the high heat resistant spore forming food isolate B. subtilis A163 were used in this study [19]. Both strains were sporulated in shake flasks containing 3-(N-morpholino) propane sulfonic acid (MOPS) (Sigma-Aldrich, St. Louis, MI, USA) buffered defined liquid medium [20]. In brief, a single colony from a Lysogeny broth (LB) [21] agar plate was inoculated in 5 mL LB liquid medium and cultivated until its exponential phase at 37 ℃ and 200 rpm. The exponentially growing cells were then subjected to overnight growth in MOPS medium in a series of dilutions. The dilution with exponential growth was selected and 1 mL was transferred into 100 mL of MOPS medium and allowed to sporulate for 72 h. Spores were harvested and purified with Histodenz (Sigma-Aldrich, St. Louis, MI, USA) gradient centrifugation [22]. Vegetative cells were harvested from LB medium in the exponential phase. Three biological replicates of each strain were harvested for both spores and cells and stored at −80 ℃ for further experiments.
2.2. Heat Resistance and DPA Measurement in Spores
Heat resistance of spores was tested at 85 ℃ and 98 ℃ following an established protocol [9]. One ml of spores with an OD600 of 2 (~2 × 108 spores/mL) was heat activated at 70 ℃ for 30 min in a water bath. After being placed on ice for 15 min, spores were injected into a metal screwcap tube with 9 mL sterile milli-Q water pre-warmed for 20 min in a glycerol bath (85 ℃ or 98 ℃). The metal tube was then kept at 85 ℃ (or 98 ℃) for another 10 min, after which, the tube was cooled on ice. The fraction of surviving spores after heat treatment was estimated by counting the number of colonies formed on LB plates. Three biological replicates were performed for each strain tested at both 85 ℃ and 98 ℃.
Spore DPA content was calculated as μg of DPA per mg dry weight of spores. The protocol of DPA measurement was modified from [23]. One ml of spores with an OD600 of 2 from each strain was suspended in a buffer containing 0.3 mM (NH4)2SO4, 6.6 mM KH2PO4, 15 mM NaCl, 59.5 mM NaHCO3 and 35.2 mM Na2HPO4. For total DPA measurement, the suspended spores were autoclaved at 121 ℃ for 15 min. After incubation, the sample was centrifuged at 15,000 rpm for 2 min and 10 µL supernatant was added to 115 µL of buffer 2 (1 mM Tris, 150 mM NaCl) with and without 0.8 mM terbium chloride (Sigma-Aldrich, St. Louis, MI, USA). After 15 min of incubation, the fluorescence of the samples was measured using a Synergy Mx microplate reader (BioTek; 270-nm excitation; 545-nm reading; gain, 100) (Bad Friedrichshall, Germany). The background fluorescence (without terbium, incubated at 37 ℃) was subtracted from that of all the samples. A calibration curve of 0–125 µg/mL DPA (2,6-pyridinedicarboxylic acid) (Sigma-Aldrich, St. Louis, MI, USA) was used to calculate DPA concentrations of the sample. Samples incubated at 98 ℃ for 1–6 h were also used to measure the amount of DPA released at various heating times. Spore dry weights were determined by weighing overnight freeze-dried spores. Three biological replicates were measured for all conditions.
2.3. Proteome Databases and Comparison of Protein Sequences
Amino acid sequences encoded in the genome of B. subtilis A163 were acquired from [24] with accession no. JSXS00000000. The UniProt proteome database UP000001570 was used for B. subtilis PY79 [25]. Every protein sequence within UP000001570 was searched against the database of B. subtilis A163 to find the best match(es) using NCBI BLAST+ BLASTP (Galaxy version 0.3.3) embedded in the web-based platform Galaxy Europe (https://usegalaxy.eu/, accessed at 5 January 2021) with the E-value set at 0.00001 [26,27,28]. The protein sequences encoded in the spoVA2mob operon in B. subtilis B4417 were acquired from NCBI with the reference sequence NZ_LJSM01000045.1.
2.4. Data Acquisition for Proteomic Analysis
Processing of samples and fractionation of every trypsin digested sample into 10 fractions was done following the protocol described by Tu et al. [29]. Every fraction was reconstituted in 0.1% formic acid in water and 200 ng equivalent (set by measuring absorbance at a wavelength of 205 nm [30]) was injected by a Ultimate 3000 RSLCnano UHPLC system (Thermo Scientific, Germeringen, Germany) onto a 75 ¼m × 250 mm analytical column (C18, 1.6 ¼m particle size, Aurora, Ionopticks, Australia) kept at 50 ℃ at 400 nL/min for 15 min in 3% solvent B before being separated by a multi-step gradient (Solvent A: 0.1% formic acid in water, Solvent B: 0.1% formic acid in acetonitrile) to 5% B at 16 min, 17% B at 38 min, 25% B at 43 min, 34% B at 46 min, 99% B at 47 min held until 54 min returning to initial conditions at 55 min equilibrating until 80 min.
Eluting peptides were sprayed by the emitter coupled to the column into a captive spray source (Bruker, Bremen, Germany) with a capillary voltage of 1.5 kV, a source gas flow of 3 L/min of pure nitrogen and a dry temperature setting of 180 ℃, attached to a timsTOF pro (Bruker, Bremen, Germany) trapped ion mobility, quadrupole, time of flight mass spectrometer. The timsTOF was operated in PASEF mode of acquisition. The TOF scan range was 100–1700 m/z with a tims range of 0.6–1.6 V·s/cm2. In PASEF mode a filter was applied to the m/z and ion mobility plane to select features most likely representing peptide precursors, the quad isolation width was 2 Th at 700 m/z and 3 Th at 800 m/z, and the collision energy was ramped from 20–59 eV over the tims scan range to generate fragmentation spectra. A total number of 10 PASEF MS/MS scans scheduled with a total cycle time of 1.16 s, scheduling target intensity 2 × 104 and intensity threshold of 2.5 × 103 and a charge state range of 0–5 were used. Active exclusion was on (release after 0.4 min), reconsidering precursors if ratio current/previous intensity >4.
2.5. Data Processing
Generated data for spores (of two strains) and cells (of two strains) were processed with MaxQuant (Version 1.6.14, Martinsried, Germany) in two separate analyses [31]. 10 fractions from the same sample were set as one experiment. Proteome databases for B. subtilis A163 and B. subtilis PY79 were included in the analysis. The proteolytic enzyme used was trypsin/p, and the maximum missed cleavages was set to 2. Carbamidomethyl (C) was set as fixed modification with variable modifications of Oxidation (M) and Acetyl (Protein N-term). The type of Group specific parameters was set as TIMS-DDA. The rest of the parameters were set using the default. Since two databases were used in the analysis, the quantified values of proteins from two strains with high percentage of identity were sometimes reported as two separate proteins in the output proteinGroup.txt (vide infra). To quantitatively compare the homologous protein from two strains, we re-assembled the identified peptides in the evidence.txt file and quantified the protein amounts using the R software package iq [32]; the R-script and evidence.txt files used can be found in Supplementary File S1. The minimum number of peptides for the quantification was 2. The differentially presented proteins in cells and spores were determined by using R/Bioconductor software package limma [33]. DAVID Bioinformatics Resources tool (version 6.8) was used to retrieve the UniProt keyword enrichment of the differentially presented proteins [34,35]. The protein list of coat proteins was retrieved from SubtiWiki (http://subtiwiki.uni-goettingen.de/, accessed at 5 January 2021) [36].
3. Results and Discussion
3.1. Heat Resistance and DPA Measurement of B. subtilis Spores
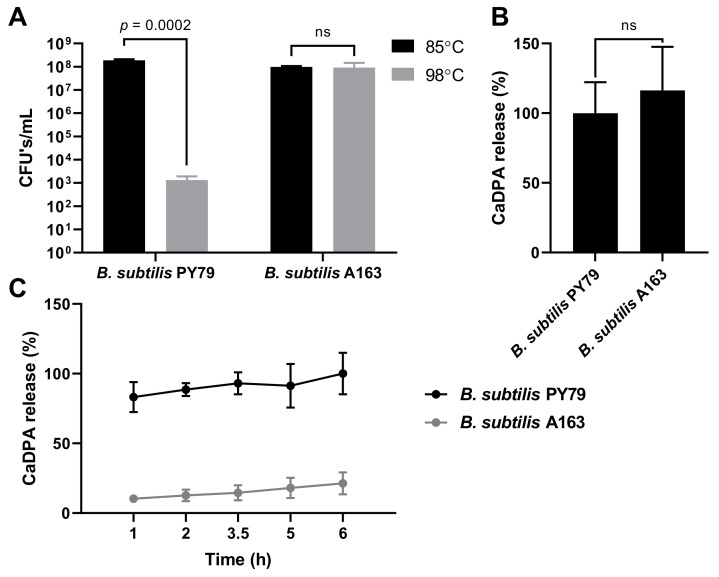
Spores of B. subtilis A163 are reported to show extreme wet heat resistance [11,18,19]. To confirm this, spores of B. subtilis A163 and PY79 were heat treated at 85 ℃ and 98 ℃, and as expected there was a significant decrease in surviving PY79 spores treated at 98 ℃, but no such difference for A163 spores (Figure 1A). Our measurements also showed that while B. subtilis A163 spores gave slightly higher values for DPA than PY79 spores, the difference was not statistically significant (Figure 1B). This latter finding is consistent with the essentially identical DPA levels found recently in B. subtilis with and without transposon Tn1546 [15]. However, the rate of release of DPA during heat treatment of A163 spores was much slower than from PY79 spores (Figure 1C). Treatment of spores of PY79 spores at 98 ℃ resulted in release of ~80% DPA at 1 h and almost all DPA at 5 h. However, only trace amounts of DPA were released from A163 spores during the first hour of heat treatment and only ~20% of the DPA was released at 6 h. This finding confirms a previous report [19], that A163 spores need much higher temperatures to release their DPA than low wet heat resistance spores. Killing of low wet heat resistance spores by wet heat appears due to damage to key spore proteins, and this takes place before loss of DPA presumably due to spore inner membrane (IM) damage but prevents the outgrowth of spores [37,38]. However, the high wet heat resistant A163 spores have features to maintain the native state of key spore proteins as well is the IM impermeability during heat treatment at higher temperatures.
Figure 1.
Heat resistance test and DPA content of B. subtilis spores. The standard deviation is shown in the graphs. Statistical significance was determined using Student’s t-test. ns, not significant. (A) Numbers of colonies formed on LB agar plates of B. subtilis spores wet heat treated at 85 ℃ and 98 ℃ for 10 min. (B) Amount of CaDPA released by B. subtilis spores that were autoclaved at 121 ℃. The amount of CaDPA released by spores was calculated as % of CaDPA released by B. subtilis PY79. (C) CaDPA released by B. subtilis spores heat treated at 98 ℃ for 1–6 h. The amount of CaDPA released by spores was calculated as % of the total CaDPA.
3.2. Identification and Quantification of Proteomes of Spores and Cells
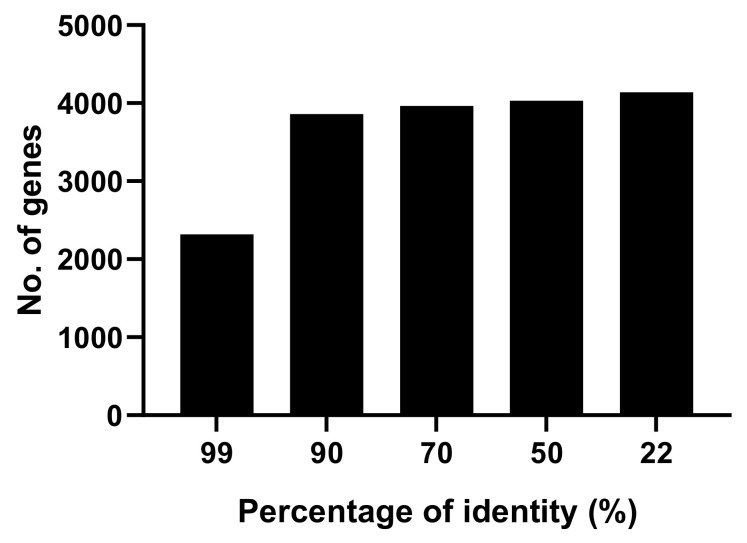
One important field in the study of spore forming bacteria is identification and quantification of the spore and growing cell proteome. In this study, we investigated the proteome of both spores and cells of B. subtilis A163. 2011 and 1901 proteins were separately identified in at least two of three biological replicates of spores and cells of B. subtilis A163, while with B. subtilis PY79, 2170 spore proteins and 2045 cellular proteins were identified. Lists of identified proteins in spores and cells can be found in Supplementary Tables S1 and S2. In terms of identification of proteins from the spoVA2mob operon in B. subtilis A163 spores, five proteins were identified (Table 1), excluding the protein with both a predicted DUF 421 domain and a DUF 1657 domain (2Duf protein), which is thought to be the most important one in the spoVA2mob operon [12], as well as the SpoVAEb protein. In addition, homologs for the two DUF 1657 domain-containing proteins and SpoVAC2mob were also identified. This could be because multiple copies of spoVA2mob were present in B. subtilis A163 [12]. To compare proteomes of spores or cells of two strains, we first checked how much similarity there was between the protein sequences of the two strains. Among 4800 protein coding genes of B. subtilis A163, amino acid sequences of 4141 genes show a minimum 22% of identity with the lab strain, and 4030 genes show more than 50% identity (Figure 2). Quite a number of proteins from the two strains show a high percentage of identity, indicating that these proteins could be considered homologs. Coat proteins identified in B. subtilis spores are shown in Table 2. SpoVM, CotU, CotR and YjdH have no homologous proteins found in B. subtilis A163. SpoVM is a key protein for the proper assembly of the spore coat [17]. Two homologous genes were found for oxdD, yjqC and cotF in B. subtilis A163, but only one homolog of CotF and two homologs of YjqC were identified. For the proteins involved in germination and the endogenous SpoVA channel [5], some germinant receptor proteins, most notably GerB proteins, were only identified in B. subtilis PY79, but not in B. subtilis A163 (Table 3). Moreover, strain specific proteins were identified in both strains. Among the B. subtilis A163 specific spore proteins, none of them show predicted functions except an alpha-glucosidase (protein id = KIL30593.1).
Table 1.
spoVA2mob proteins identified in B. subtilis A163 spores.
| Genes | Identifier in B. subtilis B4417 a | Identifier in B. subtilis A163 b | Percentage of Identity (%) c | Number of Peptides |
|---|---|---|---|---|
| DUF1657 | prot_WP_009336483.1_679 | prot_KIL30783.1_1 | 100 | 27 |
| prot_KIL30530.1_3 | 80.882 | 17 | ||
| spoVAD2mob | prot_WP_013352386.1_681 | prot_KIL30785.1_3 | 100 | 8 |
| spoVAC2mob | prot_WP_013352385.1_682 | prot_KIL30782.1_3 | 100 | 5 |
| prot_KIL32093.1_7 | 97.183 | 11 | ||
| Yhcn/YlaJ | prot_WP_017697692.1_683 | prot_KIL30781.1_2 | 100 | 9 |
| DUF1657 | prot_WP_009336488.1_684 | prot_KIL30780.1_1 | 100 | 17 |
| prot_KIL32090.1_4 | 91.176 | 16 |
aB. subtilis 168 carrying the spoVA2mob operon [12]. b identified by searching protein sequences of spoVA2mob operon of B. subtilis B4417 against the protein sequences of B. subtilis A163. c, percentage of identity of the proteins between B. subtilis B4417 and B. subtilis A163.
Figure 2.
Amino acid sequence comparisons between B. subtilis strains. Every protein sequence in the genome of B. subtilis PY79 was searched against the database containing all the protein sequences of B. subtilis A163. The match with the highest percentage of identity was included in the figure.
Table 2.
Identified coat proteins in spores of B. subtilis PY79 and A163.
| Proteins | UniProt IDs | Homologous Proteins in B. subtilis A163 | Percentage of Identity (%) | Number of Peptides | |
|---|---|---|---|---|---|
| B. subtilis A163 | B. subtilis PY79 | ||||
| SpoIVA | P35149 | prot_KIL33240.1_118 | 99.797 | 131 | 90 |
| SpoVID | P37963 | prot_KIL31245.1_33 | 95.549 | 22 | 9 |
| SpoVM | P37817 | NA | NA | NA | 2 |
| YaaH | P37531 | prot_KIL32101.1_8 | 96.721 | 117 | 132 |
| YuzC | O32089 | prot_KIL29514.1_5 | 96.721 | 13 | 14 |
| CotE | P14016 | prot_KIL29844.1_246 | 100 | 58 | 53 |
| CotM | Q45058 | prot_KIL31177.1_55 | 98.387 | 5 | 5 |
| CotO | O31622 | prot_KIL30325.1_87 | 99.111 | 9 | 8 |
| YhjR | O07572 | prot_KIL33619.1_38 | 100 | 21 | 24 |
| YknT | O31700 | prot_KIL30843.1_42 | 98.754 | 2 | 4 |
| YncD | P94494 | prot_KIL31326.1_4 | 99.746 | 40 | 38 |
| CotZ | Q08312 | prot_KIL30327.1_89 | 100 | 46 | 35 |
| CwlJ | P42249 | prot_KIL33394.1_41 | 95.775 | 36 | 26 |
| YisY | O06734 | prot_KIL33591.1_10 | 98.134 | 81 | 73 |
| YsxE | P37964 | prot_KIL31244.1_32 | 98.534 | 10 | 4 |
| YutH | O32123 | prot_KIL29568.1_59 | 99.11 | 16 | 2 |
| CotT | P11863 | prot_KIL30718.1_123 | 98.78 | 48 | 31 |
| YybI | P37495 | prot_KIL31595.1_71 | 93.893 | 47 | 51 |
| CotA | P07788 | prot_KIL30080.1_5 | 99.61 | 170 | 166 |
| CotB | P07789 | prot_KIL31029.1_37 | 68.116 | 4 | 61 |
| CotG | P39801 | prot_KIL31027.1_35 | 92.308 | 86 | 45 |
| CotP | P96698 | prot_KIL30034.1_53 | 97.203 | 24 | 14 |
| CotQ | O06997 | prot_KIL33096.1_104 (a, b) | 25 | 0 | 111 |
| CotS | P46914 | prot_KIL33875.1_18 | 99.145 | 133 | 123 |
| CotW | Q08310 | prot_KIL30330.1_92 | 98.095 | 22 | 25 |
| LipC | P42969 | prot_KIL29460.1_9 | 100 | 72 | 55 |
| OxdD | O34767 | prot_KIL33540.1_11 (a) | 94.231 | 0 | 70 |
| prot_KIL33541.1_12 (a) | 99.642 | 0 | |||
| Tgl | P40746 | prot_KIL32205.1_19 | 99.184 | 56 | 40 |
| YjqC | O34423 | prot_KIL32111.1_3 (b) | 34.426 | 151 | 46 |
| prot_KIL30071.1_90 | 51.163 | 1 | |||
| YjzB | O34891 | prot_KIL30279.1_41 | 96.104 | 1 | 4 |
| YmaG | O31793 | prot_KIL29859.1_261 | 96.703 | 7 | 19 |
| YppG | P50835 | prot_KIL33183.1_61 | 97.6 | 4 | 6 |
| YtxO | P46916 | prot_KIL33874.1_17 | 95.804 | 52 | 47 |
| YxeE | P54944 | prot_KIL32257.1_32 | 100 | 17 | 15 |
| CotU | O31802 | NA | NA | NA | 27 |
| CgeA | P42089 | prot_KIL31959.1_13 | 96.241 | 12 | 8 |
| CgeB | P42090 | prot_KIL31960.1_14 | 94.386 | 11 | 3 |
| CgeC | P42091 | prot_KIL31958.1_12 | 98.02 | 2 | 3 |
| CgeE | P42093 | prot_KIL31956.1_10 | 99.228 | 14 | 21 |
| CmpA | P14204 | prot_KIL33555.1_2 | 100 | 2 | 10 |
| CotC | P07790 | prot_KIL31334.1_12 | 100 | 48 | 30 |
| CotF | P23261 | prot_KIL31586.1_62 (a) | 100 | 0 | 51 |
| prot_KIL31587.1_63 | 94.643 | 3 | |||
| CotH | Q45535 | prot_KIL31028.1_36 | 97.238 | 101 | 77 |
| CotI | O34656 | prot_KIL33877.1_20 | 98.3 | 115 | 107 |
| CotJA | Q45536 | prot_KIL30149.1_74 | 98.78 | 67 | 53 |
| CotJB | Q45537 | prot_KIL30150.1_75 | 100 | 10 | 12 |
| CotJC | Q45538 | prot_KIL30151.1_76 | 100 | 56 | 41 |
| CotR | O06996 | NA | NA | NA | 70 |
| CotSA | P46915 | prot_KIL33876.1_19 | 99.469 | 185 | 116 |
| CotX | Q08313 | prot_KIL30329.1_91 | 100 | 72 | 48 |
| CotY | Q08311 | prot_KIL30328.1_90 | 100 | 50 | 39 |
| GerQ | P39620 | prot_KIL31830.1_12 | 98.895 | 21 | 17 |
| GerT | Q7WY67 | prot_KIL32533.1_42 | 95.541 | 33 | 7 |
| SafA | O32062 | prot_KIL32613.1_30 | 98.45 | 77 | 74 |
| SpsB | P39622 | prot_KIL31833.1_15 | 98.911 | 27 | 23 |
| YabG | P37548 | prot_KIL32152.1_41 | 98.276 | 67 | 54 |
| YdhD | O05495 | prot_KIL30052.1_71 | 99.048 | 65 | 53 |
| YgaK | Q796Y5 | prot_KIL30263.1_25 (a, b) | 24.691 | 0 | 49 |
| YhbB | O31589 | prot_KIL31183.1_5 | 99.016 | 39 | 43 |
| YheC | O07544 | prot_KIL33710.1_129 | 100 | 48 | 2 |
| YjdH | O31649 | NA | NA | NA | 19 |
| YkvP | O31681 | prot_KIL33950.1_72 | 99.248 | 59 | 49 |
| YobN | O34363 | prot_KIL30953.1_14 | 98.536 | 26 | 14 |
| YodI | O34654 | prot_KIL32546.1_55 | 95.181 | 10 | 11 |
| YpeP | P54164 | prot_KIL33154.1_32 | 99.558 | 13 | 17 |
| YqfT | P54477 | prot_KIL32864.1_5 | 100 | 14 | 18 |
NA, not available, no homologous proteins were found in B. subtilis A163 through searching using BLASTP; (a), not identified in the spore proteome, but showing a high percentage of identity with the coat protein; (b), low percentage of identity, more research is necessary.
Table 3.
Identified B. subtilis PY79 and A163 proteins involved in germination.
| Proteins | UniProt IDs | Homologous proteins in B. subtilis A163 | Percentage of Identity (%) | Number of Peptides | |
|---|---|---|---|---|---|
| B. subtilis A163 | B. subtilis PY79 | ||||
| GerAA | P07868 | prot_KIL32731.1_49 | 97.303 | 14 | 24 |
| GerAB | P07869 | prot_KIL32730.1_48 (a) | 98.082 | 0 | 3 |
| GerAC | P07870 | prot_KIL32729.1_47 | 94.906 | 19 | 30 |
| GerBA | P39569 | prot_KIL31057.1_65 (a) | 98.324 | 0 | 15 |
| GerBC | P39571 | prot_KIL31055.1_63 (a) | 97.861 | 0 | 27 |
| GerKA | P49939 | prot_KIL29364.1_41 | 98.162 | 9 | 15 |
| GerKB | P49940 | prot_KIL29362.1_39 | 96.783 | 1 | 3 |
| GerKC | P49941 | prot_KIL29363.1_40 (a, b) | 24.378 | 0 | 6 |
| GerD | P16450 | prot_KIL31648.1_3 | 100 | 31 | 52 |
| GerPA | O06721 | prot_KIL33608.1_27 | 98.63 | 6 | 9 |
| GerPB | O06720 | prot_KIL33609.1_28 | 100 | 6 | 9 |
| GerPC | O06719 | prot_KIL33610.1_29 | 99.024 | 9 | 3 |
| GerPD | O06718 | prot_KIL33611.1_30 (a) | 100 | 0 | 1 |
| GerPE | O06717 | prot_KIL33612.1_31 | 99.115 | 5 | 1 |
| GerPF | O06716 | prot_KIL33613.1_32 | 100 | 3 | 4 |
| CwlJ | P42249 | prot_KIL33394.1_41 | 95.775 | 36 | 26 |
| SleB | P50739 | prot_KIL33255.1_133 | 90.12 | 46 | 49 |
| SpoVAA | P40866 | prot_KIL33311.1_189 | 99.515 | 1 | 1 |
| SpoVAC | P40868 | prot_KIL33309.1_187 | 99.333 | 13 | 10 |
| SpoVAD | P40869 | prot_KIL33308.1_186 | 99.408 | 94 | 89 |
| SpoVAEa | P40870 | prot_KIL33306.1_184 | 100 | 13 | 11 |
| SpoVAF | P31845 | prot_KIL33305.1_183 | 99.189 | 31 | 27 |
(a), not identified in the spore proteome, but showing a high percentage of identity with the protein; (b), low percentage of identity, more research is necessary.
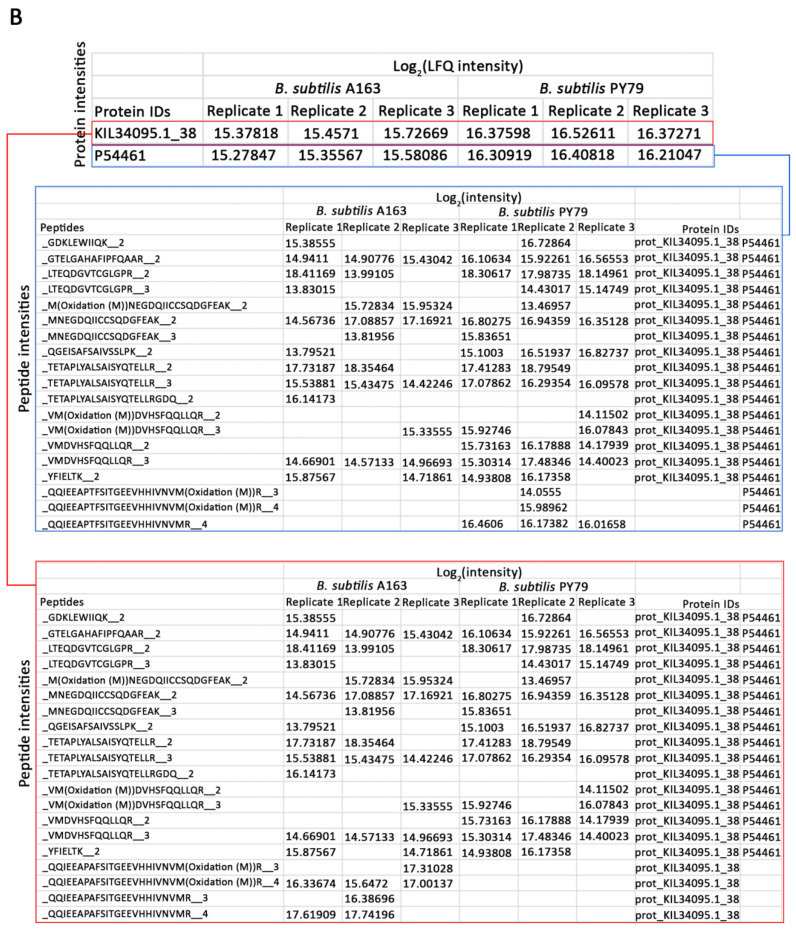
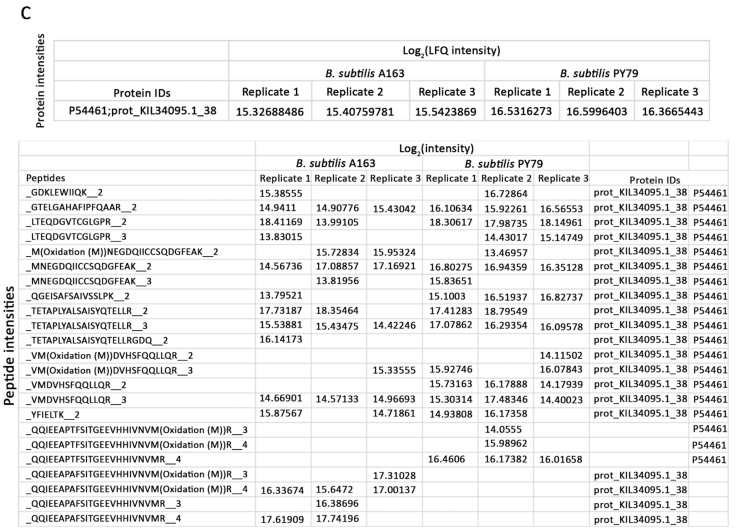
Since two databases were used in this study, homologous proteins from the two strains were often reported as two separate results, for example RsmE (ribosomal RNA small subunit methyltransferase E, Uniprot ID P54461) identified in the cellular proteome. In a comparison of amino acid sequences, RsmE from the two strains had more than 99% identity with one amino acid difference, T18A (Figure 3A). However, two proteins were in the output as two items with their own quantitative values (Figure 3B). By checking their peptide composition, we found they both contain shared peptides which can be identified from either of the two proteins, and specific peptides caused by the T18A change. Quantification of RsmE using either of the two outputs may result in an incorrect conclusion. To overcome this issue, we re-assembled the identified peptides to include both the shared peptides and specific peptides and calculated the protein abundances accordingly [32]. In the new output, proteins from two strains having shared peptides were treated as homologous proteins for the moment and their protein identifiers were both shown in the column of Protein IDs (such as RsmE in Figure 3C). In total, 1312 and 1276 proteins were quantified between two strains in spores and cells, respectively, with at least two quantified values in each strain (Tables S1 and S2).
Figure 3.
Quantitative comparison of RsmE peptide levels in growing B. subtilis PY79 and A163 cells. prot_KIL34095.1_38, identifier of the homologous protein of RsmE in B. subtilis A163; LFQ, Relative label-free quantification [39]. (A) Alignment of amino acids of RsmE between two strains. (B) Protein intensities of RsmE and their identified peptides in the default output. (C) Protein intensities of RsmE and their peptide components in the new output.
Among the proteins quantitated, proteins between two strains with an identity higher than 50% were subjected to further analysis. Some proteins have multiple homologs identified in B. subtilis A163, such as YjqC, but only one homolog in our data is quantitively compared with the protein in B. subtilis PY79. That is because in a comparison of one protein between two samples, the quantification algorithm requires that some peptides identified in one sample must be also identified in the other sample [39]. In our data, since not enough peptides were identified for the other homologs, this makes them impossible to quantitively analyze. In the quantified spore proteins, 39 proteins were found to be highly abundant in B. subtilis A163, while 69 were low abundance (Figure 4A). For the analysis of cellular proteins, 32 and 61 proteins in B. subtilis A163 were present at high and low abundance, respectively (Figure 4A). In the known spore coat proteins retrieved from SubtiWiki [36], YmaG and CgeA were low abundance and CotJC, CotH, CotSA, SpoVID and GerT were high abundance in spores of B. subtilis A163. CgeA is a protein located in the spore crust, the outermost layer, and is considered to play a role in crust glycosylation [40]. SpoVID and CotH are essential for spore coat morphogenesis, and a spoVID mutant fails to encase the spore inner and outer coat layers [41,42]. cotH mutant spores have normal heat resistance but are deficient in several coat proteins [43]. CotJC upregulation was observed in spores from a sporulation that was kinA-induced, and these spores had higher wet heat resistance than when sporulation was induced by nutrient depletion [29]. However, how increased levels of SpoVID, CotH and CotJC affect spore resistance is not known. GerT is also a component of the spore coat and ΔgerT spores respond poorly to multiple germinants [44]. For the small acid soluble proteins and the proteins involved in spore germination (germinant receptors, SpoVA channel proteins, SleB and CwlJ) [5], none were quantified to be more or less abundant in the two strains analyzed (Table S2). For proteins encoded in the spoVA2mob operon, none of them are present in PY79 strain and thus were not quantitatively compared with any proteins identified in PY79 strain.
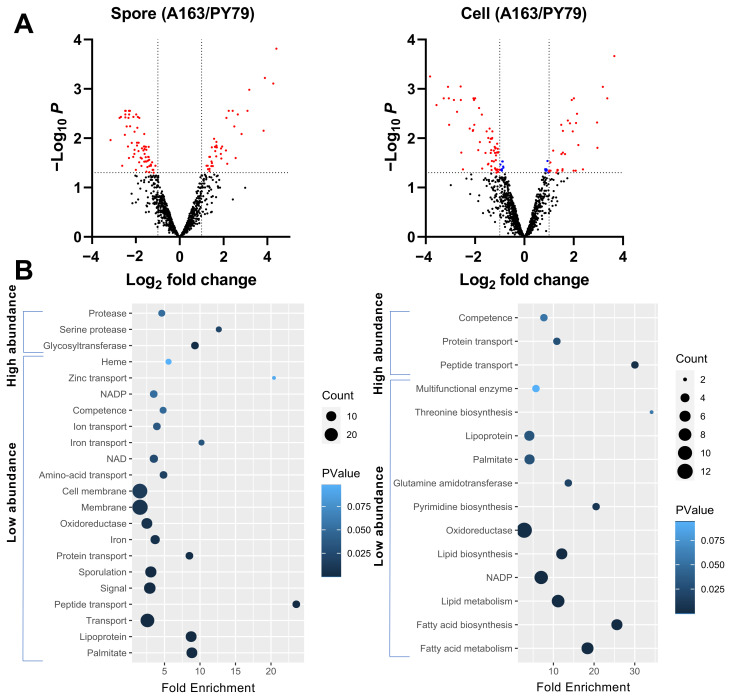
Figure 4.
Quantitative comparison of proteomes in spores and cells of B. subtilis PY79 and A163. (A) Volcano plots of the quantified proteins in spores of the two strains and the proteome comparison of their correspondent cells. Log2 fold changes smaller than 0 (or larger than 0) indicate proteins with low (or high) abundance in B. subtilis A163. Dots in red indicate proteins in B. subtilis A163 that were differentially present more than twofold with p < 0.05. Dots in blue indicate proteins that were present with Scheme 0. but less than twofold. (B) Uniprot categories enrichment of the differentially presented proteins in spores and cells of B. subtilis A163. The fold enrichment is defined as the ratio of two proportions. The first proportion is the quantified proteins belonging to a UniProt category divided by all high- (or low-) abundant proteins. The second proportion is all proteins belonging to the UniProt category in the genome divided by the total proteins in the genome. The size of the dots is indicative of the number of quantified proteins (Count) belonging to a particular term, as shown in the legend. The color of the dots is corresponding to the Fisher exact p-value (PValue), again as shown in the legend.
The Uniprot terms enriched from the most differentially presented spore and cellular proteins are shown in Figure 4B. Glycosyltransferases and proteases are enriched in the high abundance spore proteins of B. subtilis A163. Of the glycosyltransferases, YtcC is a product of the ytcABC operon, which could be involved in the extensive glycosylation of the spore surface [45]. YdhE plays roles in the resistance to bacterial toxins [46]. The pyrimidine biosynthetic (pyr) gene cluster includes the gene for PyrE [47], one of the high abundance glycosyltransferases. The last high abundance glycosyltransferase is the coat protein CotSA [48]. Among the proteases, IspA is an intracellular serine protease, and an ispA null mutant showed a decreased sporulation in at least one medium [49]. While AprE is one of the major extracellular alkaline proteases [50], serine protease YtrC has been reported to be in the spore IM fraction and to play a pivotal role in spore germination [51,52]. Proteins enriched in Uniprot terms sporulation and (cell) membrane are the major group among the low abundance spore proteins of B. subtilis A163. The proteins enriched in sporulation are listed in Table 4. Their contribution to spore resistance is unknown. Among them, SpoIIIAG, YabP, OppA, OppB, OppC, OppF, DppE and PbpE are also membrane proteins. High abundance proteins in cells of B. subtilis A163 are enriched in the transport of proteins and peptides, as well as competence. Proteins involved in biosynthesis and metabolism of fatty acids and lipids, as well as oxidoreductase, are enriched in the low-abundance cellular proteins of B. subtilis A163.
Table 4.
Proteins enriched in the Uniprot term of sporulation.
| Proteins | Descriptions | References |
|---|---|---|
| SpoIIIAG | a key component of a feeding tube apparatus creating a direct conduit between the developing forespore and the mother cell | [53,54] |
| Spo0M | regulating progress of sporulation and expression of Spo0A, but the mechanisms is still unknow | [55,56] |
| SpoVIF | involved in assembly of spore coat proteins that have roles in lysozyme resistance | [57] |
| YabP | a coat-associated protein | [58] |
| SplB | UV resistance of spores, DNA repair in spore germination | [59] |
| SinR | the master regulator of biofilm formation | [60] |
| OppA, OppB, OppC, OppF, DppE | the ATP binding cassette (ABC) transporter systems | [61] |
| PbpE | penicillin-binding protein PBP 4 | [62,63] |
| YraD | forespore-specific sporulation protein, similar to spore coat protein | [64] |
Multiple factors can contribute to the wet heat resistance of spores [65]. Among them, a spoVA2mob operon is considered to play roles in elevation of their resistance to heat and pressure. Measurement of the DPA content of spores of B. subtilis A163, the parental strain containing the spoVA2mob operon, indicated that these spores do not contain statistically significantly higher levels of DPA than those of a low resistance strain, and recent work has also found that the core water content of spores with the spoVA2mob operon is identical in a strain lacking this operon [15]. Of the seven proteins encoded in the spoVA2mob operon, we identified five in A163 spores, but none were the 2Duf protein thought to be of most importance in these spores high heat resistance or SpoVAEb. Furthermore, a number of germinant receptors were not identified in spores of B. subtilis A163. A practical approach might be trying to focus on identification of the proteome of the spore IM, as this analysis has been done on B. subtilis strain 1A700 [51]. On the other hand, the rate of DPA release from spores of B. subtilis A163 during heat treatment is low, although the mechanism preventing faster DPA release during heat treatment is unknown. Presumably this is due to the integrity and impermeability of the IM and protection by coat layers. In addition, the A163 spore proteome contains proteins specific to this strain, but with unknown function. The location of these proteins in spores and their contribution to spore resistance are also unknown. In addition, some proteins have multiple homologs identified in B. subtilis A163, for example coat protein YiqC. However, no homologs were found in B. subtilis A163 for proteins important for spore morphogenesis, such as SpoVM. What proteins would supplement the function of SpoVM or how the spore completes the coat encasement without SpoVM homologs would certainly be worth investigating. In addition, high and low abundant A163 spore proteins were revealed for the coat layers and a number of the Uniprot categories for both cellular and spore proteins. Those could also play some role in the observed high thermal resistance of A163 spores.
4. Conclusions
B. subtilis A163 arouses the interest of researchers due to its ability to produce high heat resistant spores. In this study, we found the release of spore DPA from B. subtilis A163 at 98 ℃ was much slower than that from B. subtilis PY79 spores. How the spore prevented the DPA from rapidly being released during heat treatment and if this is related with the high heat resistance of A163 spores is unknown. Through the extensive study of the proteomes of B. subtilis A163 and PY79 spores and cells, the proteomic differences of the two strains are revealed. This provides novel insights on the putative molecular basis of spore high wet heat resistance. Open questions include whether 2Duf is really not expressed during the B. subtilis A163 life-cycle, and more generally, how the proteins encoded by the spoVA2mob operon contribute to the observed high wet heat resistance of B. subtilis A163 spores.
Acknowledgments
We thank Winfried Roseboom and Henk L. Dekker from the laboratory for mass spectrometry of biomolecules, University of Amsterdam, for expert technical assistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/9/3/667/s1, Table S1: proteins identified and quantified in Bacillus subtilis cells; Table S2: proteins identified and quantified in Bacillus subtilis spores; File S1: evidence.txt files and R-scripts. The raw proteomic data of this study are available in MassIVE with access number MSV000087080.
Author Contributions
Conceptualization, S.B. and G.K.; formal analysis, Z.T.; writing—original draft preparation, Z.T.; writing—review and editing, S.B., G.K. and P.S.; visualization, Z.T.; supervision, S.B. and G.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Scholarship Council, grant number 201606170125. The APC was funded by Laboratory for Molecular Biology and Microbial Food Safety, University of Amsterdam.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oomes S., Van Zuijlen A.C.M., Hehenkamp J.O., Witsenboer H., Van der Vossen J., Brul S. The characterisation of Bacillus spores occurring in the manufacturing of (low acid) canned products. Int. J. Food Microbiol. 2007;120:85–94. doi: 10.1016/j.ijfoodmicro.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Rosenkvist H., Hansen Å. Contamination profiles and characterisation of Bacillus species in wheat bread and raw materials for bread production. Int. J. Food Microbiol. 1995;26:353–363. doi: 10.1016/0168-1605(94)00147-X. [DOI] [PubMed] [Google Scholar]
- 3.Scheldeman P., Pil A., Herman L., De Vos P., Heyndrickx M. Incidence and diversity of potentially highly heat-resistant spores isolated at dairy farms. Appl. Environ. Microbiol. 2005;71:1480–1494. doi: 10.1128/AEM.71.3.1480-1494.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Setlow P. Spore resistance properties. Microbiol. Spectr. 2014;2 doi: 10.1128/microbiolspec.TBS-0003-2012. [DOI] [PubMed] [Google Scholar]
- 5.Setlow P. Summer meeting 2013–when the sleepers wake: The germination of spores of Bacillus species. J. Appl. Microbiol. 2013;115:1251–1268. doi: 10.1111/jam.12343. [DOI] [PubMed] [Google Scholar]
- 6.Henriques A.O., Moran C.P. Structure, assembly, and function of the spore surface layers. Annu. Rev. Microbiol. 2007;61:555–588. doi: 10.1146/annurev.micro.61.080706.093224. [DOI] [PubMed] [Google Scholar]
- 7.Rose R., Setlow B., Monroe A., Mallozzi M., Driks A., Setlow P. Comparison of the properties of Bacillus subtilis spores made in liquid or on agar plates. J. Appl. Microbiol. 2007;103:691–699. doi: 10.1111/j.1365-2672.2007.03297.x. [DOI] [PubMed] [Google Scholar]
- 8.Melly E., Genest P.C., Gilmore M.E., Little S., Popham D.L., Driks A., Setlow P. Analysis of the properties of spores of Bacillus subtilis prepared at different temperatures. J. Appl. Microbiol. 2002;92:1105–1115. doi: 10.1046/j.1365-2672.2002.01644.x. [DOI] [PubMed] [Google Scholar]
- 9.Abhyankar W.R., Kamphorst K., Swarge B.N., van Veen H., van der Wel N.N., Brul S., de Koster C.G., de Koning L.J. The influence of sporulation conditions on the spore coat protein composition of Bacillus subtilis spores. Front. Microbiol. 2016;7:1636. doi: 10.3389/fmicb.2016.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isticato R., Lanzilli M., Petrillo C., Donadio G., Baccigalupi L., Ricca E. Bacillus subtilis builds structurally and functionally different spores in response to the temperature of growth. Environ. Microbiol. 2020;22:170–182. doi: 10.1111/1462-2920.14835. [DOI] [PubMed] [Google Scholar]
- 11.Berendsen E.M., Zwietering M.H., Kuipers O.P., Wells-Bennik M.H. Two distinct groups within the Bacillus subtilis group display significantly different spore heat resistance properties. Food Microbiol. 2015;45:18–25. doi: 10.1016/j.fm.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Berendsen E.M., Boekhorst J., Kuipers O.P., Wells-Bennik M.H. A mobile genetic element profoundly increases heat resistance of bacterial spores. ISME J. 2016;10:2633–2642. doi: 10.1038/ismej.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z., Schottroff F., Simpson D.J., Gänzle M.G. The copy number of the spoVA2mob operon determines pressure resistance of Bacillus endospores. Appl. Environ. Microbiol. 2019;85:e01596-19. doi: 10.1128/AEM.01596-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berendsen E.M., Koning R.A., Boekhorst J., de Jong A., Kuipers O.P., Wells-Bennik M.H. High-level heat resistance of spores of Bacillus amyloliquefaciens and Bacillus licheniformis results from the presence of a spoVA operon in a Tn1546 transposon. Front. Microbiol. 2016;7:1912. doi: 10.3389/fmicb.2016.01912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Y., Korza G., DeMarco A.M., Kuipers O.P., Li Y., Setlow P. Properties of spores of Bacillus subtilis with or without a transposon that decreases spore germination and increases spore wet heat resistance. Unpublished. [DOI] [PubMed]
- 16.Youngman P., Perkins J.B., Losick R. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid. 1984;12:1–9. doi: 10.1016/0147-619X(84)90061-1. [DOI] [PubMed] [Google Scholar]
- 17.Ramamurthi K.S., Clapham K.R., Losick R. Peptide anchoring spore coat assembly to the outer forespore membrane in Bacillus subtilis. Mol. Microbiol. 2006;62:1547–1557. doi: 10.1111/j.1365-2958.2006.05468.x. [DOI] [PubMed] [Google Scholar]
- 18.Brul S., van Beilen J., Caspers M., O’Brien A., de Koster C., Oomes S., Smelt J., Kort R., Ter Beek A. Challenges and advances in systems biology analysis of Bacillus spore physiology; molecular differences between an extreme heat resistant spore forming Bacillus subtilis food isolate and a laboratory strain. Food Microbiol. 2011;28:221–227. doi: 10.1016/j.fm.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Kort R., O’brien A.C., Van Stokkum I.H., Oomes S.J., Crielaard W., Hellingwerf K.J., Brul S. Assessment of heat resistance of bacterial spores from food product isolates by fluorescence monitoring of dipicolinic acid release. Appl. Environ. Microbiol. 2005;71:3556–3564. doi: 10.1128/AEM.71.7.3556-3564.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abhyankar W., Beek A.T., Dekker H., Kort R., Brul S., de Koster C.G. Gel-free proteomic identification of the Bacillus subtilis insoluble spore coat protein fraction. Proteomics. 2011;11:4541–4550. doi: 10.1002/pmic.201100003. [DOI] [PubMed] [Google Scholar]
- 21.Bertani G. Studies on Lysogenesis I. J. Bacteriol. 1951;62:293–300. doi: 10.1128/JB.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh S., Korza G., Maciejewski M., Setlow P. Analysis of metabolism in dormant spores of Bacillus species by 31P nuclear magnetic resonance analysis of low-molecular-weight compounds. J. Bacteriol. 2015;197:992–1001. doi: 10.1128/JB.02520-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnelly M.L., Fimlaid K.A., Shen A. Characterization of Clostridium difficile spores lacking either SpoVAC or dipicolinic acid synthetase. J. Bacteriol. 2016;198:1694–1707. doi: 10.1128/JB.00986-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berendsen E.M., Wells-Bennik M.H., Krawczyk A.O., de Jong A., van Heel A., Eijlander R.T., Kuipers O.P. Draft genome sequences of 10 Bacillus subtilis strains that form spores with high or low heat resistance. Genome Announc. 2016;4 doi: 10.1128/genomeA.00124-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunst F., Ogasawara N., Moszer I., Albertini A.M., Alloni G.O., Azevedo V., Bertero M.G., Bessières P., Bolotin A., Borchert S. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 26.Jalili V., Afgan E., Gu Q., Clements D., Blankenberg D., Goecks J., Taylor J., Nekrutenko A. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2020 update. Nucleic Acids Res. 2020;48:W395–W402. doi: 10.1093/nar/gkaa434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., Madden T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cock P.J., Chilton J.M., Grüning B., Johnson J.E., Soranzo N. NCBI BLAST+ integrated into Galaxy. Gigascience. 2015;4:s13742-015. doi: 10.1186/s13742-015-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tu Z., RAbhyankar W., NSwarge B., van der Wel N., Kramer G., Brul S., de Koning L.J. Artificial sporulation induction (ASI) by kinA overexpression affects the proteomes and properties of Bacillus subtilis spores. Int. J. Mol. Sci. 2020;21:4315. doi: 10.3390/ijms21124315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scopes R.K. Measurement of protein by spectrophotometry at 205 nm. Anal. Biochem. 1974;59:277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- 31.Tyanova S., Temu T., Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016;11:2301. doi: 10.1038/nprot.2016.136. [DOI] [PubMed] [Google Scholar]
- 32.Pham T.V., Henneman A.A., Jimenez C.R. Iq: An R package to estimate relative protein abundances from ion quantification in DIA-MS-based proteomics. Bioinformatics. 2020;36:2611–2613. doi: 10.1093/bioinformatics/btz961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritchie M.E., Phipson B., Wu D.I., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang D.W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 36.Zhu B., Stülke J. SubtiWiki in 2018: From genes and proteins to functional network annotation of the model organism Bacillus subtilis. Nucleic Acids Res. 2018;46:D743–D748. doi: 10.1093/nar/gkx908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coleman W.H., Chen D., Li Y., Cowan A.E., Setlow P. How moist heat kills spores of Bacillus subtilis. J. Bacteriol. 2007;189:8458–8466. doi: 10.1128/JB.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coleman W.H., Zhang P., Li Y.-Q., Setlow P. Mechanism of killing of spores of Bacillus cereus and Bacillus megaterium by wet heat. Lett. Appl. Microbiol. 2010;50:507–514. doi: 10.1111/j.1472-765X.2010.02827.x. [DOI] [PubMed] [Google Scholar]
- 39.Cox J., Hein M.Y., Luber C.A., Paron I., Nagaraj N., Mann M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom. 2014;13:2513–2526. doi: 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartels J., Blüher A., López Castellanos S., Richter M., Günther M., Mascher T. The Bacillus subtilis endospore crust: Protein interaction network, architecture and glycosylation state of a potential glycoprotein layer. Mol. Microbiol. 2019;112:1576–1592. doi: 10.1111/mmi.14381. [DOI] [PubMed] [Google Scholar]
- 41.Beall B., Driks A., Losick R., Moran C.P. Cloning and characterization of a gene required for assembly of the Bacillus subtilis spore coat. J. Bacteriol. 1993;175:1705–1716. doi: 10.1128/JB.175.6.1705-1716.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nunes F., Fernandes C., Freitas C., Marini E., Serrano M., Moran Jr C.P., Eichenberger P., Henriques A.O. SpoVID functions as a non-competitive hub that connects the modules for assembly of the inner and outer spore coat layers in Bacillus subtilis. Mol. Microbiol. 2018;110:576–595. doi: 10.1111/mmi.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naclerio G., Baccigalupi L., Zilhao R., De Felice M., Ricca E. Bacillus subtilis spore coat assembly requires cotH gene expression. J. Bacteriol. 1996;178:4375–4380. doi: 10.1128/JB.178.15.4375-4380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferguson C.C., Camp A.H., Losick R. GerT, a newly discovered germination gene under the control of the sporulation transcription factor σK in Bacillus subtilis. J. Bacteriol. 2007;189:7681–7689. doi: 10.1128/JB.01053-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steil L., Serrano M., Henriques A.O., Völker U. Genome-wide analysis of temporally regulated and compartment-specific gene expression in sporulating cells of Bacillus subtilis. Microbiology. 2005;151:399–420. doi: 10.1099/mic.0.27493-0. [DOI] [PubMed] [Google Scholar]
- 46.Thierbach S., Sartor P., Yücel O., Fetzner S. Efficient modification of the Pseudomonas aeruginosa toxin 2-heptyl-1-hydroxyquinolin-4-one by three Bacillus glycosyltransferases with broad substrate ranges. J. Biotechnol. 2020;308:74–81. doi: 10.1016/j.jbiotec.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Turner R.J., Lu Y., Switzer R.L. Regulation of the Bacillus subtilis pyrimidine biosynthetic (pyr) gene cluster by an autogenous transcriptional attenuation mechanism. J. Bacteriol. 1994;176:3708–3722. doi: 10.1128/JB.176.12.3708-3722.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takamatsu H., Kodama T., Watabe K. Assembly of the CotSA coat protein into spores requires CotS in Bacillus subtilis. FEMS Microbiol. Lett. 1999;174:201–206. doi: 10.1111/j.1574-6968.1999.tb13569.x. [DOI] [PubMed] [Google Scholar]
- 49.Koide Y., Nakamura A., Uozumi T., Beppu T. Cloning and sequencing of the major intracellular serine protease gene of Bacillus subtilis. J. Bacteriol. 1986;167:110–116. doi: 10.1128/JB.167.1.110-116.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han X., Shiwa Y., Itoh M., Suzuki T., Yoshikawa H., Nakagawa T., Nagano H. Molecular cloning and sequence analysis of an extracellular protease from four Bacillus subtilis strains. Biosci. Biotechnol. Biochem. 2013;77:870–873. doi: 10.1271/bbb.120920. [DOI] [PubMed] [Google Scholar]
- 51.Zheng L., Abhyankar W., Ouwerling N., Dekker H.L., van Veen H., van der Wel N.N., Roseboom W., de Koning L.J., Brul S., de Koster C.G. Bacillus subtilis spore inner membrane proteome. J. Proteome Res. 2016;15:585–594. doi: 10.1021/acs.jproteome.5b00976. [DOI] [PubMed] [Google Scholar]
- 52.Bernhards C.B., Chen Y., Toutkoushian H., Popham D.L. HtrC is involved in proteolysis of YpeB during germination of Bacillus anthracis and Bacillus subtilis spores. J. Bacteriol. 2015;197:326–336. doi: 10.1128/JB.02344-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doan T., Morlot C., Meisner J., Serrano M., Henriques A.O., Moran C.P., Jr., Rudner D.Z. Novel secretion apparatus maintains spore integrity and developmental gene expression in Bacillus subtilis. PLoS Genet. 2009;5:e1000566. doi: 10.1371/journal.pgen.1000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodrigues C.D., Henry X., Neumann E., Kurauskas V., Bellard L., Fichou Y., Schanda P., Schoehn G., Rudner D.Z., Morlot C. A ring-shaped conduit connects the mother cell and forespore during sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA. 2016;113:11585–11590. doi: 10.1073/pnas.1609604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vega-Cabrera L.A., Guerrero A., Rodríguez-Mejía J.L., Tabche M.L., Wood C.D., Gutierrez-Rios R.-M., Merino E., Pardo-Lopez L. Analysis of Spo0M function in Bacillus subtilis. PLoS ONE. 2017;12:e0172737. doi: 10.1371/journal.pone.0172737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han W.-D., Kawamoto S., Hosoya Y., Fujita M., Sadaie Y., Suzuki K., Ohashi Y., Kawamura F., Ochi K. A novel sporulation-control gene (spo0M) of Bacillus subtilis with a σH-regulated promoter. Gene. 1998;217:31–40. doi: 10.1016/S0378-1119(98)00378-3. [DOI] [PubMed] [Google Scholar]
- 57.Kuwana R., Yamamura S., Ikejiri H., Kobayashi K., Ogasawara N., Asai K., Sadaie Y., Takamatsu H., Watabe K. Bacillus subtilis spoVIF (yjcC) gene, involved in coat assembly and spore resistance. Microbiology. 2003;149:3011–3021. doi: 10.1099/mic.0.26432-0. [DOI] [PubMed] [Google Scholar]
- 58.Van Ooij C., Eichenberger P., Losick R. Dynamic patterns of subcellular protein localization during spore coat morphogenesis in Bacillus subtilis. J. Bacteriol. 2004;186:4441–4448. doi: 10.1128/JB.186.14.4441-4448.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rebeil R., Sun Y., Chooback L., Pedraza-Reyes M., Kinsland C., Begley T.P., Nicholson W.L. Spore photoproduct lyase from Bacillus subtilis spores is a novel iron-sulfur DNA repair enzyme which shares features with proteins such as class III anaerobic ribonucleotide reductases and pyruvate-formate lyases. J. Bacteriol. 1998;180:4879–4885. doi: 10.1128/JB.180.18.4879-4885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Newman J.A., Rodrigues C., Lewis R.J. Molecular basis of the activity of SinR protein, the master regulator of biofilm formation in Bacillus subtilis. J. Biol. Chem. 2013;288:10766–10778. doi: 10.1074/jbc.M113.455592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quentin Y., Fichant G., Denizot F. Inventory, assembly and analysis of Bacillus subtilis ABC transport systems. J. Mol. Biol. 1999;287:467–484. doi: 10.1006/jmbi.1999.2624. [DOI] [PubMed] [Google Scholar]
- 62.Popham D.L., Setlow P. Cloning, nucleotide sequence, and regulation of the Bacillus subtilis pbpE operon, which codes for penicillin-binding protein 4* and an apparent amino acid racemase. J. Bacteriol. 1993;175:2917–2925. doi: 10.1128/JB.175.10.2917-2925.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scheffers D.-J. Dynamic localization of penicillin-binding proteins during spore development in Bacillus subtilis. Microbiology. 2005;151:999–1012. doi: 10.1099/mic.0.27692-0. [DOI] [PubMed] [Google Scholar]
- 64.Wang S.T., Setlow B., Conlon E.M., Lyon J.L., Imamura D., Sato T., Setlow P., Losick R., Eichenberger P. The forespore line of gene expression in Bacillus subtilis. J. Mol. Biol. 2006;358:16–37. doi: 10.1016/j.jmb.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 65.Bressuire-Isoard C., Broussolle V., Carlin F. Sporulation environment influences spore properties in Bacillus: Evidence and insights on underlying molecular and physiological mechanisms. FEMS Microbiol. Rev. 2018;42:614–626. doi: 10.1093/femsre/fuy021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.