Abstract
Social exclusion refers to the experience of being disregarded or rejected by others and has wide-ranging negative consequences for well-being and cognition. Cyberball, a game where a ball is virtually tossed between players, then leads to the exclusion of the research participant, is a common method used to examine the experience of social exclusion. The neural correlates of social exclusion remain a topic of debate, particularly with regards to the role of the dorsal anterior cingulate cortex (dACC) and the concept of social pain. Here we conducted a quantitative meta-analysis using activation likelihood estimation (ALE) to identify brain activity reliably engaged by social exclusion during Cyberball task performance (Studies = 53; total N = 1,817 participants). Results revealed consistent recruitment in ventral anterior cingulate and posterior cingulate cortex, inferior and superior frontal gyri, posterior insula, and occipital pole. No reliable activity was observed in dACC. Using a probabilistic atlas to define dACC, fewer than 15% of studies reported peak coordinates in dACC. Meta-analytic connectivity mapping suggests patterns of co-activation are consistent with the topography of the default network. Reverse inference for cognition associated with reliable Cyberball activity computed in Neurosynth revealed social exclusion to be associated with cognitive terms Social, Autobiographical, Mental States, and Theory of Mind. Taken together, these findings highlight the role of the default network in social exclusion and warns against interpretations of the dACC as a key region involved in the experience of social exclusion in humans.
Keywords: Meta-analysis, Cyberball, Social exclusion, Default network, Meta-analytic connectivity modeling, Functional MRI
1. Introduction
Exclusion from social participation is an all too common, yet psychologically painful, facet of the human experience. Being bullied by peers at school, discrimination at the workplace, and rejection from a romantic partner are all experiences that can lead a person to feel the sting of social exclusion. This sensitivity to social exclusion is deeply rooted in a need for social connectedness (Baumeister and Leary, 1995; Williams, 2009). Consequently, the brain has developed systems to efficiently recognize and respond to signs of social exclusion across a range of situations (Cacioppo and Hawkley, 2009; Eisenberger et al., 2003; Fisher et al., 2010; Masten et al., 2011b). Due to its pervasiveness and importance for human functioning, social neuroscientists have sought to understand the underlying neural processes involved in reactions to social exclusion.
Previous neuroimaging studies have examined the neural correlates of social exclusion. These studies vary in their approach, but one of the most commonly employed paradigms used to evoke feelings of social exclusions in an experimental setting is the Cyberball task. Cyberball is a computerized virtual ball-tossing game played between the participant and other virtual players (Williams et al., 2000). The traditional Cyberball paradigm involves two rounds: an “inclusion” round during which the ball is received and tossed equally among all players, subsequently followed by an “exclusion” round during which the other players no longer pass the ball to the participant, thereby eliciting feelings of social exclusion. In their seminal study, Eisenberger et al. (2003) used a Cyberball task to investigate the neural response to social exclusion. Results from this study showed increased activity in the dorsal anterior cingulate (dACC), anterior insula, and right ventral prefrontal cortex during the exclusion round relative to the inclusion round (Eisenberger et al., 2003). Critically, increased activity in the dACC and anterior insula were shown to correlate with self-reports of social distress after exclusion. Based on prior work demonstrating activity of the dACC during the experience of physical pain (Rainville et al., 1997; Singer et al., 2004), this finding was interpreted to suggest that social exclusion is experienced as ‘painful’ and led to the hypothesis of overlap in the neural circuitry underlying social pain and physical pain (Eisenberger et al., 2003; Eisenberger, 2012a–b; Lieberman and Eisenberger, 2015).
Subsequent studies have since substantiated this claim. Activation of the dACC has been reported during Cyberball (Beeney et al., 2011; Dewall et al., 2010; Lieberman and Eisenberger, 2015; Onoda et al., 2010) and other social exclusion paradigms (O’Connor et al., 2008; Sebastian et al., 2010). Similar findings have also been observed during third person (Beeney et al., 2011; Meyer et al., 2013) or recollected experiences of social exclusion (Kross et al., 2011). However, findings from several studies suggest that the dACC is not specific to the experience of social or physical pain, but instead responds to various cognitive and emotional events (Somerville et al., 2006; Wager et al., 2016; Kragel et al., 2018; Perini et al., 2018). Other studies have shown that the emotional responses to social exclusion involves the subgenual subdivision of the anterior cingulate cortex rather than the dACC (Masten et al., 2009; Bolling et al., 2011b), hinting at dissociable neural representations for physical and social pain (Woo et al., 2014). Thus, while the dACC has been highlighted as key region within the literature, the lack of consistent correspondence between the neural correlates of social and physical pain have led to questions regarding the association between social exclusion and dACC.
Earlier meta-analyses of functional imaging studies aimed at identifying reliable neural correlates of social exclusion have also provided inconclusive results. When restricting the analysis to the anterior cingulate, one meta-analysis showed involvement of the dACC during social exclusion (Rotge et al., 2015). Yet, when examining across studies of social exclusion, irrespective of dACC reported activity, other meta-analytic studies have failed to find reliable dACC activity (Cacioppo et al., 2013; Vijayakumar et al., 2017). Moreover, when focusing specifically on neuroimaging studies of social exclusion using the Cyberball task, the dACC did not emerge as a region that was reliably engaged across 29 studies (Vijayakumar et al., 2017). In contrast, more ventral anterior cingulate cortex (vACC) as well as ventral prefrontal cortex and orbitofrontal cortex were more reliably recruited across past meta-analyses (Cacioppo et al., 2013; Vijayakumar et al., 2017). Further, regions of the default network have also been implicated in mentalizing about the intentions of other people, both during (Bolling et al., 2011a; Onoda et al., 2009; Wagels et al., 2017) and after (Powers et al., 2013) social exclusion. Therefore, engagement of this network may constitute an important component of the intrapersonal and interpersonal processes of social exclusion (Kawamoto et al., 2015). However, the extent to which the default network is engaged in social exclusion requires further investigation.
The present study aims to identify areas of convergence in functional activity and co-activation patterns of brain regions engaged during social exclusion measured during Cyberball. Using coordinate-based activation likelihood estimation (ALE) meta-analysis (Eickhoff et al., 2009, 2012), we identify reliable whole-brain activation patterns of social exclusion across neuroimaging studies using traditional and alternating (interspersed sequences of inclusion and exclusion) Cyberball designs. This study extends prior meta-analyses on social exclusion (Cacioppo et al., 2013; Vijayakumar et al., 2017) in several ways: First, we use meta-analytic connectivity modeling (MACM) (Eickhoff et al., 2011; Laird et al., 2009) to characterize the functional connectivity profile of regions identified in the ALE analysis. Second, we use Neurosynth (Yarkoni et al., 2011) to meta-analytically decode the cognitive processes associated with the identified neural patterns from the ALE analysis. Finally, we directly investigate whether dACC, a core node in the hypothesized common substrate of physical and social pain, is reliably engaged by Cyberball. Taking this approach allows us to not only delineate brain regions that have consistently been associated with social exclusion, but it can provide new insights into putative neural networks associated with social exclusion, and decode the psychological processes of these brain regions using valid reverse inference, in the largest sample of studies currently available.
2. Methods
2.1. Literature search and study selection
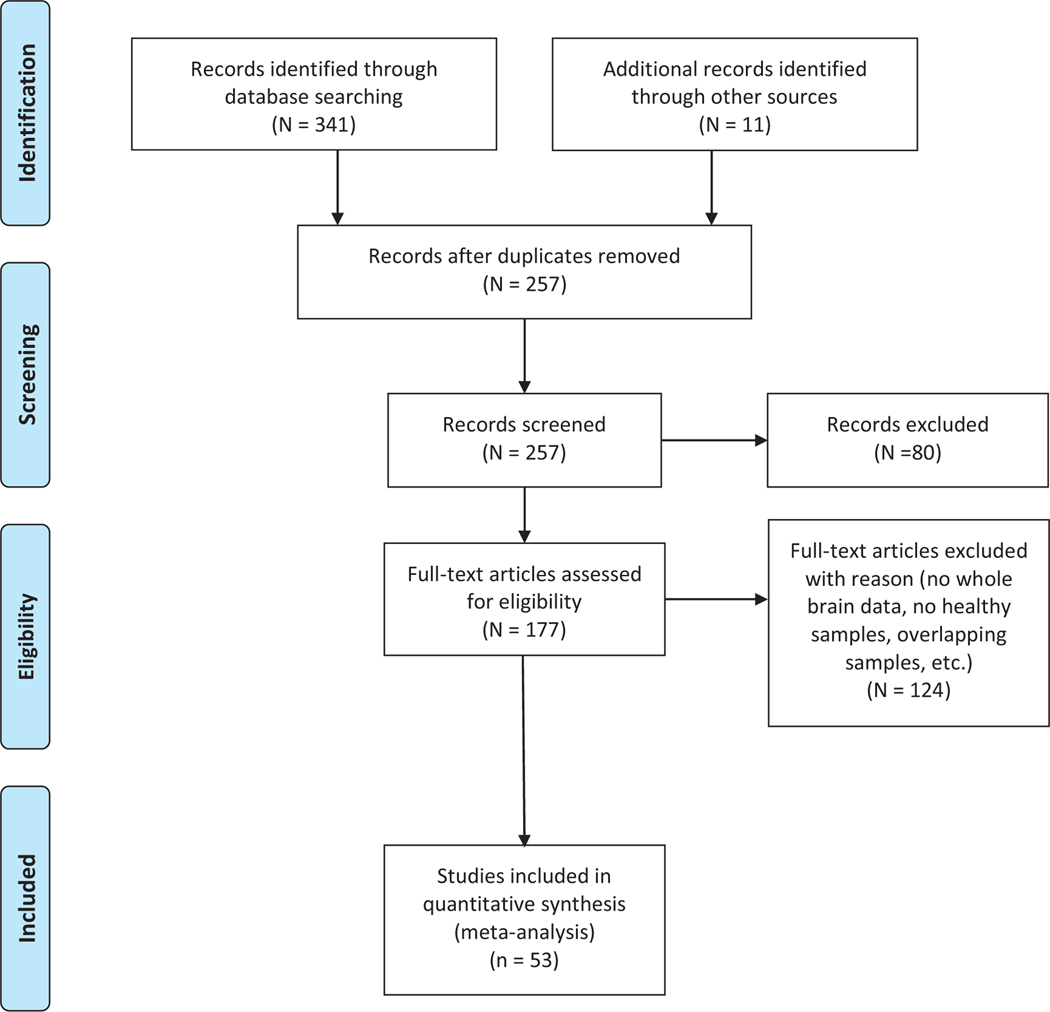
We performed a systematic review of functional magnetic resonance imaging studies investigating the neural correlates of social exclusion using Cyberball. All articles in the literature published from October 10th, 2003 to August 19th, 2020 were considered for this meta-analysis. We used PubMed/MEDLINE, and PsychINFO online databases to search for articles with abstracts, titles, and keywords using the following search string: (social rejection OR social exclusion OR ostracism) AND (MRI OR fMRI OR functional magnetic resonance imaging OR brain imaging). The search yielded 341 articles. Reference lists of relevant articles were manually searched for additional publications not captured in the online database searches yielding 257 non-duplicate articles.
Studies were included if they met the following criteria: 1) used Cyberball behavioral paradigm as an experimental manipulation for social exclusion; 2) were empirical investigations (i.e. not review articles); 3) they employed fMRI; 4) reported group main effects of an exclusion/rejection condition relative to an inclusion/acceptance condition; 5) studied healthy subjects; and 6) used whole-brain analyses with reported Montreal Neurologic Institute (MNI) or Talairach coordinates. A flow chart illustration of the literature review and study selection process can be viewed in Fig. 1. Following the criteria defined above, 53 studies were included in the present study. It should be noted that 7 studies included in our final list involved participants watching others being excluded (referred to hereafter as others-exclusion; Beeney et al., 2011; Masten et al., 2011b; Meyer et al., 2013; Novembre et al., 2015; van der Meulen et al., 2017; Tousignant et al., 2018; Lelieveeld et al., 2020), and two studies included in combined the whole-brain results for their clinical and healthy controls (Domsalla et al., 2014, van Harmelen et al., 2014). Analyses excluding these 9 studies are also provided.
Fig. 1.
Flowchart of article selection, following PRISMA guidelines. Adapted from (Moher, 2009).
2.2. Coordinate based meta-analysis
2.2.1. Activation likelihood estimation (ALE) analysis
A coordinate-base meta-analysis of fMRI studies using Cyberball was conducted with the revised version of the ALE algorithm (Eickhoff et al., 2009, 2012). The software package GingerALE (3.0.2; www.brainmap.org/ale) was used to perform two analyses on coordinates from the studies identified by the literature search (Eickhoff et al., 2012; Laird et al., 2009). Coordinates from studies reporting in Talairach space were converted to MNI space using the FSL transformation applied in GingerALE (Eickhoff et al., 2012). ALE computes the statistical spatial convergence of activation coordinates (foci) across studies. The algorithm models this convergence by creating a 3-dimensional Gaussian distribution representing the spatial uncertainty around each coordinate. The width of the distribution is weighted by the number of participants for each study, such that studies with large sample sizes have smaller Gaussian distributions thereby reflecting a more reliable approximation of the true activation. Once a model of the brain activation map is computed for each study, the maps are aggregated to identify areas of spatial convergence between activation foci that are greater than would be expected by chance.
To better control for the false-positive rates, the ALE image was thresholded using two different thresholds. The first employed a conservative threshold (p < 0.05 FWE; 5000 permutations, p < 0.001 cluster forming threshold). A second, more liberal threshold used a cluster forming threshold of p < 0.01, a cluster-based family-wise error (FWE) corrected threshold of p < 0.05, and 5,000 permutations (Eickhoff et al., 2012). Significant clusters using the more conservative threshold were then used as seeds to perform a region-to-whole-brain co-activation meta-analysis (MACM; Eickhoff et al., 2011; Laird et al., 2009).
2.2.2. Analysis
Five meta-analyses were performed using GingerALE: (1) full sample (53 studies, 1,817 participants); (2) traditional Cyberball design (29 studies, 1,021 participants); (3) adult samples (33 studies, 1,094 participants); (4) alternating Cyberball design (17 studies; 565 participants); and (5) studies reporting statistically significant increased self-reported distress after exclusion (20 studies; 632 participants). Other than the meta-analysis on the full sample, all sub-analyse (2–5) did not include others-exclusion studies. For the full sample, we also provide results omitting 9 studies (7 studies of others-exclusion; 2 studies with combined whole brain results for healthy and clinical samples). The current recommendations for ALE meta-analyses is to include a minimum of 17–20 studies to obtain sufficient power to detect valid results from ALE analysis and to prevent results from being driven by a single experiment (Eickhoff et al., 2016; Müller et al.,2018). All meta-analyses satisfy this recommendation. To examine the effects of study design, we also performed a contrast analysis between the traditional and alternating Cyberball design. Although we were interested in examining the effects of age, due to the limited number of studies for the developmental sample (n = 13), a contrast analysis between age groups was not included due to insufficient power.
2.2.3. Meta-analytic connectivity modeling (MACM)
To provide a more comprehensive view of the co-activation pattern of brain regions associated with Cyberball task, we conducted MACM analyses for each ALE clusters. MACM allows for generating whole brain co-activation patterns for a given predefined region of interest across a range of experimental neuroimaging tasks and paradigms. Analogous to seed-based connectivity analysis of resting sate fMRI data, MACM assumes that regions that consistently coactivate across experiments can be pooled to create a map of functionally connected networks. Importantly, this approach is able to capture brain regions which are functionally connected, but that may also be part of an indirect network (Robinson et al., 2010). MACM leverages the BrianMap database (www.brainmap.org), a large online repository of human neuroimaging studies, to reveal brain regions that consistently activate together above chance with a given predefined region of interests across a large set of neuroimaging experiments (Eickhoff et al., 2011; Laird et al., 2009). We created six different brain masks reflecting the six significant clusters obtained from the ALE meta-analysis cluster image from the full sample. Binarized brain masks for each cluster were generated using Nilearn (https://nilearn.github.io/index.html; Abraham et al., 2014) on the basis of the voxel assignment corresponding to the ALE cluster they belong to. Sleuth (version 3.0.4, www.brainmap.org/sleuth) was used to search the BrainMap database for foci within each ALE cluster mask. Searches were conducted to include studies that reported increased activation. The search criteria were limited to statistical contrasts that reported activations (i.e. task > baseline) in non-clinical populations. Studies that reported peak activation coordinates within each significant ALE cluster were assessed to establish each cluster’s whole-brain co-activation pattern (cluster-level FWE < 0.05; p -value < 0.001; 5000 permutations).
2.3. Neurosynth cognitive decoder
After determining reliable activation patterns, we meta-analytically decoded the cognitive terms associated with this resulting ALE map from the full sample of 53 studies. Neurosynth is a meta-analytic tool that contains a database for over 14,000 functional neuroimage studies. The brain activation patterns and peak signal coordinates in the database are paired with associated cognitive terms (Yarkoni et al., 2011; https://neurosynth.org). Taking a reverse inference approach, the Neurosynth decoder function was used to compare the activation pattern in our ALE map with those of all neuroimaging studies in the database. To do this, we first uploaded an unthresholded z-statistics map to NeuroVault which is a repository for neuroimaging studies. The Neurosynth decoder function is an integrated feature within NeuroVault, and was used it to compute a voxel-wise Pearson correlation coefficient between our ALE map each of the term-based z-statistics maps extracted from Neurosynth. The cognitive profile corresponding to the activation pattern from the meta-analysis was determined by identifying the most likely cognitive terms given activation in the ALE map. This produced a list of 1,335 terms, each with a correlation score to indicates the relative strength of association with our ALE map. The top 20 terms (excluding all anatomical, redundant, and methodologic terms) were ranked by the correlation strength between the brain regions reliably engaged during social exclusion and Neurosynth maps, and visualized as a word cloud. The ALE map archived in NeuroVault (https://neurovault.org/collections/6199/) and can be used to generate the complete list of terms.
2.4. dACC study count
To compare the frequency with which published studies report dACC peak coordinates, we defined the boundaries of a dACC ROI using the Harvard Oxford probabilistic template (cingulate [anterior division] and paracingulate gyri posterior to the genu of the corpus callosum, p > 0). Foci were clustered into four categories based on the Harvard Oxford atlas: 1) studies reporting non-dACC peaks localized outside the dACC ROI, 2) studies reporting foci with the anatomical label dACC, but the coordinate fell outside of the ROI, 3) studies reporting dACC peaks that fell within the ROI, and 4) studies reporting foci that fell within the dACC ROI but were not given the dACC anatomical label.
3. Results
3.1. Meta-analysis on the full sample of Cyberball studies
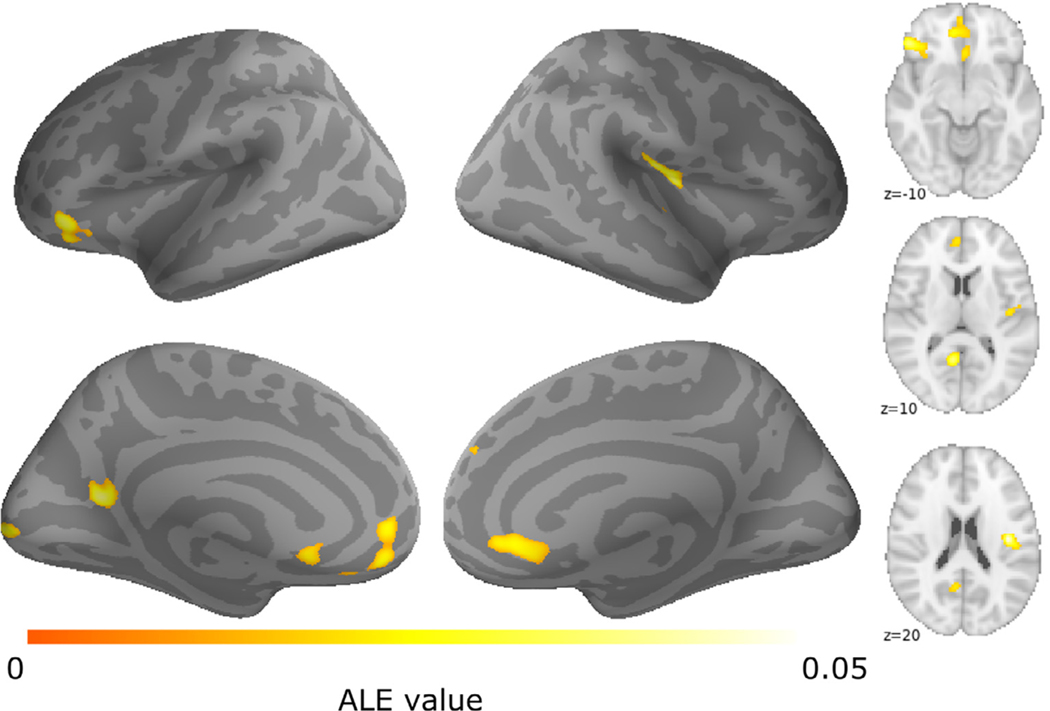
Reliable patterns of brain activity were examined in 53 studies of Cyberball, revealing six clusters of activity (Table 2; Fig. 2). On the medial aspect of the frontal lobe, we found bilateral activation of vACC, extending anteriorly towards the ventral and medial prefrontal cortices. Cyberball exclusion reliably activated the right posterior insula, right superior frontal gyrus, left IFG, left posterior cingulate cortex (PCC), and left occipital pole. All ALE results images are archived in NeuroVault (https://neurovault.org/collections/6199/).
Table 2.
Note: Results of the full sample ALE meta-analysis. For each cluster, brain region label, hemisphere, MNI coordinates, ALE maxima, cluster size (mm3), and number of studies are provided. vACC = ventral anterior cingulate cortex; IFG = inferior frontal gyrus; PCC = posterior cingulate cortex; RSC = retrosplenial cortex; SFG = superior frontal gyrus; L = left hemisphere; R = right hemisphere.
| Brain regions | Hemi | x | y | z | ALE max. | Cluster Number | Volume (mm3) | N Studies (foci) |
|---|---|---|---|---|---|---|---|---|
| vACC | L | −2 | 42 | −14 | 0.03 | 1 | 5,384 | 21(34) |
| Posterior insula | R | 40 | −14 | 18 | 0.06 | 2 | 2,296 | 11(15) |
| IFG | L | −48 | 34 | −10 | 0.04 | 3 | 2,264 | 11(13) |
| PCC/RSC | L | −8 | −56 | 12 | 0.04 | 4 | 1,523 | 8(8) |
| Occipital pole | L | −12 | −94 | 4 | 0.04 | 5 | 1,112 | 6(8) |
| SFG | R | 2 | 50 | 30 | 0.02 | 6 | 848 | 5(8) |
Fig. 2.
Results of cyberball social exclusion ALE meta-analysis. Brain areas showing consistent activation during social exclusion across (a) the full sample of Cyberball studies included in the meta-analysis (n = 53).
Similar results were also observed when using a more liberal threshold (see Supplementary Figure 1). When omitting the others-exclusion studies and the 2 studies that combined the whole-brain results of their healthy and clinical samples, all clusters except for the superior frontal gyrus remained (Supplementary Table 1).
3.2. Meta-analysis of Cyberball design
The next meta-analyses focused on Cyberball design to examine whether restricting the analysis to studies using the traditional Cyberball design (one round of inclusion followed by one round of exclusion) or the alternating design (repeated alternating blocks of inclusion and exclusion) to induce social exclusion might highlight a different activation pattern than that identified using the full Cyberball sample. Across all studies using the traditional design (n = 29; subjects = 1,021; foci = 300), ALE analysis identified a similar pattern of convergence as observed for the full sample. Social exclusion using the traditional Cyberball design was associated with activity in three clusters identified in the full sample: left inferior frontal gyrus extending to the anterior insula, left occipital pole, and right superior frontal gyrus (see Supplementary Figure 1, Supplementary Figure 2 and Supplemental Table 2). The alternating design (n = 17, subjects = 565; foci = 170) was associated with the remaining two clusters identified in the full sample: the left vACC and right posterior insula. We did additionally find a cluster in the right central opercular cortex (see Supplementary Figure 1, Supplemental Figure 2 and Supplemental Table 2).
Contrast analyses between the traditional and alternating designs revealed reliably reported activity in left anterior insula for the traditional compared to the alternating design. The right central and parietal opercular cortex showed more reliable activation in the alternating relative to the traditional design (Supplementary Table 2).
3.3. Meta-analysis across studies of adults
To examine potential developmental effects in social exclusion, we conducted a meta-analysis of Cyberball studies using an adult population (n = 33; subjects = 1,094; foci = 350). The adult sample showed reliable activity in PCC, posterior insula, and subgenual and vACC (see Supplementary Figure 1, Supplementary Figure 3 and Supplementary Table 3). A preliminary analysis of children (Age less than 18 years old; n = 13; subjects = 480; foci = 121) is provided in supplemental material (Supplementary Figure 3 and Supplementary Table 3).
3.4. Meta-analysis of self-reported distress
Further sub-analyses focused only on those studies where participants reported greater subjective experience of distress following exclusion (n = 20; subjects = 632; foci = 184) revealed similar clusters as for the full sample. Social exclusion in this sub-sample was associated with engagement of the vACC, and bilateral IFG (see Supplementary Figure 1, Supplementary Figure 4 and Supplementary Table 4).
3.5. Functional connectivity of the derived ALE-clusters—MACM analysis
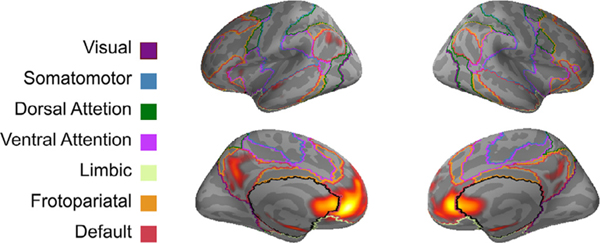
To characterize the reliable activation associated with social exclusion, we examined the functional co-activation of the ALE map for the full sample with other brain regions. We performed MACM analyses to obtain cluster-specific connectivity maps that represent brain regions that coactivate with the largest and most reliable ALE cluster for the full sample (cluster1-bilateral vACC). The co-activation based meta-analytic map for cluster 1 is shown in Fig. 3 and corresponding peak maxima are reported in Table 3. ALE analysis examining the whole-brain co-activation pattern associated with the vACC showed co-activation with anterior and posterior cortical midline structures. Specifically, bilateral ventromedial prefrontal cortex extending towards the subgenual portion of the pregenual ACC, left superior frontal gyrus, and bilaterally in the PCC. The ACC cluster also coactivated with the left inferior parietal lobule, bilateral parahippocampal gyrus, and middle temporal gyrus. Furthermore, when depicted in conjunction with Yeo 7-Network atlas (Yeo et al., 2011), we show in Fig. 3 that the co-activation pattern for the ACC cluster aligns with the default network in medial prefrontal, as well as medial and lateral parietal cortex and temporal cortex. Supplementary Figures 5–9 and Supplementary Tables 5–9 show MACM results for clusters 5–6. MACM results images are archived in NeuroVault (https://neurovault.org/collections/6199/)
Fig. 3.
MACM map of co-activation of the vACC cluster (cluster 1) derived from the full sample ALE meta-analysis. Results represent the brain areas that significantly coactivate with brain regions that are most reliably recruited during social exclusion (p < 0.001, FWE cluster-level corrected at p < 0.05). The functional connectivity map for the vACC cluster is juxtaposed with outlines of the Yeo 7-Network atlas (Yeo et al., 2011). MACM co-activation pattern (yellow/red) overlap with the default network and portions the limbic network. See Table 3 for coordinates.
Table 3.
Cluster 1 MACM result: clusters of functional co-activation associated with ALE cluster 1 (vACC-medial prefrontal cortex) from the full sample ALE meta-analysis. Brain region label, hemisphere, MNI coordinates, ALE value, and cluster size (mm3).
| Brain region | Hemi | x | y | z | ALE max. | Cluster Number | Volume (mm3) |
|---|---|---|---|---|---|---|---|
| vACC | L | −2 | 46 | −12 | 0.30 | 1 | 48,264 |
| dorsal PCC | L | −2 | −52 | 29 | 0.10 | 2 | 14,312 |
| Parahippocampal gyrus | L | −20 | −8 | −20 | 0.10 | 3 | 5,104 |
| Inferior parietal lobule | L | −42 | −76 | 34 | 0.08 | 4 | 5,080 |
| Parahippocampal gyrus | R | 22 | −6 | −20 | 0.10 | 5 | 4,792 |
| Orbitofrontal cortex | L | −40 | 24 | −14 | 0.06 | 6 | 3,408 |
| Superior frontal gyrus | L | −22 | 32 | 44 | 0.07 | 7 | 3,408 |
| Middle temporal gyrus | L | −56 | −10 | −18 | 0.07 | 8 | 1,488 |
Note. L, Left; R, Right. MACM-cluster thresholded at p < 0.001 corrected for multiple comparisons using cluster-level FWE correction at p < 0.05. x, y, z coordinates provided in MNI space.
3.6. Cognitive terms associated activation
To provide valid reverse inference into the cognitive processes associated with the meta-analytic map for Cyberball, we performed functional decoding of the ALE results from the full sample. The Neurosynth decoder function was used assess the similarity of the activation of the unthresholded ALE map with statistical maps generated for the entire set of terms included in the Neurosynth database. The top 20 Neurosynth cognitive terms with the highest correlation values are listed in Supplementary Table 10 and visualized as a word cloud in Fig. 4. The emerging pattern of ALE activation for the full sample was more associated with social- and self- cognition, as well as reward-related terms, such as: social, autobiographical, referential, mental states, reward, theory mind, value. The highest correlation was observed for the term social (r = 0.18).
Fig. 4.
Decoding of the ALE map for the full sample using Neurosynth decoder. The decoder was used to compare the unthresholded ALE map (full sample) with statistical maps generated by Neurosynth across a wide range of terms (1,335 terms). Depicted above is the word cloud showing the top 20 relevant cognitive terms that correlated with the pattern of activation for social exclusion. Font size represents relative correlation strength of that term to the full sample Cyberball meta-analytic results.
3.7. dACC study count
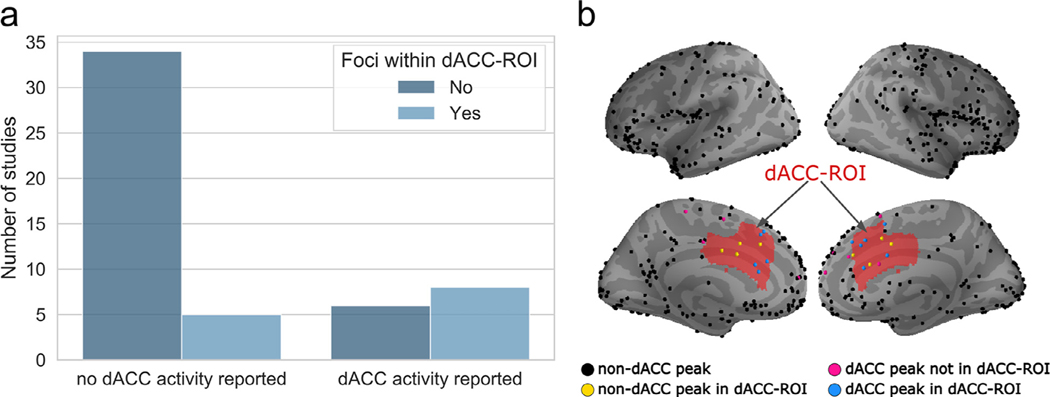
Contrary to previous reports (Eisenberger, 2012a; Lieberman & Eisenberger, 2015), ALE results for the full sample and traditional sub-sample of Cyberball studies did not show reliable activation of the dACC. To further examine this inconsistency, we used a probabilistic atlas of the dACC to quantify the number of studies with reported peak voxels in the dACC. Less than one quarter of neuroimaging studies included in this meta-analysis reported anatomical labels for peak coordinates as the dACC (Fig. 5a). Of the 14 studies reporting dACC activity, the locations for nearly half of these studies (n = 6; Fig. 5a) did not have peak voxels located within the boundaries of the dACC (Fig. 5b). Four studies report coordinates within the dACC but provide a different anatomical label (e.g. medial prefrontal cortex).
Fig. 5.
Study count of reported dACC activity across all studies listed in Table 1. (a) A qualitative assessment comparing the number of studies that reported no peak activation in the dACC relative to studies that reported peak activation in the dACC during Cyberball social exclusion. Using a dACC-ROI map (created from the Harvard-Oxford probabilistic atlas) to cross-reference foci locations, studies with foci located outside of the dACC-ROI are portrayed in dark blue; those with foci located within the dACC-ROI in light blue. (b) Activation foci reported by studies included in the full sample ALE analysis are plotted on the brain surface. The red shaded area represents boundaries of a dACC ROI map. Non-dACC reported foci are color-coded base on whether they were located outside (black) or inside (yellow) of the ROI. Similarly, reported foci that were anatomical labeled as dACC located outside (pink) and inside (blue) the ROI are shown.
4. Discussion
The present study conducted a coordinate based meta-analysis of social exclusion neuroimaging studies using the Cyberball paradigm. Using ALE, we found that social exclusion reliably engages bilateral vACC, right posterior insula, right superior frontal gyrus, left inferior frontal gyrus, left PCC, and left occipital pole. Similar patterns of activation were observed when restricting the analysis to studies to account for variable experimental manipulations and participant cohorts. Using a MACM approach to functionally characterize the pattern of co-activation from our ALE results, we demonstrate functional covariance of brain activity consistent with the topography of the default network. Implementing valid reverse inference with Neurosynth, Cyberball activity was associated with social- and self-related cognitive terms, consistent with the functional role of the default network in cognition (Andrews–Hanna, Smallwood and Spreng, 2014). While the neural response to social exclusion has been conceptualized within a social pain framework, strongly implicating the dACC, we found no converging evidence supporting dACC activation during social exclusion.
The meta-analysis results for the full sample indicate that several brain regions distributed broadly along the medial and lateral prefrontal cortices are consistently activated during Cyberball. In the prefrontal cortices, we found a large bilateral ventromedial prefrontal cluster including the pregenual and subgenual portions of the ACC (e.g. vACC). The ventral sub-region of the ACC has often been implicated in studies on emotion (Somerville et al., 2006). Increased activity in the vACC is associated with greater rejection sensitivity (Burklund et al., 2007), self-reported distress during social exclusion (Rotge et al., 2015), and engagement of this region may reflect emotional processing of negative emotions induced by social exclusion (Bolling et al., 2011b; Sebastian et al., 2011). We also identified three additional clusters—one in the right posterior insula, a second in the right superior frontal gyrus, and a third in bilateral inferior frontal gyrus. The posterior insula is implicated in mediating sensorimotor processes of exteroceptive and interoceptive information (Chang et al., 2013; Craig, 2002; Wager et al., 2004; Uddin, 2015), the inferior frontal gyrus plays a role in top-down cognitive control (Badre and Wagner, 2007), and the superior frontal gyrus which is encompassed within the dorsal medial prefrontal cortex commonly implicated in social-reflective tasks such as making judgements about others (Andrews-Hanna et la., 2014). The activation of these regions during social exclusion are linked to affective response and cognitive regulation of feelings of social exclusion (Bolling et al., 2011b; Sebastian et al., 2011; Rotge et al., 2015). We also identified clusters in left PCC and left occipital pole. The PCC has strong anatomical connections with ventromedial prefrontal areas, and is a core hub of the default network (Andrews-Hanna et al., 2010). Together with the ventromedial areas, inferior and superior frontal gyri, these regions are part of the default network and are functionally integrated regions that support a wide range of self-generated cognitive processes, such as mentalizing and autobiographical recollection (Spreng et al., 2009; Andrews-Hanna et al., 2014). These default network regions are also responsive to visual social information during goal-oriented tasks (Spreng et al., 2014).
Neural correlates of social exclusion may be impacted by differences in methodological approaches, such as task design and participant populations of social rejection. Factors related to task design (Somerville et al., 2006; Rotge et al., 2015) and age-related differences in rejection sensitivity (Somerville et al., 2006), and self-reported distress (Rotge et al., 2015) can differentially impact neural activity during exclusion. When restricting our analyses to Cyberball studies using the traditional and alternating designs, adult sample, and studies that reported significant increases in subjective distress after exclusion, we found similar clusters of activation as seen in the full sample.
Extending previous meta-analyses of neuroimaging studies using Cyberball, we conducting a MACM analysis with the observed bilateral vACC cluster as a seed region. The resulting meta-analytic functional connectivity map largely overlapped with regions of the default network (encompassing the medial prefrontal cortex, superior frontal gyrus, PCC, inferior parietal lobule, and hippocampus) and the orbitofrontal cortex, an extended region of the default network (Uddin, Yeo, Spreng, 2019). These results suggest that the functional co-activation pattern observed for this social exclusion cluster is spatially coherent with the default network.
Our results extend prior work by demonstrating that the functional characterization of regions reliably engaged during Cyberball coactivate with the default network which is known to be critical for reflective cognitive processes (Andrews–Hanna et al., 2014). They also add to a body of work linking social exclusion to a network of brain regions that are distinct from that previously identified in the extant literature on social pain. Using multivoxel pattern analysis, Woo et al. (2014) demonstrated that while the experience of social rejection and physical pain may engage similar brain areas, these experiences evoke dissociable functional connectivity patterns. When examining whole-brain network dynamics during Cyberball, social exclusion is associated with increased within-network connectivity of the default network (Schmälzle et al., 2017). The functional connectivity map derived for the vACC cluster results is largely consistent with this finding. Furthermore, our findings parallel results from seed-based connectivity showing increased connectivity between the vACC and default network brain regions during social exclusion (Bolling et al., 2011a).
Using cognitive decoding to characterize the emerging pattern of ALE activation, we show that the cognitive processes primarily associated with the identified neural patterns relate to both social and self-referential cognitive terms, mentalizing and mental inference, and valence terms. The decoding results showed a small association between social exclusion task activity and ‘pain’ and ‘painful’. Overall, these terms may capture spontaneous situational thoughts such as, “Why are they leaving me out?”, which include mental state attribution and self-reference, along with the emotional experience of pain. Given that social exclusion is a complex phenomenon, this result underscores that the interplay between social cognitive and affective processing is an important component used to navigate a potentially challenging interpersonal situation. This may be particularly relevant in light of work pointing to increased emotion regulation processes following social exclusion (DeWall et al., 2011) and differential neural responsivity to positive and negative social feedback in regions implicated in social-affective processing (i.e. vACC) (Jankowski et al., 2018; Morese et al., 2019; Powers et al., 2013; Wagels et al., 2017). Collectively, our results highlight the importance of the default network in the experience of social exclusion by virtue of this network’s involvement in self, social and emotional processes (Andrews–Hanna et al., 2014). Extending from this, a population neuroscience study has implicated the default network as central to the experience of loneliness (Spreng et al., 2020).
The neuroimaging literature on social exclusion has emphasized the role of the dACC in social pain, particularly given the association between activation in this region and subjective ratings of distress. The present ALE results show that social exclusion does not reliably engage the dACC, even when we lowered the statistical threshold for significance. One possibility for the lack of dACC engagement might be due to the task. Our results are limited to the Cyberball paradigm, yet other types of social exclusions paradigms have reported dACC activation (Gündel et al., 2003; O’Connor et al., 2008; Fisher et al., 2010; Kross et al., 2011; see Eisenberger et al., 2012b for review). Different paradigms might not evoke the same level of distress. Indeed, a prior meta-analysis on social exclusion did find that the Cyberball task showing less dACC activity compared to other social exclusion tasks (Rotge et al., 2015). Taking this into consideration, when we restricted our analysis to studies where there was a significant increase in participants’ self-reported distress, we still found no reliable dACC activity.
The dACC is often used by various studies as a region of interest, occasionally without providing any supplementary whole-brain analysis (Chester et al., 2014; Chester and DeWall, 2016; Dewall et al., 2010; Kashdan et al., 2014). It is possible that early observed effects (e.g. Eisenberger et al., 2003), with small samples by current standards, may have introduced a confirmation bias towards dACC, thereby obscuring findings of other brain regions that are more reliably recruited during social exclusion. Our findings underscore potential bias with the misattribution of observed peaks to the dACC, alongside the relatively sparse number of peaks within dACC.
The studies that report no dACC activity attribute the lack of replication to various factors such as differences in methodological approach (modified Cyberball, event-related design) or study population (adolescents versus adults) (Masten et al., 2011a; Masten et al., 2009; Moor et al., 2012; Sebastian et al., 2011). Others discuss their findings in terms of support for the ventral portion of the ACC’s involvement in indexing negative affect and the dorsal portion being involved in cognitive control (Onoda et al., 2009; Shackman et al., 2011; Wagels et al., 2017). An alternate predominant view of dACC activation for social exclusion may have motivated bias in subsequent reports to fit their results in the social pain framework. Indeed, some studies without whole brain dACC effects conduct additional region of interest analysis on the dACC (Asscheman et al., 2019) or lowered the statistical threshold for significance (Bollings et al., 2011b). Analyses investigating neural correlations with self-reported distress can more directly speak to the participant’s neural response to the experience of social exclusion. However, correlations between distress and the dACC have been inconsistent (Masten et al., 2009; Onoda et al., 2009; Masten et al., 2011a; DeWall et al., 2012; Kawamoto et al., 2012; Moor et al., 2012; Eisenberger, 2012a; Will et al., 2015). As the field moves forward in characterizing the neural correlates of social exclusion, it is critically important that reliable observations of brain activity be considered above confirmation bias.
The main advantage of taking a coordinate-based meta-analytic approach is that it is data-driven and is considered a robust method for unbiased integration of previously published functional neuroimaging literature. However, some important limitations should be acknowledged. First, we pooled across neuroimaging studies using the Cyberball task (both traditional and alternating design). We did not include studies utilizing other social exclusion paradigms. While our findings are largely consistent with previous meta-analyses which did include analyses using other social exclusion paradigms (Cacioppo et al., 2013; Vijayakumar et al., 2017), inferences regarding the meaning of the findings may not be generalizable to other social exclusion paradigms (i.e. romantic rejection, viewing visual stimuli of rejection). In pooling peak coordinates reported in published activation tables, the shape and extent of the primary result clusters in a volume may not be well-characterized. This will likely result in imperfect alignment of activity across studies. However, in the absence of consistently archived results images, coordinate based meta-analysis remains the most effective approach to amalgamating neuroimaging finds across studies. Another important limitation is that while we did provide a sub-analysis focused on studies where there was statistical evidence of greater subjective distress following exclusion, this analysis does not directly speak to how social distress correlates with brain activity. We recommend that future studies perform whole-brain regression analyses with self-reported distress to more directly identify which brain regions are involved in the affective response to social exclusion. Finally, the Neurosynth decoder is constrained by the term-based maps in the database (Yarkoni et al., 2011). Neurosynth automatically extract high frequency terms from the abstract of each study in the database, which can impact the specificity of cognitive terms. While the terms from the cognitive decoding complement our interpretation of an association between social exclusion and default network recruitment, we do not claim that there is a unique role for activity in any of the brain regions identified in this meta-analysis and cognitive terms obtained from our analysis. The correlation values represent how well the spatial distribution of activation associated with each term in the database matches the reliable activation patterns of our ALE result map. Despite these limitations, Neurosynth represents a powerful tool for decoding cognitive terms and has been shown to have high sensitivity and specificity for identify distinct neural networks (see Rubin et al., 2017; Yarkoni et al.,2011). The functional characterization results are useful for developing hypotheses that provide a better fit to the data, and allow the field to move forward towards a better understanding of the neural and cognitive-affective basis of social exclusion.
In summary, the current meta-analysis of Cyberball reveals a reliable pattern of brain activation distributed across medial prefrontal cortex, inferior and superior frontal gyri, posterior cingulate cortex and posterior insula. This pattern largely overlaps with the default network, and is associated with self-referential processes, mentalizing and valence related terms. Together, these results provide evidence for a primary role of the default network in the experience of social exclusion.
Supplementary Material
Table 1.
Cyberball studies meeting inclusion criteria.
| No. | Reference | N | Gender ratio (F/M) |
Age M(sd) | Cyberball design | Age group | Target of exclusion | Increased self-reported distress after exclusion |
N (foci) | dACC activity reported | Peaks in dACC ROI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ascheman et al., 2019 | 55 | (0/55) | 10.40(0.74) | Traditional | Developmental | Self | yes | 6 | no | no |
| 2 | Bach et al., 2019 | 21 | (2/19) | 1: 38.53(6.5) 2: 38.19(8.1) | Alternating | Adult | Self | yes | 9 | no | no |
| 3 | Beeney et al., 2011 | 20 | (20/0) | 24.6(5.8) | Traditional | Adult | Others | n/a | 9 | yes | yes |
| 4 | Bolling et al., 2011a | 21 | (6/15) | 12.90(2.59) | Alternating | Developmental | Self | yesb | 10 | no | no |
| 5 | Bolling et al., 2011b | 23 | (12/11) | 24.0(3.81) | Alternating (picture) | Adult | Self | no | 12 | no | no |
| 6 | Bolling et al., 2012 | 20 | (9/11) | 24.99 (3.91) | Alternating (picture) | Adult | Self | yesb | 12 | no | no |
| 7 | Bolling et al., 2015 | 15 | (6/9) | 1: 12.96(2.7) 2: 12.36(4.2) 3: 11.88 (3.2) | Alternating | Developmental | Self | yesb | 12 | no | yes |
| 8 | Bolling et al., 2016 | 20 | (10/10) | 12.61(2.5) | Alternating | Developmental | Self | yesb | 12 | no | no |
| 9 | Bonenberger et al., 2015 | 21 | (21/0) | 22.2(3.38) | Traditional | Adult | Self | no | 8 | no | no |
| 10 | Cheng et al., 2020 | 69 | (36/33) | 14.2(1.5) | Alternating | Developmental | Self | yesb | 4 | no | yes |
| 11 | Chester et al., 2018 | 60 | (38/22) | 20.28(2.77) | Traditional | Adult | Self | no | 19 | no | no |
| 12 | Cogoni et al., 2018 | 36 | (19/17) | 23.2(3.51) | Alternating (video) | Adult | Self | no | 14 | no | no |
| 13 | Domsalla et al., 2014 | 20 | (20/ 0) | 1: 29(2) 2: 28.7(7.8) | Alternating | Adult | Self | yesb | 12 | no | no |
| 14 | de Water et al., 2017 | 31 | (17/14) | 14.49(1.06) | Alternating | Developmental | Self | yes | 7 | no | yes |
| 15 | DeWall et al., 2012 | 25 | (16/9) | Undergraduate | Traditional | Adult | Self | yesb | 15 | no | no |
| 16 | Eisenberger et al., 2003 | 13 | (9/4) | Undergraduate | Traditional | Adult | Self | yes | 4 | yes | yes |
| 17 | Falk et al., 2014 | 36 | (36/0) | 16.8(0.47) | Traditional | Developmental | Self | yes | 6 | no | no |
| 18 | Gilman et al., 2016 | 42 | (22/20) | 1: 21.5(1.9) 2: 20.6(2.5) | Traditional | Adult | Self | yesb | 5 | no | no |
| 19 | Gonzalez et al., 2015 | 85 | (45/40) | 24.5(1.35) | Traditional | Adult | Self | no | 6 | yes | yes |
| 20 | Gradin et al., 2012 | 16 | (9/7) | 1: 40.87(11.72) 2: 41.23(11.78) | Alternating | Adult | Self | yesb | 3 | no | no |
| 21 | Hanlon et al., 2019 | 25 | (14/11) | 1: 33.5(6) 2: 38.1(6.1) | Traditional | Adult | Self | yes | 1 | yes | yes |
| 22 | Heeren et al., 2017 | 23 | (23/0) | 1: 24.96(6/6) 2: 25.30(5.62) | Traditional (events) | Adult | Self | yes | 5 | yes | yes |
| 23 | Karramans et al., 2011 | 15 | (20/5) | 22 (19–33) | Traditional | Adult | Self | yesb | 13 | no | no |
| 24 | Kawamoto et al., 2012 | 22 | (19/3) | 20.7(1.7) | Alternating (events) | Adult | Self | yes | 12 | yes | yes |
| 25 | Le et al., 2020 | 64 | (33/31) | 47.1(16.3) | Traditional | Adult | Self | no | 27 | yes | yes |
| 26 | Lelieveld et al., 2012 | 30 | (16/14) | 20.00(1.05) | Traditional | Adult | Self | no | 3 | no | no |
| 27 | Lelieveld et al., 2020 | 43 | (25/18) | 20.95(1.89) | Traditional | Adult | Others | n/a | 3 | yes | no |
| 28 | Luo et al., 2016 | 42 | (21/21) | 1: 20.38(1.7) 2: 20.38(1.12) | Traditional | Adult | Self | yes | 16 | yes | yes |
| 29 | Malejko et al., 2018 | 17 | (17/0) | 1: 23(4.26) 2: 23.3(4.13) 3: 28.7(4.59) | Traditional | Adult | Self | yesb | 11 | no | no |
| 30 | Masten et al., 2010 | 23 | (14/9) | 13.0(no sd) | Traditional | Developmental | Self | no | 4 | no | no |
| 31 | Masten et al., 2011a | 18 | (9/9) | 21.4(no sd) | Traditional | Adult | Self | yesb | 15 | no | no |
| 32 | Masten et al., 2011b | 18 | (9/9) | 20.22(no sd) | Traditional | Adult | Others | n/a | 5 | no | no |
| 33 | Masten et al., 2011c | 17 | (2/15) | 1:14.0(2.4) 2:13.6(2.5) | Traditional | Developmental | Self | yes | 19 | no | no |
| 34 | Maurage et al., 2012 | 22 | (0/22) | 1: 45.1(10.69) 2: 47.2(11.04) | Traditional | Adult | Self | yes | 7 | yes | yes |
| 35 | McIver et al., 2018 | 45 | (36/9) | 17.7(0.60) | Traditional | Developmental | Self | yesb | 11 | yes | yes |
| 36 | Meyer et al., 2013 | 16 | (12/4) | 21.69(2.12) | Traditional (picture) | Adult | Others | n/a | 7 | yes | yes |
| 37 | Moor et al., 2010 | 53 | (31/22) | 1: 11.8(.87) 2: 15.74(0.74) 3: 20.38(0.85) | Traditional (events) | Developmental | Self | yes | 9 | no | no |
| 38 | Nishiyama et al., 2015 | 46 | (29/17) | 19.85(no sd) | Traditional | Adult | Self | yes | 14 | no | no |
| 39 | Novembre et al., 2015 | 23 | (23/0) | 22.4(2.0) | Alternating | Adult | Self & Others | n/a | 13 | no | yes |
| 40 | Olié et al., 2017 | 28 | (28/0) | 1: 38.9 (no sd) 2: 41 (no sd) 3: 36 (no sd) | Traditional | Adult | Self | yesb | 1 | no | no |
| 41 | Onoda et al., 2009 | 26 | (15/11) | 21.7(1.3) | Traditional | Adult | Self | yes | 2 | no | no |
| 42 | Preller et al., 2016 | 21 | (9/12) | 26.48(4.76) | Traditional | Adult | Self | no | 26 | yes | yes |
| 43 | Puetz et al., 2016 | 51 | (26/25) | 1:10.6(1.75) 2:10.38(1.67) | Traditional | Developmental | Self | yesb | 2 | no | no |
| 44 | Radke et al., 2018 | 80 | (40/40) | 1: 24.38(3.37) 2: 24.69(3.85) | Alternating | Adult | Self | no | 5 | no | no |
| 45 | Sebastian et al., 2011 | 35 | (35/0) | 1: 15.44(0.81) 2: 28.70(3.91) | Alternating | Developmental & Adult | Self | yes | 12 | no | no |
| 46 | Tousignant et al., 2018 | 40 | (20/20) | 1: 14.25(1.33) 2: 24.25(1.97) | Alternating (picture) | Developmental & Adult | Others | n/a | 9 | yes | yes |
| 47 | van den Berg et al., 2018 | 72 | (44/28) | 36.2(16.17) | Alternating | Adult | Self | no | 11 | no | yes |
| 48 | van der Meulen et al., 2017 | 71 | (39/32) | 1: 8.15(1.06) 2: 8.23(0.67) 3: 8.21(0.71) | Traditional | Developmental | Others | n/a | 29 | no | no |
| 49 | van Harmelen et al., 2014 | 46 | (34/12) | 1: 18.31(1.95) 2: 18.85(0.25) | Traditional | Adult | Self | yes | 8 | no | no |
| 50 | Wagels et al., 2017 | 40 | (20/20) | 27.80(7.86) | Alternating | Adult | Self | no | 20 | no | no |
| 51 | Will et al., 2015 | 28 | (16/12) | 20.7(1.97) | Traditional (event) | Adult | Self | yes | 13 | no | no |
| 52 | Will et al., 2016 | 44 | (18/26) | 14.0(0.70) | Traditional (event) | Developmental | Self | yes | 19 | no | no |
| 53 | Wudarczyk et al., 2015 | 24 | (10/14) | 24.33(2.91) | Alternating | Adult | Self | yes | 3 | no | no |
Note: For Age M(sd), bolded items represent group(s) whose data was included in the meta-analysis. “Alternating” involves Cyberball design with alternating inclusion and exclusion blocks. Modifications made in the Cyberball design are indicated in parentheses (e.g. using an event-related design, providing a picture of a person to represent the other Cyberball players, etc.). “Self” involves participants being excluded while “Others” involves participants watching friends or strangers being excluded.
There is a discrepancy between the total number of subjects listed and the gender ratio listed in this reference.
Studies that provided average self-reported distress scores but did not perform any statistically assessment of social distress after exclusion.
Acknowledgements
This project was supported in part by the Natural Sciences and Engineering Research Council of Canada, Canadian Institute of Health Research, the National Institute of Aging (AG068563), and the Canada First Research Excellence Fund, awarded to McGill University for the Healthy Brains, Healthy Lives initiative. R.N.S is a Research Scholar supported by Fonds de recherche du Québec – Santé.
Footnotes
Data and code availability statement
All data are accessible within the studies cited in Table 1. Extracted coordinates are available from either author upon request.
All data were analyzed with software publicly available from http://www.brainmap.org/ and https://neurosynth.org/.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2020.117666.
Credit author statement
Mwilambwe-Tshilobo: Conceptualization, Methodology, Investigation, Data Curation, Formal analysis, Visualization, Writing
Spreng: Conceptualization, Methodology, Writing, Supervision
References
- Abraham F Pedregosa, Eickenberg M, Gervais P, Mueller A, Kossaifi, Gramfort A, Thirion B, Varoquaux G, 2014. Machine learning for neuroimaging with scikit-learn. Front. Neuroinform 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL, 2010. Evidence for the default network’s role in spontaneous cognition. J. Neurophysiol 104 (1), 322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN, 2014. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci 1316 (1), 29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asscheman JS, Koot S, Ma I, Buil JM, Krabbendam L, Cillessen AHN, van Lier PAC, 2019. Heightened neural sensitivity to social exclusion in boys with a history of low peer preference during primary school. Develop. Cogn. Neurosci 38, 100673. doi: 10.1016/j.dcn.2019.100673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach P, Frischknecht U, Bungert M, Karl D, Vollmert C, Vollstadt-Klein S, Lis S, Kiefer F, Hermann D, 2019. Effects of social exclusion and physical pain in chronic opioid maintenance treatment: FMRI correlates. Eur. Neuropsychopharmacol 29 (2), 291–305. doi: 10.1016/j.euroneuro.2018.11.1109. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD, 2007. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 45 (13), 2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR, 1995. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychol. Bull 117 (3), 479–529. doi: 10.1037//0033-2909.117.3.497. [DOI] [PubMed] [Google Scholar]
- Beeney JE, Franklin RG Jr, Levy KN, Adams RB Jr, 2011. I feel your pain: emotional closeness modulates neural responses to empathically experienced rejection. Soc. Neurosci 6 (4), 369–376. doi: 10.1080/17470919.2011.557245. [DOI] [PubMed] [Google Scholar]
- Bolling DZ, Pelphrey KA, Vander Wyk BC, 2015. Trait-level temporal lobe hypoactivation to social exclusion in unaffected siblings of children and adolescents with autism spectrum disorders. Develop. Cogn. Neurosci 13, 75–83. doi: 10.1016/j.dcn.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pelphrey KA, Vander Wyk BC, 2016. Unlike adults, children and adolescents show predominantly increased neural activation to social exclusion by members of the opposite gender. Soc. Neurosci 11 (5), 475–486. doi: 10.1080/17470919.2015.1117019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, Mayes LC, Pelphrey KA, 2011a. Development of neural systems for processing social exclusion from childhood to adolescence. Dev. Sci 14 (6), 1431–1444. doi: 10.1111/j.1467-7687.2011.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McPartland JC, Mayes LC, Pelphrey KA, 2011b. Dissociable brain mechanisms for processing social exclusion and rule violation. Neuroimage 54 (3), 2462–2471. doi: 10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonenberger M, Plener PL, Groschwitz RC, Gron G, Abler B, 2015. Polymorphism in the micro -opioid receptor gene (OPRM1) modulates neural processing of physical pain, social rejection and error processing. Exp. Brain Res 233 (9), 2517–2526. doi: 10.1007/s00221-015-4322-9. [DOI] [PubMed] [Google Scholar]
- Burklund LJ, Eisenberger NI, Lieberman MD, 2007. The face of rejection: Rejection sensitivity moderates dorsal anterior cingulate activity to disapproving facial expressions. Soc. Neurosci 2 (3–4), 238–253. doi: 10.1080/7470910701391711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo S, Frum C, Asp E, Weiss RM, Lewis JW, Cacioppo JT, 2013. A quantitative meta-analysis of functional imaging studies of social rejection. Sci. Rep 3, 2027. doi: 10.1038/srep02027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, 2009. Perceived social isolation and cognition. Trends Cogn. Sci 13 (10), 447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG, 2013. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb. Cortex 23 (3), 739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester DS, DeWall CN, 2016. Sound the alarm: the effect of narcissism on retaliatory aggression is moderated by dACC reactivity to rejection. J. Pers 84 (3), 361–368. doi: 10.1111/jopy.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester DS, Eisenberger NI, Pond RS, Richman SB, Bushman BJ, DeWall CN, 2014. The interactive effect of social pain and executive functioning on aggression: an fMRI experiment. Soc. Cogn. Affect. Neurosci 9 (5), 699–704. doi: 10.1093/scan/nst038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester DS, Lynam DR, Milich R, DeWall CN, 2018. Neural mechanisms of the rejection-aggression link. Soc. Cogn. Affect. Neurosci 13 (5), 501–512. doi: 10.1093/scan/nsy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD., 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3 (8), 655–666. doi: 10.1038/nrn894, [DOI] [PubMed] [Google Scholar]
- Cogoni C, Carnaghi A, Silani G, 2018. Reduced empathic responses for sexually objectified women: an fMRI investigation. Cortex 99, 258–272. doi: 10.1016/j.cortex.2017.11.020. [DOI] [PubMed] [Google Scholar]
- de Water E, Mies GW, Ma I, Mennes M, Cillessen AHN, Scheres A, 2017. Neural responses to social exclusion in adolescents: Effects of peer status. Cortex 92, 32–43. doi: 10.1016/j.cortex.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Dewall CN, Macdonald G, Webster GD, Masten CL, Baumeister RF, Powell C, Combs D, Schurtz DR, Stillman TF, Tice DM, Eisenberger NI, 2010. Acetaminophen reduces social pain: Behavioral and neural evidence. Psychol. Sci 21 (7), 931–937. doi: 10.1177/0956797610374741. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Masten CL, Powell C, Combs D, Schurtz DR, Eisenberger NI, 2012. Do neural responses to rejection depend on attachment style? An fMRI study. Soc. Cogn. Affect. Neurosci 7 (2), 184–192. doi: 10.1093/scan/nsq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWall CN, Twenge JM, Koole SL, Baumeister RF, Marquez A, Reid MW, 2011. Automatic emotion regulation after social exclusion: tuning to positivity. Emotion 11 (3), 623–636. doi: 10.1037/a0023534. [DOI] [PubMed] [Google Scholar]
- Domsalla M, Koppe G, Niedtfeld I, Vollstädt-Klein S, Schmahl C, Bohus M, Lis S, 2014. Cerebral processing of social rejection in patients with borderline personality disorder. Soc. Cogn. Affect. Neurosci 9 (11), 1789–1797. doi: 10.1093/scan/nst176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT, 2011. Coactivation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57 (3), 938–949. doi: 10.1016/j.neuroimage.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT, 2009. Coordinate-based ALE meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp 30 (9), 2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT, 2012. Activation likelihood estimation meta-analysis revisited. NeuroImage 59 (3), 2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Nichols TE, Laird AR, et al. , 2016. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 137, 70–85. doi: 10.1016/j.neuroimage.2016.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, 2012a. The neural bases of social pain: Evidence for shared representations with physical pain. Psychosom. Med 74 (2), 126–135. doi: 10.1097/PSY.0b013e3182464dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, 2012b. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat. Rev. Neurosci 13 (6), 421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD, 2003. Does rejection hurt? An fMRI study of social exclusion. Science 302 (5643), 290. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Falk EB, Cascio CN, O’Donnell MB, Carp J, Tinney FJ Jr, Bingham CR, Shope JT, Ouimet MC, Pradhan AK, Simons-Morton BG, 2014. Neural responses to exclusion predict susceptibility to social influence. J. Adolescent Health 54 (5 Suppl), S22–S31. doi: 10.1016/j.jadohealth.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher HE, Brown LL, Aron A, Strong G, Mashek D, 2010. Reward, addiction, and emotion regulation systems associated with rejection in love. J. Neurophysiol 104 (1), 51–60. doi: 10.1152/jn.00784.2009. [DOI] [PubMed] [Google Scholar]
- Lelieveld GJ, Harris LT, van Dillen LF, 2020. Jumping on the ‘bad’wagon? How group membership influences responses to the social exclusion of others. Soc. Cogn. Affect. Neurosci 15 (5), 571–586. doi: 10.1093/scan/nsaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Curran MT, Calderon V, Schuster RM, Evins AE, 2016. Altered neural processing to social exclusion in young adult marijuana users. biological psychiatry. Cogn. Neurosci. Neuroimaging 1 (2), 152–159. doi: 10.1016/j.bpsc.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MZ, Beckes L, Chango J, Allen JP, Coan JA, 2015. Adolescent neighborhood quality predicts adult dACC response to social exclusion. Soc. Cogn. Affect.Neurosci 10 (7), 921–928. doi: 10.1093/scan/nsu137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gündel H, O’Connor M-F, Littrell L, Fort C, Lane RD, 2003. Functional Neuroanatomy of Grief: An fMRI Study. Am. J. Psychiat 160 (11), 1946–1953. doi: 10.1176/appi.ajp.160.11.1946. [DOI] [PubMed] [Google Scholar]
- Gradin VB, Waiter G, Kumar P, Stickle C, Milders M, Matthews K, Reid I, Hall J, Steele JD, 2012. Abnormal neural responses to social exclusion in schizophrenia. PLoS One 7 (8). doi: 10.1371/journal.pone.0042608, [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Heeren A, Dricot L, Billieux J, Philippot P, Grynberg D, Timary P, Maurage P, 2017. Correlates of social exclusion in social anxiety disorder: an fMRI study. Sci. Rep 7 (1), 260. doi: 10.1038/s41598-017-00310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski KF, Batres J, Scott H, Smyda G, Pfeifer JH, Quevedo K, 2018. Feeling left out: depressed adolescents may atypically recruit emotional salience and regulation networks during social exclusion. Soc. Cogn. Affect. Neurosci 13 (8), 863–876. doi: 10.1093/scan/nsy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan TB, Dewall CN, Masten CL, Pond RSJ, Powell C, Combs D, Schurtz DR, Farmer AS, 2014. Who is most vulnerable to social rejection? The toxic combination of low self-esteem and lack of negative emotion differentiation on neural responses to rejection. PLoS One 9 (3), e90651. doi: 10.1371/journal.pone.0090651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T, Onoda K, Nakashima KI, Nittono H, Yamaguchi S, Ura M, 2012. Is dorsal anterior cingulate cortex activation in response to social exclusion due to expectancy violation? An fMRI study. Front. Evol. Neurosci 4, 11. doi: 10.3389/fnevo.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T, Ura M, Nittono H, 2015. Intrapersonal and interpersonal processes of social exclusion. Front. Neurosci 9, 62. doi: 10.3389/fnins.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel PA, Kano M, Oudenhove LV, Ly HG, Dupont P, Rubio A, Delon-Martin C, Bonaz BL, Manuck SB, Gianaros PJ, Ceko M, Losin EAR, Woo C-W, Nichols TE, Wager TD, 2018. Generalizable representations of pain, cognitive control, and negative emotion in medial frontal cortex. Nat. Neurosci 21 (2), 283–289. doi: 10.1038/s41593-017-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD, 2011. Social rejection shares somatosensory representations with physical pain. PNAS 108 (15), 6270–6275. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT, 2009. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J. Neurosci 29 (46), 14496–14505. doi: 10.1523/JNEU-ROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TM, Zhornitsky S, Wang W, Li CR, 2020. Perceived burdensomeness and neural responses to ostracism in the Cyberball task. J. Psychiatr. Res 130, 1–8. doi: 10.1016/j.jpsychires.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld G-J, Moor BG, Crone EA, Karremans JC, Beest I.van, 2012. A penny for your pain? The financial compensation of social pain after exclusion. Soc. Psychol. Personality Sci doi: 10.1177/1948550612446661. [DOI] [Google Scholar]
- Lelieveld GJ, Harris LT, van Dillen LF, 2020. Jumping on the ‘bad’wagon? How group membership influences responses to the social exclusion of others. Soc. Cogn. Affect. Neurosci 15 (5), 571–586. doi: 10.1093/scan/nsaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, 2015. The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. PNAS 112 (49), 15250–15255. doi: 10.1073/pnas.1515083112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Yu D, Han S, 2016. Genetic and neural correlates of romantic relationship satisfaction. Soc. Cogn. Affect. Neurosci 11 (2), 337–348. doi: 10.1093/scan/nsv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malejko K, Neff D, Brown R, Plener PL, Bonenberger M, Abler B, Graf H, 2018. Neural correlates of social inclusion in borderline personality disorder. Front. Psychiatry 9. doi: 10.3389/fpsyt.2018.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Colich NL, Rudie JD, Bookheimer SY, Eisenberger NI, Dapretto M, 2011a. An fMRI investigation of responses to peer rejection in adolescents with autism spectrum disorders. Develop. Cogn. Neurosci 1 (3), 260–270. doi: 10.1016/j.dcn.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M, 2009. Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Soc. Cogn. Affect. Neurosci 4 (2), 143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Morelli SA, Eisenberger NI, 2011b. An fMRI investigation of empathy for “social pain” and subsequent prosocial behavior. Neuroimage 55 (1), 381–388. doi: 10.1016/j.neuroimage.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Eisenberger NI, 2010. An fMRI Investigation of attributing negative social treatment to racial discrimination. J. Cogn. Neurosci 23 (5), 1042– 1051. doi: 10.1162/jocn.2010.21520. [DOI] [PubMed] [Google Scholar]
- Maurage P, Joassin F, Philippot P, Heeren A, Vermeulen N, Mahau P, Delperdange C, Corneille O, Luminet O, de Timary P, 2012. Disrupted regulation of social exclusion in alcohol-dependence: an fMRI study. Neuropsychopharmacology 37 (9), 2067–2075. doi: 10.1038/npp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver TA, Bosma RL, Sandre A, Goegan S, Klassen JA, Chiarella J, Booij L, Craig W, 2018. Peer victimization is associated with neural response to social exclusion. Merrill Palmer Q 64 (1), 135–161. doi: 10.13110/merrpalmquar1982.64.1.0135. [DOI] [Google Scholar]
- Meyer ML, Masten CL, Ma Y, Wang C, Shi Z, Eisenberger NI, Han S, 2013. Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Soc.l Cogn. Affect. Neurosci 8 (4), 446–454. doi: 10.1093/scan/nss019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med 151 (4), 264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Moor BG, Guroglu B, Op de Macks ZA, Rombouts SARB, Van der Molen MW, Crone EA, 2012. Social exclusion and punishment of excluders: Neural correlates and developmental trajectories. Neuroimage 59 (1), 708–717 c. [DOI] [PubMed] [Google Scholar]
- Moor BG, van Leijenhorst L, Rombouts SARB, Crone EA, Van der Molen MW, 2010. Do you like me? Neural correlates of social evaluation and developmental trajectories. Soc. Neurosci 5 (5–6), 461–482. [DOI] [PubMed] [Google Scholar]
- Morese R, Lamm C, Bosco MF, Valentini MC, Silani G, 2019. Social support modulates the neural correlates underlying social exclusion. Soc. Cogn. Affect. Neurosci doi: 10.1093/scan/nsz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller VI, Cieslik EC, Laird AR, et al. , 2018. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev 84, 151–161. doi: 10.1016/j.neubiorev.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y, Okamoto Y, Kunisato Y, Okada G, Yoshimura S, Kanai Y, Yamamura T, Yoshino A, Jinnin R, Takagaki K, Onoda K, Yamawaki S, 2015. FMRI study of social anxiety during social ostracism with and without emotional support. PLoS One 10 (5). doi: 10.1371/journal.pone.0127426, e0127426–e0127426https://doi.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novembre G, Zanon M, Silani G, 2015. Empathy for social exclusion involves the sensory-discriminative component of pain: a within-subject fMRI study. Soc. Cogn. Affect. Neurosci 10 (2), 153–164. doi: 10.1093/scan/nsu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M-F, Wellisch DK, Stanton AL, Eisenberger NI, Irwin MR, Lieberman MD, 2008. Craving love? Enduring grief activates brain’s reward center. Neuroimage 42 (2), 969–972. doi: 10.1016/j.neuroimage.2008.04.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olié E, Jollant F, Deverdun J, de Champfleur NM, Cyprien F, Le Bars E, Mura T, Bonafe A, Courtet P, 2017. The experience of social exclusion in women with a history of suicidal acts: a neuroimaging study. Sci. Rep 7 (1), 89. doi: 10.1038/s41598-017-00211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S, 2009. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Soc. Neurosci 4 (5), 443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Yoshimura S, Yamawaki S, Yamaguchi S, Ura M, 2010. Does low selfesteem enhance social pain? The relationship between trait self-esteem and anterior cingulate cortex activation induced by ostracism. Soc. Cogn. Affect. Neur 5 (4), 385–391. doi: 10.1093/scan/nsq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini I, Gustafsson PA, Hamilton JP, Kampe R, Zetterqvist M, Heilig M, 2018. The salience of self, not social pain, is encoded by dorsal anterior cingulate and insula. Sci. Rep 8 (1), 6165. doi: 10.1038/s41598-018-24658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KE, Wagner DD, Norris CJ, Heatherton TF, 2013. Socially excluded individuals fail to recruit medial prefrontal cortex for negative social scenes. Soc. Cogn. Affect. Neurosci 8 (2), 151–157. doi: 10.1093/scan/nsr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Pokorny T, Hock A, Kraehenmann R, Stämpfli P, Seifritz E, Scheidegger M, Vollenweider FX, 2016. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. PNAS 113 (18), 5119–5124. doi: 10.1073/pnas.1524187113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puetz VB, Viding E, Palmer A, Kelly PA, Lickley R, Koutoufa I, Sebastian CL, McCrory EJ, 2016. Altered neural response to rejection-related words in children exposed to maltreatment. J. Child Psychol. Psychiatry, Allied Disciplines 57 (10), 1165–1173. doi: 10.1111/jcpp.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke S, Seidel EM, Boubela RN, Thaler H, Metzler H, Kryspin-Exner I, Moser E, Habel U, Derntl B, 2018. Immediate and delayed neuroendocrine responses to social exclusion in males and females. Psychoneuroendocrinology 93, 56–64. doi: 10.1016/j.psyneuen.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC, 1997. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science (New York, N.Y.) 277 (5328), 968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT, 2010. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum. Brain Mapp 31 (2), 173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotge J-Y, Lemogne C, Hinfray S, Huguet P, Grynszpan O, Tartour E, George N, Fossati P, 2015. A meta-analysis of the anterior cingulate contribution to social pain. Soc. Cogn. Affect. Neurosci 10 (1), 19–27. doi: 10.1093/scan/nsu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin TN, Koyejo O, Gorgolewski KJ, Jones MN, Poldrack RA, Yarkoni T, 2017. Decoding brain activity using a large-scale probabilistic functional-anatomical atlas of human cognition. PLoS Comput Biol 13 (10), e1005649. doi: 10.1371/journal.pcbi.1005649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmälzle R, O’Donnell MB, Garcia JO, Cascio CN, Bayer J, Bassett DS, Vettel JM, Falk EB, 2017. Brain connectivity dynamics during social interaction reflect social network structure. Proc. Natl. Acad. Sci 114 (20), 5153–5158. doi: 10.1073/pnas.1616130114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian CL, Tan GCY, Roiser JP, Viding E, Dumontheil I, Blakemore S-J, 2011. Developmental influences on the neural bases of responses to social rejection: Implications of social neuroscience for education. Neuroimage 57 (3), 686–694. doi: 10.1016/j.neuroimage.2010.09.063. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, Blakemore SJ., 2010. Social brain development and the affective consequences of ostracism in adolescence [published correction appears in Brain Cogn. 2013 Oct;83(1):92]. Brain Cogn 72 (1), 134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ, 2011. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci 12 (3), 154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD, 2004. Empathy for pain involves the affective but not sensory components of pain. Science (New York, N.Y.) 303 (5661), 1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM, 2006. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat. Neurosci 9 (8), 1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Dimas E, Mwilambwe-Tshilobo L, Dagher A, Koellinger P, Nave G, Ong A, Kernbach J, Wiecki T, Ge T, Li Y, Holmes AJ, Yeo BTT, Turner GR, Dunbar RIM, Bzdok D, 2020. The default network of the human brain is associated with perceived social isolation. Nature Communications 11, 6393. doi: 10.1038/s41467-020-20039-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, DuPre E, Selarka D, Garcia J, Gojkovic S, Mildner J, Luh W-M, Turner GR, 2014. Goal-congruent default network activity facilitates cognitive control. J. Neurosci 34, 14108–14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN, 2009. The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: A quantitative meta-analysis. J. Cogn. Neurosci 21, 489–510. [DOI] [PubMed] [Google Scholar]
- Tousignant B, Eugène F, Sirois K, Jackson PL, 2018. Difference in neural response to social exclusion observation and subsequent altruism between adolescents and adults. Neuropsychologia 116 (Pt A), 15–25. doi: 10.1016/j.neuropsychologia.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, 2015. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci 16 (1), 55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Yeo B, Spreng RN, 2019. Towards a universal taxonomy of macro-scale functional human brain networks. Brain Topogr. 32 (6), 926–942. doi: 10.1007/s10548-019-00744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg LJM, Tollenaar MS, Pittner K, Compier-de Block LHCG, Buisman RSM, van IJzendoorn MH, Elzinga BM, 2018. Pass it on? The neural responses to rejection in the context of a family study on maltreatment. Soc. Cogn. Affect. Neurosci 13 (6), 616–627. doi: 10.1093/scan/nsy035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen M, Steinbeis N, Achterberg M, Bilo E, van den Bulk BG, van IJzendoorn MH, Crone EA, 2017. The neural correlates of dealing with social exclusion in childhood. Neuropsychologia 103, 29–37. doi: 10.1016/j.neuropsychologia.2017.07.008. [DOI] [PubMed] [Google Scholar]
- van Harmelen A-L, Hauber K, Gunther Moor B, Spinhoven P, Boon AE, Crone EA, Elzinga BM, 2014. Childhood emotional maltreatment severity is associated with dorsal medial prefrontal cortex responsivity to social exclusion in young adults. PLoS One 9 (1). doi: 10.1371/journal.pone.0085107, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N, Cheng TW, Pfeifer JH, 2017. Neural correlates of social exclusion across ages: a coordinate-based meta-analysis of functional MRI studies. Neuroimage 153, 359–368. doi: 10.1016/j.neuroimage.2017.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ,.., Cohen JD, 2004. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 303 (5661), 1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Botvinick MM, Chang LJ, Coghill RC, Davis KD, Iannetti GD, Poldrack RA, Shackman AJ, Yarkoni T, 2016. Pain in the ACC? P. Natl. Acad. Sci 113 (18), E2474–E2475. doi: 10.1073/pnas.1600282113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagels L, Bergs R, Clemens B, Bauchmuller M, Gur RC, Schneider F, Habel U, Kohn N, 2017. Contextual exclusion processing: an fMRI study of rejection in a performance-related context. Brain Imaging Behavior 11 (3), 874–886. doi: 10.1007/s11682-016-9561-2. [DOI] [PubMed] [Google Scholar]
- Will GJ, Crone EA, Güroğlu B, 2015. Acting on social exclusion: neural correlates of punishment and forgiveness of excluders. Soc. Cogn. Affect. Neurosci 10 (2), 209–218. doi: 10.1093/scan/nsu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will G-J, van Lier PAC, Crone EA, Güroğlu B, 2016. Chronic childhood peer rejection is associated with heightened neural responses to social exclusion during adolescence. J. Abnorm. Child Psychol 44 (1), 43–55. doi: 10.1007/s10802-015-9983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD, 2009. Chapter 6 ostracism: a temporal need-threat model. In: Advances in Experimental Social Psychology, Vol. 41. Academic Press, pp. 275–314. doi: 10.1016/S0065-2601(08)00406-1. [DOI] [Google Scholar]
- Williams KD, Cheung CKT, Choi W, 2000. Cyberostracism: Effects of being ignored over the Internet. J. Pers. Soc. Psychol 79 (5), 748–792. doi: 10.1037/0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Woo C-W, Koban L, Kross E, Lindquist MA, Banich MT, Ruzic L, Andrews-Hanna JR, Wager TD, 2014. Separate neural representations for physical pain and social rejection. Nat. Commun 5, 5380. doi: 10.1038/ncomms6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wudarczyk OA, Kohn N, Bergs R, Gur RE, Turetsky B, Schneider F, Habel U, 2015. Chemosensory anxiety cues moderate the experience of social exclusion—an fMRI investigation with Cyberball. Front. Psychol 6, 1475. doi: 10.3389/fp-syg.2015.01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Essen DCV, Wager TD, 2011. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8 (8), 665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL, 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol 106 (3), 1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.