CLINICAL PRESENTATION
A 50-year-old Latin American female patient complained of a painful lesion in the hard palate on a telephone consultation. The patient was isolated at home after receiving the diagnosis of coronavirus disease 19 (COVID-19), confirmed by the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA via polymerase chain reaction. The diagnosis was made approximately 8 days before the consultation. The only symptom related to COVID-19 was a persistent headache for 3 days, and no other medication was prescribed besides nonopioid analgesics. Her past medical history showed that she had type 2 diabetes. Because of the quarantine period, the patient was not able to go to the dental office and decided to send pictures of the lesion to the dentist. The patient reported no history of recent dental treatment or any type of trauma and reported wearing a removable partial denture. She described a chronologic sequence of the events, as follows: A small lesion appeared on the hard palate, with no symptoms, and she described it as an ulcer. At this point, she stopped wearing the prosthetic appliance by her own decision; 5 days later, she noted that the lesion increased in size and was accompanied by mild pain symptoms in the area of the lesion. However, on day 7, the pain became more severe and irradiated across the entire palate and middle third of the face. Examination of the clinical photographs taken 8 days after receiving the COVID-19 diagnosis and revealed a deep ulcerated lesion located at the center of the hard palate (over the midline). The bone was exposed, and the lesion measured around 2 cm at the widest diameter. The lesion was surrounded by an erythematous halo, the borders appeared lobulated, and focal areas were swollen (Figure 1 ).
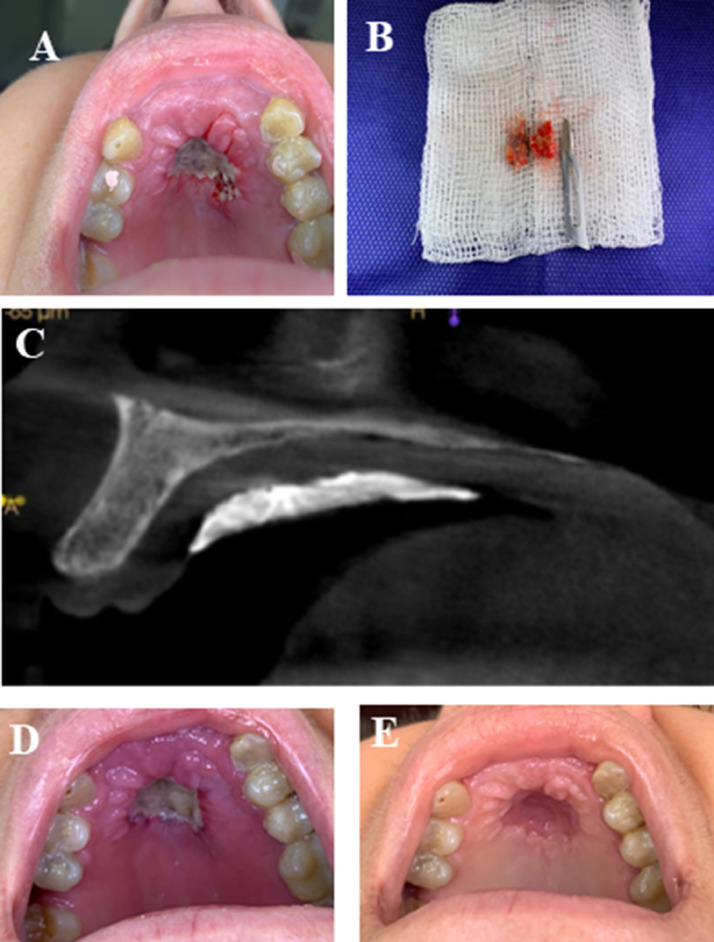
Fig. 1.
Clinical and imaging findings. (A) Clinical aspect of the lesion just after the incisional biopsy procedure, revealing a deep ulcerated lesion, with bone exposure in the hard palate. (B) Three fragments measuring about 0.5 × 0.3 × 0.1 cm to 1.0 × 1.0 × 0.5 cm removed during incisional biopsy. All of the material collected was sent for histopathological analysis. (C) Cone beam computed tomography scan taken immediately following incisional biopsy. No significant alteration was noted in the maxillary bone. Hyperdense image covering the soft tissue corresponds to surgical cement applied for homeostasis during the biopsy procedure. (D) Clinical presentation 7 days after incisional biopsy. (E) Ten days following discharge from hospitalization and approximately 60 days after initial evaluation. The lesion appears to be resolving and the patient is asymptomatic.
DIFFERENTIAL DIAGNOSIS
Inflammatory conditions
Among several reactive and inflammatory conditions, necrotizing sialometaplasia (NSM) is known to present initially as an ulcer in the palate. This destructive inflammatory benign disease locally affects the salivary glands. People with NSM can feel pain or be asymptomatic, so it might be clinically and pathologically difficult to distinguish NSM from certain malignancies.1 Although NSM usually appears on the palate, there are reports of this condition in other intraoral sites, such as the retromolar area, labial mucosa, tongue, floor of the mouth, and buccal mucosa.1 In our case, NSM was first considered because the patient reported that the lesion started with a wound that did not heal. NSM frequently affects the palatal minor salivary glands, which might be related to the use of removable partial denture. Although the patient did not report whether the denture was ill-fitted and whether its use was interrupted in the first approach, one could assume that the prosthetic appliance could be related to the lesion. Moreover, it is unknown whether the rapid progression of the lesion and worsening of its appearance could be related to COVID-19 disease, because an exacerbation of the symptoms could occur mostly because of the inflammatory systemic condition. Besides NSM, other inflammatory conditions were not considered for differential diagnosis, mostly because of the rapid progression of the lesion and bone exposure after 7 days.
Malignant neoplasms
Malignant lesions were implied in the differential diagnosis in this case. Salivary gland tumors were considered because the lesion was located in the palate. These tumors are uncommon and account for 3% to 5% of all neoplasms in the head and neck region. A systematic review by Galdirs et al. showed that the majority of tumors in the minor salivary glands were malignant.2 In this present case, the lesion's location in the hard palate was the only feature favoring the hypothesis of a malignant salivary gland neoplasm. Another aspect was observed: The borders of the lesion were raised and had a rolled appearance, suggesting an indurated (i.e., firm, hard) process and some neoplastic lesions could ulcerate as they progress. However, the rapid evolution and the absence of a mass (as seen in the pictures) were crucial to consider neoplasm as a less probable option in the differential diagnosis. Because of the impossibility to perform imaging exams at that time owing to the isolation of the patient, clinical presentation was the only source of information for diagnosis. Other hypothesis of malignant lesion for the present case was the possibility of a lymphoma. Lymphomas in the oral cavity account for 3% of all malignant tumors of the head and neck, and they clinically present as a mass, with ulceration and with or without superficial necrosis.3 Regarding this information and clinical presentation, this hypothesis was included in the diagnosis list, considering that the palate is the most common intraoral location for extranodal malignant lymphomas.
Infections of soft tissue
Infectious diseases were the most emphasized group of lesions for differential diagnosis. Fungal diseases along with lesions in the oral cavity commonly occur in some regions of Latin America, especially in Brazil. The patient of this case lives in a countryside town. Oral manifestations related to a pathogenic microorganism infection were considered in the differential diagnosis list as follows: paracoccidioidomycosis, mucormycosis, and mucocutaneous leishmaniasis. de Castro Costa et al. reviewed cases of female patients with paracoccidioidomycosis and found that Brazilian white women aged 40 to 50 years were the most affected individuals, with the possible occurrence of a single painful lesion.4 Almeida et al. concluded that the clinical presentation of oral leishmaniasis is unusual, which underlies the importance of considering leishmaniasis in the differential diagnosis of oral lesions, especially in endemic areas.5 According to a review study, the incidence of mucormycosis in South America is increasing: It appeared in 41% of 143 Brazilian patients. The mean age of the patients was 40.0 years (43.0 in Brazil, 37.5 in the other countries of South America).6 In the case reported here, both clinical condition and prevalence rates were considered for inclusion of fungal and parasite diseases in the differential diagnosis.
Bone infections
Because of bone exposure in this case, bone infections were also considered. At this point, we needed more information from the patient to determine whether to include bone necrosis in the diagnosis. Medication-related osteonecrosis of the jaws is a bone necrosis induced by antiangiogenic and/or antiresorptive drugs, such as bisphosphonates, bevacizumab, and denosumab. The most common clinical presentation includes exposure of bone surfaces in the maxilla, local pain, sequestrum formation, and secondary local infection.7 To distinguish the lesion from other delayed healing conditions, we reviewed the patient's medical history to discard the possibility of previous use of bisphosphonates, bevacizumab, and denosumab. On a second call consultation, which was guided by a structured questionnaire, the patient reinforced the information that she was not on antiresorptive treatment; thus, the hypothesis of medication-related osteonecrosis of the jaws was rejected. Osteomyelitis was also considered. Osteomyelitis is an inflammatory, usually infectious bone marrow condition with a wide range of clinical presentations; it arises more often in the mandible than in the maxilla. Some symptoms of osteomyelitis are pain, edema, fever, adynamia, and cutaneous fistula.8 We included osteomyelitis in the differential diagnosis because of the acute evolution of the lesion and because the patient had COVID-19 and diabetes; little is known about the evolution of these diseases in comorbidity, because both could be worsened by a bacterial infection progression.
Oral lesions related to SARS-CoV-2 infection
The main challenge was to include COVID-19-related lesions in the diagnosis process for this case. There is scarce information on oral findings for this condition, and the literature is mostly based on a few case series published. Brandão et al. reported 8 cases of COVID-19 infection in patients who developed oral necrotic ulcers and aphthous-like ulcerations, which started early in the course of the disease. Lesions varied from clustered ulcers of 1 to 1.5 cm in diameter covered with crusts to necrotic areas,9 including painful ulcer and focal erythema/petechia. Amorim dos Santos et al. conducted a systematic review and reported that oral mucosal lesions are more likely to present as co-infections or secondary manifestations with multiple clinical aspects.10 In this specific case, COVID-19 oral manifestations were included in the spectrum of possible diagnosis, although co-infection was likely to be considered. The patient reported mild symptoms of COVID-19 disease (i.e., no fever, only headache and some discomfort) and, interestingly, no complaints of taste loss.
DIAGNOSIS
Until the end of the mandatory isolation period, the patient was prescribed antibiotics (amoxicillin with potassium clavulanate for 7 days) along with a nonsteroidal anti-inflammatory drug and tramadol (centrally acting analgesic) because of the intense pain. Antiseptic mouthwashes were also indicated 3 times a day. During isolation, the patient reported one episode in which she received intravenous medication in an emergency medical service to control the pain. After this period, the patient attended the dental office for intraoral examination. Imaging exams were requested, and an incisional biopsy was performed under local anesthesia. Next, the patient underwent a cone beam computed tomography scan. Tomographic images revealed a maxilla with normal contour, without signs of significant eroded areas. She was advised to seek a consultation with a physician to check her diabetes status. A fasting blood sugar test revealed a glucose level of 218 mg/dL. The patient signed a written informed consent form for the present study.
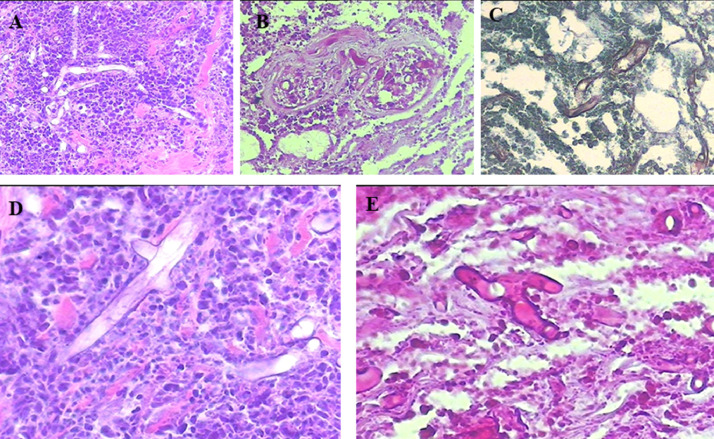
Histologic examination of the biopsy showed an ulcerated lesion with coagulative necrosis, hemorrhage, and abundant neutrophils. In the necrotic material, especially in blood vessels walls, there were many large nonseptate hyphae with thin walls and branches at 90°, which were well visualized with hematoxylin and eosin staining (Figure 2 ). These hyphae were evidenced with Grocott's methenamine (Figure 2) and special stains for fungi-like periodic acid–Schiff (Figure 2). The morphologic diagnosis of mucormycosis was confirmed. A high-resolution version of the examined slides is available as eSlides VM06234, VM06235, and VM06236 for assessment via Virtual Microscope.
Fig. 2.
Histopathological findings. (A) and (D) Heavy mixed acute and chronic inflammatory infiltrate with necrosis and large nonseptate, thin-walled fungal hyphae that branch at a 90° angle. The microorganisms are aggregated around blood vessels. (A) Original magnification 20 ×, (D) original magnification 40 × . High-resolution versions of these images are available as eSlide VM06234. (B) and (E) Hyphae stained with periodic acid–Schiff. (B) original magnification 20 ×, (E) original magnification 40 × . High-resolution versions of these images are available as eSlide VM06235. (C) Grocott's methenamine. High-resolution versions of this image are available as eSlide VM06236.
MANAGEMENT AND FOLLOW-UP
After the diagnosis of mucormycosis, the patient was quickly referred for hospitalization to start treatment. At the hospital, a series of tests was carried out to identify whether the disease had compromised the lungs and other organs. Imaging exams revealed no other affected sites. The patient started the treatment with intravenous amphotericin B in association with hydrocortisone. After 10 days of intravenous medication, superficial debridement of the lesion was conducted. In addition, the patient presented a respiratory allergic reaction, so an antihistamine therapy was introduced. Diabetes was also controlled during hospitalization. After approximately 1½ months of hospitalization, the patient was sent home with no pain or any other symptoms. The patient attended the dental office for clinical evaluation of the hard palate, which seemed recovered 10 days after discharge from the hospital.
DISCUSSION
Mucormycosis is a rare and potentially lethal invasive fungal infection caused by saprophytic aerobic fungi Rhizopus, Rhizomucor, and Cunninghamella genera of the or order Mucorales, now called Rhidopodaceae, which colonize the oral and nasal mucosa and paranasal sinuses.11 The disease usually evolves rapidly in immunocompromised or debilitated patients; that is, those with uncontrolled diabetes, uncontrolled human immunodeficiency virus infection, malignant hematological diseases, and solid organ transplantation. This disease rarely affects patients with no underlying condition.12 The epidemiology of mucormycosis is difficult to determine, mostly because of its rarity.13 However, owing to the increasing number of immunocompromised patients, aging of the population, and better health care with higher overall survival rates, there has been an increase in the incidence of this disease in the last decades.12
Disease transmission seems to occur mainly via inhalation of spores from environmental sources.13 Mucormycosis usually presents a worse outcome compared to other invasive fungal infections. The most common clinical presentation of mucormycosis is sinusitis (rhinocerebral infection), followed by pulmonary, cutaneous/subcutaneous, and disseminated diseases.13 Tissue necrosis is the hallmark of mucormycosis, resulting from angioinvasion and subsequent vascular thrombosis.13 , 14 Oral manifestations of mucormycosis usually include bone exposure and necrosis, which demands histopathological examination to confirm the diagnosis because of its nonspecific features and possible similarities to bacterial osteomyelitis, trauma, and iatrogenic infections.14
Oral mucormycosis without any other systemically compromised organs is quite uncommon. In our case, the patient had both a debilitating condition resulting from poorly controlled diabetes and acute inflammatory immune response due to COVID-19. Other case reports of oral manifestations related to mucormycosis identified triggering or predisposing factors for local and systemic conditions. Rajashri et al. reported oral mucormycosis in a patient with diabetes after tooth extraction.14 Srivastava et al. reported a case of mucormycotic osteomyelitis in the nasomaxillary zygomatic complex, resulting from a trauma in a 42-year-old male patient with poorly controlled diabetes.11 Vahabzadeh-Hagh et al. described a case of invasive oral tongue mucormycosis after orthotopic liver transplant due to a history of hepatitis C cirrhosis and hepatocellular carcinoma.15 Boras et al. reported a case of oral mucormycosis and aspergillosis in a patient with acute leukemia.16 All of these cases of oral mucormycosis share a debilitating condition, and we initially considered COVID-19 as one of these, besides diabetes.
At present, there are only 3 studies investigating the possible correlation between COVID-19 and mucormycosis. Hanley et al. conducted a postmortem study involving 10 patients with fatal COVID-19 and found the case of a 22-year-old man with disseminated mucormycosis affecting lymph nodes, heart, brain, and kidney.17 Werthman-Ehrenreich reported the case of a 33-year-old woman with altered mental status and proptosis who was ultimately diagnosed with COVID-19 along with invasive rhino-orbital-cerebral mucormycosis, which led to an orbital compartment syndrome.18 In a review study on the association between COVID-19 and fungal infection, Song et al. suggested that invasive mycoses are more likely to occur in patients with COVID-19, especially those who are severely ill or immunocompromised.19 According to their study, the most important risk factors of invasive mucormycosis in these patients are trauma, diabetes, glucocorticoid use, hemopoietic malignancies, prolonged neutropenia, allogeneic hematopoietic stem cell transplant, and solid organ transplant.
Co-infections in patients with COVID-19 are an emerging concern, especially because of their complex diagnosis, severity, and increased mortality.20 In a systematic review, Rawson et al. reported that 8% of patients experienced bacterial or fungal co-infection during hospitalization.21 Co-infections can primarily occur through the following: When the pathogenic organism is carried by patients themselves before the viral infection, when patients have an underlying chronic infection, or when the patient is hospitalized.20 Oral lesions in patients with COVID-19 seem more likely to be induced by co-infections, immunity impairment, and adverse reactions instead of direct SARS-CoV-2 infection.10
Regarding COVID-19, some studies also revealed the presence of oral necrotizing lesions among affected patients. Patel and Woolley reported the case of a 35-year-old patient with suspected COVID-19 and diagnosis of necrotizing gingivitis.22 The main signs found were severe halitosis, nonprovoked bleeding from the gingival sulcus, and necrotic interdental papillae. Singh et al., in their case series, reported the case of a 71-year-old woman diagnosed with COVID-19 who was admitted to the intensive care unit for intubation because of respiratory distress.23 The patient presented discoloration on the upper lip and tongue, which progressed to swelling, full discoloration, and finally necrosis with dark eschar formation. Brandão et al. reported 3 cases of patients with COVID-19 in the eighth and ninth decades of life who presented aphthous-like lesions and superficial necrotic areas in the hard palate, upper and lower lips, and tongue.9 Those authors suggested that the high prevalence of oral necrotic lesions in patients with COVID-19 may be because of the increased permeability of the cell walls to pathogens and to the viral replication in the oral mucosa lining provoked by the infection of oral tissues.
In the context of severe acute respiratory syndrome (SARS), which is primarily caused by a coronavirus type, a possible relationship with avascular osteonecrosis (AVN) has been suggested. Hong and Du evaluated diagnostic imaging exams of 67 patients with SARS with pain in the large joints during or after hospitalization and 28 patients were identified with AVN (41%).24 Those authors believed that administration of corticosteroids (which is frequently prescribed to severely ill patients) in patients with SARS could be the major predisposing factor for AVN development and recommended that steroids should be administrated cautiously. Considering that the disease can be severe in patients with COVID-19, it is important to know the possible effects of the medicines being prescribed to these patients to avoid major systemic complications.
Early diagnosis is the best way to manage oral mucormycosis, along with reversal of the underlying predisposing risk factors and systemic disorders, surgical debridement, and prompt administration of active antifungal agents.16 Because patients with COVID-19 may need to quarantine and socially isolate, tele-healthcare and/or tele-dentistry can be the best way to provide early diagnosis. Initial evaluation of pictures and clinical images is crucial to start the diagnosis process. In our case, the pictures of the patient along with her medical history and major complaints allowed us to recognize her oral condition and conduct the differential diagnosis. Martins et al. reported that literature evidence supports the use of tele-consultation and tele-education as effective alternatives in this period of pandemic to avoid the spread of SARS-CoV-2 and its collateral damage. The authors also emphasized that dental care delivery needs to be redesigned.25
To the best of our knowledge, this is the first case report of a patient with COVID-19 with oral mucormycosis. Mucormycosis, along with other deep fungal infections, should be considered as an important outcome of SARS-CoV-2 infection. Because COVID-19 is still under investigation, all possible associations with the disease should be reported. In addition, the possibility of obtaining information from non-in-person consultation could be an alternative for patients in quarantine.
References
- 1.Ledesma-Montes C, Garcés-Ortíz M, Salcido-García JF, Hernández-Flores F. Review of the literature on necrotizing sialometaplasia and case presentation. Quintessence Int. 2015;46:67–72. doi: 10.3290/j.qi.a32633. [DOI] [PubMed] [Google Scholar]
- 2.Galdirs TM, Kappler M, Reich W, Eckert AW. Current aspects of salivary gland tumors—a systematic review of the literature. GMS Interdiscip Plast Reconstr Surg DGPW. 2019;8:Doc12. doi: 10.3205/iprs000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Camargo AR, Duarte BF, Lisboa ML, et al. Immunophenotyping for diagnosis of oral lesions: is it an important tool? Int J Med Pharm Case Reports. 2020;13:16–23. [Google Scholar]
- 4.de Castro Costa M, de Carvalho MM, Sperandio FF, et al. Oral paracoccidioidomycosis affecting women: a systematic review. Mycoses. 2021;64:108–122. doi: 10.1111/myc.13194. [DOI] [PubMed] [Google Scholar]
- 5.Almeida TFA, da Silveira EM, dos Santos CRR, León JE, Mesquita ATM. Exclusive primary lesion of oral leishmaniasis with immunohistochemical diagnosis. Head Neck Pathol. 2016;10:533–537. doi: 10.1007/s12105-016-0732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nucci M, Engelhardt M, Hamed K. Mucormycosis in South America: a review of 143 reported cases. Mycoses. 2019;62:730–738. doi: 10.1111/myc.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabelo GD, Assunção JNR, Chavassieux P, Haroldo Arid S, Alves FA, Lemos CA. Bisphosphonate-related osteonecrosis of the jaws and its array of manifestations. J Maxillofac Oral Surg. 2015;14:699–705. doi: 10.1007/s12663-014-0707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lima ALL, Oliveira PR, Carvalho VC, et al. Recommendations for the treatment of osteomyelitis. Braz J Infect Dis. 2014;18:526–534. doi: 10.1016/j.bjid.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandão TB, Gueiros LA, Melo TS, et al. Oral lesions in patients with SARS-CoV-2 infection: could the oral cavity be a target organ? Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;131:e45–e51. doi: 10.1016/j.oooo.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amorim dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral manifestations in patients with COVID-19: a living systematic review. J Dent Res. 2021;100:141–154. doi: 10.1177/0022034520957289. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava D, Mishra S, Chandra L, Passi D. Mucormycotic osteomyelitis of maxilla following maxillofacial trauma: the disease of the diseased. J Family Med Prim Care. 2019;8:748–750. doi: 10.4103/jfmpc.jfmpc_410_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 13.Skiada A, Lass-Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56:S93–S101. doi: 10.1093/mmy/myx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajashri R, Muthusekhar MR, Kumar SP. Mucormycosis following tooth extraction in a diabetic patient: a case report. Cureus. 2020;12:8–14. doi: 10.7759/cureus.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vahabzadeh-Hagh AM, Chao KY, Blackwell KE. Invasive oral tongue mucormycosis rapidly presenting after orthotopic liver transplant. Ear Nose Throat J. 2019;98:268–270. doi: 10.1177/0145561319840535. [DOI] [PubMed] [Google Scholar]
- 16.Boras VV, Jurlina M, Brailo V, et al. Oral mucormycosis and aspergillosis in the patient with acute leukemia. Acta Stomatol Croat. 2019;53:274–277. doi: 10.15644/asc53/3/9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2021;42 doi: 10.1016/j.ajem.2020.09.032. 264.e5-264.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;185:599–606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox MJ, Loman N, Bogaert D, O'Grady J. Co-infections: potentially lethal and unexplored in COVID-19. Lancet Microbe. 2020;1:e11. doi: 10.1016/S2666-5247(20)30009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel J, Woolley J. Necrotizing periodontal disease: oral manifestation of COVID-19. Oral Dis. 2021;27(suppl 3):768–769. doi: 10.1111/odi.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh C, Tay J, Shoqirat N. Skin and mucosal damage in patients diagnosed with COVID-19 a case report. J Wound Ostomy Continence Nurs. 2020;47:435–438. doi: 10.1097/WON.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong N, Du XK. Avascular necrosis of bone in severe acute respiratory syndrome. Clin Radiol. 2004;59:602–608. doi: 10.1016/j.crad.2003.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martins MD, Carrard VC, dos Santos CM, Hugo FN. COVID-19—are telehealth and tele-education the answers to keep the ball rolling in dentistry? Oral Dis. 2020 doi: 10.1111/odi.13527. Epub ahead of print. PMID: 32615648; PMCID: PMC7361312. [DOI] [PMC free article] [PubMed] [Google Scholar]