Abstract
Externalizing disorders have been extensively linked to substance use problems. However, less is known about whether genetic factors underpinning externalizing disorders and environmental features interact to predict substance use disorders (i.e., marijuana abuse and dependence) among urban, African-Americans. We examined whether polygenic risk scores for conduct disorder (CD PRS) and attention-deficit hyperactivity disorder (ADHD PRS) interacted with contextual factors (i.e., parental monitoring, community disadvantage) to influence risk for marijuana use disorders in a sample of African-American youth. Participants (N=1,050; 44.2% male) were initially recruited for an elementary school-based universal prevention trial in a Mid-Atlantic city and followed through age 20. Participants reported on their parental monitoring in sixth grade and whether they were diagnosed with marijuana abuse or dependence at age 20. Blood or saliva samples were genotyped using the Affymetrix 6.0 microarrays. The CD and ADHD PRS were created based on genome-wide association studies conducted by Dick et al. (2010) and Demontis et al. (2017), respectively. Community disadvantage was calculated based on census data when participants were in sixth grade. There was an interaction between the CD PRS and community disadvantage such that a higher CD PRS was associated with greater risk for a marijuana use disorder at higher levels of neighborhood disadvantage. This finding should be interpreted with caution owing to the number of significance tests performed. Implications for etiological models and future research directions are presented.
Keywords: marijuana abuse and dependence, conduct disorder polygenic risk score, attention-deficit hyperactivity disorder polygenic risk score, parental monitoring, community disadvantage
1. Introduction
Marijuana abuse and dependence have been associated with a number of negative sequelae, including psychiatric disorders (e.g., major depression), reduced educational attainment, and unemployment (Kosty et al., 2017; Pacek et al., 2012). As of 2013, marijuana use disorders have disproportionately affected African-American adults, with 4.6% meeting diagnostic criteria for a marijuana use disorder compared to 2.7% and 2.8% of Caucasian and Hispanic adults, respectively (Hasin et al., 2015). Substantial changes in policy and public opinion surrounding marijuana use have prompted several states to legalize marijuana, resulting in African-American young adults having greater access to this substance. This is a source of concern given that about a third of people who use marijuana will develop a marijuana use disorder (Hasin et al., 2015). Given the negative outcomes associated with marijuana use disorders, an examination of individual and environmental factors that are associated with marijuana use disorders may serve to inform preventive and early interventions among African-Americans.
Externalizing symptoms and disorders have been positively predictive of more frequent marijuana use (Chabrol & Saint-Martin, 2009; McAdams et al., 2012) and disorders (Farmer et al., 2014). Two externalizing disorders that have been predictive of marijuana use disorders are conduct disorder (CD), defined as a consistent pattern of antisocial and disruptive behaviors that often violate social norms, and attention-deficit hyperactivity disorder (ADHD), a syndrome characterized by marked inattention, impulsivity, and hyperactivity (American Psychiatric Association, 2000; Bidwell et al., 2014; Fergusson & Boden, 2008; Grant et al., 2015). Youth with higher CD symptoms tend to be higher in novelty seeking, less responsive to punishment socialization techniques, and may be more likely to affiliate with deviant peers, all of which may predispose these youth to using marijuana more heavily (Lahey and Waldman, 2012). Moreover, youth with higher ADHD symptoms may exhibit lower levels of executive functioning, higher levels of disinhibition, and elevated risk taking; these characteristics may similarly confer risk for more frequent marijuana use and potentially the development of marijuana abuse and dependence (Du Rietz et al., 2018; Miranda et al., 2016).
Studies examining the relationship between CD and ADHD symptoms and marijuana use disorders have typically examined CD and ADHD symptoms phenotypically. However, genetic factors associated with CD and ADHD symptoms may also play a role in the development of marijuana use disorders. CD, ADHD, and marijuana use disorders may be a part of a larger externalizing syndrome, which is supported by work indicating strong, positive correlations between these conditions (Carragher et al., 2014; Harty et al., 2015; Korhonen et al., 2012; Krueger et al. 2005; Miles et al., 2002). For example, using a twin study paradigm, Korhonen et al., 2012 found that 49% of the covariance between externalizing behaviors and drug use initiation (e.g., cannabis use) was due to common genetic features. Thus, it is possible that the genetic architecture of CD and ADHD symptoms is associated with risk for marijuana use disorders, although it is unclear whether this is the case based on extant research.
Molecular genetic studies represent an effort to identify genes that account for the heritability estimates generated by quantitative genetic studies. An advantage of the molecular genetic approach is that such knowledge may shed light on neurobiological mechanisms underlying phenotypes. The polygenic risk score (PRS) approach represents one of the more common molecular genetic strategies to understand the genetic architecture underlying substance use and disorders (Dick et al., 2010). A PRS is created by aggregating multiple genetic variants, identified through genome-wide association (GWA) scans, to produce a genetic score reflective of a particular phenotype (Dick et al., 2010). The consideration of polygenic influences that are associated with marijuana use disorders is consistent with substantial evidence that numerous genetic variants associated with psychiatric outcomes (i.e., CD and ADHD) likely influence liability for substance use problems (Hines et al., 2014). In terms of studies that have examined the association between CD and ADHD polygenic influences and marijuana use disorders, no studies to our knowledge have examined these relations. Available work indicates that higher polygenic loading for (a) CD was associated with increased risk for alcohol dependence; and (b) ADHD was associated with higher levels of alcohol and tobacco use in samples of individuals of predominantly European ancestry (Dick et al., 2010; Du Rietz et al., 2018). However, it is unclear whether these PRS are related to other substance use problems, such as marijuana use disorders, among African-American adults.
Although polygenic influences of CD and ADHD may influence risk for marijuana use disorders, not all individuals higher in these PRS will develop a disorder. Indeed, the development of substance use disorders is often dependent on both individual and contextual factors (Burdzovic Andreas & Watson, 2016; Hill et al., 2011). This is supported by a number of psychiatric and molecular genetics studies indicating that genetic loading for different disorders interacts with environmental factors to influence heterogeneity in risk for psychopathology (Bronfenbrenner, 1994; Caspi & Moffitt, 2006; Dodge, 2009). Consistent with work supporting the notion that individual and environmental features interact with each other, proximal environmental factors (i.e., parental monitoring) may influence risk for marijuana use disorders among individuals with different genetic loading for CD and ADHD. Parents higher in monitoring may structure their children’s time and encourage youth disclosure, which may attenuate youth’s risk for engaging in illicit drug use, especially during middle childhood when youth often have greater unsupervised time in new settings (Dishion and McMahon, 1998). Higher parental supervision has been linked to youth being offered marijuana less frequently, and less subsequent marijuana use (Lakon et al., 2015; Siegel et al., 2014). Although parental monitoring may reduce risk for marijuana use disorders, it is unclear whether this parenting behavior influences African-American youth with different CD and ADHD genetic loadings. Among African-American youth with higher genetic loading for CD and ADHD, parental monitoring may inhibit these youth from seeking out high-risk environments and/or affiliating with substance-using peers, which may set youth on a trajectory towards reduced risk for a marijuana use disorder in adulthood.
The consideration of distal contextual factors (i.e., community disadvantage) in conjunction with polygenic influences is also paramount when considering risk for marijuana use disorders. Indeed, higher levels of neighborhood disadvantage are positively predictive of more frequent marijuana use (Furr-Holden et al., 2014; Reboussin et al., 2015). In more impoverished communities, youth may have greater exposure to illicit drugs, may be more likely to be offered drugs, and perceive drug use as normative (Gilliard-Matthews et al., 2015; Wallace et al., 2017). Minimal informal social controls, less resident consensus regarding appropriate standards for youth behavior, and prevalent availability of substances may enable the development of a marijuana use disorder. This may be particularly true among youth higher in CD symptoms who are more likely to be offered drugs, and less likely to refuse drugs upon being offered them (Burdzovic Andreas et al., 2016; Burdzovic Andreas and Pape, 2015; Rosenberg and Anthony, 2001). Exposure to communities higher in disadvantage characterized by greater sales and rates of drug use may also facilitate heavy marijuana use among individuals higher in ADHD symptoms, given their propensity for risk taking and poorer impulse control (Wallace & Muroff, 2002). However, it is uncertain whether community disadvantage exacerbates risk for marijuana use disorders among African-American youth with a higher CD and ADHD PRS. Consistent with diathesis-stress and G x E triggering models, contextual stressors, such as community disadvantage, may promote the expression of a genetic diathesis (i.e., higher genetic loading for CD and ADHD) (Shanahan & Hofer, 2005), though research is wanting.
An additional limitation of extant work is the failure to consider sex differences in the relations between environmental factors, genetic influences of externalizing disorders, and marijuana use disorders. Among urban, African-American adolescents, lower levels of parental knowledge of youth’s whereabouts predicted more frequent marijuana use among adolescent males, but not females (Tebes et al., 2011). In addition, differences in the base rates of marijuana abuse and dependence have been observed in predominantly European samples, with male adolescents displaying greater rates of marijuana use disorders relative to females (Young et al., 2002). Differences in the incidence rates of marijuana use disorders and the possibility that contextual effects operate differently among males compared to females underscore the importance of examining sex differences to assist in the development of interventions aimed at attenuating problematic marijuana use.
In the present study, we sought to address a number of gaps in the literature. First, we examined whether externalizing disorders PRS (i.e., CD and ADHD) were associated with marijuana abuse and dependence among young adults. Second, we examined whether proximal (i.e., parental monitoring) and distal (i.e., community disadvantage) contextual factors moderated the relation between CD and ADHD polygenic influences and marijuana use disorders. Third, we examined whether there were sex differences in the effects of contextual factors and polygenic influences on marijuana abuse and dependence. Fourth, we examined relations between PRS, contextual factors, and marijuana use disorders in a sample of African-American young adults, a population that may experience a number of contextual stressors that may exacerbate risk for heavy marijuana use (Galea et al., 2005).
2. Method
2.1. Participants
The study’s analytic sample was drawn from three cohorts of participants in a series of randomized controlled trials of elementary-school-based universal prevention interventions. The trials were administered in a single urban school district in the mid-Atlantic region of the U.S. The purpose of the interventions was to improve academic achievement, reduce disruptive behaviors, and promote positive outcomes in adulthood. Participants were followed from first grade to young adulthood (age ~ 20). Three-thousand and one-hundred and nine individuals were available for recruitment in first grade. Of the 3,109 participants available in first grade, 1,349 provided a successfully assayed DNA sample and reported on whether they met diagnostic criteria for marijuana abuse and dependence at age 20. Owing to the relatively small proportion of European-Americans in the study sample (<25%) and to further reduce the possibility of population stratification (described in more detail in the supplementary materials), we restricted the sample to only African-American participants, resulting in a final sample of 1,050 individuals. Participant demographic information for the analytic sample is outlined in Table 1.
Table 1.
Sample characteristics
| Characteristic | N (%) |
|---|---|
| Sex | |
| Male | 461 (43.9%) |
| Female | 589 (56.1%) |
| Free Reduced Lunch | |
| Yes | 804 (76.6%) |
| No | 212 (20.2%) |
| Intervention | |
| Yes | 545 (51.9%) |
| No | 505 (48.1%) |
| Education | |
| < High School Education | 269 (25.6%) |
| High School Diploma or GEDa | 417 (40.2%) |
| Vocational Training/College | 352 (33.9%) |
| Income | |
| <$10,000 | 579 (72.0%) |
| $10,000-$20,000 | 127 (15.8%) |
| >$20,000 | 98 (12.2%) |
| Cohort Identification | |
| Cohort 1 | 345 (32.9%) |
| Cohort 2 | 295 (28.1%) |
| Cohort 3 | 411 (39.0%) |
GED =General Education Degree.
With respect to differences in first grade demographic characteristics between the analytic sample and those African Americans not assessed at age 20 and/or those who did not provide DNA, the only significant difference (p ≤ .05) was that the analytic sample had a significantly greater proportion of females than the whole sample. There were also no significant differences with respect to the proportion of participants who received an intervention.
2.2. Measures
2.2.1. Marijuana Abuse and Dependence.
In cohorts 1 and 2, the Composite International Diagnostic Interview-University of Michigan Version (CIDI-UM; Kessler et al., 1994), modified to yield Diagnostic and Statistical Manual-IV diagnoses (American Psychiatric Association, 1994) was used to determine past year marijuana abuse and dependence at age 20. In cohort 3, the 2007 National Survey on Drug Use & Health (NSDUH) was used to assess past year marijuana abuse and dependence at age 20 (Substance Abuse Mental Health Services Administration, 2001). Both the CIDI-UM and NSDUH are structured interviews that specify the exact wording and sequence of questions and provide several categories for categorizing respondents’ replies. Marijuana abuse and dependence diagnoses were derived in accord with DSM-IV criteria using a computerized scoring algorithm. We created a dichotomous variable (marijuana abuse or dependence = 1; no marijuana abuse or dependence = 0) to reflect individuals who met diagnostic criteria for a marijuana use disorder.
2.2.2. Parent Monitoring.
When participants were in sixth grade, the Structured Interview of Parent Management Skills and Practices Youth-Version (SIPMSP) was used to assess parental monitoring (Capaldi and Patterson, 1989) in cohorts 1, 2, and 3. Sample items are “How often would your parents or a sitter know if you came home late or on weekends?” and “How often before you go out do you tell your parents when you will be back?” Seven items are rated on a scale from 1 (all of the time) to 5 (never). Items were reverse scored. An average parental monitoring score was calculated with higher scores reflecting more monitoring. Capaldi and Patterson (1989) report adequate internal consistency and test-retest reliability for the monitoring subscale.
2.2.3. Community Disadvantage.
All available sixth grade participants’ home addresses were geocoded (N =82.5%). The rationale for the choice of sixth grade as the point in development to assess community disadvantage was that it preceded the onset of marijuana use and disorder for the entire sample and was concurrent with the first opportunities to use marijuana. The community disadvantage score was calculated using census-tract level items from the 1990 and 2000 Decennial census (U.S. Census, 2009). The items used to create the index include the percentages of (a) adults 25 years or older with a college degree, (b) owner-occupied housing, (c) households with incomes below the federal poverty threshold, and (d) female-headed households with children. The following formula was used to generate the index: {[(c / 10 + d / 10) – (a / 10 + b / 10)] / 4} (Ross & Mirowsky, 2001). Higher scores reflect higher disadvantage.
2.2.4. Discovery Sample for CD.
A GWA for CD symptoms was conducted by Dick et al. (2010). This analysis included participants from the Study of Addiction: Genes and Environment (SAGE), which were drawn from three separate cohorts: the Collaborative Study on the Genetics of Alcoholism (COGA), the Collaborative Study on the Genetics of Nicotine Dependence (COGEND), and the Family Study of Cocaine Dependence (FSCD) (Dick et al., 2010). The participants included in the GWA met diagnostic criteria for alcohol dependence. Participants (N=3,963) were ethnically diverse adults and retrospectively reported on their CD symptoms. Single nucleotide polymorphisms (SNPs) that were significantly associated with CD symptoms were included in the PRS (Dick et al., 2010).
2.2.5. Discovery Sample for ADHD.
A GWA of ADHD symptoms was conducted by Demontis et al. (2017). This GWA included 20,182 individuals who met diagnostic criteria for ADHD and were drawn from 12 diverse cohorts, such as 23andme, Early Genetics and Life Course Epidemiology Consortium (EAGLE), Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH), and the Yale-Penn Study (Demontis et al., 2017). Depending upon the cohort, ADHD symptoms were ascertained through clinical interviews, medical records, and/or self-reported questionnaires. As with the CD PRS, only SNPs that were significantly associated with ADHD symptoms were included in the ADHD PRS.
2.2.6. Polygenic Risk Score Generation for CD and ADHD.
Based on the results from the discovery GWAS referenced above, our GWA panels contained 245,017 (26.7%) and 681,794 (8.4%) directly genotyped SNPs from the CD and ADHD discovery sample results, respectively. After imputation, 812,174 (88.5%) SNPs from the CD discovery sample and 6,141,434 (75.3%) SNPs from the ADHD discovery sample were available in the current sample. The reference panels included all available populations. Palindromic (A/T or C/G) SNPs were excluded, as methods for properly orienting multiple datasets are error-prone. To account for linkage disequilibrium (correlation at markers close together along a chromosome), two rounds of the LD-based results clumping were run in PLINK 2.0 (Chang et al., 2015) against the HapMap Phase III Release 2 Build 36 reference panel (The International HapMap 3 Consortium, 2010), resulting in 203,867 selected SNPs for the CD PRS and 171,780 for the ADHD PRS. Based on the p-values below our chosen threshold of 0.05, 20,810 and 20,062 SNPs were included in the CD and ADHD PRS, respectively. Raw scores were generated in the PRC imputed dosage dataset in PLINK 2.0 (Purcell et al., 2007). Mean imputation was done for missing genotypes and alleles were weighted by the effect sizes from the discovery GWA. Principal components analysis was used to create the population stratification control variables in PLINK 2.0 (Chang et al., 2015). This process uses an orthogonal transformation to reduce the multi-dimensional genome-wide SNP data into a smaller number of principal components. We used all the available measured SNPs (roughly 900,000) to generate these components. Ten principal components were identified that sufficiently accounted for population stratification in the sample. Each raw PRS was regressed on the ten ancestry principal components. The z-scored residuals from these regressions are the continuous ancestry-adjusted (or corrected) scores we used in the primary analyses. Additional information regarding the creation of the CD and ADHD PRS can be found in the supplementary materials.
2.2.7. Statistical Analyses
Bivariate correlations and descriptive statistics were conducted to investigate the relations among predictor and dependent variables using SPSS Version 24 (IBM, 2013). Missing data were imputed using the using the expectation-maximization (EM) algorithm. Logistic regressions were conducted to investigate the main effects of the predictors (participant sex, intervention status, the CD and ADHD PRS, parental monitoring, community disadvantage) and interaction terms (CD PRS (or ADHD PRS) × parental monitoring (or community disadvantage). The independent variables were mean-centered, with the exception of the PRS which were standardized. Regression analyses were run in a step-wise fashion. Step 1 included the main effects of the covariates and Step 2 included the covariates with the interaction terms.
Significant interaction terms and simple slopes were plotted using an automated spreadsheet (Dawson, 2014). For significant interactions, post-hoc probing involved computing new moderator variables at the mean and ± 1 SD from the mean of community disadvantage and parental monitoring (Holmbeck, 2002). Interaction terms were generated that included these variables. Post hoc regressions involved entry of the covariates (e.g., participant sex, the PRS), the contextual variable at the mean and ± 1 SD from the mean, and the contextual variable × PRS interaction. The unstandardized betas (at the mean and ± 1 SD from the moderator mean) were plotted.
We also examined whether there were sex differences in the interaction between PRS and contextual factors on marijuana use disorders. Two, three-way interaction terms were created that included participant sex × contextual variable (e.g., parental monitoring) × the PRS (e.g., ADHD PRS). Step 1 included the main effects of the study variables considered in the previous regressions. Step 2 included three two-way interactions (participant sex × PRS; participant sex × contextual variable; PRS × contextual variable). Step 3 included the PRS × contextual variable × participant sex interaction.
3. Results
Means, SDs, ns, and bivariate correlations are presented in Table 2. Six percent of the sample (n=63) reported meeting diagnostic criteria for marijuana abuse or dependence. About nine percent of males (n=41) and 3.7% (n=22) of females reported a marijuana use disorder.
Table 2.
Bivariate correlations, means, standard deviations, and n’s of study variables
| Variable | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1. CD PRSa | -- | |||
| 2. ADHD PRSb | −.02 | -- | ||
| 3. Parental Monitoring | −.05 | −.07* | -- | |
| 4. Community Disadvantage | .06 | −.04 | −.16** | -- |
| M | .01c | .00c | 4.46 | −.72 |
| SD | 1.03 | .98 | .51 | 1.09 |
| N | 1050 | 1050 | 851 | 866 |
p <.05;
p <.001.
CD PRS = Conduct disorder polygenic risk score.
ADHD PRS = Attention-deficit hyperactivity polygenic risk score.
The means and standard deviations represented here are from the residualized PRS.
3.1. CD PRS Results
There was a main effect of participant sex such that males were more likely to meet diagnostic criteria for marijuana use disorders (top of Table 3). The CD PRS × community disadvantage interaction was associated with marijuana use disorders (Figure 1); however, the CD PRS × parental monitoring interaction was not (see Figure 2 in the supplementary materials). The simple slope for higher community disadvantage was significant, but not for average or low community disadvantage. In communities higher in disadvantage, participants with a higher CD PRS had a greater log odds of a marijuana use disorder than participants with a lower CD PRS. In average or lower community disadvantage contexts, youth’s likelihood of meeting diagnostic criteria for a marijuana use disorder did not differ based on participants’ CD PRS.
Table 3.
Summary of 2-way regression analyses involving the polygenic risk scores, parental monitoring, and community disadvantage in predicting marijuana use disorders
| Variable | Step 1 | Step 2 | ||||
|---|---|---|---|---|---|---|
| ORa (95% CIb) | p-value | Cox & Snell R2 | OR (95% CI) | p-value | Cox & Snell R2 | |
| CD PRSc | ||||||
| Parental Monitoring | .015 | .015 | ||||
| Sex | .40 (.23 – .68) | .001 | .40 (.23 – .68) | .001 | ||
| Intervention Status | .83 (.50 – 1.40) | .487 | .83 (.50 – 1.40) | .488 | ||
| CD PRS | 1.13 (.88 – 1.44) | .356 | 1.12 (.87 – 1.44) | .371 | ||
| Parental Monitoring | .71 (.43 – 1.18) | .181 | .71 (.43 – 1.19) | .198 | ||
| CD PRS × Parental Monitoring | .95 (.56 – 1.61) | .860 | ||||
| Community Disadvantage | .014 | .017 | ||||
| Sex | .39 (.23 – .67) | .001 | .39 (.23 – .67) | .001 | ||
| Intervention Status | .81 (.48 – 1.36) | .425 | .79 (.47 – 1.33) | .375 | ||
| CD PRS | 1.13 (.88 – 1.45) | .335 | 1.14 (.89 – 1.47) | .305 | ||
| Community Disadvantage | 1.08 (.83 – 1.40) | .562 | 1.02 (.78 – 1.34) | .876 | ||
| CD PRS × Community Disadvantage | 1.31 (1.00 – 1.72) | .049 | ||||
| ADHD PRSd | ||||||
| Parental Monitoring | .014 | .017 | ||||
| Sex | .40 (.23 – .68) | .001 | .40 (.23 – .68) | .001 | ||
| Intervention Status | .83 (.49 – 1.39) | .479 | .86 (.51 – 1.45) | .563 | ||
| ADHD PRS | 1.01 (.78 – 1.32) | .916 | .98 (.75 – 1.29) | .899 | ||
| Parental Monitoring | .70 (.42 – 1.16) | .166 | .74 (.44 – 1.25) | .264 | ||
| ADHD PRS × Parental Monitoring | .65 (.39 – 1.08) | .094 | ||||
| Community Disadvantage | .013 | .016 | ||||
| Sex | .39 (.23 – .66) | .001 | .38 (.22 – .66) | <.005 | ||
| Intervention Status | .81 (.48 – 1.36) | .422 | .81 (.48 – 1.35) | .414 | ||
| ADHD PRS | 1.03 (.79 – 1.34) | .834 | 1.04 (.80 – 1.35) | .788 | ||
| Community Disadvantage | 1.09 (.84 – 1.42) | .519 | 1.10 (.85 – 1.43) | .478 | ||
| ADHD PRS × Community Disadvantage | 1.26 (.98 – 1.63) | .078 | ||||
OR = Odds ratio.
CI = Confidence interval.
CD PRS = Conduct disorder polygenic risk score.
ADHD PRS = Attention-deficit hyperactivity disorder polygenic risk score.
Fig. 1.
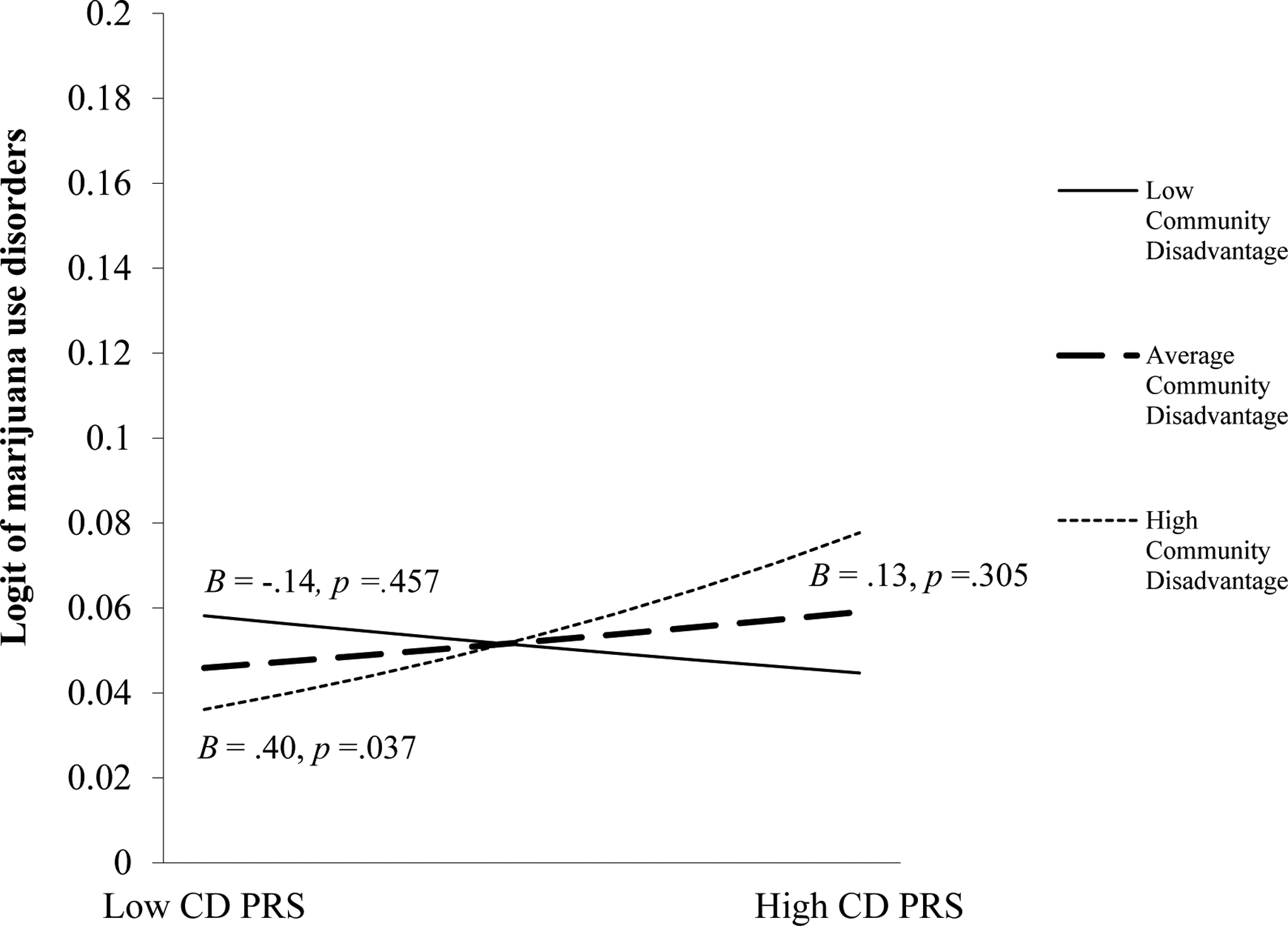
Association between the CD PRS and marijuana use disorders at low, average, and high community disadvantage levels.
Note. The y-axis of the figure was truncated to improve visibility of the slopes and corresponding p-values.
The CD PRS × community disadvantage × participant sex interaction showed a trend for significance (p =.075), but the CD PRS × parental monitoring × participant sex interaction did not (top of Table 4). Given the trend for significance, we split the sample by participant sex. When exposed to higher community disadvantage, males with a higher CD PRS exhibited a greater log odds for a marijuana use disorder (B =.64, p =.017). However, in the context of average (B =.14, p =.396) and low community disadvantage (B = −.35, p =.124), males’ log odds of marijuana abuse or dependence was not influenced by their genetic loading for CD. Among females, the slopes for high, average, and low community disadvantage were not significant.
Table 4.
Summary of 3-way regression analyses involving participant sex, the polygenic risk scores, and contextual factors in predicting marijuana use disorders
| Variable | Step 1 | Step 2 | ||||
|---|---|---|---|---|---|---|
| ORa (95% CIb) | p-value | Cox & Snell R2 | OR (95% CI) | p-value | Cox & Snell R2 | |
| CD PRSc | ||||||
| Parental Monitoring | .015 | .015 | ||||
| Sex | .39 (.23 – .67) | .001 | .39 (.23 – .67) | .001 | ||
| Intervention Status | .83 (.50 – 1.40) | .486 | .83 (.50 – 1.40) | .487 | ||
| CD PRS | 1.14 (.87 – 1.50) | .354 | 1.14 (.87 – 1.50) | .354 | ||
| Parental Monitoring | .68 (.38 – 1.20) | .182 | .68 (.38 – 1.21) | .188 | ||
| CD PRS × Parental Monitoring | .96 (.57 – 1.62) | .870 | .96 (.54 – 1.71) | .881 | ||
| Sex × CD PRS | 1.07 (.63 – 1.80) | .804 | 1.07 (.63 – 1.81) | .805 | ||
| Sex × Parental Monitoring | .81 (.28 – 2.37) | .700 | .81 (.27 – 2.45) | .708 | ||
| Sex × Parental Monitoring × CD PRS | 1.00 (.33 – 3.02) | .996 | ||||
| Community Disadvantage | .018 | .021 | ||||
| Sex | .39 (.23 – .67) | .001 | .44 (.25 – .76) | .004 | ||
| Intervention Status | .79 (.47 – 1.33) | .379 | .79 (.47 – 1.33) | .378 | ||
| CD PRS | 1.15 (.88 – 1.52) | .310 | 1.17 (.89 – 1.54) | .269 | ||
| Community Disadvantage | .99 (.74 – 1.32) | .929 | 1.01 (.76 – 1.34) | .970 | ||
| CD PRS × Community Disadvantage | 1.32 (1.00 – 1.73) | .048 | 1.24 (.93 – 1.64) | .141 | ||
| Sex × CD PRS | 1.05 (.62 – 1.78) | .860 | 1.02 (.60 – 1.74) | .930 | ||
| Sex × Community Disadvantage | .81 (.47 – 1.39) | .449 | .91 (.52 – 1.59) | .746 | ||
| Sex × Community Disadvantage × CD PRS | .61 (.35 – 1.05) | .075 | ||||
| ADHD PRSd | ||||||
| Parental Monitoring | .017 | .017 | ||||
| Sex | .39 (.23 – .68) | .001 | .39 (.22 – .68) | .001 | ||
| Intervention Status | .85 (.50 – 1.44) | .545 | .85 (.50 – 1.43) | .537 | ||
| ADHD PRS | 1.01 (.76 – 1.35) | .928 | 1.01 (.76 – 1.35) | .927 | ||
| Parental Monitoring | .71 (.40 – 1.27) | .249 | .72 (.40 – 1.30) | .273 | ||
| ADHD PRS × Parental Monitoring | .64 (.38 – 1.06) | .084 | .61 (.34 – 1.11) | .107 | ||
| Sex × ADHD PRS | 1.20 (.69 – 2.08) | .525 | 1.19 (.68 – 2.07) | .539 | ||
| Sex × Parental Monitoring | .84 (.28 – 2.48) | .746 | .87 (.28 – 2.66) | .802 | ||
| Sex × Parental Monitoring × ADHD PRS | .86 (.28 – 2.67) | .791 | ||||
| Community Disadvantage | .016 | .017 | ||||
| Sex | .39 (.23 – .66) | .001 | .40 (.23 – .68) | .001 | ||
| Intervention Status | .81 (.48 – 1.36) | .421 | .80 (.48 – 1.36) | .412 | ||
| ADHD PRS | 1.06 (.80 – 1.41) | .682 | 1.07 (.81 – 1.42) | .636 | ||
| Community Disadvantage | 1.06 (.80 – 1.40) | .702 | 1.07 (.81 – 1.41) | .644 | ||
| ADHD PRS × Community Disadvantage | 1.27 (.97 – 1.65) | .078 | 1.22 (.93 – 1.62) | .155 | ||
| Sex × ADHD PRS | 1.12 (.64 – 1.94) | .693 | 1.11 (.64 – 1.93) | .706 | ||
| Sex × Community Disadvantage | .79 (.46 – 1.36) | .391 | .79 (.46 – 1.36) | .401 | ||
| Sex × Community Disadvantage × ADHD PRS | .81 (.47 – 1.39) | .443 | ||||
OR = Odds ratio.
CI = Confidence interval.
CD PRS = Conduct disorder polygenic risk score.
ADHD PRS = Attention-deficit hyperactivity disorder polygenic risk score.
3.2. ADHD PRS Results
The ADHD PRS was not associated with marijuana use disorders (bottom of Table 3). The ADHD PRS × community disadvantage (p =.094) and ADHD PRS × parental monitoring (p =.078) interactions showed trends for significance. Both the 3-way interactions involving participant sex, the ADHD PRS, and the contextual variable were not significant (bottom of Table 4).
4. Discussion
Contemporary models of substance use indicate that the interaction of individual and contextual factors likely influence the development of substance use disorders (Shanahan and Hofer, 2005). Although higher phenotypic externalizing symptoms, such as CD and ADHD, are positively associated with and predictive of marijuana abuse and dependence (e.g., Bidwell et al., 2014; Grant et al., 2015), it is unclear whether such an association is also true for CD and ADHD polygenic genotypes. Moreover, while parental monitoring and community disadvantage have been identified as predictors of marijuana use (e.g., Reboussin et al., 2015; Siegel et al., 2014), there is a dearth of work examining whether these contextual factors interact with CD and ADHD polygenic risk sores to influence risk for marijuana use disorders in adulthood. Accordingly, using an urban, African-American sample, the present study examined whether a CD and ADHD PRS, and the interaction of these scores with parental monitoring and community disadvantage was significantly associated with marijuana use disorders.
We found that in the context of higher community disadvantage, participants with a higher CD genetic loading exhibited greater risk for a marijuana use disorder. These findings are consistent with theoretical and empirical models indicating that the combination of genetic and contextual factors may have a greater impact on substance use disorders than when genetic liabilities and environmental factors are considered alone (Shanahan and Hofer, 2005). Neighborhoods higher in disadvantage characterized by greater illicit sales of drugs and reduced access to health and social services may facilitate marijuana use disorders among individuals with a higher CD PRS given their potential propensity for rule-breaking and norm violations (Lahey & Waldman, 2012). This may be especially true in young adulthood, given that this developmental period is associated with increased independence and decreases in oversight by parents (Arnett, 2007). It is also possible that youth with higher CD genetic loading may be lower in fearfulness and may seek out deviant, substance-using peers that frequent more disadvantaged neighborhoods where marijuana may be more available. Future research should identify intermediate phenotypes associated with the CD PRS, which may help clarify the mechanisms through which higher CD genetic loading influences human behavior in the context of more disadvantaged communities.
Upon splitting the sample, the interaction between genetic propensity for CD and community disadvantage was significant among males, but not females. In particular, males with greater CD genetic loading were at elevated risk for a marijuana use disorder when exposed to higher levels of neighborhood disadvantage. One explanation for this is that the base rates of marijuana abuse and dependence were greater among males relative to females in the sample. It is also possible that females may experience a greater fear of victimization in more disordered communities relative to males (Schafer et al., 2006) and thus, may avoid spending time in these neighborhoods. Future research should examine the mechanisms through which males higher in CD genetic loading are at greater risk for a marijuana use disorder in more disadvantaged areas, and other contextual factors that influence the development of marijuana use disorders among females.
Whereas evidence of G x E interactions was found, the main effects of the CD and ADHD PRS on marijuana use disorders were not significant. The lack of an association between marijuana use disorders and the CD and ADHD PRS is consistent with a number of molecular genetic studies indicating modest main effects of genetic variants on substance disorder outcomes (Hines et al., 2014). Future work should examine whether the findings presented here generalize to other samples and whether higher polygenic load for CD and ADHD confers risk for subclinical marijuana use disorder symptoms and other substance use disorders (e.g., alcohol abuse and dependence). In addition, future work should investigate whether genetic loading for cannabis use is associated with marijuana abuse and dependence.
The main effects of community disadvantage and parental monitoring on marijuana abuse and dependence were not significant. These environmental features may play less of a role in the development of marijuana use disorders and may have a greater impact on less severe forms of marijuana involvement. Interpersonal interactions with romantic partners and friends, as well as experiences in the college/work environment, may be more influential in terms of the development of marijuana use disorders in young adulthood, given increased exposure to extrafamilial influences that characterize this developmental period (Arnett, 2009). At age 20, parental monitoring may not have had an effect on marijuana abuse and dependence as participants may have been living outside the home, pursuing postsecondary education or training, or working full-time; as such, their parents may have less of an opportunity to supervise them and prevent them from using marijuana heavily.
The main study findings should be interpreted with caution. In total, eight sets of analyses were run. Using a Bonferroni correction, the p-value for the community disadvantage and CD PRS interaction (p =.049) would not have met the necessary threshold required to be significant (p =.006). Moreover, there are several limitations of the current study to acknowledge. Both the CD and ADHD PRS were largely derived from cohorts that included a limited number of African-Americans (Dick et al., 2010; Demontis et al., 2017). Some work indicates that the genetic variants that play a role in the etiology of substance use disorders, such as marijuana abuse and dependence, among European samples may be of low frequency in African-American populations. In particular, individuals of African ancestry tend to have greater genetic diversity, increasing the likelihood that genetic markers that play a role in the etiology of substance use among individuals of African descent may not be observed or may be in low linkage disequilibrium with the variants causing the association signal in European descent populations (Dick et al., 2017). However, some work suggests that the allelic architecture for substance use disorders (i.e., alcohol dependence) is similar across European and African populations. For example, work by Brick et al. (2017) indicated that there was genetic overlap between a subset of genome-wide SNPs (rg =.77, p =.030) that contributed to additive genetic variance of alcohol dependence in samples of both European and African ancestry. Other research has found that some genes involved in the pathology of alcohol dependence are shared among African-Americans and European-Americans (Gelernter et al., 2014). These findings suggest that the genetic pathways underpinning alcohol dependence may be shared, which may generalize to marijuana abuse and dependence, though further study is needed. An additional limitation is that the discovery samples included in the GWA on CD include a number of cohorts that met diagnostic criteria for substance use disorders (Dick et al., 2010). It is thus possible that the genetic variants associated with CD may not be specific to conduct problems. Future GWAS examining genetic factors related to CD should incorporate community samples that may be less likely to have comorbid substance use problems.
Despite these limitations, the current study makes a number of contributions to the literature. We examined genetic loading for two externalizing disorders (i.e., CD and ADHD) in relation to marijuana use disorders. The consideration of genetic factors associated with these two disorders allowed us to identify whether genetic loading for externalizing disorders more generally is associated with marijuana abuse and dependence, or whether liability for marijuana use disorders is specific to CD or ADHD genetic factors. In addition, we examined the interplay between contextual factors (i.e., parental monitoring and community disadvantage) and polygenic influences of CD and ADHD in a socioeconomically disadvantaged, African-American sample, a population that may experience more frequent and severe environmental stressors that intensify their risk for a substance use disorder (Galea et al., 2005). In terms of next steps, genetic network and pathway analyses will be used to investigate whether specific biological pathways are statistically enriched in terms of the SNPs making up our CD and ADHD PRS (O’Dushlaine et al. 2015).
Supplementary Material
References
- 1000 Genomes Project Consortium, 2010. A map of human genome variation from population-scale sequencing. Nature 467, 1061–73. 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 1994. Diagnostic and statistical manual of mental disorders (4th ed). Washington, DC, US: Author. [Google Scholar]
- American Psychiatric Association, 2000. Diagnostic and statistical manual – text revision Washington, DC: Author. [Google Scholar]
- Arnett JJ, 2007. Emerging Adulthood: What is it, and what is it good for? Child Dev. Perspect 1, 68–73. 10.1111/j.1750-8606.2007.00016.x [DOI] [Google Scholar]
- Bidwell LC, Henry EA, Willcutt EG, Kinnear MK, Ito TA, 2014. Childhood and current ADHD symptom dimensions are associated with more severe cannabis outcomes in college students. Drug Alcohol Depend 135, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brick LA, Keller MC, Knopik VS, Mcgeary JE, Palmer RHC, 2017. Shared additive genetic variation for alcohol dependence among subjects of African and European ancestry. Addict. Biol 10.1111/adb.12578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U, 1994. Ecological models of human development. Readings Dev. Child https://doi.org/www.psy.cmu.edu/~siegler/35bronfebrenner94 [Google Scholar]
- Burdzovic Andreas J, Pape H, 2015. Who receives cannabis use offers: A general population study of adolescents. Drug Alcohol Depend 156, 150–156. 10.1016/j.drugalcdep.2015.09.009 [DOI] [PubMed] [Google Scholar]
- Burdzovic Andreas J, Pape H, Bretteville-Jensen AL, 2016. Who are the adolescents saying “No” to cannabis offers. Drug Alcohol Depend 163, 64–70. 10.1016/j.drugalcdep.2016.03.025 [DOI] [PubMed] [Google Scholar]
- Burdzovic Andreas J, Watson MW, 2016. Person-environment interactions and adolescent substance use: the role of sensation seeking and perceived neighborhood risk. J. Child Adolesc. Subst. Abus 25, 438–447. 10.1080/1067828X.2015.1066722 [DOI] [Google Scholar]
- Byck GR, Bolland J, Dick D, Ashbeck AW, & Mustanski BS, 2013. Prevalence of mental health disorders among low-income African American adolescents. Soc Psychiatry and Psychiatr. Epidemiol 48, 1555–1567. 10.1007/s00127-013-0657-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi DM, Patterson GR, 1989. Psychometric properties of fourteen latent constructs from the Oregon Youth Study NY, NY, US: Springer-Verlag. [Google Scholar]
- Carragher N, Krueger RF, Eaton NR, Markon KE, Keyes KM, Blanco C, Saha TD, Hasin DS, 2014. ADHD and the externalizing spectrum: direct comparison of categorical, continuous, and hybrid models of liability in a nationally representative sample. Soc. Psychiatry Psychiatr. Epidemiol 49, 1307–1317. 10.1007/s00127-013-0770-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, 2006. Gene-environment interactions in psychiatry: Joining forces with neuroscience. Nat. Rev. Neurosci 7, 583–590. 10.1038/nrn1925 [DOI] [PubMed] [Google Scholar]
- Chabrol H, Saint-Martin C, 2009. Cannabis use and delinquent behaviors in high-school students. Addict. Behav 34, 187–189. 10.1016/j.addbeh.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ, 2015. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4, 1–16. 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson JF, 2014. Moderation in management research: What, why, when and how. J Bus Psychol 29, 1–19. 10.1007/s10869-013-9308-7 [DOI] [Google Scholar]
- Delaneau O, Zagury J-F, Marchini J, 2013. Improved whole-chromosome phasing for disease and population genetic studies. Nature Methods 10, 5–6. 10.1038/nmeth.2307 [DOI] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, Belliveau R, Bybjerg-Grauholm J, Bækved-Hansen M, Cerrato F, Chambert K, Churchhouse C, Dumont A, Eriksson N, Gandal M, Goldstein J, Grove J, Hansen CS, Hauberg M, Hollegaard M, Howrigan DP, Huang H, Maller J, Martin AR, Moran J, Pallesen J, Palmer DS, Pedersen CB, Pedersen MG, Poterba T, Poulsen JB, Ripke S, Robinson EB, Satterstrom FK, Stevens C, Turley P, Won H, Andreassen OA, Burton C, Boomsma D, Cormand B, Dalsgaard S, Franke B, Gelernter J, Geschwind D, Hakonarson H, Haavik J, Kranzler H, Kuntsi J, Langley K, Lesch K-P, Middeldorp C, Reif A, Rohde LA, Roussos P, Schachar R, Sklar P, Sonuga-Barke E, Sullivan PF, Thapar A, Tung J, Waldman I, Nordentoft M, Hougaard DM, Werge T, Mors O, Mortensen PB, Daly MJ, Faraone SV, Børglum AD, Neale BM, 2017. Discovery of the first genome-wide significant risk loci for adhd. BioRxiv 1–43. 10.1101/145581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Krueger RF, Edwards A, Agrawal A, Lynskey M, Lin P, Schuckit M, Hesselbrock V, Nurnberger J, Almasy L, Porjesz B, Edenberg HJ, Bucholz K, Kramer J, Kuperman S, Bierut L, 2010. Genome-wide association study of conduct disorder symptomatology. Mol. Psychiatry 16, 800–808. 10.1038/mp.2010.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Barr P, Guy M, Nasim A, Scott D, 2017. Review: Genetic research on alcohol use outcomes in African American populations: A review of the literature, associated challenges, and implications. Am. J. Addict 26, 486–493. 10.1111/ajad.12495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishion TJ, McMahon RJ, 1998. Parental monitoring and the prevention of child and adolescent problem behavior: a conceptual and empirical formulation. Clin. Child Fam. Psychol. Rev 1, 61–75. 10.1023/A:1021800432380 [DOI] [PubMed] [Google Scholar]
- Dodge KA, 2009. Mechanisms of Gene-Environment Interaction Effects in the Development of Conduct Disorder. Perspect. Psychol. Sci 4, 408–414. 10.1111/j.1745-6924.2009.01147.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Rietz E, Coleman J, Glanville K, Choi SW, O’Reilly PF, Kuntsi J, 2018. Association of polygenic risk for attention-deficit/hyperactivity disorder with co-occurring traits and disorders. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 1–9. 10.1016/j.bpsc.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer RF, Seeley JR, Kosty DB, Gau JM, Duncan SC, 2014. Internalizing and externalizing psychopathology as predictors of cannabis use disorder onset during adolescence and early adulthood during adolescence and early adulthood. Journal of Abnormal Psychology 29, 541–551. 10.1037/adb0000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, 2008. Cannabis use and adult ADHD symptoms. Drug Alcohol Depend 95, 90–96. 10.1016/j.drugalcdep.2007.12.012 [DOI] [PubMed] [Google Scholar]
- Furr-Holden CDM, Lee MH, Johnson R, Milam AJ, Duncan A, Reboussin BA, Leaf PJ, Ialongo NS, 2014. Neighborhood environment and marijuana use in urban young adults. Prev. Sci 16, 268–278. 10.1007/s11121-014-0497-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea S, Rudenstine S, Vlahov D, 2005. Drug use, misuse, and the urban environment. Drug Alcohol Rev 24, 127–136. 10.1080/09595230500102509 [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH, Anton R, Preuss UW, Ridinger M, Rujescu D, Wodarz N, Zill P, Zhao H, Farrer LA, 2014. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol. Psychiatry 19, 41–49. 10.1038/mp.2013.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliard-Matthews S, Stevens R, Nilsen M, Dunaev J, 2015. “You see it everywhere. It’s just natural.”: Contextualizing the role of peers, family, and neighborhood in initial substance use. Deviant Behav 36, 492–509. 10.1080/01639625.2014.944068 [DOI] [Google Scholar]
- Graham JW, 2009. Missing data analysis: Making it work in the real world. Annu. Rev. Psychol 60, 549–576. 10.1146/annurev.psych.58.110405.085530 [DOI] [PubMed] [Google Scholar]
- Grant JD, Lynskey MT, Madden PAF, Nelson EC, Few LR, Bucholz KK, Statham DJ, Martin NG, Heath AC, Agrawal A, 2015. The role of conduct disorder in the relationship between alcohol, nicotine and cannabis use disorders. Psychol. Med 45, 3505–3515. 10.1017/S0033291715001518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty SC, Pedersen SL, Gnagy EM, Pelham WE, Molina BSG, 2015. ADHD and marijuana-use expectancies in young adulthood. Subst. Use Misuse 50, 1470–1478. 10.3109/10826084.2015.1018545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan J, Smith SM, Huang B, Grant BF, 2015. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry 72, 1235. 10.1001/jamapsychiatry.2015.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines LA, Morley KI, Mackie C, & Lynskey M, 2014. Genetic and environmental interplay in adolescent substance use disorder. Cur Addict Rep 2, 122–129. 10.1007/s40429-015-0049-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KG, Hawkins JD, Bailey JA, Catalano RF, Robert D, 2011. Person-environment interaction in the prediction of alcohol abuse and alcohol dependence in adulthood. Drug Alcohol Dep 110, 62–69. 10.1016/j.drugalcdep.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck GN, 2002. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. J Pediatr Psychol 27, 87–96. 10.1093/jpepsy/27.1.87 [DOI] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, Marchini J, 2009. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PloS Genetics 5, e1000529. 10.1371/journal.pgen.1000529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0 Armonk, NY: IBM Corp. [Google Scholar]
- Kessler R, McGonagle K, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen H-U, Kendler K, 1994. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Arch Gen Psychiatry 51, 8–19. 10.1001/archpsyc.1994.03950010008002 [DOI] [PubMed] [Google Scholar]
- Kessler R, Wittchen H-U, Abelson JM, Mcgonagle K, Schwarz N, Kendler KS, Knauper B, Zhao S, 1998. Methodological studies of the Composite International Diagnostic Interview (CITI) in the US National Comorbidity Study. International Journal of Methods in Psychiatric Research 7, 33–55. 10.1002/mpr.33 [DOI] [Google Scholar]
- King SM, Iacono WG, McGue M, 2004. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction 99, 1548–1559. 10.1111/j.1360-0443.2004.00893.x [DOI] [PubMed] [Google Scholar]
- Korhonen T, van Leeuwen AP, Reijneveld SA, Ormel J, Verhulst FC, Huizink AC, 2010. Externalizing behavior problems and cigarette smoking as predictors of cannabis use: The TRAILS Study. J. Am. Acad. Child Adolesc. Psychiatry 49, 61–69. 10.1097/00004583-201001000-00010 [DOI] [PubMed] [Google Scholar]
- Kosty DB, Seeley JR, Farmer RF, Stevens JJ, Lewinsohn PM, 2017. Trajectories of cannabis use disorder: risk factors, clinical characteristics and outcomes. Addiction 112, 279–287. 10.1111/add.13557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG, 2005. Externalizing psychopathology in adulthood: A dimensional-spectrum conceptualization and its implications for DSM-V. J. Abnorm. Psychol 114, 537–550. 10.1037/0021-843X.114.4.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Waldman ID, 2012. Annual research review: Phenotypic and causal structure of conduct disorder in the broader context of prevalent forms of psychopathology. J. Child Psychol. Psychiatry Allied Discip 53, 536–557. 10.1111/j.1469-7610.2011.02509.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakon CM, Wang C, Butts CT, Jose R, Timberlake DS, Hipp JR, 2015. A dynamic model of adolescent friendship networks, Parental influences, and smoking. J. Youth Adolesc 44, 1767–1786. 10.1007/s10964-014-0187-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CC, 1969. Population subdivision with respect to multiple alleles. Ann Hum Genet 33, 23–29. Retrieved from https://www-ncbi-nlm-nih-gov.proxy1.library.jhu.edu/pubmed/5821316 [DOI] [PubMed] [Google Scholar]
- Liu J, Lewinger JP, Gilliland FD, Gauderman WJ, Conti DV, 2013. Confounding and heterogeneity in genetic association studies with admixed populations. Am. J. Epidemiol 177, 351–360. 10.1093/aje/kws234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams T, Rowe R, Rijsdijk F, Maughan B, Eley TC, 2012. The covariation of antisocial behavior and substance use in adolescence: a behavioral genetic perspective. J. Res. Adolesc 22, 100–112. 10.1111/j.1532-7795.2011.00758.x [DOI] [Google Scholar]
- Meaney MJ, 2010. Epigenetics and the biological basis of gene x environment interactions. J Am Acad Child Adolesc Psychiatry 49, 752–771. 10.1016/j.jaac.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Miles DR, van den Bree MBM, Pickens RW, 2002. Sex differences in shared genetic and environmental influences between conduct disorder symptoms and marijuana use in adolescents. Am. J. Med. Genet 114, 159–168. 10.1002/ajmg.10178 [DOI] [PubMed] [Google Scholar]
- Miranda A, Colomer C, Berenguer C, Roselló R, Roselló B, 2016. Substance use in young adults with ADHD: Comorbidity and symptoms of inattention and hyperactivity/impulsivity. Int. J. Clin. Heal. Psychol 16, 157–165. 10.1016/j.ijchp.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Network and Pathway Analysis Subgroup of the Psychiatric Genomics Consortium, 2015. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci 18, 199–209. https://doi.10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Malcolm RJ, Martins SS, 2012. Race/ethnicity differences between alcohol, marijuana, and co-occurring alcohol and marijuana use disorders and their association with public health and social problems using a national sample. Am. J. Addict 21, 435–444. 10.1111/j.1521-0391.2012.00249.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico AM, Bird G, Gabbay FH, & Uhl GR, 1996. D2 dopamine receptor gene taqI A1 and B1 restriction fragment length polymorphisms: Enhanced frequencies in psychostimulant-preferring polysubstance abusers. Biol. Psychiatry 40, 776–784. 10.1016/0006-3223(95)00483-1 [DOI] [PubMed] [Google Scholar]
- Pritchard JK, & Rosenberg NA, 1999. Use of unlinked genetic markers to detect population stratification in association studies. Am. J. Hum. Genet, 65, 220–228. 10.1086/302449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC, 2007. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet 81, 559–575. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Morris N, Kang SJ, Li M, Tayo B, Lyon H, Hirschhorn J, Cooper RS, Zhu X, 2010. Interrogating local population structure for fine mapping in genome-wide association studies. Bioinformatics 26, 2961–2968. 10.1093/bioinformatics/btq560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboussin BA, Green KM, Milam AJ, Furr-Holden DM, Johnson RM, Ialongo NS, 2015. The role of neighborhood in urban black adolescent marijuana use. Drug Alcohol Depend 154, 69–75. 10.1016/j.drugalcdep.2015.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg MF, Anthony JC, 2001. Aggressive behavior and opportunities to purchase drugs. Drug Alcohol Depend 63, 245–252. 10.1016/S0376-8716(00)00213-1 [DOI] [PubMed] [Google Scholar]
- Ross CE, Mirowsky J, 2001. Neighborhood disadvantage, disorder, and health. Journal of Health and Social Behavior 42, 258–276. 10.2307/3090214 [DOI] [PubMed] [Google Scholar]
- Salas-Wright CP, Oh S, Goings TC, Vaughn MG, 2017. Trends in perceived access to marijuana among adolescents in the United States: 2002–2015. J. Stud. Alcohol Drugs 78. 10.15288/jsad.2017.78.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JA, Huebner BM, Bynum TS, 2006. Fear of crime and criminal victimization: Gender-based contrasts. J. Crim. Justice 34, 285–301. 10.1016/j.jcrimjus.2006.03.003 [DOI] [Google Scholar]
- Shanahan MJ, Hofer SM, 2005. Social context in gene – environment interactions : Retrospect and Prospect. J Gerontol B Psychol Sci Soc Sci 60, 65–76. Retrieved from https://www-ncbi-nlm-nih-gov.proxy1.library.jhu.edu/pubmed/?term=Social+Context+in+Gene%E2%80%93Environment+Interactions%3A+Retrospect+and+Prospect [DOI] [PubMed] [Google Scholar]
- Shelton K, Lifford K, Fowler T, Rice F, Neale M, Harold G, Thapar A, Van Den Bree M, 2007. The association between conduct problems and the initiation and progression of marijuana use during adolescence: A genetic analysis across time. Behav. Genet 37, 314–325. 10.1007/s10519-006-9124-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JT, Crano WD, Alvaro EM, Lac A, Hackett JD, Hohman ZP, 2014. Differentiating common predictors and outcomes of marijuana initiation: A retrospective longitudinal analysis. Subst. Use Misuse 49, 30–40. 10.3109/10826084.2013.817427 [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB, 1992. The structured clinical interview for DSM-III-R (SCID). Arch Gen Psychiatry 49, 624–629. 10.1001/archpsyc.1992.01820080032005 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Administration, 2001. 2007 National Survey on Drug Use and Health Questionnaire Retrieved from: http://datafiles.samhsa.gov/study/national-survey-drug-use-and-health-nsduh-2007-nid1353
- Tebes JK, Cook EC, Vanderploeg JJ, Feinn R, Chinman MJ, Shepard JK, Brabham T, Connell CM, 2011. Parental knowledge and substance use among African American adolescents: Influence of gender and grade level. J. Child Fam. Stud 20, 406–413. 10.1007/s10826-010-9406-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The International HapMap 3 Consortium, 2010. Integrating common and rare genetic variation in diverse human populations. Nature 467, 52–58. 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. American Community Survey 5-year estimates: 2005–2009 (2009). Retrieved from http://www.census.gov/programssurveys/acs/data/summary-file.2009.html.
- Wallace SA, Neilands TB, Phillips KS, 2017. Neighborhood context, psychological outlook, and risk behaviors among urban African American youth. Cultur. Divers. Ethnic Minor. Psychol 23, 59–69. 10.1037/cdp0000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JM, Muroff JR, 2002. Preventing substance abuse among african american children and youth : race differences in risk factor exposure and vulnerability. J. Prim. Prev 22, 235–261. 10.1023/A:1013617721016 [DOI] [Google Scholar]
- Young SE, Corley RP, Stallings MC, Rhee SH, Crowley TJ, Hewitt JK, 2002. Substance use, abuse and dependence in adolescence: Prevalence, symptom profiles and correlates. Drug Alcohol Depend 68, 309–322. 10.1016/S0376-8716(02)00225-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


