Abstract
Objective:
To quantify the risk of hyperkalemia and acute kidney injury (AKI) when spironolactone use is added on to loop diuretic use among patients with heart failure, and to evaluate whether the risk is modified by level of kidney function.
Methods:
We identified 17,110 patients with heart failure treated with loop diuretics between January 1, 2004, and December 31, 2016 within the Geisinger Health System. We estimated the incidence of hyperkalemia and AKI associated with spironolactone initiation, and used target trial emulation methods to minimize confounding by indication.
Results:
During a mean follow-up of 134 mo, 3229 of 17,110 patients (18.9%) initiated spironolactone. Incidence rates of hyperkalemia and AKI in patients using spironolactone with a loop diuretic were 2.9 and 10.1 events per 1000 person-months, respectively. In propensity scoreematched analyses, spironolactone initiation was associated with higher hyperkalemia and AKI risk compared with loop alone (hazard ratio, 1.69; 95% CI, 1.35 to 2.10; P<.001, and hazard ratio, 1.12; 95% CI, 1.00 to 1.26; P=.04, respectively). There were no differences in the relative risk of either outcome associated with spironolactone by level of kidney function.
Conclusion:
The addition of spironolactone to loop diuretics in patients with heart failure was associated with higher risk of hyperkalemia and AKI; these risks must be weighed against the potential benefits of spironolactone.
Spironolactone is an aldosterone antagonist indicated for the treatment of New York Heart Association functional class III-IV heart failure with reduced ejection (HFrEF).1 Concomitant spironolactone and loop diuretic use decreased heart failure hospitalization and mortality among patients with HFrEF in the landmark Randomized Aldactone Evaluation Study (RALES) study.2 Evidence for spironolactone’s effectiveness among patients with heart failure with preserved ejection (HFpEF) is less established,3 but data from the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial suggest a benefit for spironolactone in HFpEF, with a protective effect for heart failure hospitalization but not mortality.4 Nevertheless, any benefits associated with spironolactone must be weighed in the context of its known serious risks, specifically hyperkalemia and acute kidney injury (AKI).1,5–13
Most prior clinical trials of spironolactone focused on patient populations with relatively preserved kidney function. In both RALES and TOPCAT, patients with estimated glomerular filtration rate (eGFR) less than 30 mL/min per 1.73 m2, serum creatinine (SCr) greater than 2.5 mg/dL, and serum potassium (K) greater than 5.0 mEq/L were excluded.2,4 In addition, patients’ SCr and K levels were regularly monitored, allowing for dose adjustments and discontinuation that may have mitigated the risk of hyperkalemia and AKI. The risks of spironolactone in less-controlled, real-world settings are not well understood, particularly among those with reduced kidney function who are already at higher risk for hyperkalemia and AKI.
Despite current clinical guidelines recommending aldosterone antagonists only at eGFR greater than 30 mL/min per 1.73 m2,14,15 patients are prescribed spironolactone across all levels of eGFR.16 Randomized controlled trials are ongoing to better understand whether aldosterone antagonists provide cardiovascular and renal benefit in patients with advanced chronic kidney disease (CKD).17 Given the existing use, and the potential for expanded use in patients with HFpEF and lower kidney function, the real-world safety profile of spironolactone must be assured. Because spironolactone is commonly used in addition to loop diuretics and the latter may also modify the risks of hyperkalemia and AKI, we quantified the absolute and relative risks of hyperkalemia and AKI associated with spironolactone use among a population of patients with heart failure on loop diuretics and evaluated for differential risk by level of kidney function.
METHODS
Data Source and Population
We used integrated electronic health record (EHR) data from the Geisinger Health System (Geisinger) in Pennsylvania. These EHR data include inpatient and outpatient records for patients receiving their primary care at Geisinger, as well as medication orders, medication reconciliation, and laboratory results. We included patients age 18 years or older with a heart failure diagnosis code from inpatient or outpatient records, and a subsequent outpatient prescription for a loop diuretic from January 1, 2004, to December 31, 2016 (Supplemental Table 1, available online at http://www.mayoclinicproceedings.org). We excluded patients with end-stage renal disease, those with spironolactone use before the first loop diuretic order in the data, and patients without an antecedent SCr or K level. This study was approved by the Johns Hopkins University Institutional Review Board and the Geisinger Medical Center Institutional Review Board.
Study Designs and Exposure Definitions
To determine the incidence of hyperkalemia and AKI during real-world use of spironolactone in heart failure, we performed an as-treated analysis, with patients’ time at risk (T0) starting at the first loop diuretic prescription after their initial heart failure diagnosis. We classified time at risk according to time-varying loop diuretic and spironolactone use (single-ingredient spironolactone or combination spironolactone with hydrochlorothiazide). In the continuous use periods, we allowed for a 30-day gap between the end of one prescription and the start of the next for the same medication, and included a 15-day “washout” period at the end of a continuous use episode where outcomes could still be observed. Primary exposure groups in the time-varying, as-treated analysis were thus loop diuretic use without spironolactone (loop alone), spironolactone use without a loop diuretic (spironolactone alone), concomitant use of both a loop diuretic and spironolactone, and no use of either drug.
To strengthen evidence for a causal relationship between spironolactone and hyperkalemia and AKI we performed a target trial emulation with an intention-to-treat (ITT) design.18–21 This method, particularly when combined with propensity score matching, helps to minimize confounding by indication, allowing for a direct assessment of the risks associated with spironolactone in comparable patients. We used the ITT principle (ie, treatment assignment/status at baseline is carried forward regardless of subsequent changes to treatment) to analyze a series of “trials” where each trial represents a fixed time window when a patient may or may not begin a specific treatment regimen. To mimic a hypothetical trial selection process, enrollment criteria in each trial (cohort years: [1] 2004e2006, [2] 2007e2009, [3] 2010e2012, and [4] 2013e2016) included heart failure diagnosis, loop diuretic prescription order, no prior spironolactone order, and K level less than 5.0 mEq/L. Within each trial, patients who received an initial spironolactone prescription were compared with eligible patients who did not. Time at risk (T0) began on the date of the first spironolactone prescription for spironolactone users (treatment), and a random loop prescription during that trial period for controls. Control patients were eligible to be treatment or control patients in subsequent trials, whereas treatment patients were no longer eligible due to their previous spironolactone use. In the final analysis, the trial cohorts were pooled to create a combined dataset.
Study Outcomes
In both the as-treated and ITT analyses, patients were followed until the outcomes of interest (hyperkalemia and AKI), death, last recorded health care encounter date (including inpatient, outpatient, laboratory, or medication order dates), or the end of the study period (December 31, 2016), whichever came first. We defined hyperkalemia by inpatient International Classification of Disease, Ninth Revision (ICD-9) or ICD-10 codes 276.7 or E87.5, respectively, and AKI by inpatient ICD-9 or ICD-10 codes 584.9 or N17.9, respectively.
Kidney Function, Potassium, and Other Covariates
We defined eGFR based on outpatient SCr and the Chronic Kidney Disease Epidemiology Collaboration equation.22 We classified eGFR using four primary categories (≥90 mL/min per 1.73 m2; 60 to 89 mL/min per 1.73 m2; 30 to 59 mL/min per 1.73 m2; and <30 mL/min per 1.73 m2), and as linear splines with a knot at 60 mL/min per 1.73 m2. We classified serum K levels using three categories (<3.5 mEq/L; 3.5 to 4.9 mEq/L; and >5.0 mEq/L), and as linear splines with knots at 3.5 mEq/L and 4.9 mEq/L; in the ITT analysis, only one knot was used (3.5 mEq/L). Baseline SCr and K were considered the closest measurements to T0 within the window of 365 days before to 7 days after that date. If there was no available outpatient SCr or K, we used available inpatient SCr or K within the same window.
Other medications were captured as prescriptions that overlapped T0 and were modeled as time-fixed variables; the exception was angiotensin-converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB) use, which was modeled as a time-varying variable in the as-treated analysis. We defined comorbidities using ICD-9/10 diagnosis codes in the EHR (Supplemental Table 1). We also calculated patients’ Charlson comorbidity index (CCI) score.23,24
Statistical Analysis
We calculated frequencies, means, and proportions of the primary analytic cohorts’ characteristics at T0, including demographics, comorbidities, and prior medication use. We also stratified cohort characteristics by ever/never spironolactone initiators (in as-treated analyses), and cohort “trial” years (in ITT analyses), and we assessed for trends using logistic and linear regression for binary and continuous variables, respectively. We calculated incidence rates for hyperkalemia and AKI (per 1000 PMs) by as-treated exposure groups, both overall and stratified by time-varying eGFR category. We also calculated and plotted cumulative incidence of hyperkalemia and AKI over 3 years after T0 in the ITT analyses, incorporating the competing event of death, and stratifying by eGFR.
We used time-to-event Fine and Gray regression models to estimate subdistribution hazards ratios (sHR) accounting for the competing risk of death.25 Comparing exposure groups in as-treated analyses, we used unadjusted and fully adjusted models with time-fixed covariates, as well as adjusted models where eGFR, K, and ACEi/ARB use were included as time-varying covariates. In as-treated analyses, we tested for an interaction between time-varying eGFR (linear spline) and exposure group. In ITT analyses comparing treatment with control groups, we used unadjusted and fully adjusted models, as well as adjusted models weighted by the inverse probability of treatment weighting (IPTW), and 1:1, “nearest-neighbor” propensity score (PS) matched analyses using calipers of 0.014 (0.2 times the standard deviation of log-transformed PS); after matching, all standardized mean differences were less than 0.10. In the ITT analyses, we also tested for an interaction between T0 eGFR (linear spline) and treatment. We used robust variance estimators to account for within-person correlation. To assess for heterogeneity of effect among cohort trial years in the ITT analyses, we tested for an interaction between trial and treatment status, and found no statistically significant interaction.
We adjusted final models for age, sex, race (non-white), eGFR (linear splines), K (linear splines), time with heart failure, year of first loop order, CCI, history of hyperkalemia, history of AKI, cardiovascular disease (CVD), peripheral artery disease (PAD), diabetes mellitus, cancer, cirrhosis, ascites, atrial fibrillation, proteinuria, and prior use of anticoagulants, ACEi, ARB, other antihypertensives (combined: beta-blocker, calcium channel blockers, vasodilators), antiarrhythmics, cardiac glycosides, statins, thiazide diuretics, and potassium-sparing diuretics (excluding spironolactone). ITT analyses were additionally adjusted by time between the first loop order and T0. We used the same covariates to calculate the propensity scores, and we calculated stabilized weights for IPTW analyses.
All analyses were conducted using Stata 15.1 (StataCorp; College Station, TX) and SAS 9.4 (SAS Institute Inc; Cary, NC).
RESULTS
Study Population
There were 17,110 patients with heart failure who used loop diuretics during the study period; 50.5% were female (n=8635), 2.4% were non-White (n=414), and the mean ± SD age was 73.2 ± 13.0 years (Table 1). Diabetes (46.6%; n=7977) and CVD (66.3%; n=11,347) were common, as was the use of ACEi/ARB (80.2%; n=13,726), statins (66.2%; n=11,331), anticoagulants (59.8%; n=10,233), and other antihypertensives (89.5%; n=15,308). Roughly 19% (n=3229) went on to initiate spironolactone over a mean follow-up of 134 months; these patients were younger and had higher mean eGFRs at baseline compared with never initiators (P<.001 for both).
TABLE 1.
| Overall | Never initiators | Ever initiatorsc | P | |
|---|---|---|---|---|
| N (total) | 17,110 | 13,881 (81.1) | 3229 (18.9) | |
| Female | 8635 (50.5) | 7189 (51.8) | 1446 (44.8) | <.001 |
| Non-White | 414 (2.4) | 313 (2.3) | 101 (3.1) | <.001 |
| Mean age, y | 73.2±13.0 | 74.2±12.6 | 68.8±13.6 | <.001 |
| Mean CCI | 7.1±2.8 | 7.2±2.8 | 6.5±2.8 | <.001 |
| Baseline labs | ||||
| Mean K, mg/dL | 4.3±0.5 | 4.3±0.5 | 4.3±0.6 | <.001 |
| Mean SCr, mEq/L | 1.2±0.6 | 1.2±0.6 | 1.1±0.5 | <.001 |
| Kidney function, mL/min per 1.73 m2 | ||||
| Mean eGFR | 61.8±23.9 | 60.9±23.8 | 65.8±23.8 | <.001 |
| ≥90 | 2404 (14.1) | 1821 (13.1) | 583 (18.1) | <.001 |
| 60–89 | 6487 (37.9) | 518 (37.4) | 1302 (40.3) | <.001 |
| 30–59 | 6728 (39.3) | 5579 (40.2) | 1149 (35.6) | <.001 |
| <30 | 1491 (8.7) | 1296 (9.3) | 195 (6.0) | <.001 |
| Comorbidities | ||||
| Mean time with HF, mod | 177.3±464.5 | 184.3±476.6 | 147.0±406.9 | <.001 |
| CVDe | 11,347 (66.3) | 9256 (66.7) | 2091 (64.8) | .04 |
| Hyperkalemia | 1123 (6.6) | 937 (6.8) | 186 (5.8) | .04 |
| PAD | 3755 (21.9) | 3086 (22.2) | 669 (20.7) | .06 |
| Diabetes | 7977 (46.6) | 6433 (46.3) | 1544 (47.8) | .13 |
| Cancer | 3178 (18.6) | 2677 (19.3) | 501 (15.5) | <.001 |
| Cirrhosis | 302 (1.8) | 122 (0.9) | 180 (5.6) | <.001 |
| Ascites | 310 (1.8) | 151 (1.1) | 159 (4.9) | <.001 |
| AKI | 3284 (19.2) | 2743 (19.8) | 541 (16.8) | <.001 |
| Atrial fibrillation | 6745 (39.4) | 5481 (39.5) | 1264 (39.1) | .72 |
| Proteinuriaf | 1113 (6.5) | 915 (6.6) | 198 (6.1) | .34 |
| Medications | ||||
| ACEi or ARB | 13,726 (80.2) | 10,743 (77.4) | 2983 (92.4) | <.001 |
| Other antihypertensive | 15,308 (89.5) | 12,270 (88.4) | 3038 (94.1) | <.001 |
| Anticoagulants | 10,233 (59.8) | 8126 (58.5) | 2107 (65.3) | <.001 |
| Arrhythmic | 3776 (22.1) | 2821 (20.3) | 955 (29.6) | <.001 |
| Cardiac glycoside | 3223 (18.8) | 2223 (16.0) | 1000 (31.0) | <.001 |
| Statin | 11,331 (66.2) | 9100 (65.6) | 2231 (69.1) | <.001 |
| Thiazide diuretic | 5605 (32.8) | 4260 (30.7) | 1345 (41.7) | <.001 |
| Other K-sparing diuretic | 567 (3.3) | 413 (3.0) | 154 (4.8) | <.001 |
ACEi = angiotensin-converting enzyme inhibitor; AKI = acute kidney injury; ARB = angiotensin II receptor blockers; CCI = Charlson comorbidity index; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; HF = heart failure; K = potassium; PAD = peripheral artery disease; SCr = serum creatinine; T0 = baseline is the first loop diuretic order after initial heart failure diagnosis.
Values are n (%); mean values are shown ± SD.
Ever initiators include those who ever took spironolactone, even if it did not overlap with a loop diuretic.
Time with HF is the mean time between initial HF diagnosis and first loop prescription after the initial HF diagnosis.
CVD is a composite category including cerebrovascular disease/stroke, coronary artery disease, and myocardial infarction.
Proteinuria was defined using diagnosis codes.
Real-World Incidence of Hyperkalemia and AKI
There were 995 hyperkalemia events (7287 deaths) in 681,944 PMs of observation time. Overall, incidence rates (IRs) per 1000 PMs were highest for those on spironolactone without a loop diuretic (IR, 3.3), followed by concomitant loop and spironolactone (IR, 2.9), loop without spironolactone (IR, 1.4), and no use of either (IR, 1.3) (Table 2). In adjusted models with time-varying eGFR, K, and ACEi/ARB use, both concomitant loop and spironolactone, and spironolactone alone, were associated with a more than two-fold increase in the risk of hyperkalemia (sHR, 2.06; 95% CI, 1.70 to 2.49; P<.001 and sHR, 2.28; 95% CI, 1.40 to 3.69; P<.001, respectively) compared with loop use without spironolactone (Table 3).
TABLE 2.
Incidence of Hyperkalemia and Acute Kidney Injury Stratified by Exposure Groups and Kidney Function (As-Treated Analysis)a
| eGFR category (mL/min per 1.73 m2) | Hyperkalemia |
Acute kidney injury |
||
|---|---|---|---|---|
| Total PM | Incidence rate per 1000 PM | Total PM | Incidence rate per 1000 PM | |
| Loop aloneb | ||||
| ≥90 | 49,638 | 0.6 | 48,137 | 2.9 |
| 60–89 | 153,154 | 0.7 | 146,177 | 4.0 |
| 30–59 | 189,436 | 1.3 | 170,912 | 8.7 |
| <30 | 41,979 | 4.8 | 34,601 | 21.4 |
| Overall | 434,207 | 1.4 | 399,826 | 7.4 |
| Nonec | ||||
| ≥90 | 26,176 | 0.5 | 24,774 | 2.0 |
| 60–89 | 73,115 | 0.6 | 68,324 | 2.7 |
| 30–59 | 72,634 | 1.2 | 62,737 | 6.2 |
| <30 | 20,214 | 4.7 | 14,384 | 10.9 |
| Overall | 192,140 | 1.3 | 170,220 | 4.6 |
| Spironolactone aloned | ||||
| ≥90 | 1161 | 1.7 | 1124 | 3.6 |
| 60–89 | 2701 | 1.9 | 2457 | 2.9 |
| 30–59 | 2743 | 5.1 | 2371 | 7.2 |
| <30 | 376 | 5.3 | 243 | 20.6 |
| Overall | 6980 | 3.3 | 6196 | 5.3 |
| Loop and spironolactonee | ||||
| ≥90 | 7666 | 1.6 | 7335 | 4.6 |
| 60–89 | 16,420 | 1.6 | 15,289 | 7.7 |
| 30–59 | 21,334 | 3.3 | 18,695 | 11.3 |
| <30 | 3196 | 10.3 | 2531 | 32.0 |
| Overall | 48,617 | 2.9 | 43,853 | 10.1 |
eGFR = estimated glomerular filtration rate; PM = person-month.
“Loop alone” represents use of a loop diuretic without spironolactone.
“None” is no use of either spironolactone or a loop diuretic.
“Spironolactone alone” represents spironolactone use without a loop diuretic.
“Loop and spironolactone” represents concomitant use of a loop diuretic and spironolactone.
TABLE 3.
Risk of Hyperkalemia and Acute Kidney Injury Comparing Diuretic Use Groups (As-Treated Analysis)a
| Hyperkalemia |
Acute Kidney Injury |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted (95% CI) |
P | Fully adjusted (time-fixed) (95% CI) |
P | Fully adjusted (time-fixed) (95% CI) |
P | Unadjusted (95% CI) | P | Fully adjusted (time-fixed) (95% CI) |
P | Fully adjusted (time-fixed) (95% CI) |
P | |
| Loop aloneb | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| Nonec | 0.59 (0.50–0.70) | <.001 | 0.59 (0.49–0.70) | <.001 | 0.61 (0.51–0.73) | <.001 | 0.44 (0.40–0.48) | <.001 | 0.43 (0.39–0.47) | <.001 | 0.45 (0.41–0.49) | <.001 |
| Spironolactone aloned | 2.55 (1.66–3.92) | <.001 | 2.83 (1.82–4.39) | <.001 | 2.28 (1.40–3.69) | <.001 | 0.79 (0.55–1.13) | .20 | 0.81 (0.57–1.17) | .26 | 0.77 (0.52–1.13) | .18 |
| Loop and spironolactonee | 2.30 (1.91–2.77) | <.001 | 2.36 (1.94–2.86) | <.001 | 2.06 (1.70–2.49) | <.001 | 1.46 (1.32–1.62) | <.001 | 1.45 (1.30–1.62) | <.001 | 1.37 (1.23–1.53) | <.001 |
Data shown are subdistribution hazard ratios with loop as the reference. Models were adjusted by age, sex, race, estimated glomerular filtration rate (eGFR), potassium (K), time with heart failure, year of first loop order, Charlson comorbidity index, history of hyperkalemia, acute kidney injury, cardiovascular disease, peripheral artery disease, diabetes mellitus, cancer, cirrhosis, ascites, atrial fibrillation, proteinuria, and prior use of anticoagulants, angiotensin-converting enzyme inhibitor (ACEi), angiotensin II receptor blockers (ARB), other antihypertensives, antiarrhythmics, cardiac glycosides, statins, thiazide diuretics, and K-sparing diuretics. Time-varying variables in fully adjusted models were eGFR, K, and combined ACEi or ARB use.
“Loop alone” represents use of a loop diuretic without spironolactone.
“None” is no use of either spironolactone or a loop diuretic.
“Spironolactone alone” represents spironolactone use without a loop diuretic.
“Loop and spironolactone” represents concomitant use of a loop diuretic and spironolactone.
There were 4212 AKI events (5387 deaths) in 620,094 PMs of observation time. In contrast with trends in hyperkalemia, IRs per 1000 PMs for AKI were highest for those on concomitant loop and spironolactone (IR, 10.1), followed by loop without spironolactone (IR, 7.4), spironolactone without a loop (IR, 5.3), and no use of either (IR, 4.6). In adjusted models with time-varying covariates, concomitant loop and spironolactone was associated with a 37% increase in the risk of AKI (sHR, 1.37; 95% CI, 1.23 to 1.53; P<.001) compared with loop use without spironolactone, but there was no statistically significant difference in risk with spironolactone alone without the use of a loop diuretic (sHR, 0.77; 95% CI, 0.52 to 1.13; P=.18).
The absolute risks of hyperkalemia and AKI were higher in lower eGFR categories, but there were no consistent differences in the relative risks associated with spironolactone/loop diuretic therapy versus loop therapy alone by eGFR level in the as-treated analysis (hyperkalemia: P for interaction P=.63, and P=.61 for eGFR levels below and above 60 mL/min per 1.73 m2, respectively; AKI: P for interaction P=.33, and P=.92 for eGFR levels below and above 60 mL/min per 1.73 m2, respectively).
Target Trial Emulation Assessing Add-on Spironolactone Therapy
In the pooled target trial emulation, there were 24,127 patients (treatment group [concomitant loop and spironolactone] = 2,000; control group [loop without spironolactone] = 22,127). More than half (51.8%, n=12,499) were female, and mean ± SD age was 73.8 ± 12.7 y (Supplemental Table 2, available online at http://www.mayoclinicproceedings.org).
There were 1197 hyperkalemia events (163 in the treatment group) (Table 4), with a 1-year cumulative incidence of 1.7% and 4.0% in the control and treatment groups, respectively. Cumulative incidence of hyperkalemia at 1 year was lowest for patients with T0 eGFR greater than or equal to 60 mL/min per 1.73m2 in the control group (1.2%), followed by those with eGFR greater than or equal to 60 mL/min per 1.73m2 in the treatment group and those with eGFR less than 60 mL/min per 1.73m2 in the control group (both 2.1%); those with eGFR less than 60 mL/min per 1.73 m2 in the treatment group had the highest cumulative incidence (6.3%) (Figure 1). In the fully adjusted model (Figure 2), treatment was associated with an increase in the risk of hyperkalemia compared with control (sHR, 1.75; 95% CI, 1.46 to 2.09; P<.001), and this was similar when using IPTW (sHR, 1.58; 95% CI, 1.28 to 1.94; P<.001) and 1:1 PS-matching (n=1976 in each group; sHR, 1.69; 95% CI, 1.35 to 2.10; P<.001).
TABLE 4.
Hyperkalemia and Acute Kidney Injury Events for Treatment and Control Groups by Cohort “Trial” Year (Intention-to-Treat Analysis)a
| Cohort “trial” year | Loop and spironolactone (treated) |
Loop alone (control) |
Hyperkalemia |
Acute kidney injury |
||
|---|---|---|---|---|---|---|
| Total events | Events in treated | Total events | Events in treated | |||
| 2004–2006 | 166 | 1938 | 125 | 18 | 477 | 45 |
| 2007–2009 | 376 | 4500 | 301 | 39 | 1274 | 110 |
| 2010–2012 | 554 | 6300 | 371 | 40 | 1799 | 163 |
| 2013–2016 | 904 | 9389 | 400 | 66 | 2032 | 242 |
| Pooled | 2000 | 22,127 | 1197 | 163 | 5582 | 560 |
Treatment group refers to patients using loop and spironolactone concomitantly (loop and spironolactone). Control group refers to patients using loop without spironolactone. Control group patients can be included in subsequent cohort “trial” years as a treatment or control, but treatment group patients are not eligible for subsequent cohort “trial” years. Patients were excluded if baseline potassium level was greater than 5.0 mEq/L.
FIGURE 1.
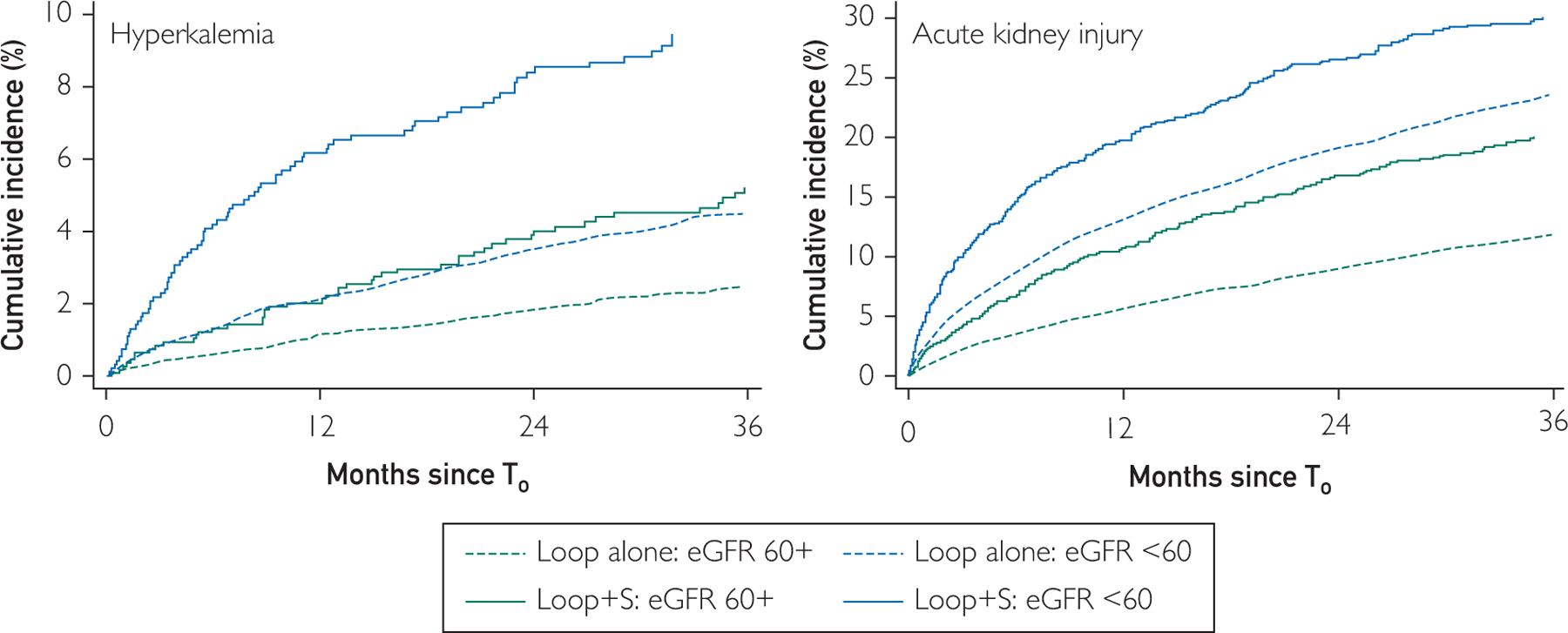
Estimated glomerular filtration rate (eGFR). Cumulative incidence estimates account for the competing risk of death. Loop alone refers to the control group. Loop and spironolactone (Loop + S) refers to the treatment group. eGFR units are mL/min per 1.73 m2. T0 for treatment group = initiation of spironolactone; T0 for treatment group = random loop order date in relevant cohort “trial” years. For hyperkalemia, 1-year cumulative incidence was 1.7% and 4.0% in control and treatment group, respectively. For AKI, 1-year cumulative incidence was 9.5% and 14.9% in control and treatment group, respectively.
FIGURE 2.
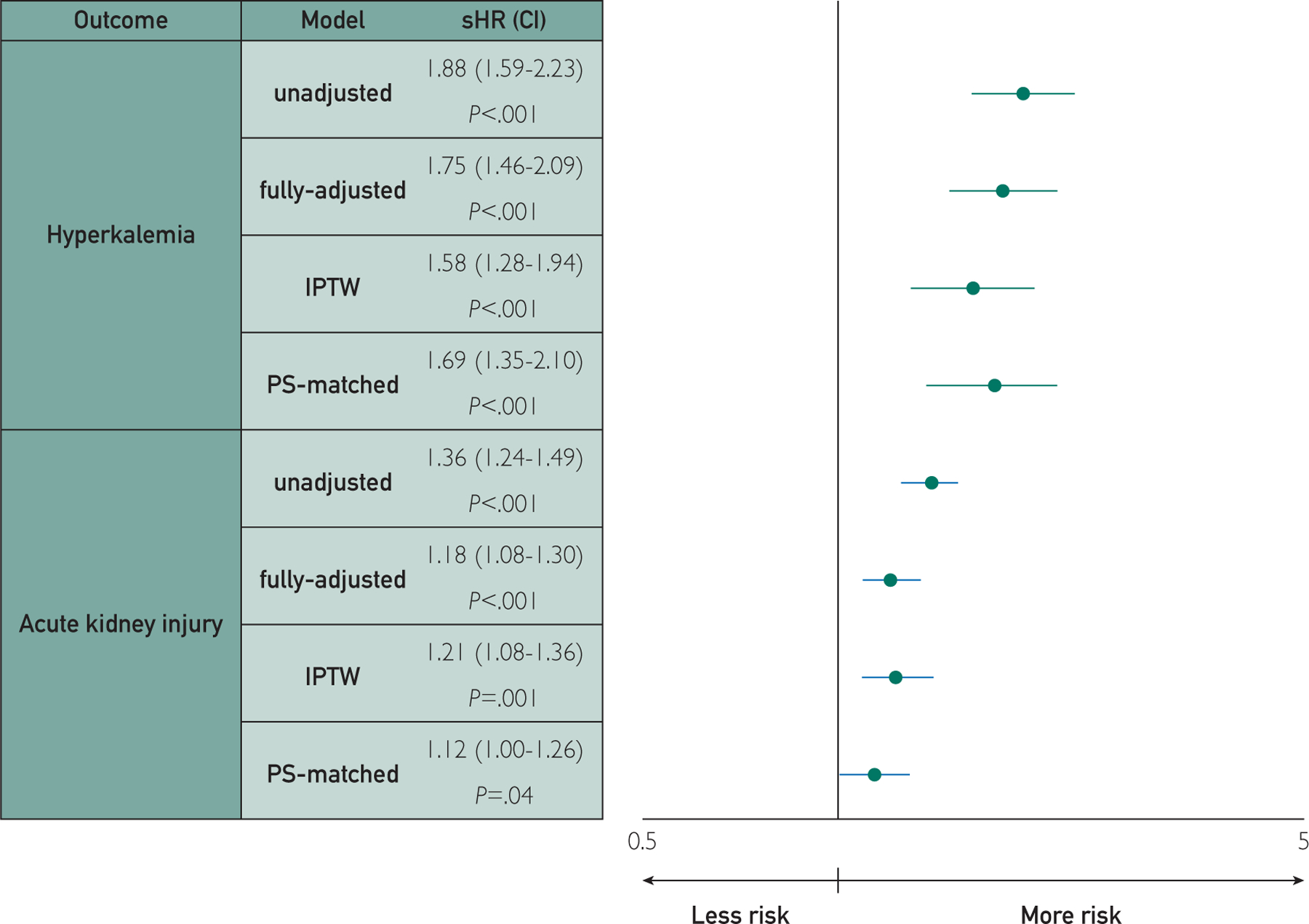
Inverse probability of treatment weights (IPTW); propensity score (PS); subdistributional hazard ratio (sHR); figure plotted on log scale; models were adjusted by age, sex, race, estimated glomerular filtration rate, potassium, time with heart failure relative to first loop prescription order, year of first loop order, time since first loop order, Charlson comorbidity index, history of hyperkalemia, acute kidney injury, cardiovascular disease, peripheral artery disease, diabetes mellitus, cancer, cirrhosis, ascites, atrial fibrillation, proteinuria, and prior use of anticoagulants, angiotensin-converting enzyme inhibitor, angiotensin II receptor blockers, other antihypertensives, antiarrhythmics, cardiac glycosides, statins, thiazide diuretics, and potassium-sparing diuretics.
There were 5582 AKI events (560 in the treatment group), with a 1-year cumulative incidence of 9.5% and 14.9% in the control and treatment groups, respectively. Similar to hyperkalemia, cumulative incidence of AKI at 1 year was lowest for patients with T0 eGFR greater than or equal to 60 mL/min per 1.73 m2 in the control group (5.8%), followed by those with eGFR greater than or equal to 60 mL/min per 1.73 m2 in the treatment group (10.8%), and those with eGFR less than 60 mL/min per 1.73 m2 in the control group (13.1%); those with eGFR less than 60 mL/min per 1.73 m2 in the treatment group had the highest cumulative incidence (20.2%). In the fully adjusted model, treatment was associated with an increased risk of AKI compared with control (sHR, 1.18; 95% CI, 1.08 to 1.30; P<.001) and this was similar when using 1:1 PS-matching (n=1976 in each group; sHR, 1.12; 95% CI, 1.00 to 1.26; P=.04), and IPTW (sHR, 1.21; 95% CI, 1.08 to 1.36; P=.001).
Similar to the as-treated analysis, there were no differences in the relative risks of hyperkalemia or AKI associated with spironolactone/loop diuretic therapy versus loop therapy alone by eGFR in the ITT analysis (hyperkalemia: P for interaction P=.31 and P=.43 for eGFR levels below and above 60 mL/min per 1.73 m2, respectively; AKI: P for interaction P=.93 and P=.46 for eGFR levels below and above 60 mL/min per 1.73 m2, respectively).
DISCUSSION
In this large, real-world cohort of patients with heart failure prescribed loop diuretics, approximately one in five were prescribed spironolactone. The cohort was at high risk for hyperkalemia and AKI, with the highest rates among patients with eGFR less than 60 mL/min per 1.73 m2, and those prescribed spironolactone. In a propensity-matched, target trial emulation, we observed a 69% increased risk of hyperkalemia with the addition of spironolactone compared with using loop diuretics alone, and a 12% increased risk of AKI. The relative risks of hyperkalemia and AKI were not modified by renal function in either analysis. These findings are important because relatively little is known about the real-world safety of spironolactone, particularly among a broader heart failure population (HFrEF and HFpEF) across the spectrum of kidney function.
A more complete understanding of spironolactone-associated hyperkalemia and AKI is critical for utilization to expand to higher risk patients excluded from the clinical trials. In RALES, the pivotal study that established spironolactone’s effectiveness in HFrEF, the cumulative incidence of hyperkalemia was very modest (~2% in the spironolactone group vs ~1% in placebo among randomized patients over the study period),2 likely due to the selection of a lower-risk study population by excluding patients based on their SCr and K, and regular monitoring thereafter. Lee et al (2013)26 found similar crude rates of both hyperkalemia and AKI when restricting their real-world systolic heart failure study cohort to align with the RALES inclusion. Similar inclusion criteria and monitoring were applied in the TOPCAT trial among patients with HFpEF, but cumulative incidence of hyperkalemia was higher (~19% in the spironolactone group vs ~9% in placebo among randomized patients over the study period) than what was reported in RALES, and drug discontinuation due to “abnormal renal function” was higher for spironolactone patients versus placebo patients (3.9% vs 2.3%, respectively; P=.006).4 Our study results were more consistent with the TOPCAT trial and other studies that included various heart failure sub-populations such as older women and after an acute myocardial infarction showing more pronounced risks with spironolactone use as compared with RALES, except that AKI was more common in our cohort overall.10,27,28
As expected, the absolute risk of both hyperkalemia and AKI with spironolactone use increased with lower eGFR; however, we observed no difference in relative risk for hyperkalemia or AKI associated with spironolactone by level of kidney function (interactions not significant in as-treated or ITT). While differences in risk by kidney function could not be explored in RALES and TOPCAT because those with higher SCr were excluded, other studies have observed relatively infrequent and mild spironolactone-associated increases in SCr and K among those with reduced kidney function.9,29–34 Similar to ACEi and ARB medications, studies have shown an early reduction in eGFR with spironolactone in the setting of CKD and diabetic nephropathy, but this appears to be transient, with little evidence of a persistent reduction in the context of longer-term use.9,10,26,35–37 Also, among those with heart failure potentially indicated for spironolactone, there are many other common risk factors which can all contribute to hyperkalemia risk independent of one’s kidney function such as older age, diabetes mellitus, volume-depleting illness, and use of ACEi, ARB, and other medications, making spironolactone’s safety challenging to study, particularly in observational study settings.5–7,38,39 Of note, several ongoing clinical trials are evaluating spironolactone’s safety and efficacy in patients with CKD, including those with end-stage renal disease.
More data are needed to better understand whether spironolactone’s risks outweigh prospective benefits in patients who are currently exposed. Spironolactone is used among patients with various comorbidities, at many levels of SCr and K, and with exposure to various concomitant medications that can all make hyperkalemia or AKI more likely. In general, aldosterone antagonists are not recommended in heart failure patients with eGFR less than 30 mL/min per 1.73 m2 by the American Heart Association,14,15 yet these patients have limited therapeutic options and may derive benefit from its pleiotropic pharmacologic properties. Outside of its cardiovascular benefit,31–34,40–42 some data suggest spironolactone may also slow the progression of CKD.29,36,37,39,41,43–47 We observed a marked increase in the incidence of primary safety outcomes in patients with spironolactone use at lower eGFRs, but routine monitoring of SCr and K is warranted at all levels of kidney function. With a better understanding of spironolactone’s safety profile in heart failure, ideal candidates for spironolactone can be chosen with the risks for hyperkalemia and AKI balanced accordingly. Additionally, while K binding therapies are available for the treatment of chronic hyperkalemia, there is still a need for novel therapeutics for the treatment of AKI given its prevalence and complex pathophysiology.
Strengths and Limitations
Our study has several notable strengths. We used a large, integrated EHR system with access to all primary care patient records across the health system, including laboratory, inpatient, and outpatient data. The Geisinger primary care system generally has little attrition, with many years of detailed records. Mortality data were also available. Our study also had some limitations. Patients were relatively homogeneous with respect to race and ethnicity. We used ICD-9/10 diagnosis codes for comorbidities and outcomes; particularly for hyperkalemia and AKI, these codes are likely to select for the most severe cases.48,49 Because most heart failure diagnosis codes are for unspecified heart failure, we could not differentiate between reduced or preserved ejection fraction heart failure.
CONCLUSION
Spironolactone use was relatively uncommon among patients on loop diuretics with heart failure. Concomitant use of spironolactone and a loop diuretic was associated with an increased risk of hyperkalemia and, to a lesser extent, AKI compared with loop diuretic use without spironolactone, with no evidence of risk modification across the spectrum of eGFR. Spironolactone has known benefits and risks in narrower patient populations, and this study offers insight into its safety profile under real-world conditions.
Supplementary Material
ACKNOWLEDGMENTS
Some data reported here were supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US government. The funding sources had no role in the design and conduct of the study, analysis or interpretation of the data, and preparation or final approval of the manuscript prior to publication.
Grant Support: Dr Secora was supported by grant T32 HL007024 from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH). Dr Gram is supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01 DK100446 and R01 DK115534). Dr Alexander is supported by 1 U01 FD004977–02 from the U.S. Food and Drug Administration (FDA). Dr Chang is supported by a grant from the NIDDK (K23 DK106515). Dr Shin is supported by a grant from the NIDDK (K01 DK121825). Dr. Inker is supported by grants from NIDDK (R01DK115534 and U01DK085689), and funding from Retrophin, Omeros, Dialysis Clinics, Inc., and Reata Pharmaceuticals for research and contracts to Tufts Medical Center.
Abbreviations and Acronyms:
- ACEi
angiotensin-converting enzyme inhibitor
- ARB
angiotensin II receptor blockers
- AKI
acute kidney injury
- CVD
cardiovascular disease
- CCI
Charlson comorbidity index
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- EHR
electronic health record
- HFpEF
heart failure with preserved ejection
- HFrEF
heart failure with reduced ejection
- ITT
intention-to-treat
- K
potassium
- PAD
peripheral artery disease
- PS
propensity score
- RALES
Randomized Aldactone Evaluation Study
- SCr
serum creatinine
- sHR
subdistribution hazard ratios
- TOPCAT
Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist
Footnotes
Potential Competing Interests: Dr Shin is a principal investigator of a grant provided to the Johns Hopkins Bloomberg School of Public Health from Merck. Dr Alexander is past Chair of the US Food and Drug Administration’s Peripheral and Central Nervous System Advisory Committee; has served as a paid advisor to IQVIA; serves as a consultant and holds equity in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and is a member of OptumRx’s National P&T Committee. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies. Dr. Inker has consulting agreements with Tricida and Omeros Corp. The remaining authors report no potential competing interests.
SUPPLEMENTAL ONLINE MATERIAL
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Contributor Information
Alex M. Secora, Johns Hopkins University Bloomberg School of Public Health, Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins University, Baltimore, MD.
Jung-Im Shin, Johns Hopkins University Bloomberg School of Public Health, Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins University, Baltimore, MD.
Yao Qiao, Johns Hopkins University Bloomberg School of Public Health, Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins University, Baltimore, MD.
G. Caleb Alexander, Johns Hopkins University Bloomberg School of Public Health, Division of General Internal Medicine, Department of Medicine, Johns Hopkins Medicine, Baltimore, MD.
Alex R. Chang, Division of Nephrology, Geisinger Health System, Danville, PA.
Leslie A. Inker, Tufts Medical Center, Department of Internal Medicine, Division of Nephrology, Boston, MA.
Josef Coresh, Johns Hopkins University Bloomberg School of Public Health, Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins University, Baltimore, MD.
Morgan E. Grams, Johns Hopkins University Bloomberg School of Public Health, Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins University, Baltimore, MD, Division of Nephrology, Department of Medicine, Johns Hopkins Medicine, Baltimore, MD.
REFERENCES
- 1.Aldactone label (Spironolactone) https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/012151s075lbl.pdf. Accessed August 22, 2019.
- 2.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. randomized aldactone evaluation study investigators. N Engl J Med 1999;341(10):709–717. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Zhang X, Dong M, et al. Effects of spironolactone in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2018;97(35): e11942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370(15):1383–1392. [DOI] [PubMed] [Google Scholar]
- 5.Sica DA. Hyperkalemia risk in chronic kidney disease: Deterrent to the use of aldosterone receptor antagonism or not. Hypertension 2009;53(5):749–750. [DOI] [PubMed] [Google Scholar]
- 6.Sica DA. Diuretic use in renal disease. Nat Rev Nephrol 2011; 8(2):100–109. [DOI] [PubMed] [Google Scholar]
- 7.Dolovich L, Gavura S, Pottie K. Hyperkalemia associated with spironolactone therapy. Can Fam Physician 2005;51(3):357–360. [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz CS, Cruz LS, Silva GR, Marcilio de Souza CA. Incidence and predictors of development of acute renal failure related to treatment of congestive heart failure with ACE inhibitors. Nephron Clin Pract 2007;105(2):c77–c83. [DOI] [PubMed] [Google Scholar]
- 9.Edwards NC, Steeds RP, Chue CD, Stewart PM, Ferro CJ, Townend JN. The safety and tolerability of spironolactone in patients with mild to moderate chronic kidney disease. Br J Clin Pharmacol 2012;73(3):447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamirisa KP, Aaronson KD, Koelling TM. Spironolactone-induced renal insufficiency and hyperkalemia in patients with heart failure. Am Heart J 2004;148(6):971–978. [DOI] [PubMed] [Google Scholar]
- 11.Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int 2004;66(1):1–9. [DOI] [PubMed] [Google Scholar]
- 12.Wei L, Struthers AD, Fahey T, Watson AD, Macdonald TM. Spironolactone use and renal toxicity: population based longitudinal analysis. BMJ 2010;340:c1768. [DOI] [PubMed] [Google Scholar]
- 13.Svensson M, Gustafsson F, Galatius S, Hildebrandt PR, Atar D. How prevalent is hyperkalemia and renal dysfunction during treatment with spironolactone in patients with congestive heart failure? J Card Fail 2004;10(4):297–303. [DOI] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. J Card Fail 2017;23(8):628–651. [DOI] [PubMed] [Google Scholar]
- 15.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2013;62(16):e147–e239. [DOI] [PubMed] [Google Scholar]
- 16.Dev S, Lacy ME, Masoudi FA, Wu WC. Temporal trends and hospital variation in mineralocorticoid receptor antagonist use in veterans discharged with heart failure. J Am Heart Assoc 2015;4(12):e002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NCT03020303; NCT03682497; NCT01848639; NCT02901184. ClinicalTrials.gov Accessed April 9, 2019.
- 18.Robins J. The analysis of randomized and nonrandomized AIDS treatment trials using a new approach to causal inference in longitudinal studies. In: Freeman Sechrest H, Mulley A, eds. Health Services Research Methodology: A Focus on AIDS Washington, DC: US Public Health Service, National Center for Health Services Research; 1989:113–159. [Google Scholar]
- 19.Hernán MA, Robins JM, García Rodríguez LA. Discussion on “statistical issues arising in the women’s health initiative. Biometrics 2005;61(4):922–930. [DOI] [PubMed] [Google Scholar]
- 20.Danaei G, Rodriguez LA, Cantero OF, Logan R, Hernan MA. Observational data for comparative effectiveness research: An emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat Methods Med Res 2013; 22(1):70–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernan MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: An application to post-menopausal hormone therapy and coronary heart disease. Epidemiology 2008;19(6):766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.International Society of Nephrology. KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013;3(1):1–150. [Google Scholar]
- 23.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 24.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 25.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94(446):496–509. [Google Scholar]
- 26.Lee KK, Shilane D, Hlatky MA, Yang J, Steimle AE, Go AS. Effectiveness and safety of spironolactone for systolic heart failure. Am J Cardiol 2013;112(9):1427–1432. [DOI] [PubMed] [Google Scholar]
- 27.Kurrelmeyer KM, Ashton Y, Xu J, Nagueh SF, Torre-Amione G, Deswal A. Effects of spironolactone treatment in elderly women with heart failure and preserved left ventricular ejection fraction. J Card Fail 2014;20(8):560–568. [DOI] [PubMed] [Google Scholar]
- 28.Wang TY, Vora AN, Peng SA, et al. Effectiveness and safety of aldosterone antagonist therapy use among older patients with reduced ejection fraction after acute myocardial infarction. J Am Heart Assoc 2016;5(1):e002612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int 2006;70(12):2116–2123. [DOI] [PubMed] [Google Scholar]
- 30.Flevari P, Kalogeropoulou S, Drakou A, et al. Spironolactone improves endothelial and cardiac autonomic function in non heart failure hemodialysis patients. J Hypertens 2013;31(6): 1239–1244. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto Y, Mori Y, Kageyama S, et al. Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol 2014;63(6): 528–536. [DOI] [PubMed] [Google Scholar]
- 32.Lin C, Zhang Q, Zhang H, Lin A. Long-term effects of low-dose spironolactone on chronic dialysis patients: A randomized placebo-controlled study. J Clin Hypertens (Greenwich) 2016; 18(2):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu R, Zhang Y, Zhu X, et al. Effects of mineralocorticoid receptor antagonists on left ventricular mass in chronic kidney disease patients: a systematic review and meta-analysis. Int Urol Nephrol 2016;48(9):1499–1509. [DOI] [PubMed] [Google Scholar]
- 34.Sun L, Xu B, Chen S, Liu S, He B, Jiang G. Effects of spironolactone on cardiovascularoutcomes in chronic kidney disease and end-stage renal disease patients. Int J of Clin and Exp Med 2016; 9(2):794–803. [Google Scholar]
- 35.Agrawal S, Agrawal N, Garg J, Mohandas R, Gupta T, Segal M. Heart failure and chronic kidney disease: should we use spironolactone? Am J Med Sci 2015;350(2):147–151. [DOI] [PubMed] [Google Scholar]
- 36.Kato S, Maruyama S, Makino H, et al. Anti-albuminuric effects of spironolactone in patients with type 2 diabetic nephropathy: A multicenter, randomized clinical trial. Clin Exp Nephrol 2015; 19(6):1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schjoedt KJ, Rossing K, Juhl TR, et al. Beneficial impact of spironolactone in diabetic nephropathy. Kidney Int 2005;68(6):2829–2836. [DOI] [PubMed] [Google Scholar]
- 38.British Hypertension Society. Loop and potassium-sparing diuretics: their use in hypertension. Prescriber 2011;22(7):13–15. [Google Scholar]
- 39.Ponda MP, Hostetter TH. Aldosterone antagonism in chronic kidney disease. CJASN 2006;1(4):668–677. [DOI] [PubMed] [Google Scholar]
- 40.Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: A randomized controlled trial. J Am Coll Cardiol 2009;54(6):505–512. [DOI] [PubMed] [Google Scholar]
- 41.Oxlund CS, Cangemi C, Henriksen JE, et al. Low-dose spironolactone reduces plasma fibulin-1 levels in patients with type 2 diabetes and resistant hypertension. J Hum Hypertens 2015; 29(1):28–32. [DOI] [PubMed] [Google Scholar]
- 42.Abolghasmi R, Taziki O. Efficacy of low dose spironolactone in chronic kidney disease with resistant hypertension. Saudi J Kidney Dis Transpl 2011;22(1):75–78. [PubMed] [Google Scholar]
- 43.Kim HY, Bae EH, Ma SK, Kim SW. Effects of spironolactone in combination with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in patients with proteinuria. Kidney Blood Press Res 2014;39(6):573–580. [DOI] [PubMed] [Google Scholar]
- 44.Luther J. Is there a new dawn for selective mineralocorticoid receptor antagonism? Current Opinion in Nephrol and Hypertension 2014;23(5):456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Momeni A, Behradmanesh MS, Kheiri S, Karami Horestani M. Evaluation of spironolactone plus hydrochlorothiazide in reducing proteinuria in type 2 diabetic nephropathy. J Renin Angiotensin Aldosterone Syst 2015;16(1):113–118. [DOI] [PubMed] [Google Scholar]
- 46.Ferreira JP, Santos M, Almeida S, Marques I, Bettencourt P, Carvalho H. The role of albuminuria as a non-invasive marker for congestive acutely decompensated chronic heart failure and the spironolactone effect in elderly Portuguese: a non-randomized trial. Nephrology (Carlton) 2014;19(3):149–156. [DOI] [PubMed] [Google Scholar]
- 47.Bertocchio JP, Warnock DG, Jaisser F. Mineralocorticoid receptor activation and blockade: An emerging paradigm in chronic kidney disease. Kidney Int 2011;79(10):1051–1060. [DOI] [PubMed] [Google Scholar]
- 48.Fleet JL, Shariff SZ, Gandhi S, Weir MA, Jain AK, Garg AX. Validity of the international classification of diseases 10th revision code for hyperkalaemia in elderly patients at presentation to an emergency department and at hospital admission. BMJ Open 2012;2(6):e002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 2014;9(4):682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


