Abstract
In Coronavirus disease 2019 (COVID-19), a decreased number of regulatory T (Treg) cells and their mediated factors lead to a hyperinflammatory state due to overactivation of the inflammatory cells and factors during the infection. In the current study, we evaluated the Nanocurcumin effects on the Treg cell population and corresponding factors in mild and severe COVID-19 patients. To investigate the Nanocurcumin effects, 80 COVID-19 patients (40 at the severe stage and 40 at the mild stage) were selected and classified into Nanocurcumin and placebo arms. In both the Nanocurcumin and placebo groups, the Treg cell frequency, the gene expression of Treg transcription factor forkhead box P3 (FoxP3), and cytokines (IL-10, IL-35, and TGF-β), as well as the serum levels of cytokines were measured before and after treatment. In both mild and severe COVID-19 patients, Nanocurcumin could considerably upregulate the frequency of Treg cells, the expression levels of FoxP3, IL-10, IL-35, and TGF-β, as well as the serum secretion levels of cytokines in the Nanocurcumin-treated group compared to the placebo group. The abovementioned factors were remarkably increased in the post-treatment with Nanocurcumin before pre-treatment conditions. By contrast, it has been observed no notable alteration in the placebo group. Our findings revealed the SinaCurcumin® effective function in a significant increase in the number of Treg cells and their mediated factors in the Nanocurcumin group than in the placebo group in both mild and severe patients. Hence, it would be an efficient therapeutic agent in rehabilitating COVID-19 infected patients.
Keywords: COVID-19, SARS-CoV2, Regulatory T cell, Nanocurcumin, Cytokine, Transcription factor
1. Introduction
Infection by SARS-CoV2 has been attributed to a broad spectrum of clinical manifestations ranging from asymptomatic course to severe conditions, including acute respiratory distress syndrome (ARDS), typical pneumonia, and multi-organ failure. Common clinical features of COVID-19 are dry cough, fever, sore throat, fatigue, and dyspnea [1,2]. Increasing evidence indicates the important role of immune system patterns in disease pathogenesis. In the course of infection, SARS-CoV2 impairs the normal responses of the immune system [3], causing lymphopenia, granulocyte and monocyte abnormalities, increased antibodies, overactivation of T helper (Th) 1 and Th17 cells, dysregulation of regulatory T (Treg) cells, and overproduction of proinflammatory cytokines, especially in severe and critical cases. Disrupted control of the immune system increases the level of inflammatory cytokines, including interleukin (IL)-1β, IL-2, IL-6, IL-7, IL-8, IL-10, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interferon gamma-induced protein 10 (IP-10), and monocyte chemoattractant protein 1 (MCP1) leading to cytokine storm, a common occurrence side effect in diseases treated by chimeric antigen receptor T (CAR-T) cells [4,5]. Cytokine storm is a causative phenomenon of hyper inflammation and respiratory system injury in SARS-CoV2 infection [6,7].
CD4+ CD25+ Foxp3+ Treg cell, a subtype of Th cells, accounts for modulation of the inflammation by producing the anti-inflammatory factors and suppressing both innate and adaptive immune responses. Treg cells have a vital role in maintaining immune tolerance and balance between inflammatory and regulatory responses. The dysfunction of Treg cells, decreased levels of their anti-inflammatory cytokines [IL-10, IL-35, and transforming growth factor (TGF)-β], and imbalance ratio of Treg/Th17 cells are other causative mechanisms of the immune system dysregulation and hyper inflammation in COVID-19 [8,9]. Concerning this, applying the therapeutic regimens to decrease the inflammatory responses, and increase the immune system regulatory responses mediated by Treg cells and their relevant factors might be applicable in mitigating the inflammation in COVID-19 patients. Recently, effective strides forward have been taken for treating the COVID-19 patients from basic research to successful clinical trials [10,11]. Despite the great level of effort, no completely effective treatments have been approved for SARS-CoV2 and several investigations have been planned or underway to obtain this aim.
Curcumin is a natural lipophilic bioactive substance extracted from the rhizome of the Curcuma longa L. (turmeric) [12]. Curcumin is characterized by various biological and pharmacological capabilities, mostly anti-inflammatory, anti-microbial, and antioxidant activities. In the last decade, the protective and therapeutic effects of curcumin have been documented in different diseases such as cancers, autoimmune and inflammatory diseases, metabolic diseases, and infections [[13], [14], [15]]. Currently, Nanocurcumin product, a nano range formulation of curcumin, has been developed to overcome the limitations of free curcumin usage with augmented biological and pharmacological functions, increased delivery and stability, as well as several advantages over curcumin [16,17].
In the current research study, we aimed to investigate the anti-inflammatory and therapeutic effects of SinaCurcumin®, a novel, and potent Nanocurcumin product on the frequency of Treg cells and their mediated factors (FoxP3, IL-10, IL-35, and TGF-β) in the Nanocurcumin-treated group compared to the placebo group of mild and severe stages of COVID-19 patients.
2. Material and methods
2.1. Study design and study population
The current study is a randomized, double-blind-placebo controlled trial study. We enrolled 40 severe COVID-19 patients admitted to the intensive care unit (ICU) and 40 mild cases of COVID-19 who were hospitalized in Imam Reza Hospital of Tabriz University of Medical Sciences. All patients were included in the study according to the randomization with a computer-generated list, based on a positive confirmed RT-PCR assay. COVID-19 patients in the severe group had extensive alveolar damage and progressive respiratory failure that their decreased oxygen levels were more severe and they need a ventilator to help them breathe due to more severe shortness of breath and pneumonia. Organ defects and weakness were more severe in more severe patients than in mild patients, as well. Also, the severe patient group was characterized by profound lymphopenia. In the severe patient group, 10% (2 out of 20) of the Nanocurcumin group and 5% (1 out of 20) of the placebo group were on ventilators that there was no significant difference between the two groups of patients. The median length of hospitalization for severe patients was 10 (7–13) days. Patients with a mild clinical presentation (absence of viral pneumonia and hypoxia) may not initially require hospitalization, and most patients will be able to manage their illness at home; however, some of them need hospitalization within 5 (4–9) days. 40 matched healthy individuals were also included in the study. To participate in the study, written informed consent was obtained from both healthy and patient groups. The ethical approval was taken from the Research Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1398.1314). The inclusion criteria of the patient group were confirmed etiological characteristics and clinical manifestations, approved positive RT-PCR test, and willingness of patients. The patients were excluded from the study if they had a history of allergic disorders, cancers (leukemia and lymphoma), chronic infections, and other underlying diseases. Accordingly, all patients and healthy controls enrolled in the study were examined in terms of chronic lung disease, renal failure, liver dysfunction, metabolic disorders, autoimmune diseases, immunodeficiency, cardiovascular diseases, gastrointestinal diseases, neurological diseases, cancers, and diabetes. None of the study subjects had the mentioned disorders and had no other underlying diseases. The demographic data and clinical manifestations of the patients have been presented in Table 1 .
Table 1.
The demographic information of mild and severe COVID-19 patients and healthy controls.
| Mild COVID-19 patients (n = 40) | Severe COVID-19 patients (n = 40) | Healthy control group (n = 40) | |
|---|---|---|---|
| Age, years | 21–73(54.2 ± 9.1) | 21–73 (54.2 ± 9.1) | 21–73 (52.4 ± 8.5) |
| Sex | |||
| Men (%) | 24 (60) | 24 (60) | 24 (60) |
| Women (%) | 16 (40) | 16 (40) | 16 (40) |
| Fever | |||
| <37.3 °C (%) | 22/40 (55.0) | 19/40 (47.5) | – |
| 37.3–38.0 °C (%) | 9/40 (22.5) | 8/40 (20.0) | – |
| 38.1–39.0 °C (%) | 7/40 (17.5) | 9/40 (22.5) | – |
| >39.0 °C (%) | 2/40 (5.0) | 4/40 (10.0) | – |
| Cough (%) | 26 (65.0) | 29 (72.5) | – |
| Headache (%) | 5 (12.5) | 6 (15.0) | – |
| Sore throat (%) | 6 (15.0) | 5 (12.5) | – |
| Dyspnea (%) | 9 (22.5) | 14 (35.0) | – |
| C-reactive protein ≥10 mg/L (%) | 23 (57.5) | 34 (85.0) | |
| Lactate dehydrogenase, U/L | – | ||
| ≤245 (%) | 27 (67.5) | 24 (60.0) | |
| >245 (%) | 15 (37.5) | 14 (35.0) | |
| Creatinine, μmol/L | – | ||
| ≤133 (%) | 35 (87.5) | 32 (40.0) | |
| >133 (%) | 5 (12.5) | 7 (17.5) | |
| White blood cell count ×109/L | – | ||
| <4 (%) | 13 (32.5) | 11 (27.5) | |
| 4–10 (%) | 18 (45) | 21 (52.5) | |
| >10 (%) | 9 (22.5) | 8 (20.0) | |
| Lymphocyte count ×109/L | – | ||
| <1·0 (%) | 27 (67.5) | 23 (57.5) | |
| ≥1·0 (%) | 13 (32.5) | 17 (42.5) | |
| Platelet count | – | ||
| <100 (%) | 23 (57.5) | 21 (52.5) | |
| ≥100 (%) | 17 (42.5) | 19 (47.5) | |
| Bilateral involvement of chest radiographs (%) | 35 (87.5) | 38 (95.0) | – |
| Mechanical ventilation | – | 3 (7.5) | – |
2.2. Intervention
To investigate the therapeutic effects of Nanocurcumin, the intervention was applied in Nanocurcumin and placebo groups. To improve the curcumin oral bioavailability, Nanocurcumin oral capsules were administered to patients. In the Nanocurcumin-treated group, mild and severe COVID-19 patients received two doses of SinaCurcumin® (Exir Nano, Tehran, Iran) in 80 mg capsule, two times for 21 days. Moreover, in the placebo group, patients took a placebo capsule two times daily for 21 days. SinaCurcumin is a registered product from curcuminoids in Iran (IRC: 1228225765) and is prescribed as a soft gelatin capsule, consisting of 80 mg of curcuminoids, as nanomicelles. Curcuminoids, the dietary polyphenols, are extracted from the dried rhizomes of Curcuma longa L. (turmeric) and contain curcumin, bisdemethoxycurcumin (BDMC), and desmethoxycurcumin (DMC), which are termed as a C3 complex [16,17]. In addition to Nanocurcumin and placebo, all COVID-19 patients received 40 mg Atorvastatin daily and bromhexine every 8 h. Additionally, hydroxychloroquine, corticosteroids, protease inhibitors, azithromycin, and broad-spectrum antibiotics, were the most use anti-COVID-19 therapeutic regimens in patients. However, because both placebo and Nanocurcumin groups took the same drugs at the same dose, the use of these drugs did not affect the study results and the only difference between the two groups was the placebo and Nanocurcumin use.
2.3. Blood collection and PBMC isolation
The sampling was conducted by the collection of 8 mL of blood samples from patients (pre-and- post-treatment with Nanocurcumin and placebo) using the heparinized syringes. The peripheral blood Mononuclear cells (PBMCs) were isolated from whole blood of experimental cases using a density-gradient technique mediated by the 1.077 g/mL standard Ficoll (lymphosep) (Biosera, UK), centrifuged at 450g for 25 min, and then washed twice by phosphate buffer saline (PBS) (Sigma-Aldrich, Schnelldorf, Germany). The harvested cells (5 × 106) were cultured in a culture medium consisting of 10% heat-inactivated fetal bovine serum, 200 mM of l-glutamine, 10 ng/mL of PMA, and 100 U/mL of penicillin (eBioscience, San Diego, CA, USA). Afterward, the isolated cells were incubated in a 5% CO2 incubator at 37 °C for 48 h. Harvested cells were utilized for cell count by flow cytometry and mRNA expression measurement by Real-time polymerase chain reaction (RT-PCR).
2.4. Cell separation and flow cytometry
To analyze the frequency alteration, flow cytometry was used to count the population of circulating Tregs among PBMCs in mild and severe COVID-19 infected patients as well as healthy subjects. Using monoclonal antibodies against the surface and intracellular markers. Subsequently, to cell surface staining, the harvested cells (1 × 106) were washed and incubated along with monoclonal antibodies against surface and intracellular antigens, including fluorescein isothiocyanate (FITC)-conjugated anti-human CD4, phycoerythrin (PE)-conjugated anti-human FoxP3, and allophycocyanin (APC)-conjugated anti-human CD25 (eBioscience) at 4 °C for 15 min. The FACS Calibur flow cytometer (BD Biosciences) and FlowJo software (Becton Dickinson, Mountain View, CA, USA) analyzed the population of stained cells.
2.5. Real-time PCR assessment
The gene expression levels of Treg cell-associated transcription factor (FoxP3) and cytokines (TGF-β, IL-10, and IL-35) were detected in isolated PBMCs of patients and controls using Real-time polymerase chain reaction (PCR) along with SYBR green approach. For this purpose, total RNA extraction was employed from isolated cells of COVID-19 patients and healthy individuals using the RNX-PLUS Solution (SinaClon, Tehran, Iran). Subsequently, the complementary DNA (cDNA) was synthesized, applying the Revert Aid™ reverse transcriptase and random hexamer primer (Thermo Fisher Scientific). Real-time PCR measured the mRNA expression levels of the abovementioned genes based on the following steps: DNA denaturation was at 95 °C for 10 s, which was repeated for 40 cycles. The annealing step was at 60 °C for 10 s, which was followed by the extension step at 72 °C for 20 s. The electrophoresis analysis was performed to prove the gene amplification and DNA sequencing, utilizing agarose gel (2%) and Biosystems (Seqlab, Gottingen, Germany). Of note, the curves were a plot employing the six standards, prepared from 10-fold serial dilutions obtained from genes concentrated samples. Data were analyzed using the 2−ΔΔCT formula, the comparative Ct method. Also, the relative expression of target genes was compared with the housekeeping gene. Here, the β-actin was a housekeeping gene. The primer sequences have been detailed in Table 2 .
Table 2.
Primer sequence.
| Gene | Primer | Sequence |
|---|---|---|
| FoxP3 | Forward | TCATCCGCTGGGCCATCCTG |
| Reverse | GTGGAAACCTCACTTCTTGGTC | |
| IL-10 | Forward | CAT CGA TTT CTT CCC TGT GAA |
| Reverse | TCTTGGAGCTTATTAAAGGCATTC | |
| IL-35 | Forward | TGCACTGCTGGAGACATCG |
| Reverse | CAAGGCACAGGGTCATCATC | |
| TGF-β | Forward | CGACTACTACGCCAAGGA |
| Reverse | GAGAGCAACACGGGTTCA | |
| β-Actin | Forward | AGAGCTACGAGCTGCCTGAC |
| Reverse | AGCACTGTGTTGGCGTACAG |
2.6. The enzyme-linked immunosorbent assay (ELISA)
Protein secretion levels of Treg-associated cytokines (TGF-β, IL-10, and IL-35) were measured in the serum samples of mild and severe COVID-19 patients in pre- and post-treatment conditions as well as healthy subjects, employing the ELISA kit (MyBioSource, Nivelles, Belgium). ELISA technique assessed the serum levels of cytokines according to the following steps: 1) 100 μL of coating antibody was added to the wells to coat the 96-well assay plates. 2) The plates were incubated at 4 °C overnight and then washed by PBS consisting of Tween-20 (0.05%). 3) To eliminate the uncoated antibodies, a blocking buffer containing 2% milk in PBS was poured into wells. 4) The plates were incubated on a shaker at room temperature for 2 h and then washed by PBS. 5) The plates were incubated with 100 μL of standards and samples for 1 h. 6) 100 μL of a biotinylated antibody was added to wells and incubated for 1 h. 7) 100 μL of the avidin-biotin-peroxidase complex was poured into wells, and followed by incubation for 30 min. 8) In the last step, the plates were incubated with 100 μL of tetramethylbenzidine (TMB) substrate for 30 min, and then the reaction was stopped. The Medgenix ELISA reader (BP-800; Biohit, Helsinki, Finland) read the absorbance values of samples and standards at 450 nm.
2.7. Statistical analysis
In the current study, SPSS PC Statistics Software (version 19.0; SPSS Inc., Chicago, IL) was applied to perform the statistical analysis. The unpaired Student's t-test was employed to analyze the statistical differences of immunologic parameters between the control group and the COVID-19 patient group. The one-way ANOVA was utilized to compare the statistical differences of immunologic parameters in patient groups between before- and after treatment. Also, the Shapiro-Wilk test controlled the proportion of data to normal ranges. Descriptive data were indicated as the mean ± SD and P < 0.05 was represented as statistically significant. The GraphPad Prism was applied to draw the graphs (version 7.00 for Windows; GraphPad Software, La Jolla, CA; www.graphpad.com).
3. Results
3.1. Treg cell frequency, gene expression level, and serum secretion level of Treg cell factors in mild and severe COVID-19 patients and controls
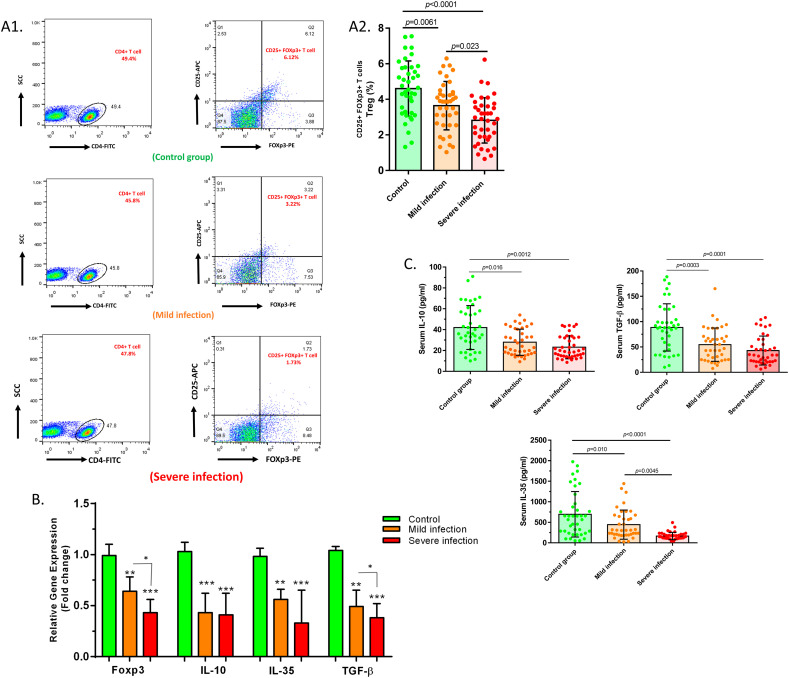
The comparison of Treg cell percentage between baseline and disease condition revealed a significant reduction of Treg cell frequency in both mild and severe 2019-nCoV patients in comparison with healthy controls (P = 0.0061 and P < 0.0001, respectively). A significantly reduced number of Treg cells was detected in severe patients compared to mild patients (P = 0.023) (Fig. 1A).
Fig. 1.
Changes in the number and mediated factors of Treg cells in mild and severe 2019-nCoV patients compared to controls. A1. The results pertaining to the flow cytometry analysis of the control group, mild patients and severe patients, which indicated the percentage of CD4+ CD25+ FoxP3+ (Treg) cells among the total CD4+ T cell population. A2. Treg cell population was notably lower in mild and severe COVID-19 infected patients in comparison with healthy subjects (P = 0.0061 and P < 0.0001, respectively). A significant reduction in Treg cell frequency was counted in severe patients compared to mild cases, as well (P = 0.023). B. The significant downregulated expression levels of FoxP3 (P = 0.0063, P = 0.0002, and P = 0.025), IL-10 (P = 0.0004, P = 0.0001, and P = 0.08), IL-35 (P = 0.0054, P = 0.0005, and P = 0.066), and TGF-β (P = 0.0036, P = 0.0001, and P = 0.038) were detected in mild patient group vs. controls, severe patient group vs. controls, and severe patients group vs. mild patient group, respectively. C. The serum secretion levels of IL-10 (P = 0.016 and P = 0.0012), IL-35 (P = 0.01 and P < 0.0001), and TGF-β (P = 0.0003 and P = 0.0001) were detected to be significantly lower in mild patient group vs. controls and severe patient group vs. controls, respectively. Mild patient group, n = 40; severe patient group, n = 40; control group, n = 40. Results are given as, mean ± SD. (+) in graphs indicates the mean. P < 0.05 describes statistically significant. COVID-19, Coronavirus disease 2019; Treg, T-regulatory cell; FoxP3, forkhead box P3; IL, interleukin; TGF-β, transforming growth factor-beta.
Data analysis illustrated the downregulated expression levels of FoxP3 (P = 0.0063, P = 0.0002, and P = 0.025), IL-10 (P = 0.0004, P = 0.0001, and P = 0.08), IL-35 (P = 0.0054, P = 0.0005, and P = 0.066), and TGF-β (P = 0.0036, P = 0.0001, and P = 0.038) in the mild patient group versus controls, severe patient group versus controls, and severe patient group versus mild patient group for each factor, respectively (Fig. 1B and Table 3 ). The cytokine secretion levels of Treg cells were also evaluated in serum samples of both COVID-19 patients and control groups using ELISA. According to findings, the concentration of IL-10 (P = 0.016 and P = 0.0012), IL-35 (P = 0.01 and P < 0.0001), and TGF-β (P = 0.0003 and P = 0.0001) were found to be significantly lower in mild patients versus controls and in severe patients versus controls, respectively. Besides, it was found that the secretion level of IL-35 was considerably decreased in severe patients than in mild cases (P = 0.0045) (Fig. 1C and Table 4 ).
Table 3.
Expression levels of Treg cell relevant factors in COVID-19 patients and control groups.
| Mild patients | Healthy controls | P value | Severe patients | Healthy controls | P value | Severe patients | Mild patients | P value | |
|---|---|---|---|---|---|---|---|---|---|
| FoxP3 | 0.64 ± 0.14 | 0.99 ± 0.11 | 0.0063 | 0.43 ± 0.13 | 0.99 ± 0.11 | 0.0002 | 0.43 ± 0.13 | 0.64 ± 0.14 | 0.02 |
| IL-10 | 0.43 ± 0.19 | 1.03 ± 0.09 | 0.0004 | 0.41 ± 0.21 | 1.03 ± 0.09 | 0.0001 | 0.41 ± 0.21 | 0.43 ± 0.19 | 0.08 |
| IL-35 | 0.56 ± 0.1 | 0.982 ± 0.08 | 0.0054 | 0.33 ± 0.32 | 0.982 ± 0.08 | 0.0005 | 0.33 ± 0.32 | 0.56 ± 0.1 | 0.06 |
| TGF-β | 0.49 ± 0.16 | 1.04 ± 0.04 | 0.0036 | 0.38 ± 0.14 | 1.04 ± 0.04 | 0.0001 | 0.38 ± 0.14 | 0.49 ± 0.16 | 0.03 |
Abbreviations: COVID-19, Coronavirus disease 2019; Treg, T-regulatory; FoxP3, forkhead box P3; IL, interleukin; TGF-β, transforming growth factor beta.
Table 4.
Secretion levels of Treg cell cytokines in COVID-19 patients and control groups.
| Mild patients | Healthy controls | P value | Severe patients | Healthy controls | P value | Severe patients | Mild patients | P value | |
|---|---|---|---|---|---|---|---|---|---|
| IL-10 | 28.54 ± 12.92 | 42.03 ± 21.14 | 0.016 | 23.50 ± 9.97 | 42.03 ± 21.14 | 0.0012 | 23.50 ± 9.97 | 28.54 ± 12.92 | 0.54 |
| IL-35 | 443.1 ± 355.2 | 695.5 ± 552.8 | 0.01 | 166 ± 89.12 | 695.5 ± 552.8 | <0.0001 | 166 ± 89.12 | 443.1 ± 355.2 | 0.004 |
| TGF-β | 54.65 ± 32.92 | 88.55 ± 46.68 | 0.0003 | 42.97 ± 28.62 | 88.55 ± 46.68 | 0.0001 | 42.97 ± 28.62 | 54.65 ± 32.92 | 0.63 |
Abbreviations: COVID-19, Coronavirus disease 2019; Treg, T-regulatory; IL, interleukin; TGF-β, transforming growth factor beta.
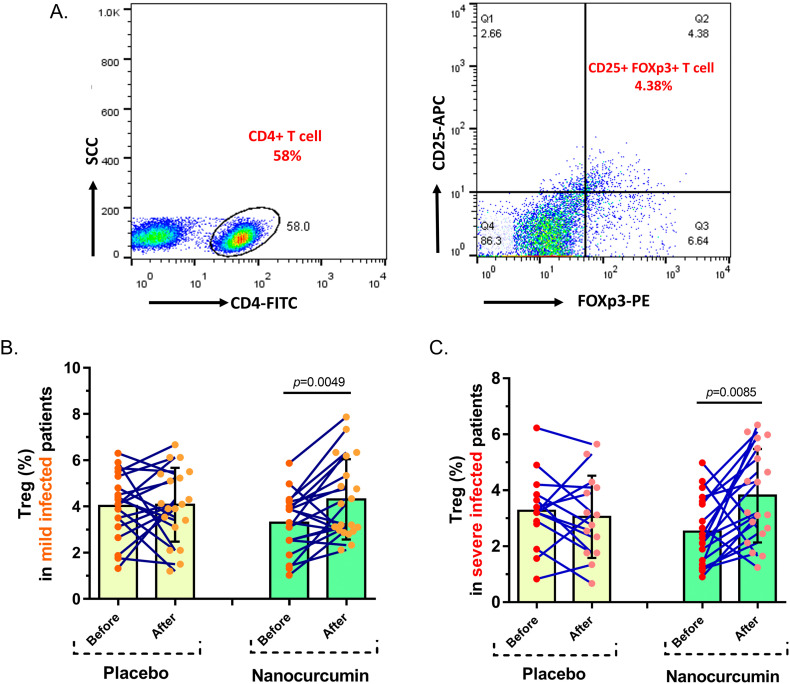
3.2. Circulating Treg cell frequency
In the mild stage of COVID-19 patients, Nanocurcumin could notably build up the number of Treg cells from 3.295% ± 1.262% pre-treatment to 4.308% ± 1.737% post-treatment with the drug (P = 0.0049); while, no notable variation was gained in the placebo group after and before treatment (4.074% ± 1.595% vs. 4.025% ± 1.424%, P = 0.9). Moreover, in severe stage COVID-19 patients, the Nanocurcumin treated group indicated a considerable elevation in the frequency of Treg cell after treatment (3.807% ± 1.669%) compared to before treatment (2.525% ± 1.219%) with Nanocurcumin (P = 0.0085). By contrast, in the placebo group, the number of Treg cells did not significantly change after and before treatment (3.053% ± 1.468% vs. 3.269% ± 1.307%, P = 0.461) (Fig. 2 ).
Fig. 2.
The number of Treg cells in Nanocurcumin and placebo-treated groups in mild and severe COVID-19 patients. A. The results pertaining to the flow cytometry analysis of the Nanocurcumin and placebo-treated patients with mild and severe COVID-19, which indicated the percentage of CD4+ CD25+ FoxP3+ (Treg) cells among the total CD4+ T cell population. B. Nanocurcumin could significantly increase the frequency of Treg cells in the Nanocurcumin-treated group in mild COVID-19 patients after treatment vs. before treatment (P = 0.0049). C. In severe patients, considerable enhance was found in the number of Tregs in Nanocurcumin-treated patients after treatment vs. before treatment (P = 0.0085). No significant differences were observed in the placebo-treated group in both mild and severe patients after and before treatment (P = 0.9 and P = 0.461, respectively). Mild patient group, n = 40; severe patient group, n = 40; Nanocurcumin treated group, n = 20; placebo-treated group, n = 20. Results are given as, mean ± SD. P < 0.05 describes statistically significant. COVID-19, Coronavirus disease 2019; Treg, T-regulatory cell.
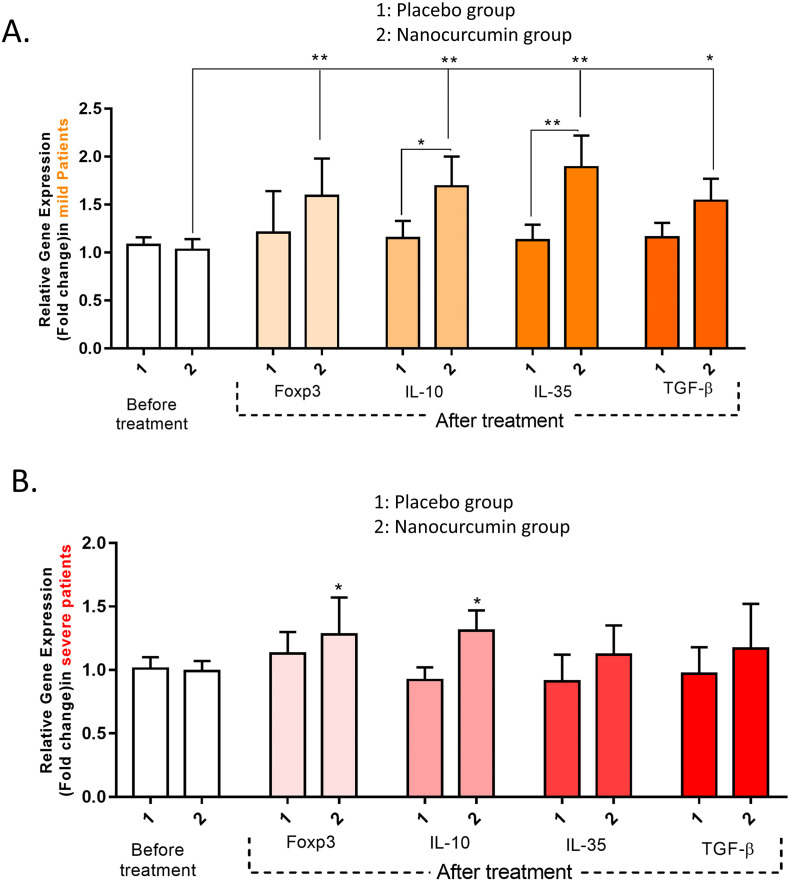
3.3. The gene expression levels of Treg cell-related factors
It was found that in mild patients receiving Nanocurcumin, the expression levels of FoxP3 (P = 0.0062), IL-10 (P = 0.0051), IL-35 (P = 0.0044), and TGF-β (P = 0.032) were significantly upregulated after treatment. Also, we found significantly upregulated gene expression levels of IL-10 (P = 0.018) and IL-35 (P = 0.0083) cytokines in Nanocurcumin-received patients when compared with placebo-received group (Fig. 3A). Similarly, a considerably higher expression level of FoxP3 (P = 0.042) and IL-10 (P = 0.045) were detected in Nanocurcumin-received patients after treatment in severe COVID-19 patients (Fig. 3B).
Fig. 3.
The gene expression levels of Treg cell transcription factor and cytokines in Nanocurcumin and placebo-treated group in mild and severe COVID-19 patients. A. In mild COVID-19 patients, the expression levels of FoxP3 (P = 0.0062), IL-10 (P = 0.0051), IL-35 (P = 0.0044), and TGF-β (P = 0.032) were significantly upregulated after treatment. Also we found significantly upregulated gene expression levels of IL-10 (P = 0.018) and IL-35 (P = 0.0083) cytokines in Nanocurcumin-received patients than in placebo-received group. B. In severe COVID-19 patients a significantly increased expression levels of FoxP3 (P = 0.042) and IL-10 (P = 0.045) were detected in Nanocurcumin received patients after treatment. Mild patient group, n = 40; severe patient group, n = 40; Nanocurcumin treated group, n = 20; placebo-treated group, n = 20. Results are given as, mean ± SD. P < 0.05 describes statistically significant. COVID-19, Coronavirus disease 2019; Treg, T-regulatory cell; FoxP3, forkhead box P3; IL, interleukin; TGF-β, transforming growth factor-beta.
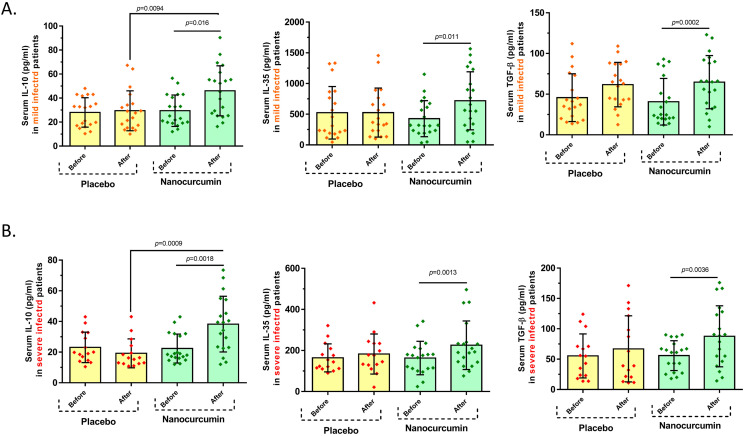
3.4. Serum secretion levels of Treg cell cytokines
Cytokine serum levels of Treg cell relevant cytokines were assessed in serum samples of mild and severe infected patients, in pre-and post-treatment with Nanocurcumin and placebo using ELISA. Based on obtained findings, in mild patients, treatment significantly enhanced the serum levels of all IL-10 (46.04 ± 20.86 vs. 29.46 ± 13.09, P = 0.016), IL-35 (718.6 ± 473 vs. 427.2 ± 293.1, P = 0.011), and TGF-β (64.79 ± 32.70 vs. 40.72 ± 28.63, P = 0.0002) cytokines when compared with pre-treatment condition in the Nanocurcumin receiving group. In contrast, no remarkable alteration in serum secretion levels of IL-10, IL-35, and TGF-β was found in the placebo group after treatment vs. before treatment. Besides, serum secretion level of IL-10 was found to be considerably higher in the Nanocurcumin patient group in comparison with the placebo group after receiving treatment (46.04 ± 20.86 vs. 29.43 ± 16.61, P = 0.0094); however, no meaningful differences were observed in secretion levels of TGF-β and IL-35 in the Nanocurcumin and placebo groups, after treatment (Fig. 4A and Table 5 ). In severe condition, Nanocurcumin significantly elevated the serum levels of IL-10 (38.24 ± 18.17 vs. 22.35 ± 9.42, P = 0.0018), IL-35 (225.5 ± 118.3 vs. 163.4 ± 81.43, P = 0.0013), and TGF-β (87.68 ± 50.27 vs. 55.87 ± 24.62, P = 0.0036) cytokines in post-treatment Nanocurcumin group when compared with pre-treatment condition. Moreover, a remarkably increased level of IL-10 concentration was detected in the Nanocurcumin-treated group in comparison with the placebo-treated group after treatment (38.24 ± 18.17 vs. 19.21 ± 9.40, P = 0.0009). On the other hand, it was found that the serum secretion levels of IL-10, IL-35, and TGF-β were not considerably higher in the placebo group post-treatment vs. pre-treatment. Moreover, no considerable alterations were found in serum secretion levels of mentioned cytokines between Nanocurcumin- and placebo-treated groups (Fig. 4B and Table 5).
Fig. 4.
The serum secretion levels of Treg cell cytokines in Nanocurcumin and placebo-treated groups in mild and severe COVID-19 patients. A. In mild COVID-19 patients, treatment notably increased the serum concentration of IL-10 (P = 0.016), IL-35 (P = 0.011), and TGF-β (P = 0.0002) cytokines in comparison with pre-treatment condition in the Nanocurcumin-treated group; however, no significant changes in serum secretion levels of IL-10, IL-35, and TGF-β were found in the placebo group after treatment vs. before treatment. Also, the serum secretion level of IL-10 was considerably higher in the Nanocurcumin-treated group than in the placebo group after treatment (P = 0.0094), but no significant differences were observed in secretion levels of IL-35 and TGF-β among Nanocurcumin and placebo-treated groups, after treatment. B. In severe patients, the secretion levels of IL-10 (P = 0.0018), IL-35 (P = 0.0013), and TGF-β (P = 0.0036) cytokines were meaningfully higher in post-treatment with Nanocurcumin in the treated group when compared with pre-treatment condition. Besides, the serum secretion level of IL-10 was found to be considerably increased in the Nanocurcumin-treated group in comparison with the placebo-treated group after treatment (P = 0.0009). No significant alterations in the serum secretion levels of cytokines were observed in the placebo-treated group after treatment vs. before treatment. Mild patient group, n = 40; severe patient group, n = 40; Nanocurcumin treated group, n = 20; placebo-treated group, n = 20. Results are given as, mean ± SD. (+) in graphs indicates the mean. P < 0.05 describes statistically significant. COVID-19, Coronavirus disease 2019; Treg, T-regulatory cell; IL, interleukin; TGF-β, transforming growth factor-beta.
Table 5.
Secretion levels of Treg cell cytokines in Nanocurcumin and placebo-treated groups of mild and severe COVID-19 patients.
| Mild COVID-19 patients | ||||||
|---|---|---|---|---|---|---|
| Nanocurcumin (n = 20) |
P value | Placebo (n = 19) |
P value | |||
| Before | After | Before | After | |||
| IL-10 | 29.46 ± 13.09 | 46.04 ± 20.86 | 0.016 | 28.04 ± 12.27 | 29.43 ± 16.61 | 0.73 |
| IL-35 | 427.2 ± 293.1 | 718.6 ± 473 | 0.011 | 523.6 ± 428 | 526.6 ± 398.7 | 0.98 |
| TGF-β | 40.72 ± 28.63 | 64.79 ± 32.70 | 0.0002 | 45.71 ± 29.36 | 61.69 ± 27.31 | 0.08 |
| Severe COVID-19 patients | ||||||
|---|---|---|---|---|---|---|
| Nanocurcumin (n = 19) |
P value | Placebo (n = 15) |
P value | |||
| Before | After | Before | After | |||
| IL-10 | 22.35 ± 9.42 | 38.24 ± 18.17 | 0.0018 | 23.09 ± 9.94 | 19.21 ± 9.40 | 0.1 |
| IL-35 | 163.4 ± 81.43 | 225.5 ± 118.3 | 0.0013 | 164 ± 68.12 | 182.9 ± 97.44 | 0.58 |
| TGF-β | 55.87 ± 24.62 | 87.68 ± 50.27 | 0.0036 | 55.34 ± 36.24 | 66.95 ± 54.44 | 0.15 |
Abbreviations: COVID-19, Coronavirus disease 2019; Treg, T-regulatory; IL, interleukin; TGF-β, transforming growth factor beta.
3.5. Clinical symptoms and laboratory findings
Clinical symptoms, including fever, cough, dyspnea, and headache were also assessed in COVID-19 patients before and after treatment with Nanocurcumin. Moreover, some laboratory findings, including White blood cell (WBC) count, lymphocyte count, platelet count, creatinine, Lactate dehydrogenase, and bilateral involvement of chest radiographs were examined in patients before and after treatment with Nanocurcumin. Treatment with Nanocurcumin was associated with statistically significant improvement of clinical symptoms in the mild and severe patient group after treatment compared to before treatment (P < 0.0001) (Table 6, Table 7 ). However, there was no significant difference in most clinical symptoms and laboratory findings in the placebo-treated group before and after treatment. In mild stage patients, the mortality rate was found to be 0% (0 out of 20) in the Nanocurcumin-treated group and 5% (1 out of 20) in the placebo-treated group. Besides, in severe patients, the Nanocurcumin group had 5% mortality rate (1 out of 20) and the placebo group had 25% mortality rate (5 out of 20). According to findings, the mortality rate was demonstrated considerably higher in the placebo-treated group than in the Nanocurcumin-treated group in patients (mild and severe) (P < 0.0001).
Table 6.
Clinical symptoms and laboratory findings of mild COVID-19 patients before and after treatment with Nanocurcumin and placebo.
| Mild COVID-19 patients |
Placebo group |
|||||
|---|---|---|---|---|---|---|
| Before treatment (n = 20) | After treatment (n = 20) | P value | Before treatment (n = 20) | After treatment (n = 19) | P value | |
| Fever (N, %) | <0.0001 | ns | ||||
| <37.3 °C | 8 (40%) | 1 (5%) | 8 (40%) | 7 (36%) | ||
| 37.3–38.0 °C | 4 (20%) | 0 (0%) | 9 (45%) | 8 (42%) | ||
| 38.1–39.0 °C | 5 (25%) | 0 (0%) | 2 (10%) | 3 (15%) | ||
| >39.0 °C | 2 (10%) | 0 (0%) | 1 (5%) | 1 (5.2%) | ||
| Cough (%) | 7 (35%) | 1 (5%) | <0.0001 | 11 (55%) | 3 (20%) | 0.004 |
| Dyspnea (%) | 6 (30%) | 2 (1%) | <0.0001 | 4 (20%) | 1 (5.2%) | 0.01 |
| C-reactive protein ≥10 mg/L (%) | 13 (65%) | 4 (20%) | <0.0001 | 9 (45%) | 7 (36%) | ns |
| Lactate dehydrogenase, U/L | <0.0001 | ns | ||||
| ≤245 (%) | 14 (70%) | 17 (85%) | 11 (55%) | 13 (65%) | ||
| >245 (%) | 6 (30%) | 3 (15%) | 9 (45%) | 6 (31%) | ||
| Creatinine, μmol/L | <0.0001 | ns | ||||
| ≤133 (%) | 15 (75%) | 17 (85%) | 16 (80%) | 17 (89%) | ||
| >133 (%) | 4 (20%) | 3 (15%) | 4 (20%) | 2 (10%) | ||
| White blood cell count | <0.0001 | ns | ||||
| ×109/L | 6 (30%) | 5 (25%) | 4 (20%) | 6 (31%) | ||
| <4 (%) | 9 (45%) | 8 (40%) | 11 (55%) | 7 (36%) | ||
| 4–10 (%) | 5 (25%) | 7 (35%) | 5 (25%) | 6 (31%) | ||
| >10 (%) | ||||||
| Lymphocyte count ×109/L | <0.0001 | 0.03 | ||||
| <1·0 (%) | 14 (70%) | 9 (45%) | 15 (75%) | 10 (52%) | ||
| ≥1·0 (%) | 6 (30%) | 11 (55%) | 5 (25%) | 9 (47%) | ||
| Platelet count | <0.0001 | ns | ||||
| <100 (%) | 12 (60%) | 7 (35%) | 11 (55%) | 5 (26%) | ||
| ≥100 (%) | 8 (40%) | 13 (65%) | 9 (45%) | 14 (73%) | ||
| Bilateral involvement of chest radiographs | 18 (90%) | 9 (45%) | <0.0001 | 17 (85%) | 8 (42%) | 0.003 |
Table 7.
Clinical symptoms and laboratory findings of severe COVID-19 patients before and after treatment with Nanocurcumin and placebo.
| Severe COVID-19 patients |
Placebo group |
|||||
|---|---|---|---|---|---|---|
| Before treatment (n = 20) | After treatment (n = 19) | P value | Before treatment (n = 20) | After treatment (n = 15) | P value | |
| Fever (N, %) | <0.0001 | ns | ||||
| <37.3 °C | 11 (55%) | 4 (20%) | 9 (45%) | 8 (53%) | ||
| 37.3–38.0 °C | 5 (25%) | 2 (10%) | 6 (30%) | 5(33%) | ||
| 38.1–39.0 °C | 3 (15%) | 0 (0%) | 2 (10%) | 1 (6.6%) | ||
| >39.0 °C | 1 (5%) | 0 (0%) | 3 (15%) | 1 (6.6%) | ||
| Cough (%) | 15 (75%) | 2 (10%) | <0.0001 | 11 (55%) | 7 (47%) | ns |
| Dyspnea (%) | 7 (35%) | 1 (5%) | <0.0001 | 9 (45%) | 2 (15%) | 0.03 |
| C-reactive protein ≥10 mg/L (%) | 17 (85%) | 5 (26%) | <0.0001 | 14 (70%) | 9 (60%) | ns |
| Lactate dehydrogenase, U/L | <0.0001 | 0.02 | ||||
| ≤245 (%) | 11 (55%) | 16 (84%) | 9 (45%) | 12 (80%) | ||
| >245 (%) | 8 (40%) | 3 (15%) | 11 (55%) | 3 (20%) | ||
| Creatinine, μmol/L | <0.0001 | ns | ||||
| ≤133 (%) | 16 (80%) | 8 (42%) | 17 (85%) | 13 (86%) | ||
| >133 (%) | 4 (20%) | 11 (57%) | 3 (20%) | 2 (13%) | ||
| White blood cell count | <0.0001 | ns | ||||
| ×109/L | 6 (25%) | 5 (26%) | 8 (40%) | 5 (33%) | ||
| <4 (%) | 10 (55%) | 9 (47%) | 9 (45%) | 6 (40%) | ||
| 4–10 (%) | 4 (20%) | 5 (26%) | 3 (15%) | 4 (26%) | ||
| >10 (%) | ||||||
| Lymphocyte count ×109/L | <0.0001 | ns | ||||
| <1·0 (%) | 15 (75%) | 10 (52%) | 14 (70%) | 8 (53%) | ||
| ≥1·0 (%) | 5 (25%) | 9 (47%) | 6 (30%) | 7 (46%) | ||
| Platelet count | <0.0001 | 0.04 | ||||
| <100 (%) | 11 (55%) | 5 (26%) | 13 (65%) | 4 (26%) | ||
| ≥100 (%) | 9 (45%) | 14 (73%) | 7 (35%) | 11 (73%) | ||
| Bilateral involvement of chest radiographs | 16 (80%) | 9 (47%) | <0.0001 | 18 (90%) | 6 (40%) | 0.001 |
| Mechanical ventilation | 2 (10%) | 0 (0%) | ns | 1 (5%) | 0 (0%) | ns |
4. Discussion
The importance of immune system responses in the COVID-19 immunopathogenesis and the lack of approved and practical treatment prompted us to investigate the therapeutic effects of a potent novel Nanocurcumin-based formulation called SinaCurcumin® in COVID-19 patients. Curcumin is a bioactive-ingredient traditional medicine that originated from the rhizomes of Curcuma longa L [18]. At the molecular level, curcumin can modulate various signaling molecules and functions on multiple targets in different cellular pathways [19,20]. Curcumin is mostly known as a modulatory agent, which can modulate inflammatory responses and inhibit infections. Interestingly, curcumin obtained much success in infections, cancers, autoimmunities, inflammatory diseases, etc. as a therapeutic or protective natural agent [12,19,21]. According to some references, curcumin has major problems such as poor bioavailability because of its poor solubility in water, a higher rate of metabolic activity, and rapid excretion from the body; whereas these restrictions have been solved in nano range formulation of curcumin, namely Nanocurcumin [22,23]. The nano-formulation of curcumin termed Nanocurcumin paved the way for some relevant restrictions to curcumin, such as bioavailability. This formulation increases dissolution rate, saturation solubility, bioavailability, and drug stability. Nanomicelles containing curcumin is a registered curcumin product (SinaCurcumin®) for oral use. Each soft gel of SinaCurcumin® contains 80 mg of curcumin as a nano micelle. The percentage of encapsulation of curcumin in this nano micelle is near to 100% and their sizes are around 10 nm. SinaCurcumin® has a significantly higher bioavailability after oral use compared to a simple powder form of curcumin with the following mechanisms. After oral administration, the soft gels of SinaCurcumin® open and pass through the small intestine in less than 15 min in the stomach. These nano micelles after reaching the small intestine could be dissolved in unstirred water layer [16,24].
Relying on the benefits of the SinaCurcumin®, including anti-inflammatory and anti-microbial functions, we were prompted to evaluate the efficacy of SinaCurcumin® on Treg cell responses in mild and severe COVID-19 infected patients.
Based on obtained findings, Nanocurcumin could significantly increase the number of Treg cells in both mild and severe infected patients in comparison with those received placebo. Moreover, the expression levels of all FoxP3, TGF-β, IL-10, and IL-35 factors were notably upregulated in Nanocurcumin-treated groups of both mild and severe patients after treatment versus before treatment; while, no considerable alterations were found in the placebo-treated group. We found that the serum levels of all IL-10, IL-35, and TGF-β cytokines were remarkably enhanced after treatment by Nanocurcumin in comparison with pre-treatment conditions. However, the placebo group showed no meaningful differences after receiving treatment in comparison to before treatment. Additionally, in both mild and severe-infected patients, a higher secretion level of IL-10 was observed in the Nanocurcumin-received group than in the placebo group, after treatment. Our findings also revealed an overall improvement in clinical symptoms of patients treated with Nanocurcumin. According to the results, Nanocurcumin could significantly mitigate the fatality in the mild and severe-treated patients, which proposes the potent therapeutic function of Nanocurcumin against SARS-CoV2 infection. Given the role of the immune system in defense against SARS-CoV2 as well as the viral immunopathogenesis, targeting immune responses such as suppressing Th1 and Th17 cell responses and augmenting the Treg cell responses can be effective in reducing the inflammation and accelerating the rehabilitation and recovery process in infected patients [25]. The reduced frequency and dysfunction of Treg cells in the course of SARS-CoV2 infection lead to disease progression, respiratory system injury, and ARDS [26,27]. Also, the enhanced number of naive T helper cells and downregulated frequency of Treg cells [mainly induced Treg (iTreg) cells] were detected in severe COVID-19 patients and proposed the essential roles of these cells in disease immunopathogenesis. Some other related studies evidenced the reduced level of peripheral Treg cells in COVID-19 patients, mostly in the severe stage than in the mild stage, which might be caused by Treg cell migration from peripheral blood to lung tissue [8,[28], [29], [30]]. Immunologically, the imbalance of the immune system, the deficiency of T-regulatory cells, and the increase of inflammatory factors play the main role in the pathogenesis of autoimmune and inflammatory diseases. This claim has been proven in various disorders such as multiple sclerosis (MS) [31,32], Behçet's disease [33], ankylosing spondylitis (AS) [28,34], preeclampsia [35], and recurrent implantation failure [36,37]. Recommendation for the use of herbal-based drugs (curcumin, apigenin, pyrocatechol, etc.) as anti-inflammatory agents, as well as other immunomodulatory drugs, including monoclonal antibodies, interferon beta, Janus Kinase Inhibitors, corticosteroids (dexamethasone, methylprednisolone, etc.), and nonsteroidal anti-inflammatory drugs (NSAIDs), highlights the importance of suppressing inflammatory responses and enhancing anti-inflammatory responses of Tregs in the course of COVID-19 infection [38,39]. There are several natural substances known to have antiviral and anti-inflammatory effects against coronaviruses and other virus types, including lycorine, flavones, quinones, fatty acids, terpenes, alkaloids, and tannins. These natural products can fight against SASR-CoV2 by inhibiting the ACE2 and TMPRSS2 receptors, as well as RNA polymerase [40]. Among herbal medicine, curcumin demonstrated potent anti-inflammatory and modulatory effects, which were discussed in many various disorders. In this point of view, some supporting findings in line with our investigation proved the immunomodulatory role of Nanocurcumin in suppressing the inflammatory responses (Th1 and Th17 cell-mediated inflammation) and upgrading the Treg cell responses in autoimmune or inflammatory diseases [41]. Also, it was found that curcumin acted as a double-edged sword in attenuating the lung injury in acute lung injury mice, on the one hand, it downregulated the proinflammatory cytokines and on the other hand, it increased the differentiation of Treg cells and the expression of IL-10 cytokine, as an anti-inflammatory cytokine [42]. Another study examined the therapeutic effect of curcumin against COVID-19 and provided promising results regarding the high affinity of curcumin to bind to viral nucleocapsid, membrane, and spike proteins, and the efficient role of curcumin as a potential natural therapeutic agent in the development of anti-SARS-CoV2 drugs [43]. In a study by Tahmasebi et al., immunomodulatory effects of Nanocurcumin were investigated on Th17 cell responses in mild and severe COVID-19 patients. Nanocurcumin could significantly reduce the frequency of Th17 cells and their related inflammatory factors (RORγt, IL-17, IL-21, IL-23, and GM-CSF) in both mild and severe COVID-19 patients [25]. Additionally, some other supportive studies have proven the anti-viral and anti-inflammatory functions of curcumin in various infections, such as human immunodeficiency virus (HIV), Human papillomavirus (HPV), Influenza, parainfluenza virus (type 3), vesicular stomatitis virus, respiratory syncytial virus (RSV), herpes simplex virus (HSV), hepatitis virus, adenovirus, and zikavirus [15,44,45]. In addition to the mentioned studies, some other related studies suggest the usage of Nanocurcumin as a potent therapeutic agent against SARS-CoV2 due to the anti-viral and anti-inflammatory activities [[46], [47], [48], [49]].
5. Conclusion
In conclusion, regarding the importance of Treg cells in suppressing inflammation and inducing the immune system homeostasis, a decreased number of Treg cells could be considered as one of the important causes of the hyperactivated immune system, hyper inflammation, and lung injury in the COVID-19 patients, mostly in severe condition. Therefore, Nanocurcumin would be effective in reducing inflammation and improving 2019-nCoV patients by upregulating the activity of Treg cells. Also, the reduction in mortality in treated patients was one of the promising results of Nanocurcumin treatment in COVID-19. We proposed that Nanocurcumin may represent a potential treatment option against SARS-CoV2; however, further research is necessary to evaluate the anti-inflammatory and immunomodulatory potential uses of this compound in COVID-19 patients.
CRediT authorship contribution statement
Conceptualization and writing original draft: Safa Tahmasebi and Balsam Qubais Saeed. Conceptualization, investigation, data curation: Alexei Valerievich Yumashev, Mohamed A. El-Esawi, and Jamshid Gholizadeh Navashenaq. Conceptualization, introducing, selecting patients and clinical assessment: Hamed Valizadeh, Javad Adigozalou and Armin Sadeghi. Revised article, review, and final edition: Saeed Aslani, Mehdi Yousefi, and Elmira Temirgalieva. Supervision, validation, and editing: Majid Ahmadi and Leila Roshangar.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be considered as a potential conflict of interest.
Acknowledgments
Acknowledgment
The authors gratefully acknowledge the financial support of this project by the Stem Cell Research Center (SCRC) at Tabriz University of Medical Sciences (grant No. 65660).
Data availability statement
Data supporting the findings in this study are immediately available upon reasonable request.
References
- 1.Tahmasebi S., Khosh E., Esmaeilzadeh A. The outlook for diagnostic purposes of the 2019-novel coronavirus disease. J. Cell. Physiol. 2020;235:9211–9229. doi: 10.1002/jcp.29804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host & Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esmaeilzadeh A., Tahmasebi S., Athari S.S. Chimeric antigen receptor -T cell therapy: applications and challenges in treatment of allergy and asthma. Biomed. Pharmacother. 2020;123 doi: 10.1016/j.biopha.2019.109685. [DOI] [PubMed] [Google Scholar]
- 5.Tahmasebi S., Elahi R., Khosh E., Esmaeilzadeh A. Programmable and multi-targeted CARs: a new breakthrough in cancer CAR-T cell therapy. Clin. Transl. Oncol. 2020:1–17. doi: 10.1007/s12094-020-02490-9. [DOI] [PubMed] [Google Scholar]
- 6.Tan M., Liu Y., Zhou R., Deng X., Li F., Liang K., et al. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020;160(3):261–268. doi: 10.1111/imm.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., et al. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduction and Targeted Therapy. 2020;5:1–8. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020:130. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infec. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghaebi M., Osali A., Valizadeh H., Roshangar L., Ahmadi M. Vaccine development and therapeutic design for 2019-nCoV/SARS-CoV-2: Challenges and chances. J. Cell. Physiol. 2020;235:9098–9109. doi: 10.1002/jcp.29771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCreary E.K., Pogue J.M. Open Forum Infectious Diseases. Oxford University Press; US: 2020. Coronavirus disease 2019 treatment: a review of early and emerging options; p. ofaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahimi H.R., Kazemi Oskuee R. Curcumin from traditional Iranian medicine to molecular medicine. Razavi Int J Med. 2014;2 [Google Scholar]
- 13.Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lelli D., Sahebkar A., Johnston T.P., Pedone C. Curcumin use in pulmonary diseases: state of the art and future perspectives. Pharmacol. Res. 2017;115:133–148. doi: 10.1016/j.phrs.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Mathew D., Hsu W.-L. Antiviral potential of curcumin. J. Funct. Foods. 2018;40:692–699. [Google Scholar]
- 16.Hatamipour M., Sahebkar A., Alavizadeh S.H., Dorri M., Jaafari M.R. Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids. Iranian journal of basic medical sciences. 2019;22:282–289. doi: 10.22038/ijbms.2019.32873.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahimi H.R., Mohammadpour A.H., Dastani M., Jaafari M.R., Abnous K., Ghayour Mobarhan M., et al. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna journal of phytomedicine. 2016;6:567–577. [PMC free article] [PubMed] [Google Scholar]
- 18.Heger M., van Golen R.F., Broekgaarden M., Michel M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol. Rev. 2014;66:222–307. doi: 10.1124/pr.110.004044. [DOI] [PubMed] [Google Scholar]
- 19.Imran M., Ullah A., Saeed F., Nadeem M., Arshad M.U., Suleria H.A.R. Cucurmin, anticancer, & antitumor perspectives: a comprehensive review. Crit. Rev. Food Sci. Nutr. 2018;58:1271–1293. doi: 10.1080/10408398.2016.1252711. [DOI] [PubMed] [Google Scholar]
- 20.Willenbacher E., Khan S.Z. 2019. Curcumin: New Insights Into an Ancient Ingredient Against Cancer; p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flora G., Gupta D., Tiwari A. Nanocurcumin: a promising therapeutic advancement over native curcumin. Crit. Rev. Ther. Drug Carrier Syst. 2013;30:331–368. doi: 10.1615/critrevtherdrugcarriersyst.2013007236. [DOI] [PubMed] [Google Scholar]
- 22.Gera M., Sharma N., Ghosh M., Huynh D.L., Lee S.J., Min T., et al. Nanoformulations of curcumin: an emerging paradigm for improved remedial application. Oncotarget. 2017;8 doi: 10.18632/oncotarget.19164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanna D.H., Saad G.R. Nanocurcumin: preparation, characterization and cytotoxic effects towards human laryngeal cancer cells. RSC Adv. 2020;10:20724–20737. doi: 10.1039/d0ra03719b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahimi H.R., Nedaeinia R., Shamloo A.S., Nikdoust S., Oskuee R.K. Novel delivery system for natural products: nano-curcumin formulations. Avicenna journal of phytomedicine. 2016;6:383. [PMC free article] [PubMed] [Google Scholar]
- 25.Tahmasebi S., El-Esawi M.A., Mahmoud Z.H., Timoshin A., Valizadeh H., Roshangar L. 2020. Immunomodulatory Effects of Nanocurcumin on Th17 Cell Responses in Mild and Severe COVID-19 Patients. [DOI] [PubMed] [Google Scholar]
- 26.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadeghi A., Tahmasebi S., Mahmood A., Kuznetsova M., Valizadeh H., Taghizadieh A., et al. Th17 and Treg cells function in SARS-CoV2 patients compared with healthy controls. J. Cell. Physiol. 2021;236:2829–2839. doi: 10.1002/jcp.30047. [DOI] [PubMed] [Google Scholar]
- 28.Ahmadi M., Hajialilo M., Dolati S., Eghbal-Fard S., Heydarlou H., Ghaebi M., et al. The effects of nanocurcumin on Treg cell responses and treatment of ankylosing spondylitis patients: a randomized, double-blind, placebo-controlled clinical trial. J. Cell. Biochem. 2020;121:103–110. doi: 10.1002/jcb.28901. [DOI] [PubMed] [Google Scholar]
- 29.Wang F., Hou H., Luo Y., Tang G., Wu S., Huang M., et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI insight. 2020;5 doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W., Su B., Pang L., Qiao L., Feng Y., Ouyang Y., et al. Vol. 17. 2020. High-dimensional immune profiling by mass cytometry revealed immunosuppression and dysfunction of immunity in COVID-19 patients; pp. 650–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolati S., Ahmadi M., Rikhtegar R., Babaloo Z., Ayromlou H., Aghebati-Maleki L., et al. Changes in Th17 cells function after nanocurcumin use to treat multiple sclerosis. Int. Immunopharmacol. 2018;61:74–81. doi: 10.1016/j.intimp.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Izadi M., Tahmasebi S., Pustokhina I., Yumashev A.V., Lakzaei T., Alvanegh A.G., et al. Changes in Th17 cells frequency and function after ozone therapy used to treat multiple sclerosis patients. Multiple Sclerosis and Related Disorders. 2020;46 doi: 10.1016/j.msard.2020.102466. [DOI] [PubMed] [Google Scholar]
- 33.Ahmadi M., Yousefi M., Abbaspour-Aghdam S., Dolati S., Aghebati-Maleki L., Eghbal-Fard S., et al. Disturbed Th17/Treg balance, cytokines, and miRNAs in peripheral blood of patients with Behcet’s disease. J. Cell. Physiol. 2019;234:3985–3994. doi: 10.1002/jcp.27207. [DOI] [PubMed] [Google Scholar]
- 34.Hajialilo M., Dolati S., Abdolmohammadi-Vahid S., Ahmadi M., Kamrani A., Eghbal-Fard S., et al. Nanocurcumin: a novel strategy in treating ankylosing spondylitis by modulating Th17 cells frequency and function. J. Cell. Biochem. 2019;120:12027–12038. doi: 10.1002/jcb.28488. [DOI] [PubMed] [Google Scholar]
- 35.Eghbal-Fard S., Yousefi M., Heydarlou H., Ahmadi M., Taghavi S., Movasaghpour A., et al. The imbalance of Th17/Treg axis involved in the pathogenesis of preeclampsia. J. Cell. Physiol. 2019;234:5106–5116. doi: 10.1002/jcp.27315. [DOI] [PubMed] [Google Scholar]
- 36.Ahmadi M., Abdolmohammadi-Vahid S., Ghaebi M., Aghebati-Maleki L., Dolati S., Farzadi L., et al. Regulatory T cells improve pregnancy rate in RIF patients after additional IVIG treatment. Syst Biol Reprod Med. 2017;63:350–359. doi: 10.1080/19396368.2017.1390007. [DOI] [PubMed] [Google Scholar]
- 37.Ahmadi M., Abdolmohamadi-Vahid S., Ghaebi M., Dolati S., Abbaspour-Aghdam S., Danaii S., et al. Sirolimus as a new drug to treat RIF patients with elevated Th17/Treg ratio: a double-blind, phase II randomized clinical trial. Int. Immunopharmacol. 2019;74 doi: 10.1016/j.intimp.2019.105730. [DOI] [PubMed] [Google Scholar]
- 38.Rana M.M. Cytokine storm in COVID-19: potential therapeutics for immunomodulation. Journal of Research in Clinical Medicine. 2020;8:38. [Google Scholar]
- 39.Rizk J.G., Kalantar-Zadeh K., Mehra M.R., Lavie C.J., Rizk Y., Forthal D.N. Pharmaco-immunomodulatory therapy in COVID-19. Drugs. 2020:1–26. doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.da Silva Antonio A., LSM Wiedemann, Veiga-Junior V.F. Natural products’ role against COVID-19. RSC Adv. 2020;10:23379–23393. doi: 10.1039/d0ra03774e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolati S., Babaloo Z., Ayromlou H., Ahmadi M., Rikhtegar R., Rostamzadeh D., et al. Nanocurcumin improves regulatory T-cell frequency and function in patients with multiple sclerosis. J. Neuroimmunol. 2019;327:15–21. doi: 10.1016/j.jneuroim.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Chai Y.-s., Y-q Chen, Lin S.-h., Xie K., Wang C.-j., Y-z Yang, et al. Curcumin regulates the differentiation of naïve CD4+ T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed. Pharmacother. 2020;125 doi: 10.1016/j.biopha.2020.109946. [DOI] [PubMed] [Google Scholar]
- 43.Suravajhala R., Parashar A., Malik B., Nagaraj A.V., Padmanaban G., Kishor P.K., et al. 2020. Comparative Docking Studies on Curcumin With COVID-19 Proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das S., Sarmah S., Lyndem S., Singha Roy A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 2020:1–18. doi: 10.1080/07391102.2020.1763201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moghadamtousi S.Z., Kadir H.A., Hassandarvish P., Tajik H., Abubakar S., Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Z., Ying Y. The inhibitory effect of curcumin on virus-induced cytokine storm and its potential use in the associated severe pneumonia. Frontiers in cell and developmental biology. 2020;8:479. doi: 10.3389/fcell.2020.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manoharan Y., Haridas V., Vasanthakumar K.C., Muthu S., Thavoorullah F.F., Shetty P. Curcumin: a wonder drug as a preventive measure for COVID19 management. Indian journal of clinical biochemistry: IJCB. 2020;35:373–375. doi: 10.1007/s12291-020-00902-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suravajhala R., Parashar A., Malik B., Nagaraj V.A., Padmanaban G., Kavi Kishor P., Polavarapu R., Suravajhala P. Comparative docking studies on curcumin with COVID-19 proteins. Preprints. 2020 doi: 10.20944/preprints202005.0439.v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zahedipour F, Hosseini SA, Sathyapalan T, Majeed M, Jamialahmadi T, Al-Rasadi K, et al. 2020. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother. Res..(n/a). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings in this study are immediately available upon reasonable request.