Abstract
β-Catenin/Wnt signaling pathway is critically regulated in a normal cell by a number of post-translational modifications. In pancreatic cancer however, aberrant activation of this pathway plays a significant role in tumor progression and metastasis. Though a number of studies have focused on understanding Wnt signaling pathway in pancreatic cancer, there has been no systematic study to evaluate molecules that may be affecting this pathway. In the current study, we used a diterpene triepoxide, triptolide, to inhibit post-translational modifications in Wnt pathway and evaluated how this compound may be affecting the intricate signaling that regulates cell proliferation in pancreatic cancer.
Our results showed that triptolide inhibits the activation of WNT1, FZD1, and disheveled (DSH) in pancreatic cancer cell lines MIA PaCa-2 and S2-VP10 by inhibiting the phosphorylation of LRP6 and simultaneously blocked translocation of β-catenin to the nucleus by inhibiting its glycosylation.
Additionally, inhibition of post-translational modification of the Wnt-signaling pathway also demonstrated regression of tumor growth in a Syngenic Tumor Implantation Model (STIM). Interestingly, these findings suggest Wnt signaling is a vital molecular pathway in pancreatic cancer and may be amenable to targeted drug therapy.
Keywords: Wnt, β-Catenin, OGT, TCF/LEF1, Pancreatic tumor, STIM model
Introduction
Pancreatic cancer ranks as the fourth most prevalent cancer causing deaths in the United States with an overall 5 year survival of less than 5 per cent. Despite advancement in surgery and chemotherapy, therapeutic resistance and both distal and local recurrence is more often the case than not [1,2]. Indeed, the past three decades spent on understanding the oncogenic pathways that fuel malignant transformation and proliferation have certainly not led to significant gains in the clinics for patients with this dismal diagnosis. One such pathway that has been relatively under-studied is the WNT signaling pathway.
Typically, the deregulation of canonical Wnt signaling pathway genes (β-catenin and Axin) are implicated in the tumorigenesis at various sites including the colon and pancreas [1,3,4]. Mutations in APC (Adenopolypous coli) and β-catenin genes have emerged as potential reasons for the aberrant activation of this pathway in colorectal and liver cancers [5–7]. According to the classical Wnt signaling pathway, the transcription regulator β-catenin is targeted for degradation by a complex formed by GSK-3β and APC. On the contrary, upregulation of WNT and frizzled (FZD) receptor inhibits the formation of destruction complex and β-catenin degradation. As a result, β-catenin translocates in to the nucleus and binds with T-cell factor (TCF) and lymphoid enhancer factor (LEF) transcriptional coactivators, thereby inducing the transcription of TCF/LEF1 responsive genes e.g. cyclin D1 and c-Myc genes. The transactivation of TCF target genes induced by Wnt/β-catenin pathway constitutes the primary transforming event in colorectal cancer (CRC) [8]. The inhibition of β-catenin reduces TCF transcriptional activity and induces apoptosis through Caspase 3 activation [9]. Interestingly, O-GlcNAc transferase (OGT) adds a single N-acetylglucosamine group to target proteins such as β-catenin using the activated donor UDP-GlcNAc, the end product of the hexosamine biosynthesis pathway [10]. Several other studies have also shown that levels of OGT and O-GlcNAcylation are upregulated in different cancers, including pancreatic cancer [11]. O-GlcNAcylation stabilizes β-catenin through direct competition with phosphorylation at threonine 41 and regulates the various physiological functions, including cell survival, cell cycle regulation and angiogenesis [12–14].
Triptolide, a diterpene epoxide from Chinese plant Tripterygium wilfordii has been shown to be effective against various malignancies, including cholangiocarcinoma [15], gastric cancer [16], osteosarcoma [17] and neuroblastoma through various mechanisms [18,19]. Moreover, triptolide also affects numerous prosurvival pathways such as HSP70 and NF-κB in a various cancers [12,20]. Besides its in-vitro efficacy in different cancers, triptolide has not made it to the clinics due to its insolubility in water. Recently, a water-soluble prodrug of triptolide (Minnelide), synthesized by the University of Minnesota has shown considerable promise in preclinical studies [21]. Its mechanism of action has been enigmatic as its surprising potency in killing cancer cells both in vitro and in vivo. Previously published data from our laboratory has suggested that a major mechanism may involve downregulation of OGT in mediating triptolide induced cell death [12].
Herein, we evaluated the effect of triptolide on β-catenin/Wnt signaling and its possible link through OGT. Our results demonstrate that triptolide inactivates β-catenin/Wnt pathway by inhibiting LRP6 phosphorylation and β-catenin-TCF/LEF1 signaling, which in turn downregulates the genes associated with cell proliferation and cell cycle, namely Cyclin D1 and c-Myc. Moreover, our results indicate that OGT may serve as an important link between triptolide mediated inhibition of β-catenin/Wnt signaling pathway in pancreatic cancer.
Materials and methods
Cell culture and treatments
The pancreatic cancer cell line MIA PaCa-2 (obtained from American Type Culture Collection) and The S2-VP10 cell lines [gift from Prof. D. Buschbaum, University of Alabama]. The BxPC-3, Capan-1 and Human pancreatic ductal epithelial cells were obtained from American Type Culture Collection. No authentication was done by the authors, but American Type Culture Collection authenticates using short tandem repeat profiling.
We used SMARTpool reagent from Dharmacon for OGT silencing. SiLRP6 and SiFZD1 were used from Cell Signaling Technology and Ambion, respectively. Transfections were done according to Banerjee et al., 2013 [12]. A rescue experiment for silencing was done by transiently transfecting pancreatic cancer lines with Recombinant (r) human LRP6 protein (R and D systems), rFZD (R and D systems) and rOGT.
Triptolide was used at a concentration of 100 nM for in vitro experiments. 10 μM MG132 (Calbiochem, San Diego, CA) was used to treat MIA PaCa-2 cells followed by the treatment of 100 nM triptolide for 12, 24 and 48 h treatments.
Quantitative real time PCR and western blotting
Primers used for WNT, FZD1, LRP6, AXIN2, GSK-3β, cyclin D, c-Myc expression analysis are listed in Supplementary Table1.
The antibodies against β-catenin (Abcam), p-β-catenin, APC (Santa Cruz), GSK3-beta, LRP6, p-LRP6 (abcam), AXIN2, anti β-TrCP, DSH, c-Myc, Cyclin D, OGT, TCF-1, α-TUBULIN (cell signaling), TBP (Abcam) and PCNA (cell signaling) were used in different fractions of cell (membrane, cytosol and nuclear) and tumor lysates.
TCF/LEF reporter assay
For β-catenin-TCF/LEF reporter assay MiaPaCa-2 cells were transfected with Tcf/Lef reporter plasmid (SA biosciences) for 1, 3, 6, 8, 12, 18, 24 and 48 h. Luciferase activity was quantified and standardized as described in Promega, dual luciferase reporter assay system.
ChIP (chromatin immunoprecipitation) assay
Tumors from six months old KPC mice and MIA PaCa-2 cell lines were treated with 0.21 mg/kg/day Minnelide and 100 nM Tpl fro 12 and 24 h respectively. ChIP was performed by using standard protocol of Pierce Agarose Chip kit (Thermo Fisher scientific). Anti-β-catenin antibody (Chip grade, Millipore) was used to immunoprecipitate the β-catenin binding regions. The ChIP results were analyzed with quantitative real time PCR by using SP5 and c-Myc promoter region primers from Millipore.
Cell viability assay
Cell viability assays followed by siRNA and triptolide treatment were determined with the Dojindo Cell Counting Kit-8 (Dojindo Molecular Technologies).
Immunoprecipitation assay
Triptolide treated Mia-PaCa2 cells lysates were incubated with anti-GSK-3β for overnight. Immunoprecipitated complex was captured by agarose beads protein G (Roche). Samples were further immunoblotted with GSK-3β, APC, β-TrCP, p-β-catenin antibodies.
Immunofluorescence and confocal microscopy
siOGT (10 nM) treated Mia-PaCa-2 cells were fixed and permeabilized. On the other hand, tumors sections from control and Minnelide treated mice was deparaffinized in xylene and hydrated through graded ethanol. Immunofluorescence images were obtained on a Nikon Eclipse Ti confocal microscope using a 100× oil immersion objective.
Animal experiments
All animal experiments were performed according to the University of Minnesota Animal Care Committee guidelines. Cre, three and six months old genetically engineered KPC (KrasG12D, P53R172HPDXCre) mice were used to study the Wnt pathway during the progression of pancreatic cancer. 0.21 mg Minnelide/kg body weight was administered intraperitoneally (IP) every day for 30 days in KPC mice. Animals were age matched in both treatment and saline group. At the end of experiment (Day 30), mice were sacrificed; tumors and pancreas were collected from each mouse, weighed and snap frozen in liquid nitrogen.
STIM (syngenic tumor implantation model)
Isolation of tumors and tumor implantation was done according to Majumder et al., 2015 [22]. After two weeks of tumor implantation, mice were treated with 0.21 mg/kg/day Minnelide by IP injection. Mice were euthanized after 6 weeks of treatment and their tumor weights and volumes were compared in Saline and Minnelide treated mice.
Statistical analysis
Values are expressed as mean ± SEM. All the experiments are performed n = 3. The significant difference between any two samples was analyzed by unpaired student t-test. Values of p < 0.05 were considered as statistically significant.
Results
Wnt pathway is up-regulated in both human and mouse pancreatic cancer cells
Comparative qRT-PCR analysis of pancreatic cancer cells lines (Capan-1, Mia PaCa-2, S2-VP10 and BxPC-3) with HPDEC (normal human pancreatic cell line) showed over-expression of Wnt/β-catenin pathway transcripts (Wnt1, Fzd1, Lrp6, Axin2, Gsk-3β and β-catenin) in MIA PaCa-2 and S2-VP10 pancreatic cancer lines suggesting activation of Wnt/β-catenin pathway during pancreatic tumorigenesis (Fig. 1A). Furthermore we saw increased nuclear transport of β-catenin in pancreatic cancer lines to support this observation (Fig. 1B).
Fig. 1.
Evidence of activation of WNT/β-catenin pathway in human and mice model of pancreatic cancer. (A) q-RT PCR analysis of Wnt pathway genes in pancreatic cancer cell lines. (B) Nuclear transport of β-catenin in various human pancreatic cancer cell lines along with HPDEC. (C) Expressional dynamics of Wnt pathway genes during onset and progression of pancreatic cancer in KPC mice (cre, three and six months old) by q-RT PCR. (D) Immunoblotting of β-catenin in cytoplasmic and nuclear protein fractions during the onset and progression of tumor as compared with control cre mice. (E) Immunofluorescence showing the β-catenin expression during the progression of pancreatic tumor. Experiments were performed n = 3 and similar results were obtained each time.
To address if Wnt pathway is involved in progression of pancreatic cancer, animals of control (mice expressing Pdx driven Cre recombinase), three months (3M) and six months (6M) old age KPC were used for the experiments. q-RT-PCR analysis showed an increase in expression of the Wnt pathway players (Wnt1, Fzd1, Lrp6, Axin2, Gsk-3β and β-catenin) in three months and six month old animals indicating a correlation of activation of Wnt pathway with tumor progression (Fig.1C). Increased nuclear expression of β-catenin protein in three and six months old KPC mice was visualized by western blotting as well and microscopy further corroborated above findings (Fig. 1D and E).
Concerted actions of LRP6 and FZD1 are required for the nuclear transport of β-catenin in pancreatic cancer cell lines
LRP6 and FZD1 are receptors and key mediators of Wnt signaling, therefore we next investigated how these proteins regulate β-catenin in pancreatic cancer. Inhibiting LRP6 and FZD1 genes using siRNA reduced the transcription of β-catenin gene (Fig. 2A). The reduction was more effective when both (LRP6 and FZD) silencing RNAs were used in the culture at the same time. Restoration of β-catenin levels upon treatment with recombinant rLRP6 and rFZD1 plasmids substantiated this observation (Fig. 2A). Furthermore, maximum reduction in the nuclear expression of β-catenin protein and TCF/LEF1 activity was detected only in cultures treated with both of the siRNAs (Fig. 2B and C). TCF/LEF1 downstream genes such as cyclin-D1 and c-Myc were also affected after silencing of LRP6 and FZD in MIA Paca-2 (Fig. 2D) and S2-VP10 cell line (Supplemtery Fig. 4). Moreover, the viability of MIA PaCa-2 (Fig. 2E) and S2-VP10 (Fig. 2F) was decreased to 40% of control upon inhibition of LRP6 and FZD1 genes. Overall, these results indicate that inhibition of LRP6 and FZD1 is associated with reduced β-catenin-TCF/LEF transcriptional activity and hence, reduced the cell proliferation.
Fig. 2.
Effect of inhibition of LRP6 and FZD1 on β-catenin-TCF signaling. (A) Transcriptional changes in β-catenin after treating MIA PaCa-2 and S2-VP10 cell lines with SiLRP6, SiFZD1 and SiLRP6+SiFZD1 for 48 h and overexpression of recombinant rLRP6 and rFZD1 plasmids for 24 h. (B) MIA PaCa-2 and S2-VP10 cytoplasmic and nuclear fractions were isolated using cytoplasmic and nuclear extraction kit. Different fractions of MIA PaCa and S2-VP10 cells were immunoblotted and probed with β-catenin antibody. (C) MIA PaCa-2 and S2-VP10 cells were transfected with TCF/LEF reporter plasmid for 18 h and treated with SiLRP6, SiFZD1 and SiLRP6+SiFZD1 for 48 h, inhibition of LRP6 and FZD were recovered using rLRP6 and rFZD1 plasmids for 24 h, luciferase activity was counted with Promega kit. (D) Expression of Cyclin D1 and c-Myc by q-RT PCR upon LRP6 and FZD silencing in MIA PaCa-2 and S2-VP10 (Supplementary Fig. 4) cell line. (E) Viability of S2-VP10 cells were measured with CCK8 after 48 h and 72 h treatment of SiLRP6, SiFZD1 and SiLRP6+SiFZD1 and after that overexpression of recombinant rLRP6 and rFZD1 plasmids for 24 h. (F) Viability of MIA PaCa-2 by CCK8. Experiment was repeated three times with similar results obtained.
Triptolide treatment inhibits nuclear translocation of β-catenin
Triptolide, a diterpene triepoxide has shown promising results against pancreatic cancer. To evaluate its effect on the Wnt signaling pathway, we treated pancreatic cancer cells with triptolide and evaluated its effect on β-catenin expression and translocation to nucleus. Our data showed that β-catenin transcripts were not affected by triptolide treatment (12, 24 and 24 h) in MIA PaCa-2 and S2-VP10 cell lines (Fig. 3A), however, its nuclear transport was inhibited by triptolide in time dependent manner (Fig. 3B).
Fig. 3.
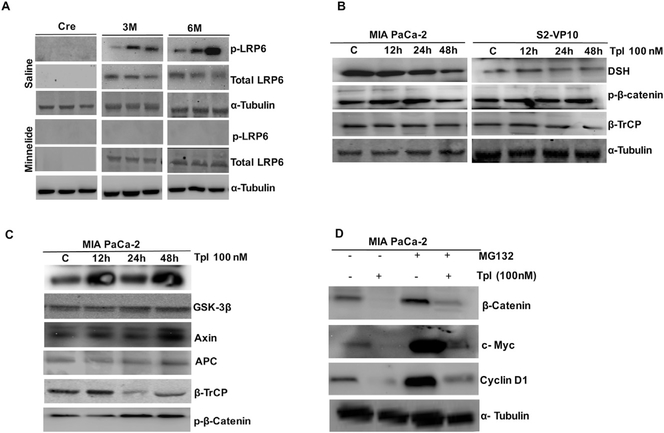
Effect of Minnelide on β-catenin/wnt pathway during the progression of pancreatic cancer. (A) MIA PaCa-2 and S2-VP10 cells were treated with 100 nM triptolide for 12, 24 and 48 h and checked the transcriptional level of β-catenin by q-RT-PCR. (B) MIA PaCa-2 and S2-VP10 cells were treated with 100 nM triptolide for 12, 24 and 48 h and their cytoplasmic and nuclear fractions were isolated and immunoblotted to check their β-catenin transport. (C) 0.21 mg/kg/day Minnelide was injected in three and six months old KPC mice and expressional dynamics of β-catenin/wnt pathway genes was studies in comparison to control cre mice. (D) Cytoplasmic and nuclear cell lysates from three and six months old KPC mice were immunoblotted and probed with anti-β-catenin. (E) Tumors from control and three and six old Minnelide treated mice were immunostained with β-catenin (green) antibody and DAPI (blue) and visualized under confocal microscope (Olympus Inc.). C: control (cre), 3M: 3 months, 6M: 6 months, Minn: Minnelide.
In vivo, Minnelide treatment reduced the RNA levels of Wnt and Fzd1, Gsk-3β, Axin2 while the Lrp6 and β-catenin transcript levels were found to be unaffected (Fig. 3C). Furthermore, Minnelide treatment also inhibited the nuclear transport of β-catenin in the tumors obtained from three month and six month old KPC mice as observed by western blotting (Fig. 3D) and confirmed by immunofluorescence (Fig. 3E).
Minnelide inhibits the LRP6 phosphorylation and increases the β-catenin degradation: plausible mechanism
Activation of β-catenin-Wnt pathway involves LRP6 phosphorylation, which in turn leads to the translocation of β-catenin in to the nucleus [23]. Our results showed increased LRP6 phosphorylation (ser1490) in three and six month old KPC mice derived tumors (Fig. 4A). Interestingly, Minnelide treatment completely abolished LRP6 phosphorylation (ser1490) (Fig. 4A). In line with this finding, triptolide treatment also inhibited LRP6 phosphorylation in MIA PaCa-2 cells at 12 h (Supplementary Fig. 1). Also, triptolide treatment in MIA PaCa-2 and S2-VP10 reduced the protein levels of DSH and increased phosphorylated β-catenin and β-TrCP levels in a time dependent manner (Fig. 4B). We further authenticated the formation of degradation complex by immunoprecipitation in MIA PaCa with anti GSK-3β. Triplotide treatment in these cells increased the interaction of APC, AXIN2 and β-TrCP with GSK-3β, thereby reflecting the degradation of β-catenin (Fig. 4C). These results suggest that triptolide treatment inhibits the expression of DSH, eventually trigger the formation of GSK-3β, APC, AXIN2 and β-TrCP complex thus, preventing the stabilization of β-catenin and activation of β-catenin-TCF transcription.
Fig. 4.
Effect of Minnelide and triptolide on β-catenin/Wnt signaling. (A) Effect of Minnelide on total LRP6 and phosphor (p) LRP6 protein levels during the tumor progression. (B) Protein levels of DSH, p-β-catenin, β-TrCP were compared at different time points of triptolide treatment in MIA PaCa-2 and S2-VP10 cell lines. (C) MIA PaCa-2 cells were treated with 100 nM triptolide for (12, 24 and 48 h) immunoprecipitated with GSK-3β and immunoblotted with GSK-3β, Axin2, APC, β-TrCP and p-β-catenin antibodies, respectively. (D) MIA PaCa-2 cells were treated with 10 μM MG-132 followed by the treatment of 100 nM triptolide for 48 h and immunoblotted with β-catenin, c-Myc and Cyclin D1. The experiment was repeated two times with similar results obtained.
In order to see the effect of triptolide on proteasomal mediated degradation and subsequent stability of β-catenin, we pretreated MIA PaCa-2 cells with MG132 to block β-catenin proteosomal degradation, which resulted in increased expression of downstream genes Cyclin D1, c-Myc (Fig. 4D). This observation implies that triptolide treatment increases the proteosomal degradation of β-catenin and inhibits downstream signaling pathway.
Triptolide disrupts β-catenin-TCF binding and downregualtion of its downstream genes in nucleus
β-Catenin does not bind to the DNA but directs TCF/LEF1 to regulate the genes responsible for cell proliferation. We performed a ChIP assay on 100 nM triptolide treated MIA PaCa-2 cells and our results showed that promoter binding activity of β-catenin with SP5 and c-Myc was decreased at 24 h (Supplementery Fig. 2). As seen in the ChIP assay, triptolide treatment in MIA PaCa-2 and S2-VP10 also decreased the TCF/LEF1 reporter activity at 24 h (Fig. 5A). Interestingly, nuclear transport of TCF-1 remained unaffected with triptolide treatment till 24 h (Fig. 5B). Consistent with this, we also observed downregulation of downstream genes such as c-Myc and Cyclin D1 in MIA PaCa-2 and S2-VP10 at 48 h of triptolide treatment that corroborated our above findings (Fig. 5C).
Fig. 5.
Effect of Minnelide on binding of β-catenin with TCF/LEF and downstream elements. (A) MIA PaCa and S2-VP10 cells were transfected with TCF/LEF1 reporter plasmid for 18 h and treated with 100 nM triptolide for 24 h, luciferase activity was measured. (B) Cytoplasmic and nuclear protein levels of TCF-1 were done in 100 nM treated S2-VP10 and MIA PaCa-2 cells. (C) S2-VP10 and MIA PaCa-2 cells were treated with 100 nM triptolide for different time points and performed western blot for Cyclin D1 and c-Myc. (D and E) ChIP assay showing the binding of β-catenin with c-Myc and SP5 promoter of TCF/LEF in control and six months old Minnelide treated group. ChIP results were validated by q-PCR. (F) Cyclin D1 and c-Myc RNA expression was measured in cre, three and six months old KPC mice as compared to control cre mice.
In vivo, Minnelide also inhibited the binding of β-catenin at the promoter region of SP5 and c-Myc in six months old KPC mice as compared to untreated (Fig. 5D and E). In addition, Minnelide further decreased expression of cell cycle genes such as Cyclin D1 and c-Myc (Fig. 4F).
Triptolide inhibits β-catenin/TCF-1 signaling through OGT
Our laboratory showed that triptolide treatment downregulates OGT (12). To show if triptolide treatment attenuates β-catenin-TCF1 signaling through downregulation of OGT, MIA-PaCa cells were pretreated with 10 nM siOGT for 48 h, immunoprecipitated and developed the blot with anti-O-GlcNAc antibody (Fig. 6A). The O-GlcNac modification at β-catenin was decreased in siOGT and triptolide treated samples as compared to controls. Our findings suggest that β-catenin is modified by O-GlcNAc addition and triptolide altered the O-GlcNAc levels of β-catenin by inhibiting its glycosylation levels.
Fig. 6.
Effect of inhibition of OGT on β-catenin/Wnt signaling. (A) Immunoprecipitation showing β-catenin was glycosylated with O-GlcNAc modification and reduced after silencing of OGT. S: soluble fraction, IP: immunoprecipitated pellet. (B) Immunoblotting showing the β-catenin transport from cytoplasm to nucleus after silencing the OGT for 24 h. (C) Immunofluorescence showing the reduced transport of β-catenin after inhibition of OGT as compared to control in MIA PaCa-2 cells. (D) Overexpression of recombinant (r) OGT abrogates triptolide mediated inhibition of nuclear transport of β-catenin. (E) MIA PaCA-2 cells showing the reduced TCF/LEF1 reporter activity after inhibition of SiOGT as compared to control. (F) Immunoblotting showing the expression of Cyclin D1 and c-Myc after inhibition of OGT as compared to controls.
β-Catenin translocation in to nucleus was also affected with OGT inhibition in MIA PaCa-2 cancer cells at 24 h, as observed by western blotting (Fig. 6B) and immunofluorescence (Fig. 6C). To confirm triptolide mediated inhibition of β-catenin translocation is indeed through OGT, we overexpressed OGT in MIA PaCa-2 cells for 24 h. Overexpression of OGT in triptolide treated cells restored the β-catenin translocation in nucleus inhibited by triptolide (Fig. 6D). Furthermore, OGT inhibition reduced TCF/LEF1 reporter activity and their downstream genes such as Cyclin D1 and c-Myc (Fig. 6E and F). This indicates the inhibition of OGT interferes in β-catenin nuclear transport, which eventually suppresses the expression of TCF/LEF1 mediated downstream genes.
Minnelide inhibits the growth of syngenic pancreatic tumor by inhibiting β-catenin-Wnt signaling
Minnelide substantially reduced the tumor weight (Fig. 7A) and tumor volume (Fig. 7B) at endpoint in STIM mice. Furthermore, Minnelide treatment downregulated the expression of Wnt, Fzd, Gsk-3β and Axin however, expression of LRP6 and β-catenin remained unchanged (Fig. 7C). Minnelide treatment also inhibited nuclear translocation of β-catenin as compared to control (Fig. 7D). Our results demonstrated that Minnelide treatment inhibited TCF-1, Cyclin D, c-Myc, OGT levels while at the same time showed increased cleavage of caspase-3 in Minnelide treated STIM mice when compared to controls (Fig. 7E). Interestingly, Minnelide treatment reduced the interaction of β-catenin and TCF interaction in the nucleus (Fig. 7F). These observations clearly indicate that suppression of tumor growth with Minnelide was indeed associated with in vivo inhibition of β-catenin/Wnt pathway.
Fig. 7.
Minnelide inhibits the growth of STIM pancreatic tumor by inhibiting β-catenin/Wnt signaling. (A) Comparing tumor weights in control and 0.21 mg/kg Minnelide treated groups of STIM model. (B) Minnelide treated and untreated groups were compared for their tumor volumes. (C) q-RT PCR showing the expressional dynamics of β-catenin/Wnt pathway genes in six weeks of STIM model. (D) Tumors from control and treated group were homogenized and fractionated in to membrane, cytosol and nucleus lysates, subjected to immunoblotting by using anti-β-catenin. (E) Representative blots showed the effect of Minnelide on protein levels of TCF-1, Cyclin D1, c-Myc, GSK-3β, Cl-caspase-3 and OGT. (F) Immunofluorescence showing the interaction of β-catenin and TCF-1 in the nucleus in both control and Minnelide treated group.
Discussion
Aberrant activation of Wnt/β-catenin pathway proteins have been suggested to be involved in various human cancers including pancreatic cancer [2,24,25]. In recent years, drug discovery platforms have facilitated several new agents, gaining attention in directly targeting the β-catenin and its downstream partners [TCF/LEF1] to improve target therapy of cancer [3].
Pancreatic cancer is a devastating disease with no known therapy that can significantly improve survival. Over last 10 years, our group has been investigating water-soluble analog of triptolide (a diterpene triepoxide derived from a Chinese herb). Owing to its limited solubility, we have also developed a water soluble prodrug of triptolide, Minnelide [21]. Minnelide is currently under Phase I clinical trial for GI malignancies and is showing promising results. Even though significant advances have been made in order to understand the mechanism of action of Minnelide, we still do not have a full understanding of the pathways affected by this compound. Our early reports demonstrated that Minnelide mediated inhibition of pancreatic cancer cells was associated with reduced expression of HSP70 and NFκB [26–28]. In this study we have evaluated the effect of Minnelide/triptolide on Wnt signaling pathway. Our current observation showed that triptolide and its prodrug Minnelide inhibited the β-catenin/Wnt signaling, thereby inhibiting downstream transcriptional genes cyclin D1 and c-Myc, which are important for cell cycle and cell proliferation. Furthermore, Minnelide treatment decreased the transport of β-catenin and its interaction with TCF/LEF1, which is important to activate the transcription of cell proliferation genes as well as increased cleaved caspase 3 levels indicating apoptotic cell death in these tumor cells. Moreover, Minnelide mediated inhibition of pancreatic tumor growth was indeed associated with downregulation of β-catenin/Wnt signaling.
Previous published reports showed the downregulation of oncogene β-catenin by several molecules inhibits the growth of breast and colon cancer cells [29,30]. In agreement with the above studies, our results also indicate that triptolide inhibited the expression of DvI-1, which in turns activated the Axin/GSK-3β/APC destruction complex, increased phosphorylation of β-catenin, inhibited its interaction with TCF/LEF1 and eventually reduced the expression of cell cycle and proliferation genes. Recent literature also shows that aberrant activation of Wnt/β-catenin signaling inhibits the activation of GSK-3β, which leads to the accumulation of β-catenin in the cytolplasm and activates the transcription of β-catenin-TCF mediated cell cycle and proliferation genes [12,31]. Another study showed that AV-65, a novel Wnt/β-catenin inhibitor reduced expression of downstream genes such as c-Myc and cyclin D1 and eventually growth of myeloma cells [31]. Our findings are in conformity with these observations as Minnelide treatment inhibited the interaction of β-catenin with TCF-1, further downregulating the expression levels of c-Myc and cyclin D-1 and leading to apoptosis in pancreatic cancer cells.
LRP6 phosphorylation has been shown as a major event in activating β-catenin mediated Wnt signaling in colorectal and other cancers [32,33]. A natural polyphenol compound, Rottlerin was shown to decrease LRP6 expression and its phosphorylation in prostate and breast cancer cell lines. Similarly, our study also showed that triptolide (as well as Minnelide in vivo) inhibited the phosphorylation of LRP6, even if the expression of LRP6 did not change. Phosphorylation levels of LRP6 in KPC mice during disease progression were also linked to the transport of β-catenin into the nucleus. Importantly, silencing of LRP6 and FZD1 highlighted the potential concerted actions of these mediators towards the expression and nuclear transport of β-catenin, evident by increased TCF/LEF activity and concomitant upregulation of c-myc and cyclin D. Mutations in various components of Wnt pathway like in APC, AXIN2 and β-catenin has been shown to lead to aberrant activation of β-catenin levels in the nucleus [34–36]. These mutational changes prevent β-catenin phosphorylation and its proteosomal degradation. Our recent in-vitro findings demonstated that triptolide activated the components of β-catenin destruction complex by enhancing the proteosomal degradation of β-catenin.
O-GlcNAc transferase (OGT) adds a single N-acetylglucosamine group to target proteins using the activated donor UDP-GlcNAc, an end product of the hexosamine biosynthesis pathway (10). OGT has been shown to be overexpressed in many types of cancer such as colon cancer and breast cancer [13,37]. Published reports showed the OGT targets β-catenin in colon cancer [13]. Interestingly, few studies have talked about how the overexpression of OGT in cancer regulates β-catenin translocation [38]. Previously, our lab showed that triptolide treatment inhibited the OGT transcript, protein levels and decreased total O-GlcNAc in human pancreatic cancer cell lines [12]. We found that reduced OGT levels inhibited the ingress of β-catenin in the nucleus and interestingly overexpression of OGT was able to rescue triptolide mediated inhibition of β-catenin in MIA PaCa-2 cell lines. This confirmed that triptolide mediated inhibition of β-catenin translocation in nucleus is probably through OGT. It can be summarized that triptolide affects O-GlcNAc modification of β-catenin, preventing its translocation in to nucleus. This results in reduced interaction of β-catenin with TCF/LEF1 and further downstream molecules such as cyclin D1 and c-Myc. In addition, published reports showed the role of GlcNAcylation in promoting breast cancer malignancy [37]. Our results imply that OGT regulates β-catenin/TCF-LEF1 signaling in promoting pancreatic tumor progression. This was consistent with the findings that administration of Minnelide suppressed the growth of pancreatic tumors in STIM mice. Tumors derived from Minnelide treated mice showed reduced levels of TCF-1, cyclin D1, c-Myc and OGT as well as increased cleaved caspase 3. We have summarized these findings in Supplementary Fig. 3 with regards to the mechanism of action of triptolide in mediating cell death via the Wnt/β-Catenin pathway.
In conclusion, our study shows that triptolide and Minnelide mediated inhibition of β-catenin/Wnt signaling in pancreatic cancer results in apoptosis which is modulated via OGT.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Sundaram Ramakrishnan for his valuable suggestions and comments in development of this work.
Dr. Nipun Merchant, Michael VanSaun and Fanuel Messaggio helped in revising the manuscript.
Financial support
National Institutes of Health (NIH) grant CA124723 and CA170946 (to A. Saluja) and Minneamrita Therapeutics, LLC (to A. Saluja).
Footnotes
Disclosures
The University of Minnesota has a patent for Minnelide™ (which has been licensed to Minneamrita Therapeutics LLC, Moline, IL). AS has ownership interests (including patents) and is a consultant/advisory board member for Minneamrita Therapeutics LLC. SB is a consultant for Minneamrita Therapeutics. This relationship is managed by University of Miami according to its conflict of interest policy. The other authors have nothing to disclose.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.canlet.2016.11.026.
References
- [1].Zeng G, Germinaro M, Micsenyi A, Monga NK, Bell A, Sood A, et al. , Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma, Neoplasia 8 (2006) 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang L, Heidt DG, Lee CJ, Yang H, Logsdon CD, Zhang L, et al. , Oncogenic function of ATDC in pancreatic cancer through Wnt pathway activation and beta-catenin stabilization, Cancer Cell 15 (2009) 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pramanik KC, Fofaria NM, Gupta P, Ranjan A, Kim SH, Srivastava SK, Inhibition of beta-catenin signaling suppresses pancreatic tumor growth by disrupting nuclear beta-catenin/TCF-1 complex: critical role of STAT-3, Oncotarget 6 (2015) 11561–11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Heiser PW, Cano DA, Landsman L, Kim GE, Kench JG, Klimstra DS, et al. , Stabilization of beta-catenin induces pancreas tumor formation, Gastroenterology 135 (2008) 1288–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wu ZQ, Brabletz T, Fearon E, Willis AL, Hu CY, Li XY, et al. , Canonical Wnt suppressor, Axin2, promotes colon carcinoma oncogenic activity, Proc. Natl. Acad. Sci. U S A. 109 (2012) 11312–11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cairo S, Armengol C, De Reynies A, Wei Y, Thomas E, Renard CA, et al. , Hepatic stem-like phenotype and interplay of Wnt/beta-catenin and Myc signaling in aggressive childhood liver cancer, Cancer Cell 14 (2008) 471–484. [DOI] [PubMed] [Google Scholar]
- [7].Segditsas S, Tomlinson I, Colorectal cancer and genetic alterations in the Wnt pathway, Oncogene 25 (2006) 7531–7537. [DOI] [PubMed] [Google Scholar]
- [8].van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, et al. , The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells, Cell 111 (2002) 241–250. [DOI] [PubMed] [Google Scholar]
- [9].Veeramachaneni NK, Kubokura H, Lin L, Pippin JA, Patterson GA, Drebin JA, et al. , Down-regulation of beta catenin inhibits the growth of esophageal carcinoma cells, J. Thorac. Cardiovasc. Surg. 127 (2004) 92–98. [DOI] [PubMed] [Google Scholar]
- [10].Sclabas GM, Uwagawa T, Schmidt C, Hess KR, Evans DB, Abbruzzese JL, et al. , Nuclear factor kappa B activation is a potential target for preventing pancreatic carcinoma by aspirin, Cancer 103 (2005) 2485–2490. [DOI] [PubMed] [Google Scholar]
- [11].Fardini Y, Dehennaut V, Lefebvre T, Issad T, O-GlcNAcylation: a new cancer hallmark? Front. Endocrinol. (Lausanne) 4 (2013) 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Banerjee S, Sangwan V, McGinn O, Chugh R, Dudeja V, Vickers SM, et al. , Triptolide-induced cell death in pancreatic cancer is mediated by O-GlcNAc modification of transcription factor Sp1, J. Biol. Chem. 288 (2013) 33927–33938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Olivier-Van Stichelen S, Dehennaut V, Buzy A, Zachayus JL, Guinez C, Mir AM, et al. , O-GlcNAcylation stabilizes beta-catenin through direct competition with phosphorylation at threonine 41, FASEB J. 28 (2014) 3325–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Olivier-Van Stichelen S, Drougat L, Dehennaut V, El Yazidi-Belkoura I, Guinez C, Mir AM, et al. , Serum-stimulated cell cycle entry promotes ncOGT synthesis required for cyclin D expression, Oncogenesis 1 (2012) e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tengchaisri T, Chawengkirttikul R, Rachaphaew N, Reutrakul V, Sangsuwan R, Sirisinha S, Antitumor activity of triptolide against cholangiocarcinoma growth in vitro and in hamsters, Cancer Lett. 133 (1998) 169–175. [DOI] [PubMed] [Google Scholar]
- [16].Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, et al. , Triptolide inhibits the growth and metastasis of solid tumors, Mol. Cancer Ther. 2 (2003) 65–72. [PubMed] [Google Scholar]
- [17].Banerjee S, Thayanithy V, Sangwan V, Mackenzie TN, Saluja AK, Subramanian S, Minnelide reduces tumor burden in preclinical models of osteosarcoma, Cancer Lett. 335 (2013) 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Antonoff MB, Chugh R, Borja-Cacho D, Dudeja V, Clawson KA, Skube SJ, et al. , Triptolide therapy for neuroblastoma decreases cell viability in vitro and inhibits tumor growth in vivo, Surgery 146 (2009) 282–290. [DOI] [PubMed] [Google Scholar]
- [19].Krosch TC, Sangwan V, Banerjee S, Mujumdar N, Dudeja V, Saluja AK, et al. , Triptolide-mediated cell death in neuroblastoma occurs by both apoptosis and autophagy pathways and results in inhibition of nuclear factor-kappa B activity, Am. J. Surg. 205 (2013) 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu L, Salnikov AV, Bauer N, Aleksandrowicz E, Labsch S, Nwaeburu C, et al. , Triptolide reverses hypoxia-induced epithelial-mesenchymal transition and stem-like features in pancreatic cancer by NF-kappaB downregulation, Int. J. Cancer 134 (2014) 2489–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, et al. , A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer,, Sci. Transl. Med. 4 (2012), 156ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Majumder K, Arora N, Modi S, Chugh R, Nomura A, Giri B, et al. , A novel immunocompetent mouse model of pancreatic cancer with robust stroma: a valuable tool for preclinical evaluation of new therapies, J. Gastrointest. Surg. 20 (2016) 53–65 discussion 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, et al. , Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions, Development 135 (2008) 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pasca di Magliano M, Biankin AV, Heiser PW, Cano DA, Gutierrez PJ, Deramaudt T, et al. , Common activation of canonical Wnt signaling in pancreatic adenocarcinoma, PLoS One 2 (2007) e1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang Y, Morris J.P.t., Yan W, Schofield HK, Gurney A, Simeone DM, et al. , Canonical Wnt signaling is required for pancreatic carcinogenesis, Cancer Res. 73 (2013) 4909–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dudeja V, Mujumdar N, Phillips P, Chugh R, Borja-Cacho D, Dawra RK, et al. , Heat shock protein 70 inhibits apoptosis in cancer cells through simultaneous and independent mechanisms, Gastroenterology 136 (2009) 1772–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, et al. , Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70, Cancer Res. 67 (2007) 9407–9416. [DOI] [PubMed] [Google Scholar]
- [28].Dakeng S, Duangmano S, Jiratchariyakul W, U.P. Y, Bogler O, Patmasiriwat P, Inhibition of Wnt signaling by cucurbitacin B in breast cancer cells: reduction of Wnt-associated proteins and reduced translocation of galectin-3-mediated beta-catenin to the nucleus, J. Cell Biochem. 113 (2012) 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Verma UN, Surabhi RM, Schmaltieg A, Becerra C, Gaynor RB, Small interfering RNAs directed against beta-catenin inhibit the in vitro and in vivo growth of colon cancer cells, Clin. Cancer Res. 9 (2003) 1291–1300. [PubMed] [Google Scholar]
- [30].Gan XQ, Wang JY, Xi Y, Wu ZL, Li YP, Li L, Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading to stabilization of beta-catenin-TCF interaction, J. Cell Biol. 180 (2008) 1087–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yao H, Ashihara E, Strovel JW, Nakagawa Y, Kuroda J, Nagao R, et al. , AV-65, a novel Wnt/beta-catenin signal inhibitor, successfully suppresses progression of multiple myeloma in a mouse model, Blood Cancer J. 1 (2011) e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Khan Z, Vijayakumar S, de la Torre TV, Rotolo S, Bafico A, Analysis of endogenous LRP6 function reveals a novel feedback mechanism by which Wnt negatively regulates its receptor, Mol. Cell Biol. 27 (2007) 7291–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lu W, Lin C, Li Y, Rottlerin induces Wnt co-receptor LRP6 degradation and suppresses both Wnt/beta-catenin and mTORC1 signaling in prostate and breast cancer cells, Cell Signal 26 (2014) 1303–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu Y, Patel L, Mills GB, Lu KH, Sood AK, Ding L, et al. , Clinical significance of CTNNB1 mutation and Wnt pathway activation in endometrioid endometrial carcinoma, J. Natl. Cancer Inst. 106 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Polakis P, Wnt signaling and cancer, Genes Dev. 14 (2000) 1837–1851. [PubMed] [Google Scholar]
- [36].Hajra KM, Fearon ER, Cadherin and catenin alterations in human cancer, Genes Chromosom. Cancer 34 (2002) 255–268. [DOI] [PubMed] [Google Scholar]
- [37].Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C, et al. , GlcNAcylation plays an essential role in breast cancer metastasis, Cancer Res. 70 (2010) 6344–6351. [DOI] [PubMed] [Google Scholar]
- [38].Sayat R, Leber B, Grubac V, Wiltshire L, Persad S, O-GlcNAc-glycosylation of beta-catenin regulates its nuclear localization and transcriptional activity, Exp. Cell Res. 314 (2008) 2774–2787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.