Abstract
Aims
Periprosthetic joint infections (PJIs) and osteomyelitis are clinical challenges that are difficult to eradicate. Well-characterized large animal models necessary for testing and validating new treatment strategies for these conditions are lacking. The purpose of this study was to develop a rabbit model of chronic PJI in the distal femur.
Methods
Fresh suspensions of Staphylococcus aureus (ATCC 25923) were prepared in phosphate-buffered saline (PBS) (1 × 109 colony-forming units (CFUs)/ml). Periprosthetic osteomyelitis in female New Zealand white rabbits was induced by intraosseous injection of planktonic bacterial suspension into a predrilled bone tunnel prior to implant screw placement, examined at five and 28 days (n = 5/group) after surgery, and compared to a control aseptic screw group. Radiographs were obtained weekly, and blood was collected to measure ESR, CRP, and white blood cell (WBC) counts. Bone samples and implanted screws were harvested on day 28, and processed for histological analysis and viability assay of bacteria, respectively.
Results
Intraosseous periprosthetic introduction of planktonic bacteria induced an acute rise in ESR and CRP that subsided by day 14, and resulted in radiologically evident periprosthetic osteolysis by day 28 accompanied by elevated WBC counts and histological evidence of bacteria in the bone tunnels after screw removal. The aseptic screw group induced no increase in ESR, and no lysis developed around the implants. Bacterial viability was confirmed by implant sonication fluid culture.
Conclusion
Intraosseous periprosthetic introduction of planktonic bacteria reliably induces survivable chronic PJI in rabbits.
Cite this article: Bone Joint Res 2021;10(3):156–165.
Keywords: Periprosthetic osteomyelitis, Rabbit model, Staphylococcus aureus, Femur implant, Low mortality rate
Article focus
Development of a periprosthetic osteomyelitis model in rabbit.
Key messages
This study reports successful development of periprosthetic osteomyelitis in a rabbit model.
Strengths and limitations
The reported model has many features in common with the clinical presentation of chronic periprosthetic joint infection (PJI), including an acute phase followed by subclinical persistence of infection.
The model suffers no mortality, is easily replicated, and can be used to test the safety and efficacy of various therapeutic treatments.
A limitation is the absence of several important clinical analyses routinely used to define PJI.
Introduction
The occurrence of infections that develop around orthopaedic implants is reported to be 1% to 5%,1 and are associated with significant morbidity, disability, and healthcare costs.2-8 Despite increased mechanistic understanding, improved diagnostic techniques, and treatment algorithms, periprosthetic joint infections (PJIs) remain a major challenge.9 Continued improvement in the prevention, diagnosis, and treatment of implant-related infections is critical.
Diagnosis of PJI, defined here as inflammation of bone caused by an infectious organism in the vicinity of hardware associated with a joint repair of arthroplasty that leads to progressive bone destruction and loss, is based on: 1) clinical history and examination; 2) laboratory test results, including leucocyte counts,10 ESR,10,11 and CRP10,11 or α-defensin levels; 3) diagnostic imaging; 4) microbiological analysis; and 5) histopathology.12 Clinically, the most common pathogen identified in cases of PJI is Staphylococcus aureus, 2,13 including methicillin-sensitive and methicillin-resistant strains.6 Once the pathogen is identified, the first line of treatment for many orthopaedic infections involves debridement of necrotic tissue, potential removal of hardware, and extensive irrigation and antibiotic treatment administered orally, intravenously, or locally via antibiotic beads.14,15 These infections include cases of PJI and osteomyelitis, which are highly resistant to antibiotic treatment due to the development of bacterial biofilms.16-19 As a result, repeated surgical debridement and prolonged use of systemic antibiotics are often required.20,21 Multiple surgical treatments and debridements increase the already high risk of poor functional outcomes, morbidity, and mortality.22-24 There is a need in orthopaedics to provide a lasting and less invasive form of antimicrobial prophylaxis and treatment for PJI.
To gain a better understanding of periprosthetic osteomyelitis development, several animal models have been employed, including rodents (mice and rats) and larger animals such as rabbits, pigs, goats, and dogs.6 Rodents and larger animals have unique advantages and disadvantages. The rabbit is the most popular model for the study of PJI because it is docile and affordable, and is large enough to both employ common orthopaedic procedures including hardware placement and heavy enough such that mechanical forces impact outcomes.25 In addition, rabbit bone and joint biology26 and immunology27,28 are similar to humans such that infection susceptibility, pathogenesis, and immune response28,29 are close to the human condition, which are important parameters when assessing biofilm formation and the efficacy of antimicrobial therapies for clinical use. The methods to model PJI have been variable in the literature, utilizing implants and methods unique to the hardware or treatment being tested and making comparison between studies difficult. Importantly, most studies focused on periprosthetic osteomyelitis development or antimicrobial treatment methods are short in time frame, and report mostly on final outcome in terms of bacterial load and bone degeneration, not disease progression and attaining a chronic state.
We were interested in developing a reliable model of chronic PJI that developed in a manner similar to the human condition, progressing from an acute to chronic state. A specific weakness of the rabbit model is its susceptibility to stress, duodenal ulcers, and secondary conditions that introduce confounding factors to the study of osteomyelitis. A challenge was then to deliver bacteria in a way that will reliably lead to chronic PJI development as observed in the clinic, but not create an infection so severe that the animals cannot survive for a long duration in the perioperative period.
The purpose of this study was to develop and characterize a rabbit model of chronic PJI using common radiological and clinical markers.
Methods
Ethical review committee statement
All experiments were performed at an accredited veterinary facility according to protocols approved by the local University of Pittsburgh Institutional Animal Care and Use Committee (IACUC; protocol 19024514) and the Defense Department Animal Care and Use Review Office (ACURO) of the USA Army Medical Research and Development Command (USAAMRDC; protocol DM140372), and according to the Animal Welfare act and the recommendations of the Office of Laboratory Animal Welfare (OLAW). The full ARRIVE guideline checklist was submitted to the journal in order to demonstrate compliance with ARRIVE guidelines.
Bacteria preparation
S. aureus (ATCC 25923; American Type Culture Collection, Manassas, Virginia, USA) was grown in tryptic soy broth (TSB; Neogen, Lansing, Michigan, USA) medium for 16 hours at 37°C, and bacterial concentrations were estimated spectrophotometrically, where an A600 of 1.0 corresponds to ∼6.6 × 108 colony-forming units (CFUs)/ml, as determined by a separate standard curve relating the A600 and CFU. Briefly, 1 ml of 16-hour bacterial culture was collected, centrifuged (5,000 rpm × three minutes), supernatant discarded, and the pellet resuspended in 1 ml of phosphate-buffered saline (PBS), from which 50 µl was removed and diluted with 950 µl PBS (required to place the optical density (OD) reading within the linear range of the spectrophotometer). The overnight bacterial culture was adjusted using PBS and 1 µl of 1 × 109 CFUs of S. aureus/ml was used in the experiment.
Postoperative animal care
After surgery, rabbits were singley housed in standard cages under standard conditions (provided sufficient space, controlled environment, and free access to food, water, and enrichment) in a Biosafety Level 2 (BSL2) facility and monitored twice a day for 28 days. For infected rabbits, body temperature and mass were measured once before surgery and treatments (antibiotics and/or analgesic) administered twice daily until animals stabilized and were deemed 'recovered' according to lameness, body condition, and behaviour, all monitored twice daily. Thereafter, temperature, mass, lameness, body condition, and behaviour were monitored every 48 hours. To avoid loss of animals due to weight loss (i.e. ≥ 20% in the course of the study), individualized diets supplemented with fresh vegetables, fruits, and Critical Care (Oxbow Animal Health, Omaha, Nebraska, USA) were provided, and forced feeding by facility technicians and authors (HY, SK, PNM, ADCMF, PGA) was initiated at 17% weight loss as necessary (one rabbit in the chronic PJI group was force-fed for one day).
Blood assays
The blood sample of each rabbit was collected from their ear vein at 3, 5, 7, 14, 21, and 28 days after surgery. Whole blood was collected in commercially available anticoagulant treated tubes. ESRs were determined using the DISPETTE 2 kit (Fisher Scientific, Cambridge, Massachusetts, USA) within 24 hours of blood collection. CRP levels were determined by removing cells from the plasma by centrifugation for 15 minutes at 2,000 xg and performing an enzyme-linked immunosorbent assay (ELISA) for rabbit CRP (PTX1) (Abcam, Cambridge, Massachusetts, USA) according to the manufacturer's instructions. CRP, ESR, and white blood cells (WBCs) are well known established markers for infections. In the early development of our model, highly elevated ESR and CRP levels were consistently observed between days 3 and 5, therefore we chose to measure WBC on days 5 and 28 (study endpoint). WBC counts and differentials were ascertained from a blood smear that was prepared on a standard ColorFrost Plus slide and stained with polychromic stains using the DipQuick stain kit (ThermoFisher, Waltham, Massachusetts, USA). After drying, WBCs were counted under a microscope (Olympus BH2 with a 100× oil immersion objective; Olympus America, Center Valley, Pennsylvania, USA) by an experienced veterinary technician (ADCMF).
Radiographs
Radiological images captured with a Fidex veterinary scanner (Animage, Pleasanton, California, USA) were obtained from rabbits weekly after surgery (five timepoints: 0, 7, 14, 21, and 28 days). At the end of the experimental period, both operative and contralateral nonoperative knees from each rabbit were harvested and X-rayed. A total of five radiographs per timepoint were evaluated independently, in blinded fashion, by two study members (SK and PGA) using a modified scoring scale originally reported by An and Friedman30 assessing: 1) periosteal reaction; 2) osteolysis; 3) deformity; 4) sequestrum formation; 5) spontaneous fracture; and 6) general impression. Parameters 1 to 4 were assessed for each sample on the following scale: 0 (absent), 1 (mild), 2 (moderate), and 3 (severe). Parameters 5 and 6 were judged with 0 (absent) or 1 (present). The maximum score to be achieved was 14 (4 parameter × 3 points = max. 12 points + parameters 6 and 7 = max. 2 points).31
Histology
Harvested knees were fixed in 10% formalin for five days at 4°C with shaking, and were transferred and stored in 70% ethanol. Before decalcification, screws were removed without disturbing the surrounding tissue. The condyles were separated from each other and the femoral shaft using a fine-toothed saw. Samples were then placed into 10% (v/v) neutral buffered paraformaldehyde (Electron Microscopy Sciences, Hatfield, Pennsylvania, USA) for an additional 24 hours. All samples were decalcified by submerging the tissue in Decal (StatLab, McKinney, Texas, USA) for 24 hours with gentle shaking at 4°C, changing the solution every three days for seven days. Samples were then dehydrated through a graded ethanol series, equilibrated in xylene, and embedded in paraffin. Samples were sectioned medial-to-lateral at 6 µm thickness to obtain anatomical sagittal sections perpendicular to the orientation of the screw. Sections from the superficial, middle, and deep regions of the bone tunnel were mounted on Superfrost Ultraplus O positively charged slides, deparaffinized with Histoclear (National Diagnostics, Atlanta, Georgia, USA), rehydrated, and stained by Harris Hematoxylin (Sigma-Aldrich, St. Louis, Missouri, USA) and Eosin Y (Leica Biosystems, Buffalo Grove, Illinois, USA) or a modified Gram’s method for bacteria32 (Sigma-Aldrich). These were then cover-slipped and digitally imaged using an Infinity3 CCD camera (Teledyne-Lumenera, Ottawa, Canada), mounted on an IX-81 microscope (Olympus) or a DP74 CCD camera (Olympus) mounted on a SZX16 microscope (Olympus), and processed with CelSens imaging software (Olympus).
Bacterial viability assay
To verify bacterial viability after 28 days in this model, a separate cohort of three rabbits was prepared. At 28 days, implanted screws were carefully removed in a sterile manner without disturbing the surrounding tissue and placed in a 1.5 ml plastic sample tube containing 1 ml of PBS. To confirm the viability and bioactivity of bacteria on the implant surface, the implanted screws were sonicated at 40 Hz for 20 minutes at room temperature (Model 3510; Branson Ultrasonics, Danbury, Connecticut, USA) and the sonicate collected. The eluent and the sonicate collected from the implanted screws were spread upon TSB agar plates and cultured for 16 hours at 37°C for colony counting. The viability of bacteria around the implant was confirmed by colony forming activity detected on bacterial culture plates. After 16 hours of culture, bacterial colonies were evident (Supplementary Figure a).
Statistical analysis
The statistical significance (p-values) of mean values in a two-sample comparison was determined using the paired t-test (Microsoft Excel; Microsoft, Redmond, Washington, USA). Data are presented as mean and SD. Using an α of 0.05 (confidence interval of 95%), statistical significance was set at p < 0.05.
Results
Vital signs and mortality
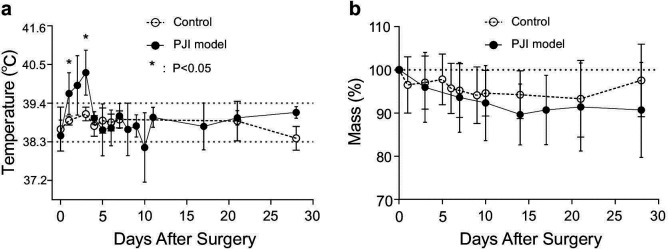
All rabbits in the chronic PJI model and control groups survived 28 days. Rabbits in the chronic PJI group had elevated temperatures (mean 40.3°C (SD 0.6)) compared to controls measured at a mean 39.1°C (SD 0.2) at 72 hours after surgery (a mean elevation of +1.2°C (SD 0.6); p < 0.05, paired t-test). Animals in the control group were never febrile (Figure 1a). Maximum weight loss in the chronic PJI group was 17% after surgery (Figure 1b). No mortality occurred in either experimental group.
Fig. 1.
Physiological markers of illness. a) Temperature and b) body mass of rabbits in the two groups of rabbits after surgery (n = 5/group). *p < 0.05 versus control aseptic group, paired t-test. PJI, periprosthetic joint infection.
Blood test results
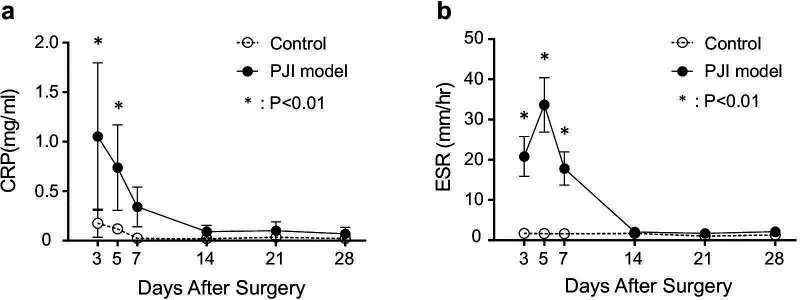
Rabbits in the chronic PJI group developed elevated CRP (Figure 2a) and ESR (Figure 2b) day 3 after surgery, which returned to normal by day 14. All animals in the control group had normal ESR values and slightly elevated CRP values that were significantly lower than osteomyelitis values.33,34 Taken together with the body mass and temperature profiles, these data indicate an early acute phase of the infection, lasting approximately 14 days, followed by a less symptomatic chronic phase. Laboratory values of WBC differentials revealed leukocytosis in the chronic PJI group. Total WBC counts were elevated on both day 5 and day 28 in the chronic PJI group over the control group (Figure 3), and timepoints chosen for mean height of infection (day 5) and end point analysis (pre-euthanasia, day 28). As shown in Table I, in comparison to the control group, rabbits in the chronic PJI group possessed abnormal WBC differentials, including increases in neutrophil count on day 5 and day 28 over control values, and elevated monocytes on day 5 and day 28 over controls that remained unchanged within normal ranges (Table I).35,36 Lymphocyte counts for the chronic PJI group remained in the normal range,35,36 but were significantly lower on day 5 and day 28 than controls in this experiment. These laboratory values indicated a persistent infection and inflammation in the inoculated animals.
Fig. 2.
Haematological evidence of inflammation. a) CRP and b) ESR values in the two groups of rabbits (n = 5/group; (n = 4)). *p < 0.01 versus control aseptic screw group, paired t-test. PJI, periprosthetic joint infection.
Fig. 3.
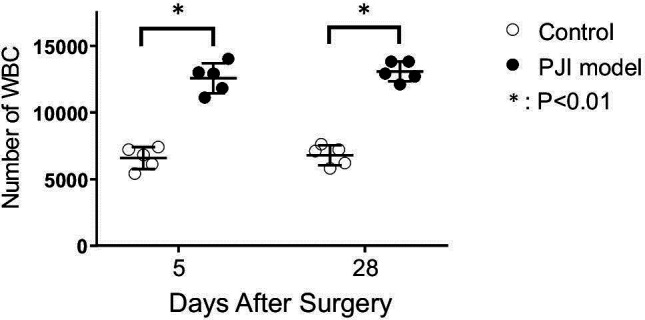
Haematological evidence of infection. White blood cell (WBC) counts in the two groups of rabbits (n = 5/group). *p < 0.01 versus control aseptic screw group, paired t-test. PJI, periprosthetic joint infection.
Table I.
Prevalence of selected white blood cells five and 28 days after surgery.
| Time point | Mean white blood cells count, % (SD) | ||||
|---|---|---|---|---|---|
| Neutrophils | Lymphocytes | Monocytes | Eosinophils | Band neutrophils | |
| Typical value34-36 | 34 to 43 | 39 to 68 | 1.0 to 9.0 | 0.1 to 2.0 | 0.0 |
| Day 5 | |||||
| Control | 32.0 (5.2) | 65.4 (4.6) | 2.4 (1.1) | 0.2 (0.4) | 0.0 |
| PJI model | 51.8 (5.6)* | 39.2 (4.0)* | 8.0 (2.9)* | 0.4 (0.9) | 0.4 (0.5) |
| Day 28 | |||||
| Control | 34.6 (5.6) | 62.0 (5.3) | 3.4 (1.8) | 0.0 | 0.0 |
| PJI model | 51.6 (2.7)* | 35.0 (5.3)* | 11.4 (2.9)* | 1.0 (0.7)* | 0.8 (0.8) |
p < 0.05 compared to control group on the corresponding day.
PJI, periprosthetic joint infection.
Radiographs
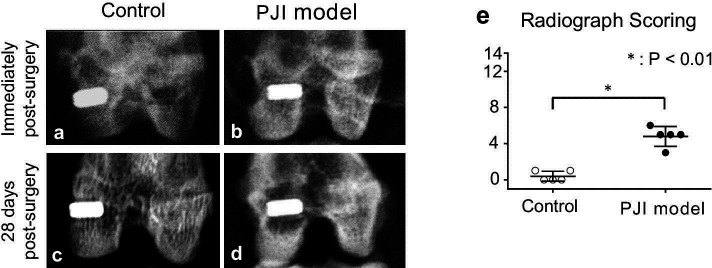
Postoperative radiographs revealed no fractures in the bone surrounding the implant (Figures 4a and 4b). After 28 days, no radiolucency or other radiological parameters of osteolysis were observed surrounding the bone tunnel in the control group (Figure 4c). In contrast, large areas of radiolucency were observed in 100% of the animals in the osteomyelitis group (Figure 4d) as compared to controls (Figure 4c). Qualitative assessment according to An and Friedman30 of the chronic PJI group showed a significantly higher score (p < 0.001, paired t-test) than that of the control group (Figure 4e).
Fig. 4.
Radiological evidence of osteomyelitis. Representative radiographs of rabbit knees and implants immediately after surgery (a and b) and 28 days after surgery (c and d) in the control aseptic screw group (a and c) and chronic periprosthetic joint infection (PJI) group (b and d). e) Radiological scoring of rabbits in the control and PJI groups at 28 days (n = 5/group). *p < 0.01 versus control aseptic screw group, paired t-test.
Histology
Haematoxylin and eosin staining revealed fibrous tissue lining the bone tunnel in the chronic PJI group, while normal trabecular bone structure and bone marrow were preserved in the control group (Figures 5a and 5b). Gram staining revealed no Gram-positive bacteria, such as S. aureus (Figures 5c and 5e), while the fibrous tissue in chronic PJI samples stained positively with Gram stain (Figure 5d). High magnification revealed Gram-positive particles 1 μm to 2 μm in diameter characteristic of S. aureus cells (Figure 5f), providing histological indication of biofilm formation in this model.
Fig. 5.
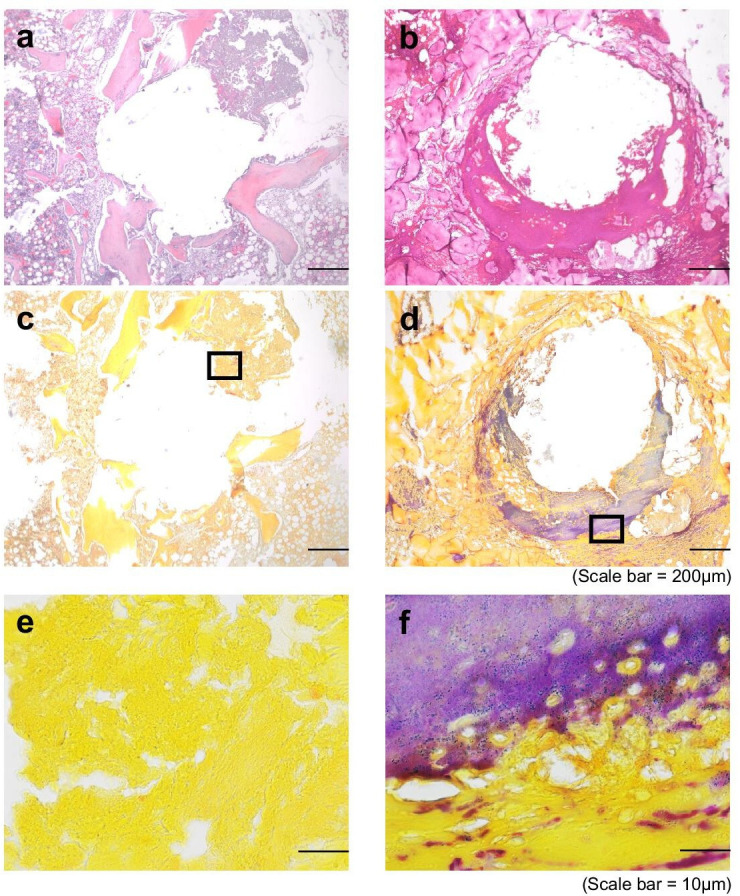
Histological evidence of osteomyelitis. Serial sections from a representative sample in the (a, c, e) control aseptic screw group and (b, d, f) chronic periprosthetic joint infection (PJI) group were stained either with haematoxylin and eosin (H&E) (a, b) and imaged at 40× (scale bar = 200 μm), or with Gram’s stain (c-f) imaged at 40× (c, d; scale bar = 200 μm) or 100× (e,f; scale bar = 10 μm).
Discussion
PJI is a large clinical challenge with limited solutions.37 Treatment of these infections has not changed or improved appreciably in the past several decades, reflecting the lack of consensus in PJI terminology, outcome evaluation, study design, and reporting as well as inadequate clinical relevance of current in vitro and in vivo models. It has been noted that well-characterized large animal models necessary for testing and validation of new treatment strategies for periprosthetic osteomyelitis are lacking.6 The purpose of this study was to develop a rabbit model of femoral chronic PJI in the distal femur that has characteristics of clinical, chronic PJI. Here, we report that intraosseous delivery of planktonic bacteria into a predrilled bone tunnel followed by headless screw insertion and bone wax sealing consistently results in survivable, chronic PJI in the rabbit model. In this model, there is an acute phase of infection in which the animal suffers high temperature, loss of appetite, and weight loss. These clinical values normalized after the first week of inoculation, a pattern that was reflected in the serum ESR and CRP values as well. Together these data suggest the ability of the immune system to control the infection.38 The blood test results also indicate leukocytosis, specifically increased total neutrophil and elevated monocyte counts.35,36 Reduction in lymphocyte percentage has been observed in other studies of leporine responses to infection.36 This leukocytosis and the detectable increase in immature neutrophils (in the rabbit model) indicate active infections.39 Radiography showed that by day 28, 100% of the animals in the experimental group suffered bone infection as defined by increased radiolucency around the implant, which is a hallmark of PJI.40-42 Histological analysis of explanted bone from the inoculated group revealed fibrous tissue formation within the bone tunnel that stained positively on Gram’s stain in serial sections, whereas healthy bone formation was observed in the aseptic screw group.32 The positive Gram’s stain was coincident with particles characteristic of bacteria in size. In addition, the viability of bacteria after 28 days of inoculation with a separate cohort was demonstrated using ex vivo bacterial culture. Together these results strongly suggest the evidence of PJI.
A variety of animal models have been used to study PJI and osteomyelitis in the experimental setting, including rodents (mice and rats) and large animals such as rabbits, pigs, goats, and dogs.6,43 In the literature, the rabbit is among the most frequently used models in the study of PJI and osteomyelitis. It is most frequently used to study therapeutic interventions for the treatment of acute and chronic infections. Readouts are frequently focused on survival, bone lesions, and healing through imaging techniques and bacterial culture at an endpoint.6,44 Interestingly, the study of PJI and osteomyelitis pathogenesis and biofim formation is most often performed in rodents, which are much more affordable to maintain, have well-characterized immune systems, and are amenable to a plethora of genetic and imaging tools. However, rodent models are limited by their small size and the presentation of the disease is very different than in humans. For example, rodents rarely become ill at the onset of PJI or osteomyelitis, often forming local abscesses as opposed to succumbing to sepsis.6,45 This difference in outcomes reflects fundamental differences in the immune responses between rodents, rabbits, and humans that may have significant consequences when developing treatment for clinical translation. Literature indicates that rabbits are preferred not just for size and affordability,6,25 but also for well-characterized immune response to infections, where rabbits are phylogenetically more closely related to humans than rodents.27,46 As the model chosen for this study will subsequently be used to test therapeutic interventions for chronic (and acute) PJI, the rabbit presented a sufficiently large, affordable, and physiologically similar system to humans for this and future studies.
In this study, we have characterized PJI disease progression in the rabbit over a four-week period, demonstrating that the animals suffer acute illness in the first week, characterized by high fever, weight loss, and elevated WBC, ESR, and CRP values, but become survivable with systemic antibiotic treatment. In the absence of antibiotics, the animals would have died. However, the antibiotics are not sufficient to resolve the infection, leading to periprosthetic osteomyelitis that was demonstrated by radiography and histology. This scenario suggests a subsequent subclinical, chronic phase, which is much more akin to illness observed with PJI and in the clinic.16,17 Thus, the data indicate that we have successfully developed a leporine model of chronic, periprosthetic osteomyelitis that has many of the same clinical outcomes observed in humans.
In the process of developing the periprosthetic osteomyelitis model reported here, other methods of induction were tested (Supplementary Material). Each cohort included five rabbits and all failed due to high mortality rate and excessive weight loss (over 20%) despite intensive care (i.e. antibiotics injection, force feeding, etc.) (Supplementary Table i and Supplementary Figure b). In our experience, intra-articular bacterial injection at concentrations of 100 μl of 2.0 × 107 CFUs/ml and 100 μl of 2.0 × 106 CFUs/ml caused 100% mortality. Our observations indicate that the animals suffered acute illness characteristic of sepsis (undiagnosed), and although it is possible that lower initial bacterial loads might have reduced the mortality rate, rabbits generally do not tolerate intra-articular infection well. To this point, a previously reported rabbit osteomyelitis model described by Darouiche et al1 is comparable to the osteomyelitis model reported here. Darouiche et al1 induced osteomyelitis with an intra-articular injection of 500 CFUs S. aureus, and evaluated the effectiveness of their novel antimicrobial-coated devices in the prevention of osteomyelitis. Despite a very short endpoint of seven days, three rabbits died and the clinical state of the survivors was not published. In that study and in most other previous publications, rabbit infection models are developed for the evaluation of new antibiotic treatments or implant in comparison to conservative treatment.25,47-50 As a result, the timepoints are short, and the question of whether the artificially induced joint infection or acute osteomyelitis can or does progress to chronic osteomyelitis is not addressed. In addition, many models use study-specific or novel implants and procedures, making them difficult to replicate. A strength of this study is the use of 'off-the-shelf' materials and methods that are easily replicated and permit 100% survivorship over 28 days with appropriate medication and diet. After these 28 days, we observe clear evidence of PJI that will facilitate future studies of osteomyelitis treatment and biofilm development.
However, there are several limitations to our study. First, the purpose of this study was to develop a chronic PJI model reflecting the clinical progression of the disease. However, the intolerance of the rabbits for intra-articular infection with placement of an intra-articular implant as performed by our group required bacterial inoculation in the bone tunnel, which may not reflect the clinical mechanism of PJI. Second, two major criteria of PJI as described by Parvizi et al12 and Gehrke et al51 were not verified, namely synovial WBC and synovial polymorphonuclear leucocyte counts. Third, the method of sample preparation for histological analysis, paraffin embedding, and standard sectioning rendered the evaluation of biofilm formation on the implant surface and bone interface impossible, such that the viability of the bacteria at the time of harvest and the possibility of nosocomial/contaminant infections around the implant cannot be ruled out.
We conclude that intraosseous delivery of planktonic bacteria into the bone tunnel followed by headless screw insertion and bone wax sealing consistently results in survivable, chronic PJI in the rabbit model. Such a model can be useful not only in testing the efficacy of antimicrobial approaches for clinical trial, but also provide insights on PJI disease progression.
Author contributions
H. Yagi: Conducted the experiments, Provided the animal care, Processed the samples, Collected and analyzed the data, Wrote and edited the paper.
S. Kihara: Conducted the in vivo experiments (surgeries), Processed the samples, Collected and analyzed the data, Wrote and edited the paper.
P. N. Mittwede: Conducted the in vivo experiments (surgeries), Processed the samples, Collected and analyzed the data, Wrote and edited the paper.
P. L. Maher: Conducted the in vivo experiments (surgeries), Processed the samples, Collected and analyzed the data, Reviewed the paper.
A. C. Rothenberg: Generated ideas for the project, Performed preliminary in vitro studies, Analyzed the data, Reviewed the paper.
A. D. C. M. Falcione: Provided and supervised the animal care, Processed the samples, Analyzed the data.
A. Chen: Generated ideas for the project, Performed the preliminary in vitro studies, Reviewed the paper.
K. L. Urish: Analyzed the data, Edited the manuscript.
R. S. Tuan: Conceptualized and supervised the project, Analyzed the data, Edited the paper.
P. G. Alexander: Supervised the project, Carried out the experiments, Analyzed the data, Wrote and edited the paper.
H. Yagi and S. Kihara are joint first authors.
R. S. Tuan and P. G. Alexander are joint senior authors.
Funding statement
This study was funded by the US Department of Defense, Congressionally Directed Medical Research Programs, Military Infectious Diseases Applied Research Award Number: W81XWH-15-2-0011. K. L. Urish reports a personal grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), related to this study.No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Acknowledgements
Many thanks to the Division of Laboratory Animal Research at the University of Pittsburgh for their patience and collaboration in the successful completion of this study.
A. Chen generated the ideas and performed the preliminary in vitro studies while at the University of Pittsburgh School of Medicine, and reviewed the paper while at Brigham and Women's Hospital.
Supplementary material
Image of representative bacterial cultures to confirm the presence of viable bacteria, description of other means of periprosthetic osteomyelitis induction that were tested prior to the model reported here, table comparing preliminary models tested in the development of the final chronic periprosthetic osteomyelitis model, and Kaplan-Meier graph comparing all models of periprosthetic osteomyelitis tested. The E10 full ARRIVE guideline checklist is also included to demonstrate compliance with ARRIVE guidelines.
© 2021 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 1. Darouiche RO, Mansouri MD, Zakarevicz D, Alsharif A, Landon GC. In vivo efficacy of antimicrobial-coated devices. J Bone Joint Surg Am. 2007;89-A(4):792–797. [DOI] [PubMed] [Google Scholar]
- 2. Hotchen AJ, Dudareva M, Ferguson JY, Sendi P, McNally MA. The Bach classification of long bone osteomyelitis. Bone Joint Res. 2019;8(10):459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hackett DJ, Rothenberg AC, Chen AF, et al. The economic significance of orthopaedic infections. J Am Acad Orthop Surg. 2015;23 Suppl:S1–S7. [DOI] [PubMed] [Google Scholar]
- 4. Malizos KN. Global Forum: The Burden of Bone and Joint Infections: A Growing Demand for More Resources. J Bone Joint Surg Am. 2017;99-A(5):e20. [DOI] [PubMed] [Google Scholar]
- 5. Willey M, Karam M. Impact of infection on fracture fixation. Orthop Clin North Am. 2016;47(2):357–364. [DOI] [PubMed] [Google Scholar]
- 6. Reizner W, Hunter JG, O'Malley NT, Southgate RD, Schwarz EM, Kates SL. A systematic review of animal models for Staphylococcus aureus osteomyelitis. Eur Cell Mater. 2014;27:196–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465–475. [DOI] [PubMed] [Google Scholar]
- 8. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96-A(8):624–630. [DOI] [PubMed] [Google Scholar]
- 9. Chen AF, Nana AD, Nelson SB, McLaren A. What's new in musculoskeletal infection: update across orthopaedic subspecialties. J Bone Joint Surg Am. 2017;99-A(14):1232–1243. [DOI] [PubMed] [Google Scholar]
- 10. van den Kieboom J, Bosch P, Plate JDJ, et al. Diagnostic accuracy of serum inflammatory markers in late fracture-related infection: a systematic review and meta-analysis. Bone Joint J. 2018;100-B(12):1542–1550. [DOI] [PubMed] [Google Scholar]
- 11. Metsemakers WJ, Morgenstern M, McNally MA, et al. Fracture-related infection: a consensus on definition from an international expert group. Injury. 2018;49(3):505–510. [DOI] [PubMed] [Google Scholar]
- 12. Parvizi J, Tan TL, Goswami K, et al. Definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;2018(33):1309–1314. [DOI] [PubMed] [Google Scholar]
- 13. Kremers HM, Nwojo ME, Ransom JE, Wood-Wentz CM, Melton LJ, Huddleston PM. Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. J Bone Joint Surg Am. 2015;97-A(10):837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Rev. 2019;4(7):482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goswami K, Parvizi J, Maxwell Courtney P. Current recommendations for the diagnosis of acute and chronic PJI for hip and Knee-Cell counts, alpha-defensin, leukocyte esterase, next-generation sequencing. Curr Rev Musculoskelet Med. 2018;11(3):428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacqueline C, Caillon J. Impact of bacterial biofilm on the treatment of prosthetic joint infections. J Antimicrob Chemother. 2014;69 Suppl 1:i37–i40. [DOI] [PubMed] [Google Scholar]
- 17. Zimmerli W, Sendi P. Orthopaedic biofilm infections. APMIS. 2017;125(4):353–364. [DOI] [PubMed] [Google Scholar]
- 18. Zhou J, Zhou XG, Wang JW, Zhou H, Dong J. Treatment of osteomyelitis defects by a vancomycin-loaded gelatin/β-tricalcium phosphate composite scaffold. Bone Joint Res. 2018;7(1):46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Urish KL, DeMuth PW, Kwan BW, et al. Antibiotic-tolerant Staphylococcus aureus Biofilm Persists on Arthroplasty Materials. Clin Orthop Relat Res. 2016;474(7):1649–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beck-Broichsitter BE, Smeets R, Heiland M. Current concepts in pathogenesis of acute and chronic osteomyelitis. Curr Opin Infect Dis. 2015;28(3):240–245. [DOI] [PubMed] [Google Scholar]
- 21. Mauffrey C, Herbert B, Young H, Wilson ML, Hake M, Stahel PF. The role of biofilm on orthopaedic implants: the "Holy Grail" of post-traumatic infection management? Eur J Trauma Emerg Surg. 2016;42(4):411–416. [DOI] [PubMed] [Google Scholar]
- 22. Grammatopoulos G, Bolduc M-E, Atkins BL, et al. Functional outcome of debridement, antibiotics and implant retention in periprosthetic joint infection involving the hip: a case-control study. Bone Joint J. 2017;99-B(5):614–622. [DOI] [PubMed] [Google Scholar]
- 23. Berend KR, Lombardi AV, Morris MJ, Bergeson AG, Adams JB, Sneller MA. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res. 2013;471(2):510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645–1654. [DOI] [PubMed] [Google Scholar]
- 25. Bottagisio M, Coman C, Lovati AB. Animal models of orthopaedic infections. A review of rabbit models used to induce long bone bacterial infections. J Med Microbiol. 2019;68(4):506–537. [DOI] [PubMed] [Google Scholar]
- 26. Mäkitaipale J, Sievänen H, Laitinen-Vapaavuori O. Tibial bone density, cross-sectional geometry and strength in Finnish pet rabbits: a peripheral quantitative computed tomography study. Vet Rec. 2018;183(12):382. [DOI] [PubMed] [Google Scholar]
- 27. Graur D, Duret L, Gouy M. Phylogenetic position of the order Lagomorpha (rabbits, hares and allies). Nature. 1996;379(6563):333–335. [DOI] [PubMed] [Google Scholar]
- 28. Esteves PJ, Abrantes J, Baldauf H-M, et al. The wide utility of rabbits as models of human diseases. Exp Mol Med. 2018;50(5):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gatin L, Saleh-Mghir A, Massin P, Crémieux A-C. Critical analysis of experimental models of periprosthetic joint infection. Orthop Traumatol Surg Res. 2015;101(7):851–855. [DOI] [PubMed] [Google Scholar]
- 30. An YH, Friedman RJ. Animal models of orthopedic implant infection. J Invest Surg. 1998;11(2):139–146. [DOI] [PubMed] [Google Scholar]
- 31. Lucke M, Schmidmaier G, Sadoni S, et al. A new model of implant-related osteomyelitis in rats. J Biomed Mater Res B Appl Biomater. 2003;67(1):593–602. [DOI] [PubMed] [Google Scholar]
- 32. Becerra SC, Roy DC, Sanchez CJ, Christy RJ, Burmeister DM. An optimized staining technique for the detection of gram positive and gram negative bacteria within tissue. BMC Res Notes. 2016;9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McLaughlin RM, Fish RE, Newcomer CE. Clinical biochemistry and hematology. : Manning PJ, Ringler DH. The Biology of the Laboratory Rabbit. Second ed. San Diego, California: Academic Press, 1994:111–124. [Google Scholar]
- 34. Ferreira FS, Barretto FL, Fabres A, Silveira LS, Carvalho CB. Cardiac markers in five different breeds of rabbits (Oryctolagus cuniculus Linnaeus, 1758) used for cardiovascular research. Pesq Vet Bras. 2016;36(8):737–742. [Google Scholar]
- 35. Hewitt CD, Innes DJ, Savory J, Wills MR. Normal biochemical and hematological values in New Zealand white rabbits. Clin Chem. 1989;35(8):1777–1779. [PubMed] [Google Scholar]
- 36. Toth LA, Krueger JM. Hematologic effects of exposure to three infective agents in rabbits. J Am Vet Med Assoc. 1989;195(7):981–986. [PubMed] [Google Scholar]
- 37. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386–394. [DOI] [PubMed] [Google Scholar]
- 38. Bauer TW, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88-A:869–882. [DOI] [PubMed] [Google Scholar]
- 39. Deniset JF, Kubes P. Recent advances in understanding neutrophils. F1000Res. 2016;5:2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mariani BD, Martin DS, Chen AF, Yagi H, Lin SS, Tuan RS. Polymerase chain reaction molecular diagnostic technology for monitoring chronic osteomyelitis. J Exp Orthop. 2014;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simpfendorfer CS. Radiologic approach to musculoskeletal infections. Infect Dis Clin North Am. 2017;31(2):299–324. [DOI] [PubMed] [Google Scholar]
- 42. Zimmerli W. Clinical presentation and treatment of orthopaedic implant-associated infection. J Intern Med. 2014;276(2):111–119. [DOI] [PubMed] [Google Scholar]
- 43. Hanberg P, Lund A, Søballe K, Bue M. Single-dose pharmacokinetics of meropenem in porcine cancellous bone determined by microdialysis: An animal study. Bone Joint Res. 2019;8(7):342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alvarez H, Castro C, Moujir L, et al. Efficacy of ciprofloxacin implants in treating experimental osteomyelitis. J Biomed Mater Res B Appl Biomater. 2008;85(1):93–104. [DOI] [PubMed] [Google Scholar]
- 45. Niska JA, Meganck JA, Pribaz JR, et al. Monitoring bacterial burden, inflammation and bone damage longitudinally using optical and μCT imaging in an orthopaedic implant infection in mice. PLoS One. 2012;7(10):e47397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tao L, Reese TA. Making mouse models that reflect human immune responses. Trends Immunol. 2017;38(3):181–193. [DOI] [PubMed] [Google Scholar]
- 47. Jensen LK, Koch J, Dich-Jorgensen K, et al. Novel porcine model of implant-associated osteomyelitis: a comprehensive analysis of local, regional, and systemic response. J Orthop Res. 2017;35(10):2211–2221. [DOI] [PubMed] [Google Scholar]
- 48. Arens D, Wilke M, Calabro L, et al. A rabbit humerus model of plating and nailing osteosynthesis with and without Staphylococcus aureus osteomyelitis. eCM. 2015;30:148–162. [DOI] [PubMed] [Google Scholar]
- 49. Zhang X, Ma Y-F, Wang L, et al. A rabbit model of implant-related osteomyelitis inoculated with biofilm after open femoral fracture. Exp Ther Med. 2017;14(5):4995–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zahar A, Kocsis G, Citak M, et al. Use of antibiotic-impregnated bone grafts in a rabbit osteomyelitis model. THC. 2017;25(5):929–938. [DOI] [PubMed] [Google Scholar]
- 51. Gehrke T, Lausmann C, Citak M. Still fighting prosthetic joint infection after knee replacement. Lancet Infect Dis. 2019;19(6):560. [DOI] [PubMed] [Google Scholar]