Abstract
Background
Atrial fibrillation (AF) is the most common sustained arrhythmia, with growing evidence identifying obesity as an important risk factor for the development of AF. Although defective atrial myocyte excitability due to stress-induced remodeling of ion channels is commonly observed in the setting of AF, little is known about the mechanistic link between obesity and AF. Recent studies have identified increased cardiac late sodium current (INa,L) downstream of calmodulin-dependent kinase II (CaMKII) activation as an important driver of AF susceptibility.
Methods
Here, we investigated a possible role for CaMKII-dependent INa,L in obesity-induced AF using wild-type (WT) and whole-body knock-in mice that ablates phosphorylation of the Nav1.5 sodium channel and prevents augmentation of the late sodium current (S571A; SA mice).
Results
A high-fat diet (HFD) increased susceptibility to arrhythmias in WT mice, while SA mice were protected from this effect. Unexpectedly, SA mice had improved glucose homeostasis and decreased body weight compared to WT mice. However, SA mice also had reduced food consumption compared to WT mice. Controlling for food consumption through pair feeding of WT and SA mice abrogated differences in weight gain and AF inducibility, but not atrial fibrosis, premature atrial contractions or metabolic capacity.
Conclusions
These data demonstrate a novel role for CaMKII-dependent regulation of Nav1.5 in mediating susceptibility to arrhythmias and whole-body metabolism under conditions of diet-induced obesity.
Subject terms: Cardiovascular biology, Metabolism
Introduction
Atrial fibrillation (AF) affects ~3 million people in the USA alone [1] with an expected incidence of 15 million by 2050 [2]. AF is highly correlated with multiple risk factors including heart failure, age, obesity, and type 2 diabetes (https://www.cdc.gov/obesity/adult/causes.html) [3–5]. Among these, the growing incidence in obesity (https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight) has been identified as a major risk factor for AF [1, 6, 7] and is implicated in 17.9% of all AF cases [7]. Although the incidence of AF and obesity is rapidly increasing worldwide, the causative link between the two pathologies is not clear.
It is well-established that atrial excitability undergoes dramatic changes in the setting of AF, which further exacerbates the substrate for atrial arrhythmia (atrial remodeling). Studies have shown that defects in atrial myocyte Ca2+ handling are important in atrial remodeling, leading to aberrant membrane excitability and dysregulation of critical Ca2+-dependent signaling pathways [8, 9]. Previous work from our group and others showed that calmodulin protein kinase II (CaMKII) dependent regulation of voltage-gated sodium channels (Nav) is a critical determinant of AF susceptibility in animal and humans [10–16]. Specifically, CaMKII phosphorylates the alpha subunit of cardiac Nav (Nav1.5) at Ser571 in the DI-DII linker, leading to increased pathogenic late current (INa,L), which promotes arrhythmogenic action potential after depolarizations and further disrupts intracellular Ca2+ handling [8–10, 15]. This increase in INa,L has been observed in animal models and patients with AF, and drugs that target INa,L have shown promise as therapeutic targets for AF. Importantly, whole-body knock-in mice lacking the Ser571 site in Nav1.5 (SA mice) have reduced AF inducibility [8].
Obesity increases pericardial fat mass, induces left atrial remodeling, and is a major risk factor for AF [17–21]. Although INa,L has been recognized as an important driver of AF, the impact of obesity on INa,L has not been studied. Here, we investigated whether diet-induced obesity increased susceptibility to AF in wild-type (WT) mice, and whether the SA knock-in mouse model had reduced susceptibility to AF, even in the presence of a high-fat diet. Similar to previous studies [22, 23], we found that diet-induced obesity increased AF susceptibility compared to age-matched, chow-fed WT mice. Ablation of CaMKII-dependent phosphorylation of Nav1.5 was protective against the development of AF under conditions of diet-induced obesity. Surprisingly, we also found that the SA allele improved glucose metabolism, and reduced body weight gain associated with HFD, likely due to decreased food intake. Mitigating the difference in food intake by pair-feeding WT mice to SA mice reduced atrial arrhythmia events in WT mice, but the improvements in glucose metabolism were still maintained. Taken together, these data highlight the novel role of Nav1.5 in mediating susceptibility to AF and whole-body glucose metabolism under conditions of obesity.
Methods
Animals
Male, 18–24-week-old C57BL/6 mice from Jackson Laboratory (WT mice) or Scn5a knock-in mice with a Ser-to-Ala mutation at Ser571 of the cardiac voltage-gated channel Nav1.5 (SA mice) were used for all experiments [10]. Animals were housed at room temperature (22 °C) on a 12-h light/dark cycle. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health following protocols approved by the IACUC at The Ohio State University. Animals were euthanized using isoflurane and cervical dislocation followed by collection of tissue or cell isolation.
High-fat diet and pair feeding
WT and SA mice were fed a chow (20% kcal from fat; Teklad) or high-fat diet (60% kcal from fat; Research Diets Inc.) for 6 or 12 weeks. WT and SA mice were fed ad libitum throughout the study unless otherwise indicated. In a subset of experiments, pair-feeding of WT and SA mice was performed by measuring daily food intake of SA mice and calculating the difference in food consumption from the previous day. Each WT mouse was randomly paired to a specific SA mouse and fed the calculated food consumption for that SA mouse each day for 6 weeks.
Atrial arrhythmia susceptibility
To measure susceptibility to atrial arrhythmia events in vivo, subsurface ECGs were obtained from anesthetized mice using subcutaneous ECG leads in the lead II configuration and recording software (Powerlab, ADInstruments). Mice were anesthetized using 2% isoflurane with an oxygen flow rate of 1.0 L/min, and then placed in laying position on a heating pad to maintain body temperature. 1.5% isoflurane was used to maintain anesthesia. Recordings were taken at baseline for 3 min, with epinephrine (1.5 mg/kg, intraperitoneal) for 3 min, and with caffeine (120 mg/kg, intraperitoneal) for 10 min. LabChart (ADInstruments) was used to analyze the data for premature atrial contractions (PACs) in the 10 min following epi/caffeine injection (PACs/10 min), as well as for the severity of atrial tachycardia or fibrillation (AT/AF). AT was defined as at least three premature p-waves in rapid succession (i.e., three premature p-waves within 100 ms), while AF was defined as a period of R–R variability without discernible p-waves. The AT/AF score was assigned based on the duration of AT/AF during the 10 min following epi/caffeine injection, with a score of 0 corresponding to no incidence of AT/AF, a score of 1 to <1 s of AT/AF, a score of 2 to 1–10 s of AT/AF, a score of 3 to 10–60 s of AT/AF, and a score of 4 to >1 min of AT/AF. A subset of WT mice fed a high-fat diet was injected with mexiletine (25 mg/kg) or saline 15 min before baseline ECG recording began.
Echocardiography
To assess left atrial remodeling, echocardiographic images were obtained from anesthetized mice following 6 weeks of HFD. Mice were anesthetized using 2% isoflurane, and 1.5% isoflurane was used to maintain anesthesia. Left atrial diameter was measured along the parasternal long axis view using a Vevo2100 (VisualSonics) system with the MS-400 transducer.
Histology and imaging
Left atria were fixed in neutral buffered 10% formalin, processed routinely into paraffin, and then sectioned serially at 5 µm. Sections were stained using Masson’s Trichrome to examine the amount of interstitial and perivascular fibrosis, wheat germ agglutinin Alexa488 Conjugate to evaluate cell cross-sectional area, and TUNEL kits to examine the amount of apoptosis. Fibrosis was quantified using a custom-built MATLAB program [24]. Cell cross-sectional area was evaluated using ImageJ.
Body composition and metabolic testing
Body weight was measured using an OHAUS NV212 scale. Body fat and lean mass were measured using an EchoMRI instrument (EchoMRI LLC) with canola oil calibration [25]. Glucose tolerance testing was performed after a 12-h fast with drinking water available ad libitum. Blood glucose was assessed at baseline by a tail vein prick. Glucose was administered by intraperitoneal injection (2 g glucose/kg body weight or per kg lean mass) at 0 min, and the tail vein prick was used to measure blood glucose levels at 15, 30, 60, and 120 min post injection [25]. Insulin tolerance testing was performed following a 2 h fast with drinking water ad lib. Baseline blood glucose levels were measured using a tail vein prick. Insulin was administered by intraperitoneal injection (1 unit per kg body weight) at 0 min. Blood glucose levels were measured at 10, 15, 30, 45, and 60 min post injection. If at any time a mouse dropped below 40 mg/dL glucose, they were given an intraperitoneal injection of 200 μL of 20% glucose (0.1 g/mL) and subsequently removed from the test. Pyruvate tolerance testing was performed after a 12-h fast with drinking water available ad libitum. Baseline blood glucose levels were measured using a tail vein prick. Pyruvate was administered intraperitoneally (2 g sodium pyruvate/kg body weight) at 0 min and blood glucose levels were measured 15, 30, 45, 60, and 90 min post injection [26].
Comprehensive lab animal monitoring system
The Comprehensive Lab Animal Monitoring System (Oxymax Opto-M3; Columbus Instruments) was used to measure activity level, volume of O2 consumption, volume of CO2 production, and heat production. Total energy expenditure of mice was calculated as described previously [27]. Data were collected over 48 h; 24 h in the fed state and 24 h in the fasted state.
Quantitative PCR
Tissue processing and quantitative PCR (qPCR) were performed as previously described [28]. Sigma-Aldrich custom primers were used for genes of interest with the sequences shown in Supplementary Table 1. All qPCR gene expression was normalized to the housekeeping gene GAPDH.
Western blotting
Tissue processing and immunoblotting were performed as previously described [26]. The GAPDH (Fitzgerald; 10R-G109A), phosphorylated CaMKII at Thr286/287 (pCaMKII) (Thermo Fisher; MA1-047), and CaMKII (Badrilla; A010-56AP) antibodies were commercially sourced, and phosphorylated Nav1.5 at Ser571 (pNav1.5) and Nav1.5 antibodies were custom generated, as described previously [10, 15]. All immunoblotting data were normalized to GAPDH.
Statistical analysis
GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. The sample sizes in each experiment are provided in figure legends. Only ill and/or wounded animals were excluded from the analyses (n = 2 in this study). The data are presented as means ± SEM. Statistical significance was defined as P < 0.05 and determined by two-tailed t-test or one- or two-way ANOVA, with Tukey and Bonferroni post hoc analysis.
Results
SA mice are resistant to weight gain and atrial arrhythmias induced by high-fat diet
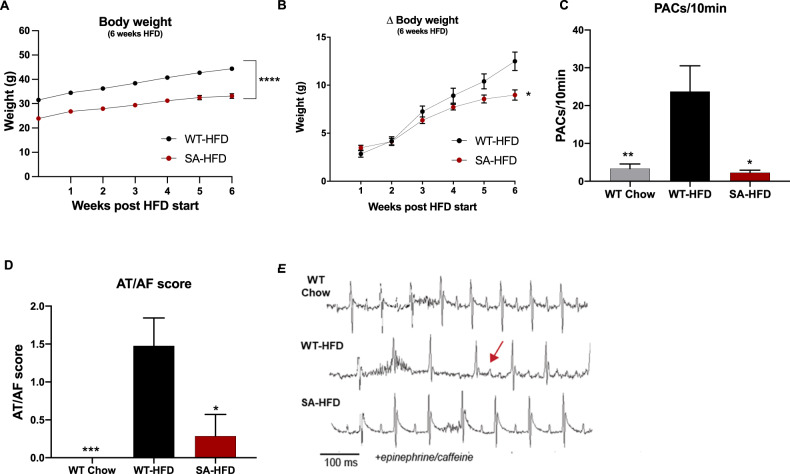
To investigate the role of diet-induced obesity on susceptibility to AF, WT and SA mice were fed a high-fat diet for 6 weeks (WT-HFD and SA-HFD, respectively). SA-HFD mice had reduced total body weight and body weight gain compared to WT-HFD mice (Fig. 1A, B).
Fig. 1. SA knock-in allele confers resistance to body weight gain and reduces susceptibility to atrial fibrillation on a high-fat diet.
A Body weight of WT-HFD and SA-HFD mice after 6 weeks of HFD; B changes in body weight for WT-HFD and SA-HFD mice after 6 weeks of HFD. Data are presented as means ± SEM (WT-HFD n = 27, SA-HFD n = 13; *p < 0.05, ****p < 0.0001 vs WT-HFD). C Density of pre-atrial contractions; D severity of AT/AF based on a score of 0 (none) to 4 (severe); and E representative ECGs following injection of epinephrine (1.5 mg/kg) and caffeine (120 mg/kg). Data are presented as means ± SEM (WT Chow n = 22, WT-HFD n = 23, SA-HFD n = 14; *p < 0.05, **p < 0.01, ***p < 0.001 vs WT-HFD).
To determine the effects of HFD on susceptibility to atrial arrhythmia, WT-HFD mice, age-matched chow-fed WT (WT Chow) mice, and SA-HFD mice underwent adrenergic challenge using epi/caffeine, and changes in the incidence of atrial arrhythmia events [PACs and atrial tachycardia/fibrillation (AT/AF)] were measured. WT-HFD mice had increased incidence and severity of PACs and AT/AF compared to WT Chow mice (Fig. 1C–E). Interestingly, SA-HFD mice were resistant to the HFD-induced increase in atrial arrhythmia events (Fig. 1C–E). Similar to their response on a chow diet [8], SA mice had little to no PACs or AT/AF events even after 6 weeks of a high-fat diet. Consistent with a role for CaMKII-dependent phosphorylation of Nav1.5 in atrial arrhythmia, elevated levels of phosphorylated/activated CaMKII (pCaMKII) and phosphorylated Nav1.5 at S571 (pNav1.5) were observed in whole heart lysates from WT-HFD compared to WT Chow without any difference in total Nav1.5 (Sup. Figure 1A). Interestingly, SA-HFD hearts were resistant to the increase in pCaMKII observed in WT-HFD (Supplementary Fig. 1B) (pNav1.5 was not evaluated in SA-HFD due to the loss of antibody epitope as previously reported [10]). Overall, these data indicate that preventing CaMKII-dependent phosphorylation of Nav1.5 confers resistance to body weight gain and reduces susceptibility to AF under conditions of diet-induced obesity.
Inhibition of the late sodium current with Mexiletine reduces susceptibility to arrhythmias
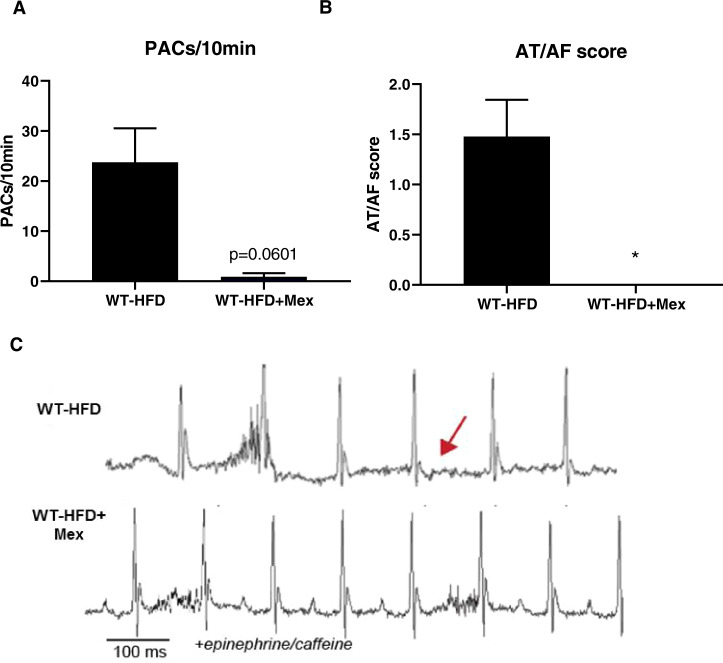
Given that increased INa,L leads to atrial arrhythmias in high-fat fed mice, we assessed the effect of the Na+ channel blocker mexiletine on arrhythmia susceptibility in WT-HFD mice [8]. Consistent with the hypothesis that increased INa,L downstream of CaMKII-dependent phosphorylation of Nav1.5 increases susceptibility to obesity-induced AF, treatment with mexiletine eliminated PACs and AT/AF in WT-HFD mice (Fig. 2A–C). These data provide the first evidence that dysregulation of voltage-gated sodium channels contributes to HFD-induced AF.
Fig. 2. Inhibition of INa,L with Mexiletine reduces susceptibility to atrial fibrillation under conditions of diet-induced obesity.
A Density of pre-atrial contractions; B severity of AT/AF based on a score of 0 (none) to 4 (severe) following injection of epinephrine (1.5 mg/kg) and caffeine (120 mg/kg); and C representative EKGs from 6-week high-fat diet mice injected with saline or mexiletine followed by exposure to epinephrine/caffeine. Data are presented as means ± SEM (WT-HFD n = 23, WT + HFD + Mex n = 8) (*p < 0.05 vs WT-HFD).
SA mice are resistant to HFD-induced cardiac remodeling
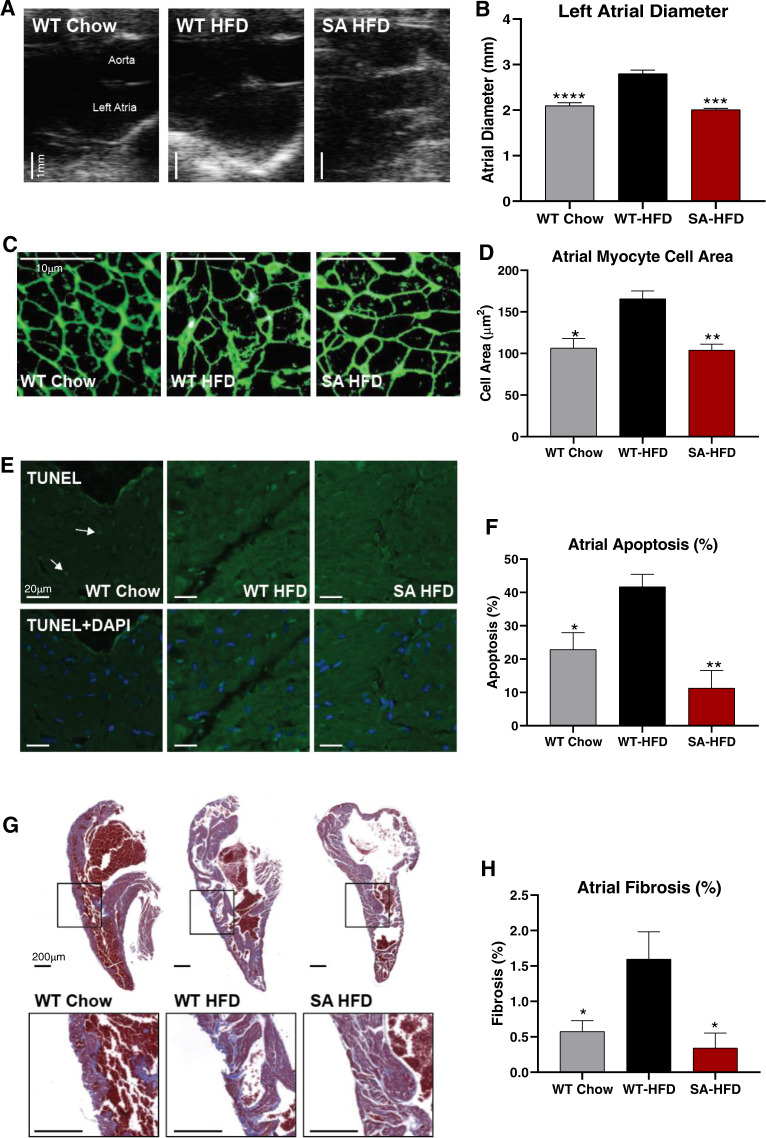
Previous studies have shown that obesity or a high-fat diet contribute to atrial remodeling and susceptibility to arrhythmias [17, 19, 21]. To investigate whether phospho-ablation of Nav1.5 protects against HFD-induced atrial remodeling, atrial size and structure were analyzed by echocardiography and immunohistochemistry. Echocardiography data revealed that HFD increased atrial chamber size in WT-HFD, but not SA-HFD mice. In fact, SA-HFD mice and WT Chow mice had similar atrial chamber size (Fig. 3A, B). HFD also increased atrial myocyte cross-sectional area in WT, but not SA mice (Fig. 3C, D). These data indicate that HFD induces pathological atrial hypertrophy in WT mice, while SA mice are resistant to this adverse remodeling.
Fig. 3. SA knock-in mouse model is resistant to high-fat diet-induced cardiac remodeling.
A Representative echocardiograms (Scale bar 1 mm) and B quantification of left atrial diameters. Data are presented as means ± SEM (WT Chow n = 15, WT-HFD n = 15, SA-HFD n = 4; ***p < 0.001, ****p < 0.0001 vs WT-HFD). C Left atrial section cell membranes stained with Wheat germ agglutinin (Scale bar 10 μm) and D Cross-sectional areas measured from atrial cells to determine cell areas changes. Data are presented as means ± SEM (n = 3/group; *p < 0.05, **p < 0.01 vs WT-HFD). E TUNEL staining of left atrial sections to determine apoptosis (hot spots = nuclei with DNA fragmentation due to apoptosis; Arrows = toward hot spots; DAPI stain = location of all nuclei) (Scale bar 20 μm) and F Percentage of cells undergoing apoptosis. Data are presented as means ± SEM (WT Chow n = 4, WT-HFD n = 4; SA-HFD n = 3; *p < 0.05, **p < 0.01 vs WT-HFD). G Masson’s Trichrome staining of Left atrial sections to indicate fibrosis levels relative to normal cardiac tissue (Scale bar 200 μm) and H percentage of fibrotic tissue. Data are presented as means ± SEM (WT Chow n = 6, WT-HFD n = 6; SA-HFD n = 4; *p < 0.05 vs WT-HFD).
In addition to pathological remodeling, AF is associated with increased apoptosis and tissue fibrosis. To determine if a high-fat diet affected atrial myocyte apoptosis and fibrosis, TUNEL staining was performed in WT Chow, WT-HFD, and SA-HFD mice. WT-HFD mice had increased apoptosis (Fig. 3E, F) and fibrosis (Fig. 3G, H) compared to WT Chow mice. Moreover, SA mice were resistant to the HFD-induced increase in apoptosis and fibrosis (Fig. 3E–H). These data indicate that phosphorylation of Nav1.5 is an important contributor to the development of HFD-induced atrial apoptosis and fibrosis. Together, these data demonstrate that phospho-ablation of the Nav1.5 channel negates HFD-induced atrial remodeling and arrhythmias.
SA mice have improved metabolic capacity
SA mice are protected from HFD-induced arrhythmias and atrial remodeling (Figs. 1 and 3). It is important to note that these findings are confounded by significant differences in body weight between the WT-HFD and SA-HFD mice (Fig. 1), which are also present in WT and SA chow-fed mice (Supplementary Fig. 2A). This difference is likely due to altered food consumption as SA mice consumed significantly less food compared to WT mice on a chow diet (Supplementary Fig. 2B). Glucose tolerance was improved in SA chow-fed mice compared to WT chow-fed mice when injected with glucose based on either body weight (Supplementary Fig. 2C, D) or lean mass (Supplementary Fig. 2E, F). There was no difference in insulin tolerance or pyruvate tolerance between SA and WT mice on a chow diet (Supplementary Fig. 2G–J). These data indicate that attenuation of INa,L can affect metabolic capacity even on a chow diet.
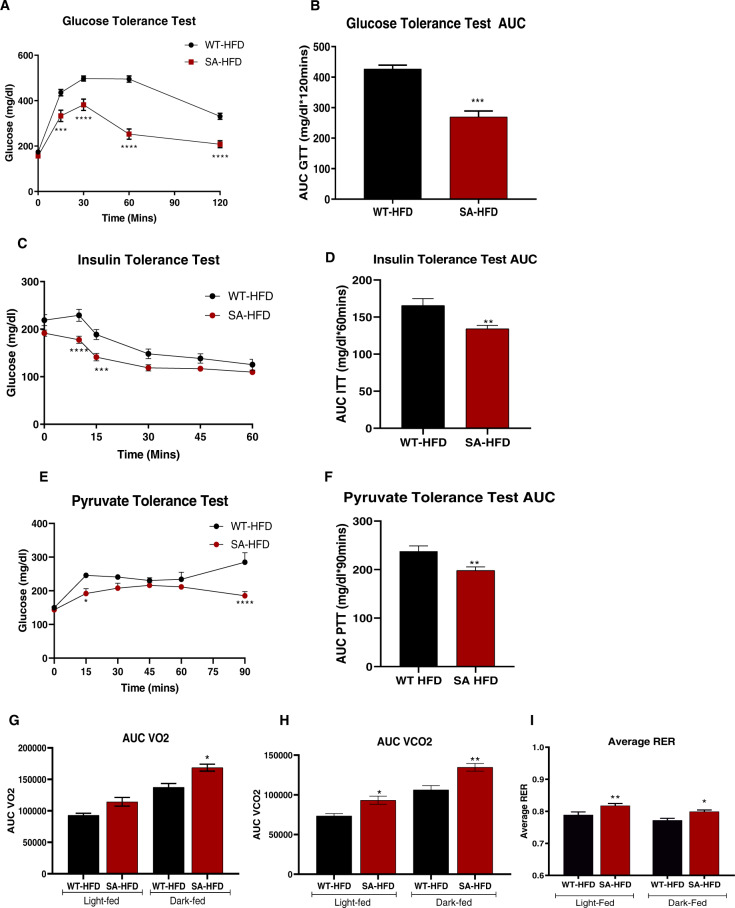
SA-HFD mice were protected from diet-induced obesity and AF. Since metabolic dysfunction is a co-morbidity for obesity and AF, we investigated the effects of a high-fat diet on the metabolic health of SA mice. After 6 weeks of a high-fat diet, SA mice (SA-HFD) had a preserved glucose tolerance when injected with glucose based on body weight (Fig. 4A, B) or lean mass (Supplementary Fig. 3A, B), preserved insulin tolerance (Fig. 4C, D), and pyruvate tolerance (Fig. 4E, F) compared to high-fat fed wild-type mice (WT-HFD). SA-HFD mice had reduced fat mass and lean mass after 6 weeks of HFD compared to WT-HFD mice (Supplementary Fig. 3C–F). We measured metabolic capacity after 12 weeks of a high-fat diet via indirect calorimetry and determined that SA mice had increased VO2, VCO2, and respiratory exchange ratio (RER) (Fig. 4G–I), indicating that these mice predominantly used carbohydrates as a fuel source. These data indicate that preventing CaMKII-dependent phosphorylation of Nav1.5 leads to improved metabolic health in addition to having a protective effect on atrial remodeling and reduced susceptibility to arrhythmia.
Fig. 4. SA knock-in mouse model has improved metabolic capacity under conditions of diet-induced obesity.
A Glucose tolerance test excursion curve and B glucose tolerance test area under curve after 6 weeks of HFD. Data are presented as means ± SEM (n = 5/group; **p < 0.01, ***p < 0.001, ****p < 0.0001 vs WT-HFD). C Insulin tolerance test excursion curve and D insulin tolerance test area under curve after 6 weeks of HFD. Data are presented as means ± SEM (n = 5/group; **p < 0.01, ***p < 0.001, ****p < 0.0001 vs WT-HFD). E Pyruvate tolerance test excursion curve and F pyruvate tolerance test area under curve after 6 weeks of HFD. Data are presented as means ± SEM (WT-HFD n = 5, SA-HFD n = 9; *p < 0.05, **p < 0.01, ****p < 0.0001 vs WT-HFD). G Volume of O2 consumption H volume of CO2 production, and I respiratory exchange ratio of mice was measured and calculated using CLAMS monitoring system as described previously after 12 weeks of HFD [27]. Data are presented as means ± SEM (WT-HFD n = 5, SA-HFD n = 4; *p < 0.05, **p < 0.01 vs WT-HFD).
Since the SA mice are a whole-body knock-in mouse, we investigated gene expression changes in several tissues to determine a potential role in mediating whole-body metabolic health. Expression of genes involved in mitochondrial metabolism, fatty acid oxidation, and glucose metabolism were measured in the liver, tibialis anterior (TA) skeletal muscle, interscapular brown adipose tissue (iBAT), inguinal subcutaneous white adipose tissue (ingWAT), and the small intestine. These tissues were selected for their documented roles in metabolism, and specifically glucose metabolism [25, 29, 30]. The small intestine was investigated because it expresses Nav1.5 in rodents and humans [31–33]. Select genes related to mitochondrial metabolism and fatty acid oxidation were increased in the liver, TA, iBAT, ingWAT, and small intestine of SA-HFD mice compared to WT-HFD mice (Supplementary Fig. 4A–O). Interestingly, there were no changes in expression of genes involved in glucose metabolism in any of the tissues measured (Supplementary Fig. 4A–O). These data indicate that some genes involved in mitochondrial and fatty acid metabolism are altered in multiple peripheral tissues in the SA mice and may contribute to whole-body changes in metabolism.
Differences in body weight account for improved AT/AF susceptibility, but not metabolism in SA-HFD mice
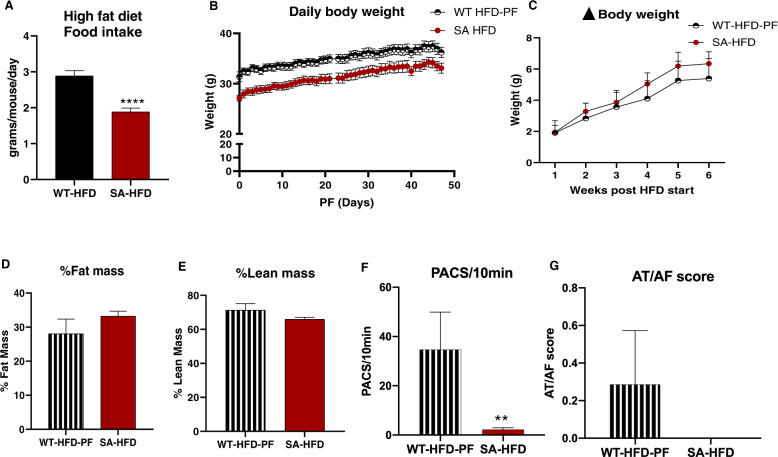
SA-HFD mice have reduced susceptibility to arrhythmias and improved metabolic health compared to WT-HFD mice. It is important to note that these findings are confounded by significant differences in body weight (Fig. 1A, B). Similar to a chow-fed mice, SA mice had reduced food intake compared to WT mice on HFD (Supplementary Fig. 2B, Fig. 5A). To determine if the differences in food intake were the primary driver for the difference in body weight, resistance to arrhythmia development, and improved metabolic health in SA-HFD mice, we investigated a separate cohort of mice where food intake in WT-HFD mice was paired to that of SA-HFD mice (WT-HFD-PF) (Supplementary Fig. 5A). Pair-feeding blunted the HFD-induced weight gain observed in ad libitum fed WT mice (Fig. 5B, C; Supplementary Fig. 5B, C). Pair-feeding also abolished the differences in fat mass (Supplementary Fig. 5D), percent fat mass (Fig. 5D), and percent lean mass (Fig. 5E), but not total lean mass (Supplementary Fig. 5E), between WT-HFD and SA-HFD mice. In parallel, pair-feeding reduced the difference in fibrosis between the both groups compared to WT-HFD, although a significant increase in fibrosis was still apparent in WT-HFD-PF compared to SA-HFD (Supplementary Fig. 5F, G).
Fig. 5. Attenuating body weight gain on HFD partially mitigates susceptibility to AF in wild-type mice compared to SA knock-in mice.
A Average daily food intake of WT-HFD and SA-HFD mice on a high-fat diet. Data are presented as means ± SEM (WT-HFD n = 15, SA-HFD n = 5; ****p < 0.0001 vs WT-HFD). Wild-type mice were pair-fed (WT-HFD-PF) the same amount of HFD as SA-HFD mice for 6 weeks and B body weight C changes in body weight measured. D changes in percent fat and E lean mass measured using EchoMRI. Data are presented as means ± SEM (WT-HFD-PF n = 9, SA-HFD n = 10). F Density of pre-atrial contractions and G severity of AT/AF based on a score of 0 (none) to 4 (severe) measured following injection of epinephrine (1.5 mg/kg) and caffeine (120 mg/kg). Data are presented as means ± SEM (WT-HFD-PF n = 7, SA-HFD n = 14; **p < 0.01 vs WT-HFD-PF).
To determine if susceptibility to AT/AF was primarily in response to changes in body weight, WT-HFD-PF mice were compared to ad libitum fed WT-HFD mice, WT Chow mice (Fig. 1C, D), and SA-HFD mice. While pair-feeding did not eliminate differences in PACs between WT-HFD-PF and SA-HFD mice (Fig. 5F), pair-feeding reduced incidence of AT/AF in WT-HFD-PF mice (Fig. 5G). WT-HFD-PF mice had a lower AT/AF score compared to WT-HFD mice (Figs. 1D and 5G), with no significant differences in AT/AF score between WT-HFD-PF, WT Chow, or SA-HFD mice (Figs. 1D and 5G). These data indicate that the SA allele reduces severity of atrial arrhythmia events dependent on food consumption and weight gain.
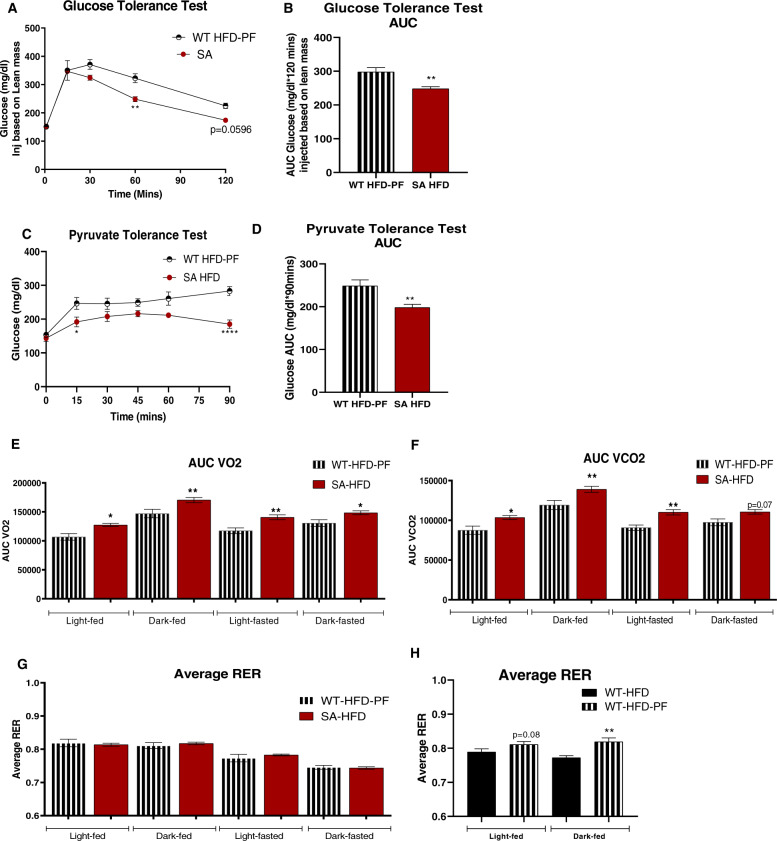
To determine if the observed metabolic improvements were dependent on body weight in SA-HFD mice, we investigated the metabolic health of WT-HFD-PF mice compared to SA-HFD mice. To account for the slight differences in body weight, mice were injected with glucose based on lean mass and glucose tolerance was measured. Even when injected based on lean mass, glucose tolerance was improved in SA-HFD compared to WT-HFD-PF mice (Fig. 6A, B). Insulin tolerance was not different between groups (Supplementary Fig. 5H, I), but pyruvate tolerance was improved in SA-HFD mice, indicating that SA-HFD mice had improved gluconeogenic activity compared to WT-HFD-PF mice (Fig. 6C, D). Together these data indicate that the effects of improved glucose and pyruvate tolerance were not dependent on body weight. Indirect calorimetry measurements revealed that SA-HFD mice had increased VO2 and VCO2 compared to WT-HFD-PF mice, but with no difference in RER (Fig. 6E–G). Interestingly, WT-HFD-PF mice had increased RER compared to ad libitum WT-HFD mice (Fig. 6H), indicating a shift to increased carbohydrate utilization in these mice, similar to SA-HFD mice. Taken together, these data indicate that SA-HFD mice have systemic improvements in metabolic health compared to WT-HFD-PF mice that are independent of body weight.
Fig. 6. Attenuating body weight gain on HFD does not affect metabolic capacity of wild-type mice compared to SA knock-in mice.
A Glucose tolerance test excursion curve and B area under curve for glucose tolerance after 6 weeks of pair-feeding. C Pyruvate tolerance excursion curve and D area under curve for pyruvate tolerance after 6 weeks of pair-feeding. Data are presented as means ± SEM (WT-HFD-PF n = 9, SA-HFD n = 10; *p < 0.05, **p < 0.01, ****p < 0.0001 vs WT-HFD-PF). E Volume of O2 consumption; F volume of CO2 production; G, H respiratory exchange ratio of mice measured using CLAMS Monitoring system after 6 weeks of pair feeding [27]. Data are presented as means ± SEM WT-HFD n = 5, WT-HFD-PF n = 9, SA-HFD n = 10; *p < 0.05, **p < 0.01 vs WT-HFD-PF (E–G) and vs WT-HFD (H).
Discussion
In this study, we investigated the link between CaMKII-dependent regulation of Nav1.5, pathogenic late current (INa,L), diet-induced obesity, and AF, taking advantage of our phospho-ablated SA knock-in mouse model lacking the CaMKII phosphorylation site on Nav1.5, which attenuates INa,L. Diet-induced obesity resulted in increased arrhythmias and obesity-induced atrial remodeling (fibrosis, pathological hypertrophy, apoptosis) in WT mice, but phospho-ablation of Nav1.5 in the SA knock-in mice conferred resistance to obesity-induced arrhythmias and atrial remodeling. Moreover, phospho-ablation of Nav1.5 resulted in improved metabolic capacity in mice, independent of body weight. This highlights a novel role for the late Na+ current to modulate whole-body metabolism.
Dysregulation of Nav1.5 is frequently reported in animal models of AF and human patients [8, 34–37]. Our group and others have demonstrated that dysregulation of Nav1.5 mediates atrial arrhythmogenesis downstream of CaMKII activation [8, 10, 12]. Of specific interest, previous work has shown that in addition to other targets involved in AF pathogenesis, CaMKII phosphorylates Nav1.5 at Ser571 in both atrial and ventricular myocytes, directly augmenting INa,L and disrupting intracellular homeostasis of both Na+ and Ca2+ [8, 10, 38]. Dysregulation of Nav1.5 Ser571 has been observed in multiple cardiac disease states, including heart failure, ischemia/reperfusion, and AF [38–40]. In addition, drugs like ranolazine that target INa,L have been successfully employed to treat AF [34, 41, 42]. However, the role of INa,L in stress-induced AF, such as that resulting from aging, heart failure, and obesity, remains unclear. An outstanding question to be answered going forward is how does a high-fat diet promotes CaMKII/Nav1.5 dysregulation and atrial structural/electrical remodeling to set the stage for increased susceptibility to atrial arrhythmias? Previous work has shown increased CaMKII activity with a high-fat diet downstream of increased oxidative stress [43]. At the same time, accumulation of epicardial adipose tissue has been linked to atrial remodeling through paracrine signaling [44, 45]. Thus it is likely that changes in the volume/composition of fat depots adjacent to the atria may drive the remodeling process and arrhythmia, although further investigation is needed.
Here, we determined that HFD increased atrial arrhythmia burden in WT mice, but preventing augmentation of INa,L in SA reduced susceptibility to the development of arrhythmias. Surprisingly, SA mice also had an improved metabolic profile compared to WT-HFD mice, independent of their body weight. These improvements were similar to previous studies investigating the role of the INa,L inhibitor ranolazine. The HARMONY and RAFFAELLO trials have shown that ranolazine decreases AF burden and recurrence in AF patients [46, 47]. In addition, pre-clinical and clinical studies have reported that ranolazine induces weight loss and improves glycemia in patients with type 2 diabetes and coronary heart disease [48–50]. Although the mechanism remains unclear, it has been proposed that ranolazine may alter metabolism by reducing glucagon release from the pancreas, or by modulating fatty acid uptake, oxidation, and gluconeogenesis in the liver [51–53]. Based on our findings, it is possible that metabolic benefits of Nav1.5 phospho-ablation and ranolazine are mediated through similar mechanisms.
While Nav1.5 is predominantly expressed in the heart, Nav1.5 is also found in other tissues [54, 55]. The impact of Nav1.5 on glucose metabolism in tissues other than the heart has not been studied. Since the SA mouse model is a whole-body knock-in model, effects on peripherally expressed Nav1.5 could mediate the observed metabolic benefits. Analysis of genes in the liver, TA, iBAT, ingWAT, and small intestine indicates some changes to mitochondrial and glucose metabolism in SA-HFD mice that warrant further studies to better determine their role in improving metabolic capacity.
It is important to note that SA mice had lower food intake on HFD compared to WT mice which reduced the weight gain in these animals. Attenuating body weight gain by pair-feeding WT and SA mice eliminated differences in HFD-induced AT/AF but not PACs in WT mice compared to SA mice. This indicates that reduced body weight gain likely reduces the severity of the AF disease state, but not arrhythmogenic triggers factors like PACs. In contrast, improved glucose metabolism was still observed in SA-HFD mice compared to WT-HFD-PF, suggesting a direct effect of Nav1.5 on metabolism. Future studies should investigate the impact of therapeutic treatments such as ranolazine on food intake in patients, as well as mechanisms behind decreased food intake and its role in metabolic effects of Nav1.5. Moreover, it will be of value to highlight the relative importance of modulating cardiac-specific changes in Nav1.5 activity, relative to extra-cardiac Nav1.5. Interestingly, the use of mexiletine successfully reduced AF burden in WT-HFD mice with just 15 min of pre-treatment, suggesting a critical importance of direct electrophysiologic action of Ser571 in acutely contributing to AF burden. It will be interesting to see how this differs from the chronic contributions of Ser571 to the structural changes of atrial hypertrophy and fibrosis and how such changes incorporate extra-cardiac Nav1.5. It is also worth noting that cardiac-specific transgenic models have altered systemic energy homeostasis, suggesting that modulation of cardiac Nav1.5 alone may be sufficient to induce non-cardiac changes in metabolic regulation and energy storage [56].
Overall, these data demonstrate a previously understudied interaction between the CaMKII/Nav1.5 pathway, AF, and obesity. Phospho-ablation of the Nav1.5 site, Ser571, that regulates INa,L reduces AF susceptibility by attenuating weight gain and improves whole-body glucose metabolism independent of body weight. Together, these data establish Nav1.5 as an important driver for whole-body metabolic health and reiterate its importance in arrhythmic susceptibility under conditions of diet-induced obesity. These data also highlight the need to better elucidate the mechanistic basis for observed changes to metabolism and arrhythmia susceptibility with future studies. This study provides greater understanding of the interplay between co-morbidities like cardiovascular diseases, obesity, type 2 diabetes and their underlying effectors. Analysis of the mechanistic aspects of these diseases, especially the role of the late Na+ current in this context, can further the understanding and treatment of these co-morbidities in humans.
Supplementary information
Supplemental Figure Legends, Supplemental Tables
Acknowledgments
Funding
This work was supported by the National Institutes of Health [grant numbers R01-HL135096 to TJH; R01-HL134824 to TJH and PJM; R35-HL135754 to PJM; and R01-HL138738 and R01-AG060542 to KIS], American Heart Association [Postdoctoral fellowship to AGS], TriFit Challenge Grant from the Ross Heart Hospital and Dorothy M. Davis Heart and Lung Research Institute [to KIS and TJH].
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Revati S. Dewal, Amara Greer-Short, Cemantha Lane
Contributor Information
Thomas J. Hund, Email: Thomas.Hund@osumc.edu
Kristin I. Stanford, Email: Kristin.Stanford@osumc.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41366-021-00742-4.
References
- 1.Vyas V, Lambiase P. Obesity and atrial fibrillation: epidemiology, pathophysiology and novel therapeutic opportunities. Arrhythm Electrophysiol Rev. 2019;8:28–36. doi: 10.15420/aer.2018.76.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Brandes A, Smit MD, Nguyen BO, Rienstra M, Van Gelder IC. Risk factor management in atrial fibrillation. Arrhythm Electrophysiol Rev. 2018;7:118–27. doi: 10.15420/aer.2018.18.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nalliah CJ, Sanders P, Kottkamp H, Kalman JM. The role of obesity in atrial fibrillation. Eur Heart J. 2015;37:1565–72. doi: 10.1093/eurheartj/ehv486. [DOI] [PubMed] [Google Scholar]
- 5.Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. 2017;120:1501–17. doi: 10.1161/CIRCRESAHA.117.309732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega FB, et al. An Overview and Update on Obesity and the Obesity Paradox in Cardiovascular Diseases. Prog. Cardiovasc. Dis. 2018;61:142–50. doi: 10.1016/j.pcad.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–8. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greer-Short A, Musa H, Alsina KM, Ni L, Word TA, Reynolds JO, et al. Calmodulin kinase II regulates atrial myocyte late sodium current, calcium handling, and atrial arrhythmia. Heart rhythm. 2020;17:503–11. doi: 10.1016/j.hrthm.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Xiong F, Qi XY, Xiao J, Villeneuve L, Abu-Taha I, et al. Altered calcium-handling produces reentry-promoting action potential alternans in atrial fibrillation-remodeled hearts. JCI insight. 2020. [DOI] [PMC free article] [PubMed]
- 10.Glynn P, Musa H, Wu X, Unudurthi SD, Little S, Qian L, et al. Voltage-Gated Sodium Channel Phosphorylation at Ser571 Regulates Late Current, Arrhythmia, and Cardiac Function In Vivo. Circulation. 2015;132:567–77. doi: 10.1161/CIRCULATIONAHA.114.015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koval OM, Snyder JS, Wolf RM, Pavlovicz RE, Glynn P, Curran J, et al. Ca2+/calmodulin-dependent protein kinase II-based regulation of voltage-gated Na+ channel in cardiac disease. Circulation. 2012;126:2084–94. doi: 10.1161/CIRCULATIONAHA.112.105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer TH, Herting J, Mason FE, Hartmann N, Watanabe S, Nikolaev VO, et al. Late INa increases diastolic SR-Ca2+-leak in atrial myocardium by activating PKA and CaMKII. Cardiovascular research. 2015;107:184–96. doi: 10.1093/cvr/cvv153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sag CM, Mallwitz A, Wagner S, Hartmann N, Schotola H, Fischer TH, et al. Enhanced late INa induces proarrhythmogenic SR Ca leak in a CaMKII-dependent manner. J Mol Cell Cardiol. 2014;76:94–105. doi: 10.1016/j.yjmcc.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Toischer K, Hartmann N, Wagner S, Fischer TH, Herting J, Danner BC, et al. Role of late sodium current as a potential arrhythmogenic mechanism in the progression of pressure-induced heart disease. J Mol Cell Cardiol. 2013;61:111–22. doi: 10.1016/j.yjmcc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, et al. A beta(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Investig. 2010;120:3508–19. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Investig. 2006;116:3127–38. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nalliah CJ, Sanders P, Kalman JM. The impact of diet and lifestyle on atrial fibrillation. Curr Cardiol Rep. 2018;20:137. doi: 10.1007/s11886-018-1082-8. [DOI] [PubMed] [Google Scholar]
- 18.Mahajan R, Nelson A, Pathak RK, Middeldorp ME, Wong CX, Twomey DJ, et al. Electroanatomical Remodeling of the Atria in Obesity: Impact of Adjacent Epicardial Fat. JACC Clin Electrophysiol. 2018;4:1529–40. doi: 10.1016/j.jacep.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Liu C, Li G, Laukkanen JA, Hao L, Zhao Q, Zhang J, et al. Overweight and obesity are associated with cardiac adverse structure remodeling in Chinese elderly with hypertension. Scientific reports. 2019;9:17896.. doi: 10.1038/s41598-019-54359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batal O, Schoenhagen P, Shao M, Ayyad AE, Van Wagoner DR, Halliburton SS, et al. Left atrial epicardial adiposity and atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:230–6. doi: 10.1161/CIRCEP.110.957241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shuai W, Kong B, Fu H, Shen C, Jiang X, Huang H. MD1 Deficiency Promotes Inflammatory Atrial Remodelling Induced by High-Fat Diets. Can J Cardiol. 2019;35:208–16. doi: 10.1016/j.cjca.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Zhang F, Hartnett S, Sample A, Schnack S, Li Y. High fat diet induced alterations of atrial electrical activities in mice. Am J Cardiovasc Dis. 2016;6:1–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Kondo H, Abe I, Gotoh K, Fukui A, Takanari H, Ishii Y, et al. Interleukin 10 Treatment Ameliorates High-Fat Diet-Induced Inflammatory Atrial Remodeling and Fibrillation. Circ Arrhythm Electrophysiol. 2018;11:e006040.. doi: 10.1161/CIRCEP.117.006040. [DOI] [PubMed] [Google Scholar]
- 24.Gratz D, Winkle AJ, Dalic A, Unudurthi SD, Hund TJ. Computational tools for automated histological image analysis and quantification in cardiac tissue. MethodsX. 2020;7:22–34. doi: 10.1016/j.mex.2019.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–23. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes. 2015;64:2002–14. doi: 10.2337/db14-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albarado DC, McClaine J, Stephens JM, Mynatt RL, Ye J, Bannon AW, et al. Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice. Endocrinology. 2004;145:243–52. doi: 10.1210/en.2003-0452. [DOI] [PubMed] [Google Scholar]
- 28.Lessard SJ, Rivas DA, Alves-Wagner AB, Hirshman MF, Gallagher IJ, Constantin-Teodosiu D, et al. Resistance to aerobic exercise training causes metabolic dysfunction and reveals novel exercise-regulated signaling networks. Diabetes. 2013;62:2717–27. doi: 10.2337/db13-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toyoda T, An D, Witczak CA, Koh HJ, Hirshman MF, Fujii N, et al. Myo1c regulates glucose uptake in mouse skeletal muscle. J Biol Chem. 2011;286:4133–40. doi: 10.1074/jbc.M110.174938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trevellin E, Scorzeto M, Olivieri M, Granzotto M, Valerio A, Tedesco L, et al. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes. 2014;63:2800–11. doi: 10.2337/db13-1234. [DOI] [PubMed] [Google Scholar]
- 31.Beyder A, Mazzone A, Strege PR, Tester DJ, Saito YA, Bernard CE, et al. Loss-of-function of the voltage-gated sodium channel NaV1.5 (channelopathies) in patients with irritable bowel syndrome. Gastroenterology. 2014;146:1659–68. doi: 10.1053/j.gastro.2014.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beyder A, Gibbons SJ, Mazzone A, Strege PR, Saravanaperumal SA, Sha L, et al. Expression and function of the Scn5a-encoded voltage-gated sodium channel NaV 1.5 in the rat jejunum. Neurogastroenterol Motil. 2016;28:64–73. doi: 10.1111/nmo.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veerman CC, Wilde AA, Lodder EM. The cardiac sodium channel gene SCN5A and its gene product NaV1.5: Role in physiology and pathophysiology. Gene. 2015;573:177–87. doi: 10.1016/j.gene.2015.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, et al. Altered Na(+) currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol. 2010;55:2330–42. doi: 10.1016/j.jacc.2009.12.055. [DOI] [PubMed] [Google Scholar]
- 35.Kumar K, Nearing BD, Carvas M, Nascimento BC, Acar M, Belardinelli L, et al. Ranolazine exerts potent effects on atrial electrical properties and abbreviates atrial fibrillation duration in the intact porcine heart. J Cardiovasc Electrophysiol. 2009;20:796–802. doi: 10.1111/j.1540-8167.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- 36.Makiyama T, Akao M, Shizuta S, Doi T, Nishiyama K, Oka Y, et al. A novel SCN5A gain-of-function mutation M1875T associated with familial atrial fibrillation. J Am Coll Cardiol. 2008;52:1326–34. doi: 10.1016/j.jacc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–57. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grandi E, Herren AW. CaMKII-dependent regulation of cardiac Na(+) homeostasis. Front Pharmacol. 2014;5:41. doi: 10.3389/fphar.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howard T, Greer-Short A, Satroplus T, Patel N, Nassal D, Mohler PJ, et al. CaMKII-dependent late Na(+) current increases electrical dispersion and arrhythmia in ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2018;315:H794–801. doi: 10.1152/ajpheart.00197.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herren AW, Weber DM, Rigor RR, Margulies KB, Phinney BS, Bers DM. CaMKII Phosphorylation of Na(V)1.5: Novel in Vitro Sites Identified by Mass Spectrometry and Reduced S516 Phosphorylation in Human Heart Failure. J Proteome Res. 2015;14:2298–311. doi: 10.1021/acs.jproteome.5b00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burashnikov A, Antzelevitch C. Role of late sodium channel current block in the management of atrial fibrillation. Cardiovasc Drugs Ther. 2013;27:79–89. doi: 10.1007/s10557-012-6421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aidonidis I, Doulas K, Hatziefthimiou A, Tagarakis G, Simopoulos V, Rizos I, et al. Ranolazine-induced postrepolarization refractoriness suppresses induction of atrial flutter and fibrillation in anesthetized rabbits. J Cardiovasc Pharmacol Ther. 2013;18:94–101. doi: 10.1177/1074248412453874. [DOI] [PubMed] [Google Scholar]
- 43.Joseph LC, Avula UMR, Wan EY, Reyes MV, Lakkadi KR, Subramanyam P, et al. Dietary Saturated Fat Promotes Arrhythmia by Activating NOX2 (NADPH Oxidase 2) Circ Arrhythm Electrophysiol. 2019;12:e007573.. doi: 10.1161/CIRCEP.119.007573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J. 2015;36:795–805a. doi: 10.1093/eurheartj/eht099. [DOI] [PubMed] [Google Scholar]
- 45.Nalliah CJ, Bell JR, Raaijmakers AJA, Waddell HM, Wells SP, Bernasochi GB, et al. Epicardial Adipose Tissue Accumulation Confers Atrial Conduction Abnormality. J Am Coll Cardiol. 2020;76:1197–211. doi: 10.1016/j.jacc.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 46.Reiffel JA, Camm AJ, Belardinelli L, Zeng D, Karwatowska-Prokopczuk E, Olmsted A, et al. The HARMONY Trial: Combined Ranolazine and Dronedarone in the Management of Paroxysmal Atrial Fibrillation: Mechanistic and Therapeutic Synergism. Circ Arrhythm Electrophysiol. 2015;8:1048–56. [DOI] [PubMed]
- 47.De Ferrari GM, Maier LS, Mont L, Schwartz PJ, Simonis G, Leschke M, et al. Ranolazine in the treatment of atrial fibrillation: Results of the dose-ranging RAFFAELLO (Ranolazine in Atrial Fibrillation Following An ELectricaL CardiOversion) study. Heart rhythm. 2015;12:872–8. [DOI] [PubMed]
- 48.Ghosh GC, Ghosh RK, Bandyopadhyay D, Chatterjee K, Aneja A. Ranolazine: multifaceted role beyond coronary artery disease, a recent perspective. Heart Views. 2018;19:88–98. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_18_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teoh IH, Banerjee M. Effect of ranolazine on glycaemia in adults with and without diabetes: a meta-analysis of randomised controlled trials. Open Heart. 2018;5:e000706. doi: 10.1136/openhrt-2017-000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caminiti G, Fossati C, Battaglia D, Massaro R, Rosano G, Volterrani M. Ranolazine improves insulin resistance in non-diabetic patients with coronary heart disease. A pilot study. Int J Cardiol. 2016;219:127–9. [DOI] [PubMed]
- 51.Lisi D, Andrews E, Parry C, Hill C, Ombengi D, Ling H. The Effect of Ranolazine on Glycemic Control: a Narrative Review to Define the Target Population. Cardiovasc Drugs Ther. 2019;33:755–61. doi: 10.1007/s10557-019-06917-6. [DOI] [PubMed] [Google Scholar]
- 52.Al Batran R, Gopal K, Aburasayn H, Eshreif A, Almutairi M, Greenwell AA, et al. The antianginal ranolazine mitigates obesity-induced nonalcoholic fatty liver disease and increases hepatic pyruvate dehydrogenase activity. JCI insight. 2019:4. [DOI] [PMC free article] [PubMed]
- 53.Rizzetto R, Rocchetti M, Sala L, Ronchi C, Villa A, Ferrandi M, et al. Late sodium current (INaL) in pancreatic beta-cells. Pflugers Arch. 2015;467:1757–68. doi: 10.1007/s00424-014-1613-0. [DOI] [PubMed] [Google Scholar]
- 54.Strege PR, Mazzone A, Bernard CE, Neshatian L, Gibbons SJ, Saito YA, et al. Irritable bowel syndrome patients have SCN5A channelopathies that lead to decreased NaV1.5 current and mechanosensitivity. Am J Physiol Gastrointest Liver Physiol. 2018;314:G494–503. doi: 10.1152/ajpgi.00016.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazzone A, Strege PR, Tester DJ, Bernard CE, Faulkner G, De Giorgio R, et al. A mutation in telethonin alters Nav1.5 function. J Biol Chem. 2008;283:16537–44. doi: 10.1074/jbc.M801744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amoasii L, Holland W, Sanchez-Ortiz E, Baskin KK, Pearson M, Burgess SC, et al. A MED13-dependent skeletal muscle gene program controls systemic glucose homeostasis and hepatic metabolism. Gens Dev. 2016;30:434–46. doi: 10.1101/gad.273128.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure Legends, Supplemental Tables