Abstract
Fibrotic lung changes are well-known complications of SARS, MERS, and ARDS from other causes and are anticipated in recovered COVID patients. However, there is limited data so far showing a temporal relationship between lung changes on imaging in the acute phase and follow-up imaging after recovery from the infection. We present 12 patients who demonstrate the development of interstitial lung changes and pulmonary fibrosis in the same distribution and pattern as the acute phase findings, up to 6 months after the acute infection, demonstrating a direct relationship between these changes and COVID-19 pneumonia.
Keywords: COVID-19, Fibrosis, Recovered, Temporal imaging, HRCT
1. Introduction
As the world continues to grapple with the COVID-19 pandemic and the acute toll it has taken on our health system, there are expected long-term implications on recovered patients. Anecdotal evidence suggests that recuperated patients continue to suffer from fatigue, cognitive slowing, body aches, and difficulty breathing. Long-term studies with SARS, MERS, and ARDS have shown fibrotic lung changes in recovered patients and these are anticipated in COVID-19 patients as well.1 , 2 Given the substantial number of people affected, the burden of subsequent interstitial lung disease may be significant. We present 12 patients who demonstrate the development of interstitial lung changes and pulmonary fibrosis in the same distribution as the acute phase findings.3 , 4
2. Description
This was a HIPAA-compliant institutional review board-exempt retrospective review of patients with confirmed COVID pneumonia who were admitted into our urban multicenter health system with waived requirement of informed patient consent. Our Picture Archive and Communication System (PACS) was searched for all chest CT reports containing the words “COVID+ honeycombing”,5 “COVID+ interstitial (89)”, “COVID+ bronchiectasis (65)”, “COVID+ cystic (37)” and “COVID+ fibrosis (42)” from 03/01/2020 to 10/09/2020 yielding a total of 245 cases. Images were reviewed by two radiologists (AG, 2 years' experience; PL a cardiothoracic radiologist with 14 years' experience). Patients with known severe interstitial lung disease, without sequential acute phase and follow-up imaging, and without laboratory confirmation of COVID-19 were excluded. 12 patients meeting these criteria were identified. Pertinent patient information was gathered including demographics, comorbidities, risk factors for interstitial lung disease, disease course during hospitalization, and disease outcome. The most recent pre-infection imaging, acute infectious phase imaging, and recovery phase imaging findings of these patients was then compared and reviewed. Pertinent changes on CT included the following as defined by the Fleischner Society: ground-glass opacity (GGO), reticular abnormality, traction bronchiectasis, architectural distortion, honeycombing and other (non-emphysematous) cystic change.4 Additionally, pulmonary fibrosis was defined as architectural distortion with traction bronchiectasis or honeycombing or both.4 The extent of opacities (0–100%), geographic distribution (central, subpleural or both), and lung zone involvement (upper, mid, lower) was assessed during the acute infection and in follow-up imaging after recovery. Demographic data and other relevant history are summarized in Supplementary Table 1, clinical course of infection is summarized in Supplementary Table 2, time intervals between symptom onset and the reviewed studies are summarized in Supplementary Table 3, and imaging findings in the acute and follow-up phases are summarized in Tables 4 and 5.
The average age of the patients identified was 65.1 years (35–89, median 66) with 8 males and 4 females. 7 of these patients were former smokers, none were active smokers. No other risk factors for ILD were identified in any patient except remote occupational asbestos exposure in 1 patient and known mild ILD with ANCA vasculitis in another. All patients were serologically confirmed to have COVID-19, 2 by the presence of antibodies, and the remaining by viral RNA detection by PCR/NAAT. 6/12 patients had required invasive mechanical ventilation, of which 4 developed severe ARDS requiring eventual tracheostomy, 3 of these also required ECMO. The remaining 5/12 patients had received supplemental oxygen and one remained stable on room air. 7/12 patients required supplemental oxygen upon discharge. Of the patients who had severe ARDS, one ultimately died of the disease, the other remained dependent on the tracheostomy and was discharged to a long-term care facility, the third to a short-term rehabilitation facility after being decannulated, and the fourth remains in the hospital.
9 patients had either CT or radiographic imaging of the chest predating the infection which demonstrated lack of lung disease. In the peak phase of infection, typical appearances of COVID-19 pneumonia, including GGOs, consolidation, and crazy-paving pattern in a predominantly peripheral and subpleural distribution were seen in all 12 patients, using the Radiologic Society of North America (RSNA) published reporting criteria.6 Follow-up imaging was obtained at an average of 92.5 days after symptom onset (range 14–196 days) and demonstrated interstitial lung changes and pulmonary fibrosis as defined by the Fleischner Society in the same distribution as the acute changes with accompanying improvement in GGO and consolidation.3 , 4 In the follow-up post-recovery phase, all patients (12/12) had mild persistent GGOs, reticular abnormality, bronchiectasis and architectural distortion; 7/12 had honeycombing or other (non-emphysematous) cystic change (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6 ). In all patients (12/12), the distribution of the changes was both central and subpleural, and occurred in all lung zones (upper, mid, lower). 11/12 patients had 40% or greater of the lung affected by COVID-19 during the acute phase of the infection.
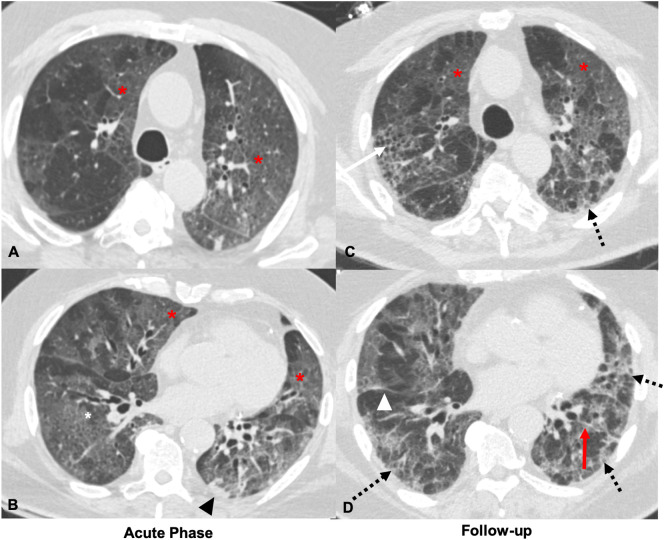
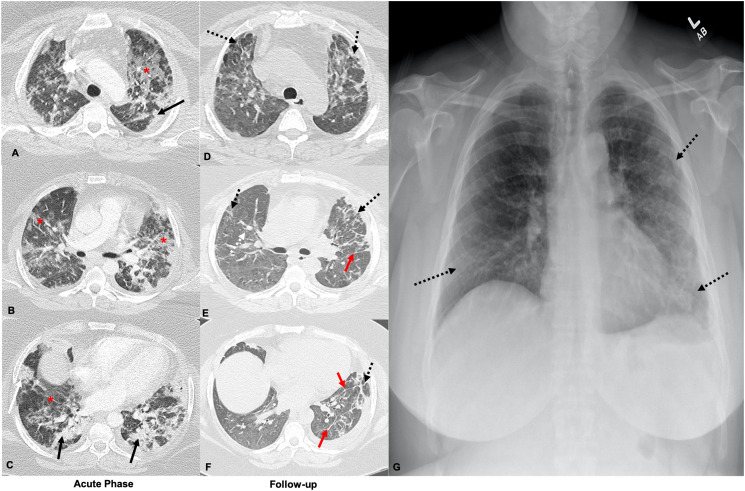
Fig. 1.
72-Year-old male who is a former smoker and has a remote history of asbestos exposure. Axial CT images obtained 1 day after reported symptom onset through the upper (A) and mid (B) lungs during the acute phase demonstrate multifocal ground glass opacities with adjacent interlobular lines in a ‘crazy-paving’ pattern (red *), with mild linear atelectasis in the left lower lobe (black arrowhead). Axial CT images through similar levels 14 days later (C and D) show improving ground glass opacities (red *) with new focal right upper lobe honeycombing (black arrow), volume loss in the right lower lobe with displacement of the right major fissure (white arrowhead), left lower lobe parenchymal band (red arrow), and developing bilateral peripheral reticular opacities (dashed black arrows). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
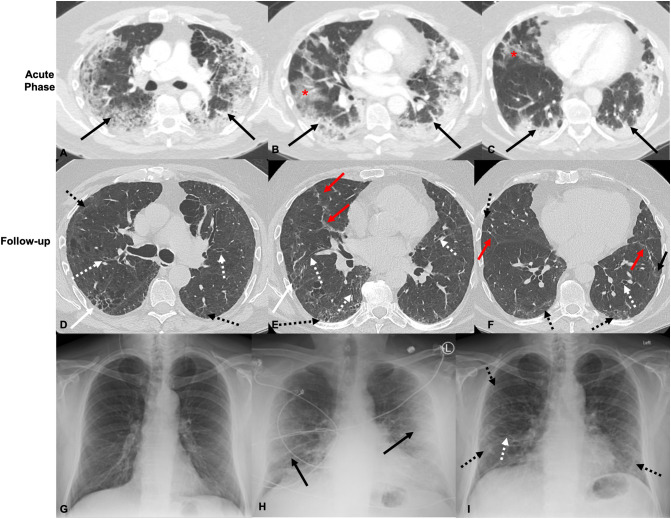
Fig. 2.
66-Year-old male who is a former smoker. Axial CT images obtained 15 days after reported symptom onset show bilateral peripheral ground glass (red *) and consolidative opacities (black arrows) during active COVID-19 infection in the upper (A), mid (B), and lower (C) lung zones. Axial CT images through the upper (D), mid (E), and lower (F) lung zones at the same level over 4 months after COVID-19 infection show new bilateral peripheral interstitial changes with increased reticular opacities (dashed black arrows), parenchymal bands (red arrows), and focal cystic change with increased reticulation in the posterior right upper lobe (white arrows). There are areas of increased bronchiectasis (dashed white arrows). Baseline PA radiograph 1 year prior to COVID-19 infection demonstrating clear lungs (G). AP Radiograph during known COVID-19 infection demonstrating bilateral peripheral opacities (white arrows) (H). PA Radiograph 2 months after COVID-19 infection demonstrating new prominent interstitial linear and reticular opacities in the lung periphery (dashed black arrows), increased bronchiectasis (dashed white arrows) with overall volume loss (I). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
89-Year-old female who is a former smoker. Axial CT images obtained 8 days after symptom onset showing ‘crazy paving’ with ground glass opacities in the upper and mid lungs during acute COVID infection (A and B) (red *). 66 days after symptom onset, the same areas in the upper and mid lungs (C and D) demonstrate prominent peripheral reticular opacities (dashed black arrows), bronchiectasis and bronchiolectasis (dashed white arrows), and parenchymal bands (red arrows). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
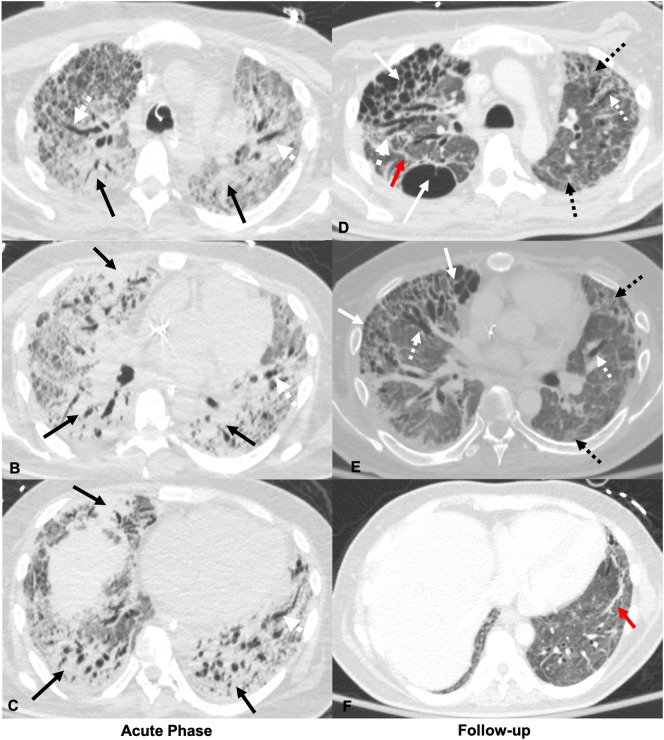
Fig. 4.
35-Year-old male with diabetes mellitus type 1. Axial CT images obtained at initial presentation, 40 days after symptom onset, through the upper (A), mid (B), and lower (C) lungs show diffuse dense consolidation (solid black arrows), diffuse ground glass opacities with prominent septal markings (red *), and traction bronchiectasis (dashed white arrows) during active COVID-19 infection. Axial CT through the upper (D), mid (E), and lower (F) lobes at the same level 114 days after symptom onset demonstrate resolution of the previously seen consolidation, significantly improved ground glass opacities, and development of bilateral peripheral cystic changes and a larger posterior right upper lobe cyst (solid white arrows), peripheral reticular opacities (dashed black arrows), parenchymal bands (red arrows), and multifocal areas of traction bronchiectasis and bronchiolectasis (dashed white arrows). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
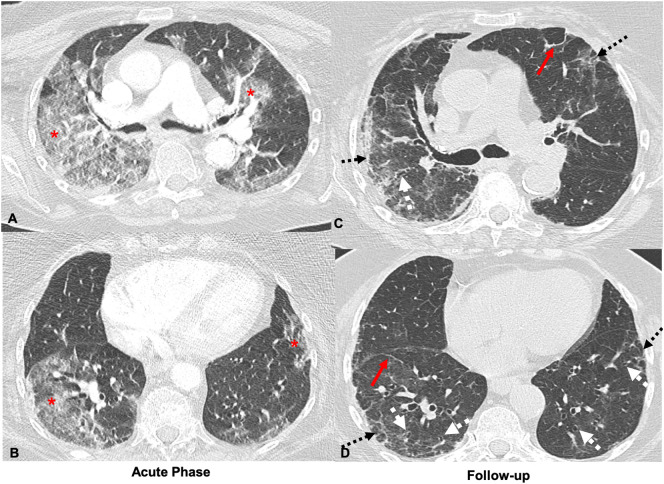
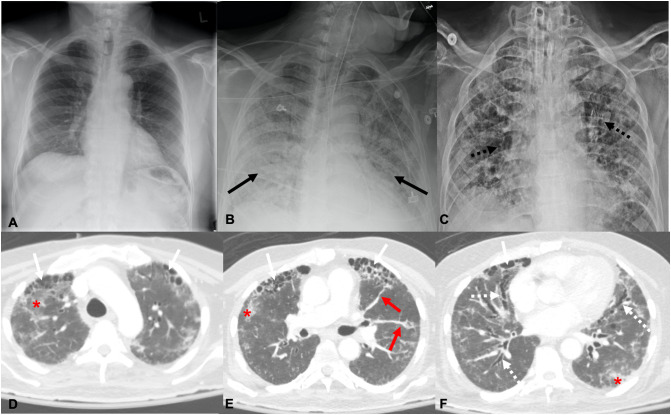
Fig. 5.
43-Year-old female with no history of smoking. Axial CT images obtained 13 days after symptom onset show bilateral ground glass (red *) and consolidative opacities (black arrows) during active COVID-19 infection through the upper (A), mid (B), and lower (C) lungs. Axial CT through the upper (D), mid (E), and lower (F) lobes at the same level 84 days after symptom onset demonstrate improving ground glass opacities with development of bilateral peripheral reticular opacities (dashed black arrows), parenchymal bands (red arrows) and adjacent architectural distortion. A chest radiograph obtained over a month after the CT demonstrates prominent interstitial markings throughout the lungs (black arrows) (G). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
76-Year-old male who is a former smoker. A chest radiograph obtained in Jun 2018 (A) demonstrates clear lung fields. A radiograph obtained about a month after symptom onset (B) demonstrates diffuse, confluent opacities throughout the lungs (solid black arrows) with clinically confirmed severe ARDS requiring intubation and eventual tracheostomy. Radiograph obtained 146 days after symptom onset shows volume loss and extensive interstitial pulmonary fibrotic changes (C). Axial CT images of the upper (D), mid (E), and lower (F) lung fields obtained 146 days after symptom onset demonstrate development of honeycombing (solid white arrows), traction bronchiectasis (dashed white arrows), parenchymal bands (solid red arrows), with persistent mild ground glass opacities (red *). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
These cases demonstrate imaging manifestations of interstitial changes and pulmonary fibrosis in 12 patients following COVID-19 infection, occurring in the same geographic distribution as the acute phase findings, up to approximately 6 and one-half months after the onset of symptoms. This provides further insight into the natural history of COVID-19 pneumonia and the potential development of interstitial lung disease that may last beyond clinical recovery, especially in patients who had severe acute pneumonia. The lack of other risk factors for interstitial lung disease, corresponding geographic distributions as acute changes, and the temporal relationship between the findings do indicate that pulmonary fibrosis can develop after COVID-19 pneumonia.
In the acute phase, COVID-19 pneumonia usually presents with bilateral, ill-defined predominantly subpleural and peripheral GGOs most commonly in the lower lobes. Also seen are consolidation, GGOs with reticulation leading to crazy paving, microvascular dilation, fibrotic streaks, subpleural lines, solid nodules, and pleural effusions.[7], [8], [9], [10] Acute phase findings are seen to peak during days 6–14 of the infection.10 , 11 At 3 weeks, Liu et al., found most of the patients had no residual lung disease, but over 40% had persistent GGOs or a fibrous stripe. They also observed that younger patients were more likely to have complete resolution of imaging findings.9 In another study by Liu et al., similar observations were made with most of the patients demonstrating resolution of findings by Week 5 but those with more severe pneumonia having persistent GGOs.10 Preliminary work by Fang et al. studying lung changes up to 56.1 ± 7.7 days similarly demonstrated gradually decreasing ground glass opacities and increasing reticulation and interlobular thickening; they also noted a slowing down of resolution of fibrotic changes by day 49.12 Yu et al. showed similar results and postulate that older or immunocompromised patients and those with worse respiratory status are more likely to develop fibrosis.5 Tissue sampling in deceased patients has shown fibroblast proliferation, airspace obliteration, and micro-honeycombing.13
It has been seen in ARDS survivors that 5-year lung changes are mild, often in the non-dependent areas of the lungs, and not associated with the initial disease course.14 SARS patients had similar outcomes with most recovering pulmonary function and resolution of imaging features within 2 years from the infection.15 In MERS, patients who had fibrotic lung changes within 1 year after infection had more severe acute pneumonia with a greater number of ICU admission days, positive imaging findings, older age, and higher lactate dehydrogenase levels.16 , 17 Data so far shows recovered COVID-19 patients have persistent deficits in lung function, particularly diffusion capacity and restrictive ventilatory defects, which are associated with disease severity.18 While there is not enough information to predict the natural course of interstitial changes and pulmonary fibrosis in COVID-19 patients, our preliminary data suggests that follow-up imaging post discharge, whether with radiography and/or HRCT, should be considered in recovered patients with persistent pulmonary symptoms that may be explained by fibrosis, particularly those who had positive imaging findings during the acute phase of infection. Additionally, patients with positive imaging findings during the acute phase should also be considered for early pulmonary rehabilitation in anticipation of developing fibrosis.
All our patients required hospitalization for COVID-19, and 11/12 patients had 40% or more of the lung affected by COVID-19 during the acute phase of infection, suggesting that those with greater disease severity and more extensive involvement during the acute phase may be more likely to develop fibrosis. Patients with <50% and predominantly peripheral involvement were more likely to have a better disease outcome and lesser chronic involvement. The extent or distribution of changes in the acute phase was not associated with the development of honeycombing specifically. 9 of the 12 patients had CTs during the acute phase, of which 6 demonstrated severe interstitial changes, only 3 of these 6 patients developed honeycombing but all had other imaging features of fibrosis (Supplementary Tables 4 and 5). All 5 patients who had >50% parenchymal involvement in the recovered phase suffered from significant comorbidities including BMI > 30 (3/5), diabetes mellitus type 1 (1/5), and Hepatitis B (1/5), all proinflammatory conditions, and one patient was also on immunosuppressants.
Given the predilection of this virus towards an older population and those with more comorbidities associated with a higher “pro-inflammatory” state, which are independent risk factors for idiopathic pulmonary fibrosis as well, the development of fibrotic lung changes in recovered COVID patients could be far more substantial than seen with SARS, MERS, or other recovered ARDS patients.19 Further work is required in the recovered COVID population to identify patients at risk of developing fibrosis, the impact on their pulmonary function, and if such changes are persistent as this would significantly alter not only post-recovery follow-up and management but also acute phase management in order to mitigate the development of permanent lung damage.
Funding
None.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinimag.2021.03.030.
Appendix A. Supplementary data
Supplementary tables
References
- 1.Spagnolo P., Balestro E., Aliberti S., et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020;8(8):750–752. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ojo A.S., Balogun S.A., Williams O.T., Ojo O.S. Pulmonary fibrosis in COVID-19 survivors: predictive factors and risk reduction strategies. Pulm Med. 2020;2020:6175964. doi: 10.1155/2020/6175964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Muller N.L., Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 4.Hatabu H., Hunninghake G.M., Richeldi L., et al. Interstitial lung abnormalities detected incidentally on CT: a position paper from the Fleischner Society. Lancet Respir Med. 2020;8(7):726–737. doi: 10.1016/S2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu M., Liu Y., Xu D., Zhang R., Lan L., Xu H. Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J Radiol. 2020;21(6):746–755. doi: 10.3348/kjr.2020.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson S., Kay F.U., Abbara S., et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - secondary publication. J Thorac Imaging. 2020;35(4):219–227. doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou S., Wang Y., Zhu T., Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;214(6):1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 8.Shi H., Han X., Jiang N., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D., Zhang W., Pan F., et al. The pulmonary sequalae in discharged patients with COVID-19: a short-term observational study. Respir Res. 2020;21(1):125. doi: 10.1186/s12931-020-01385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X., Zhou H., Zhou Y., et al. Temporal radiographic changes in COVID-19 patients: relationship to disease severity and viral clearance. Sci Rep. 2020;10(1):10263. doi: 10.1038/s41598-020-66895-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Dong C., Hu Y., et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020;296(2):E55–E64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang Y., Zhou J., Ding X., Ling G., Yu S. Pulmonary fibrosis in critical ill patients recovered from COVID-19 pneumonia: Preliminary experience. Am J Emerg Med. 2020;38(10):2134–2138. doi: 10.1016/j.ajem.2020.05.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grillo F., Barisione E., Ball L., Mastracci L., Fiocca R. Lung fibrosis: an undervalued finding in COVID-19 pathological series. Lancet Infect Dis. 2021;21(4):e72. doi: 10.1016/S1473-3099(20)30582-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcox M.E., Patsios D., Murphy G., et al. Radiologic outcomes at 5 years after severe ARDS. Chest. 2013;143(4):920–926. doi: 10.1378/chest.12-0685. [DOI] [PubMed] [Google Scholar]
- 15.Zhang P., Li J., Liu H., et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020;8:8. doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das K.M., Lee E.Y., Singh R., et al. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging. 2017;27(3):342–349. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang Il Jun M., Park Wan Beom, Kim Gayeon, et al. Long-term respiratory complication in patients with Middle East respiratory syndrome: 1-year follow-up after the 2015 outbreak in South Korea. Open Forum Infect Dis. 2017;4(1) [Google Scholar]
- 18.Mo X., Jian W., Su Z., et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55(6) doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8(8):807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables