Abstract
Purpose
To determine the incidence of and timing and predictors for progression from pre-plus to plus disease, based on evaluation of images.
Methods
Two trained readers independently evaluated posterior pole images of infants from 13 North American centers for pre-plus/plus disease, stage, and zone of retinopathy of prematurity (ROP). Discrepancies between readers were adjudicated. To be eligible for analysis, eyes had to have at least two imaging sessions, the earlier one with pre-plus disease.
Results
Of 681 eyes of 444 infants with pre-plus first detected at mean postmenstrual age (PMA) of 35.5 ± 2.1 weeks, 54 (7.9%) progressed to plus disease at a mean PMA of 37.6 ± 2.4 weeks with the mean interval for progression of 2.7 weeks (range, 0.4–8.9 weeks). Progression rate was higher for eyes with larger number of quadrants of pre-plus (44% for eyes with four quadrants vs 4% with one quadrant [P < 0.0001]), earlier PMA with pre-plus (18% for 32 weeks’ PMA vs 3% for PMA of >37 weeks [P = 0.02]), higher ROP stage (12% for stage 3, 2.5% for no ROP [P < 0.0001]), lower ROP zone (24% for zone I, 6% for zone II or no ROP [P < 0.0001]) at the time of first pre-plus detection.
Conclusions
Based on image evaluation, 8% of eyes progressed from pre-plus to plus disease at a mean interval of 3 weeks. Pre-plus in multiple quadrants, higher stages of ROP, and lower zones of ROP were associated with higher risk of progression. Image evaluation for pre-plus may help in the identification of high-risk eyes for developing plus disease.
Retinopathy of prematurity (ROP) is a leading cause of blindness in children.1–3 Plus disease, an important component of the clinical description of ROP, is characterized as sufficient vascular dilation and tortuosity in at least two quadrants of the posterior pole and is currently a major indication for treatment-requiring ROP.4,5 In 2005, the International Committee for the Classification of ROP extended the plus disease to pre-plus,5 to describe the intermediate stage between normal and plus disease, in which vessel dilation and tortuosity are abnormal yet not enough to be diagnosed as plus disease.5 The clinical diagnoses of pre-plus and plus disease are based on a set of reference images and therefore tend to be subjective with substantial variability across pediatric ophthalmologists and retinal specialists.6–9 However, vessel dilation and tortuosity are continuous measures used to assess pre-plus and plus disease. In recent years, posterior retinal images have been used to aid the diagnosis of pre-plus or plus disease. Our previous research has shown that image grading by nonphysician trained readers can detect almost all plus and pre-plus disease (sensitivity, 94%) with good specificity (81%).10 However, limited data are available describing the progression from pre-plus to plus disease based on image evaluation.11,12 The purpose of this study was to determine the rate of and timing and predictors for the progression from pre-plus to plus disease based on image evaluation.
Subjects and Methods
This study is a secondary analysis of data from the Telemedicine Approaches to Evaluating Acute-Phase Retinopathy of Prematurity (e-ROP) Study, a multicenter observational cohort study supported by the National Eye Institute of the National Institutes of Health to evaluate the validity, reliability, feasibility, safety, and relative cost-effectiveness of a digital imaging system to identify infants with referral-warranted ROP (ie, zone I ROP, stage 3 ROP or worse, or plus disease). The details of the design, methods, and results from the e-ROP Study have been published.13–19 Salient features of the study and image grading for determining pre-plus or plus disease are described here.
Infants with birth weight of <1,251 g were enrolled from 13 clinical centers in North America. Informed consent was obtained from the parent/guardian of eligible infants. The study protocol and informed consent process were approved by institutional review boards of participating clinical centers.
In addition to clinical examination by study-certified ophthalmologists, infants underwent a series of imaging sessions of the posterior pole and four peripheral quadrants for each eye using the RetCam Shuttle (Clarity Medical Systems, Pleasanton, CA). Imaging sessions began at 32 weeks’ postmenstrual age (PMA), and follow-up imaging continued as clinically indicated by until the examining ophthalmologist noted any of the following: mature retinal vessels, immature zone III on 2 sessions at least 7 days apart, ROP regressed or regressing on 2 visits at least 7 days apart, treatment for severe ROP, or if the infant reached 40 weeks’ PMA with no ROP or only stage 1 or 2 ROP. Image sets from each eye were uploaded to a secure web-based portal for remote evaluation. Following the standard grading protocol, two trained nonphysician readers in the central image reading center at the University of Pennsylvania independently evaluated images for posterior pole vessel status (normal, pre-plus, or plus), stage (1, 2, 3, 4, 5), and zone of ROP (I, II, III), with discrepancies adjudicated by a reading supervisor. For each quadrant of the posterior pole, trained readers determined whether the posterior pole vessels were normal or sufficiently abnormal based on vessel dilation and tortuosity to be designated as pre-plus or plus disease. A circular area of the retina within a radius of 3.5 disk diameters from the center of the disk was used to identify the dilation and tortuosity of arterioles and venules for grading (normal, pre-plus, plus). Plus disease was defined as severe dilation and tortuosity of arterioles and venules that was equal to or greater than that of the reference images of the International Classification of Retinopathy of Prematurity (ICROP) in two or more quadrants. Pre-plus disease was defined as vascular dilation or tortuosity that was not normal yet insufficient for the diagnosis of plus disease.4,5 If plus disease was present only in one quadrant, the eye was considered to have only pre-plus. Posterior pole vessels that did not demonstrate dilation and tortuosity were graded as normal.5 The trained readers determined zone of vascularization or the zone with morphologic features consistent with ROP. ROP was determined by the presence of a demarcation line (stage 1), a ridge (stage 2), extraretinal neovascularization (stage 3), or retinal detachment (stage 4 or 5). When the image was not of sufficient quality to grade posterior pole abnormality or specific ROP features, it was recorded as cannot grade. The discrepancies between two readers were adjudicated by an ophthalmologist reading supervisor.13,14 Readers and adjudicator were masked to results of all diagnostic examinations, previous gradings of both eyes, current grading of the fellow eye, and demographic data. Trained readers had very good reproducibility for grading posterior pole vessel normality (normal, pre-plus, plus), with percent agreement of 80% (weighted κ = 0.60) for inter-reader agreement and 86% (weighted κ = 0.73) for intra-reader agreement.14
Statistical Analysis
Descriptive analyses were performed for eyes with pre-plus disease and eyes that progressed from pre-plus to plus disease using mean, standard deviation, median, and range for continuous measures and percentage for categorical measures. Comparisons of demographics between infants with versus without progression to plus disease were performed using two-sample t test for continuous measures and the Fisher exact test for categorical measures. We evaluated associations of ocular characteristics from image evaluation with progression from pre-plus to plus using univariate and multivariate logistic regression models, and inter-eye correlation was accounted for by using generalized estimating equations. All statistical analyses were made using SAS v9.4 (SAS Institute Inc, Cary, NC) and were two-sided. A P value of <0.05 was considered statistically significant.
Results
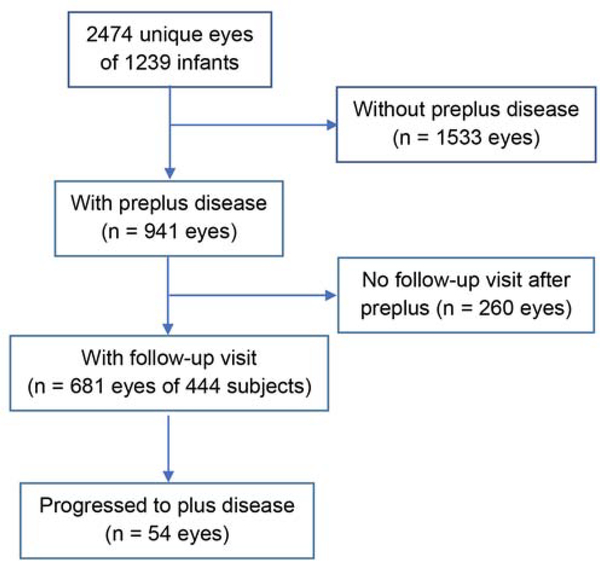
A total of 2,474 eyes of 1,239 infants with retinal images were included, 941 (38.0%) of which had pre-plus disease based on image evaluation. After excluding 260 pre-plus eyes (10.5%) without follow-up image session for determining progression to plus disease, 681 pre-plus eyes of 444 infants (mean birth weight, 773 g; mean gestational age [GA] of 26 weeks) had at least one follow-up session and were eligible for the pre-plus progression analysis (Figure 1). Of these 681 pre-plus eyes with a mean follow-up of 3.7 ± 2.2 weeks after the first diagnosis of pre-plus, 54 eyes of 41 infants (7.9%) progressed to plus, 356 eyes (52.2%) remained pre-plus at the last study examination (Figure 2A), and 271 eyes (39.8%) regressed to normal-appearing posterior pole vessels (Figure 2B). The demographics of infants with or those without progression to plus disease were shown in Table 1. The infants with progression from pre-plus to plus disease had lower mean birth weight (660 vs 760 g [P < 0.0001]), and lower GA (25 vs 26 weeks [P < 0.0001]) than infants without progression to plus disease (Table 1). Of all 681 eyes with pre-plus, the mean PMA was 35.5 ± 2.1 weeks (range, 32–42 weeks) at first pre-plus detection by images. Of the 54 eyes in which pre-plus progressed to plus disease, the mean PMA was 34.8 ± 2.0 weeks (range, 32–40 weeks) at first pre-plus detection by images and 37.6 ± 2.4 weeks (range, 33–41 weeks) at plus disease detection (Table 2).
FIG 1.
Eyes eligible for the analysis. Eligible eyes are those that had pre-plus disease in at least one image session followed by at least another image session for determining the progression to plus disease.
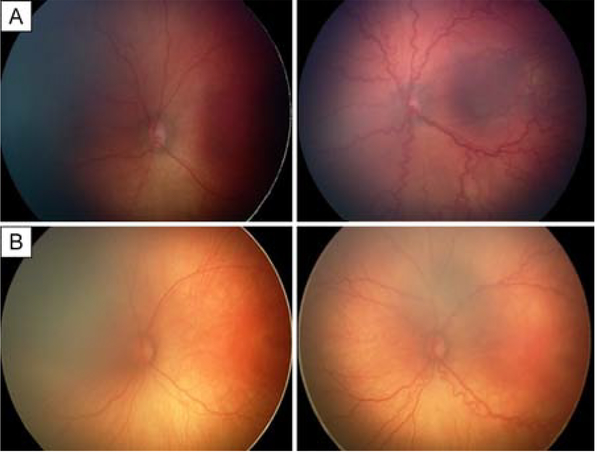
FIG 2.
Example of eyes that progressed from pre-plus to plus in 2 weeks (A) and a pre-plus eye that did not progress to plus in 3 weeks (B). Plus disease was defined as severe dilation and tortuosity of arterioles and venules that was equal to or greater than that of the reference images of the International Classification of Retinopathy of Prematurity (ICROP) in two or more quadrants; pre-plus disease, as vascular dilation or tortuosity that was not normal yet insufficient for the diagnosis of plus disease based on the ICROP reference images.
Table 1.
Demographics of infants with and without progression from pre-plus to plus disease
| Demographic characteristics | All infants with pre-plus (n = 444 infants) | Infants without progression to plus (n = 403 infants) | Infants progress to plus disease (n = 41 infants) | P value |
|---|---|---|---|---|
| Birth weight, g | <0.0001 | |||
| Mean ± SD | 773 ± 197 | 783 ± 200 | 676 ± 134 | |
| Median (Q1, Q3) | 748 (630, 900) | 760 (640, 917) | 660 (590, 775) | |
| Min, Max | 330, 1240 | 330, 1240 | 400, 1020 | |
| Gestational age, weeks | <0.0001 | |||
| Mean ± SD | 26 ± 1.8 | 26 ± 1.9 | 25 ± 1.2 | |
| Median (Q1, Q3) | 26 (24.7, 27.1) | 26 (24.7, 27.3) | 25 (24.1, 25.9) | |
| Min, Max | 23.0, 34.0 | 23.0, 34.0 | 23.1, 27.7 | |
| Sex, no. (%) | 0.61 | |||
| Female | 201 (45.3) | 184 (45.7) | 17 (41.5) | |
| Male | 243 (54.7) | 219 (54.3) | 24 (58.5) | |
| Race, no. (%) | 0.48 | |||
| White | 254 (57.2) | 230 (57.1) | 24 (58.5) | |
| Black | 126 (28.4) | 117 (29.0) | 9 (22.0) | |
| Other | 64 (14.4) | 56 (13.9) | 8 (19.5) | |
| Ethnicity, no. (%) | 0.17 | |||
| Hispanic | 64 (14.4) | 61 (15.1) | 3 (7.3) | |
| Non-Hispanic | 380 (85.6) | 342 (84.9) | 38 (92.7) | |
Table 2.
Distribution of postmenstrual age at its first detection of pre-plus and progression from pre-plus to plus disease in retinal images
| PMA, weeks | All eyes with pre-plus, no. (%), n = 681 eyes |
Eyes that progressed from pre-plus to plus disease, no. (%), n = 54 eyes |
|
|---|---|---|---|
| At first detection of pre-plus | At first detection of pre-plus | At the progression from pre-plus to plus | |
| 32 | 57 (8.4) | 10 (18.5) | 0 (0) |
| 33 | 124 (18.2) | 10 (18.5) | 3(5.6) |
| 34 | 104 (15.3) | 8 (11.1) | 2(3.7) |
| 35 | 126 (18.5) | 12 (22.2) | 9 (16.7) |
| 36 | 104 (15.3) | 8 (14.8) | 10 (18.5) |
| 37 | 64 (9.4) | 5 (9.3) | 6 (11.1) |
| 38 | 62 (9.1) | 2 (3.7) | 6 (11.1) |
| 39 | 20 (1.6) | 0 (0.0) | 6 (11.1) |
| 40 | 11 (1.6) | 1 (1.9) | 6 (11.1) |
| 41 | 7 (1.0) | 0 (0.0) | 6 (11.1) |
| 42 | 2 (0.3) | 0 (0.0) | 0 (0) |
| Mean ± SD | 35.5 (2.1) | 34.8 (2.0) | 37.6 (2.4) |
| Median (IQR) | 35.4 (33.9, 36.9) | 35.1 (33.1, 36.3) | 37.1 (35.6, 39.7) |
IQR, interquartile range; PMA, postmenstrual age; SD, standard deviation.
The results for associations between ocular characteristics at the first detection of pre-plus and progression to plus disease are provided in Table 3. On univariate analysis, the progression rate was higher in eyes with a greater number of quadrants of pre-plus (44% for eyes with four quadrants vs 4% with one quadrant [P < 0.0001]), earlier PMA (18% for 32 weeks’ PMA vs 3% for PMA >37 weeks [P = 0.02]), higher ROP stage (13% for stage 3, 8% for stage 2, and 2.4% for no ROP [P < 0.0001]), and a lower zone of ROP (24% for zone I, 6% for zone II ROP or no ROP [P < 0.0001]). See Table 3. In multivariate analysis, lower GA (P = 0.01), greater number of quadrants of pre-plus (P = 0.004) and higher stage and lower zone of ROP at pre-plus (P = 0.04) were independently associated with higher rate of progression from pre-plus to plus disease (Table 4).
Table 3.
Ocular characteristics at the first detection of pre-plus and associations with progression from pre-plus to plus disease based on image evaluation
| Ocular characteristics at first detection of pre-plus | Eyes with pre-plus disease, no., n = 681 | Eyes progressed from pre-plus to plus disease, no. (%), n = 54 | P value |
|---|---|---|---|
| Number of quadrants with pre-plus | <0.0001 | ||
| 1 | 424 | 17 (4.0%) | |
| 2 | 248 | 33 (13.3%) | |
| 4 | 9 | 4 (44.4%) | |
| PMA weeks at the first detection of pre-plus | 0.02 | ||
| 32 | 57 | 10 (17.5%) | |
| 33 | 124 | 10 (8.1%) | |
| 34 | 104 | 6 (5.8%) | |
| 35 | 126 | 12 (9.5%) | |
| 36 | 104 | 8 (7.7%) | |
| 37 | 64 | 5 (7.8%) | |
| >37 | 102 | 23 (2.9%) | |
| Stage at the first detection of pre-plus | <0.0001 | ||
| No ROP | 206 | 5 (2.4%) | |
| Stage 2 | 222 | 17 (7.7%) | |
| Stage 3 | 253 | 32 (12.6%) | |
| Zone at the first detection of pre-plus | <0.0001 | ||
| No ROP | 206 | 5 (2.4%) | |
| Zone II ROP | 391 | 29 (7.4%) | |
| Zone I ROP | 84 | 20 (23.8%) | |
| Combination of stage and zone at the first detection of pre-plus | <0.0001 | ||
| No ROP | 206 | 5 (2.4%) | |
| Stage 2, zone I | 26 | 6 (23.1%) | |
| Stage 2, zone II | 196 | 11 (5.6%) | |
| Stage 3, zone I | 58 | 14 (24.1%) | |
| Stage 3, zone II | 195 | 18 (9.2%) | |
PMA, postmenstrual age.
Table 4.
Multivariate analysis of predictors for progression from pre-plus to plus disease
| Predictors | Eyes with pre-plus, no., n = 681 | Eyes progressed from pre-plus to plus disease, no. (%), n = 54 | OR (95% CI) | P value |
|---|---|---|---|---|
| GA per week decrease | 1.33 (1.07–1.65) | 0.01 | ||
| GA, weeks (as categorical value) | 0.01 | |||
| 23 | 76 | 9 (11.8) | 4.28 (0.92–19.8) | |
| 24 | 159 | 18 (11.3) | 4.68 (1.23–17.7) | |
| 25 | 135 | 15 (11.1) | 5.19 (1.22–20.2) | |
| 26 | 133 | 9 (6.8) | 3.01 (0.73–12.3) | |
| ≥27 | 168 | 3 (1.9) | 1.00 | |
| Number of quadrants with pre-plus at the first detection of pre-plus | 0.004 | |||
| 1 | 424 | 17 (4.0) | 1.00 | |
| 2 | 248 | 33 (13.3) | 2.88 (1.40–5.91) | |
| 4 | 9 | 4 (44.4) | 13.5 (2.96–61.7) | |
| Combination of ROP stage and zone at the time of first pre-plus | 0.04 | |||
| No ROP | 206 | 5 (2.4) | 1.00 | |
| Stage 2, zone I | 26 | 6 (23.1) | 6.43 (1.88–21.9) | |
| Stage 2, zone II | 196 | 11 (5.6) | 1.54 (0.51–4.65) | |
| Stage 3, zone I | 58 | 14 (24.1) | 4.76 (1.25–18.2) | |
| Stage 3, zone II | 195 | 18 (9.2) | 1.96 (0.61–6.33) | |
CI, confidence interval; GA, gestational age; OR, odds ratio.
Of the 54 eyes with progression from pre-plus to plus disease, the mean time interval between first detection of pre-plus pre-plus and first detection of plus disease was 2.7 ± 1.7 weeks (range, 0.4–8.9). Time between first detection of pre-plus and first detection of plus disease varied with ROP stage and zone (Table 5), with mean intervals of 3.9 weeks in eyes with stage 2 zone II ROP and 2.2 weeks for eyes with stage 3 zone II ROP (P = 0.01).
Table 5.
Time intervals for progression from pre-plus to plus disease by combination of stage and zone
| Combination of stage and zone at first detection of pre-plus | Time interval, weeks, between first detection of pre-plus and first detection of plus disease | |||
|---|---|---|---|---|
| # eyes progressed to plus | Mean ± SD | Median (IQR) | Minimum, maximum | |
| No ROP | 5 | 1.9 ± 0.7 | 2 (1.7, 2.0) | 1, 3 |
| Stage 2, zone 1 | 6 | 2.9 ± 1.2 | 2.9 (2, 4) | 1.3, 4.0 |
| Stage 2, zone II | 11 | 3.9 ± 2.2 | 3 (3, 4) | 1, 8.9 |
| Stage 3, zone I | 14 | 2.8 ± 1.8 | 3 (2, 5) | 1, 7 |
| Stage 3, zone II | 18 | 2.2 ± 1.3 | 2 (1, 3) | 0.6, 5.0 |
| All combined | 54 | 2.7 ± 1.7 | 2.5 (1.6, 3.3) | 0.4, 8.9 |
IQR, interquartile range; SD, standard deviation.
Discussion
Image evaluation in e-ROP showed that around 8% of eyes with an initial diagnosis of pre-plus disease progressed to plus disease with a mean interval of 3 weeks. Lower gestational age, more quadrants with pre-plus, higher stage and lower zone of ROP at first detection of pre-plus were independently associated with higher risk of progression from pre-plus to plus disease. These findings suggest that documenting characteristics of pre-plus and ROP using image evaluation may help identify eyes that are more likely to develop plus disease. Because plus disease is currently a major clinical feature indicating the need for the treatment of severe ROP and delay of treatment for severe ROP can lead to the poor vision outcome,20 eyes with these high-risk features should be monitored closely for early detection of plus disease and prompt ROP treatment if indicated.
Consistent with our study, several previous studies demonstrated that presence of pre-plus or features of pre-plus were associated with development of plus disease.11,21 Wallace and colleagues21 studied 214 premature infants and found that in 10 eyes with pre-plus at 33–34 weeks’ PMA, 7 eyes (70%) subsequently underwent laser treatment, whereas of 154 eyes without pre-plus at 33–34 weeks’ PMA, only 14 (9%) required laser treatment. This association remained significant even after adjustment by birth weight, GA, and ROP stage and zone. Ghodasra and colleagues11 compared the posterior pole vessel dilation and tortuosity in images between 11 pre-plus eyes that progressed to plus and 19 pre-plus eyes that regressed spontaneously and found that eyes that progressed to plus had wider vessels and were more tortuous than eyes that did not progress; they concluded that digital image analysis can be useful for predicting which pre-plus ROP eyes would require treatment.
Because presence and characteristics of pre-plus are predictive for the progression to plus disease, image analysis tools (such as ROPtool,22 CAIAR,23 RISA24) for identifying and quantifying the pre-plus disease characteristics based on vessel dilation and tortuosity have been developed for determining the risk of progression to plus disease.20 Future research to further evaluate and validate these objective tools for recognizing the presence of pre-plus disease and for quantifying the severity of pre-plus disease will likely help identify eyes that will develop plus disease, thus improving the detection of eyes with plus disease for timely treatment.
This is the first and the largest cohort study using retinal images for evaluating the incidence and timing of first detection of pre-plus and the progression from pre-plus to plus disease. Our study found that pre-plus disease is common (38%) in eyes of premature infants, and about 8% of pre-plus progressed to plus disease. We found that pre-plus disease was first detected in images at median of 35 weeks’ PMA and could progress to plus disease at a median of 3 weeks. This information on the incidence and timing of pre-plus disease and plus disease is important for developing screening program for using retinal images to monitor posterior pole vessel changes and detect plus disease.
Our study found that eyes with higher ROP stage and/or lower zone at the time of first detection of pre-plus were independently associated with higher risk of progression of pre-plus to plus. This finding is consistent with the previous clinical observations that plus disease typically occurred in eyes with advanced ROP.20,25 We found that larger number of quadrants with pre-plus at the time of first detection of pre-plus was associated with higher risk of progression from pre-plus to plus. In eyes with only one quadrant with pre-plus, 4% progressed to plus disease; the progression increased to 13% in eyes with two quadrants of pre-plus, and progression to plus disease reached 44% in eyes with four quadrants noted to have pre-plus disease. Although this finding is not surprising, given that presence of abnormal dilation and tortuosity be present in at least two quadrants to diagnose plus disease,26 it is helpful for identifying eyes at higher risk of progression to plus disease.
The strengths of our study are the large sample size from multiclinical centers and the independent grading of pre-plus disease and plus disease by two trained graders, with discrepancies adjudicated by a senior reader following the standard grading protocol in the central reading center. However, the study is limited by the fact that the grading of pre-plus and plus disease is subjective. Future studies using semiautomated grading of retinal vessels for detecting pre-plus or plus disease will address this limitation. Also, because infants had different patterns of follow-up after pre-plus in retinal images, some eyes remained pre-plus disease without further follow-up to observe regression to normal or progression to plus disease. Because the timing of imaging was based on when the clinical examination was performed, the time of first pre-plus detection on images was not necessarily exactly when pre-plus first developed, and can be delayed if image quality was poor. Finally, because these images were taken with a contact imaging system, if too much pressure was placed on the eye, the diagnosis of pre-plus and/or plus disease might be under-called.27,28 Despite these limitations, our findings demonstrate the importance of image evaluation in determining the presence and characteristics of pre-plus disease at its first diagnosis, which can make screening and timely detection of plus disease more effective and may contribute to improved treatment outcomes of severe ROP.
Supplementary Material
Acknowledgments
Supported by National Eye Institute of the National Institutes of Health, Department of Health and Human Services. U10 EY017014 and R21EY025686.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res 2013; 74 Suppl 1:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blencowe H, Moxon S, Gilbert C. Update on blindness due to retinopathy of prematurity globally and in India. Indian Pediatr 2016; 53 Suppl 2:S89–S92. [PubMed] [Google Scholar]
- 3.Gilbert C Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev 2008;84:77–82. [DOI] [PubMed] [Google Scholar]
- 4.Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol 1984;102:1130–34. [DOI] [PubMed] [Google Scholar]
- 5.The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123:991–9. [DOI] [PubMed] [Google Scholar]
- 6.Chiang MF, Gelman R, Jiang L, Martinez-Perez ME, Du YE, Flynn JT. Plus disease in retinopathy of prematurity: an analysis of diagnostic performance. Trans Am Ophthalmol Soc 2007;105:73–84. [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang MF, Jiang L, Gelman R, Du YE, Flynn JT. Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmol 2007;125:875–80. [DOI] [PubMed] [Google Scholar]
- 8.Chiang MF, Keenan JD, Du YE, et al. Assessment of image-based technology: impact of referral cutoff on accuracy and reliability of remote retinopathy of prematurity diagnosis. AMIA Annu Symp Proc 2005:126–30. [PMC free article] [PubMed] [Google Scholar]
- 9.Cook A, White S, Batterbury M, Clark D. Ocular growth and refractive error development in premature infants without retinopathy of prematurity. Invest Ophthalmol Vis Sci 2003;44:953–60. [DOI] [PubMed] [Google Scholar]
- 10.Cheng QE, Daniel E, Pan W, et al. Plus disease in Telemedicine Approaches to Evaluating Acute-Phase ROP (e-ROP) Study: characteristics, predictors, and accuracy of image grading. Ophthalmology 2019;126:868–75. [DOI] [PubMed] [Google Scholar]
- 11.Ghodasra DH, Karp KA, Ying GS, et al. Risk stratification of preplus retinopathy of prematurity by semiautomated analysis of digital images. Arch Ophthalmol 2010; 128:719–23. [DOI] [PubMed] [Google Scholar]
- 12.Wallace DK, Freedman SF, Hartnett ME, Quinn GE. Predictive value of pre-plus disease in retinopathy of prematurity. Arch Ophthalmol 2011;129:591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel E, Pan W, Quinn GE, Smith E, Baumritter A, Ying GS. Single grading vs double grading with adjudication in the telemedicine approaches to evaluating acute-phase retinopathy of prematurity (e-ROP) study. J AAPOS 2018;22:32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel E, Quinn GE, Hildebrand PL, et al. Validated system for centralized grading of retinopathy of prematurity: Telemedicine Approaches to Evaluating Acute-Phase Retinopathy of Prematurity (e-ROP) Study. JAMA Ophthalmol 2015;133:675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn GE, Ying GS, Daniel E, et al. Validity of a telemedicine system for the evaluation of acute-phase retinopathy of prematurity. JAMA Ophthalmol 2014;132:1178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinn GE, Ying GS, Pan W, Baumritter A, Daniel E. detection of potentially severe retinopathy of prematurity by remote image grading. JAMA Ophthalmol 2017;135:982–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying GS, Pan W, Quinn GE, Daniel E, Repka MX, Baumritter A. Intereye agreement of retinopathy of prematurity from image evaluation in the Telemedicine Approaches to Evaluating of Acute-Phase ROP (e-ROP) Study. Ophthalmol Retina 2017;1:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying GS, Quinn GE, Wade KC, Repka MX, Baumritter A, Daniel E. Predictors for the development of referral-warranted retinopathy of prematurity in the telemedicine approaches to evaluating acute-phase retinopathy of prematurity (e-ROP) study. JAMA Ophthalmol 2015;133:304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ying GS, VanderVeen D, Daniel E, Quinn GE, Baumritter A. Risk score for predicting treatment-requiring retinopathy of prematurity (ROP) in the Telemedicine Approaches to Evaluating Acute-Phase ROP Study. Ophthalmology 2016;123:2176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davitt BV, Wallace DK. Plus disease. Surv Ophthalmol 2009;54:663–70. [DOI] [PubMed] [Google Scholar]
- 21.Wallace DK, Freedman SF, Hartnett ME, Quinn GE. Predictive value of pre-plus disease in retinopathy of prematurity. Arch Ophthalmol 2011;129:591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace DK, Zhao Z, Freedman SF. A pilot study using “ROPtool” to quantify plus disease in retinopathy of prematurity. J AAPOS 2007;11:381–7. [DOI] [PubMed] [Google Scholar]
- 23.Wilson CM, Cocker KD, Moseley MJ, et al. Computerized analysis of retinal vessel width and tortuosity in premature infants. Invest Ophthalmol Vis Sci 2008;49:3577–85. [DOI] [PubMed] [Google Scholar]
- 24.Swanson C, Cocker KD, Parker KH, Moseley MJ, Fielder AR. Semiautomated computer analysis of vessel growth in preterm infants without and with ROP. Br J Ophthalmol 2003;87:1474–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaffer DB, Palmer EA, Plotsky DF, et al. ; The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Prognostic factors in the natural course of retinopathy of prematurity. Ophthalmology 1993;100:230–37. [DOI] [PubMed] [Google Scholar]
- 26.Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics 2000;105:295–310. [DOI] [PubMed] [Google Scholar]
- 27.Zepeda-Romero LC, Martinez-Perez ME, Ramirez-Ortiz MA, Gutierrez-Padilla JA. RetCam compression artifact can mask plus disease. Eye (Lond) 2009;23:2266–7. [DOI] [PubMed] [Google Scholar]
- 28.Zepeda-Romero LC, Martinez-Perez ME, Ruiz-Velasco S, Ramirez-Ortiz MA, Gutierrez-Padilla JA. Temporary morphological changes in plus disease induced during contact digital imaging. Eye (Lond) 2011;25:1337–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.