Abstract
Purpose.
Health-related quality of life (HRQOL) among older cancer survivors can be impaired by factors such as treatment, comorbidities, and social challenges. These HRQOL impairments may be especially pronounced in rural areas, where older adults have higher cancer burden and more comorbidities and risk factors for poor health. This study aimed to assess rural-urban differences in HRQOL for older cancer survivors and controls.
Methods.
Data came from Surveillance, Epidemiology, and End Results-Medicare Health Outcomes Survey (SEER-MHOS), which links cancer incidence from 18 U.S. population-based cancer registries to survey data for Medicare Advantage Organization enrollees (1998–2014). HRQOL measures were 8 standardized subscales and 2 global summary measures. We matched (2:1) controls to breast, colorectal, lung, and prostate cancer survivors, creating an analytic dataset of 271,640 participants (ages 65+). HRQOL measures were analyzed with linear regression models including multiplicative interaction terms (rurality by cancer status), controlling for sociodemographics, cohort, and multimorbidities.
Results.
HRQOL scores were higher in urban than rural areas (e.g., global physical component summary score for breast cancer survivors: urban mean=38.7, standard error [SE]=0.08; rural mean=37.9, SE=0.32; p<.05), and were generally lower among cancer survivors compared to controls. Rural cancer survivors had particularly poor vitality (colorectal: p=.05), social functioning (lung: p=.05), role limitation-physical (prostate: p<.01), role limitation-emotional (prostate: p<.01), and global mental component summary (prostate: p=.02).
Conclusion.
Supportive interventions are needed to increase physical, social, and emotional HRQOL among older cancer survivors in rural areas. These interventions could target cancer-related stigma (particularly for lung and prostate cancers) and/or access to screening, treatment, and ancillary healthcare resources.
Keywords: Health-related quality of life, cancer survivorship, older adults, rural, urban, health disparities
Demographic trends [1] and improvements in cancer diagnosis and treatment [2] have resulted in increasing numbers of older adult cancer survivors. Health-related quality of life (HRQOL) is a multidimensional construct reflecting physical, psychological, and social well-being related to health [3]. HRQOL has been an area of research for cancer survivors for at least 25 years, especially as survival after cancer treatment has become longer [4].
Compared to younger cancer survivors, older cancer survivors experience multiple challenges to their HRQOL, including more comorbidities (e.g., depression, anemia) [5, 6]. Rural areas have greater proportions of older residents [7], and they also have elevated cancer rates [8, 9]. HRQOL among cancer survivors in rural areas is poorer than among other cancer survivors [10–12]. However, to our knowledge, no national studies have examined rural-urban differences in HRQOL among older cancer survivors compared to people who have not had cancer.
HRQOL is an important patient-reported outcome in its own right and due to its relationship with other clinical outcomes [13–17]. HRQOL among cancer survivors varies by health behaviors [18], social support [19], and cancer treatment [20], all of which may differ for people living in urban areas versus rural areas [21–24]. For example, smoking is more common in rural than urban areas [25], including among cancer survivors [26], and is associated with lower HRQOL among cancer survivors [18]. Similarly, definitive treatment is less common in rural than urban areas [22, 27], which could impair HRQOL. Thus, a clear understanding of the impact of rurality on HRQOL among cancer survivors is needed.
In this study, we used a large, national dataset linking cancer outcomes data from the National Cancer Institute (NCI)’s Surveillance, Epidemiology, and End Results (SEER) program [28] to HRQOL survey data from Medicare Advantage Organization (MAO) enrollees to examine the impact of rurality on HRQOL for older cancer survivors versus matched controls. We hypothesized that case-control differences in HRQOL would be greater for participants in rural than urban areas. As we anticipate the “silver tsunami” [1] of older cancer survivors in the coming years, these results can proactively identify additional needs for clinical, public health, and policy interventions to support HRQOL among survivors in rural areas.
Materials and Methods
Data source
SEER-Medicare Health Outcomes Survey (SEER-MHOS) is a dataset linking SEER cancer incidence data with survey responses from MHOS [29]. SEER is a collection of 18 population-based cancer registries [28], covering about 28% of the U.S. population [30]. Cancer cases diagnosed through 2013 were included in the current analysis. MHOS is a population-based survey measuring HRQOL, health issues, functioning, and demographics among MAO enrollees. Each year, MHOS is administered to a random sample of beneficiaries in MAO plans that have at least 500 enrollees. The survey is available in English, Spanish, Chinese, and Russian, and participants complete the survey via a mailed questionnaire or telephone-administered interview. We used SEER-MHOS data collected from 1998 to 2014 [29]. Collecting data on cancer cases through 2013 and MHOS through 2014 increases the opportunity for recently-diagnosed patients to complete the MHOS survey post-diagnosis. NCI and Centers for Medicare and Medicaid Services (CMS) collaboratively link individuals in SEER and MHOS using identifiers from the SEER-Medicare dataset [31]. Additional details on SEER-MHOS are available at https://healthcaredelivery.cancer.gov/seer-mhos/.
Analytic sample
In the current analysis, we limited the sample to participants aged 65 years or older with a history of cancer before the baseline survey (“cancer survivors”) and participants who had never been diagnosed with cancer (“controls”). Response rates for the baseline survey were 64%−72% across cohorts [31].
Cancer survivors were limited to participants who were diagnosed with one of the four most common types of cancer [32] prior to completing a baseline survey: breast (females only; n=28,398), colorectal (n=19,131), lung (n=16,491), or prostate (males only; n=31,607).
Controls were participants who (1) had no cancer diagnosis recorded in SEER and (1) did not self-report a cancer diagnosis on the survey. We performed a 2:1 match of controls to cancer survivors (respectively) based on sex (if applicable), age, race/ethnicity, and MHOS cohort. Each control participant could serve as a control in one or more cancer type groups (i.e., some non-cancer participants served as controls in two or more cancer patient groups), resulting in a sample of 176,013 controls (total n=271,640).
Measures
Dependent variables: HRQOL measures.
HRQOL was assessed using two tools: SF-36 [33] for years 1998–2005, and VR-12 [34] for years 2006–2014. Scores on the two HRQOL tools were standardized with a bridging algorithm to ensure comparability, as described elsewhere [35]. Resulting HRQOL scores ranged from 0 to 100, with a population mean of 50 and a standard deviation of 10, based on 1990 population norms, with higher scores indicating better HRQOL. We gathered standardized scores on ten HRQOL domains (Supplementary Table S1), including eight subscales: (1) physical functioning; (2) role limitation-physical; (3) pain; (4) general health; (5) vitality; (6) social functioning; (7) role limitation-emotional; and (8) emotional well-being; and two global summary measures: (9) physical component summary and (10) mental component summary. These subscales and global summary measures have demonstrated adequate psychometric properties in other studies [35, 36].
Independent variable: Rurality.
We assessed rurality based on county of residence at the time of MHOS using the U.S. Department of Agriculture’s rural-urban continuum codes [37], which are based on population size, urbanization, and proximity to a metropolitan area. Per previous research studies [38], we coded counties with scores of 1–3 (metropolitan) as urban and counties with scores of 4–9 (non-metropolitan) as rural.
Control variables.
From SEER-MHOS, we gathered several control variables that could affect the relationship between rurality and HRQOL: sex (male vs. female); race/ethnicity (non-Hispanic white; non-Hispanic black; Hispanic; vs. other); annual household income ($50,000 or more; less than $50,000; vs. missing); educational attainment (high school degree or more vs. less than a high school degree); marital status (married; divorced/separated; widowed; vs. never married); Census region (Northeast; Midwest; South; vs. West); age (in years); and a multimorbidity index [39] summing selected self-reported comorbidities [40] (i.e., 1 point for each: angina/pectoris/coronary artery disease; arthritis; congestive heart failure; Crohn’s disease/ulcerative colitis/inflammatory bowel disease; diabetes/high blood sugar/sugar in urine; emphysema/asthma/COPD; hypertension; myocardial infarction; stroke). We also controlled for MHOS cohort year.
We summarized additional characteristics among the cancer survivors: cancer stage (in situ, localized, regional, distant, local+regional [prostate cancer only], or unstaged); time between cancer diagnosis and completing MHOS (in months); and characteristics of their census tract (percent of residents living in households below the federal poverty line and population density (residents per square mile)).
Statistical analysis
First, we generated descriptive statistics for the study variables. Next, we estimated the unadjusted mean and standard error (SE) of HRQOL subscale and global summary scores for each group (controls, breast cancer survivors, colorectal cancer survivors, lung cancer survivors, prostate cancer survivors). Using two-sample t-tests, we evaluated differences in these scores across levels of rurality.
Then, we used linear regression models to examine differences in HRQOL subscale and global summary scores for each group of cancer survivors compared to their matched controls, stratified by rurality. From these models, we gathered first the unadjusted differences (i.e., beta coefficients) using bivariate linear regression, and then the adjusted differences using multivariable linear regression, adjusting for the control variables described above. Finally, we reran the multivariable linear regression models adding multiplicative interaction terms for cancer status and rurality to test whether the difference in HRQOL subscale and global summary scores for cancer survivors versus controls varied for rural versus urban participants. We probed selected models with statistically-significant interaction terms (Wald chi-square p<.10) by estimating the adjusted marginal means for the HRQOL scores separately for urban controls, urban cancer survivors, rural controls, and rural cancer survivors. Models estimated robust standard errors to prevent against bias in case of heteroscedasticity.
All analyses were conducted using SAS version 9.4 (Cary, NC) and, except where noted, used a two-sided p value of .05. The SEER-MHOS dataset is sponsored by NCI and CMS (https://healthcaredelivery.cancer.gov/seer-mhos/). The proposal for the current analysis was reviewed and approved by the SEER-MHOS team at NCI. The study was exempt from institutional review board review because it involved analysis of previously-collected, deidentified data.
Results
A summary of participant characteristics appears in Table 1. Overall, 5.7% of participants were living in rural areas at the time of the survey (controls: 5.7%; breast cancer survivors: 5.6%; colorectal cancer survivors: 5.9%; lung cancer survivors: 5.3%; prostate cancer survivors: 5.6%). Most participants were non-Hispanic white (77.2%), had at least a high school degree (71.8%), and were married (58.0%). The average age was 74.70 years (SE=0.01), with a score of 1.92 (SE=<0.01) on the multimorbidity index. Among cancer survivors, the average time between cancer diagnosis and completing the survey was 83 months (SE=0.54) for breast cancer, 65 (SE=0.60) for colorectal cancer, 27 (SE=0.45) for lung cancer, and 59 (SE=0.37) for prostate cancer.
Table 1.
Descriptive statistics for overall sample and by group, SEER-MHOS, 1998–2014 (n=271,640).
| All participants | Controls | Breast cancer survivors | Colorectal cancer survivors | Lung cancer survivors | Prostate cancer survivors | |
|---|---|---|---|---|---|---|
| n=271,640 | n=176,013 | n=28,398 | n=19,131 | n=16,491 | n=31,607 | |
| % | % | % | % | % | % | |
| Rurality1 | ||||||
| Urban | 94.3 | 94.3 | 94.4 | 94.1 | 94.7 | 94.5 |
| Rural | 5.7 | 5.7 | 5.6 | 5.9 | 5.3 | 5.6 |
| Sex | ||||||
| Male | 51.0 | 50.9 | -- | 48.3 | 50.4 | 100.0 |
| Female | 49.0 | 49.1 | 100.0 | 51.7 | 49.6 | -- |
| Race/ethnicity | ||||||
| Non-Hispanic white | 77.2 | 79.2 | 74.4 | 72.2 | 77.8 | 70.9 |
| Non-Hispanic black | 8.3 | 7.6 | 8.6 | 9.1 | 8.8 | 11.3 |
| Hispanic | 6.7 | 6.0 | 7.5 | 8.2 | 5.4 | 9.3 |
| Other | 7.8 | 7.1 | 9.4 | 10.6 | 8.1 | 8.5 |
| Annual household income | ||||||
| $50,000 or more | 11.8 | 11.0 | 10.9 | 11.2 | 8.6 | 18.7 |
| Less than $50,000 | 68.3 | 69.0 | 66.8 | 67.8 | 70.9 | 64.7 |
| Missing | 20.0 | 20.0 | 22.3 | 21.0 | 20.5 | 16.6 |
| Educational attainment | ||||||
| High school degree or more | 71.8 | 71.0 | 77.4 | 69.9 | 67.2 | 74.4 |
| Less than a high school degree | 28.3 | 29.0 | 22.6 | 30.1 | 32.8 | 25.6 |
| Marital status | ||||||
| Married | 58.0 | 58.2 | 43.9 | 54.3 | 53.5 | 74.4 |
| Divorced/separated | 11.9 | 11.4 | 14.8 | 11.6 | 15.6 | 10.2 |
| Widowed | 26.4 | 26.6 | 37.3 | 30.4 | 27.7 | 12.1 |
| Never married | 3.7 | 3.8 | 4.1 | 3.8 | 3.2 | 3.4 |
| Census region | ||||||
| Northeast | 18.9 | 21.4 | 12.7 | 15.3 | 14.5 | 14.3 |
| Midwest | 18.0 | 22.6 | 9.2 | 8.9 | 9.5 | 8.6 |
| South | 24.8 | 27.4 | 18.4 | 18.7 | 22.3 | 19.7 |
| West | 38.3 | 28.7 | 59.7 | 57.1 | 53.8 | 57.4 |
| Cancer stage | ||||||
| In situ | -- | -- | 16.4 | 5.6 | 1.8 | 0.8 |
| Localized | -- | -- | 55.7 | 44.4 | 26.9 | 4.2 |
| Regional | -- | -- | 19.1 | 30.5 | 22.9 | 1.5 |
| Distant | -- | -- | 2.5 | 8.2 | 34.8 | 3.3 |
| Local+Regional | -- | -- | -- | -- | -- | 84.4 |
| Unstaged | -- | -- | 6.3 | 7.9 | 10.0 | 5.8 |
| mean(SE) | mean(SE) | mean(SE) | mean(SE) | mean(SE) | mean(SE) | |
| Age, years (range: 65–108) | 74.70(0.01) | 74.74(0.02) | 74.39(0.05) | 75.93(0.06) | 73.30(0.05) | 74.86(0.04) |
| Multimorbidity index (range: 0–9)2 | 1.92(0.00) | 1.92(0.0) | 1.91(0.01) | 1.92(0.01) | 2.06(0.01) | 1.84(0.01) |
| Time since diagnosis, months (range: 0–470) | -- | -- | 83.04(0.54) | 64.57(0.60) | 26.60(0.45) | 59.33(0.37) |
| % census tract residents in poverty (range: 0–100) | -- | -- | 13.76(0.08) | 14.03(0.10) | 14.29(0.11) | 13.71(0.07) |
| Census tract population density (range: 0–211,402) | -- | -- | 4113(41.9) | 4165(53.0) | 3882(56.2) | 4052(38.0) |
Participants were classified as urban if they lived in a county with a USDA rural-urban continuum code [37] of 1, 2, or 3 at the time of MHOS, and they were classified as rural if they lived in a county with a code of 4 or greater.
Multimorbidity index summed self-reported comorbidities including angina, stroke, chronic obstructive pulmonary disease, diabetes, hypertension, gastrointestinal disease, and arthritis.
Note. Percentages may not sum to 100% due to rounding and missingness.
Unadjusted (overall and case-control) differences in HRQOL by rurality
Participants tended to have the highest scores on the global mental component summary measure and the lowest scores on the physical functioning subscale or global physical component summary measure (Table 2). Generally, unadjusted HRQOL subscale and global summary scores were higher for participants in urban than rural areas. For example, scores on the physical functioning subscale were higher for urban than rural participants for lung cancer survivors (urban: mean=37.1, SE=0.11; rural: mean=35.3, SE=0.47; p<.001) and prostate cancer survivors (urban: mean=41.6, SE=0.08; rural: mean=40.0, SE=0.32; p<.001).
Table 2.
Unadjusted mean scores on health-related quality of life (HRQOL) subscales, stratified by rurality and cancer history, SEER-MHOS, 1998–2014 (n=271,640).
| Urban | |||||||||||||||
| Controls | Breast cancer survivors | Colorectal cancer survivors | Lung cancer survivors | Prostate cancer survivors | |||||||||||
| Subscale | mean | SE | mean | SE | mean | SE | mean | SE | mean | SE | |||||
| Physical functioning | 39.7 | 0.03 | 38.0 | 0.08 | 38.7 | 0.10 | 37.1 | 0.11 | 41.6 | 0.08 | |||||
| Role limitation-physical | 42.3 | 0.03 | 41.2 | 0.08 | 41.4 | 0.10 | 40.2 | 0.10 | 42.8 | 0.07 | |||||
| Pain | 44.0 | 0.03 | 42.8 | 0.07 | 43.9 | 0.09 | 42.7 | 0.10 | 44.9 | 0.07 | |||||
| General health | 44.7 | 0.03 | 44.0 | 0.07 | 43.7 | 0.09 | 41.8 | 0.10 | 44.6 | 0.07 | |||||
| Vitality | 47.4 | 0.03 | 46.4 | 0.07 | 46.9 | 0.09 | 45.2 | 0.09 | 48.3 | 0.07 | |||||
| Social functioning | 46.9 | 0.03 | 46.0 | 0.08 | 46.2 | 0.10 | 45.1 | 0.11 | 47.4 | 0.07 | |||||
| Role limitation-emotional | 47.7 | 0.03 | 47.0 | 0.08 | 47.2 | 0.09 | 46.3 | 0.10 | 48.2 | 0.07 | |||||
| Emotional well-being | 50.5 | 0.03 | 49.8 | 0.07 | 50.3 | 0.08 | 49.1 | 0.09 | 51.4 | 0.06 | |||||
| Physical component summary | 40.1 | 0.03 | 38.7 | 0.08 | 39.3 | 0.09 | 37.7 | 0.10 | 41.1 | 0.07 | |||||
| Mental component summary | 51.9 | 0.03 | 51.4 | 0.07 | 51.6 | 0.08 | 50.7 | 0.09 | 52.3 | 0.06 | |||||
| Rural | |||||||||||||||
| Controls | Breast cancer survivors | Colorectal cancer survivors | Lung cancer survivors | Prostate cancer survivors | |||||||||||
| Subscale | mean | SE | p | mean | SE | p | mean | SE | p | mean | SE | p | mean | SE | p |
| Physical functioning | 38.7 | 0.14 | *** | 37.7 | 0.35 | 38.1 | 0.41 | 35.3 | 0.47 | *** | 40.0 | 0.32 | *** | ||
| Role limitation-physical | 41.1 | 0.13 | *** | 40.2 | 0.32 | ** | 39.9 | 0.38 | *** | 38.7 | 0.44 | *** | 40.2 | 0.31 | *** |
| Pain | 42.7 | 0.12 | *** | 41.8 | 0.29 | ** | 42.8 | 0.35 | ** | 40.7 | 0.40 | *** | 43.1 | 0.27 | *** |
| General health | 43.3 | 0.12 | *** | 43.0 | 0.30 | *** | 42.2 | 0.38 | *** | 39.5 | 0.43 | *** | 42.7 | 0.29 | *** |
| Vitality | 46.6 | 0.12 | *** | 45.7 | 0.30 | * | 46.4 | 0.36 | 43.6 | 0.42 | *** | 46.6 | 0.28 | *** | |
| Social functioning | 46.1 | 0.13 | *** | 45.6 | 0.33 | 45.3 | 0.39 | * | 43.5 | 0.47 | *** | 45.9 | 0.31 | *** | |
| Role limitation-emotional | 47.0 | 0.12 | *** | 46.4 | 0.32 | * | 46.0 | 0.38 | ** | 45.3 | 0.45 | * | 46.5 | 0.31 | *** |
| Emotional well-being | 50.1 | 0.11 | *** | 49.2 | 0.30 | 49.5 | 0.34 | * | 48.1 | 0.41 | *** | 50.1 | 0.27 | *** | |
| Physical component summary | 38.7 | 0.13 | *** | 37.9 | 0.32 | * | 38.2 | 0.37 | ** | 35.7 | 0.43 | *** | 39.1 | 0.29 | *** |
| Mental component summary | 51.5 | 0.11 | *** | 50.9 | 0.30 | 50.9 | 0.34 | * | 49.7 | 0.41 | * | 51.1 | 0.27 | *** | |
Note. P-values reflect the results of two-sample t-tests comparing mean HRQOL subscale scores for urban participants (top panel) versus rural participants [37] (bottom panel). SE=standard error.
p<.05
p<.01
p<.001.
In addition, bivariate models examining case-control differences in unadjusted HRQOL subscale and global summary scores showed that, for urban and rural participants, scores were often lower for cancer survivors compared to controls (Supplementary Table S2).
Adjusted case-control differences in HRQOL by rurality
Multivariable models examining case-control differences in adjusted HRQOL subscale and global summary scores showed that, for urban and rural participants, scores were often lower for cancer survivors compared to their matched controls, even after adjusting for relevant control variables (Table 3). For example, among urban participants, physical functioning subscale scores were significantly lower for survivors versus controls for breast cancer (diff.=−0.40, SE=0.10, p<.001) and lung cancer (diff.=−1.93, SE=0.13, p<.001). Among rural participants, the scores did not differ for the breast, colorectal, and prostate cancer groups, but they were significantly lower for lung cancer survivors versus controls (diff.=−1.88, SE=0.58, p<.01). Case-control differences were usually greatest for the lung cancer group compared to other cancer types.
Table 3.
Adjusted case-control differences in mean scores on health-related quality of life (HRQOL) subscales, stratified by rurality, SEER-MHOS, 1998–2014 (n=271,640).
| Urban participants | ||||||||||||
| Breast cancer survivors v. controls | Colorectal cancer survivors v. controls | Lung cancer survivors v. controls | Prostate cancer survivors v. controls | |||||||||
| diff. | SE | p | diff. | SE | p | diff. | SE | p | diff. | SE | p | |
| Physical functioning | −0.40 | 0.10 | *** | −0.21 | 0.12 | −1.93 | 0.13 | *** | 0.06 | 0.09 | ||
| Role limitation-physical | −0.38 | 0.01 | *** | −0.37 | 0.12 | ** | −1.42 | 0.13 | *** | −0.46 | 0.09 | *** |
| Pain | −0.16 | 0.01 | 0.18 | 0.11 | −0.42 | 0.12 | *** | −0.33 | 0.08 | *** | ||
| General health | −0.56 | 0.01 | *** | −0.67 | 0.10 | *** | −2.18 | 0.12 | *** | −0.72 | 0.08 | *** |
| Vitality | −0.47 | 0.01 | *** | −0.20 | 0.11 | −1.49 | 0.12 | *** | −0.28 | 0.08 | *** | |
| Social functioning | −0.09 | 0.10 | −0.15 | 0.12 | −0.95 | 0.13 | *** | −0.22 | 0.09 | * | ||
| Role limitation-emotional | −0.06 | 0.01 | 0.13 | 0.12 | −0.76 | 0.13 | *** | −0.21 | 0.09 | * | ||
| Emotional well-being | 0.07 | 0.09 | 0.10 | 0.11 | −0.81 | 0.12 | *** | −0.17 | 0.08 | * | ||
| Physical component summary | −0.53 | 0.09 | *** | 0.40 | 0.11 | *** | −1.70 | 0.12 | *** | −0.35 | 0.08 | *** |
| Mental component summary | 0.03 | 0.09 | 0.09 | 0.11 | −0.59 | 0.12 | *** | −0.22 | 0.08 | ** | ||
| Rural participants | ||||||||||||
| Breast cancer survivors v. controls | Colorectal cancer survivors v. controls | Lung cancer survivors v. controls | Prostate cancer survivors v. controls | |||||||||
| diff. | SE | p | diff. | SE | p | diff. | SE | p | diff. | SE | p | |
| Physical functioning | 0.22 | 0.42 | 0.35 | 0.49 | −1.88 | 0.58 | ** | −0.09 | 0.38 | |||
| Role limitation-physical | −0.62 | 0.40 | −0.10 | 0.47 | −1.29 | 0.56 | * | −1.52 | 0.38 | *** | ||
| Pain | −0.18 | 0.36 | 0.89 | 0.42 | * | −0.71 | 0.50 | −0.49 | 0.34 | |||
| General health | −0.33 | 0.35 | −0.25 | 0.43 | −2.57 | 0.51 | *** | −1.17 | 0.34 | *** | ||
| Vitality | −0.31 | 0.36 | 0.65 | 0.42 | −1.63 | 0.52 | ** | −0.63 | 0.35 | |||
| Social functioning | −0.14 | 0.41 | 0.36 | 0.46 | −1.68 | 0.57 | ** | −0.84 | 0.38 | * | ||
| Role limitation-emotional | −0.38 | 0.39 | 0.16 | 0.47 | −0.14 | 0.56 | −1.24 | 0.37 | *** | |||
| Emotional well-being | −0.43 | 0.37 | 0.58 | 0.42 | −0.73 | 0.50 | −0.97 | 0.34 | ** | |||
| Physical component summary | −0.07 | 0.38 | 0.23 | 0.44 | −1.76 | 0.53 | *** | −0.58 | 0.35 | |||
| Mental component summary | −0.45 | 0.37 | 0.47 | 0.41 | −0.58 | 0.50 | −1.00 | 0.33 | ** | |||
Note. P-values reflect the statistical significance of case-control differences in HRQOL subscale scores, evaluated using multivariable linear regression, controlling for participant sex, race/ethnicity, annual household income, educational attainment, marital status, age, multimorbidity index score, Census region, and survey cohort. Diff.=difference; SE=standard error.
p<.05
p<.01
p<.001.
Among urban participants, consistent case-control differences were observed across cancer types for two HRQOL subscales (role limitation-physical (all p<.01); general health (all p<.001)), as well as the global physical component summary score (all p<.001). That is, compared to matched controls, survivors of breast, colorectal, lung, or prostate cancer living in urban areas had significantly lower HRQOL in these domains.
Among rural participants, fewer case-control differences were observed. However, for the lung and prostate cancer groups, survivors had lower HRQOL than matched controls across three subscales: role limitation-physical (both p<.05); general health (both p<.001); and social functioning (both p<.05).
Interactions between rurality and cancer status in their association with HRQOL
We found evidence of statistical interactions between rurality and cancer status in their relationship with HRQOL in eight models; for the remaining models, the case-control differences in HRQOL did not differ by rurality.
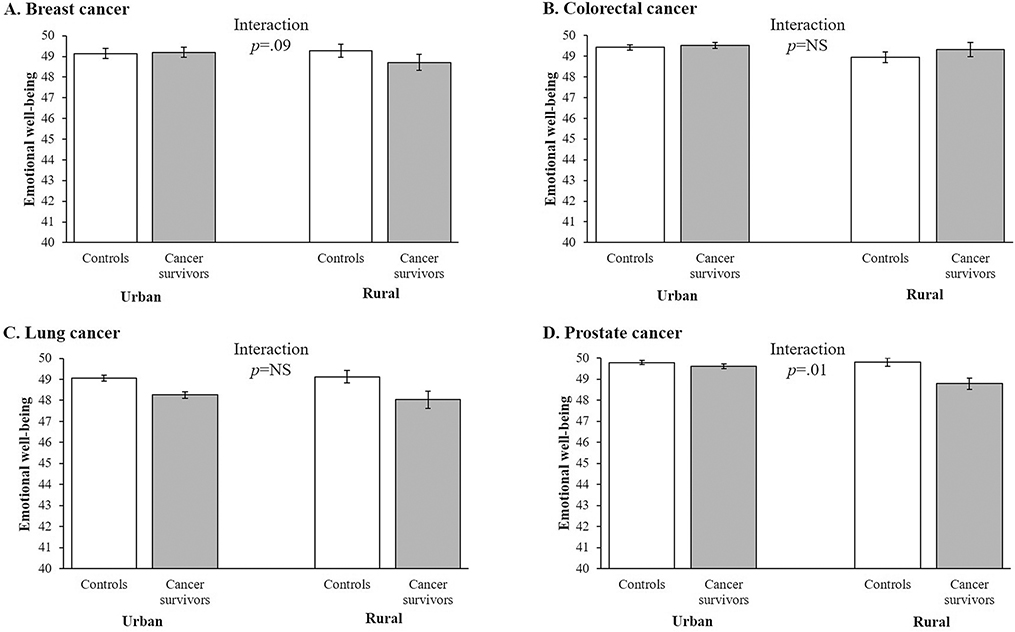
For the breast cancer group, rurality and cancer status interacted in their association with emotional well-being (interaction p=.09). Although the case-control differences on scores for this subscale did not achieve statistical significance, compared to their matched controls, urban survivors had slightly higher scores on this subscale (diff.=0.07, SE=0.09, p=not significant[NS]), while rural survivors had slightly lower scores (diff.=−0.43, SE=0.37, p=NS) (Table 3).
For the colorectal cancer group, rurality and cancer status interacted in their association with vitality (interaction p<.05). Compared to their matched controls, urban survivors had slightly lower scores on this subscale (diff.=−0.20, SE=0.11, p=NS), while rural survivors had slightly higher scores (diff.=0.65, SE=0.42, p=NS) (Table 3).
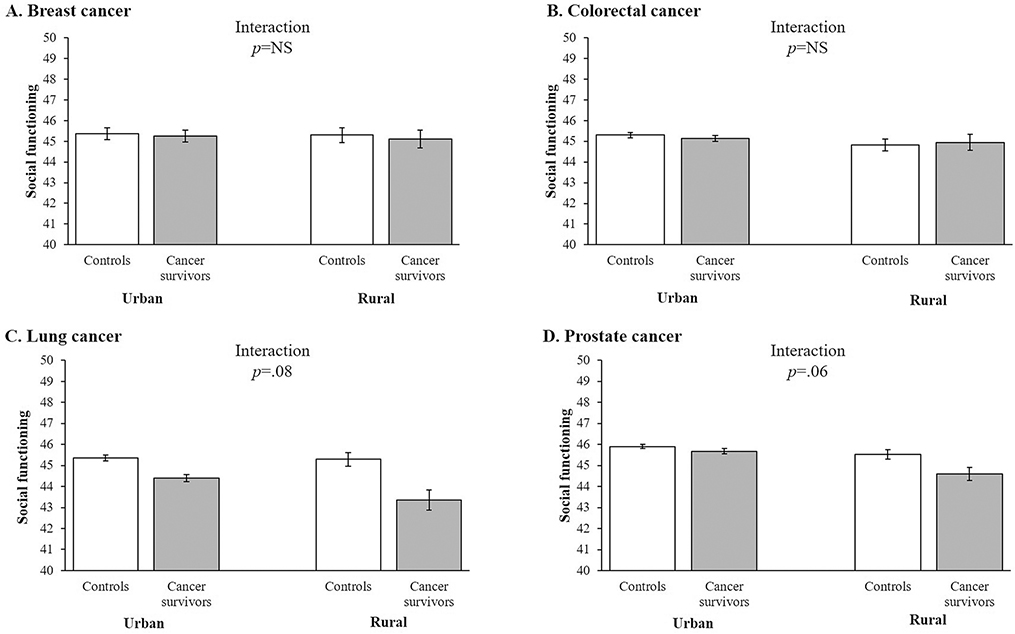
For the lung cancer group, rurality and cancer status interacted in their association with social functioning (interaction p=.05). Compared to their matched controls, both groups of survivors had lower scores on this subscale, but the case-control difference was smaller for urban survivors (diff.=−0.95, SE=0.13, p<.001) than for rural survivors (diff.=−1.68, SE=0.57, p<.01) (Table 3).
The remaining interactions were observed in the prostate cancer group for role limitation-physical (p<.01), social functioning (p=.07), role limitation-emotional (p<.01), and emotional well-being (p=.01), as well as the global mental component summary measure (p=.02). For each of these domains, prostate cancer survivors had lower scores than their matched controls, but this difference was smaller for the urban survivors than for the rural survivors. For example, urban prostate cancer survivors had scores on the role limitation-physical subscale that were 0.46 points (SE=0.09, p<.001) lower than urban controls, while rural prostate cancer survivors had scores that were 1.52 points (SE=0.38, p<.001) lower than rural controls (interaction p<.01) (Table 3).
Figures 1 and 2 illustrate the patterns of case-control differences in adjusted social functioning (Figure 1) and emotional well-being (Figure 2) subscale scores across rurality for the different cancer groups. (The remaining HRQOL subscales demonstrated statistically-significant interactions between cancer status and rurality for only one or zero cancer types; these patterns are not depicted in Figures.) For social functioning, no interaction was observed for the breast or colorectal cancer groups; however, for lung and prostate cancer, the case-control differences were larger in rural than urban areas (Figure 1) (both interaction p<.10). For emotional well-being, no interaction was observed for the colorectal or lung cancer groups; however, for breast and prostate cancer, the case-control differences were larger in rural than urban areas (Figure 2) (both interaction p<.10).
Figure 1.
Adjusted mean score for the social functioning health-related quality of life (HRQOL) subscale for controls and matched cancer survivors in urban and rural areas for (A) breast cancer (females only), (b) colorectal cancer, (c) lung cancer, and (d) prostate cancer (males only), SEER-MHOS, 1998–2014 (n=271,640). Note. Social functioning subscale has a population mean of 50 and a standard deviation of 10, based on 1990 population norms. Adjusted mean scores for the social functioning HRQOL subscale controlled for participant sex (where applicable), race/ethnicity, annual household income, educational attainment, marital status, age, multimorbidity index score, Census region, and survey cohort.
Figure 2.
Adjusted mean score for the emotional well-being health-related quality of life (HRQOL) subscale for controls and matched cancer survivors in urban and rural areas for (A) breast cancer (females only), (b) colorectal cancer, (c) lung cancer, and (d) prostate cancer (males only), SEER-MHOS, 1998–2014 (n=271,640). Note. Emotional well-being subscale has a population mean of 50 and a standard deviation of 10, based on 1990 population norms. Adjusted mean scores for the emotional well-being HRQOL subscale controlled for participant sex (where applicable), race/ethnicity, annual household income, educational attainment, marital status, age, multimorbidity index score, Census region, and survey cohort.
Discussion
In this national study of HRQOL among MAO enrollees, we found systematic differences in subscale and global summary scores among cancer survivors compared to matched controls, predominately in HRQOL domains that measure physical health. These case-control differences of up to 2 points on the HRQOL subscales were generally similar for participants living in rural versus urban areas. However, in eight instances, the reductions in HRQOL for cancer survivors differed by rurality; thus, these results provided partial support for our hypothesis that case-control differences in HRQOL among older adult patients would be greater in rural compared to urban areas, albeit limited primarily to prostate cancer survivors. These findings hold several implications for future research and public health initiatives.
Lung and prostate cancer survivors: Potential role of rural-urban differences in stigma
Lung cancer (in both rural and urban areas) was associated with greater reductions in HRQOL than other cancer types, particularly for physical health. For example, physical functioning, role limitation-physical, general health, energy/fatigue, and global physical component summary scores were lower for urban survivors versus matched controls (Table 3). Compared to the other cancer groups, lung cancer survivors had the shortest time between diagnosis and completing MHOS. Some of these lung cancer survivors may have been in active treatment or undergoing maintenance therapy [41] at the time of completing MHOS, which could have had negative impacts on their HRQOL. Cancer-related reductions in HRQOL usually diminish after treatment [42–44], which could explain the relatively smaller case-control HRQOL differences for the other cancers. Additional factors may have contributed to the large case-control differences in HRQOL for this group, including smoking [18, 45, 46]. That is, compared to their controls, lung cancer survivors who smoked may have had lower levels of HRQOL due to smoking, even before diagnosis and treatment. Patients diagnosed with lung cancer are encouraged to quit smoking to improve their clinical outcomes [47–49], and many are successful [50]. However, continuing to smoke may serve as a coping mechanism during cancer diagnosis and treatment [51], and quitting smoking does not reduce the social stigma associated with lung cancer [52]. In addition, HRQOL may be negatively impacted in the immediate aftermath of quitting [53]. Despite these issues, smoking cessation is clearly a benefit to cancer patients [54]. In this study, lung cancer survivors (particularly in rural areas, where smoking rates are higher [25]) experienced poorer social functioning (Figure 1C), perhaps due to changes in social interactions as a result of their lung cancer [55] or, potentially, their smoking cessation [56]. We were unable to examine current smoking or smoking history in this analysis because survey items assessing smoking changed over the study period, precluding meaningful analysis; future studies should test these hypotheses about the interrelationships of rurality, smoking, cancer survivorship, quitting behaviors, and HRQOL. Additional interventions for smoking cessation [57] and social functioning in the face of a cancer diagnosis are needed to maintain physical HRQOL, social HRQOL, and clinical outcomes among older lung cancer survivors, particularly in rural areas.
Several rural-urban differences emerged in HRQOL for the prostate cancer group. Prostate cancer survivors in rural areas had lower scores than survivors in urban areas for each HRQOL subscale and global summary measure (Table 2), and case-control differences in rural areas were larger than in urban areas for HRQOL measures examining physical, social, and emotional domains. Treatment for prostate cancer can have side effects including incontinence or impotence [58], but reductions in HRQOL are usually minimal when compared to age-matched controls who may not have full continence or potency [59, 60]. Prostate cancer-related HRQOL concerns may be more pronounced in rural areas for at least three reasons. First, rural (including rural Appalachian [61]) patients may be diagnosed with more advanced or aggressive prostate cancer than other patients [22, 62], which could result in rural-urban differences in treatment [63] and subsequent physical side effects [64]. Second, prostate cancer is highly stigmatized because it is “a life-threatening illness and a disease that affects sexual organs and sexual function” [65] (p. 364). Stigma and norms around gender and masculinity related to sexual function, especially in rural areas [66, 67], may create challenges for prostate cancer patients in disclosing their diagnosis [65] or seeking formal psychosocial support [68], leading to reductions in social or emotional HRQOL. Third, access to mental health services in rural areas, in general, is very poor [69, 70], and utilization of these services among rural older adults is low [71, 72]. Additionally, general attitudes towards mental health services among rural men are poor [73, 74], further contributing to rural-urban disparities in HRQOL. This combination of more advanced disease, increased stigma, and low access to mental health services may make prostate cancer survivors in rural areas particularly vulnerable to social and emotional HRQOL challenges (Figures 1D and 2D). Previous studies among rural older adults (i.e., not specific to cancer survivors) suggest that informal caregiving and support, such as support from married partners, is the most promising short-term solution to the mental health crisis in this population [71, 72, 75]. Additional research is needed to determine how effectively informal supports can attenuate physical, social, and emotional HRQOL declines among rural prostate cancer survivors.
Potential role of rural-urban differences in access to care in explaining HRQOL
Access to formal and informal healthcare resources may explain many of the rural-urban differences, or lack of differences, observed in the current study. First, few rural-urban HRQOL differences emerged for breast or colorectal cancer survivors, with more disparities observed for lung and prostate cancer survivors. Even though screening for these cancers is lower in rural areas than urban area [76–78], these modalities allow for earlier detection and improved treatment of these cancers, potentially reducing the deleterious impact of these cancers on HRQOL; that is, even though screening is imperfectly accessed in rural communities, these procedures potentially serve as equity-promoting interventions by minimizing the rural-urban disparities in HRQOL for breast and colorectal cancer survivors. Second, rural-urban disparities in access to healthcare providers may give rise to differences in treatment options and, subsequently, HRQOL. For example, medical specialists, including oncologists, are concentrated in urban areas [76, 79], which makes accessing care more challenging in terms of time and distance for rural patients [80, 81]. These challenges may explain differences in cancer treatment [22, 82, 83] (e.g., early-stage breast cancer patients in rural areas are more likely to receive a mastectomy than patients in urban areas [84]), that may impact HRQOL. Third, rural areas have lower access to ancillary healthcare resources, including mental health providers [69, 70], pain management options [85, 86], and support groups for cancer survivors [87]. However, research suggests that the high level of social connectedness in rural communities [88] may provide some buffer for the impairments in social HRQOL for cancer survivors in these areas. Additional research is needed on how access to care impacts cancer survivors differently in rural versus urban communities, and whether these impacts are similar across cancer types. Policy efforts may be crucial for increasing access to screening, treatment, and ancillary healthcare services in rural areas, while researchers should continue to explore how to leverage social connectedness in rural communities to improve HRQOL among older cancer survivors.
Strengths and limitations
This study has several strengths and limitations. In terms of strengths, we leveraged a large, national sample of MAO enrollees to examine rural-urban differences in HRQOL among older cancer survivors. Our analyses used eight subscales and two global summary measures to assess several domains of HRQOL, which allowed us to examine cancer-specific effects on different aspects of HRQOL (e.g., physical, social, and emotional). Our primary study limitation was the small sample size in rural areas; even with the large SEER-MHOS dataset, only about 5% of the sample came from rural areas. As a result, estimates of HRQOL differences in rural areas were less precise (i.e., had larger SE’s), so even when effects estimates were of similar magnitude to estimates for urban areas, they did not reach statistical significance. Future studies should continue to examine HRQOL among larger samples of rural cancer survivors. The other main study limitation was the relatively small HRQOL differences (<4-point case-control and rural-urban difference on all subscale and global summary scores). However, some of these differences surpass the minimally-important difference for clinical significance (i.e., 3-point difference on subscale scores; 2-point difference on global summary scores) [89]. Further, at a population level, even small differences in HRQOL may result in large impacts on the healthcare system. Other study limitations include our inability to test all relevant variables, including health behaviors (e.g., smoking, obesity) and cancer treatment. We used a county-level measure of rurality, but other geographic scales (e.g., census tract [90]) may offer greater insight into the local relevance of rurality for cancer survivors. However, census tract-level rurality information was not available for controls in the SEER-MHOS dataset [91]. Finally, the SEER-MHOS sample is national, but it is not representative of all older populations or all MAO enrollees; caution should be used in generalizing these findings to other populations.
In conclusion, we found differences in HRQOL scores for older cancer survivors compared to their matched controls, with some differences pronounced in rural areas. Cancer stigma, particularly for lung and prostate cancer survivors, and access to screening, treatment, and ancillary healthcare resources in rural areas will continue to challenge HRQOL in these communities. Additional research as well as clinical, public health, and policy interventions are needed to reduce disparities in physical, social, and emotional HRQOL in rural compared to urban areas, especially as the number of cancer survivors continues to grow [1].
Supplementary Material
Acknowledgments:
This work was completed as part of authors’ official duty at the National Institutes of Health (Z99 CA999999). No external financial support was provided. The authors declare no potential conflicts of interest. Data collection was approved by the Centers for Medicare and Medicaid Services, and the proposal for the current analysis was reviewed and approved by the SEER-MHOS team at NCI. Information on data access is available at https://healthcaredelivery.cancer.gov/seer-mhos/obtain/.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- [1].Bluethmann SM, Mariotto AB, and Rowland JH, “Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States,” Cancer Epidemiol Biomarkers Prev, vol. 25, no. 7, pp. 1029–36, July 2016, doi: 10.1158/1055-9965.Epi-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cho H, Mariotto AB, Schwartz LM, Luo J, and Woloshin S, “When do changes in cancer survival mean progress? The insight from population incidence and mortality,” Journal of the National Cancer Institute, vol. 2014, no. 49, pp. 187–197, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guyatt GH, Feeny DH, and Patrick DL, “Measuring health-related quality of life,” Annals of Internal Medicine, vol. 118, no. 8, pp. 622–629, 1993. [DOI] [PubMed] [Google Scholar]
- [4].Ferrell BR, Dow KH, and Grant M, “Measurement of the quality of life in cancer survivors,” Qual Life Res, vol. 4, no. 6, pp. 523–31, December 1995, doi: 10.1007/bf00634747. [DOI] [PubMed] [Google Scholar]
- [5].Naeim A, Aapro M, Subbarao R, and Balducci L, “Supportive care considerations for older adults with cancer,” Journal of Clinical Oncology, vol. 32, no. 24, pp. 2627–34, August 20 2014, doi: 10.1200/jco.2014.55.3065. [DOI] [PubMed] [Google Scholar]
- [6].Siegel R et al. , “Cancer treatment and survivorship statistics, 2012,” CA Cancer J Clin, vol. 62, no. 4, pp. 220–41, Jul-Aug 2012, doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- [7].Meit M et al. , “The 2014 update of the rural-urban chartbook,” Rural Health Reform Policy Research Center, 2014. [Google Scholar]
- [8].Blake KD, Moss JL, Gaysynsky A, Srinivasan S, and Croyle RT, “Making the case for investment in rural cancer control: An analysis of rural cancer incidence, mortality, and funding trends,” Cancer Epidemiol Biomarkers Prev, vol. 26, no. 7, pp. 992–997, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Henley SJ, Anderson RN, Thomas CC, Massetti GM, Peaker B, and Richardson LC, “Invasive Cancer Incidence, 2004–2013, and Deaths, 2006–2015, in Nonmetropolitan and Metropolitan Counties - United States,” Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C. : 2002), vol. 66, no. 14, pp. 1–13, July 7 2017, doi: 10.15585/mmwr.ss6614a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beck SL, Towsley GL, Caserta MS, Lindau K, and Dudley WN, “Symptom experiences and quality of life of rural and urban older adult cancer survivors,” Cancer nursing, vol. 32, no. 5, pp. 359–69, Sep-Oct 2009, doi: 10.1097/NCC.0b013e3181a52533. [DOI] [PubMed] [Google Scholar]
- [11].Reid-Arndt SA and Cox CR, “Does rurality affect quality of life following treatment for breast cancer?,” J Rural Health, vol. 26, no. 4, pp. 402–5, Fall 2010, doi: 10.1111/j.1748-0361.2010.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Weaver KE, Geiger AM, Lu L, and Case LD, “Rural-urban disparities in health status among US cancer survivors,” (in eng), Cancer, vol. 119, no. 5, pp. 1050–7, March 1 2013, doi: 10.1002/cncr.27840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Djarv T, Metcalfe C, Avery KN, Lagergren P, and Blazeby JM, “Prognostic value of changes in health-related quality of life scores during curative treatment for esophagogastric cancer,” (in eng), Journal of clinical oncology : official journal of the American Society of Clinical Oncology, vol. 28, no. 10, pp. 1666–70, April 1 2010, doi: 10.1200/jco.2009.23.5143. [DOI] [PubMed] [Google Scholar]
- [14].Chen J, Ou L, and Hollis SJ, “A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting,” (in eng), BMC Health Serv Res, vol. 13, p. 211, June 11 2013, doi: 10.1186/1472-6963-13-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Basch E et al. , “Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology,” (in eng), Journal of clinical oncology : official journal of the American Society of Clinical Oncology, vol. 30, no. 34, pp. 4249–55, December 1 2012, doi: 10.1200/jco.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- [16].Velikova G et al. , “Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial,” (in eng), Journal of clinical oncology : official journal of the American Society of Clinical Oncology, vol. 22, no. 4, pp. 714–24, February 15 2004, doi: 10.1200/jco.2004.06.078. [DOI] [PubMed] [Google Scholar]
- [17].Greenhalgh J and Meadows K, “The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: a literature review,” (in eng), Journal of evaluation in clinical practice, vol. 5, no. 4, pp. 401–16, November 1999. [DOI] [PubMed] [Google Scholar]
- [18].Blanchard CM, Courneya KS, Stein K, and Society AC, “Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II,” Journal of Clinical Oncology, vol. 26, no. 13, pp. 2198–2204, 2008, doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- [19].Leung J, Pachana NA, and McLaughlin D, “Social support and health-related quality of life in women with breast cancer: a longitudinal study,” Psycho-oncology, vol. 23, no. 9, pp. 1014–1020, 2014. [DOI] [PubMed] [Google Scholar]
- [20].Eltahir Y et al. , “Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures,” Plastic and Reconstructive Surgery, vol. 132, no. 2, pp. 201e–209e, 2013. [DOI] [PubMed] [Google Scholar]
- [21].Boscoe FP et al. , “Geographic proximity to treatment for early stage breast cancer and likelihood of mastectomy,” The Breast, vol. 20, no. 4, pp. 324–328, 2011. [DOI] [PubMed] [Google Scholar]
- [22].Baldwin LM, Andrilla CHA, Porter MP, Rosenblatt RA, Patel S, and Doescher MP, “Treatment of early-stage prostate cancer among rural and urban patients,” Cancer, vol. 119, no. 16, pp. 3067–3075, 2013. [DOI] [PubMed] [Google Scholar]
- [23].Trivedi T, Liu J, Probst J, Merchant A, Jhones S, and Martin AB, “Obesity and obesity-related behaviors among rural and urban adults in the USA,” (in eng), Rural Remote Health, vol. 15, no. 4, p. 3267, Oct-Dec 2015. [PubMed] [Google Scholar]
- [24].Bettencourt BA, Schlegel RJ, Talley AE, and Molix LA, “The breast cancer experience of rural women: a literature review,” Psycho-oncology, vol. 16, no. 10, pp. 875–887, 2007. [DOI] [PubMed] [Google Scholar]
- [25].Roberts ME et al. , “Rural tobacco use across the United States: how rural and urban areas differ, broken down by census regions and divisions,” Health & Place, vol. 39, pp. 153–159, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Weaver KE, Palmer N, Lu L, Case LD, and Geiger AM, “Rural–urban differences in health behaviors and implications for health status among US cancer survivors,” Cancer Causes & Control, vol. 24, no. 8, pp. 1481–1490, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Goff BA et al. , “Predictors of comprehensive surgical treatment in patients with ovarian cancer,” (in eng), Cancer, vol. 109, no. 10, pp. 2031–42, May 15 2007, doi: 10.1002/cncr.22604. [DOI] [PubMed] [Google Scholar]
- [28].National Cancer Institute. “Surveillance, Epidemiology, and End Results Program.” http://seer.cancer.gov/ (accessed 2020).
- [29].SEER-Medicare. “Documentation for the patient entitlement and diagnosis summary file (PEDSF).” https://healthcaredelivery.cancer.gov/seermedicare/aboutdata/PEDSF.pdf (accessed 2020).
- [30].National Cancer Institute. “Number of persons by race and Hispanic ethnicity for SEER participants (2010 Census data).” https://seer.cancer.gov/registries/data.html (accessed 2020).
- [31].Ambs A, Warren JL, Bellizzi KM, Topor M, Haffer SC, and Clauser SB, “Overview of the SEER--Medicare Health Outcomes Survey linked dataset,” (in eng), Health care financing review, vol. 29, no. 4, pp. 5–21, Summer 2008. [PMC free article] [PubMed] [Google Scholar]
- [32].Siegel RL, Miller KD, and Jemal A, “Cancer statistics, 2018,” CA Cancer J Clin, vol. 68, no. 1, pp. 7–30, January 2018, doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- [33].Stewart AL and Ware JE, Measuring functioning and well-being: the medical outcomes study approach. Duke University Press, 1992. [Google Scholar]
- [34].Selim AJ et al. , “Updated US population standard for the Veterans RAND 12-item Health Survey (VR-12),” Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation, vol. 18, no. 1, pp. 43–52, 2009. [DOI] [PubMed] [Google Scholar]
- [35].Selim A, Rogers W, Qian S, Rothendler JA, Kent EE, and Kazis LE, “A new algorithm to build bridges between two patient-reported health outcome instruments: the MOS SF-36(R) and the VR-12 Health Survey,” (in eng), Qual Life Res, vol. 27, no. 8, pp. 2195–2206, August 2018, doi: 10.1007/s11136-018-1850-3. [DOI] [PubMed] [Google Scholar]
- [36].Jenkinson C, Wright L, and Coulter A, “Criterion validity and reliability of the SF-36 in a population sample,” Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation, vol. 3, no. 1, pp. 7–12, 1994. [DOI] [PubMed] [Google Scholar]
- [37].United States Department of Agriculture. “Rural-Urban Continuum Codes: Overview.” http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx (accessed 2020).
- [38].Meilleur A, Subramanian SV, Plascak JJ, Fisher JL, Paskett ED, and Lamont EB, “Rural residence and cancer outcomes in the United States: issues and challenges,” Cancer Epidemiology, Biomarkers & Prevention, vol. 22, no. 10, pp. 1657–1667, 2013, doi: 10.1158/1055-9965.EPI-13-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Austin SR, Wong YN, Uzzo RG, Beck JR, and Egleston BL, “Why Summary Comorbidity Measures Such As the Charlson Comorbidity Index and Elixhauser Score Work,” (in eng), Med Care, vol. 53, no. 9, pp. e65–72, September 2015, doi: 10.1097/MLR.0b013e318297429c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Smith AW et al. , “Cancer, comorbidities, and health-related quality of life of older adults,” (in eng), Health care financing review, vol. 29, no. 4, pp. 41–56, Summer 2008. [PMC free article] [PubMed] [Google Scholar]
- [41].Coate LE and Shepherd FA, “Maintenance therapy in advanced non-small cell lung cancer: evolution, tolerability and outcomes,” (in eng), Therapeutic advances in medical oncology, vol. 3, no. 3, pp. 139–57, May 2011, doi: 10.1177/1758834011399306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Quach C, Sanoff HK, Williams GR, Lyons JC, and Reeve BB, “Impact of colorectal cancer diagnosis and treatment on health-related quality of life among older Americans: a population-based, case-control study,” (in eng), Cancer, vol. 121, no. 6, pp. 943–50, March 15 2015, doi: 10.1002/cncr.29125. [DOI] [PubMed] [Google Scholar]
- [43].Stover AM, Mayer DK, Muss H, Wheeler SB, Lyons JC, and Reeve BB, “Quality of life changes during the pre- to postdiagnosis period and treatment-related recovery time in older women with breast cancer,” (in eng), Cancer, vol. 120, no. 12, pp. 1881–9, June 15 2014, doi: 10.1002/cncr.28649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Reeve BB et al. , “Impact of diagnosis and treatment of clinically localized prostate cancer on health-related quality of life for older Americans: a population-based study,” (in eng), Cancer, vol. 118, no. 22, pp. 5679–87, November 15 2012, doi: 10.1002/cncr.27578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wilson D, Parsons J, and Wakefield M, “The health-related quality-of-life of never smokers, ex-smokers, and light, moderate, and heavy smokers,” (in eng), Prev Med, vol. 29, no. 3, pp. 139–44, September 1999, doi: 10.1006/pmed.1999.0523. [DOI] [PubMed] [Google Scholar]
- [46].Mulder I, Tijhuis M, Smit HA, and Kromhout D, “Smoking cessation and quality of life: the effect of amount of smoking and time since quitting,” (in eng), Prev Med, vol. 33, no. 6, pp. 653–60, December 2001, doi: 10.1006/pmed.2001.0941. [DOI] [PubMed] [Google Scholar]
- [47].Lung Cancer Alliance. “Quitting smoking after diagnosis.” https://lungcanceralliance.org/what-is-lung-cancer/coping-with-diagnosis/quitting-smoking-after-diagnosis/ (accessed 2019). [Google Scholar]
- [48].Parsons A, Daley A, Begh R, and Aveyard P, “Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis,” (in eng), Bmj, vol. 340, p. b5569, January 21 2010, doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mason DP et al. , “Impact of smoking cessation before resection of lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database study,” (in eng), The Annals of thoracic surgery, vol. 88, no. 2, pp. 362–70; discussion 370–1, August 2009, doi: 10.1016/j.athoracsur.2009.04.035. [DOI] [PubMed] [Google Scholar]
- [50].Gritz ER, Nisenbaum R, Elashoff RE, and Holmes EC, “Smoking behavior following diagnosis in patients with stage I non-small cell lung cancer,” (in eng), Cancer causes & control : CCC, vol. 2, no. 2, pp. 105–12, March 1991. [DOI] [PubMed] [Google Scholar]
- [51].Duffy SA, Louzon SA, and Gritz ER, “Why do cancer patients smoke and what can providers do about it?,” (in eng), Community oncology, vol. 9, no. 11, pp. 344–352, November 1 2012, doi: 10.1016/j.cmonc.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chapple A, Ziebland S, and McPherson A, “Stigma, shame, and blame experienced by patients with lung cancer: qualitative study,” (in eng), Bmj, vol. 328, no. 7454, p. 1470, June 19 2004, doi: 10.1136/bmj.38111.639734.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Coste J, Quinquis L, D’Almeida S, and Audureau E, “Smoking and health-related quality of life in the general population. Independent relationships and large differences according to patterns and quantity of smoking and to gender,” (in eng), PloS one, vol. 9, no. 3, p. e91562, 2014, doi: 10.1371/journal.pone.0091562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cataldo JK, Dubey S, and Prochaska JJ, “Smoking cessation: an integral part of lung cancer treatment,” (in eng), Oncology, vol. 78, no. 5–6, pp. 289–301, 2010, doi: 10.1159/000319937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kelley DE, Kent EE, Litzelman K, Mollica MA, and Rowland JH, “Dyadic associations between perceived social support and cancer patient and caregiver health: An actor-partner interdependence modeling approach,” (in eng), Psychooncology, vol. 28, no. 7, pp. 1453–1460, July 2019, doi: 10.1002/pon.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Christakis NA and Fowler JH, “The collective dynamics of smoking in a large social network,” (in eng), The New England journal of medicine, vol. 358, no. 21, pp. 2249–58, May 22 2008, doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Maxwell CJ and Hirdes JP, “The prevalence of smoking and implications for quality of life among the community-based elderly,” (in eng), Am J Prev Med, vol. 9, no. 6, pp. 338–45, Nov-Dec 1993. [PubMed] [Google Scholar]
- [58].American Cancer Society. “Considering prostate cancer treatment options.” https://www.cancer.org/cancer/prostate-cancer/treating/considering-options.html (accessed 2019).
- [59].Litwin MS et al. , “Quality-of-life outcomes in men treated for localized prostate cancer,” (in eng), Jama, vol. 273, no. 2, pp. 129–35, January 11 1995, doi: 10.1001/jama.273.2.129. [DOI] [PubMed] [Google Scholar]
- [60].Penson DF et al. , “General quality of life 2 years following treatment for prostate cancer: what influences outcomes? Results from the prostate cancer outcomes study,” (in eng), Journal of clinical oncology : official journal of the American Society of Clinical Oncology, vol. 21, no. 6, pp. 1147–54, March 15 2003, doi: 10.1200/jco.2003.07.139. [DOI] [PubMed] [Google Scholar]
- [61].McDonald AC et al. , “Aggressive prostate cancer among men in Pennsylvania: Differences between Appalachian and non-Appalachian counties,” ed: AACR, 2019.
- [62].Jemal A, Ward E, Wu X, Martin HJ, McLaughlin CC, and Thun MJ, “Geographic patterns of prostate cancer mortality and variations in access to medical care in the United States,” (in eng), Cancer Epidemiol Biomarkers Prev, vol. 14, no. 3, pp. 590–5, March 2005, doi: 10.1158/1055-9965.Epi-04-0522. [DOI] [PubMed] [Google Scholar]
- [63].Steenland K et al. , “The effect of race and rural residence on prostate cancer treatment choice among men in Georgia,” (in eng), Urology, vol. 77, no. 3, pp. 581–7, March 2011, doi: 10.1016/j.urology.2010.10.020. [DOI] [PubMed] [Google Scholar]
- [64].Litwin MS, “The resilience of men: quality of life after prostate cancer,” (in eng), Nature reviews. Urology, vol. 16, no. 6, pp. 334–335, June 2019, doi: 10.1038/s41585-019-0176-4. [DOI] [PubMed] [Google Scholar]
- [65].Holland JC, Kelly BJ, and Weinberger MI, “Why psychosocial care is difficult to integrate into routine cancer care: stigma is the elephant in the room,” (in eng), Journal of the National Comprehensive Cancer Network : JNCCN, vol. 8, no. 4, pp. 362–6, April 2010. [DOI] [PubMed] [Google Scholar]
- [66].Pedersen VH, Armes J, and Ream E, “Perceptions of prostate cancer in Black African and Black Caribbean men: a systematic review of the literature,” (in eng), Psychooncology, vol. 21, no. 5, pp. 457–68, May 2012, doi: 10.1002/pon.2043. [DOI] [PubMed] [Google Scholar]
- [67].Oliffe J, “Health behaviors, prostate cancer, and masculinities: a life course perspective,” Men and Masculinities, vol. 11, no. 3, pp. 346–366, 2009. [Google Scholar]
- [68].Kelley S, DeCourtney C, and Thorsness J, “Development and evaluation of a support program for prostate cancer survivors in Alaska,” (in eng), International journal of circumpolar health, vol. 74, p. 28605, 2015, doi: 10.3402/ijch.v74.28605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Cummings JR, Allen L, Clennon J, Ji X, and Druss BG, “Geographic Access to Specialty Mental Health Care Across High- and Low-Income US Communities,” (in eng), JAMA psychiatry, vol. 74, no. 5, pp. 476–484, May 1 2017, doi: 10.1001/jamapsychiatry.2017.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].McCord CE, Elliott TR, Brossart DF, and Castillo LG, “Addressing mental health issues in rural areas,” in Rural Populations and Health: Determinants, Disparities, and Solutions, Crosby RA, Wendel ML, Vanderpool RC, and Casey BR Eds. San Francisco, CA: Jossey-Bass, 2012, pp. 323–340. [Google Scholar]
- [71].Neese JB, Abraham IL, and Buckwalter KC, “Utilization of mental health services among rural elderly,” (in eng), Archives of psychiatric nursing, vol. 13, no. 1, pp. 30–40, February 1999. [DOI] [PubMed] [Google Scholar]
- [72].Chalifoux Z, Neese JB, Buckwalter KC, Litwak E, and Abraham IL, “Mental health services for rural elderly: innovative service strategies,” (in eng), Community mental health journal, vol. 32, no. 5, pp. 463–80, October 1996. [DOI] [PubMed] [Google Scholar]
- [73].Jones AR, Cook TM, and Wang J, “Rural-urban differences in stigma against depression and agreement with health professionals about treatment,” (in eng), J Affect Disord, vol. 134, no. 1–3, pp. 145–50, November 2011, doi: 10.1016/j.jad.2011.05.013. [DOI] [PubMed] [Google Scholar]
- [74].Judd F et al. , “Help-seeking by rural residents for mental health problems: the importance of agrarian values,” (in eng), Aust N Z J Psychiatry, vol. 40, no. 9, pp. 769–76, September 2006, doi: 10.1080/j.1440-1614.2006.01882.x. [DOI] [PubMed] [Google Scholar]
- [75].Revicki DA and Mitchell JP, “Strain, social support, and mental health in rural elderly individuals,” (in eng), Journal of gerontology, vol. 45, no. 6, pp. S267–74, November 1990. [DOI] [PubMed] [Google Scholar]
- [76].Moss JL, Liu B, and Feuer EJ, “Urban/Rural Differences in Breast and Cervical Cancer Incidence: The Mediating Roles of Socioeconomic Status and Provider Density,” Womens Health Issues, vol. 27, no. 6, pp. 683–691, Nov-Dec 2017, doi: 10.1016/j.whi.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Davis MM et al. , “Geographic and population-level disparities in colorectal cancer testing: A multilevel analysis of Medicaid and commercial claims data,” Preventive Medicine, vol. 101, pp. 44–52, 2017, doi: S0091-7435(17)30164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Cole AM, Jackson JE, and Doescher M, “Urban–rural disparities in colorectal cancer screening: cross-sectional analysis of 1998–2005 data from the Centers for Disease Control’s Behavioral Risk Factor Surveillance Study,” Cancer Medicine, vol. 1, no. 3, pp. 350–356, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Aboagye JK, Kaiser HE, and Hayanga AJ, “Rural-urban differences in access to specialist providers of colorectal cancer care in the United States: a physician workforce issue,” JAMA Surgery, vol. 149, no. 6, pp. 537–543, 2014. [DOI] [PubMed] [Google Scholar]
- [80].Loree JM et al. , “Impact of Travel Distance and Urban-Rural Status on the Multidisciplinary Management of Rectal Cancer,” The Journal of Rural Health, 2016. [DOI] [PubMed] [Google Scholar]
- [81].Arcury TA, Gesler WM, Preisser JS, Sherman J, Spencer J, and Perin J, “The effects of geography and spatial behavior on health care utilization among the residents of a rural region,” Health Services Research, vol. 40, no. 1, pp. 135–155, 2005, doi: HESR346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mollica MA et al. , “Patient experiences of cancer care: scoping review, future directions, and introduction of a new data resource: Surveillance Epidemiology and End Results-Consumer Assessment of Healthcare Providers and Systems (SEER-CAHPS),” Patient Experience Journal, vol. 4, no. 1, pp. 103–121, 2017. [Google Scholar]
- [83].Cary C, Odisho AY, and Cooperberg MR, “Variation in prostate cancer treatment associated with population density of the county of residence,” Prostate Cancer and Prostatic Diseases, 2016, doi: 10.1038/pcan.2015.65. [DOI] [PubMed] [Google Scholar]
- [84].Jacobs LK, Kelley KA, Rosson GD, Detrani ME, and Chang DC, “Disparities in urban and rural mastectomy populations : the effects of patient- and county-level factors on likelihood of receipt of mastectomy,” (in eng), Annals of surgical oncology, vol. 15, no. 10, pp. 2644–52, October 2008, doi: 10.1245/s10434-008-0053-5. [DOI] [PubMed] [Google Scholar]
- [85].Eaton LH, Langford DJ, Meins AR, Rue T, Tauben DJ, and Doorenbos AZ, “Use of Self-management Interventions for Chronic Pain Management: A Comparison between Rural and Nonrural Residents,” (in eng), Pain management nursing : official journal of the American Society of Pain Management Nurses, vol. 19, no. 1, pp. 8–13, February 2018, doi: 10.1016/j.pmn.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Currie M, Philip LJ, and Roberts A, “Attitudes towards the use and acceptance of eHealth technologies: a case study of older adults living with chronic pain and implications for rural healthcare,” (in eng), BMC Health Serv Res, vol. 15, p. 162, April 16 2015, doi: 10.1186/s12913-015-0825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wilson SE, Andersen MR, and Meischke H, “Meeting the needs of rural breast cancer survivors: what still needs to be done?,” (in eng), Journal of women’s health & gender-based medicine, vol. 9, no. 6, pp. 667–77, Jul-Aug 2000, doi: 10.1089/15246090050118198. [DOI] [PubMed] [Google Scholar]
- [88].Black AR, Cook JL, Murry VM, and Cutrona CE, “Ties that bind: implications of social support for rural, partnered African American women’s health functioning,” (in eng), Womens Health Issues, vol. 15, no. 5, pp. 216–23, Sep-Oct 2005, doi: 10.1016/j.whi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- [89].Hays RD, Farivar SS, and Liu H, “Approaches and recommendations for estimating minimally important differences for health-related quality of life measures,” (in eng), Copd, vol. 2, no. 1, pp. 63–7, March 2005. [DOI] [PubMed] [Google Scholar]
- [90].WWAMI Rural Health Research Center. “RUCA data: Using RUCA data.” http://depts.washington.edu/uwruca/ruca-uses.php (accessed 2020). [Google Scholar]
- [91].Moss JL, Stinchcomb DG, and Yu M, “Providing Higher Resolution Indicators of Rurality in the Surveillance, Epidemiology, and End Results (SEER) Database: Implications for Patient Privacy and Research,” (in eng), Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, vol. 28, no. 9, pp. 1409–1416, 2019, doi: 10.1158/1055-9965.EPI-19-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.