Abstract
Purpose:
To describe symptom clusters based on severity of co-occurring symptoms among adults with multiple sclerosis (MS) by age groups and to further examine symptom clusters as a correlate of quality of life (QOL) by age groups.
Methods:
This cross-sectional study enrolled persons with MS between 20–79 years of age who completed measures of fatigue, depression, anxiety, sleep quality and QOL using the 36-Item Short Form Health Survey. Bivariate correlation and partial correlation analyses examined associations among symptoms, QOL, and MS characteristics. K-means cluster analyses determined symptom clusters among the full sample and pre-determined age groups (i.e., 20–39, 40–59, and 60–79). One-way ANOVAs examined differences in QOL among clusters for the overall sample and by age groups.
Results:
Among the overall sample of 205 participants, symptoms were significantly correlated with QOL and three distinct clusters were identified and differentiated by the magnitude of symptom experience (i.e., mild, moderate, and severe). Results were consistent among young and middle-aged adults; however, among older adults two severe sleep problem clusters were identified that were distinguished by moderate versus severe fatigue, depression, and anxiety. ANOVAs among the overall sample indicated that the three symptom clusters varied significantly for both physical component scores, F(2, 202)=12.03, p<.001, η2=.10, and mental component scores, F(2, 202)=137.92, p<.001, η2=.58; severe symptom cluster was associated with worse QOL. Patterns in the age subgroup ANOVAs were consistent.
Conclusions:
Given the strong association between severity of symptom clusters and QOL, approaches for targeting co-occurring symptoms are critically needed.
Keywords: fatigue, depression, anxiety, sleep, multiple sclerosis
Plain English summary
Multiple sclerosis (MS) is a prevalent neurological disease affecting an estimated 1 million adults in the United States. People with MS experience debilitating symptoms such as fatigue, depression, anxiety, and sleep problems that often occur together or “cluster.” As the MS population is living longer, more research is examining the impact of aging within MS. This study examined clusters of co-occurring symptoms in young, middle-aged, and older adults with MS and if more severe symptom clustering was related to poor quality of life. This study indicated that among young and middle-aged adults there were groups (clusters) that experience mild, moderate, or severe symptoms of fatigue, depression, anxiety, and sleep together, but in the older adult groups sleep problems are prevalent and though there were two groups who reported severe sleep problems differentiated by either mild or severe fatigue, depression, and anxiety. Symptom cluster group was associated with mental quality of life in all age groups and physical quality of life in middle-aged and older adults, wherein the severe symptom cluster group had the worst quality of life. Findings from this study suggest that treatments might target and improve multiple symptoms for helping people manage the effects of MS on QOL.
Introduction
Multiple sclerosis (MS) is an immune-mediated, non-traumatic disease of the central nervous system with an estimated prevalence of one million adults in the United States [1, 2]. Recent epidemiological data further indicate that the majority of persons with MS are over 45 years old, with the highest prevalence being in the 55–64 year old age group [2]. Older adults with MS experience greater cognitive dysfunction and mobility disability, and engage in less physically active than middle-aged and young adults with MS [3–6]. There may be further changes in expression of symptom and quality of life (QOL) with increasing age in MS, and this supports the further examination of symptoms and QOL to determine if the lived experience of aging with MS is associated with distinct symptom and QOL profiles.
Common symptoms reported by persons with MS include fatigue, depression, anxiety, and poor sleep quality. Fatigue is considered one of, if not the most, common and debilitating symptoms experienced by persons with MS based on a prevalence of ~75% reporting clinically significant symptoms at some point during disease course [7]. Indeed, a recent international study of 2,318 persons with MS reported that clinically significant fatigue is prevalent in 66% of cases [8]. One recent systematic review reported the prevalence of anxiety and depression in MS is 22% and 24%, respectively; these rates are significantly higher than those in the general population [9]. Another recent literature review reported sleep disturbances in approximately 60% of persons with MS [10]. There is mixed evidence regarding aging-related differences in symptoms in MS [11, 12]. For example, the prevalence of depression among older adults in the general population is lower than the prevalence among young and middle-aged adults [13]. However, one review highlighted mixed findings among persons with MS, in which some studies reported an increase in prevalence of depression as age increases whereas other studies reported decreases in prevalence of depression as age increases [11]. This supports continued investigation of symptom expression by age group in persons with MS, perhaps based on the concept of symptom clustering and its link with the anchor of QOL.
Symptom clusters refer to “three or more concurrent symptoms (e.g., pain, fatigue, sleep insufficiency) that are related to each other” [14]. This concept reflects that symptoms rarely occur in isolation, but rather co-occur and result in a broader symptom experience in chronic diseases such as MS. Symptom cluster research began in the cancer literature with a focus on examining symptom clusters by intensity or severity and possible treatment protocols [15]. The concept of symptom clusters was then extended into other populations with chronic diseases given a common experience of multiple co-occurring severe symptoms that may have a downstream impact on QOL, independence, and participation. Symptom clusters in MS have included various symptoms such as pain, fatigue, depression, cognitive functioning, sleep quality, and irritability and consistently identified cluster variation based on mild, moderate, or severe symptom experience [16–20]. For example, a preliminary study among persons with MS examined the symptom cluster of fatigue, depression, and pain and the clustering was differentially associated with QOL; the symptom cluster group with the highest fatigue, depression, and pain symptoms had the lowest QOL [16]. Another study compared the exploratory power of clusters consisting of three versus five symptoms in persons with MS and determined both represented significant symptom clusters and both were associated with physical activity wherein individuals with more severe symptoms were less physically active[18]. The latter study highlights a need for further research regarding various interrelated symptoms that significantly affect health and wellbeing and may serve as a guide for more effective treatment strategies that address common co-occurring symptoms.
To date, we are unaware of focal research that has examined MS symptom clusters and QOL by age groups. By comparison, focal research examining symptom clusters and QOL among older adults has been conducted in other populations with chronic disease. One study among breast cancer survivors over the age of 65 identified associations between QOL and depression, sleep, musculoskeletal and hormonal symptom clusters [22]. Another study of adults with osteoarthritis over the age of 50 reported a symptom cluster of pain, depression, and fatigue that was associated with QOL [23]. Such a pattern of results indicated consistent associations between symptom clusters and QOL among older adults with chronic diseases. Additionally, several studies in the cancer literature indicate differences in symptom clusters between younger and older adults [24, 25]. For example, a symptom cluster of pain, nausea, and appetite loss in older adults (i.e., participants > 60 years old) included additional symptoms (i.e., fatigue and drowsiness) among younger adults with metastatic cancer [24]. Collectively, such observations support examining potential differences in symptoms clusters between older and younger adults with other chronic diseases.
The overall purpose of this study was to examine differences in QOL based on symptom clusters in three age groups of adults with MS; the age groups were selected based on benchmarks that represent discrete life stages in persons with MS that have been utilized in previous research [3, 4]. The study addressed three distinct purposes (i) describe symptom clusters based on severity of co-occurrence among fatigue, depression, anxiety, and sleep quality in persons with MS (ii) compare symptom clusters between young, middle-aged, and older adults with MS (i.e., 20–39, 40–59, and 60–79 years of age) and (iii) examine symptom clusters as a correlate of QOL by age group. We hypothesized that symptom clusters would differ by age group such that symptoms of fatigue, depression, anxiety, and sleep would cluster among younger and middle-aged adults, whereas fatigue and sleep would be the primary symptoms clustering among older adults. Additionally, we hypothesized that symptom clusters would manifest that differ in QOL by symptom severity (i.e., more severe symptoms, lower QOL) and the largest magnitude differences between symptom clusters and QOL among the older adult group given older adults are experiencing declines in mental and physical functioning related to MS disease and aging. That hypothesis is further based on heterogeneity in prevalence of anxiety and depression among older adults in the general population and MS reported in previous studies [13]. Such an investigation may inform future behavioral intervention research targeting age-specific concurrent symptoms for improving QOL by age group among persons living with MS.
Methods
Participants
Participants were recruited through flyers advertising the study and distributed in the local community, the National MS Society, local MS chapter, the Lakeshore Foundation, and through the University of Alabama at Birmingham (UAB) i2b2 database. Interested participants were prompted to contact the research team by telephone or e-mail for a telephone screening. Telephone screening included an overview of the study procedures and questions assessing the inclusion criteria: (1) age between 20–79 years; (2) ambulatory with or without assistance; (3) willingness to complete all testing procedures; (4) relapse free for at least 30 days. Upon satisfaction of the inclusion/exclusion criteria, all participants were then categorized into pre-defined age groups (i.e., 20–39, 40–59, 60–79 years of age), affording the opportunity to examine symptom clusters by age group in adults with MS. Age groups were chosen based on the recommendation that groupings represent unique stages of adult life in MS and these further align with previous research in persons with MS [3, 4] and the changing age demography of the MS population [2].
Measures
Fatigue.
The Fatigue Severity Scale (FSS) measured subjective fatigue severity and its impact [26]. The FSS includes 9 questions examining fatigue in the past week. The items were rated on a scale between 1–7, with 1 indicating “strongly disagree” and 7 indicating “strongly agree.” The overall score was the average of item scores with a range between 1 and 7. FSS scores above 4 are indicative of severe MS-related fatigue [27]. There is evidence of reliability and validity of the FSS among persons with MS [26, 28].
Anxiety and Depression.
Anxiety and depression were measured using the Hospital Anxiety and Depression Scale (HADS) [29]. The 14-item questionnaire includes 7 items assessing depressive symptoms (HADS-D) and 7 items assessing anxiety symptoms (HADS-A). The items are rated on a scale between 0 and 3, with 0 indicating “not at all” and 3 indicating “most of the time.” Scores were summed per subscale and ranged between 0–21, and the score of 11 has been suggested as the optimum cutoff score for identifying significant symptoms for both subscales in persons with MS [30]. There is evidence of reliability and validity of the HADS among persons with MS [31].
Sleep Quality.
The Pittsburgh Sleep Quality Index (PSQI) assessed the quality of one’s sleep during the past month [32]. Seven component scores were calculated ranging from 0–3 and component scores were then summed, yielding the global PSQI score between 0–21. High global PSQI scores reflected worse sleep quality and scores greater than 5 indicated significant poor sleep quality. There is evidence of internal consistency, reliability, and validity of PSQI scores in various populations [33].
Quality of Life.
The 36-item Medical Outcomes Study Short-Form Health Survey (SF-36) provided a self-report measure of health-related quality of life (HRQOL) [34]. The items yield two summary scores between 0–100 representing physical and mental component scores (PCS; MCS) where higher scores indicate better health status [34]. The validity and reliability of SF-36 scores is well established among persons with MS [35].
Demographic & Clinical Characteristics.
Participants self-reported age, sex, race, marital status, employment status, education, and income as well as disease duration and clinical course. Self-report disability status was measured using the single-item Patient Determined Disease Steps (PDDS) for inclusion as a covariate with scores ranging from 0–8 [36]. The PDDS has been validated in persons with MS [37].
Procedures
All study procedures were approved by a University Institutional Review Board, and participants provided written informed consent prior to beginning study procedures. Questionnaires were completed during a single laboratory visit in a quiet, private room. Participants received remuneration for completing the study.
Statistical Analyses
Data analyses were conducted using SPSS Statistics version 25 (IBM, Inc., Armonk, NY). Descriptive statistics are reported as mean±SD, unless otherwise noted (e.g., median). Baseline differences in demographic and clinical characteristics among age groups (20–39, 40–59, & 60–79 years of age) were examined using ANOVA and Chi-square difference tests as appropriate. First, bivariate correlation analyses were performed for examining correlations among symptoms (fatigue, depression, anxiety, and sleep quality), HRQOL (mental and physical), and MS clinical characteristics (PDDS & MS clinical course) followed by partial correlation analyses controlling for significant MS clinical characteristics. This preliminary step provides a foundation regarding potential interrelation among symptoms and association with QOL. We performed a K-means cluster analysis as a method of clustering the sample of persons of MS into three subgroups based on reported experiences with the four symptoms (fatigue, depression, anxiety, and sleep quality). The three subgroup model was chosen based on previous literature indicating symptom clusters of mild, moderate, and severe symptoms among persons with MS [16–18, 21] and K-means was chosen as the most suitable method when analyzing larger sample sizes [38]. Standard criteria for K-means cluster were applied, namely maximum iterations=10 and convergence criteria=0. The cluster analysis was conducted for the overall sample first and then by age groups. One-way ANOVAs were then conducted for examining differences in HRQOL among identified clusters for the overall sample and separately by age groups. This same approach of data analysis has been undertaken in previous research on symptom clusters in MS [18, 20–21] based on standard guidelines for symptom cluster research [39–40].
Results
Participants
The study enrolled 217 persons with MS; however, only participants with complete data on all measures were included in the analyses (N=205). Demographic and clinical characteristics and scores on all outcomes of interest (i.e., FSS, HADS-D, HADS-A, PSQI, & SF-36) for the final sample of 205 persons with MS are provided in Table 1. The sample included 57 participants between 20–39 years of age in “young adults group,” 89 participants between 40–59 years of age in “middle-aged adults group,” and 59 participants between 60–79 years of age in “older adults group.” Significant differences were observed among groups regarding age and disease duration (p=.001), and there were significant differences among groups in employment status (p=.001), marital status (p=.02), race (p=.001), and scores on the SF-36 PCS (p=.008).
Table 1.
Sample Demographics and Clinical Characteristics
| Variable, units | Overall Sample n=205 | Group 1 Age 20–39 N=57 | Group 2 Age 40–59 N=89 | Group 3 Age 60–79 N=59 |
|---|---|---|---|---|
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | |
| Age, years*** | 49.4±13.2 | 33.0±4.7 | 49.4±5.6 | 65.3±4.3 |
| MS Duration, years*** | 12.8±9.4 | 5.7±5.0 | 11.9±7.3 | 21.0±9.4 |
| Median(IQR) | Median(IQR) | Median(IQR) | Median(IQR) | |
| PDDS | 1.0(3.0) | 1.0(3.0) | 1.0(2.75) | 3.0(4.0) |
| Sex | n(%) | n(%) | n(%) | n(%) |
| Female | 154(75.5) | 43(76.8) | 66(74.2) | 45(76.3) |
| Male | 50(24.5) | 13(23.2) | 23(25.8) | 14(23.7) |
| Marital Status* | ||||
| Married | 125(61.0) | 27(47.4) | 55(61.8) | 43(72.9) |
| Single/Divorced/Widowed | 80(39.0) | 30(52.6) | 34(38.2) | 16(27.1) |
| Employed*** | ||||
| Yes | 116(57.1) | 44(78.6) | 57(64.8) | 15(25.4) |
| No | 87(42.9) | 12(21.4) | 31(35.2) | 44(74.6) |
| Race*** | ||||
| Caucasian | 133(64.9) | 26(45.6) | 57(64.0) | 50(84.7) |
| African American | 63(30.7) | 25(43.9) | 29(32.6) | 9(15.3) |
| Other | 9(4.4) | 6(10.5) | 3(3.4) | 0(0) |
| Education | ||||
| High School-Some College | 88(42.9) | 24(42.1) | 35(39.3) | 29(49.2) |
| College Graduate or More | 117(57.1) | 33(57.9) | 54(60.7) | 30(50.8) |
| Annual Household Income | ||||
| $40,000 or Less | 70(34.1) | 25(43.9) | 27(30.3) | 18(30.5) |
| Greater than $40,000 | 135(65.9) | 32(56.1) | 62(69.7) | 41(69.5) |
| MS Clinical Course | ||||
| RRMS | 183(89.3) | 51(89.5) | 82(92.1) | 50(84.7) |
| Progressive | 22(10.7) | 6(10.5) | 7(7.9) | 9(15.3) |
| Symptoms | ||||
| FSS | 4.4(1.6) | 4.2(1.5) | 4.4(1.7) | 4.7(1.5) |
| HADS-Anxiety | 6.5(4.1) | 6.8(4.4) | 6.3(4.0) | 6.3(4.1) |
| HADS-Depression | 5.7(4.1) | 5.6(4.4) | 5.8(4.0) | 5.5(4.0) |
| PSQI Global | 8.2(4.1) | 7.8(4.2) | 8.2(4.2) | 8.3(4.0) |
| SF-36 PCS** | 41.3(11.3) | 44.2(9.6) | 41.9(12.0) | 37.8(10.9) |
| SF-36 MCS | 48.6(11.5) | 46.4(12.2) | 48.3(11.7) | 51.0(10.2) |
Note. Symptoms data are presented as mean (SD); PDDS: Patient Determined Disease Steps; RRMS: Relapsing Remitting Multiple Sclerosis; FSS: Fatigue Severity Scale; HADS: Hospital Anxiety and Depression Scale; PSQI: Pittsburg Sleep Quality Index; PCS: Physical Component Score; MCS: Mental Component Score;
p< .05,
p< .01,
p< .001
Bivariate Correlations
The bivariate correlations among FSS, HADS-D, HADS-A, PSQI, SF-36, disability status (PDDS), and MS clinical course (relapsing remitting or progressive) are provided in Table 2. Correlations were moderate or strong in magnitude among all symptoms and between each symptom and HRQOL measures (both MCS and PCS). PDDS was significantly correlated with PCS, FSS, HADS-D, and HADS-A. MS clinical course was significantly correlated with PCS, HADS-A, PSQI and PDDS.
Table 2.
Correlations Among Quality of Life, Symptom Variables, and MS Clinical Characteristics
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. Physical Component Score | – | |||||||
| 2. Mental Component Score | .13 | – | ||||||
| 3. Fatigue | −.57*** | −.35*** | – | |||||
| 4. Depression | −.42*** | −.70*** | .39*** | – | ||||
| 5. Anxiety | −.26*** | −.75*** | .39*** | .60*** | – | |||
| 6. Sleep | −.30*** | −.49*** | .31*** | .47*** | .48*** | – | ||
| 7. Disability Status (PDDS) | −.69*** | −.08 | .42*** | .35*** | .19* | .17 | – | |
| 8. MS Clinical Course | .17* | .13 | −.14 | −.17* | −.15* | −.15* | −.34*** | – |
Partial Correlations
The partial correlation analyses controlling for disability status (PDDS) and MS clinical course are provide in Table 3. All correlation coefficients slightly decreased, however the strength and direction of correlations among symptoms and HRQOL measures remained consistent.
Table 3.
Partial Correlations Among Quality of Life and Symptom Variables Controlling for Disability Status and MS Clinical Course
| Measure | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1. Physical Component Score | – | ||||
| 2. Mental Component Score | .09 | – | |||
| 3. Fatigue | −.44*** | −.34*** | – | ||
| 4. Depression | −.28*** | −.70*** | .29*** | – | |
| 5. Anxiety | −.22** | −.75*** | .38*** | .58*** | – |
| 6. Sleep | −.29*** | −.48*** | .27*** | .43*** | .45*** |
Cluster Analysis
Overall sample.
The cluster analysis for the overall sample of 205 participants identified 3 subgroups or clusters of differing experiences with the four symptoms. The clusters are consistent with groups having mild, moderate, and severe symptoms as depicted in Figure 1. Ninety-six participants (47%) were classified into the mild symptoms cluster, 73 (36%) into the moderate symptoms cluster, and 36 (18%) in the severe symptoms cluster. Cluster group demographic and clinical characteristics are presented in Table 4.
Fig. 1.

Cluster Analysis Results Overall Sample
Table 4.
Cluster Group Demographics and Clinical Characteristics
| Variable, units | Mild Symptom Cluster N=96 | Moderate Symptom Cluster N=73 | Severe Symptom Cluster N=36 |
|---|---|---|---|
| Mean±SD | Mean±SD | Mean±SD | |
| Age, years*** | 49.7±13.2 | 49.3±13.0 | 48.6±13.7 |
| MS Duration, years*** | 13.4±9.5 | 13.0±9.7 | 10.5±8.5 |
| Median(IQR) | Median(IQR) | Median(IQR) | |
| PDDS | 1.0(3.0) | 2.0(3.0) | 2.0(4.0) |
| Sex | n(%) | n(%) | n(%) |
| Female | 75(78.9) | 50(68.5) | 29(80.6) |
| Male | 20(21.1) | 23(31.5) | 7(19.4) |
| Marital Status* | |||
| Married | 70(72.9) | 38(52.1) | 17(47.2) |
| Single/Divorced/Widowed | 26(27.1) | 35(47.9) | 19(52.8) |
| Employed | |||
| Yes | 64(66.7) | 40(55.6) | 12(34.3) |
| No | 32(33.3) | 32(44.4) | 23(65.7) |
| Race*** | |||
| Caucasian | 66(69.5) | 46(63.0) | 21(58.3) |
| African American | 25(26.3) | 25(34.2) | 13(36.1) |
| Other | 4(3.2) | 2(2.7) | 2(5.6) |
| Education | |||
| High School-Some College | 31(32.2) | 36(42.1) | 21(58.3) |
| College Graduate or More | 65(67.7) | 37(57.9) | 15(41.7) |
| Annual Household Income | |||
| $40,000 or Less | 17(18.3) | 33(45.8) | 21(58.3) |
| Greater than $40,000 | 76(81.7) | 39(54.2) | 15(41.7) |
| MS Clinical Course | |||
| RRMS | 91(94.8) | 61(83.6) | 31(86.1) |
| Progressive | 5(5.2) | 12(16.4) | 5(13.9) |
Note. PDDS: Patient Determined Disease Steps; RRMS: Relapsing Remitting Multiple Sclerosis
Age groups.
Subgroup analyses by age group are depicted in Figures 2–4. The clusters per age group were generally comparable with the overall sample in yielding three interpretable clusters, yet the cluster meaning differed among the three age groups. Among young and middle-aged adults, mild, moderate, and severe symptom clusters were observed consistent with the overall group findings.
Fig. 2.
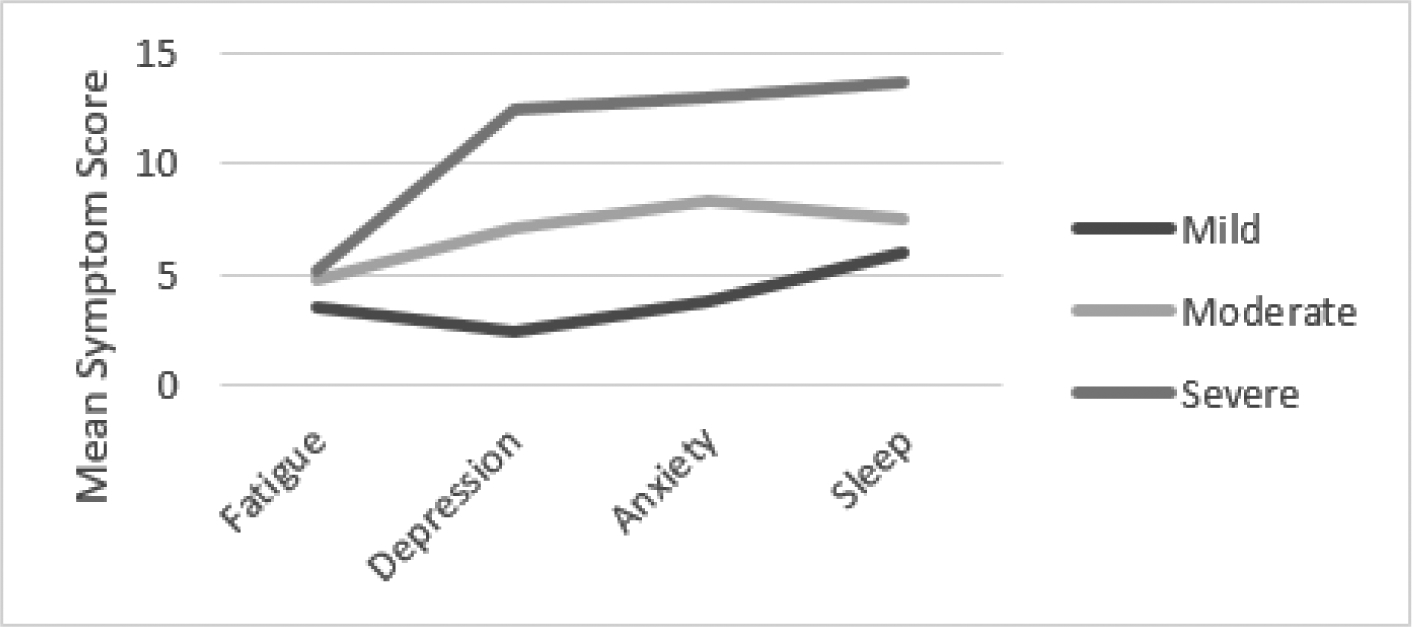
Cluster Analysis Results Young Adults (20–39 years old)
Fig. 4.
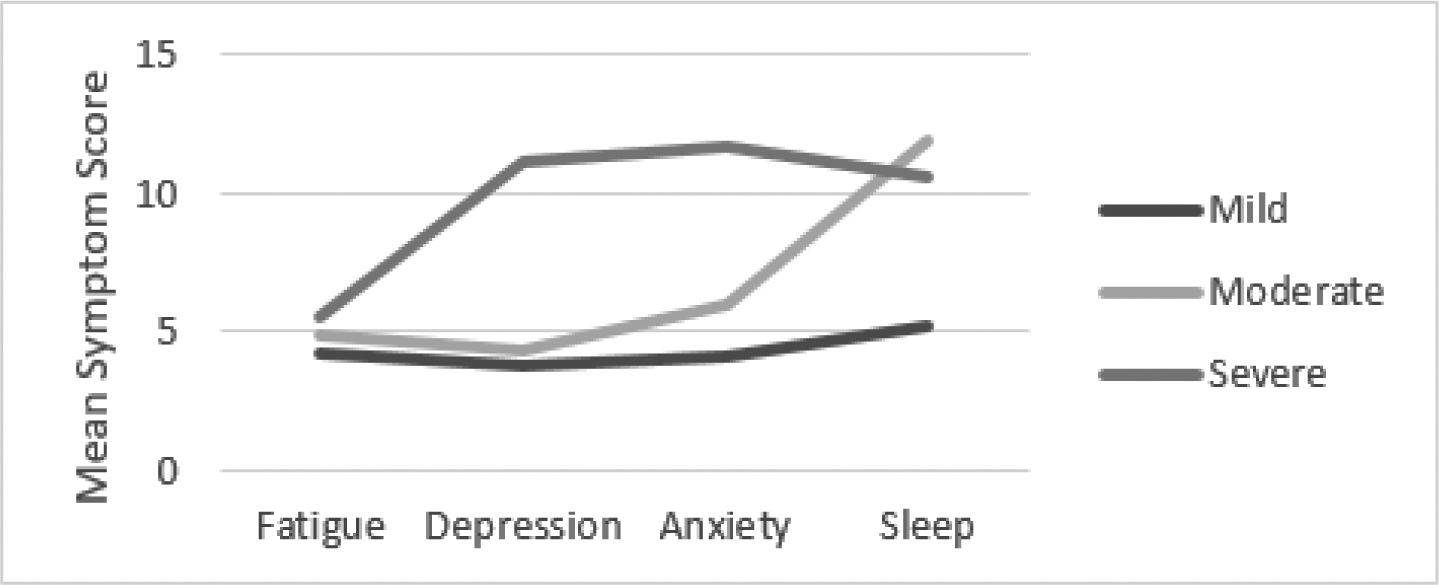
Cluster Analysis Results Older Adults (60–79 years old)
Among older adults, there appear to be two symptom clusters with severe sleep symptoms that are heterogeneous regarding fatigue, depression, and sleep (moderate versus severe). Among young adults, 29 (51%) were classified in the mild symptoms cluster, 18 (32%) in the moderate symptom cluster, and 10 (18%) in the severe symptom cluster. Among middle-aged adults, 44 (49%) were classified in the mild symptoms cluster, 34 (38%) in the moderate symptom cluster, and 11 (12%) in the severe symptom cluster. Among older adults, 29 (49%) were classified in the mild symptom cluster, 17 (29%) were classified in the moderate fatigue, depression, anxiety and poor sleep cluster, and 13 (22%) in the severe symptom cluster.
Clinical Significance of Symptoms
Overall sample.
Clinical significance of symptoms varied among clusters. The overall sample FSS mean for mild symptom cluster was 3.9, and approached the cutoff score of 4. Among the moderate and severe clusters, the FSS mean was higher with mean scores of 4.7 and 5.5, respectively. All clusters exceeded the cutoff score of 5 for PSQI global indicating sleep problems; however, the means increased with 6.0 for the mild cluster, 8.2 for the moderate cluster, and 13.8 for the severe cluster. Regarding both Depression and Anxiety, only the severe group demonstrated HADS scores above the cutoff score of 11.
Age groups.
Clinical significance of symptoms varied across age group clusters. Among older and middle-aged adults, all clusters met or exceeded the FSS cutoff score; however, among young adults, the mild symptom cluster mean FSS score was 3.6. All clusters exceeded the PSQI cutoff score of 5, indicating sleep problems with magnitude of problems increasing with symptom cluster severity. Except for older adults (HADS-D mean= 10.5), the means for the severe cluster group met the cutoff score of 11 which is indicative of clinically significant symptoms of depression.
QOL
The ANOVA analyses for the overall sample indicated that the three identified symptom clusters varied significantly for both PCS, F(2, 202)= 12.03, p <.001, η2=.10, and MCS, F(2, 202)= 137.92, p <.001, η2=.58. Eta-squared values indicate that symptom cluster group membership differences were higher for MCS than PCS. Significant differences were consistent among middle-aged adults (PCS F(2, 86)= 9.51, p <.001, η2=. 18, and MCS, F(2, 86)= 51.33, p <.001, η2=.54) and older adults (PCS F(2, 56)= 3.96, p <.05, η2=.12, and MCS, F(2, 56)= 36.10, p <.001, η2=.48). Among young adults group clusters, significant differences in MCS F(2, 54)= 55.31, p <.001, η2=.67, but not PCS F(2, 54)= 2.28, p =.14, η2=.07 were identified. We further highlight expected direction of differences in which participants in mild symptom cluster groups reported highest HRQOL (Table 5). The differences in HRQOL were of larger magnitude for MCS than PCS and all cluster group means were below the population average of 50 indicating poor HRQOL, except MCS in the mild cluster group.
Table 5.
Overall Sample and Age Group Mean Physical and Mental Health-Related Quality of Life
| Group (n) | Physical Component Score Mean±SD | Mental Component Score Mean±SD |
|---|---|---|
| Overall Sample (205) | ||
| Mild Cluster (96) | 44.9±9.5 | 56.5±5.5 |
| Moderate Cluster (73) | 39.7±11.3 | 45.9±9.0 |
| Severe Cluster (36) | 35.1±12.5 | 32.7±8.9 |
| Young Adults (57) | ||
| Mild Cluster (29) | 46.0±8.4 | 55.2±5.1 |
| Moderate Cluster (18) | 44.0±10.2 | 41.6±10.0 |
| Severe Cluster (10) | 39.0±10.9 | 29.2±6.1 |
| Middle-Aged Adults (89) | ||
| Mild Cluster (44) | 45.8±10.8 | 55.8±5.4 |
| Moderate Cluster (34) | 40.6±11.2 | 44.6±10.5 |
| Severe Cluster (11) | 29.9±11.2 | 30.1±8.0 |
| Older Adults (59) | ||
| Mild Cluster (29) | 41.4±10.9 | 56.4±7.2 |
| Moderate Cluster (17) | 36.1±10.6 | 51.5±8.2 |
| Severe Cluster (13) | 32.0±9.0 | 38.4±7.2 |
Discussion
This study examined fatigue, depression, anxiety, and sleep quality as a symptom cluster among persons with MS overall and by age groups, and then examined the association with physical and mental HRQOL. The cluster analysis identified three sub-groups whose experiences with symptoms were consistent with mild, moderate, and severe symptomology in the overall sample, and some differences in the clustering of symptoms per age group – the older age group demonstrated two clusters with severe sleep problems differentiated by severity of fatigue, depression, and anxiety symptoms. Participants in the mild symptoms cluster reported the highest HRQOL and those in the severe cluster reported the lowest HRQOL – this was particularly apparent for mental HRQOL. This study provides the first examination of symptoms clusters in persons with MS by age groups as strong correlates of mental HRQOL, and such evidence regarding the impact of multiple debilitating symptoms on QOL in MS may inform the design of behavioral interventions for comprehensive symptom management.
Mild, moderate, and severe symptom cluster groups were identified in the overall sample, and these aligned with previous literature regarding symptom clusters in MS [16–21]. The established mild, moderate, and severe symptom clusters were consistent among young adults and middle-aged adults groups; however, within the older adult group, composition of clusters was inconsistent with two severe sleep problem clusters. Sleep problems are prevalent among persons with MS whereby 70–87% of participants report poor overall sleep quality indicative of significant sleep problems [41–43]. Importantly, sleep disorders can exacerbate other symptoms of MS, including fatigue and depression [10]. Systematic examination of sleep problems by age groups in MS are yet to be examined, despite sleep problems representing a consistently reported issue among older adults of the general population [44]. Drawing from the general population literature, age-related sleep problems may be associated with physiological changes over the lifespan that lead to decreases in sleep latency, great proportion of sleep shallower cycles (Stage 1–2 versus REM), and higher prevalence of sleep disorders such as sleep breathing disorders, insomnia, restless legs syndrome, and rapid eye movement behavior disorder [45]. Therefore, given the compounding impact of aging and MS disease on sleep, further examination is needed regarding physiological changes that impact sleep quality across the lifespan in MS and approaches for improving overall sleep.
The presence of frequent or intense symptoms in MS has been consistently associated with worse QOL [43, 46, 47]. The results from this study demonstrate that symptom cluster group severity is associated with HRQOL by age groups in adults with MS; however, differences were significantly higher for mental HRQOL. One study of symptoms and HRQOL reported bladder dysfunction and sleep quality were related to mental HRQOL, whereas age, disability status, and sleep quality were related to physical HRQOL [47]. Another study reported differential predictors of HRQOL, whereby physical HRQOL was predicted by age, female sex, disability status, time since last relapse, and MS treatment status and mental HRQOL was predicted by occupational status, inpatient/outpatient status, time since last relapse, and MS treatment status [48]. Therefore, further consideration of sociodemographic and MS clinical characteristics, such as age, is warranted for examining predictors of HRQOL in MS. Nevertheless, this study highlights the potential for improving mental HRQOL by targeting co-occurring fatigue, depression, anxiety, and sleep symptoms.
The evidence of symptom clusters and the consistent association with QOL in this study and previous literature provide support for prospective benefits of holistic symptom management [16–21]. One evidence-based approach for improving fatigue, mood, sleep, and QOL is physical activity [49, 50]. Further, there is evidence describing associations among symptom clusters with physical activity in MS [17, 21]. Physical activity represents a particularly attractive option as it demonstrates the potential to target symptom clusters using a singular approach, as opposed to established individual treatments for individual symptoms such as Cognitive Behavioral Therapy for treating depression. By extension, future physical activity research may focus on the development and testing of symptom-severity tailored interventions. For example, such an approach might involve stepwise intervention models in which individuals with mild-moderate symptoms are prescribed a physical activity intervention, whereas individuals with severe symptoms are prescribed physical activity plus additional counseling resources given the likelihood of co-occurring debilitating symptoms that necessitate rigorous approaches. Collectively, health behavior change is a promising avenue for holistic MS symptom management warranting innovative strategies targeting clusters of symptoms that may improve overall QOL.
This study is not without limitations. The cross-sectional design of this study limits causal inferences regarding the directionality of associations among symptoms and QOL by age groups in persons with MS. The study of symptom clusters in MS is relatively new and there is no universally-agreed upon set of symptoms for generating clusters or protocol for statistically determining symptom clusters [39]. This study did not include other measures that may be related to the symptoms included in previous research such as pain and cognitive functioning that may be included in future research. Further, this sample was diverse in terms of race and socioeconomic status, and may not be representative of the larger MS population, as participants all resided in the Southeastern United States.
Conclusions
This study provides novel evidence for symptom clusters in MS by age groups indicating that fatigue, depression, anxiety, and sleep quality co-occur in young, middle-aged, and older adults. Such symptom clusters negatively impact HRQOL, particularly mental components, among young, middle, and older adults living with MS. This extends previous research regarding symptom clusters with MS and underscores the opportunity for identifying approaches for improving co-occurring symptoms in MS by age group.
Fig. 3.
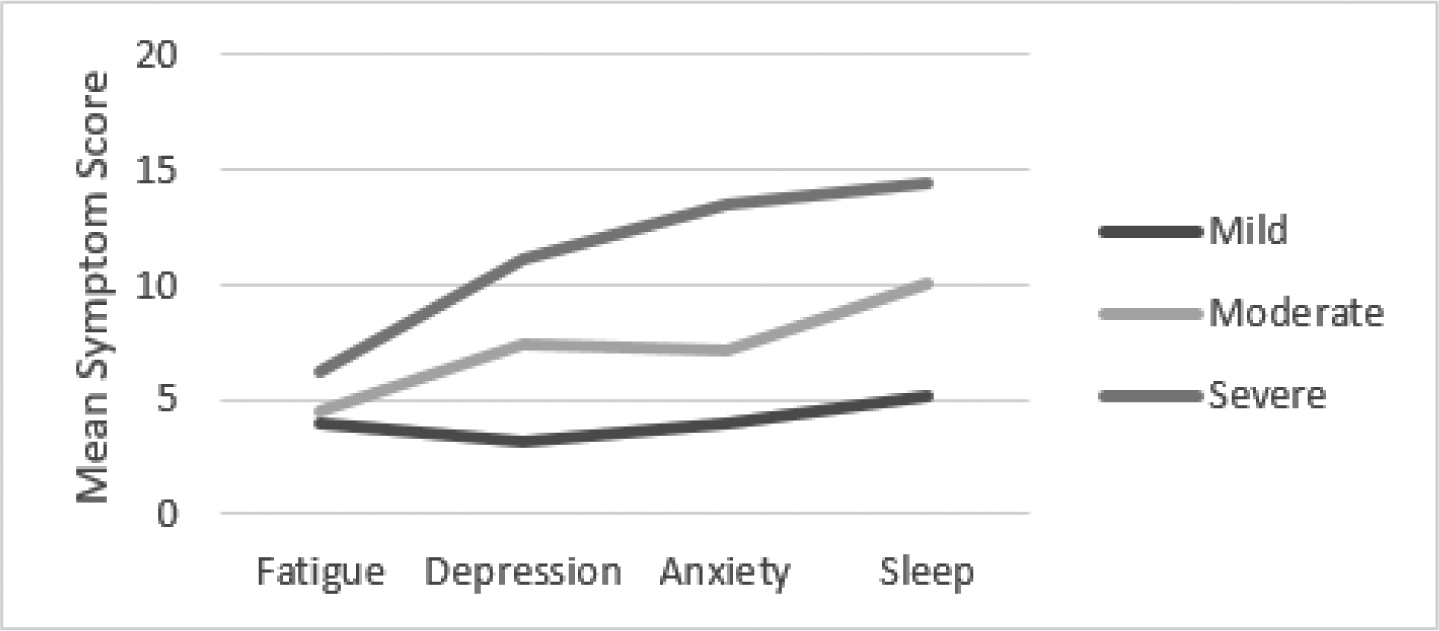
Cluster Analysis Results Middle-Aged Adults (40–59 years old)
Funding:
Funding for this study was provided by the National Multiple Sclerosis Society [CA-1708-29059]. Research reported in this publication was supported, in part, by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health [F32HD101214; F31HD097903] and the National Heart, Lung, and Blood Institute of the National Institutes of Health [T32HL105349]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest/Competing interests: The authors declare that they have no conflict of interest.
Ethics approval: The study was approved by University of Alabama at Birmingham Institutional Review Board and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate: Informed consent was obtained from all individual participants included in the study.
Consent for publication: N/A
Availability of data and material: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability: N/A
References
- 1.Compston A, & Coles A (2008). Multiple sclerosis. Lancet, 372(9648), 1502–1517. 10.1016/s0140-6736(08)61620-7 [DOI] [PubMed] [Google Scholar]
- 2.Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA, et al. (2019). The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology, 92(10), e1029–e1040. doi: 10.1212/wnl.0000000000007035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klaren RE, Sebastiao E, Chiu CY, Kinnett-Hopkins D, McAuley E, & Motl RW Levels and rates of physical activity in older adults with multiple sclerosis. Aging and Disease, 7(3), 278. 10.14336/AD.2015.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baird JF, Cederberg KL, Sikes EM, Silveira SL, Jeng B, Sasaki JE, et al. (2019). Physical activity and walking performance across the lifespan among adults with multiple sclerosis. Multiple Sclerosis and Related Disorders, 35, 36–41. 10.1016/j.msard.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 5.Baird JF, Cederberg KL, Sikes EM, Jeng B, Sasaki JE, Sandroff BM, & Motl RW (2019). Changes in cognitive performance with age in adults with multiple sclerosis. Cognitive and Behavioral Neurology, 32(3), 201–207. 10.1097/WNN.0000000000000200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bollaert RE, & Motl RW (2019). Physical and cognitive functions, physical activity, and sedentary behavior in older adults with multiple sclerosis. Journal of Geriatric Physical Therapy, 42(4), 304–312. https://10.1519/JPT.0000000000000163 [DOI] [PubMed] [Google Scholar]
- 7.Krupp L (2006). Fatigue is intrinsic to multiple sclerosis (MS) and is the most commonly reported symptom of the disease. Multiple Sclerosis Journal, 12(4), 367–368. 10.1191/135248506ms1373ed [DOI] [PubMed] [Google Scholar]
- 8.Weiland TJ, Jelinek GA, Marck CH, Hadgkiss EJ, van der Meer DM, Pereira NG, & Taylor KL (2015). Clinically significant fatigue: prevalence and associated factors in an international sample of adults with multiple sclerosis recruited via the internet. PLoS One, 10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrie RA, Reingold S, Cohen J, Stuve O, Trojano M, Sorensen PS, et al. (2015). The incidence and prevalence of psychiatric disorders in multiple sclerosis: A systematic review. Multiple Sclerosis Journal, 21(3), 305–317. 10.1177/1352458514564487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakkas GK, Giannaki CD, Karatzaferi C, & Manconi M (2019). Sleep Abnormalities in Multiple Sclerosis. Current Treatment Options in Neurology, 21(1), 4. 10.1007/s11940-019-0544-7 [DOI] [PubMed] [Google Scholar]
- 11.Gray V, & Arnett P (2014). Aging with multiple sclerosis: cognitive, emotional and neuropathological considerations. Neurodegenerative Disease Management, 4(2), 187–194. 10.2217/nmt.14.12 [DOI] [PubMed] [Google Scholar]
- 12.Kneebone II, Dunmore EC, & Evans E (2003). Symptoms of depression in older adults with multiple sclerosis (MS): comparison with a matched sample of younger adults. Aging Mental Health, 7(3), 182–185. 10.1080/1360786031000101148 [DOI] [PubMed] [Google Scholar]
- 13.Jorm AF (2000). Does old age reduce the risk of anxiety and depression? A review of epidemiological studies across the adult life span. Psychological Medicine, 30(1), 11–22. 10.1017/s0033291799001452 [DOI] [PubMed] [Google Scholar]
- 14.Dodd MJ, Miaskowski C, & Lee KA (2004). Occurrence of symptom clusters. JNCI Monographs, 2004(32), 76–78. [DOI] [PubMed] [Google Scholar]
- 15.Barsevick A (2016). Defining the symptom cluster: how far have we come?. Seminars in oncology nursing, 32(4), 334–350. 10.1016/j.soncn.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 16.Motl RW, & McAuley E (2010). Symptom cluster and quality of life: preliminary evidence in multiple sclerosis. The Journal of Neuroscience Nursing, 42(4), 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motl RW, Suh Y, & Weikert M (2010). Symptom cluster and quality of life in multiple sclerosis. J Pain Symptom Management, 39(6), 1025–1032. 10.1016/j.jpainsymman.2009.11.312 [DOI] [PubMed] [Google Scholar]
- 18.Motl RW, Weikert M, Suh Y, & Dlugonski D (2010). Symptom cluster and physical activity in relapsing-remitting multiple sclerosis. Research in Nursing & Health, 33(5), 398–412. [DOI] [PubMed] [Google Scholar]
- 19.Newland PK, Fearing A, Riley M, & Neath A (2012). Symptom clusters in women with relapsing-remitting multiple sclerosis. Journal of Neuroscience Nursing, 44(2), 66–71. [DOI] [PubMed] [Google Scholar]
- 20.Shahrbanian S, Duquette P, Kuspinar A, & Mayo NE (2015). Contribution of symptom clusters to multiple sclerosis consequences. Quality of Life Research, 24(3), 617–629. [DOI] [PubMed] [Google Scholar]
- 21.Motl RW, & McAuley E (2009). Symptom cluster as a predictor of physical activity in multiple sclerosis: preliminary evidence. Journal of Pain Symptom Management, 38(2), 270–280. [DOI] [PubMed] [Google Scholar]
- 22.Roiland RA, & Heidrich SM (2011). Symptom clusters and quality of life in older adult breast cancer survivors. Oncology Nursing Forum, 38(6), 672–680. 10.1188/11.ONF.672-680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins JB, & McCoy TP (2015). Symptom clusters, functional status, and quality of life in older adults with osteoarthritis. Orthopaedic Nursing, 34(1), 36–42; quiz 43–34. 10.1097/nor.0000000000000112 [DOI] [PubMed] [Google Scholar]
- 24.Cheung WY, Le LW, Gagliese L, & Zimmermann C (2011). Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Supportive Care in Cancer, 19(3), 417–423. 10.1007/s00520-010-0865-2 [DOI] [PubMed] [Google Scholar]
- 25.Yates P, Miaskowski C, Cataldo JK, Paul SM, Cooper BA, Alexander K, et al. (2015). Differences in Composition of Symptom Clusters Between Older and Younger Oncology Patients. Journal of Pain Symptom Management, 49(6), 1025–1034. 10.1016/j.jpainsymman.2014.11.296 [DOI] [PubMed] [Google Scholar]
- 26.Krupp LB, LaRocca NG, Muir-Nash J, & Steinberg AD (1989). The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology, 46(10), 1121–1123. [DOI] [PubMed] [Google Scholar]
- 27.Andreasen A, Stenager E, & Dalgas U (2011). The effect of exercise therapy on fatigue in multiple sclerosis. Multiple Sclerosis Journal, 17(9), 1041–1054. [DOI] [PubMed] [Google Scholar]
- 28.Learmonth Y, Dlugonski D, Pilutti L, Sandroff B, Klaren R, & Motl RW (2013). Psychometric properties of the fatigue severity scale and the modified fatigue impact scale. Journal of Neurolgical Sciences, 331(1–2), 102–107. [DOI] [PubMed] [Google Scholar]
- 29.Zigmond AS, & Snaith RP (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. [DOI] [PubMed] [Google Scholar]
- 30.Watson TM, Ford E, Worthington E, & Lincoln NB (2014). Validation of mood measures for people with multiple sclerosis. International Journal of MS Care, 16(2), 105–109. 10.7224/1537-2073.2013-013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marrie RA, Zhang L, Lix LM, Graff LA, Walker JR, Fisk JD, et al. (2018). The validity and reliability of screening measures for depression and anxiety disorders in multiple sclerosis. Multiple Sclerosis and Related Disorders, 20, 9–15. [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter JS, & Andrykowski MA (1998). Psychometric evaluation of the Pittsburgh Sleep Quality Index. Journal of Psychosomatic Research, 45(1), 5–13. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE Jr, & Sherbourne CD (1992). The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care, 473–483. [PubMed] [Google Scholar]
- 35.Vickrey B, Hays RD, Harooni R, Myers LW, & Ellison GW (1995). A health-related quality of life measure for multiple sclerosis. Quality of Life Research, 4(3), 187–206. [DOI] [PubMed] [Google Scholar]
- 36.Hohol M, Orav E, & Weiner H (1995). Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology, 45(2), 251–255. [DOI] [PubMed] [Google Scholar]
- 37.Learmonth YC, Motl RW, Sandroff BM, Pula JH, & Cadavid D (2013). Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurology, 13(1), 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarstedt M, & Mooi E (2014). Cluster Analysis. In Wainrib BR (Ed.), A concise guide to market research. The Process, Data, and, Methods Using IBM SPSS Statistics (pp. 274–324). New York: Springer. [Google Scholar]
- 39.Kim HJ, & Abraham IL (2008). Statistical approaches to modeling symptom clusters in cancer patients. Cancer nursing, 31(5), E1–E10. https://10.1097/01.NCC.0000305757.58615.c8 [DOI] [PubMed] [Google Scholar]
- 40.Barsevick AM, Whitmer K, Nail LM, Beck SL, & Dudley WN (2006). Symptom cluster research: conceptual, design, measurement, and analysis issues. Journal of Pain Symptom Management, 31(1), 85–95. [DOI] [PubMed] [Google Scholar]
- 41.Ghaem H, & Haghighi AB (2008). The impact of disability, fatigue and sleep quality on the quality of life in multiple sclerosis. Annals of Indian Academy of Neurology, 11(4), 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marrie RA, Reider N, Cohen J, Trojano M, Sorensen PS, Cutter G, et al. (2015). A systematic review of the incidence and prevalence of sleep disorders and seizure disorders in multiple sclerosis. Multiple Sclerosis Journal, 21(3), 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabrizi FM, & Radfar M (2015). Fatigue, sleep quality, and disability in relation to quality of life in multiple sclerosis. International Journal of MS Care, 17(6), 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landry GJ, Best JR, & Liu-Ambrose T (2015). Measuring sleep quality in older adults: a comparison using subjective and objective methods. Frontiers in Aging Neuroscience, 7, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Espiritu JRD (2008). Aging-related sleep changes. Clinics in Geriatric Medicine, 24(1), 1–14. [DOI] [PubMed] [Google Scholar]
- 46.Benedict RH, Wahlig E, Bakshi R, Fishman I, Munschauer F, Zivadinov R, et al. (2005). Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. Journal of the Neurological Sciences, 231(1–2), 29–34. [DOI] [PubMed] [Google Scholar]
- 47.Merlino G, Fratticci L, Lenchig C, Valente M, Cargnelutti D, Picello M, et al. (2009). Prevalence of ‘poor sleep’among patients with multiple sclerosis: an independent predictor of mental and physical status. Sleep Medicine, 10(1), 26–34. [DOI] [PubMed] [Google Scholar]
- 48.Fernández O, Baumstarck-Barrau K, Simeoni M-C, Auquier P, & MusiQoL study group. (2011). Patient characteristics and determinants of quality of life in an international population with multiple sclerosis: assessment using the MusiQoL and SF-36 questionnaires. Multiple Sclerosis Journal, 17(10), 1238–1249. [DOI] [PubMed] [Google Scholar]
- 49.Motl RW, McAuley E, Snook EM, & Gliottoni RC (2009). Physical activity and quality of life in multiple sclerosis: intermediary roles of disability, fatigue, mood, pain, self-efficacy and social support. Psychology, Health & Medicine, 14(1), 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Motl RW, & Sandroff BM (2015). Benefits of exercise training in multiple sclerosis. Current Neurology and Neuroscience Reports, 15(9), 62. [DOI] [PubMed] [Google Scholar]


