Abstract
Rheumatologists increasingly receive consults for patients treated with immune checkpoint inhibitors (ICIs) for cancer. ICIs can cause inflammatory syndromes known as immune-related adverse events (irAEs). Several rheumatologic irAEs are reported including inflammatory arthritis, polymyalgia rheumatica, and myositis. For patients who present with musculoskeletal symptoms while on ICI therapy, it is important to have an algorithm for evaluation. The differential diagnosis includes a range of musculoskeletal syndromes like crystalline arthritis, mechanical issues, and osteoarthritis, in addition to irAEs. After diagnosing a rheumatologic irAE, rheumatologists must work with the patient and their oncologist to form a treatment plan. Treatment of irAEs is guided by severity. Evidence for management is limited to observational studies. Inflammatory arthritis and polymyalgia rheumatica are treated with non-steroidal anti-inflammatory drugs in mild cases, corticosteroids for moderate to severe cases, and sometimes require other disease modifying anti-rheumatic drugs. Myositis due to ICIs can be accompanied by myocarditis or myasthenia gravis. Corticosteroids and holding the ICI are usually required to treat myositis; some patients with severe myositis need intravenous immunoglobulin or plasmapheresis. Further research is needed to optimize treatment of irAEs that does not compromise the anti-tumor effect of ICIs.
Background:
Cancer immunotherapy has been a substantial breakthrough for treating patients with a variety of malignancies. The most commonly used class of cancer immunotherapy, immune checkpoint inhibitors (ICI), block immune regulatory interactions allowing for increased T cell activation and an anti-tumor immune response1–3. Currently approved ICIs block cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), or programmed death-ligand 1 (PD-L1), but investigations are underway focusing on a variety of other positive and negative regulatory targets4. ICIs are used for an ever-expanding list of tumor types. Initially, ICIs were used only in advanced stage cancer, but now adjuvant therapy in melanoma5 is approved, and neoadjuvant therapy has shown efficacy for non-small lung cancer6. ICIs first came to the attention of rheumatologists in the mid-2010s due to their side effect profile. ICIs can cause inflammatory adverse events termed immune-related adverse events (irAEs), likely due to non-tumor-specific immunological activation. IrAEs can affect nearly any tissue type including the skin, gastrointestinal tract, nervous system, lungs, endocrine organs, and musculoskeletal structures7. Some irAEs like dermatitis and thyroid disease are common and not life threatening, while others like myocarditis are uncommon but often fatal8. Many irAEs share similarities to classic autoimmune diseases, but there are key differences in clinical presentations, treatment, and long term outcomes9.
Rheumatologic irAEs were not appreciated as early in the history of ICIs as some other irAEs, but are increasingly noted. IrAEs with phenotypes similar to rheumatoid arthritis, spondylo-arthritis, polymyalgia rheumatica (PMR), giant cell arteritis (GCA), myositis, ANCA-associated vasculitis, scleroderma, and other rheumatic diseases have been described10. Epidemiologic data remains limited, particularly for rare irAEs like vasculitis and scleroderma. Inflammatory arthritis, however, may occur in 3 to 7% of patients treated with ICIs11,12. Over 40% of oncology patients are eligible for treatment with ICIs, thus the number of patients who may experience rheumatologic irAEs from ICI treatment is substantial13. Studies have yet to define risk factors for development of specific rheumatic irAEs. IrAEs are generally more common in patients treated with combination anti-PD-1/anti-CTLA-4 blockade than with monotherapies14. Certain irAEs like rash, colitis, and hypophysitis are more common with CTLA-4 blockade, while pneumonitis and hypothyroidism are more common with PD-1 blockade15. Rheumatic irAEs may persist after cessation of ICI therapy as seen in inflammatory arthritis16. Persistence of inflammatory arthritis was associated with combination ICI therapy regimens, longer duration of ICI therapy, and having multiple irAEs16.
The coexisting cancer and goal of immunologic activation against the tumor complicate treatment of irAEs. Physicians must balance relieving inflammation in the organ affected by the irAE with not impairing the anti-tumor response to ICI. Treatment of irAEs usually starts with corticosteroids and may require holding ICI therapy. On a positive note, irAEs seem to be a positive prognostic factor for tumor response in different tumor types17.
The clinical scenario above highlights several issues that arise in referrals for suspected rheumatologic irAEs. Non-rheumatology providers may not be familiar with musculoskeletal and neurologic examinations or with associated symptoms that can accompany inflammatory arthritis, PMR/GCA, myositis, or other rheumatic syndromes. This issue is further complicated in the era of COVID-19, when telemedicine makes physical examination more difficult or impossible for certain maneuvers. Also, patients with cancer are often older adults and may have comorbidities such as osteoarthritis (OA) that complicate the picture. As a result, rheumatologists must consider a broad differential diagnosis for patients on ICIs as we will detail in the next section.
Approach:
History:
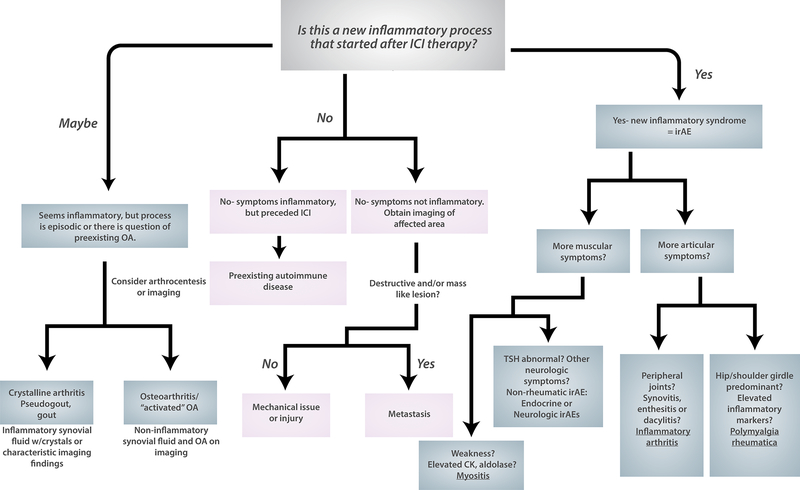
An important first step is to determine whether the symptoms are related to ICI therapy. This is a key branch point in our evaluation algorithm (Figure 1). Establishing a temporal relationship can usually be determined by history and careful review of the medical record, including primary care and oncology notes. For symptoms that were present before ICI therapy, the patient should be assessed for an underlying autoimmune or mechanical issue based on history and physical examination.
Figure 1:
Approach to differential diagnosis for musculoskeletal symptoms in patients on immune checkpoint inhibitor therapy for cancer. Key branch points are whether symptoms are temporally related to ICI therapy and whether there is a persistent inflammatory syndrome present. If a patient has a new inflammatory syndrome that has only started since ICI therapy, this is an irAE (right side of algorithm). An in depth history and physical examination can localize the problem to muscles and joints. From there a diagnosis of inflammatory arthritis, PMR, or myositis can be made. Alternatively, patients may experience weakness and/or muscle pain in endocrine irAEs like thyroiditis or neurologic irAEs like myasthenia gravis. In patients who do not have a new inflammatory process (center of algorithm), they may have a preexisting autoimmune disease or a non-inflammatory musculoskeletal condition. Imaging will be important in the non-inflammatory musculoskeletal conditions to evaluate for metastasis. Finally, on the left side fo the algorithm there are relatively common conditions, crystalline arthritis and osteoarthritis that may be related to ICI therapy but are not as clearly irAEs.
ICI: immune checkpoint inhibitor. irAE: immune-related adverse event. OA: osteoarthritis.
It is important to remember, however, that patients can have injuries and other mechanical issues unrelated to ICI therapy during their treatment. Even if the symptoms clearly started while on ICI therapy, physicians should consider a full differential diagnosis. There are reports of patients experiencing “activated osteoarthritis” with more pain and swelling at joints previously affected by OA18,19. Crystalline arthritis, particularly calcium pyrophosphate disease20,21, is also reported in patients on ICIs. We ask patients about all past musculoskeletal, inflammatory, and autoimmune diagnoses with particular attention to prior injuries and surgeries, OA, and crystalline arthritis. Any trauma or change in physical activity preceding the symptoms should be elicited. Family history may be helpful. If there is a significant history of autoimmunity in the family, the patient may have an underlying autoimmune disease that is unmasked or may be more likely to develop irAEs. The stage of the patient’s cancer and how well they are responding to treatment may also suggest whether metastatic disease could be causing symptoms22.
For patients in whom an irAE is suspected and no alternate cause of symptoms is found, determining the type of irAE is the next step. Pain may be present in the joints in inflammatory arthritis or PMR or in the muscles/fascia in myositis or eosinophilic fasciitis due to ICIs. Fatigue may be present in rheumatologic irAEs, but is also seen in endocrine irAEs and in ICI-treated patients without a defined irAE23. Weakness could reflect myositis, but also myasthenia gravis, neuropathy, or untreated thyroid disease, all of which can occur as irAEs.
Taking note of the ICI regimen and whether the patient has experienced non-rheumatologic irAEs can be clarifying. As previously mentioned, combination CTLA-4/PD-1 blockade typically has the highest rate of irAEs, so a high level of suspicion should be maintained in patients on these regimens. In studies of inflammatory arthritis as an irAE, over half experience additional irAEs19. For patients with suspected myositis, history should also evaluate for symptoms of myasthenia or myocarditis as these irAEs can accompany each other24,25.
Finally, irAEs may present after ICI therapy cessation26. For patients who have been on ICIs in the last 1–2 years, irAEs remain on the differential diagnosis.
Physical examination:
When possible, patients should be evaluated in person so a full physical examination can be performed. A musculoskeletal and neurologic examination are critical. If the patient has significant peripheral synovitis and/or enthesitis, dactylitis, or bursitis then inflammatory arthritis is most likely. In PMR, many patients lack peripheral inflammatory arthritis, though there are more reports of peripheral arthritis in PMR associated with ICIs than in the traditional disease19. Patients with PMR may have limited range of motion in the hips and shoulders or tenderness to palpation. A strength examination of the proximal and distal muscles can help determine whether myositis is present. A neurologic examination should also evaluate for possible signs of myasthenia gravis like ptosis and signs of bulbar weakness. The physician should assess for signs of myocarditis such as tachycardia, arrhythmia, or volume overload. Noting other irAEs like rashes, dry mouth, or dry eyes can also be helpful.
Diagnostic testing:
Laboratory testing may reveal elevated erythrocyte sedimentation rate and/or C-reactive protein in inflammatory arthritis, PMR, or myositis. If inflammatory markers are elevated, they can be followed for improvement with treatment of the irAE. In myositis as an irAE, CK has been elevated from 1000 to over 15,000 units/mL27. Troponin should also be checked given the overlap of myocarditis with myositis. Autoantibodies are sometimes present in rheumatologic irAEs but at much lower rates than in traditional rheumatic diseases. Less than 10% of patients with inflammatory arthritis due to ICIs had a positive RF or anti-CCP in one systematic review19. Similarly, the majority of patients with myositis from ICIs do not have myositis-specific autoantibodies27. There have been reports, however, of anti-striated muscle antibodies and anti-acetylcholine receptor antibodies in patients with myositis from ICIs28,29. If myositis or myasthenia gravis is suspected, obtaining anti-acetylcholine receptor antibodies is reasonable.
Joint fluid aspiration in inflammatory arthritis has shown WBC counts > 1000 (cells/ml) with a neutrophil predominance30,31. Synovial fluid can be helpful in confirming inflammatory arthritis or assessing for crystalline disease and should be obtained when possible.
Ultrasound can demonstrate objective evidence of inflammatory arthritis including synovitis with doppler signal, tenosynovitis, and enthesophytes31. MRI shows synovitis, tenosynovitis, bone marrow edema, and erosions 32. In PMR, ultrasound may show subdeltoid, subacromial bursitis, trochanteric bursitis, or biceps tenosynovitis33,34. Ultrasound and MRI may also suggest other diagnoses like crystalline arthritis, OA, and non-inflammatory musculoskeletal conditions.
If myositis is suspected, EMG, MRI, and muscle biopsy have all been used to support the diagnosis. As in traditional forms of myositis, EMGs show a myopathic pattern, which may demonstrate abnormal spontaneous activity (irritable myopathy)27. MRI can show muscle and/or fascial inflammation35. Myofascial inflammation was actually more common than synovitis in one MRI study of patients on ICIs with musculoskeletal complaints so should be considered when there is pain around the joint rather than centered on the joint.35 Muscle biopsies have shown two major patterns: endomysial lymphocytic inflammation and perivascular macrophagic infiltrate, both of which can be accompanied by necrosis24,27,36,37.
Managing rheumatologic irAEs:
When a diagnosis of a rheumatologic irAE is confirmed, there are several questions for rheumatologists to consider. First, the plan for future oncology treatment should be determined. This may greatly influence how non-life threatening irAEs are managed. If the ICI is going to be stopped, there may be more flexibility in terms of immunosuppression. If chemotherapy or targeted agents are to be started, this needs to be considered in treatment decision-making. If the ICI will be continued or restarted, the acceptable dose of concurrent corticosteroids should be discussed with the treating oncologist. Next, having a conversation with the patient about their goals in irAE treatment is crucial. Patients have different perspectives based on personal values and cancer prognosis that may affect how aggressive they wish to be in treating irAEs38.
The approaches to managing inflammatory arthritis and PMR are similar. Corticosteroids are first line therapy for those who do not improve with NSAIDs and/or intraarticular steroid injections. For those who cannot taper steroids or require steroid-sparing agents, ICIs are often held or stopped. For those who can taper off steroids or have symptomatic control with prednisone 10 mg daily or equivalent, ICIs may be given concurrently at the discretion of the oncologist. Steroid-sparing agents have included hydroxychloroquine, sulfasalazine, methotrexate, TNF-inhibitors, and IL-6R inhibitors (discussed in more detail in evidence section below).
Myositis can be life threatening and thus has a different approach. The ICI is almost always held if not discontinued when myositis is diagnosed. Hospitalized patients may receive higher dose IV steroids, while outpatients may start prednisone 1 mg/kg or equivalent. Treatment options are discussed in more detail below.
Treatment, Evidence:
The evidence for evaluation and treatment of rheumatic irAEs is primarily from prospective and retrospective cohort studies, case series, and case reports (Table 1). There are a few systematic reviews that have combined the observational data, but the limited quality of some primary data should be considered in interpreting these studies.
Table 1:
Selected studies for epidemiology, evaluation, and management of rheumatologic irAEs
| Author, year (citation) | Study population | N | Results | Comments |
|---|---|---|---|---|
| Inflammatory arthritis | ||||
| Braaten et al. 2020 | ICI-induced IA with at least one follow-up after ICI cessation | 60 | Risk factors for persistent IA after ICI cessation: longer duration ICI, combo ICI therapy. Persistent IA may associate with better tumor response. Patients treated with DMARDs/biologic no worse tumor prognosis. MTX, LEF, SSA, HCQ, TNF-I. | Biased toward patients surviving long enough to have follow up after ICI cessation and for patients engaged in rheumatology care (likely more severe IA). |
| Kim et al. 2017. | ICI-induced IA treated with tocilizuma. | 3 | All patients with symptomatic improvement of IA. One had durable anti-tumor response while on tocilizumab for 18 months. | All 3 patients had melanoma. Difficult to make conclusions with N=3 regarding any effect on tumor response. |
| Subedi et al. 2020. | ICI-treated patients referred for rheumatology consult for IA | 8 | Tenosynovitis, synovitis of wrists and hands most common, also saw bone marrow edema and erosions in 3 patients | Retrospective review- MRI may identify those with high risk IA, larger prospective study needed. |
| Roberts et al. 2019. | Patients with ICI-induced IA treated with HCQ first-line | 11 | Only one patient needed methotrexate, none required biologics. Five patients received corticosteroids for IA or other irAEs in addition. Seven patients had resolution of joint pain. | Small N, but HCQ safe and effective in this population. Deserves additional study given favorable safety profile of HCQ. |
| Buder-Bakhaya et al. 2018. | Patients treated with pembrolizumab or nivolumab (+/− ipilimumab) for metastatic cutaneous malignancy | 26 (arthralgia) | Arthralgia common- 13%. Arthritis in 5–7.6%, depending on whether you count activated OA as inflammatory arthritis. 40% with arthritis needed corticosteroids and 20% other immunosuppression. | Arthralgia can be managed with NSAIDs while those with objective evidence of inflammatory arthritis more often needed corticosteroids. Raises ?: how to classify those with known OA and synovitis on imaging in the setting of ICI. |
| Cappelli et al 2018 | Patients with ICI-induced IA treated with anti-PD-1/PD-L1 monotherapy or anti-PD-1/anti-CTLA-4 combination therapy | 30 | Combo therapy: more likely to present with knee arthritis, to have higher CRP, to have other irAEs. TNF-I and methotrexate treated patients with prior tumor response had no tumor progression. | Since earlier in time (less recognition of IA as irAE) and all patients referred to rheum, likely represents more severe IA (80% needed corticosteroids). Follow up time only up to 16 months for evaluating tumor response. |
| Inflammatory arthritis and PMR (same paper) | ||||
| Ghosh et al. 2020 | Patients with IA or PMR due to ICI therapy | 294 (IA) 78 (PMR) |
Median time to onset of arthritis 4 months. Polyarthritis most common joint involvement pattern. Less than 10% positive for RF or anti-CCP. 45% needed additional immunomodulatory therapy beyond steroids. | Systematic literature review of observational studies including case reports. Incomplete data for arthritis joint patterns, serologies, arthritis outcomes based on what was included in the primary studies. Most recent SLR focusing on arthritis. |
| Belkhir et al. 2017 | Patients with seropositive (RF or anti-CCP) IA or PMR after ICI therapy | 4 (PMR) 6 (RA) |
In patients with RA, 5/6 needed steroids, 3 needed DMARD. PMR steroid dosing: prednisone 20–60 mg daily. | Small case series. Seropositive patients are the minority in ICI-induced IA. 2/3 patients with pre-ICI serum already had anti-CCP. |
| PMR | ||||
| Calabrese et al. 2019 | Patients with PMR due to ICI therapy | 20 (case series) 29 (systematic review) |
Case series and SLR. 94% received glucocorticoids (prednisone 7.5 to 60 mg daily). In case series, 30% of patients had normal inflammatory markers. | Attempted to evaluate for EULAR/ACR classification criteria but incomplete data for many cases in SLR. Whether traditional classification criteria should be used for ICI-induced disease is a question. |
| Van der Geest et. Al 2020 | Patients with PMR due to ICI therapy | 6 | Imaging study, 6 with US and 5 with PET. Uptake on PET in shoulders, hip joints, greater trochanters, sternoclavicular joints, interspinous bursae. | Small N, but useful concept given many oncology patients regularly have PET scans for tumor evaluation. |
| Myositis | ||||
| Touat et al. 2018 | Patients with metastatic cancer and myositis due to ICIs | 10 | Heterogeneity in muscle involvement: proximal pattern most common but some with ocular/bulbar/neck primarily. Corticosteroids from 1 mg/kg to 1000 mg IV methylpred used for treatment; 3 needed IVIG or PLEX. | Small number of patients but many have EMG, muscle biopsy data which is useful in describing ICI-induced myositis. |
| Matas-Garcia et al. 2020 | Patients receiving ICI with biopsy-proven inflammatory myopathy | 9 | Some muscle necrosis in all 9, perimysial/perivascular inflammatory infiltrate >> endomysial inflammatory infiltrate. Prednisone 0.5 mg/kg- 2000 mg IV per day, with 5 needing IVIG. | Small N, but helpful data on biopsy characteristics, treatments and outcomes in biopsy-proven myositis. |
| More than one irAE (epidemiology, cancer outcomes) | ||||
| Richter et al. 2019 | Retrospective single center study of ICI treated patients | 43 with rheum irAE | 2% ICI-treated patients developed IA. 71% of those with rheum irAE needed immunosuppression. Only 12% required ICI discontinuation. 2 patients died of myositis. | Likely underestimates prevalence of rheum irAE, particularly mild. IA most common rheumatic irAE. |
| Allenbach et al. 2020 | Rheumatic irAE reported to WHO pharmacovigilance database | 465 (myositis) 606 (IA) 76 (PMR) |
Fatality rate for myositis 24%. Arthritis, myositis more common in those treated with ICI combination therapy. | WHO database requires active reporting, so likely biased toward more severe events. No laboratory or imaging data to confirm diagnoses available. |
| Kostine et al. 2018 | Patients who received ICIs at a single center | N= 524 9 (IA) 11 (PMR) |
19 patients required glucocorticoids, two required methotrexate. Patients with rheum irAEs higher tumor response than those without. |
Only those referred to rheumatology were diagnosed with rheum irAE likely biasing to more severe disease. Collected from 2015–2017 when less awareness of rheum irAEs. |
| Angelopoulou et al. 2020. | Literature review of MSK irAEs | 209 (IA) 51 (myositis) 44 (PMR) |
Prevalence rate of MSK irAEs of 6% in prospective studies. 70% of patients needed corticosteroids and 18% treated with DMARDs. | Question about search technique given fewer cases found than prior SLRs. |
Anti-CCP: anti-cycliic citrullinated peptide antibodies. DMARD: disease modifying anti-rheumatic drug. ICI: immune checkpoint inhibitor. IA: inflammatory arthritis. irAE: immune related adverse event. MSK: musculoskeletal. OA: osteoarthritis. PMR: polymyalgia rheumatica. RA: rheumatoid arthritis. RF: rheumatoid factor. SLR: systematic literature review. MTX: methotrexate. LEF: leflunoide. SSA: sulfasalazine. HCQ: hydroxychloroquine. TNF-I: tumor necrosis factor alpha inhibitors.
General principals of treating irAEs/Oncology guidelines
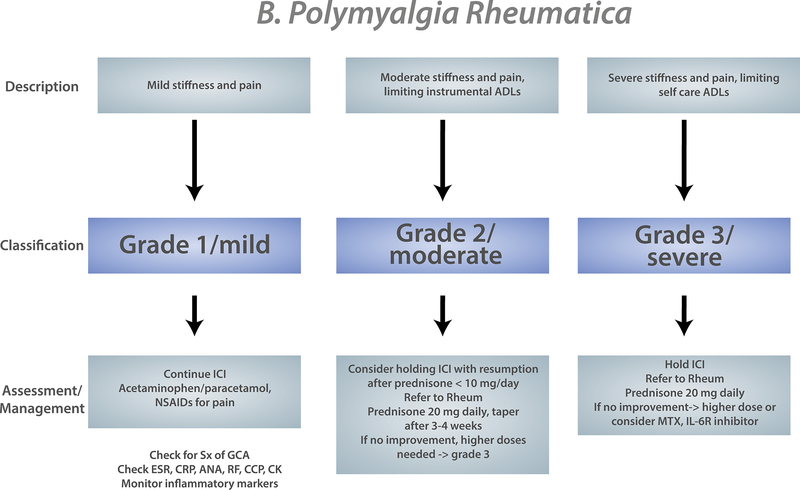
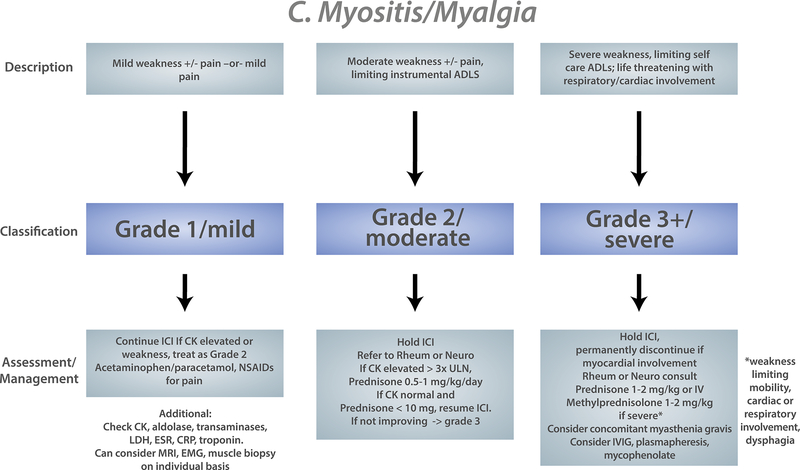
It is important to understand how referring oncologists conceptualize irAE management and treatment. The Common Terminology Criteria for Adverse Events (CTCAE) is used to grade severity of adverse events in oncology clinical trials and serves as the measure of severity in management and treatment guidelines39. For rheumatologic/musculoskeletal irAEs the CTCAE has several limitations. For example, to have arthritis of grade 3 severity, patients must be hindered in performing their self-care activities of daily living or have irreversible joint damage. Having CTCAE grade 3 or higher is often classified as a “severe” irAE, so musculoskeletal irAEs do not often meet this criterion. Several organizations publish guidelines for the evaluation and management of irAEs including the American Society of Clinical Oncologists (ASCO)40, the European Society for Medical Oncology (ESMO)41, the National Comprehensive Cancer Network (NCCN)42, and the Society for Immunotherapy of Cancer (SITC)43. Most of these guidelines are based on expert consensus. The NCCN guidelines are updated based on literature review every 6 months. We have collated recommendations from all four major oncology guidelines into a general algorithm so rheumatologists can understand what oncologists will be referencing in managing inflammatory arthritis, PMR, and myositis (Figure 2). The European League Against Rheumatism (EULAR) has also published a document of overarching principals to guide diagnosis and management of rheumatic irAEs44. Like oncology guidelines, this is based on expert consensus.
Figure 2:
Composite oncology evaluation/treatment algorithm for musculoskeletal irAEs based on American Society of Clinical Oncology (ASCO)/National Comprehensive Cancer Network (NCCN)/European Society for Medical Oncology/Society for the Immunotherapy of Cancer guidelines for A) Inflammatory arthritis, B) Polymyalgia rheumatica, C) Myositis. For PMR and Myositis only ASCO and NCCN include these entities in their guidelines. Severity (grade) of the irAE is determined by the severity of symptoms and effect on daily function. Different evaluation and management strategies are recommended based on grade.
ADL: activities of daily living. ICI: immune checkpoint inhibitor. IL-6R inhibitor: IL-6 receptor inhibitor
Inflammatory arthritis
Some patients with mild symptoms will need only NSAIDs for treatment or an intraarticular steroid injection. Generally, patients presenting to rheumatology for inflammatory arthritis will require systemic corticosteroids for grade 2 symptoms (Figure 2A). In one systematic review, 74% of patients with inflammatory arthritis were treated with systemic corticosteroids19 (Table 1). The dosing of corticosteroids reported has ranged from 5–20 mg prednisone or equivalent daily up to prednisone 1 mg/kg or more18,45. The length of a steroid taper is variable due to the heterogeneity in severity of ICI-induced inflammatory arthritis. Some patients will be continued on ICIs, and others stopped. Even among those who stop ICIs, more than 40% may still have symptoms 6 months after cessation16 (Table 1).
Methotrexate has been commonly used as a csDMARD to treat ICI-induced inflammatory arthritis, particularly in patients with grade 2 symptoms who cannot taper steroids or grade 3 symptoms19 (Figure 2A). Some oncologists have cited the approved regimen of pemetrexed (a chemotherapy agent similar to methotrexate) with pembrolizumab and carboplatin in lung cancer as a reason they are comfortable with methotrexate not impairing tumor response to ICI.
Hydroxychloroquine has also been used as a steroid-sparing agent. In one study of 11 patients from Canada hydroxychloroquine was used early in the course of ICI-induced inflammatory arthritis with good symptomatic control in the majority of patients46. For patients with mild but persistent symptoms hydroxychloroquine would be a potential treatment. Sulfasalazine and leflunomide have also been used, but less commonly.
TNF-inhibitors are the most commonly used class of biologic DMARDs. Infliximab was used early in ICI therapy to treat colitis due to ipilimumab. Short-term use of infliximab (1–3 doses) has not affected tumor response in melanoma47. Adalimumab, etanercept, and infliximab have been used to treat ICI-induced inflammatory arthritis16,19 (Figure 2A).
A small case series of three patients with inflammatory arthritis treated with tocilizumab was published in 201748. One patient in this series continued to receive tocilizumab along with ICI therapy. Giving biologic DMARDs concurrently with ICIs was initially avoided, but has become a more common practice. Recent data show successful colitis treatment with infliximab without stopping the ICI49.
Due to limited numbers, data on tumor progression and csDMARD/bDMARD usage is primarily analyzed by grouping drugs of different mechanisms together. In one study of 60 patients there was no difference in tumor outcomes in those who required csDMARDs/bDMARDs and those who did not16.
The data are even more limited regarding biologics beyond TNF-inhibitors and IL-6R inhibitors. There is a case report of tumor progression on secukinumab when used for psoriasis,50 while another case of pembrolizumab-induced psoriasis was treated successfully in this way without progression51. Apremilast has been used in a patient with PsA52 and in patients with psoriasis53 with success.
PMR
There are limited data on management of patients with PMR. A systematic literature review of musculoskeletal irAEs that included “polymyalgia rheumatica” in the search terms found only 78 patients in the published literature19; another literature review found 54 cases54 (Table 1). The mainstay of treatment is corticosteroids (required in 94% of patients in one study33). Dosing of steroids has been somewhat different from traditional PMR. Of 46 patients who needed steroids in one study, 17 of them (37%) required more than prednisone 20 mg daily for symptomatic control. Dosing of corticosteroids up to 60 mg daily has been reported33.
There is even more limited experience with steroid-sparing agents in ICI-induced PMR. In one study, five patients out of 49 needed additional immunosuppression33. Two were treated with tocilizumab, and three others were treated with methotrexate or hydroxychloroquine. A case of PMR from ICIs treated with infliximab has also been reported55.
Myositis
Corticosteroids are the first line therapy for myositis due to ICIs. Initial dosing can range from 0.5 mg/kg prednisone daily up to 2000 mg IV methylprednisolone24,27. Myositis is a clinical scenario where initial aggressive therapy with high dose immunosuppression is often indicated. With inflammatory arthritis and polymyalgia rheumatica, there is not the same risk of death or rapid morbidity, so a step up approach is more commonly used.
Intravenous immunoglobulin (IVIg) has been used in ICI-induced myositis24,27 (Table 1). IVIg has been used successfully for other irAEs such as myasthenia gravis56, limbic encephalitis57, pure red cell aplasia58, and immune-mediated thrombocytopenia59.
Case reports and series have described plasmapheresis (PLEX) for severe myositis and for patients with myasthenia and/or myocarditis overlap27,24,60. Outcomes have been variable with the use of PLEX, with some patients making a recovery and others dying of respiratory failure despite PLEX. IVIg or PLEX are also used when there is myasthenia overlap.
When patients have myocarditis in addition to myositis, additional therapies have been attempted. Tocilizumab was used successfully in one case of myositis/myocarditis overlap refractory to corticosteroids61. Abatacept has also been used in this scenario62.
Managing preexisting autoimmune disease during ICI therapy
Patients with preexisting autoimmune diseases were excluded from the original clinical trials evaluating ICIs. However, with many regimens now approved as standard of care, patients with autoimmune disease and cancer are increasingly receiving ICIs in clinical practice. Several retrospective studies and systematic literature reviews of observational studies have evaluated flares and de novo irAEs in patients with preexisting autoimmune disease (Table 2). Overall, patients with preexisting autoimmune disease can generally be treated with ICIs and do not have adverse events severe enough to require ICI treatment cessation. About 40–50% of patients with autoimmune disease will have a flare63–65. It is important to note that this data is based primarily on patients with psoriasis, rheumatoid arthritis, and psoriatic arthritis and not vasculitis, scleroderma, or SLE. For more severe multisystem autoimmune diseases, the safety profile is less clear. The rate of flare may be higher in anti-PD-1/PD-L1 agents, while de novo irAEs may be higher in anti-CTLA-4 agents64. Most studies have combined autoimmune diseases of all types, including those limited to one organ like psoriasis and autoimmune thyroid disease. One study of 22 RA patients showed flares happened commonly (in 55%), but only one patient had to discontinue ICIs due to a flare or adverse event66.
Table 2:
Selected studies for the evaluation and management of preexisting autoimmune disease while on ICI therapy
| Author, year (citation) | Study population | N | Results | Comments |
|---|---|---|---|---|
| Tison et al. 2020 | Patients with preexisting autoimmune disease treated with ICI for cancer | 112 | Most common diagnoses: PsO/PsA, RA, IBD. Flares in 47%, other irAEs in 42%. Shorter progression free survival in those who were on immunosuppression at ICI start. | Multicenter retrospective study from France. Raises concerns about effect of immunosuppression on tumor response. |
| Efuni et al. 2020 | Patients with RA treated with ICI for cancer | 22 | Flares in 55%, other irAEs in 32%, mostly managed with steroids only 1 discontinued ICI | Retrospective review, but encouraging for successful treatment of patients with RA with ICIs. |
| Abdel-Wahab et al. 2018 | Patients with preexisting autoimmune disease treated with ICI for cancer | 123 | PsO/PsA, RA: most common diagnoses. More flares with anti-PD-1/PD-L1 agents, more de novo irAEs with ipilimumab. 17% of patients discontinued ICI due to flare or irAEs. | Systematic literature review that included case reports, caution in interpreting numerical values for flare as likely biased toward more flares/irAEs in case reports. |
| Danlos et al. 2018 | Patients from registry for anti-PD-1 treated pts who had preexisting autoimmune disease | 45 | Vitiligo, PsO most common diagnoses. Less than 15% of patients on immunomodulatory therapy at ICI start. | Not many patients with systemic autoimmune disease or on immunosuppressive therapy at start of ICI, so not applicable to many rheum patients. |
| Menzies et al. 2017 | Patients with preexisting autoimmune disease or prior severe irAE treated with anti-PD-1 for melanoma | 52 (preexisting autoimmune) | RA most common (N=13). 38% on immunosuppression at start of ICI. 38% had flare, 29% de novo irAEs. Lower tumor response rate if on immunosuppression at ICI start. | Small numbers for each autoimmune disease and heterogeneous immunosuppressive treatment. With small numbers and heterogeneity, hard to interpret tumor response data. |
| Johnson et al. 2016 | Patients with preexisting autoimmune disease treated with ipilimumab for melanoma | 30 | RA, PsO, IBD were most common. 13 patients were receiving steroid or csDMARD at start of ICI. 50% had flare and/or irAEs. | Small numbers for each autoimmune disease. Ipilimumab monotherapy now uncommon since newer agents/regimens approved. |
IBD: inflammatory bowel disease. ICI: immune checkpoint inhibitor. irAE: immune related adverse event. RA: rheumatoid arthritis. csDMARD: conventional synthetic disease modifying anti-rheumatic drug. PsA: psoriatic arthritis. PsO: psoriasis.
The question of how to manage patients with preexisting autoimmune disease in terms of steroids and other forms of immunosuppression is still under debate. There is data that suggest immunosuppression with steroids or steroid-sparing immunosuppression at ICI start is associated with worsened tumor response65,67. Given the concern over affecting tumor response and lack of data on the effects of specific agents, minimizing the amount of immunosuppression at the time of ICI start and treating flares with short courses of steroids as needed is a reasonable approach.
Discussion:
Returning to our initial case: The 65-year-old man with new musculoskeletal symptoms on ICI therapy presented to your clinic for an appointment. On further history, he tells you that he started having symptoms about 6 months after ICI therapy started. First, his right knee was sore and it was difficult to kneel due to stiffness and pain in the knee. He ignored this symptom initially since he had experienced pain in his left knee before his joint replacement. Next, his ankles became painful and swollen. Finally, he developed stiffness and pain in his MCPs and PIPs which impaired his grip and fine motor tasks. The hand symptoms which limited his ability to do buttons and dress himself prompted him to reach out to his oncologist. It was about 11 months since his first dose of ICI therapy. On examination, he had synovitis of the small joints of hands, ankles and bilateral knee effusions with some warmth along with right knee crepitus. His strength was full. Laboratory studies showed elevated ESR and CRP with a negative rheumatoid factor and negative anti-CCP antibodies. He was diagnosed with inflammatory arthritis due to ICI therapy. You have a discussion with his oncologist who plans to hold the next dose of ICI and a prednisone taper is started at 40 mg daily. When the patient tapered to 15 mg daily, however, symptoms returned. Methotrexate was started as a steroid sparing agent with plans by his oncologist to restart ICI therapy when his inflammatory arthritis was stable.
IrAEs represent a new group of multisystem inflammatory disorders that rheumatologists will encounter for the foreseeable future. The patient described at the beginning of this article could have had inflammatory arthritis, PMR, or myositis/fasciitis due to ICI treatment depending on further history and physical examination. Currently, however, there is limited prospective data and no data from randomized clinical trials to guide management of all of these rheumatic irAEs.
Before trials for management of rheumatic irAEs can commence, there must be an agreed upon taxonomy for types of irAEs and understanding of different phenotypes within categories. Additionally, the CTCAE used by oncologists to classify and grade irAEs could be improved to better reflect rheumatic irAEs and their impacts on patients. As new types of immunotherapy and combination regimens are tested and approved new irAEs may be discovered and improving the ability for signal detection by oncologists is important.
A first priority is high quality prospective observational studies. These studies can provide insight into how to define rheumatic irAEs for trial inclusion and what indices can be used to monitor disease activity and response to therapeutics. It may be that existing outcome measures from traditional rheumatic diseases are useful in irAEs, but this has not been systematically studied.
Once case definitions and outcome measures are defined, clinical trials can begin to address several questions that come up in the treatment of irAEs. The first question is the dosing of initial steroids and whether we should aim to step up if lack or response or step down from a higher steroid dose as induction. Right now, it is unclear which will lead to lower cumulative steroid dosing and whether short-term higher dose steroids have a different immunologic effect on tumor response than longer-term low dose steroids. Types of immunosuppression beyond corticosteroids have been taken from treatments for traditional rheumatic disease like RA and dermatomyositis and applied to similar irAEs. Additional clinical research priorities for inflammatory arthritis, PMR and myositis due to ICIs are outlined in Table 3.
Table 3:
Key clinical research questions for rheumatic irAEs
| Research area | Key clinical research questions |
|---|---|
| Rheumatic irAEs (general) | -Should corticosteroids be started at a high dose with a taper or started at a low dose with step up therapy? -How early should csDMARDs and biologic DMARDs be utilized in treatment? -Do all rheumatic irAEs have the possibility of becoming chronic processes or is this unique to inflammatory arthritis? |
| Inflammatory arthritis | -What are the phenotypes of inflammatory arthritis and do they correlate with response to particular treatments and cancer prognosis? -What is the role of osteoarthritis in inflammatory arthritis due to ICIs? Can an increase in osteoarthritis pain be caused by ICIs and does this represent a new process? -What imaging modality is most sensitive and specific for inflammatory arthritis due to ICIs? |
| Polymyalgia rheumatica | -What is the optimal starting corticosteroid dose and does it differ from traditional polymyalgia rheumatica? -Should patients with PMR-like symptoms and peripheral synovitis be classified as PMR or inflammatory arthritis? -What is the prevalence of giant cell arteritis in those who develop polymyalgia rheumatica from ICIs? |
| Myositis | -What is the optimal evaluation for patients with suspected myositis (e.g. EMG, MRI, muscle biopsy)? -What is the best starting dose for corticosteroids? -What is the optimal steroid sparing agent? -In which cases is it safe to rechallenge patients with ICI? -Are there risk factors present before ICI therapy is started for myositis? If so, should patients with these risk factors have enhanced monitoring? |
One trial, NIVO-AID, run through the Cancer Therapeutics Evaluation Program (CTEP), a part of the National Cancer Institute, is evaluating the use of nivolumab for patients with solid tumors and underlying autoimmune diseases [NCT03656627]. To our knowledge there are not active trials specifically evaluating treatments for rheumatic irAEs.
It is critical to define the biology of irAEs, which may allow for risk stratification at ICI initiation and inform therapeutic choices guided by mechanism. Given the diversity of irAEs in terms of presentation, severity, and response to treatments, the pathogenesis of irAEs is likely heterogeneous. Limited data has been published for particular irAEs that may be relevant to pathogenesis. For example, genetic studies in insulin-dependent diabetes associated with ICIs and in inflammatory arthritis suggest there may be similar HLA associations68,69. Interestingly, particular HLA haplotypes have also been associated with better or worse tumor response to ICIs70. Perturbations in the microbiome have been linked to development of colitis as an irAE71. Elevated baseline levels of IL-17 have also been associated with development of severe colitis72. An intriguing question relevant to pathogenesis is why there have been minimal cases of lupus-like syndromes with irAEs. Drug-induced lupus is a well described entity and can be associated with many medications. Thus far, cutaneous forms of lupus, most commonly subacute cutaneous lupus, are described in association with ICIs, but systemic lupus less frequently73–75.
Ultimately, high-quality clinical data collection coupled with translational science will allow patients with rheumatic irAEs to be treated effectively. Until more data is available, discussing the limitations in our knowledge with referring oncologists and patients will allow for shared decision-making in managing irAEs.
Clinical Challenge:
You receive a consult from a local oncologist. A 65-year-old man with a history of knee osteoarthritis s/p left total knee arthroplasty who is being treated with nivolumab (anti-PD-1 monoclonal antibody) for non-small cell lung cancer has new symptoms of severe aching in his arms and legs with limited mobility. The oncologist does not localize symptoms to the joints and/or muscles, but notes that the patient is having trouble doing chores around the house such as cleaning and cooking.
Acknowledgments
Financial support: LCC was supported by NIAMS K23-AR075872.
Financial interests: LCC: research funding Bristol-Myers Squibb. COB: research funding from Bristol-Myers Squibb.
References:
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer cell. 2015;27(4):450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8(9):1069–1086. [DOI] [PubMed] [Google Scholar]
- 4.Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baetz TD, Fletcher GG, Knight G, et al. Systemic adjuvant therapy for adult patients at high risk for recurrent melanoma: A systematic review. Cancer treatment reviews. 2020;87:102032. [DOI] [PubMed] [Google Scholar]
- 6.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med. 2018;378(21):1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378(2):158–168. [DOI] [PubMed] [Google Scholar]
- 8.Allenbach Y, Anquetil C, Manouchehri A, et al. Immune checkpoint inhibitor-induced myositis, the earliest and most lethal complication among rheumatic and musculoskeletal toxicities. Autoimmunity reviews. 2020:102586. [DOI] [PubMed] [Google Scholar]
- 9.Calabrese LH, Calabrese C, Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nature reviews Rheumatology. 2018;14(10):569–579. [DOI] [PubMed] [Google Scholar]
- 10.Cappelli LC, Gutierrez AK, Bingham CO 3rd, Shah AA. Rheumatic and Musculoskeletal Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors: A Systematic Review of the Literature. Arthritis care & research. 2017;69(11):1751–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buder-Bakhaya K, Benesova K, Schulz C, et al. Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies. Cancer immunology, immunotherapy : CII. 2018;67(2):175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kostine M, Rouxel L, Barnetche T, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Annals of the rheumatic diseases. 2018;77(3):393–398. [DOI] [PubMed] [Google Scholar]
- 13.Haslam A, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA network open. 2019;2(5):e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chai QQ, Du JY, Zhu J, Wu B. The Differences in the Safety and Tolerability of Immune Checkpoint Inhibitors as Treatment for Non-Small Cell Lung Cancer and Melanoma: Network Meta-Analysis and Systematic Review. Front Pharmacol. 2019;10:1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2017;28(10):2377–2385. [DOI] [PubMed] [Google Scholar]
- 16.Braaten TJ, Brahmer JR, Forde PM, et al. Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing P, Zhang F, Wang G, et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: a systematic review and meta-analysis. Journal for immunotherapy of cancer. 2019;7(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buder-Bakhaya K, Benesova K, Schulz C, et al. Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies. Cancer immunology, immunotherapy : CII. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh N, Tiongson MD, Stewart C, et al. Checkpoint Inhibitor-Associated Arthritis: A Systematic Review of Case Reports and Case Series. J Clin Rheumatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim ST, Bittar M, Kim HJ, et al. Recurrent pseudogout after therapy with immune checkpoint inhibitors: a case report with immunoprofiling of synovial fluid at each flare. Journal for immunotherapy of cancer. 2019;7(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corominas H, Badlissi F, Shmerling RH. Crystal-induced oligoarthritis triggered by pembrolizumab, an immune checkpoint inhibitor. Joint Bone Spine. 2018;85(5):647–648. [DOI] [PubMed] [Google Scholar]
- 22.Albayda J, Bingham CO 3rd, Shah AA, Kelly RJ, Cappelli L. Metastatic joint involvement or inflammatory arthritis? A conundrum with immune checkpoint inhibitor-related adverse events. Rheumatology (Oxford, England). 2018;57(4):760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortellini A, Vitale MG, De Galitiis F, et al. Early fatigue in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: an insight from clinical practice. Journal of translational medicine. 2019;17(1):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matas-García A, Milisenda JC, Selva-O’Callaghan A, et al. Emerging PD-1 and PD-1L inhibitors-associated myopathy with a characteristic histopathological pattern. Autoimmun Rev. 2020;19(2):102455. [DOI] [PubMed] [Google Scholar]
- 25.Jeyakumar N, Etchegaray M, Henry J, et al. The Terrible Triad of Checkpoint Inhibition: A Case Report of Myasthenia Gravis, Myocarditis, and Myositis Induced by Cemiplimab in a Patient with Metastatic Cutaneous Squamous Cell Carcinoma. Case Reports Immunol. 2020;2020:5126717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. Journal for immunotherapy of cancer. 2019;7(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Touat M, Maisonobe T, Knauss S, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. 2018;91(10):e985–e994. [DOI] [PubMed] [Google Scholar]
- 28.Shah M, Tayar JH, Abdel-Wahab N, Suarez-Almazor ME. Myositis as an adverse event of immune checkpoint blockade for cancer therapy. Seminars in arthritis and rheumatism. 2019;48(4):736–740. [DOI] [PubMed] [Google Scholar]
- 29.Mammen AL, Rajan A, Pak K, et al. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Annals of the rheumatic diseases. 2019;78(1):150–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cappelli LC, Gutierrez AK, Baer AN, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Annals of the rheumatic diseases. 2017;76(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albayda J, Dein E, Shah AA, Bingham CO 3rd, Cappelli L. Sonographic Findings in Inflammatory Arthritis Secondary to Immune Checkpoint Inhibition: A Case Series. ACR Open Rheumatol. 2019;1(5):303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subedi A, Williams SG, Yao L, et al. Use of Magnetic Resonance Imaging to Identify Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis. JAMA Netw Open. 2020;3(2):e200032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calabrese C, Cappelli LC, Kostine M, Kirchner E, Braaten T, Calabrese L. Polymyalgia rheumatica-like syndrome from checkpoint inhibitor therapy: case series and systematic review of the literature. RMD open. 2019;5(1):e000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Geest KSM, Sandovici M, Rutgers A, et al. Imaging in immune checkpoint inhibitor-induced polymyalgia rheumatica. Annals of the rheumatic diseases. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daoussis D, Kraniotis P, Filippopoulou A, et al. An MRI study of immune checkpoint inhibitor-induced musculoskeletal manifestations myofasciitis is the prominent imaging finding. Rheumatology (Oxford, England). 2020;59(5):1041–1050. [DOI] [PubMed] [Google Scholar]
- 36.Vermeulen L, Depuydt CE, Weckx P, et al. Myositis as a neuromuscular complication of immune checkpoint inhibitors. Acta Neurol Belg. 2020;120(2):355–364. [DOI] [PubMed] [Google Scholar]
- 37.John S, Antonia SJ, Rose TA, et al. Progressive hypoventilation due to mixed CD8(+) and CD4(+) lymphocytic polymyositis following tremelimumab - durvalumab treatment. Journal for immunotherapy of cancer. 2017;5(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cappelli LC, Grieb SM, Shah AA, Bingham CO 3rd, Orbai AM. Immune checkpoint inhibitor-induced inflammatory arthritis: a qualitative study identifying unmet patient needs and care gaps. BMC Rheumatol. 2020;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.U.S. Department of Health and Human Services N, NCI. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf. Published 2017. Accessed 8/24/2018, 2018.
- 40.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2017;28(suppl_4):iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 42.Thompson JA, Schneider BJ, Brahmer J, et al. Management of Immunotherapy-Related Toxicities, Version 1.2019. J Natl Compr Canc Netw. 2019;17(3):255–289. [DOI] [PubMed] [Google Scholar]
- 43.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. Journal for immunotherapy of cancer. 2017;5(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kostine M, Finckh A, Bingham CO 3rd, et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Annals of the rheumatic diseases. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cappelli LC, Brahmer JR, Forde PM, et al. Clinical presentation of immune checkpoint inhibitor-induced inflammatory arthritis differs by immunotherapy regimen. Seminars in arthritis and rheumatism. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts J, Smylie M, Walker J, et al. Hydroxychloroquine is a safe and effective steroid-sparing agent for immune checkpoint inhibitor-induced inflammatory arthritis. Clinical rheumatology. 2019;38(5):1513–1519. [DOI] [PubMed] [Google Scholar]
- 47.Horvat TZ, Adel NG, Dang TO, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim ST, Tayar J, Trinh VA, et al. Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Annals of the rheumatic diseases. 2017;76(12):2061–2064. [DOI] [PubMed] [Google Scholar]
- 49.Badran YR, Cohen JV, Brastianos PK, Parikh AR, Hong TS, Dougan M. Concurrent therapy with immune checkpoint inhibitors and TNFα blockade in patients with gastrointestinal immune-related adverse events. Journal for immunotherapy of cancer. 2019;7(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Esfahani K, Miller WH Jr. Reversal of Autoimmune Toxicity and Loss of Tumor Response by Interleukin-17 Blockade. N Engl J Med. 2017;376(20):1989–1991. [DOI] [PubMed] [Google Scholar]
- 51.Monsour EP, Pothen J, Balaraman R. A Novel Approach to the Treatment of Pembrolizumab-induced Psoriasis Exacerbation: A Case Report. Cureus. 2019;11(10):e5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nigro O, Pinotti G, Gueli R, et al. Psoriatic arthritis induced by anti-PD1 and treated with apremilast: a case report and review of the literature. Immunotherapy. 2020;12(8):549–554. [DOI] [PubMed] [Google Scholar]
- 53.Fattore D, Annunziata MC, Panariello L, Marasca C, Fabbrocini G. Successful treatment of psoriasis induced by immune checkpoint inhibitors with apremilast. European journal of cancer (Oxford, England : 1990). 2019;110:107–109. [DOI] [PubMed] [Google Scholar]
- 54.Angelopoulou F, Bogdanos D, Dimitroulas T, Sakkas L, Daoussis D. Immune checkpoint inhibitor-induced musculoskeletal manifestations. Rheumatol Int. 2020. [DOI] [PubMed] [Google Scholar]
- 55.Calabrese C, Kirchner E, Kontzias K, Velcheti V, Calabrese LH. Rheumatic immune-related adverse events of checkpoint therapy for cancer: case series of a new nosological entity. RMD open. 2017;3(1):e000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Safa H, Johnson DH, Trinh VA, et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. Journal for immunotherapy of cancer. 2019;7(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung M, Jaffer M, Verma N, Mokhtari S, Ramsakal A, Peguero E. Immune checkpoint inhibitor induced anti-glutamic acid decarboxylase 65 (Anti-GAD 65) limbic encephalitis responsive to intravenous immunoglobulin and plasma exchange. J Neurol. 2020;267(4):1023–1025. [DOI] [PubMed] [Google Scholar]
- 58.Isoda A, Miyazawa Y, Tahara K, et al. Pembrolizumab-induced Pure Red Cell Aplasia Successfully Treated with Intravenous Immunoglobulin. Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shiuan E, Beckermann KE, Ozgun A, et al. Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy. Journal for immunotherapy of cancer. 2017;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xing Q, Zhang ZW, Lin QH, et al. Myositis-myasthenia gravis overlap syndrome complicated with myasthenia crisis and myocarditis associated with anti-programmed cell death-1 (sintilimab) therapy for lung adenocarcinoma. Ann Transl Med. 2020;8(5):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doms J, Prior JO, Peters S, Obeid M. Tocilizumab for refractory severe immune checkpoint inhibitor-associated myocarditis. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salem JE, Allenbach Y, Vozy A, et al. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. N Engl J Med. 2019;380(24):2377–2379. [DOI] [PubMed] [Google Scholar]
- 63.Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2017;28(2):368–376. [DOI] [PubMed] [Google Scholar]
- 64.Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann Intern Med. 2018;168(2):121–130. [DOI] [PubMed] [Google Scholar]
- 65.Tison A, Quéré G, Misery L, et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis & rheumatology (Hoboken, NJ). 2019;71(12):2100–2111. [DOI] [PubMed] [Google Scholar]
- 66.Efuni E, Cytryn S, Boland P, et al. Risk of Toxicity After Initiating Immune Checkpoint Inhibitor Treatment in Patients With Rheumatoid Arthritis. J Clin Rheumatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arbour KC, Mezquita L, Long N, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36(28):2872–2878. [DOI] [PubMed] [Google Scholar]
- 68.Cappelli LC, Dorak MT, Bettinotti MP, Bingham CO, Shah AA. Association of HLA-DRB1 shared epitope alleles and immune checkpoint inhibitor-induced inflammatory arthritis. Rheumatology (Oxford, England). 2019;58(3):476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Filette JMK, Pen JJ, Decoster L, et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol. 2019;181(3):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chowell D, Morris LGT, Grigg CM, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science (New York, NY). 2018;359(6375):582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2017;28(6):1368–1379. [DOI] [PubMed] [Google Scholar]
- 72.Tarhini AA, Zahoor H, Lin Y, et al. Baseline circulating IL-17 predicts toxicity while TGF-beta1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. Journal for immunotherapy of cancer. 2015;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michot JM, Fusellier M, Champiat S, et al. Drug-induced lupus erythematosus following immunotherapy with anti-programmed death-(ligand) 1. Annals of the rheumatic diseases. 2019;78(7):e67. [DOI] [PubMed] [Google Scholar]
- 74.Shao K, McGettigan S, Elenitsas R, Chu EY. Lupus-like cutaneous reaction following pembrolizumab: An immune-related adverse event associated with anti-PD-1 therapy. Journal of cutaneous pathology. 2018;45(1):74–77. [DOI] [PubMed] [Google Scholar]
- 75.Liu RC, Sebaratnam DF, Jackett L, Kao S, Lowe PM. Subacute cutaneous lupus erythematosus induced by nivolumab. The Australasian journal of dermatology. 2018;59(2):e152–e154. [DOI] [PubMed] [Google Scholar]