Abstract
Identifying progression is of fundamental importance to the management of glaucoma. It is also a challenge. The most sophisticated, and probably the most useful, commercially available clinical tool for identifying progression is the Guided Progression Analysis (GPA), which was initially developed to identify progression using 24–2 visual field tests. More recently it has been extended to retinal nerve fiber layer (RNFL) and ganglion cell + inner plexiform layer (GCIPL) thicknesses measured with optical coherence tomography (OCT). However, the OCT GPA requires a minimum of 3 tests to determine “possible loss (progression)” and a minimum of 4 tests to determine if the patient shows “likely loss (progression).” Thus, it is not designed to answer a fundamental question asked by both clinician and patient, namely: Did damage progress since the last visit? Some clinicians use changes in summary statistics, such as global/ average circumpapillary RNFL thickness. However, these statistics have poor sensitivity and specificity due to segmentation and alignment errors. Instead of relying on the GPA analysis or summary statistics, one needs to evaluate RNFL and GCIPL probability maps and circumpapillary OCT b–scan images. In addition, we argue that the clinician can make a better decision about suspected progression between two test days by topographically comparing the changes in the different OCT maps and images, as well as topographically comparing the changes in the visual field to the changes in the OCT probability maps.
Keywords: glaucoma, progression, optical coherence tomography, OCT, perimetry, visual fields
Précis:
For determining whether a patient has progressed between two visits based upon OCT scans, the Guided Progression Analysis (GPA) and changes in global OCT statistics are of limited value. Instead, methods based upon topographical comparisons of OCT probability maps and examination of OCT images are needed.
Identifying progression is of fundamental importance to the management of glaucoma. Among the tools available to the clinician is the visual field test obtained with static automated perimetry. However, the repeat variability of this test has been a major obstacle to successful detection of progression. Based upon years of research, it is generally accepted that multiple visual field tests are required to reliably, and statistically, establish progression.1–3 The Zeiss’ clinical report, the “Guided Progression Analysis (GPA) for 24–2 Test” (Carl Zeiss Meditec, Inc., Dublin, CA) was based upon this research. The GPA has helped overcome the variability in visual field testing, and enabled more objective judgements to be made.
The same approach has been extended to the analysis of progression using optical coherence tomography (OCT). In particular, the Zeiss GPA reports for retinal nerve fiber layer (RNFL) and ganglion cell + inner plexiform layer (GCIPL) thicknesses are, like the GPA for 24–2 test, based upon the assumption that one needs a minimum of 4 tests to detect “likely” progression.
Thus, the GPA OCT report, like the GPA 24–2 visual field report, is not designed to answer a fundamental clinical question important to both the patient and the clinician, namely: Did damage progress since the last visit? Our purpose here is to first review the OCT GPA and to illustrate how its design limits answering this fundamental question, and second to discuss approaches for answering this question, including the need to evaluate both RNFL and GCL probability maps, and to scrutinize circumpapillary OCT b-scans.
THE 24–2 VISUAL FIELD AND THE GPA
The GPA is undoubtedly the most sophisticated, and probably the most useful, clinical tool for identifying progression based upon multiple 24–2 visual fields. Figure 1 provides an example of the GPA Summary Report for the 24–2 test. The key aspect of this report is the Progression Analysis, shown within the red rectangle in panel A. It is enlarged and presented in panel B. An open triangle indicates that the sensitivity at that point on the current test (TC1) has decreased at the p<5% level as compared to the average of two baseline tests (first row in Fig. 1). In this example, 3 points met this criterion. Note, it takes 3 tests to analyze whether the sensitivity at a given point has decreased, two baseline tests and the test at TC1. If on the test at time 2 (TC2), one of the locations with an open triangle on TC1 (Fig, 1B) is also significantly less sensitive, again compared to baseline, then a half-filled triangle appears. In this example, one of the locations satisfied this criterion, as is shown by the Progression Analysis for TC2 in Fig. 1C. If that point were significant on a fifth test (TC3), again compared to the baseline tests, then a filled triangle would appear at that location. The GPA uses the Early Manifest Glaucoma Trial criteria4 for progression, namely, 3 or more half-filled locations denote “possible loss (progression)”, and 3 or more filled triangles denote “likely loss (progression)”. In other words, a minimum of 4 tests (i.e., 2 baseline tests plus 2 subsequent tests) are needed to determine “possible progression” and a minimum of 5 tests are needed to determine “likely progression”.
Figure 1.
A. The Guided Progression Analysis (GPA) Summary Report for the 24–2 Test for a patient tested on three dates. B. The Progression Analysis within the red rectangle in panel A is shown enlarged. The small triangles indicate that the sensitivity at that location is significantly lower compared to the two baseline tests. C. The Progression Analysis for the 4th test. The open triangle, as in B, indicates that the sensitivity at that location is significantly lower compared to the two baseline tests, and the half-filled triangle indicates that that location was also one of the three significant location (open triangles) on test 3 (panel B).
While the GPA approach is statistically sound, it does not answer the question: Did the eye show progression of damage since the last test? To answer this question, the clinician is left to interpret the reports for the 24–2 while taking into consideration other information such as intraocular pressure (IOP), fundus examination/photography, other risk factors (e.g., disc hemorrhage, central corneal thickness, family history), and, often, OCT data.
THE OCT AND THE GPA
The GPA and RNFL Thickness
As with the visual fields, the challenge when using OCT tests is to distinguish real loss (i.e., progression) from test-retest variability. Again, the most sophisticated, commercially available statistical approach based upon multiple tests is the Zeiss OCT GPA. Figure 2A is the top half of the GPA report for an OCT disc scan obtained on TC1 for the same eye tested as in Fig. 1. The top row shows the RNFL thickness maps in pseudo-color. The second row shows the “Change Map.” The Change Map is similar to the Progression Analysis reports in Fig. 1B,C. In the case of the OCT Change Map, an individual location is a superpixel on the RNFL thickness map. A yellow region, like the open triangle in Figs. 1B,C, indicates that for this superpixel, the third test was significantly different than the two baseline tests. This location/superpixel is said to show “possible loss.” If the same superpixel is also significantly different from baseline on the next, (4th) test, then it is coded red (“likely loss”). Figure 2B shows the top half of the report for the 4th test on TC2. The red regions contain superpixels that were significantly different from the baseline tests obtained on both TC1 and TC2. Overall, there must be at least 20 adjacent yellow or red superpixels for the change maps to be classified as “possible loss” or “likely loss”. Thus, an eye is likely progressing if a large enough region turns red and stays red. For the eye in our example, test 3 (TC1) showed yellow regions (yellow arrows, Fig. 2A) suggesting “possible loss/progression”. On test 4 (TC2), most of these same regions were now red (red arrows in Fig. 2B) suggesting “likely loss/progression”.
Figure 2.
A. The Guided Progression Analysis (GPA) Summary Report for the RNFL thickness plots (top row) from OCT cube scans centered on the disc. The Change Map is similar to the Progression Analysis in Fig. 1. In this case, yellow indicates that the third visit was significantly different than the two baseline exams. This location/superpixel is said to show “possible loss”. If the same superpixel is also significantly different from baseline on the next (4th) test, then it is coded red (“likely loss”) as shown in panel B, the GPA Report for test 4.
Thus, based upon the OCT RNFL analysis, we can now say, after a minimum of 4 tests (i.e., 2 baseline tests plus 2 subsequent tests) that this eye is “likely progressing”, as compared to baseline. But, did it progress since the last test (i.e., between TC1 and TC2)? The GPA does not provide a way to answer this question.
The GPA and GCIPL Thickness
More recently, the same logic has been applied to GCIPL thickness measures obtained from OCT cube scan centered on the fovea. Figure 3 shows Change Maps, for the same eye and test dates as in Fig. 2B. After 4 tests, the Change Maps indicate “likely progression,” as indicated by the red regions on the Change Map for test 4 (Fig. 3, lower right panel). But, again did this eye progress between test 3 (TC1: 8/25/2014) and test 4 (TC2: 2/24/2017)?
Figure 3.
A. The Guided Progression Analysis (GPA) Summary Report for the GCIPL thickness plots (top row) from OCT cube scans centered on the fovea. The Change Map is similar to the Change Map in Fig. 2.
Recent Advances in OCT and GPA
Recently, two groups have suggested substantial improvements to the OCT GPA approach. Leung and colleagues5,6 have proposed replacing the “event-based” comparison between baseline tests and the test at TC1, with a “trend-based progression analysis” (TPA). TPA uses linear regression analysis based upon all tests between Baseline 1 and TC, the current test. They demonstrated that compared to GPA, TPA detected more eyes with progressive RNFL thinning.5 This same group has also argued that progression can be detected sooner (i.e., with fewer tests) if RNFL and GCIPL results are combined either via separate analyses as in Figs. 2 and 3,7 or by using a wide-field scan and measuring the combined RNFL+GCIPL thickness.8 These are important improvements, which should be considered as modifications to the standard GPA analysis. However, they still require at least 4 tests to conclude “likely progression.”
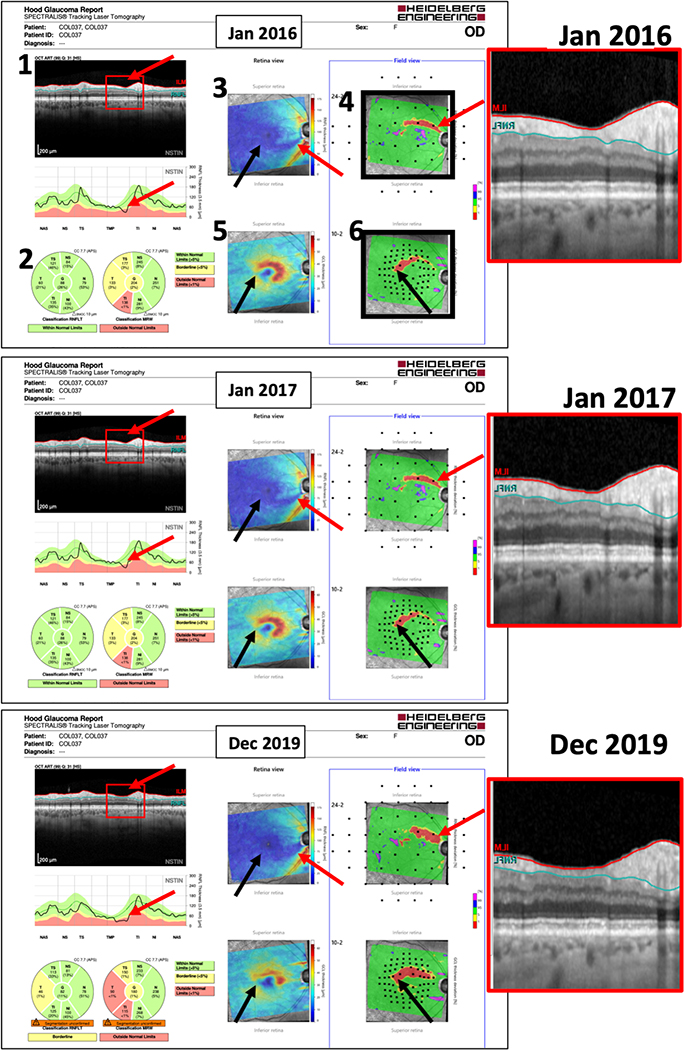
Park, Lee, Kim and colleagues9–11 have also argued for using trend-based analysis, as well as information from both OCT RFNL and GCIPL thickness measures. For reasons to be discussed below, the Lee et al.10 study is particularly important for our purposes here. They compared four methods for determining whether an eye had progressed. Figure 4A, from their study, shows the images from one study eye. Two of their methods employed the traditional GPA analysis of the RNFL and GCIPL. The thickness and change maps for these GPA methods are shown in Fig. 4A, labelled (1) (RNFL) and 2 (GCIPL). Based upon both GPA analyses, this eye showed “likely progression” by test 6, as indicated by the red regions on the change maps (red arrows in (1) and (2) of Fig. 4A). Their other two methods involved a “qualitative” judgement of progression by glaucoma experts. In method 3, they viewed the series of RNFL thickness maps, shown as (3) of Fig. 4A. In method 4, a similar judgement was made of the deviation maps, shown as (4) of Fig. 4A. In general, thickness maps and deviation maps are available from a commercial report, the Zeiss Panomap. The Lee et al. study found that expert assessment of the deviation maps had the highest accuracy, although the results were not significantly different from the traditional GPA of the RNFL (1). [Note: the “deviation” maps are “probability” maps, where the yellow and red colors refer to regions significantly different from healthy controls at the 5% (yellow) and 1% (red) levels.]
Figure 4.
A. A sample set of images used in Lee et al.10 The thickness and Change Maps for the 2 GPA methods are shown in Fig. 4A, labelled (1) (RNFL) and 2 (GCIPL). Based upon both GPA analyses, this eye showed “likely progression” by the 6th test/exam (red arrow). Methods 3 and 4 involved a judgement of progression by glaucoma experts, who viewed the series of RNFL thickness maps (3) or deviation maps from the Zeiss Panomap Report. B. The latter are shown enlarged. The colored regions indicate significantly abnormal at the 5% (yellow) and 1% (red) as compared to healthy controls. The region within the red rectangle appears to be progressing and this change can be seen on the 3rd test/exam. Reproduced and modified from eFigure 3 with permission from JAMA Ophthalmology. 2018; 136(10): 1121–1127 Copyright© 2018 American Medical Association. All rights reserved.
BUT IS THERE PROGRESSION SINCE THE LAST TEST?
The Clinical Problem
Glaucoma is usually a progressive disease if left untreated, and progression can occur despite treatment. Hence, over time changes in treatment are needed in virtually all patients with diagnosed or suspected glaucoma. Due to the irreversible nature of glaucomatous damage, clinicians do not have the time to wait for documented, confirmed progression before escalating therapy. In fact, being behind (and not ahead) of the disease has been argued to be a main reason that patients continue to go blind from glaucoma.12 Therefore, although repeating tests multiple times and waiting for confirmation has its merits, in daily practice a decision often needs to be made relative to the most recent visit(s). However, there is no standard procedure for evaluating OCT changes between two test dates, although there are at least three different general approaches employed by clinicians. We consider these three approaches next.
Viewing B-Scan Images and cpRNFL Thickness Plots.
One approach involves looking at b-scans before evaluating cpRNFL thickness plots. We have argued that, for detecting and understanding glaucoma, the clinician should closely examine b-scan images, especially circumpapillary images.13.14 Progression between two visits can also be assessed with circumpapillary b-scan images.15–18 However, in the case of progression the situation is more complicated as the b-scan images from two sessions must be aligned. There are two ways to accomplished this. First, when the circumpapillary b-scan is derived from a cube scan, the scans from two test dates can be centered in the same optic disc location after acquisition. The circumpapillary b-scan images in Fig. 5A, from a Topcon OCT instrument (Topcon, Inc. Tokyo, Japan) were derived from cube scans after centering of the optic disc. Successful alignment can be confirmed by the common location of the blood vessel shadows. Notice that a subtle local thinning of the RNFL is visible on the derived b-scan images within the red rectangle. This is easier to visualize in the inset to the right of panel A, which shows the region within the red rectangle enlarged. (See ref. 17 for more examples.)
Figure 5.
A. Derived circumpapillary b-scans from cube scans obtained at two different visits. These are the same as shown in panel 1 of B and C below. The cpRNFL thickness for the two dates are superimposed in the lower panel of A, where the faint gray curve is from time 1. The insets to the right show the portion of the panels within the red rectangle. B. A one-page report using a wide-field, swept-source OCT cube scan as input.30 The probability maps for RNFL (4) and GCIPL (6) are shown in the red rectangles. These maps are based upon the thickness maps in (3) and (5), but are shown in field view (inferior retina/superior visual field on top). The red arrows point to topographically similar locations with thinned RNFL. The black arrows point to locations of the GCIPL which are part of the same defect. B. The report for the same eye obtained 1.2 years later. The regions indicated by the arrows can be seen to have progressed. Reproduced and modified from Fig. 3 in ref. 24 with permission from Ophthalmology. 2017;124(10):1466–1474. Copyright© 2017 ARVO. All rights reserved.
A second approach involves obtaining the second circle scan at the “same” location used in the earlier scan. This is best obtained with some type of eye tracking to assure accurate placement of the second scan. Because both a circle and a cube scan must be obtained, this scanning protocol typically takes more time. However, it produces a better circumpapillary image as the circle scan can now be averaged. Figure 6 shows b-scans obtained with a circle scan and a Spectralis OCT instrument (Heidelberg Engineering, Inc., Heidelberg, Germany). There is a local thinning of the cpRNFL in the region within the red rectangle in Fig. 6A, which is visible on the b-scans (orange arrow). This defect can be better appreciated in panel B of Fig. 6, which contains enlarged images of the regions within the red rectangle in panel A. Furthermore, the thinning of the cpRNFL is clearly visible on the cpRNFL thickness plot (red arrow). Examination of the b-scans within the red rectangle indicates that the cpRNFL thinning is not due to a segmentation error, as the segmentation is similar on both test dates. (See ref 16 for more examples.)
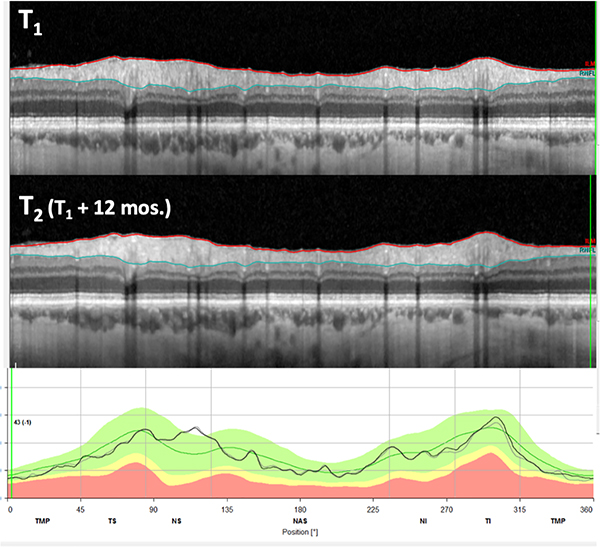
Figure 6.
A. OCT b-scan images of a circumpapillary circle scans from two tests 12 mos. apart, along with the associated circumpapillary RNFL thickness plot (lower panel) for the first (light gray) and second (black) tests. The arrows indicate a region with an abnormally thin RNFL, which appears to have progressed between the 2 test dates. B. Enlarged images of the portion of panel A within the red rectangle. C. Enlarged images of the portion of panel A within the green rectangle.
In many eyes, the lack of progression between two scan dates can be seen with the same approach. In particular, if the two cpRNFL thickness plots are essentially superimposed then it is extremely unlikely that there is significant progression. This is illustrated in Fig. 7, where the black (time 2) and gray (time 1) curves in the bottom panel of Fig. 7 are nearly superimposed.
Figure 7.
Same as in Fig. 6A for an eye in which the RNFL thickness has shown little to no change between the 2 test days. The RNFL thickness plots (lower panel) for the two visits are nearly identical.
In sum, b-scans can be used to assess progression in many eyes with glaucoma, including some with advanced glaucoma.18 However, it is likely that most of the clinicians currently looking at circumpapillary b-scan images are those using the Heidelberg Spectralis. Until recently, the b-scan images in the reports of other manufactures were either not present and/or too small to be of use. However, the images shown in Fig. 5 demonstrate that derived circumpapillary images can be of sufficient quality to detect changes, especially when used with the cpRNFL thickness plot. If clinicians start using these images, as we suggest, the manufactures will improve the quality of them either through the addition of a circle scan and/or software enhancements.
Change in Global Thickness of the Circumpapillary Retinal Nerve Fiber Layer (cpRNFL).
Many clinicians make use of summary statistics when determining the presence or absence of progression. Whether the circumpapillary b-scan image is obtained from a circle scan (Figs. 6 and 7) or derived from a cube scan (Fig. 5), all OCT instruments show a plot of the thickness of the circumpapillary (cp) RNFL as a function of distance around the disc, as shown by the black and gray curves in the lower panels of Figs. 5A, 6A, and 7. Some clinicians have advocated using changes in the average (global) thickness of this circumpapillary RNFL thickness plot. For example, the so-called “rule of 5”, based upon 95% confidence intervals,19–22 stipulates that if the global RNFL thickness (G) changes by greater than 5 um between two tests, then statistically significant progression has taken place. However, this rule has been shown to have both poor sensitivity and specificity for detecting progression.16,17,22,23 In fact, as we have recently documented, no criterion ΔG value will produce good sensitivity and specificity.16 The reason is simple. On the one hand, the ΔG produced by a significant local change in cpRNFL may be less than −2 um, while subtle changes in segmentation of the cpRNFL and/or the centering/ alignment of the circle scan can result in larger changes in ΔG.16,17 Figure 6 illustrates both problems. The ΔG for this eye was only −3 um. Thus, the defect, which is clearly seen within the red rectangle (Fig. 6A,B), would not be flagged as progression. The segmentation error within the green rectangle (Fig. 6A,C) also helped obscure the loss of cpRNFL. In any case, it is clear that one should not use ΔG measures without scrutinizing the b-scan image to assess the alignment and segmentation errors.16,17 Further, these errors severely limit the use of ΔG for the purpose of detecting progression as they are very common and often difficult to impossible to correct.16,17
Evaluating Probability and Thickness Maps
When assessing progression between visits, many clinicians now look at RNFL and GCL thickness and/or deviation/probability maps, which are available on all major OCT instruments. The Lee et al study10 discussed above supplied evidence that experts can do as well as, or better than, the GPA by viewing only deviation/probability maps. Their study also opens the possibility that experts using deviation maps may need fewer tests. For example, notice in Fig. 4A that the GPA for both the RNFL (1) and GCIPL (2) required all 6 tests to reach “likely progression” (red arrows in bottom row of Fig. 4A). Their study design did not include a determination of the number tests needed by the experts. However, in this example, progression is fairly obvious in the deviation maps well before the 6th test. This can be more easily visualized in Fig. 4B, where the images from panel (4) of Fig. 4A are enlarged. For example, consider the region within the red rectangles. Notice that the red and yellow areas in this region increase between baseline 2 (black arrow) and test 3 (red arrow), as well as between test 3 (red arrow) and test 4 (red arrow). That is, there is clear change well before the GPA criteria for “likely progression” is met on test 6.
Another example is shown in Fig. 4C, which contains the deviation maps from the Zeiss Panomaps for the eye in Figs. 1–3. There is a clear change in the deviation maps (red arrows) between Exams 3 and 4. These examples illustrate that a comparison of deviation maps may allow an assessment of progression between visits in at least some eyes.
Our group has advocated for the importance of probability maps in detecting early glaucoma.26–30 Figure 5B, C is our one-page report using a wide-field OCT cube scan as input.30 [A version of this report is available in some Topcon instruments (Topcon, Inc., Tokyo, Japan).] In Fig. 5B, C, the probability maps for RNFL (4) and GCIPL (6) are shown in the red rectangles. Although these maps are very similar to the deviation maps of the Panomap Report in Fig. 4A–C, there are 3 differences. One, the RNFL and GCIPL probability maps are shown separately in our report, while they are combined in the Panomap. Two, our probability maps are displayed in field view, i.e., rotated around the horizontal meridian to allow for a comparison with visual field. Finally, in our probability maps, the scale is continuous from 10% (green) to 0.1% (dark red), while the Panomap deviation map has two colors (yellow and red) and two probability levels.
Recently, we have presented evidence that the report in Fig. 5B,C can be used to identify progression occurring between two sessions, at least in some eyes. In particular, Wu et al.15 found that an expert assessing the reports from two test days out-performed an event-based analysis of the global cpRNFL thickness, a metric commonly used to detect change between two tests. In the Wu et al. study, experts flickered between the reports from the two test days. This approach is similar to the flickering between disc photos that has long been used to help detect progression. Figure 5B,C shows the reports for two days from Wu et al. Notice that there is an indication of progression in the region of the red arrows on the RNFL thickness and probability maps (panels 3 and 4). There is also a suggestion of progression on the GCIPL thickness and probability maps (black arrows in panels 5 and 6), as well as in the thickness of the cpRNFL seen in the region of the red arrows on the b-scan image (panel 1-upper) and cpRNFL thickness plot (panel 1- lower).
Taken together, the evidence above suggests that at least in some eyes, progression can be assessed between two sessions by an expert viewing OCT probability maps within OCT reports such as seen in Fig. 5, and Fig. 8, discussed below.
Figure 8.
A. A one-page report using a spectral domain OCT cube scan as input. The probability maps for RNFL (4) and GCIPL (6) are shown in the black rectangles. These maps are based upon the thickness maps in (3) and (5), but are shown in field view (inferior retina/superior visual field on top). The red arrows point to topographically similar locations with thinned RNFL. The black arrows point to locations of the GCIPL which are part of the same defect. B,C. The report for the same eye obtained 12 (B) and 47 (C) months later. The regions indicated by the arrows can be seen to progress. The insets to the right show enlarged images of the b-scans in panel 1 for the region within the red rectangles. Note: this report is not available in this form for use in the USA.
Structure-Structure Agreement: Combining viewing B-Scan Images and cpRNFL Thickness Plots with Probability Maps.
The need for multiple 24–2 visual field tests, and the 24–2 GPA, is due to the inherent variability in visual field measures. OCT measures, such as global thickness also show variability. However, when it comes to overcoming measurement error, the OCT has two major advantages compared to visual fields. First, by examining circle scans as in Figs. 5–8, it is often possible to identify the source of the variability (e.g., a segmentation error), and then correct for it, either by correcting the segmentation or, more practically by taking it into consideration when making a clinical judgment. Second, the different analyses of the OCT scans, as seen in the OCT reports in Figs, 5 and 8, provide more than one source of information. For example, GCL thickness/probability maps, RNFL thickness/probability maps, and circumpapillary RNFL images/thickness provide related, but different views, of possible damage. Subtle damage or progression on one of these may not be convincing. However, topographical comparison among different analyses of OCT scans should reduce the need for multiple OCT tests.
This is illustrated by the OCT reports in Figs. 5 and 8, from two different instruments and for two different patients. In these figures, there are subtle signs of progression associated with the red and black arrows on the various parts of each report. The red arrows point to locations that topographically correspond to the defect seen on the b-scan in panel 1 (upper), and the black arrows are a second location near fixation that is topographically consistent with an arcuate defect that includes the red arrows. The changes on each of these individual maps/plots by themselves might be ignored, as they are subtle. However, together they strongly suggest progression. Note that some of the most common sources of variability will affect these maps/plots differently. For example, segmentation errors that result in an artifactually larger GCL thickness by mistakenly including the RNFL will result in a RNFL thinner than expected. Other errors due to centering of scans on the disc or fovea; pathological conditions such as epiretinal membranes or retinochisis; or anatomical variations caused by variation in the shape of the fovea or location of major blood vessels, can be identified as well.
Structure-Function Comparisons:
The same argument can be made for comparing OCT (structure) and visual field (function) results. Subtle changes consistent with progression seen with either OCT or visual field tests may or may not be due to random variability. However, if these changes are in the corresponding regions of the visual field and retina, then these subtle changes together make true progress likely. Consistent with this argument, we have recently shown that comparing visual field and OCT probability maps can improve the identification of early glaucoma compared to either alone.31,32 Similarly, it should be possible to reduce the number of OCT and visual fields tests needed to identify progression by topographically comparing progressing regions on visual fields with those on OCT probability maps. In addition, these topographical structure-structure and structure-function comparisons can be enhanced with a model of RNFL projections.33
NEED FOR RESEARCH
There are at least 5 areas that need more study. First, until automated procedures and/or OCT reading centers become common, we need to develop methods for training eye care providers to make better use of the information in OCT scans. This includes examining circumpapillary b-scans in every patient. Second, there is a need for refinement and validation of so-called qualitative methods, such as those involved in evaluating the reports in Figs. 6 and 8.29,30 These methods are actually based upon extensive quantitative analyses. Thus, calling them “qualitative” is misleading. In any case, ultimately the clinician is making a decision based upon information from various sources. The best way to integrate visual field and OCT information into this decision process is still an open question. Third, we need to test our hypothesis that in many eyes a pair of OCT is sufficient to make a decision concerning progression. Fourth, for clinical trials and screening we need to search for, and validate, objective metrics for identifying glaucoma and its progression. The current methods of using summary metrics such as MD, PSD and GHT of visual fields, and global/average or regional/sector thickness of OCT RNFL and GCIPL are clearly inadequate and insufficient.13,29,.34–41 On the other hand, combining topographical related information within and between visual field and OCT probability maps may help.31,32,42 Finally, many believe that deep-learning, convolutional neural networks (CNN) may be the answer. Interestingly, the RNFL probability maps are a particularly good input to these models.35,43 However, while current work with CNN models is encouraging, much work needs to be done to make them clinically useful. Until then, we need to develop methods that allow clinicians to make decisions about changes without requiring long follow-up with multiple tests. Based upon the approaches described here, this is possible and practical even when looking at a pair of good quality OCT exams and enables detection of rapid progressors.
Acknowledgments
Supported by: NIH/ NEI grant: EY002115 and EY025253.
Financial disclosures: DCH receives lecture fees, research support and equipment from Topcon, Inc. and Heidelberg Engineering. ET received lecture fees from Topcon, Inc.; CGD receives research support from Topcon, Inc. and Heidelberg Engineering and is consultant for Carl Zeiss Meditec, Perfuse Therapeutics, Novartis, and Galimedix.
REFERENCES
- 1.Chauhan BC, Garway-Heath DF, Goñi FJ, Rossetti L, Bengtsson B, Viswanathan AC, Heijl A Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92(4):569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keltner JL, Johnson CA, Quigg JM, Cello KE, Kass MA, Gordon MO. Confirmation of visual field abnormalities in the Ocular Hypertension Treatment Study. Ocular Hypertension Treatment Study Group. Arch Ophthalmol. 2000;118(9):1187–94. [DOI] [PubMed] [Google Scholar]
- 3.Bengtsson B, Lindgren A, Heijl A, Lindgren G, Asman P, Patella M. Perimetric probability maps to separate change caused by glaucoma from that caused by cataract. Acta Ophthalmol Scand. 1997;75(2):184–8. [DOI] [PubMed] [Google Scholar]
- 4.Heijl A, Leske MC, Bengtsson B, et al. EMGT Group. Measuring visual field progression in the Early Manifest Glaucoma Trial. Acta Ophthalmol Scand. 2003;81:286–93. [DOI] [PubMed] [Google Scholar]
- 5.Yu M, Lin C, Weinreb RN, et al. Risk of visual field progression in glaucoma patients with progressive retinal nerve fiber layer thinning: a 5-year prospective study. Ophthalmol. 2016;123:1201–1210. [DOI] [PubMed] [Google Scholar]
- 6.Lin C, Mak H, Yu M, et al. Trend-based progression analysis for examination of the topography of rates of retinal nerve fiber layer thinning in glaucoma. JAMA Ophthalmol. 2017;135:189–195. [DOI] [PubMed] [Google Scholar]
- 7.Hou HW, Lin C, Leung CK. Integrating Macular Ganglion Cell Inner Plexiform Layer and Parapapillary Retinal Nerve Fiber Layer Measurements to Detect Glaucoma Progression. Ophthalmol. 2018;125(6):822–831. [DOI] [PubMed] [Google Scholar]
- 8.Wu K, Lin C, Lam AK, Chan L, Kai-Shun Leung C. Wide-field Trend-based Progression Analysis of Combined Retinal Nerve Fiber Layer and Ganglion Cell Inner Plexiform Layer Thickness: A New Paradigm to Improve Glaucoma Progression Detection. Ophthalmol. 2020; [published online ahead of print, 2020 Mar 29]. [DOI] [PubMed] [Google Scholar]
- 9.Lee WJ, Kim YK, Park KH, Jeoung JW. Trend-based analysis of ganglion cell-inner plexiform layer thickness changes on optical coherence tomography in glaucoma progression. Ophthalmol. 2017;124:1383–1391. [DOI] [PubMed] [Google Scholar]
- 10.Lee WJ, Kim TJ, Kim YK, Jeoung JW, Park KH. Serial combined wide-field optical coherence tomography maps for detection of early glaucomatous structural progression. JAMA Ophthalmol. 2018;136:1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Na Lee 2018 Lee WJ, Na KI, Ha A, Kim YK, Jeoung JW, Park KH. Combined use of retinal nerve fiber layer and ganglion cell inner plexiform layer event-based progression analysis. Am J Ophthalmol. 2018;196:65–71. [DOI] [PubMed] [Google Scholar]
- 12.Susanna R Jr, De Moraes CG, Cioffi GA, Ritch R. Why do people (still) go blind from glaucoma? Transl Vis Sci Technol. 2015;4(2):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hood DC, De Moraes CG. Challenges to the Common Clinical Paradigm for Diagnosis of Glaucomatous Damage With OCT and Visual Fields. Invest Ophthalmol Vis Sci. 2018;59(2):788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hood DC, De Moraes CG. Four questions for every clinician diagnosing and monitoring glaucoma. J. of Glaucoma. 2018;27:657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z, Weng DSD, Rajshekhar R, Thenappan A, Ritch R, Hood DC. Evaluation of a Qualitative Approach for Detecting Glaucomatous Progression Using Wide-Field Optical Coherence Tomography Scans. Trans Vis Sci Tech. 2018;May 1;7(3):5. eCollection 2018 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eguia MD, Tsamis E, Zemborain ZZ, Sun A, Percival J, De Moraes CG, Ritch R, Hood DC. Why OCT global circumpapillary retinal nerve fiber layer thickness is a poor measure of glaucomatous progression. Trans. Vis. Sci. Tech. 2020;9(11):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun A, Tsamis E, E; Eguia MD, Liebmann JM, Blumberg DM, Al-Aswad LA, Cioffi GA, DE Moraes CG, Hood DC. Global optical coherence tomography measures for detecting the progression of glaucoma have fundamental flaws. Eye. 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thenappan AA, Tsamis E Zemborain ZZ, La Bruna S, Eguia MD, Joiner DB, De Moraes CG, Hood DC. Detecting progression in advanced glaucoma: Are OCT global metrics viable measures? Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung CK, Cheung CY, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology. 2009;116(7):1257–1263. [DOI] [PubMed] [Google Scholar]
- 20.Tan BB, Natividad M, Chua KC, Yip LW. Comparison of retinal nerve fiber layer measurement between 2 spectral domain OCT instruments. J Glaucoma. 2012;21(4):266–273. [DOI] [PubMed] [Google Scholar]
- 21.Ghasia FF, El-Dairi M, Freedman SF, Rajani A, Asrani S. Reproducibility of spectral domain optical coherence tomography measurements in adult and pediatric glaucoma. J Glaucoma. 2015;24(1):55–63. [DOI] [PubMed] [Google Scholar]
- 22.Thompson AC, Jammal AA, Medeiros FA. Performance of the Rule of 5 for Detecting Glaucoma Progression between Visits with OCT. Ophthalmol Glaucoma. 2019;2(5):319–326.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatham AJ, Medeiros FA. Detecting Structural Progression in Glaucoma with Optical Coherence Tomography. Ophthalmology. 2017;124(12S): S57–S65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin JW, Sung KR, Lee GC, Durbin MK, Cheng D. Ganglion Cell-Inner Plexiform Layer Change Detected by Optical Coherence Tomography Indicates Progression in Advanced Glaucoma. Ophthalmol. 2017;124(10):1466–1474.. [DOI] [PubMed] [Google Scholar]
- 25.Ha A, Park KH. Optical Coherence Tomography for the Diagnosis and Monitoring of Glaucoma [published online ahead of print, 2019 Apr 24]. Asia Pac J Ophthalmol (Phila). 2019;8:135–145. [DOI] [PubMed] [Google Scholar]
- 26.Hood DC, Raza AS, de Moraes CGV, Liebmann JM, Ritch R. (2013) Glaucomatous damage of the macula. Prog Ret Eye Res. 2013;32, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hood DC, Raza AS (2014) On improving the use of OCT imaging for detecting glaucomatous damage. Brit J Ophthalmol. 2014;98, Suppl 2:ii1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hood DC, Slobodnick A, Raza AS, De Moraes CG, Teng CC, Ritch R. Early glaucoma involves both deep local, and shallow widespread, retinal nerve fiber damage of the macular region. Invest Ophthalmol Vis Sci. 2014;55, 632–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hood DC. (2017) Improving our understanding, and detection, of glaucomatous damage: An approach based upon optical coherence tomography (OCT). Prog Retin Eye Res. 57:46–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hood DC, De Cuir N, Blumberg DM, Liebmann J, Ravivarn Jarukasetphon, Ritch R, De Moraes CG. A single wide-field OCT protocol can provide compelling information for the diagnosis of early glaucoma Trans Vis Sci Tech. 2016;5(60):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hood DC, Tsamis E, Bommakanti N, Joiner DB, Al-Aswad LL, Blumberg DM, Cioffi GA, Liebmann JM, De Moraes CG. Structure-function agreement is better than commonly thought in eyes with early glaucoma. Invest Ophthalmol Vis Sci. 2019;60(13):4241–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsamis E, Bommakanti N, Sun A, Thakoor K, De Moraes CG, Hood DC. An automated method for assessing topographical structure-function agreement in abnormal glaucomatous regions. Trans Vis Sci Tech.. 2020;9(4):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hood DC, Zemborain ZZ, Tsamis E, De Moraes CG. Improving the Detection of Glaucoma and Its Progression: A Topographical Approach. J Glaucoma. 2020;29(8):614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grillo LM, Wang DL, Ramachandran R, et al. The 24–2 Visual Field Test Misses Central Macular Damage Confirmed by the 10–2 Visual Field Test and Optical Coherence Tomography. Transl Vis Sci Technol. 2016;5(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muhammad H, Fuchs TJ, De Cuir N, De Moraes CG, Blumberg DM, Liebmann JM, Ritch R, Hood DC. Hybrid deep learning on single wide-field optical coherence tomography scans accurately classifies glaucoma suspects. Journal of Glaucoma 2017;26(12):1086–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Moraes CG, Sun A, Jarukasetphon R, et al. Association of Macular Visual Field Measurements With Glaucoma Staging Systems. JAMA Ophthalmol. 2019;137(2):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rabiolo A, Morales E, Mohamed L, et al. Comparison of Methods to Detect and Measure Glaucomatous Visual Field Progression. Trans Vis Sci Tech. 2019;8(5):2–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hougaard JL, Heijl A, Bengtsson B. Glaucoma detection by Stratus OCT. J Glaucoma. 2007;16(3):302–306 [DOI] [PubMed] [Google Scholar]
- 39.Leal-Fonseca M, Rebolleda G, Oblanca N, Moreno-Montañes J, Muñoz-Negrete FJ. A comparison of false positives in retinal nerve fiber layer, optic nerve head and macular ganglion cell-inner plexiform layer from two spectral-domain optical coherence tomography devices. Graefes Arch Clin Exp Ophthalmol. 2014;252(2):321–330 [DOI] [PubMed] [Google Scholar]
- 40.Kim KE, Jeoung JW, Park KH, Kim DM, Kim SH. Diagnostic classification of macular ganglion cell and retinal nerve fiber layer analysis: differentiation of false-positives from glaucoma. Ophthalmol. 2015;122(3):502–510. [DOI] [PubMed] [Google Scholar]
- 41.Wang DL, Raza AS, de Moraes CG, Chen M, Alhadeff P, Jarukatsetphorn R, Ritch R, Hood DC. Central glaucomatous damage of the macula can be overlooked by conventional OCT retinal nerve fiber layer thickness analyses. Translational Visual Science & Technology. 2015;4(6):4. eCollection 2015 Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee WJ, Park KH, Seong M. Vulnerability Zone of Glaucoma Progression in Combined Wide-field Optical Coherence Tomography Event-based Progression Analysis. Invest Ophthalmol Vis Sci. 2020;61(5):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thakoor K, Li X, Tsamis E, Sajda P, Hood DC. Enhancing the Accuracy of Glaucoma Detection from OCT Probability Maps Using Convolutional Neural Networks. In Proceedings of the Annual IEEE Engineering in Medicine and Biology Conference. Berlin, Germany, July, 2019. [DOI] [PubMed] [Google Scholar]