Abstract
Despite successful research efforts aimed at understanding pain mechanisms, there is still no adequate treatment for many patients suffering from chronic pain. The contribution of neuroinflammation to chronic pain is widely acknowledged. Here we summarize findings indicating that T cells play a key role in the suppression of pain. An active contribution of the immune system to resolution of pain may explain why immunosuppressive drugs are often not sufficient to control pain. This would also imply that dysregulation of certain immune functions promote transition to chronic pain. Conversely, stimulating the endogenous immune-mediated resolution pathways may provide a potent approach to treat chronic pain.
Why study mechanisms underlying resolution of pain?
Pain in response to tissue damage or inflammation represents an important warning signal and often serves a protective purpose. Short term treatment with non-steroidal anti-inflammatory drugs (NSAIDs) and/or opioids is usually enough to manage this transient pain.
Pain control becomes much more challenging when acute pain does not resolve and transitions into a state of chronic pain (see Glossary). According to a 2016 report from the Centers for Disease Control (CDC), 20.4% of adults in the United States experienced chronic pain, and 8% suffered from high impact chronic pain (https://www.cdc.gov/mmwr/volumes/67/wr/pdfs/mm6736a2-H.pdf). Chronic pain not only reduces quality of life, but also represents a real financial burden to society since it comes with an estimated $560 billion per year in medical costs, lost productivity and disability programs (2016 estimates; https://www.cdc.gov/mmwr/volumes/67/wr/pdfs/mm6736a2-H.pdf ). Chronic pain is frequently reported after nerve damage caused by e.g. traumatic injury or diabetes, or during chronic inflammation as is the case in patients with e.g. inflammatory bowel disease, rheumatoid arthritis, or multiple sclerosis. Often the cause of chronic pain is not or only partially understood, for example in patients suffering from chronic regional pain syndrome, fibromyalgia, low back pain, or recurrent headache.
In most patients, pain caused by a transient insult such as infection, surgery or chemotherapy, resolves when the tissues heal, or chemotherapy is completed. However, in a subgroup of patients, transition to chronic pain takes place. Chemotherapy-induced peripheral neuropathy resolves in 70–75% of affected patients within weeks after completion of treatment, but in the other 25–30% of patients, the pain persists for months to years [1, 2]. Similarly, depending on the methodology used to assess pain, 20- >50% of patients develop chronic pain after surgery [3–6].
Irrespective of the cause of chronic pain, for many patients the current treatments only provide partial relief at best. In addition, even when these interventions are effective at providing symptom relief, they do not eliminate the underlying cause of the persistent or recurring pain [7, 8]. Therefore, long-term treatment is often needed with increased risk of serious side effects as well as drug-induced aggravation of pain. In addition, opioids, often the treatment of choice in case of chronic pain, represent a huge additional health risk in view of the addictive properties [9].
Despite overwhelming evidence from rodent models for a role of (neuro)inflammation in the induction and maintenance of multiple forms of chronic pain (Box 1), clinically, suppression of inflammation with NSAIDs or steroids is not sufficient to control it [7, 10]. Pain also often persists even when inflammation is successfully controlled by treatment with e.g. inhibitors of cytokine signaling in patients with autoimmune disorders such as rheumatoid arthritis [11].
Box 1:
Contribution of neuroinflammation to chronic pain
| Much of the research effort on chronic pain has focused on understanding the mechanisms that are responsible for maintaining pain and multiple key pathways have been identified. There are clear changes in the gene expression pattern in peripheral pain sensing neurons that have their cell bodies in the dorsal root ganglia [18]. These nociplastic changes contribute to the hyperexcitability of these neurons that is responsible for increased sensitivity to pain and can also lead to spontaneous firing. Blocking the activity of these peripheral pain sensing neurons suppresses pain, indicating their key role in chronic pain. |
| The excitability of these peripheral neurons and of second order pain sensing neurons in the spinal cord is regulated by multiple other cell types, including microglia and astrocytes in the spinal cord, Schwann cells in the peripheral nerve, keratinocytes in the skin, macrophages infiltrating dorsal root ganglia, nerve and spinal cord, and infiltrating lymphocytes [69]. All these cells produce mediators that can increase neuronal excitability and many of these mediators can also inhibit the activity of descending inhibition pathways. Pain promoting molecules produced and released by these cells include inflammatory mediators such as cytokines, reactive oxygen species, bradykinin, growth factors and prostaglandins, but also so-called gliotransmitters such as ATP, and glutamate that can rapidly activate neurons [69]. Preclinical studies have shown that targeting the signaling pathways engaged by these mediators can at least temporarily suppress pain, but in most cases pain does not fully resolve and continuous treatment is required [70]. We also want to emphasize that there is clear evidence for a key role of M1-type macrophages in the onset and maintenance of chronic pain in multiple models. Local depletion of the DRG/spinal cord from macrophages has been shown to prevent chronic neuropathic pain multiple models [71–73]. |
Here, we explore the concept that pharmacological immunosuppression is not enough to resolve pain because the immune system actively contributes to the pathways regulating the resolution of pain. We focus on studies indicating that T cells can promote resolution of pain and on the potential contribution of macrophages, the anti-inflammatory cytokine interlueukin-10 (IL10), and endogenous opioids (Figure 1, Key figure).
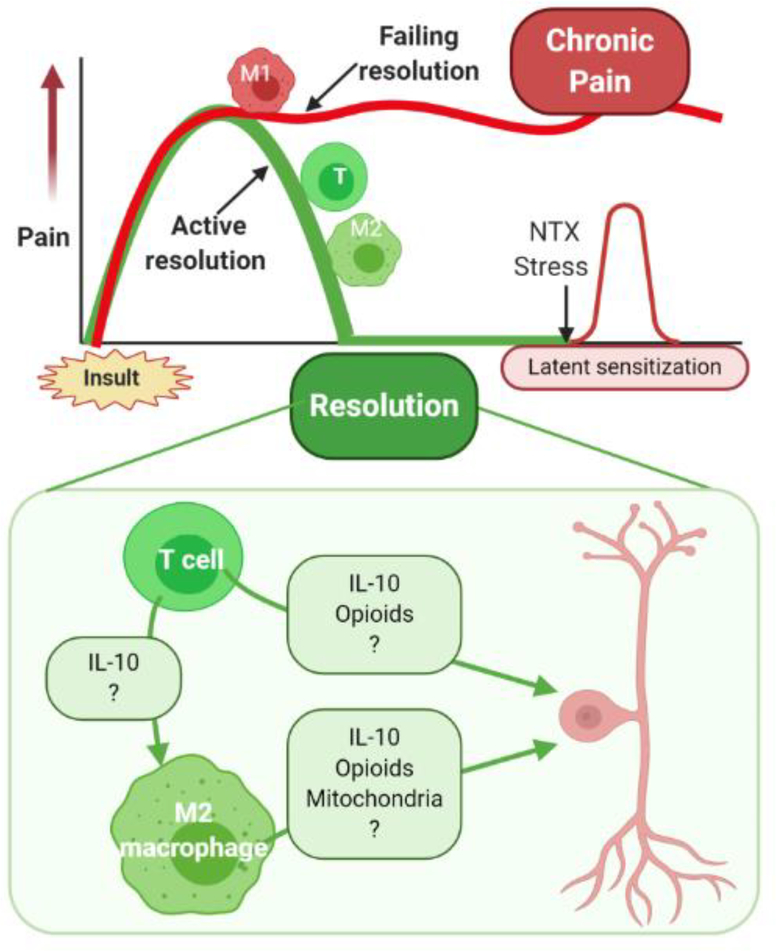
Figure 1, Key Figure: Contribution of T cells and macrophages to the resolution of pain.
Top Panel: Pain induced by transient insults such as tissue damage, inflammation, or drug treatment, does not resolve spontaneously once the driver is gone. Resolution of pain is an active regulatory process that requires the activity of T cells (T) and wound healing M2 macrophages (M2). When this resolution fails, M1 macrophages remain active and promote chronic pain. The apparent resolution of pain is the result of a novel homeostatic balance that requires active suppression, a situation known as latent sensitization.
Bottom Panel: Potential pathways contributing to T cell-mediated suppression or resolution of pain. CD4+ or CD8+ T cells suppress pain or promote the resolution of pain after recovery from inflammation, surgery or completion of chemotherapy via multiple pathways. T cells can produce the anti-inflammatory cytokine IL10 and promote the differentiation of M1 pro-inflammatory macrophages into M2 wound healing macrophages. Both T cells and M2 macrophages can release IL10 and opioid peptides that can signal to sensitized pain sensing neurons in the peripheral and central nervous system to reverse or control sensitization and resolve pain. In addition, M2 macrophages can transfer mitochondria to sensory neurons in dorsal root ganglia thereby contributing to resolution of pain. T cells and macrophages likely produce additional currently unknown factors (?) that contribute to pain resolution.
Resolution of pain is an active process
One may think that resolution of pain that occurs after resolution of the insult that caused the pain, e.g. infection, surgery or chemotherapy is a passive process in which the nervous system returns to its basal state when the tissues have healed and the driver of pain has dissipated (Figure 1). However, the existing evidence indicates that after an episode of pain hypersensitivity the nervous system does not readily return to its basal state, but transitions into a novel state coined as ‘latent sensitization’ [12, 13] or ‘hyperalgesic priming’ [14, 15]. When endogenous opioid signaling is inhibited during the phase of latent sensitization, e.g. after apparent resolution of inflammatory hyperalgesia, the pain hypersensitivity re-emerges [12]. Pain hypersensitivity also can re-emerge when mice are exposed to a mild stressor during the phase of latent sensitization, while the same stressor does not affect pain sensitivity in control mice [13, 16, 17]. Exposure of mice to a second stimulus, e.g. an inflammatory mediator during the state of hyperalgesic priming results in a prolonged and/or increased response [15]. These studies reveal that when the driver of pain (in this example inflammation) dissipates, the nervous system does not return to its basal state, but rather transitions to a novel state phase that is associated with long lasting changes in gene expression [18].
Role of T cells in the resolution of pain.
T cells are a key component of the adaptive immune system that provide help to B cells, kill virus-infected cells, regulate the activity of other T cell subsets, B cell subsets and innate immune cells, and can develop immunologic memory. All T cells express the cell surface marker CD3, and specific CD3+ subpopulations can be identified by cell surface and intracellular markers, and cytokine profiles (Box 2). Studies in mice lacking cells of the adaptive immune system, such as severe combined immunodeficient (SCID) mice, Rag1−/−, or Rag2−/− mice, or wild type mice depleted from (specific subsets of) T cells, have identified pain promoting as well as pain suppressing effects of CD3+ T cells. A pain promoting role of T cells has been reported in models of neuropathic pain induced by surgical damage to nerves and this is likely mediated at least in part by production of the cytokine interferon-γ by T helper1 (Th1) cells [19]. Some other studies did not detect a contribution of T cells (for review see [20]). These studies were mostly conducted in males. A more recent set of studies indicated that CD3+ T cells contribute to neuropathic pain response to spared nerve injury or chronic constriction injury in females but not in males [21]. However, this female T cell dependency only became apparent when at the same time the activity of microglia and macrophages was suppressed [21]. Further studies will be needed to fully characterize the potential pain promoting effects of (specific subsets of) T cells in male and female mice in multiple models.
Box 2:
Major cytokine-based subsets of T cells and macrophages
| CD4+ T cell subsets | ||
|---|---|---|
| Positive | Negative | |
| Th1 | IFNγ, IL2, TNFα | IL4 |
| Th2 | IL4, IL5, IL9, IL10, IL13, TNFα | IFNγ |
| Treg | IL4, IL10, TGFβ | |
| Th9 | IL9 | |
| Th17 | IL9, IL17, IL21, IL22 | IL4, IFNγ |
| Th22 | IL22 | IL4, IFNγ, IL9, IL17 |
| CD8+ T cell subsets | ||
| Tc1 | IFNγ, IL2,TNFα | IL4, IL5 |
| Tc2 | IL5, IL13 | IFNγ |
| Treg | IL10, TGFβ | |
| Tc9 | IL9, IL10 | |
| Tc17 | IL17, IL21 | |
| Macrophages | ||
| M1 | TNFα, IL1β, IL6, IL12, IL23, IL-27 | |
| M2a | IL10, TGFβ | |
| M2b | TNFα, IL1β, IL6, IL10 | |
| M2c | IL10, TGFβ | |
| M2d | IL10 | |
Overview of the major subsets of CD3+ T cells and macrophages based on cytokine production based on references [74] and [75]. There are additional subsets of T cells, including γδ T cells and T cell subsets can be further divided on the basis of e.g. their function as memory, effector or effector memory T cells, but this is beyond the scope.
Here we will focus on the role of (subsets of) CD3+ T cells in suppressing or counteracting pain. Extensive studies in models of inflammatory bowel disease have demonstrated that CD4+ Th1 and Th17 cells in the inflamed gut produce endogenous opioids that are released to suppress pain as well as local inflammation [22, 23]. Regulatory T cells (Treg) identified as CD4+FoxP3+ T cells can suppress neuropathic pain induced by peripheral nerve ligation and inflammatory pain in a model of neuritis [24–26]. Expansion of the CD4+ Treg cell population by treatment with CD28 superantigen reduced both the severity of neuritis and mechanical pain hypersensitivity [24]. These pain suppressing effects of Tregs are thought to be directly related to their capacity to downregulate the pro-inflammatory response in the peripheral nerve, dorsal root ganglion and/or spinal cord [24]. Studies in the antigen- and-collagen-induced model of rheumatoid arthritis showed that depletion of CD8+ T cells increased pain, indicating that not only CD4+, but also CD8+ T cells can suppress pain during chronic inflammation. Interestingly, the increase in pain in arthritic mice depleted of CD8+ T cells was independent of changes in inflammatory activity in the joint [27]. A dissociation between inflammation and pain was also reported in a model of colitis [28].
CD3+ T cells also play a key role in the resolution of pain after completion of peripheral inflammation. In WT mice, complete Freund’s adjuvant (CFA)-induced mechanical hypersensitivity resolves within 2–3 weeks. In contrast, in male and female Rag2−/− mice that do not have mature T and B cells, inflammatory pain induced by CFA is markedly prolonged [29]. Reconstitution of these Rag2−/− mice with CD3+ T lymphocytes normalizes resolution of the inflammatory pain. In line with what has been described for suppression of pain in a model of arthritis as described above [27], CD3+ T cells were required to resolve pain hypersensitivity, but not for resolution of CFA-induced paw inflammation [29]. This finding supports the hypothesis that resolution of inflammation is not sufficient to resolve pain.
Recent studies identified the contribution of CD3+ T cells to the resolution of pain after completion of chemotherapy. Treatment of WT mice with a short 2–3-day course of chemotherapeutic drugs like paclitaxel or cisplatin induces signs of peripheral neuropathy (CIPN) including mechanical allodynia, spontaneous pain, and loss of intraepidermal nerve fibers that resolve within two weeks [30, 31]. In contrast, these signs of CIPN persist for weeks to months in male and female Rag2−/− mice. Resolution is normalized when these mice are reconstituted with CD3+ T cells or CD8+ T cells. Importantly, reconstitution of Rag2−/− mice with CD4+ T cells did not normalize resolution of CIPN [30, 31]. Collectively, the findings summarized above indicate that different subsets of T cells suppress pain in different models.
Additional support for a potential key role of CD3+ T cells in the resolution of pain comes from studies on the mechanisms underlying the resolution of pain in response to several pharmacological interventions. For example, treatment of mice with neuropathic pain as induced by chronic constriction injury of the sciatic nerve (CCI) with three doses of a tumor necrosis factor receptor 2 (TNRF2) agonist, leads to resolution of mechanical allodynia without relapse for at least 3 weeks [32]. The beneficial effects of this TNFR2 agonist critically depend on the activity of CD4+ Tregs producing IL10. CD4+ Tregs also play a critical role in the suppression of mechanical allodynia in response to intrathecal administration of IL35 to mice with experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis [33]. Supporting the notion that resolution of inflammation and resolution of pain are not directly linked, the suppression of pain in response to IL35 was independent of its effect on disease severity. CD3+ T cells are also required for the reversal of CIPN in response to treatment with an inhibitor of histone de-acetylase 6 (HDAC6), a cytosolic member of the family of histone de-acetylases [34]. In WT mice, two weeks of treatment with an HDAC6 inhibitor after completion of chemotherapy normalized mechanical allodynia and spontaneous pain [34, 35]. However, treatment of Rag2−/− mice with the HDAC6 inhibitor only resolved mechanical allodynia when these mice had been reconstituted with CD3+ T cells. Importantly, the need for T cells in spontaneous resolution of pain or resolution of pain in response to the pharmacological interventions summarized here did not differ between sexes [29, 31, 32].
In summary, the existing evidence indicates that multiple subsets of T cells are part of a common regulatory pathway that promotes the resolution of pain (Figure 1). Which T cell subset and associated cytokine profile is needed for pain resolution will likely depend on model specific variables. These variables include (but are not limited to) the nature of the insult that initiated the pain (e.g. inflammation in models of inflammatory bowel disease (IBD) versus tissue damage in models of CIPN) leading to activation of T cell subsets by specific antigens, damage signals and/or other co-stimulatory factors. Other determining factors are the location of the insult, the involvement of other inflammatory cells such as microglia and monocytes/macrophages, the chemotactic activity of the microenvironment, and the migration pattern of the T cells in each situation. Future studies should identify how the different subsets of T cells that promote pain resolution are triggered, how they mediate their effect after specific activation, and where they exert their pain suppressive effects in various models of pain.
Role of opioid peptides in T cell-dependent control of pain
As mentioned above, there is a key role of endogenous opioid signaling in preventing the re-emergence of pain during the state of latent sensitization. Multiple subsets of leukocytes, including T cells and macrophages, can produce the opioid peptides such as enkephalins and endorphins [36–40]. Already in 1990, Stein and colleagues proposed that stress-induced analgesia is mediated by release of opioid peptides from leukocytes at sites of inflammation [41]. More recently, an elegant study in a model of neuropathic pain, showed that repeated application of IL4 at the site of nerve damage promoted differentiation of M2 macrophages and analgesia via production of endogenous opioids by these M2 macrophages [42].
The role of opioid production by T cells in pain control in inflammatory bowel disease is well-established (Figure 2). Effector CD4+ T cells in the inflamed gut suppress visceral pain in models of inflammatory bowel disease by production and release of enkephalins [22, 23]. These CD4+ T cells are activated by ‘classical’ major histocompatibility complex (MHC) class II-dependent exposure to bacterial antigens in the draining lymph nodes of the gut to produce and release opioid peptides upon re-exposure to these antigens in the inflamed intestinal mucosa [22, 23].
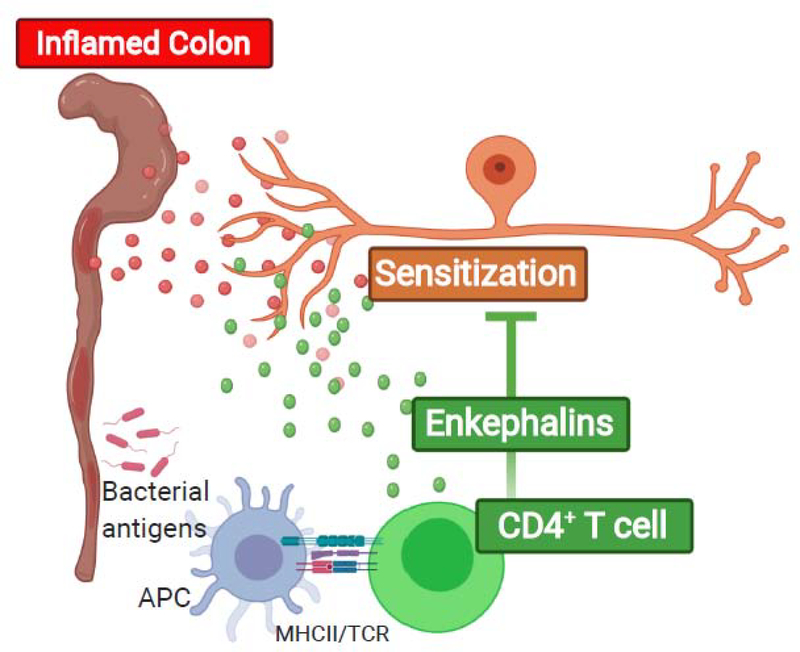
Figure 2: CD4+ T cells suppress pain in models of inflammatory bowel disorder (IBD) via release of enkephalins.
Bacterial antigens presented by antigen presenting cells via MHC class II activate CD4+ T cells to produce enkephalins. Upon exposure to the antigen in the gut, CD4+ T cells release these enkephalins that subsequently suppress pain sensitivity.
Corticotropin releasing hormone (CRH) not only induces production and release of endorphins by the pituitary but also by peripheral leukocytes [36, 43]. Local administration of CRH at the site of nerve injury or at the site of paw inflammation, reverses mechanical hypersensitivity in wild type mice via a CD3+T cell and β-endorphin-mediated pathway [36, 43–45]. Opioid peptides produced by CD3+ T cells have also been implicated in the suppression of pain during pregnancy [46]. During late pregnancy, female mice showed no evidence of chronic inflammatory pain induced by CFA, or neuropathic pain induced by spared nerve injury. Intrathecal naloxone rapidly reversed pregnancy-induced analgesia indicating ongoing engagement of endogenous opioid receptor-mediated pain suppressing pathways. In other words, interruption of ongoing analgesic activity of endogenous opioids by naloxone unmasked the existing pain hypersensitivity as a result of the previous exposure to CFA or to the spared nerve injury (SNI) model of neuropathic pain. Notably, pregnancy analgesia did not develop in mice that are deficient of CD3+ T cells and was re-instated by transfer of either CD4+ or CD8+ T cells from late pregnant mice, indicating that both T cell subsets can produce opioids during inflammatory conditions or nerve damage [46]. It remains to be determined whether production of endogenous opioid peptides by CD8+ T cell contributes to the role of these cells in resolution of pain in models of CIPN.
Role of macrophages in the resolution of pain
T cells are potent regulators of the activity and differentiation state of other cells of the immune system. In the context of the regulation of pain, macrophages deserve special attention. Macrophages can polarize into two major functional subsets: M1 and M2 (Box 2), and cytokines produced by various subsets of T cells can regulate this polarization process [47]. M1 pro-inflammatory macrophages have anti-microbial properties while M2 macrophages are involved in tissue remodeling and wound healing [47, 48]. The contribution of pro-inflammatory macrophages to chronic pain Is well established (Box 1). Recent findings indicate that M2 type macrophages can contribute to pain resolution [49, 50]. Depletion of mice from peripheral monocytes/macrophages after onset of inflammatory pain delays resolution of thermal hyperalgesia induced by intraplantar injection of the pro-inflammatory cytokine IL1β or carrageenan from 1–3 days to more than one week without affecting maximum severity of hyperalgesia [49]. Preliminary data from Eijkelkamp’s team recently showed that M2 type or tissue repair macrophages are key for the resolution of pain in these models [50]. This study showed that depletion of mice from all macrophages and monocytes prolonged mechanical allodynia, thermal hyperalgesia and spontaneous pain. Adoptive transfer of M2-type macrophages normalized resolution whereas transfer of M1-type macrophages had no effect on the resolution. An exciting and provocative finding in this study was that the M2 macrophages transfer their mitochondria to dorsal root ganglion (DRG) neurons. The transfer of mitochondria reversed neuronal metabolism from the more glycolytic state to a more respiratory state and thereby normalized neuronal excitability. In addition to this exciting novel pathway it is likely that cytokines produced by in particular M2 macrophages such as IL10 can also contribute to resolution of pain [49].
Role of endogenous IL10 production in the resolution of pain
It has been known for a long time that IL10 when provided from an exogenous source is a potent suppressor of pain, but due to poor bioavailablity even when administered locally a single injection of IL10 only provides transient pain relief [51]. The seminal work by Watkins and coworkers showed that local administration of vectors that encode IL10 lead to long term suppression of pain in multiple models and in dogs with pain due to the spontaneous development of osteoarthritis [52, 53]. A fusion protein consisting of IL10 coupled to IL4 with a linker sequence (IL4/IL10 synerkine) reversed CFA-induced inflammatory pain and four doses were sufficient to induce resolution of pain relieve without relapse [54].
Subsets of CD4+ and CD8+ T cells and macrophages are all capable of producing the anti-inflammatory cytokine IL10 (Box 2). At least some of the beneficial effects of CD4+ T cells (including T reg) in models of neuropathic pain induced by surgical damage to the nerve and in some of the inflammatory models, depends on their capacity to produce IL10 [26, 32, 55–57]. However, although resolution of CIPN is delayed when IL10 signaling is interrupted, CD8+ T from IL10 deficient mice do promote resolution of CIPN, indicating that other cells (possibly M2 macrophages) than the CD8+ T cells are the source of IL10 promoting resolution of pain in this model [30, 32, 33].
Regular exercise reduces the risk of developing chronic pain and reduces pain in individuals with a variety of chronic pain conditions [58]. Preclinical studies show similar beneficial effects of exercise on pain that are associated with increases in the percentage of M2 type macrophages in muscle [59]. Inhibition of IL10 signaling reduces the beneficial effect of exercise in a model of muscle pain [58–60]. Strenuous exercise can induce muscle pain and this is exacerbated in IL10-deficient mice [61]. IL10 produced by CD4+ Tregs mediates the long lasting suppression of neuropathic pain in response to a TNFR2 agonist [32], an adenosine 2 A (A2A) receptor agonist, or a sphingosine receptor 1 agonist [55–57]. It is very well possible that increased IL10 production by M2 macrophages contributes to the effects of these interventions on pain as well.
The most widely proposed mode of action for the beneficial effects of IL10 on pain is through suppression of inflammation. Indeed, the beneficial effects of IL10 expressing vectors on neuropathic pain in models using surgical damage to nerves, is associated with suppression of the activity of spinal cord microglia and reductions in the levels of pro-inflammatory cytokines [51, 54, 62, 63]. Notably, inhibition of IL10 signaling through intrathecal injection of a neutralizing antibody prolonged pain induced by intraplantar administration of carrageenan, whereas intraplantar administration of the anti-IL10 had no effect [64]. These findings indicate a key role for IL10 signaling at the level of the spinal cord or DRG in resolution of pain, not at the level of the peripheral site of inflammation. Consistently, knockdown of IL10 receptors in DRG and spinal cord by intrathecal injection of antisense oligonucleotides prevents exercise-induced muscle analgesia and this was attributed to a decrease in IL10 receptor expression in DRG neurons [60]. Resolution of CIPN is delayed in mice with nociceptor specific deletion of IL10R1, the ligand binding component of the heterodimer that constitutes the IL10 receptor [65]. These findings support a model in which IL10 signaling to receptors on nociceptive neurons plays promotes the normal resolution of pain induced by chemotherapy. The mechanism via which IL10 signaling to nociceptors promotes resolution of pain remains to be elucidated. In vitro, exposure of DRG neurons to IL10 for 12 hours reduced the expression of voltage gated sodium channels and reduced the density of tetratoxin (TTX) sensitive and Nav1.8 currents in DRG neurons [66]. Short term (10 min) ex vivo exposure of DRG neurons from cisplatin-treated mice suppresses the abnormal spontaneous activity of these neurons [65]. This effect is likely too rapid to be explained by changes in ion channel expression and may be mediated by post translational modification, redistribution of ion channels, or metabolic changes within neurons. IL10 has been shown to reverse the switch to the glycolytic pathway in macrophages as induced by pro-inflammatory stimuli thereby preserving oxidative respiration and maintaining an M2-type ‘wound healing’ state and this could contribute to pain resolution [67]. In addition, IL10 promotes mitophagy which prevents production of oxygen radicals, restores mitochondrial membrane potential, and oxidative respiration in macrophages [67]. If IL10 would have the same effects on mitochondrial health in nociceptors this may well be an important contributing mechanism for the resolution of pain.
Targeting T cells to promote pain resolution
The role of T cells in resolution of pain is interesting because of the capacity of T cells to develop memory that forms the basis for development of vaccination strategies. Indeed, immunization of mice with ovalbumin followed by local administration of ovalbumin to re-activate memory T cells, suppressed visceral pain in a model of colitis [68]. This suppression of visceral pain in response to re-exposure to the immunizing antigen was mediated via increased recruitment of opioid producing CD4+ effector T lymphocytes to the inflamed colon. In the same model, vaccination with Bacillus Calmette–Guérin (BCG), followed by local administration of Mycobacterium bovis also strongly reduced visceral pain without aggravating colitis [68]. This is interesting in view of the potential clinical translation because BCG vaccination is already widely used to protect against tuberculosis.
In contrast to the antigen-mediated activation of CD4+ T cells that is needed for the suppression of pain in models of IBD, the CD8+ T cell-mediated resolution of CIPN is not mediated via ‘classical’ MHC class I-mediated antigen dependent activation of these T cells. Transfer of transgenic CD8+ T cells that can only recognize an antigen (chicken ovalbumin peptide that is not present in mice) to Rag2−/− mice normalizes CIPN resolution as effectively as transfer of wild type CD8+ T cells [31]. Even though T cells do not need to be capable of recognizing a relevant antigen to promote resolution of CIPN, transfer of CD8+ T cells from mice that had already recovered from cisplatin-induced pain to Rag2−/− mice accelerated resolution or could even prevent development of cisplatin-induced pain (Figure 3). These results indicate that antigen-independent “education” of CD8+ T cells improves their capacity to promote resolution of pain [31]. If this “education” of CD8+ T cells could be performed ex vivo, it may open the possibility to develop an intervention to prevent CIPN in patients scheduled for chemotherapy. It remains to be determined whether these educated CD8+ T cells also promote resolution of other forms of pain.
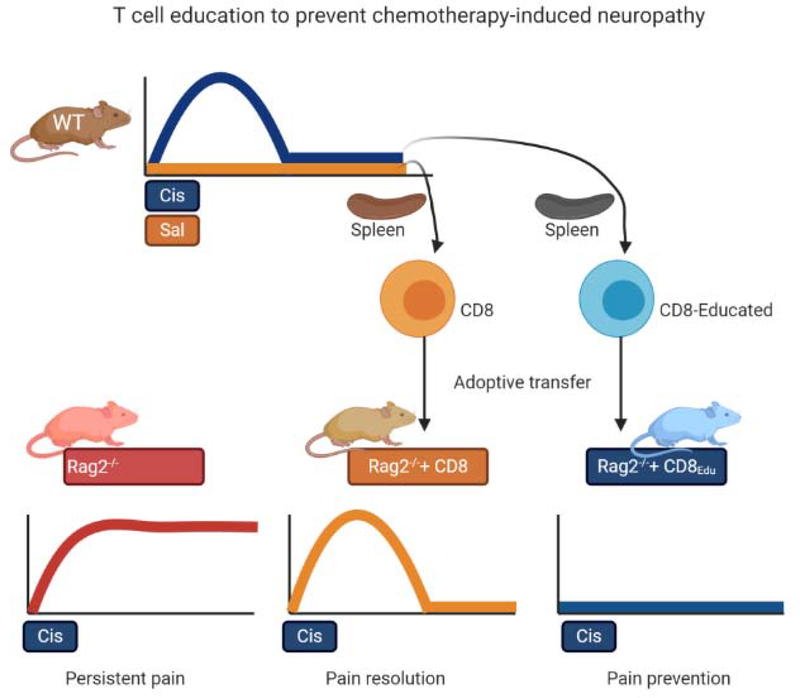
Figure 3: Education of CD8 T cells to prevent chemotherapy-induced neuropathy.
Wild type (WT) mice treated with cisplatin develop mechanical hypersensitivity that resolves after completion of chemotherapy. Rag2−/− mice that do not have mature T and B cells develop persistent in mechanical hypersensitivity in response to the same course of cisplatin (cis). Adoptive transfer of CD8+ T cells from saline (sal) treated mice to Rag2−/− mice prior to treatment with cisplatin normalizes resolution of CIPN. Adoptive transfer of CD8+ T cells obtained from WT mice that have recovered from cisplatin-induced neuropathy (Educated CD8+ T cells) prevents development of cisplatin-induced mechanical hypersensitivity in the recipient Rag2−/− mice.
Concluding remarks
We presented evidence for a role of T cells, M2 macrophages, IL10 signaling and production of endogenous opioids by T cells in the resolution or suppression of pain. We conclude that the immune system does not only contribute to the onset and maintenance of chronic pain, but also plays a key role in pain suppression or resolution. If T cell activation contributes to pain resolution, it is perhaps not surprising that pharmacologic immunosuppression is often not enough to resolve pain. Better understanding of the mechanisms via which (subsets of) T cells promote pain resolution would allow for development of novel strategies to control pain through engaging these T cell dependent pathways. Critical studies in inflammatory bowel disease have already shown that immunization strategies may be feasible. In addition, there are already some exciting examples summarized above indicating that pharmacological interventions that act via T cell-dependent pathways may have long lasting beneficial effects. There are also still many Outstanding questions. For example, does the immune system contribute to preventing re-emergence of pain during the state of latent sensitization? [12, 16, 17]. It is tempting to speculate that what we call ‘resolution of pain’ by T cells and macrophages reflects a new homeostatic balance in which re-instatement of pain is actively prevented (Figure 1).
Outstanding questions:
Can we activate the endogenous immune mediated resolution pathways to treat chronic pain? Transfer of T cells from mice that had recovered from CIPN to T cell deficient recipient mice before the start of chemotherapy prevents development of chemotherapy-induced mechanical allodynia [31]. This points to the interesting possibility that transfer of autologous T cells educated ex vivo by chemotherapy could be developed as an intervention to prevent chronic pain after cancer treatment.
Does the immune system contribute to resolution of pain-related changes in affect, such as anxiety and depression? T cells and IL10 signaling are not only required for resolution of inflammatory pain but also for resolution of depression-like behavior in response to transient peripheral inflammation [76]. In addition, transfer of T cells from mice that had been exposed to a stressor to T cell deficient mice protected the recipients against stress-induced depression-like behavior [77].
Can we develop ways to promote IL10 signaling or (endogenous) IL10 production to control chronic pain. Studies by Watkins and co-workers show that IL10 encoding vectors increase IL10 levels in the spinal cord and results from studies in dogs provide promising results for control of osteoarthritis related pain [53, 78]. Can this strategy be used to prevent or treat chronic pain? For how long is increased IL10 signaling needed, and is this safe?
Can the capacity of the peripheral blood leukocytes to produce IL10 or endogenous opioids be used to identify individuals at risk for chronic pain e.g. before surgery or chemotherapy? Will this help select patients who would benefit most from novel preventive or therapeutic interventions hat target the immune system?
Does persistent IL10 signaling to sensory neurons contribute to keeping the nervous system under control during the state of latent sensitization? Do disturbances in the immune system lead to re-emergence of pain?
Multiple fundamental questions also remain to be addressed, for example: how are the CD4+ or CD8+ T cells that promote resolution of pain activated to produce and release endogenous opioids, IL10 and/or other pain suppressing factors? Are these activation signals specific for each form of chronic pain? What is the nature of the chemotactic signal(s) that recruit T cells to the site of inflammation or tissue damage, or to the DRG, nerve, spinal cord or other sites of action? Are the effects of T cells on the nervous system direct or indirect? Do macrophages play and additional role or do they mediate or increase the pain suppressing effects of T cells? It also remains to be determined whether the activity of T cells contributes to the state of latent sensitization by releasing endogenous opioids or (promoting production of) cytokines like IL10. Once we more clearly understand which and how specific subsets of T cells are activated to promote pain resolution, we may also use these parameters as a predictive biomarker to identify individuals at risk for chronic pain e.g. before surgery or chemotherapy (see Clinicians Corner). These patients could then be selected for interventions that boost the activity of these endogenous T cell-mediated pain resolution pathways before exposing them to chemotherapy or surgery to prevent transition to chronic pain.
Clinician’s Corner.
In most cases pain caused by surgery, chemotherapy or infection resolves when tissues heal, inflammation resolves, or treatment is completed. However, a significant subgroup of patients transitions to chronic pain.
Treating a chronic pain condition still represents a major clinical challenge. It is widely accepted that persistent (neuro)inflammation drives chronic pain. However, treatment with immunosuppressive drugs like NSAIDs is often not sufficient to treat chronic pain.
The finding that pain often persists even when inflammatory activity is successfully controlled, supports the hypothesis that active immune regulation is needed for resolution of pain. If so, combining anti-inflammatory therapies with interventions that activate this pain resolving immune circuitry may well be key to developing novel treatments for chronic pain. Such combined interventions may well address the underlying cause of the persistence of the pain state rather than targeting only the symptom.
The immune system promotes resolution of pain through the activity of T cells and macrophages. These cells can produce anti-inflammatory cytokines like IL10 and opioid peptides including enkephalins and endorphins. We propose that chronic pain may develop when these immune resolution mechanisms fail. If so, predictive biomarkers for the risk of developing chronic pain could be developed by assessing the capacity of immune cells, including T cells and monocytes in the peripheral circulation, to produce pro-resolution factors like IL10 and opioids.
Studies in models of inflammatory bowel disease have shown that developing immunization strategies to suppress pain may be possible.
With respect to chemotherapy-induced peripheral neuropathies, future studies should determine whether ex vivo “education” of T cells by chemotherapy could accelerate resolution of this debilitating side effect of cancer treatment.
Highlights:
A better understanding of how resolution of acute pain is regulated uncovers novel therapeutic strategies urgently needed by patients with chronic pain.
A key role of the immune system in pain resolution may explain why anti-inflammatory drugs are often not sufficient to treat chronic pain.
T cells promote resolution of pain in males and females and there is a key contribution of M2-type macrophages and signaling by IL10 and opioids produced by these cells.
The prediction is that failure of immune-mediated resolution will lead to chronic pain. If so, stimulating endogenous immune-mediated resolution pathways should provide a novel approach to eliminate the cause of chronic pain rather than providing temporary symptom relief.
Acknowledgements:
The work of Annemieke Kavelaars and Cobi J. Heijnen is supported by grants RO1 NS116704, RO1 NS073939, RO1 CA208371, RO1 CA227064, RO1 CA 230512, and R21 NS104804 of the National Institutes of Health.
Glossary:
- Allodynia
pain due to a stimulus that does not normally provoke pain
- CD4+ or CD8+ T cells
CD4 and CD8 are cell surface markers on subsets of T cells that have specific functions. CD4 cells are also known as helper T cells, while CD8 cells are cytotoxic and suppressor T cells. Regulatory T cells are identified within both the CD4 and CD8 subsets
- Chronic pain
pain that lasts or recurs for more than three months
- Chemotherapy-induced peripheral neuropathy
pain, numbness and tingling that develops in a glove-and-stocking distribution in response to treatment of cancer patients with chemotherapeutics
- Dorsal root ganglion
contains the cell bodies of primary pain sensing neurons
- Neuropathic pain
pain caused by a lesion or disease of the somatosensory nervous system
- Nociceptor
a central or peripheral neuron of the somatosensory nervous system that is capable of encoding noxious stimuli
- Pain
an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage
- Rag1 or 2−/− mice
mice genetically deficient in the enzyme Rag1 or Rag2 do not develop mature T and B lymphocytes because both enzymes are required for rearrangement of antigen receptors
- SCID mice
Severe combined immunodeficient mice: these mice do not have mature T and B cells due to a genetic deletion on chromosome 16
- Sensitization
Increased responsiveness of pain sensing neurons to their normal input, and/or recruitment of a response to normally subthreshold inputs. Latent sensitization refers to the concept that pain can re-emerge after apparent resolution when silencing pathways are interrupted, e.g. by inhibiting opioid receptor signaling
Footnotes
Conflicts of interest:
The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Seretny M et al. (2014) Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 155 (12), 2461–70. [DOI] [PubMed] [Google Scholar]
- 2.Molassiotis A et al. (2019) Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer 19 (1), 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richebe P et al. (2018) Persistent Postsurgical Pain: Pathophysiology and Preventative Pharmacologic Considerations. Anesthesiology 129 (3), 590–607. [DOI] [PubMed] [Google Scholar]
- 4.Montes A et al. (2015) Genetic and Clinical Factors Associated with Chronic Postsurgical Pain after Hernia Repair, Hysterectomy, and Thoracotomy: A Two-year Multicenter Cohort Study. Anesthesiology 122 (5), 1123–41. [DOI] [PubMed] [Google Scholar]
- 5.Macrae WA (2008) Chronic post-surgical pain: 10 years on. Br J Anaesth 101 (1), 77–86. [DOI] [PubMed] [Google Scholar]
- 6.Lavand’homme P (2017) Transition from acute to chronic pain after surgery. Pain 158 Suppl 1, S50–S54. [DOI] [PubMed] [Google Scholar]
- 7.Salat K et al. (2018) Experimental Drugs for Neuropathic Pain. Curr Neuropharmacol 16 (8), 1193–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price TJ et al. (2018) Transition to chronic pain: opportunities for novel therapeutics. Nat Rev Neurosci 19 (7), 383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skolnick P and Volkow ND (2016) Re-energizing the Development of Pain Therapeutics in Light of the Opioid Epidemic. Neuron 92 (2), 294–297. [DOI] [PubMed] [Google Scholar]
- 10.Cherubino P et al. (2012) The management of chronic pain in important patient subgroups. Clin Drug Investig 32 Suppl 1, 35–44. [DOI] [PubMed] [Google Scholar]
- 11.Lee YC et al. (2014) Subgrouping of patients with rheumatoid arthritis based on pain, fatigue, inflammation, and psychosocial factors. Arthritis Rheumatol 66 (8), 2006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corder G et al. (2013) Constitutive mu-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science 341 (6152), 1394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivat C et al. (2007) Non-nociceptive environmental stress induces hyperalgesia, not analgesia, in pain and opioid-experienced rats. Neuropsychopharmacology 32 (10), 2217–28. [DOI] [PubMed] [Google Scholar]
- 14.Parada CA et al. (2003) Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci 17 (9), 1847–52. [DOI] [PubMed] [Google Scholar]
- 15.Reichling DB and Levine JD (2009) Critical role of nociceptor plasticity in chronic pain. Trends Neurosci 32 (12), 611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson TS and Taylor BK (2020) Targeting spinal neuropeptide Y1 receptor-expressing interneurons to alleviate chronic pain and itch. Prog Neurobiol, 101894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marvizon JC et al. (2015) Latent sensitization: a model for stress-sensitive chronic pain. Curr Protoc Neurosci 71, 9 50 1–9 50 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price TJ and Ray PR (2019) Recent advances toward understanding the mysteries of the acute to chronic pain transition. Curr Opin Physiol 11, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costigan M et al. (2009) T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci 29 (46), 14415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laumet G et al. (2019) T Cells as an Emerging Target for Chronic Pain Therapy. Front Mol Neurosci 12, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorge RE et al. (2015) Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 18 (8), 1081–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boue J et al. (2014) Endogenous regulation of visceral pain via production of opioids by colitogenic CD4(+) T cells in mice. Gastroenterology 146 (1), 166–75. [DOI] [PubMed] [Google Scholar]
- 23.Basso L et al. (2018) T-lymphocyte-derived enkephalins reduce Th1/Th17 colitis and associated pain in mice. J Gastroenterol 53 (2), 215–226. [DOI] [PubMed] [Google Scholar]
- 24.Austin PJ et al. (2012) Regulatory T cells attenuate neuropathic pain following peripheral nerve injury and experimental autoimmune neuritis. Pain 153 (9), 1916–31. [DOI] [PubMed] [Google Scholar]
- 25.Lees JG et al. (2015) Depletion of Foxp3+ regulatory T cells increases severity of mechanical allodynia and significantly alters systemic cytokine levels following peripheral nerve injury. Cytokine 71 (2), 207–14. [DOI] [PubMed] [Google Scholar]
- 26.Davoli-Ferreira M et al. (2020) Regulatory T cells counteract neuropathic pain through inhibition of the Th1 response at the site of peripheral nerve injury. Pain 161 (8), 1730–1743. [DOI] [PubMed] [Google Scholar]
- 27.Baddack-Werncke U et al. (2017) Cytotoxic T cells modulate inflammation and endogenous opioid analgesia in chronic arthritis. J Neuroinflammation 14 (1), 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basso L et al. (2020) Endogenous control of inflammatory visceral pain by T cell-derived opioids in IL-10-deficient mice. Neurogastroenterol Motil 32 (2), e13743. [DOI] [PubMed] [Google Scholar]
- 29.Laumet G et al. (2020) CD3(+) T cells are critical for the resolution of comorbid inflammatory pain and depression-like behavior. Neurobiol Pain 7, 100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krukowski K et al. (2016) CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain. J Neurosci 36 (43), 11074–11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laumet G et al. (2019) Cisplatin educates CD8+ T cells to prevent and resolve chemotherapy-induced peripheral neuropathy in mice. Pain 160 (6), 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer R et al. (2019) TNFR2 promotes Treg-mediated recovery from neuropathic pain across sexes. Proc Natl Acad Sci U S A 116 (34), 17045–17050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duffy SS et al. (2019) Regulatory T Cells and Their Derived Cytokine, Interleukin-35, Reduce Pain in Experimental Autoimmune Encephalomyelitis. J Neurosci 39 (12), 2326–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma J et al. (2019) Cell-specific role of histone deacetylase 6 in chemotherapy-induced mechanical allodynia and loss of intraepidermal nerve fibers. Pain 160 (12), 2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krukowski K et al. (2017) HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain 158 (6), 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith EM et al. (1986) Corticotropin releasing factor induction of leukocyte-derived immunoreactive ACTH and endorphins. Nature 321 (6073), 881–2. [DOI] [PubMed] [Google Scholar]
- 37.Kavelaars A et al. (1989) The role of IL-1 in the corticotropin-releasing factor and arginine-vasopressin-induced secretion of immunoreactive beta-endorphin by human peripheral blood mononuclear cells. J Immunol 142 (7), 2338–42. [PubMed] [Google Scholar]
- 38.Heijnen CJ et al. (1991) Beta-endorphin: cytokine and neuropeptide. Immunol Rev 119, 41–63. [DOI] [PubMed] [Google Scholar]
- 39.Kamphuis S et al. (1998) Role of endogenous pro-enkephalin A-derived peptides in human T cell proliferation and monocyte IL-6 production. J Neuroimmunol 84 (1), 53–60. [DOI] [PubMed] [Google Scholar]
- 40.Rittner HL et al. (2001) Opioid peptide-expressing leukocytes: identification, recruitment, and simultaneously increasing inhibition of inflammatory pain. Anesthesiology 95 (2), 500–8. [DOI] [PubMed] [Google Scholar]
- 41.Stein C et al. (1990) Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc Natl Acad Sci U S A 87 (15), 5935–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Celik MO et al. (2020) IL-4 induces M2 macrophages to produce sustained analgesia via opioids. JCI Insight 5 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kavelaars A et al. (1990) Induction of beta-endorphin secretion by lymphocytes after subcutaneous administration of corticotropin-releasing factor. Endocrinology 126 (2), 759–64. [DOI] [PubMed] [Google Scholar]
- 44.Labuz D et al. (2010) T lymphocytes containing beta-endorphin ameliorate mechanical hypersensitivity following nerve injury. Brain Behav Immun 24 (7), 1045–53. [DOI] [PubMed] [Google Scholar]
- 45.Schafer M et al. (1994) Interleukin 1 beta and corticotropin-releasing factor inhibit pain by releasing opioids from immune cells in inflamed tissue. Proc Natl Acad Sci U S A 91 (10), 4219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen SF et al. (2017) T-Cell Mediation of Pregnancy Analgesia Affecting Chronic Pain in Mice. J Neurosci 37 (41), 9819–9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray PJ et al. (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41 (1), 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mege JL et al. (2011) Macrophage polarization and bacterial infections. Curr Opin Infect Dis 24 (3), 230–4. [DOI] [PubMed] [Google Scholar]
- 49.Willemen HL et al. (2010) Microglial/macrophage GRK2 determines duration of peripheral IL-1beta-induced hyperalgesia: contribution of spinal cord CX3CR1, p38 and IL-1 signaling. Pain 150 (3), 550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raoof R et al. (2020) Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. bioRxiv, 2020.02.12.940445. [DOI] [PubMed] [Google Scholar]
- 51.Vanderwall AG and Milligan ED (2019) Cytokines in Pain: Harnessing Endogenous Anti-Inflammatory Signaling for Improved Pain Management. Front Immunol 10, 3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwilasz AJ et al. (2015) The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology 96 (Pt A), 55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watkins LR et al. (2020) Targeted interleukin-10 plasmid DNA therapy in the treatment of osteoarthritis: toxicology and pain efficacy assessments. Brain Behav Immun. [DOI] [PubMed] [Google Scholar]
- 54.Eijkelkamp N et al. (2016) IL4–10 Fusion Protein Is a Novel Drug to Treat Persistent Inflammatory Pain. J Neurosci 36 (28), 7353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwilasz AJ et al. (2019) A single peri-sciatic nerve administration of the adenosine 2A receptor agonist ATL313 produces long-lasting anti-allodynia and anti-inflammatory effects in male rats. Brain Behav Immun 76, 116–125. [DOI] [PubMed] [Google Scholar]
- 56.Kwilasz AJ et al. (2018) Sustained reversal of central neuropathic pain induced by a single intrathecal injection of adenosine A2A receptor agonists. Brain Behav Immun 69, 470–479. [DOI] [PubMed] [Google Scholar]
- 57.Chen Z et al. (2019) Sphingosine-1-phosphate receptor 1 activation in astrocytes contributes to neuropathic pain. Proc Natl Acad Sci U S A 116 (21), 10557–10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sluka KA et al. (2018) Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain 159 Suppl 1, S91–S97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leung A et al. (2016) Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing interleukin-10 in mice. Pain 157 (1), 70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alvarez P et al. (2017) Nociceptor interleukin 10 receptor 1 is critical for muscle analgesia induced by repeated bouts of eccentric exercise in the rat. Pain 158 (8), 1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borghi SM et al. (2015) Interleukin-10 limits intense acute swimming-induced muscle mechanical hyperalgesia in mice. Exp Physiol 100 (5), 531–44. [DOI] [PubMed] [Google Scholar]
- 62.Milligan ED et al. (2005) Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol Pain 1, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ledeboer A et al. (2007) Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun 21 (5), 686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willemen HL et al. (2014) Monocytes/Macrophages control resolution of transient inflammatory pain. J Pain 15 (5), 496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laumet G et al. (2020) Interleukin-10 resolves pain hypersensitivity induced by cisplatin by reversing sensory neuron hyperexcitability. Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen KF et al. (2013) Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Exp Neurol 247, 466–75. [DOI] [PubMed] [Google Scholar]
- 67.Ip WKE et al. (2017) Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 356 (6337), 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Basso L et al. (2018) Mobilization of CD4+ T lymphocytes in inflamed mucosa reduces pain in colitis mice: toward a vaccinal strategy to alleviate inflammatory visceral pain. Pain 159 (2), 331–341. [DOI] [PubMed] [Google Scholar]
- 69.Ji RR et al. (2016) Pain regulation by non-neuronal cells and inflammation. Science 354 (6312), 572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grace PM et al. (2014) Pathological pain and the neuroimmune interface. Nat Rev Immunol 14 (4), 217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu X et al. (2020) Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun 11 (1), 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang H et al. (2016) Dorsal Root Ganglion Infiltration by Macrophages Contributes to Paclitaxel Chemotherapy-Induced Peripheral Neuropathy. J Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shepherd AJ et al. (2018) Macrophage angiotensin II type 2 receptor triggers neuropathic pain. Proc Natl Acad Sci U S A 115 (34), E8057–E8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mousset CM et al. (2019) Comprehensive Phenotyping of T Cells Using Flow Cytometry. Cytometry A 95 (6), 647–654. [DOI] [PubMed] [Google Scholar]
- 75.Shapouri-Moghaddam A et al. (2018) Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 233 (9), 6425–6440. [DOI] [PubMed] [Google Scholar]
- 76.Laumet G et al. (2018) Resolution of inflammation-induced depression requires T lymphocytes and endogenous brain interleukin-10 signaling. Neuropsychopharmacology 43 (13), 2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brachman RA et al. (2015) Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J Neurosci 35 (4), 1530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milligan ED et al. (2005) Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci 21 (8), 2136–48. [DOI] [PubMed] [Google Scholar]