Abstract
The salivary pellicle, an adlayer formed by adsorption of salivary components on teeth and dental biomaterials, has direct consequences on basic outcomes of dentistry. Here, we provide an overview of salivary pellicle formation processes with a critical focus on dental biomaterials. We describe and critique the array of salivary pellicle measurement techniques. We also discuss factors that may affect salivary pellicle formation and the heterogeneity of the published literature describing salivary pellicle formation on dental biomaterials. Finally, we survey the many effects salivary pellicles have on dental biomaterials and highlight its implications on design criteria for dental biomaterials. Future investigations may lead to rationally designed dental biomaterials to control the salivary pellicle and enhance material function and patient outcomes.
Keywords: Salivary pellicle, Dental biomaterials, Surface, Protein adsorption, Dentistry
Graphical Abstract
1.0. Introduction
The role of saliva in the oral cavity ranges from remineralization [1] to lubrication [2] to digestion and beyond [3]. This is achieved through saliva’s multifunctionality, redundancy of components, and amphifunctionality [4]. Saliva is over 99% water [5] yet contains secretions including proteins and glycoproteins [6] from major salivary glands (submandibular, parotid, and sublingual) and minor salivary glands (buccal, palatal, labial, and von Ebner’s), gingival crevicular fluid, and bacterial elaborations [7]. The presence of approximately 0.3 wt% mucins, a family of glycosylated proteins, renders saliva its rheology and its unique lubrication properties [8]. An important consequence of this solute-laden (at least 1,515 unique proteins [9]) fluid in the oral cavity is the inevitable adsorption of salivary components onto surfaces such as dentition and dental biomaterials. The completely-acellular adlayer formed by adsorption of salivary components was first described in 1839 [10] and termed the salivary pellicle (SP) by C. Dawes in 1963 [11].
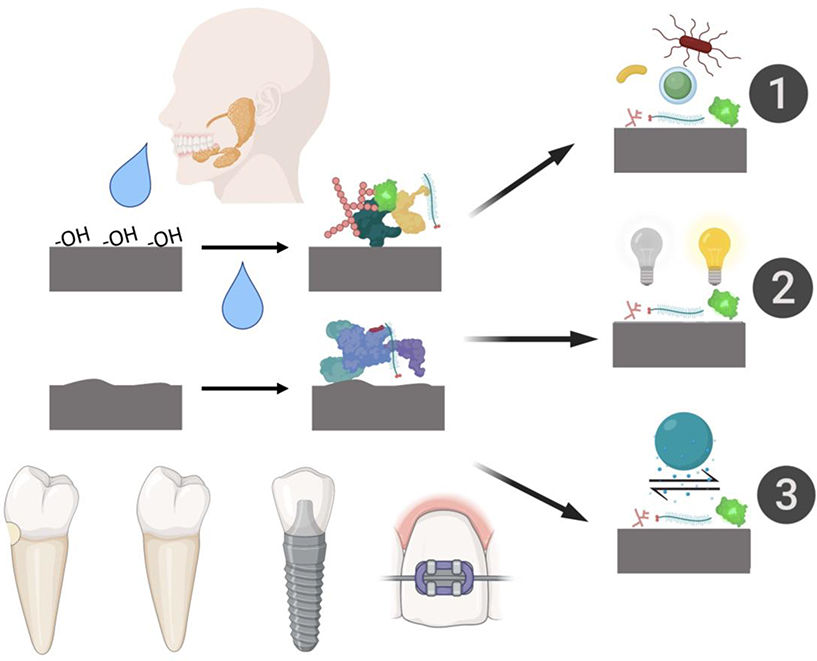
SP formation on dentition and dental biomaterials affects basic outcomes of dentistry. SP acts as an integument for tooth surfaces and dental biomaterials, protecting them from wear and aiding in speech and mastication [12,13]. SPs can reduce the demineralization rate of enamel [14] and inhibit “surface-induced” precipitation of calcium-rich species, and thus, over mineralization of tooth surfaces [15]. SPs controls cariogenic biofilm formation on dentition and dental biomaterials [6,16,17]. Furthermore, SP formation can modulate periodontal wound healing [18]. The range of interactions the SP mediates on dental biomaterial surfaces is summarized in Figure 1. As evidence of the effect on SP on dental biomaterials, 45.8% of patients develop caries during orthodontic treatment due to biofilm formation on orthodontics materials, alongside a white-spot prevalence rate of 68.4% [19]. Similarly, dental crowns may accumulate plaque that leads to caries or periodontitis; approximately 14.4% of patients developed periodontitis from crown treatment after 5 years [20]. Staining of dental veneers can lead to patient dissatisfaction and need for further treatment [21]. Modulation of bacterial adhesion to dental biomaterials surfaces through the formed SP can cause an imbalance, or dysbiosis, of the microbiota on materials surface and lead to enrichment of pathobionts [22–24].
Figure 1:
Schematic overview of dental biomaterial surface properties that can lead to differences in salivary pellicle formation and the resultant dental biomaterials outcomes this may drive. 1) Salivary pellicle composition can drive differences in biofilm bacterial content and activity. 2) Salivary pellicle formation can stain dental biomaterials. 3) Salivary pellicles can affect tribological properties and wear of dental biomaterials.
While it is clear that SPs regulate many interactions in the oral mucosa, SP formation on dental biomaterials remains less studied than SP formation on teeth. The purpose of this review is to highlight the current state of knowledge of SP formation on dental biomaterials, with a particular emphasis on methods used for studying SPs and the effects of SP formation on dental biomaterials. A better recognition and understanding of SP formation on dental biomaterials may lead to rationally designed materials that control SP formation and increase dental biomaterials clinical outcomes.
2.0. Salivary Pellicle Formation
2.1. General Characteristics of Salivary Pellicle Formation
Initial adsorption of salivary proteins and other biomolecules in saliva on any oral surface takes place within a matter of seconds [25] and grows to around 10–20 nm thick after one minute [26]. The ultimate SP thickness is about 1.3 μm at 24 hours [26]. This adsorption process is dynamic and initially adsorbed proteins tend to undergo conformation changes as later proteins adsorb [27,28]. However, protein desorption can also occur within the first minute of protein-substrate interactions for other proteinaceous fluids such as blood; this is likely true for saliva as well [29].
Adsorption of proteins generally depends on non-covalent interactions between the surfaces and molecules already sorbed on the surfaces (interactions we term throughout as material:proteinsearly and proteinsearly:proteinslate interactions, respectively) such as hydrophobic, electrostatic forces, hydrogen bonding and van der Waals forces. This complexity gives rise to the “Vroman effect” which describes changes over time of adsorbed proteins from blood onto surfaces, but can be applied to other systems, where smaller molecular weight proteins are replaced with larger, slower diffusing, higher molecular weight proteins [30]. Molecular shape affects adsorption dynamics as well. For example, globular proteins tend to have a greater ability to rearrange shape to maximize surface interactions than rod-like proteins and so have a high affinity for surfaces [31]. A simple, intuitive view of adsorption may suggest that abundant proteins in solution would adsorb in abundance compared to less abundant proteins, regardless of protein shape. However, this is not necessarily the case. Analysis of the blood proteins has shown that albumin, which is about 50% of plasma protein by weight, is under-represented on surfaces, while fibrinogen, which is about 5% of plasma protein by weight, is over-represented [32]. Similar analyzes for orally-relevant proteins are lacking to date but general principles likely still apply.
Two disparate mechanisms of SP adsorption exist. Early protein adsorption is based on material:proteinsearly interactions as opposed to later proteins which adsorb predominately based on proteinsearly:proteinslate interactions. These two phases lead to the two distinct SP layers [33]. A thinner, denser layer forms on the material and a thicker, less dense layer on top of the first layer [25]. The basal lay generally has a network structure [33] and the outer layer has a more heterotypic structure with aggregates of proteins [25]. The basal layer preferentially contains proline-rich proteins, statherins, histatins, and highly glycosylated mucins compared to the outer layer [25,34]. Interestingly, the basal portion of the SP is more resistant to mechanical wear than the outer layer [35]; this is probably due to the more tenacious non-covalent interactions between the material:proteinsearly in the basal layer versus proteinsearly:proteinslate of the outer layer.
A frequently overlooked factor in any adsorptive process, including that of SP formation, is the role of water [36]. Water is the first molecule to adsorb to a surface given its orders of magnitude smaller size than many biomolecules and relative abundance. Proteins must thus displace the highly structured, adsorbed water layer in order to adsorb to a surface [36,37] Other factors such as temperature, pH, and ionic strength of the solution can influence adsorption as well [38–40]. The SP is also known to undergo enzymatic crosslinking and proteolysis during SP formation, further complicating the role of protein:protein interactions in the formation process beyond physical interactions [41].
Substrate (dentition, dental implants, etc.) physical, chemical, and topographical properties can markedly affect SP formation. Effects of these material and surfaces properties on general protein adsorption have been thoroughly reviewed by others, see Nakanishi et al.,[42] Wang et al., [43] Rabe et al.[44], and Sterzenbach et al. [22]. Here, our scope is on reviewing the existing body of literature specific to SP formation on dental biomaterials.
2.2. Salivary Pellicle Formation on Dental Biomaterials
2.2.1. Early, Foundational Research About Salivary Pellicle Formation on Dental Biomaterials
Despite less work on SP formation performed on dental biomaterials than on dentition, a small, but robust, body of literature exists outlining the general principles of SP formation on dental biomaterials. Early pioneering work by Hannig et. al [28] showed no morphological nor thickness differences between SPs formed on enamel compared to resin composites, amalgams, and casting alloys [28] even though there are markedly different physicochemical differences between these materials. Other early work similarly showed no morphological differences between SPs on microfilled composites and glass ionomers [45]. These studies are admittedly limited due to their reliance on transmission electron microscopy (TEM) or scanning electron microscopy (SEM), which only evaluate thickness and morphological traits and not chemical or biomolecule composition. However, these techniques have been historically valuable in the understanding of SPs.
A number of other studies have been performed since these early studies, which we summarized and divided by dental biomaterial in the following sections. We also note some of the most commonly used SP analytical techniques.
2.2.2. Salivary Pellicle Formation on PMMA and Other Polymeric Dental Biomaterials
Initial work [46] showed differences in amino acid contents of SP on polyethylene terephthalate commercial dental polymers films (Mylar and Melinex ®) and glass. The SP formed on these two polyesters contained more glutamic acid and less proline compared to glass. Later work [47] using gas chromatography showed less proline and more serine on enamel SPs compared to SPs on polyvinyl chloride/polyvinyl acetate. While interesting, drawing further conclusions on specific molecular composition of the SP is limited as this characterization only provides information on amino acids composition.
Other studies have found poly(methyl methacrylate) (PMMA) denture materials had a comparable SP composition to natural dentition [48]. Two-dimensional gel electrophoresis has shown differences in protein composition between titanium, PMMA, and enamel; however, the compositional differences were not investigated beyond pI and molecular weight differences, resulting in weak biomolecule assignment [49]. Furthermore, liquid-polish coatings on PMMA have shown less protein adsorption, as determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), than uncoated PMMA [50]. Past work [51] compared the amount of SP proteins adsorbed to PMMA, polyethylene (PE), polyte-trafluoroethylene (PTFE), silicone, a silorane-based and a methacrylate-based dental composite, a glass ionomer cement, a cobalt–chromium–molybdenum alloy, and titanium. The authors showed that the hydrophobic surfaces tended to adsorb more protein but there was no relationship between the adhesion of Streptococci gordonii to water contact angles of the surfaces or amount of protein adsorbed. The authors suggested that surface chemistry affects SP formation more than simply hydrophobicity/philicity.
2.2.3. Salivary Pellicle Formation on Resin Composites
While SP compositions on different materials may appear to be similar in some circumstances, this does not mean the biological activity is similar. Hannig et al. [28] showed no differences in SPs formed on resin composites, amalgams, and casting alloys using TEM. Similarly, the same group [52] showed no differences in lysozyme activity between SPs formed on enamel, dentin, gold alloy, titanium, amalgam, feldspar ceramic, resin composite (Spectrum, Dentsply DeTrey), and PMMA. However, amylase activity was significantly lower on titanium, resin composite, and PMMA than the other tested materials. This demonstrates that while SPs may appear morphologically similar and have similar functional activities for some measures, this may not apply to all functional measures of a material.
More recent work has delineated the influence of fillers and protease inhibitors on SPs formed on experimental resins [53]. The authors showed that incorporation of fillers generally increased the abundance of SP proteins and addition of inhibitors generally increased cystatins, lysozymes, and mucins regardless of the specific inhibitor used. Other work used SDS-PAGE to investigate SP composition and concluded that human salivary low-molecular-weight mucin (MG2) proline-rich proteins and agglutinin were enriched on a glass ionomer cement compared to a resin composite (TPH Spectrum, Dentsply DeTrey) [54].
2.2.4. Salivary Pellicle Formation on Metals
Titanium alloy dental implants have been of considerable interest giving the importance the SP plays mediating peri-implant healing [55–57]. Mass spectrometry has been used to compare SP protein content on three common implant surfaces. The SP formed on microrough and highly hydrophilic SLActive® (Institut Straumann A.G.) surfaces was composed of more proteins than on less hydrophilic SLA® and smooth machined surfaces. Other results showed the microrough surfaces of SLA® and SLActive® surfaces demonstrated some adsorption specificity while the smooth machined surface showed almost no specificity [58]. Other work has shown that incorporation of niobium and zirconia in titanium alloys changes the SP protein profile compared to commercially pure titanium [59].
Other work [60] determined in vivo SP formation on titanium, copper-chromium, silver palladium copper gold alloy, and denture resin (methacrylates and dimethacrylates). The most commonly adsorbed salivary components among all groups were immunoglobulin alpha-1 heavy chain constant region, polymeric immunoglobulin receptor precursor, and albumin, among others. Finally, titanium alloy surface roughening has been shown to increase lactoferrin and MG2 adsorption compared to smoother surfaces [61].
Some work has shown compositional differences in SPs between bracket metal, ligature ring elastomer, and orthodontic bonding resin [62]. Specific results showed that bracket metal adsorbed more amylases than the other materials tested. Others [63] compared SP formation on gold, stainless steel, alumina and zirconia and found that adsorption kinetics and thickness of SP were similar on all materials.
3.0. Characterizing Salivary Pellicles
As seen, a wide variety of techniques have been used to analyze SP formed on surfaces. A summary of techniques utilized to determine SP properties on dental biomaterials and dentition is shown in Table 1 and Figure 2. These techniques reflect the range of experimental multidisciplinary backgrounds that SP investigators possess, which expand from biochemical to colloidal/interfacial sciences to clinical dentistry. An underlying difficulty in measuring or characterizing SPs is the amount of protein available in saliva to be analyzed; the SP formed on a tooth labial surface over 2 hours in vivo contains just 2.0 μg of protein [64].
Table 1:
Survey of techniques used to analyze properties of SPs formed on dental biomaterials and dental hard tissues.
| Major Technique | Saliva Source | Fluid Flow? | Substrate | Reference |
|---|---|---|---|---|
| RIfS | Collected Saliva | Injected | Titanium dioxide, DC-PEG, Laminin, and EGF coated glass | [70] |
| UPLC flight MS | Collected Saliva | Agitation | PMMA | [71] |
| SDS-Page | Collected Saliva and In Vivo | Rotating and In Vivo | HA and In Vivo | [72] |
| TEM and SDS-PAGE | In Vivo | Intra-Oral Appliance | Bovine Enamel | [35] |
| Assorted Colorimetric Assays | In Vivo | Intra-Oral Appliance | PMMA | [48] |
| QCM | Collected Saliva | Flow Across QCM | Ti and Fn bound to Ti | [73] |
| SDS-Page and AFM | Collected Saliva | None | Human enamel | [74] |
| SDS-Page and Western Blots | Collected Saliva | None | Stainless steel orthodontic bracket, bonding resin, and elastomeric ligature ring | [62] |
| CLSM | In Vivo | Intra-Oral Appliance | Bovine Enamel | [75] |
| TEM | In Vivo | Intra-Oral Appliance | Bovine Enamel | [26] |
| Immunofluorescence and Immunoelectrophoresis | In Vivo | Intra-Oral Appliance | Bovine Enamel | [76] |
| TEM | In Vivo | Intra-Oral Appliance | Array of dental biomaterials including composite resins, amalgams and PMMA. | [28] |
| QCM | Collected Saliva | Flow Across QCM | Ti and Anatase | [77] |
| LC-ESI-MS/MS | In Vivo | In Vivo | In Vivo | [78] |
| 2D-Electrophoresis and LC-MS/MS | Collected Saliva | Rocking | Ti | [79] |
| LC-ESI-MS/MS and SDS-PAGE | In Vivo | In Vivo | In Vivo | [80] |
| TEM and Gold Immunolabeling | In Vivo | Intra-Oral Appliance | Bovine Enamel | [81] |
| TEM, SEM, and Gold Immunolabeling | In Vivo | Intra-Oral Appliance | Bovine Enamel | [82] |
| TEM and gold immunolabeling and SEM | Collected Saliva | Rotary Shaker | HA and Bovine Enamel | [83] |
| Ellipsometry | Collected Saliva | None | HA | [83] |
| Ellipsometry and AFM | Collected Saliva | None | Silica | [84] |
| GLC | In Vivo | In Vivo | PMMA and In Vivo | [47] |
| SEM | Artificial saliva | None | Dental Composites | [85] |
| AFM | In Vivo | Intra-Oral Appliance | Mica, Silicon, and Graphite | [86] |
| 2D-Electrophoresis | Collected Saliva | Shaking | Ti, Extracted Enamel, and PMMA | [49] |
| FFS | Collected Saliva | None | Silica | [87] |
| AFM and FFS | Collected Saliva | None | Silica | [88] |
| SDS-PAGE | Collected Saliva | Rocking | Glass Ionomer | [54] |
| Fluorescent immunolabeling | In Vivo | Intra-Oral Appliance | Bovine Enamel | [89] |
| nLC- ESI-MS/MS and XPS | Collected Saliva | None | Ti and other implant treatments | [58] |
| QCM-D | Collected Saliva | Flow Across QCM-D | Gold, stainless steel, alumina and zirconia | [63] |
RIfS: reflectometric interference spectroscopy, UPLC flight MS: ultra performance liquid chromatography time of flight mass spectrometry, SDS-PAGE: sodium dodecyl sulphate–polyacrylamide gel electrophoresis, TEM: transmission electron microscopy, QCM: quartz crystal microbalance, AFM: atomic force microscopy, CLSM: confocal laser scanning microscopy, LC-ESI-MS/MS: liquid chromatography electrospray ionization tandem mass spectrometry, SEM: scanning electron microscopy, GLC: gas-liquid chromatography, FFS: friction force spectroscopy, QCM-D: quartz crystal microbalance with dissipation monitoring, DC-PEG: dicarboxypolyethylene glycol, EGF: epidermal growth factor, PMMA: polymethyl methacrylate, HA: hydroxyapatite, FN: fibronectin, Ti: titanium, nLC- ESI-MS/MS: nano liquid chromatography electrospray ionization tandem mass spectrometry, XPS: X-ray photoelectron spectroscopy.
Figure 2:
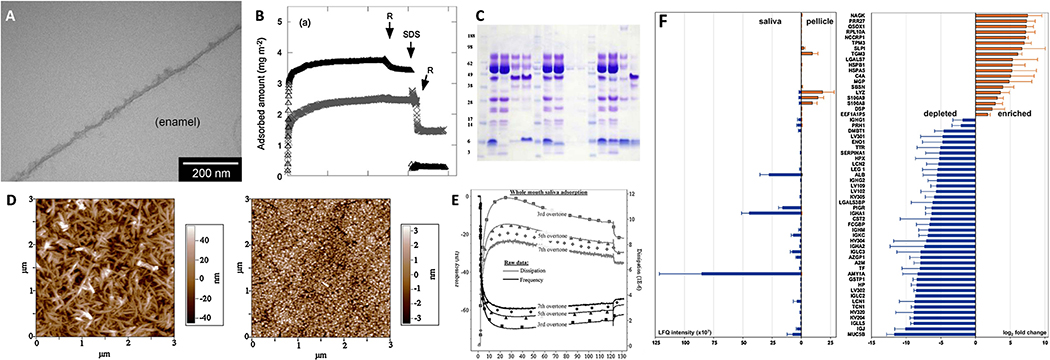
Overview of analytical techniques used to analyze SP formation. A) TEM micrograph of an in situ salivary pellicle formed on enamel after exposure to saliva at 37 °C for 90 min [65]. B) Adsorption kinetics of lactoferrin on hydrophilic silica (Δ) and hydrophobized silica (×) determined via ellipsometry [66]. C) Immunoblot following Coomassie (detects proteins) and periodic acid Schiff stain (detects glycoproteins) of salivary pellicles collected from various surfaces [67]. D) Atomic force micrographs of a hydroxyapatite surface before (left) and after (right) salivary pellicle formation [68]. E) Adsorption kinetics of saliva on hydroxyapatite via QCM-D with raw data and fitted Voigt modelled data [68]. F) Relative abundance of predominant proteins in an SP formed on feldspar ceramic compared to whole saliva using label-free quantitative nano-LC-MS/MS (liquid chromatography-mass spectrometry/mass spectrometry) [69]. All figures were reproduced with permission from the publisher.
An important factor when analyzing SPs’ composition is the method in which the analyte is removed from the surface for further analysis, such as with SDS-PAGE. Different methods reflecting a large range of protocols used by authors to obtain a sample for SDS-PAGE from dental biomaterials and enamel is found in Table 2. Generally, SDS-PAGE solution is used to wash off the adsorbed SP, though the surface may also be scrubbed or boiled. In the only study of its type, Hannig et al. [35] investigated a variety of SP removal methods from enamel, including those utilized for SDS-PAGE. Using TEM, the authors found that SDS-PAGE solution did not completely remove the SP. Indeed, only 0.6 M hydrochloric acid, 0.4% EDTA (pH 7.4), and ultrasonicated 3% sodium hypochlorite showed complete removal of the SP. This serves as a cautionary tale for researchers using methods that requires desorption of the SP. Other techniques are similarly sensitive to sample preparation. For example, electron microscopy measurements of SP are dependent on the sample dehydration method.
Table 2:
SP harvesting techniques used to obtain analyte for SDS-PAGE.
A pertinent question when designing SP experiments is whether samples should be incubated in collected saliva or placed in vivo. Only one previous study has directly addressed this question to the authors’ knowledge. Carlén et al. [72] comparad SP formation in vivo vs. in vitro. Major differences were noted between the two sampling methods. Samples worn in vivo contained a lot of albumin, while albumin was not found on in vitro incubated samples. A major source of differences between groups was dependent on where in the mouth samples were harvested from. These differences may be explained by proximity to different salivary glands and tongue shear forces [26,72,75,92,93] Variation between individuals may also be attributed to circadian rhythms and diet [74,94–96].
The range of techniques used to harvest SP in vivo is summarized in Table 3. These techniques generally involve scaling the SP onto some sort of paper where it is then transferred to perform further analyses. However, similar to SDS-PAGE techniques, the techniques used to isolate the SP can affect the results. For example, differences in collected SP glycoprotein content were shown between pre-treating SPs with 2M calcium chloride versus 5% EDTA following by mechanical scaling [97]. In short, the methodology utilized to isolate SPs formed on dental biomaterials can directly affect the results and should be rationalized beforehand and thoroughly described.
Table 3:
SP harvesting techniques used in vivo.
| Technique | Reference |
|---|---|
| Scrapped and collected on a glass wool plug | [98] |
| Swabbed with filter paper soaked in citric acid | [78] |
| Swabbed with filter paper soaked in citric acid | [99] |
| Swabbed with membrane paper | [80] |
| Scrapped and collected on a glass wool plug | [47] |
| Scrapped and collected on a glass wool plug | [100] |
| Swabbed with sodium bicarbonate soaked membrane paper | [101] |
| Swabbed with sodium bicarbonate soaked membrane paper | [64] |
| Swabbed with sodium bicarbonate soaked PVDF paper | [102] |
| Filter paper soaked in citric acid | [103] |
| Whole extracted teeth placed in hydrochloric acid and scrapped | [104] |
| Scrapped and collected on a glass wool plug | [105] |
| Scaled | [106] |
| Swabbed with calcium chloride solution on filter paper and scrapped | [97] |
| Scrapped and suctioned | [107] |
| Scrapped and placed onto PVDF | [108] |
PVDF: Polyvinylidene fluoride
4.0. Effects of Salivary Pellicles on Dental Biomaterials
A driving factor in the study of SP formation on dental biomaterials is the potential downstream effects on dental biomaterial characteristics, performance, and longevity. SP formation on dental biomaterials may reduce the biological activity of dental biomaterials because the SP covers the engineered functional domains. For example, an antibacterial dental primer and adhesive showed decreased antibacterial effects after salivary pellicle formation [109]. This reduction in biological activity holds true across a range of biomaterials, including PEGylated silicon [110], silicon with anchored antibacterial pyridinium [110], and a resin filler with an added quaternary ammonium methacrylate antibacterial monomer [111]. Biological activity that is dependent on direct interactions between cells and materials surface components, such as antibacterial monomers, may be particularly influenced by the presence of a SP. Here we summarize the known effects that SP formation has on dental biomaterials. We note that other effects exist for SP formation on dentition [112,113] but not all of these have been similarly studied on dental biomaterials.
4.1. Effects of Salivary Pellicles on Dental Biomaterial Lubrication and Wear
The formation of the SP is important in the mastication and speech production due its lubriciousness [14,114]. One group [115] measured the forces acting between an SP and silica with an atomic force microscopy (AFM) and found that the presence of a SP reduced the friction coefficient and was dependent on hydration state [116]. Tribological measurements using pig tongue and esophagus tissues has shown that unstimulated saliva is a better lubricant compared to stimulated saliva [117]; this may be due to the presence of statherin that itself reduces the interfacial tension of SPs and forms a calcium-enriched viscoelastic film [118]. Others [119] have shown that statherin and its specific confirmation played an important role in the lubriciousness caused by SPs on dental biomaterials and enamel. Sarkar, Xu, and Lee have comprehensively reviewed the role of SPs on surface-mediated mechanical properties for both dentition and biomaterials [8]. Notably, fluid saliva itself (i.e., liquid phase) plays an important role in lubricating intraoral surfaces beyond just the biomolecules present at interfaces [12]. In total, this modulation of friction due to SP may affect the performance of dental biomaterials given its relationship to mastication [14,114], speech production [14,114], wear [120], and attrition [121].
Wear, the progress loss of material due to both mechanical and bio/chemical factors, remains an active area of dental biomaterials research [122]. However, the role SPs play in dental biomaterial wear is still debated despite the intuitive role and the wealth of work examining SP-mediated tribology on oral soft tissues [113]. In fact, Hannig and Joiner [14] commented that “systematic investigations on the effect and influence of the salivary pellicle on tooth wear are lacking.” This statement still stands fifteen years later. Previous work has studied the adhesion strength between SPs and materials like compomers [123] and silica [124] which likely relates to wear modulation of SPs on dental biomaterials. Others have shown marked differences in SP binding strength between titania, hydroxyapatite, gold, zirconia, silica, and hydrophobized silica [125]. However, no studies have been performed to directly delineate the role of SPs on dental biomaterial wear.
4.2. Effects of Salivary Pellicles on Dental Biomaterial Erosion
Erosion, or the weakening of a material’s mechanical properties that make it more susceptible to mechanical forces leading to wear, contributes to dentition and dental biomaterials failure [126]. While dental biomaterial erosion is far less of a problem than natural mineralized dentition erosion, it is still a concern [127,128]. For example, erosion can lead to dissolution of the siliceous layer of glass-ionomer cements and can soften the resin phase of resin composite and cements [129].
The semi-permeable SP is important in reducing erosion because it can act as a diffusion barrier for degradative chemical agents (acids, enzymes, etc.); this function is commonly attributed to mucin, albumin, and casein [5,130]. The SP can also contain enzymes that neutralize acidic protons [131] from common erosive agents including fruit juices and gastric juices [132]. Furthermore, the SP may act as a reservoir of ions for remineralization [133]; polyanions penetrate into the SP near the base while polycations adsorb on top of the SP by interactions with mucins [134]. SP formed for just one hour can protect from orange juice erosion of dentition [75]. This anti-erosive behavior of the SP has been harnessed to create a synthetic peptide based on the natural SP to reduce erosion [135]. Clinical evidence supports the role of SP in erosion reduction as one group [136] showed differences in SP protein content based on the degree of enamel erosion. However, to date, no studies have been performed for the role of SPs on dental biomaterial erosion directly.
4.3. Effects of Salivary Pellicles on Dental Biomaterial Staining
Staining of dental biomaterials associated to SPs factors is of concern given the aesthetic demands of modern dentistry. Color is one of the most important factors determining satisfaction with one’s smile [137]. Staining generally associated with dental biomaterials is an extrinsic stain due to its localization on the surface, as opposed to intrinsic staining of the bulk of the material.
The most well studied staining element of dental biomaterials relating to SPs is chlorhexidine (CHX), a cationic bisbiguanide that is frequently used in mouthwashes and toothpastes as an antimicrobial agent [138]. The mechanisms by which CHX staining occurs are an active area of research but all involve SPs. The first proposed mechanism is nonenzymatic browning reactions or Maillard reactions [14]. This occurs when a sugars react with amino acids (from proteins in the SP, for example) and colored products are formed; this process is accelerated by CHX [139]. The second mechanism involves the denaturing of biomolecules in the SP due to CHX, exposing sulfur groups, and leading to eventual coordination with either iron or tin and a color change [140]. We note direct evidence for either of these mechanisms is mixed [14].
The other major cause of staining of dental biomaterials that relates to SPs is dietary components such as coffee, red wine, and tea [141]. Results from ellipsometry [142] have shown that black tea and red wine cause an increase in SP mass on hydroxyapatite. This is probably due to tannins that bind to proline-rich proteins found in the SP [143]. Indeed, extracted, stained teeth possess a thicker SP and have chemical differences compared to non-stained controls [144]. A similar result occurs on acrylic disks [145,146], and likely does on many other dental biomaterials as well.
An important note for in vitro testing of staining is that previous work [145] has shown differences in staining of acrylic blocks due to the use of stimulated versus un-stimulated collected saliva. This is likely due to the differences in protein content of each type of saliva. Relatively less work has focused on dental biomaterials and stain formation, mechanisms, etc. compared to enamel or hydroxyapatite except for stain removal (for example, see Jagger et al. [147]). Even less work has focused on the role of SPs in staining of dental biomaterials, though the role is clearly evident for enamel staining. Future research is needed to discern potential differences between enamel and dental biomaterials staining and the relationship to SPs.
4.4. Effects of Salivary Pellicles on Microbial Interactions
The process of initial microbial adhesion to a surface is reversible, but an emerging biofilm eventually forms [148]. These processes are dictated by the presence of a SP and specific components recognized by bacterial adhesins that bind to common SP biomolecules like amylase [149]. This topic has been previously reviewed by Sterzenbach et al. [22], Nobbs et al. [150], and Hannig et al. [151]. Recent work [63] has shown that formation of SP on a number of traditional dental biomaterials facilitated more attachment of an early colonizer of oral surfaces equally compared to bare biomaterials surfaces, which was attributed to the fact that these different biomaterials did not notably affect the physical-chemical properties of the SP. These results highlight the lack of specific surface design of classic dental biomaterials to control SP formation and SP effects in the oral environment.
5.0. Design of Dental Biomaterials That Modulate the Salivary Pellicles: Present and Future Considerations
The modification of biomaterials surfaces to control protein adsorption is commonplace for blood-contacting medical devices (see Ngo et al.’s [152] review), providing a potential source of ideas for dental biomaterial engineering. Indeed, efforts have been made to design dental biomaterials that reduce protein adsorption (as a way to reduce SP formation) and thus, hinder biofilm colonization of the designed surfaces.
A relevant example is dental resin composites and other resin-based materials used in the dental clinics to restore decayed teeth. Considering that SPs affect the composition of the colonizing biofilm [153] and that bacteria contribute to the high propensity for failure of dental restorations (more than 50% [154]), engineering polymeric dental biomaterials to minimize SP formation is meritorious. Resin composites with an added protein-repellant monomer (2-methacryloyloxyethyl phosphorylcholine; a methacrylate with a phospholipid polar group) reduced protein adsorption by 1/12th as compared to a standard formulation and, combined with an antimicrobial monomer - quaternary ammonium dimethylaminohexadecyl methacrylate (DMAHDM), produced surfaces with synergistic antibacterial properties [155,156].
Poly(2-hydroxyethyl methacrylate) and polyethylene glycol methacrylate have been used to coat PMMA, which is used in dentistry for a number of devices such as denture bases and maxillofacial prostheses, to attenuate SP formation and subsequent bacterial adhesion [157]. Dental metallic surfaces used to manufacture orthodontic bracket, implants, etc. can also benefit from control of SP formation as a means of gaining anti-biofilm activity. In one example, UV irradiation of titanium dioxide (which is the oxide naturally occurring on titanium surfaces) reduced SP mass compared to untreated surfaces [158] as photoactivation of the surfaces produced free radical and anionic oxygen species that affected protein/substrate interactions.
While one goal in mediating blood interactions with biomaterial surfaces has been to hinder protein adsorption [159], another approach has focused on the selective adsorption of blood components that can promote bioactivity. One example of this is selecting specifically for fibrinogen adsorption to decrease cytotoxicity of carbon nanotubules [160]. In a similar manner, harnessing the SP formation can represent a viable option to enhance the functionality of newly designed dental biomaterials. New dental biomaterials could be designed so they promote adsorption of particular molecules that reduce bacterial adherence, select for specific bacterial strains and/or trigger positive cell actions, such as reduced inflammation. Such an approach recognizes the seemingly inevitability of salivary fouling of the dental biomaterial and attempts to harness it. However, modulation of adsorption of specific SP components presents itself as a difficult task due to the large number of salivary proteins competing for adsorption to form the SP. Thus, this approach has been minimally explored [84]. Advances in proteomics [141][142], a deeper understanding of the structure and dynamics of SPs on synthetic substrates, and knowledge of the feedback mechanisms between SP and oral biological agents [143] should enable the design of new dental biomaterials that govern SP formation with optimal functionality.
Conclusions
The salivary pellicle acts as a mediator of interactions between surfaces in the oral cavity, including dental biomaterial surfaces, and biological and mechanical agents in the mouth. Differences in dental biomaterials have been shown to influence salivary pellicle composition and structure and define subsequent interactions critical to dental biomaterial function. Despite the relevance of the formation of SPs on dental biomaterials, many factors remain unexplored for SP composition, structure, and functions on dental biomaterials compared to dentition. Advances in this field should enable the design of better engineered dental biomaterials that harness the salivary pellicle for tailoring dental biomaterial function and improving patient outcomes in dentistry.
Highlights.
We review salivary pellicle formation on dental biomaterial surfaces.
Pellicle measurement methods and factors that affect formation are summarized.
We survey effects salivary pellicles have on dental biomaterial function.
We finally highlight implications for dental biomaterial design.
Acknowledgements
NGF acknowledges support from NIH-NIDCR T90-DE0227232 and a 3M Science and Technology Fellowship. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Interests
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- [1].Koulourides T, Feagin F, Pigman W, Remineralization of Dental Enamel By Saliva in Vitro, Ann. N. Y. Acad. Sci. 131 (1965) 751–757. doi: 10.1111/j.1749-6632.1965.tb34839.x. [DOI] [PubMed] [Google Scholar]
- [2].Bongaerts JHH, Rossetti D, Stokes JR, The Lubricating Properties of Human Whole Saliva, Tribol. Lett. 27 (2007) 277–287. doi: 10.1007/s11249-007-9232-y. [DOI] [Google Scholar]
- [3].Mandel ID, The Functions of Saliva, J. Dent. Res. 66 (1987) 623–627. doi: 10.1177/00220345870660S203. [DOI] [PubMed] [Google Scholar]
- [4].Levine MJ, Development of artificial salivas., Crit. Rev. Oral Biol. Med. 4 (1993) 279–286. doi: 10.1177/10454411930040030401. [DOI] [PubMed] [Google Scholar]
- [5].Carpenter GH, The Secretion, Components, and Properties of Saliva, Annu. Rev. Food Sci. Technol. 4 (2013) 267–276. doi: 10.1146/annurev-food-030212-182700. [DOI] [PubMed] [Google Scholar]
- [6].Lendenmann U, Grogan J, Oppenheim FG, Saliva and dental pellicle-A review, Adv. Dent. Res. 14 (2000) 22–28. doi: 10.1177/08959374000140010301. [DOI] [PubMed] [Google Scholar]
- [7].Humphrey SP, Williamson RT, A review of saliva: Normal composition, flow, and function, J. Prosthet. Dent. 85 (2001) 162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- [8].Sarkar A, Xu F, Lee S, Human saliva and model saliva at bulk to adsorbed phases – similarities and differences, Adv. Colloid Interface Sci. 273 (2019) 102034. doi: 10.1016/j.cis.2019.102034. [DOI] [PubMed] [Google Scholar]
- [9].Schweigel H, Wicht M, Schwendicke F, Salivary and pellicle proteome: A datamining analysis, Sci. Rep. 6 (2016) 1–12. doi: 10.1038/srep38882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nasmyth A, On the structure, physiology, and pathology of the persistent capsular investments and pulp of the tooth, Trans R Med Chir Soc L. 22 (1839) 310–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dawes C, The nomenclature of the integuments of enamel surface tooth, Braz. Dent. J. 115 (1963) 65–68. [Google Scholar]
- [12].Aguirre A, Mendoza B, Levine M, In vitro characterization of human salivary lubrication, Arch. Oral …. 34 (1989) 615–617. http://www.sciencedirect.com/science/article/pii/0003996989900241. [DOI] [PubMed] [Google Scholar]
- [13].Vukosavljevic D, Custodio W, Buzalaf MAR, Hara AT, Siqueira WL, Acquired pellicle as a modulator for dental erosion, Arch. Oral Biol. 59 (2014) 631–638. doi: 10.1016/j.archoralbio.2014.02.002. [DOI] [PubMed] [Google Scholar]
- [14].Hannig M, Joiner A, The Structure, Function and Properties of the Acquired Pellicle, Teeth Their Environ. 19 (2005) 29–64. doi: 10.1159/000090585. [DOI] [PubMed] [Google Scholar]
- [15].Bennick A, The binding of calcium to a salivary phosphoprotein, protein C, and comparison with calcium binding to protein A, a related salivary phosphoprotein., Biochem. J. 163 (1977) 241–5. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1164689&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Trautmann S, Barghash A, Fecher-Trost C, Schalkowsky P, Hannig C, Kirsch J, Rupf S, Keller A, Helms V, Hannig M, Proteomic Analysis of the Initial Oral Pellicle in Caries-Active and Caries-Free Individuals, Proteomics - Clin. Appl. 1800143 (2018) 1–11. doi: 10.1002/prca.201800143. [DOI] [PubMed] [Google Scholar]
- [17].Luo J, Wang Y, Wang K, Jiang W, Li X, Zhang L, Comparative proteomic analysis on acquired enamel pellicle at two time points in caries-susceptible and caries-free subjects, J. Dent. 94 (2020). doi: 10.1016/j.jdent.2020.103301. [DOI] [PubMed] [Google Scholar]
- [18].Zentner A, Heaney TG, An in vitro investigation of the role of high molecular weight human salivary sulphated glycoprotein in periodontal wound healing, J. Periodontol. 66 (1995) 944–955. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8558395. [DOI] [PubMed] [Google Scholar]
- [19].Sundararaj D, Venkatachalapathy S, Tandon A, Pereira A, Critical evaluation of incidence and prevalence of white spot lesions during fixed orthodontic appliance treatment: A meta-analysis, J. Int. Soc. Prev. Community Dent. 5 (2015) 433. doi: 10.4103/2231-0762.167719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Behr M, Zeman F, Baitinger T, Galler J, Koller M, Handel G, Rosentritt M, The Clinical Performance of Porcelain-Fused-to-Metal Precious Alloy Single Crowns: Chipping, Recurrent Caries, Periodontitis, and Loss of Retention, Int. J. Prosthodont. 27 (2014) 153–160. doi: 10.11607/ijp.3440. [DOI] [PubMed] [Google Scholar]
- [21].Yu H, Cheng S, Jiang N, Cheng H, Effects of cyclic staining on the color, translucency, surface roughness, and substance loss of contemporary adhesive resin cements, J. Prosthet. Dent. 120 (2018) 462–469. doi: 10.1016/j.prosdent.2017.10.009. [DOI] [PubMed] [Google Scholar]
- [22].Sterzenbach T, Helbig R, Hannig C, Hannig M, Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications, Clin. Oral Investig. (2020). doi: 10.1007/s00784-020-03646-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Graves DT, Corrêa JD, Silva TA, The Oral Microbiota Is Modified by Systemic Diseases, J. Dent. Res. 98 (2019) 148–156. doi: 10.1177/0022034518805739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schulz A, Lang R, Behr J, Hertel S, Reich M, Kümmerer K, Hannig M, Hannig C, Hofmann T, Targeted metabolomics of pellicle and saliva in children with different caries activity, Sci. Rep 10 (2020) 1–11. doi: 10.1038/s41598-020-57531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lindh L, Aroonsang W, Sotres J, Arnebrant T, Salivary Pellicles, in: Saliva Secret. Funct, 2014: pp. 30–39. doi: 10.1159/000358782. [DOI] [PubMed] [Google Scholar]
- [26].Hannig M, Ultrastructural investigation of pellicle morphogenesis at two different intraoral sites during a 24-h period., Clin. Oral Investig. 3 (1999) 88–95. doi: 10.1007/s007840050084. [DOI] [PubMed] [Google Scholar]
- [27].Juriaanse AC, Booij M, Arends J, te JJ. Bosch, The adsorption in vitro of purified salivary proteins on bovine dental enamel, Arch. Oral Biol. 26 (1981) 91–96. doi: 10.1016/0003-9969(81)90076-5. [DOI] [PubMed] [Google Scholar]
- [28].Hannig M, Transmission electron microscopic study of in vivo pellicle formation on dental restorative materials, Eur. J. Oral Sci. 105 (1997) 422–433. doi: 10.1111/j.1600-0722.1997.tb02139.x. [DOI] [PubMed] [Google Scholar]
- [29].Xu L-C, Bauer JW, Siedlecki CA, Proteins, platelets, and blood coagulation at biomaterial interfaces, Colloids Surfaces B Biointerfaces. 124 (2014) 49–68. doi: 10.1016/j.colsurfb.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hirsh SL, Mckenzie DR, Nosworthy NJ, Denman JA, Sezerman OU, Bilek MMM, The Vroman effect : Competitive protein exchange with dynamic multilayer protein aggregates, Colloids Surfaces B Biointerfaces. 103 (2013) 395–404. doi: 10.1016/j.colsurfb.2012.10.039. [DOI] [PubMed] [Google Scholar]
- [31].Roach P, Farrar D, Perry CC, Interpretation of protein adsorption: Surface-induced conformational changes, J. Am. Chem. Soc. 127 (2005) 8168–8173. doi: 10.1021/ja042898o. [DOI] [PubMed] [Google Scholar]
- [32].Brash JL, Horbett TA, Latour RA, Tengvall P, The blood compatibility challenge Part 2: protein adsorption phenomena governing blood reactivity, Acta Biomater. (2019). doi: 10.1016/j.actbio.2019.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cárdenas M, Arnebrandt T, Rennie A, Fragneto G, Thomas RK, Lindh L, Human saliva forms a complex film structure on alumina surfaces, Biomacromolecules. 8 (2007) 65–69. doi: 10.1021/bm060492t. [DOI] [PubMed] [Google Scholar]
- [34].Lee YH, Zimmerman JN, Custodio W, Xiao Y, Basiri T, Hatibovic-Kofman S, Siqueira WL, Proteomic Evaluation of Acquired Enamel Pellicle during In Vivo Formation, PLoS One. 8 (2013). doi: 10.1371/journal.pone.0067919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hannig M, Khanafer AK, Hoth-Hannig W, Al-Marrawi F, Açil Y, Transmission electron microscopy comparison of methods for collecting in situ formed enamel pellicle, Clin. Oral Investig. 9 (2005) 30–37. doi: 10.1007/s00784-004-0284-1. [DOI] [PubMed] [Google Scholar]
- [36].RA R, Biomaterials: Protein-surface interactions, in: Encycl. Biomater. Bioeng, 2nd ed., Informa Healthcare, New York, NY, 2008: pp. 270–84. [Google Scholar]
- [37].BD R, Role of water in biomaterials, in: Biomater. Sci. An Introd. to Mater. Med, 3rd ed., Academic Press, Waltham, MA, 2013: pp. 55–9. [Google Scholar]
- [38].Norde W, Drive forces for protein adsoprtion at solid interfaces, Macromoecular Symp. 18 (1996) 5–18. [Google Scholar]
- [39].Lee HS, Myers C, Zaidel L, Nalam PC, Caporizzo MA, Daep CA, Eckmann DM, Masters JG, Composto RJ, Competitive Adsorption of Polyelectrolytes onto and into Pellicle-Coated Hydroxyapatite Investigated by QCM-D and Force Spectroscopy, ACS Appl. Mater. Interfaces. 9 (2017) 13079–13091. doi: 10.1021/acsami.7b02774. [DOI] [PubMed] [Google Scholar]
- [40].Vassilakos N, Arnebrant T, Glantz P-O, Adsorption of whole saliva onto hydrophilic and hydrophobic solid surfaces: influence of concentration, ionic strength and pH, Eur. J. Oral Sci. 100 (1992) 346–353. doi: 10.1111/j.1600-0722.1992.tb01085.x. [DOI] [PubMed] [Google Scholar]
- [41].Yao Y, Lamkin MS, Oppenheim EG, Pellicle precursor proteins: acidic proline-rich proteins, statherin, and histatins, and their crosslinking reaction by oral transglutaminase, J. Dent. Res. 78 (1999) 1696–1703. doi: 10.1177/00220345990780110601. [DOI] [PubMed] [Google Scholar]
- [42].Nakanishi K, Sakiyama T, Imamura K, On the adsorption of proteins on solid surfaces, a common but very complicated phenomenon, J. Biosci. Bioeng. 91 (2001) 233–244. doi: 10.1016/S1389-1723(01)80127-4. [DOI] [PubMed] [Google Scholar]
- [43].Wang Z, Yan Y, Qiao L, Protein adsorption on implant metals with various deformed surfaces, Colloids Surfaces B Biointerfaces. 156 (2017) 62–70. doi: 10.1016/j.colsurfb.2017.05.015. [DOI] [PubMed] [Google Scholar]
- [44].Rabe M, Verdes D, Seeger S, Understanding protein adsorption phenomena at solid surfaces, Adv. Colloid Interface Sci. 162 (2011) 87–106. doi: 10.1016/j.cis.2010.12.007. [DOI] [PubMed] [Google Scholar]
- [45].Shahal Y, Steinberg D, Hirschfeld Z, Bronshteyn M, Kopolovic K, In vitro bacterial adherence onto pellicle-coated aesthetic restorative materials., J. Oral Rehabil. 25 (1998) 52–58. [DOI] [PubMed] [Google Scholar]
- [46].Sönju T, Glantz PO, Chemical composition of salivary integuments formed in vivo on solids with some established surface characteristics, Arch. Oral Biol. 20 (1975) 687–691. doi: 10.1016/0003-9969(75)90138-7. [DOI] [PubMed] [Google Scholar]
- [47].Oste R, Ronstrom A, Birkhed D, Edwardsson S, Stenberg M, Gas-liquid chromatographic analysis of amino acids in pellicle formed on tooth surface and plastic film in vivo, Arch. Oral Biol. 26 (1981) 635–641. http://www.ncbi.nlm.nih.gov/pubmed/6947769. [DOI] [PubMed] [Google Scholar]
- [48].Gocke R, Gerath F, von Schwanewede H, Quantitative determination of salivary components in the pellicle on PMMA denture base material, Clin. Oral Investig. 6 (2002) 227–235. doi: 10.1007/s00784-002-0176-1. [DOI] [PubMed] [Google Scholar]
- [49].Svendsen IE, Lindh L, The composition of enamel salivary films is different from the ones formed on dental materials, Biofouling. 25 (2009) 255–261. doi: 10.1080/08927010802712861. [DOI] [PubMed] [Google Scholar]
- [50].Davidi MP, Beyth N, Weiss EI, Weiss EI, Eilat Y, Feuerstein O, Sterer N, Effect of liquid-polish coating on in vitro biofilm accumulation on provisional restorations: Part 2, Quintessence Int. (Berl). 39 (2008) 45–49. [PubMed] [Google Scholar]
- [51].Schweikl H, Hiller KA, Carl U, Schweiger R, Eidt A, Ruhl S, Müller R, Schmalz G, Salivary protein adsorption and Streptococccus gordonii adhesion to dental material surfaces, Dent. Mater. 29 (2013) 1080–1089. doi: 10.1016/j.dental.2013.07.021. [DOI] [PubMed] [Google Scholar]
- [52].Hannig C, Wasser M, Becker K, Hannig M, Huber K, Attin T, Influence of different restorative materials on lysozyme and amylase activity of the salivary pelliclein situ, J. Biomed. Mater. Res. Part A. 78A (2006) 755–761. doi: 10.1002/jbm.a.30758. [DOI] [PubMed] [Google Scholar]
- [53].Pelá VT, Prakki A, Wang L, Ventura TMS, de Souza e Silva CM, Cassiano LPS, Brianezzi LFF, Leite AL, Buzalaf MAR, The influence of fillers and protease inhibitors in experimental resins in the protein profile of the acquired pellicle formed in situ on enamel-resin specimens, Arch. Oral Biol. 108 (2019) 104527. doi: 10.1016/j.archoralbio.2019.104527. [DOI] [PubMed] [Google Scholar]
- [54].Carlén A, Nikdel K, Wennerberg A, Holmberg K, Olsson J, Surface characteristics and in vitro biofilm formation on glass ionomer and composite resin, Biomaterials. 22 (2001) 481–487. doi: 10.1016/S0142-9612(00)00204-0. [DOI] [PubMed] [Google Scholar]
- [55].Han A, Tsoi JKH, Rodrigues FP, Leprince JG, Palin WM, Bacterial adhesion mechanisms on dental implant surfaces and the influencing factors, Int. J. Adhes. Adhes. 69 (2016) 58–71. doi: 10.1016/j.ijadhadh.2016.03.022. [DOI] [Google Scholar]
- [56].Bürgers R, Hahnel S, Reichert TE, Rosentritt M, Behr M, Gerlach T, Handel G, Gosau M, Adhesion of Candida albicans to various dental implant surfaces and the influence of salivary pellicle proteins, Acta Biomater. 6 (2010) 2307–2313. doi: 10.1016/j.actbio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- [57].Al-Radha ASD, Dymock D, Younes C, O’Sullivan D, Surface properties of titanium and zirconia dental implant materials and their effect on bacterial adhesion, J. Dent. 40 (2012) 146–153. doi: 10.1016/j.jdent.2011.12.006. [DOI] [PubMed] [Google Scholar]
- [58].Zuanazzi D, Xiao Y, Siqueira WL, Evaluating protein binding specificity of titanium surfaces through mass spectrometry–based proteomics, Clin. Oral Investig. (2020). doi: 10.1007/s00784-020-03548-2. [DOI] [PubMed] [Google Scholar]
- [59].Pantaroto HN, Amorim KP, Matozinho Cordeiro J, Souza JGS, Ricomini-Filho AP, Rangel EC, Ribeiro ALR, Vaz LG, Barão VAR, Proteome analysis of the salivary pellicle formed on titanium alloys containing niobium and zirconium, Biofouling. 0 (2019) 1–14. doi: 10.1080/08927014.2019.1580360. [DOI] [PubMed] [Google Scholar]
- [60].Mukai Y, Torii M, Urushibara Y, Kawai T, Takahashi Y, Maeda N, Ohkubo C, Ohshima T, Analysis of plaque microbiota and salivary proteins adhering to dental materials, J. Oral Biosci. (2020). doi: 10.1016/j.job.2020.02.003. [DOI] [PubMed] [Google Scholar]
- [61].Cavalcanti YW, Soares RV, Assis MAL, Zenóbio EG, da Silva Girundi FM, Titanium Surface Roughing Treatments contribute to Higher Interaction with Salivary Proteins MG2 and Lactoferrin, J. Contemp. Dent. Pract. 16 (2015) 141–146. doi: 10.5005/jp-journals-10024-1651. [DOI] [PubMed] [Google Scholar]
- [62].Lee SJ, Kho HS, Lee SW, Yang WS, Experimental salivary pellicles on the surface of orthodontic materials, Am. J. Orthod. Dentofac. Orthop. 119 (2001) 59–66. doi: 10.1067/mod.2001.110583. [DOI] [PubMed] [Google Scholar]
- [63].Sang T, Ye Z, Fischer NG, Skoe EP, Echeverría C, Wu J, Aparicio C, Physical-chemical interactions between dental materials surface, salivary pellicle and Streptococcus gordonii, Colloids Surfaces B Biointerfaces. 190 (2020). doi: 10.1016/j.colsurfb.2020.110938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Yao Y, Grogan J, Zehnder M, Lendenmann U, Nam B, Wu Z, Costello CE, Oppenheim FG, Compositional analysis of human acquired enamel pellicle by mass spectrometry, Arch. Oral Biol. 46 (2001) 293–303. doi: 10.1016/S0003-9969(00)00134-5. [DOI] [PubMed] [Google Scholar]
- [65].Zimmermann R, Delius J, Friedrichs J, Stehl S, Hofmann T, Hannig C, Rehage M, Werner C, Hannig M, Impact of oral astringent stimuli on surface charge and morphology of the protein-rich pellicle at the tooth–saliva interphase, Colloids Surfaces B Biointerfaces. 174 (2019) 451–458. doi: 10.1016/j.colsurfb.2018.11.028. [DOI] [PubMed] [Google Scholar]
- [66].Svendsen IE, Lindh L, Arnebrant T, Adsorption behaviour and surfactant elution of cationic salivary proteins at solid/liquid interfaces, studied by in situ ellipsometry, Colloids Surfaces B Biointerfaces. 53 (2006) 157–166. doi: 10.1016/j.colsurfb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- [67].Gibbins HL, Yakubov GE, Proctor GB, Wilson S, Carpenter GH, What interactions drive the salivary mucosal pellicle formation?, Colloids Surfaces B Biointerfaces. 120 (2014) 184–192. doi: 10.1016/j.colsurfb.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ash A, Burnett GR, Parker R, Ridout MJ, Rigby NM, Wilde PJ, Structural characterisation of parotid and whole mouth salivary pellicles adsorbed onto DPI and QCMD hydroxyapatite sensors, Colloids Surfaces B Biointerfaces. 116 (2014) 603–611. doi: 10.1016/j.colsurfb.2013.10.024. [DOI] [PubMed] [Google Scholar]
- [69].Delius J, Trautmann S, Médard G, Kuster B, Hannig M, Hofmann T, Label-free quantitative proteome analysis of the surface-bound salivary pellicle, Colloids Surfaces B Biointerfaces. 152 (2017) 68–76. doi: 10.1016/j.colsurfb.2017.01.005. [DOI] [PubMed] [Google Scholar]
- [70].Länge K, Herold M, Scheideler L, ürgen Geis-Gerstorfer J, Wendel HP, ünter Gauglitz G, Investigation of initial pellicle formation on modified titanium dioxide (TiO2) surfaces by reflectometric interference spectroscopy (RIfS) in a model system, Dent. Mater. 20 (2004) 814–822. doi: 10.1016/j.dental.2003.10.010. [DOI] [PubMed] [Google Scholar]
- [71].Custodio W, Silva WJ, Paes Leme AF, Cury JA, Del Bel Cury AA, Plasma proteins in the acquired denture pellicle enhance substrate surface free energy and Candida albicans phospholipase and proteinase activities, J. Investig. Clin. Dent. 6 (2015) 273–281. doi: 10.1111/jicd.12101. [DOI] [PubMed] [Google Scholar]
- [72].Carlén A, Börjesson AC, Nikdel K, Olsson J, Composition of pellicles formed in vivo on tooth surfaces in different parts of the dentition, and in vitro on hydroxyapatite, Caries Res. 32 (1998) 447–55. doi: 10.1159/000016486. [DOI] [PubMed] [Google Scholar]
- [73].Scheideler L, Rupp F, Wendel HP, Sathe S, Geis-Gerstorfer J, Photocoupling of fibronectin to titanium surfaces influences keratinocyte adhesion, pellicle formation and thrombogenicity, Dent. Mater. 23 (2007) 469–478. doi: 10.1016/j.dental.2006.03.005. [DOI] [PubMed] [Google Scholar]
- [74].Zeng Q, Zheng L, Zhou J, Xiao H, Zheng J, Zhou Z, Effect of alcohol stimulation on salivary pellicle formation on human tooth enamel surface and its lubricating performance, J. Mech. Behav. Biomed. Mater. 75 (2017) 567–573. doi: 10.1016/j.jmbbm.2017.05.029. [DOI] [PubMed] [Google Scholar]
- [75].Amaechi BT, Higham SM, Edgar WM, Milosevic A, Thickness of acquired salivary pellicle as a determinant of the sites of dental erosion, J. Dent. Res. 78 (1999) 1821–1828. doi: 10.1177/00220345990780120901. [DOI] [PubMed] [Google Scholar]
- [76].Kraus FW, Orstavik D, Hurst DC, Cook CH, The acquired pellicle: variability and subject-dependence of specific proteins., J. Oral Pathol. 2 (1973) 165–73. http://www.ncbi.nlm.nih.gov/pubmed/4207799. [DOI] [PubMed] [Google Scholar]
- [77].Rupp F, Haupt M, Eichler M, Doering C, Klostermann H, Scheideler L, Lachmann S, Oehr C, Wendel HP, Decker E, Geis-Gerstorfer J, Von Ohle C, Formation and photocatalytic decomposition of a pellicle on anatase surfaces, J. Dent. Res. 91 (2012) 104–109. doi: 10.1177/0022034511424901. [DOI] [PubMed] [Google Scholar]
- [78].Zimmerman JN, Custodio W, Hatibovic-Kofman S, Lee YH, Xiao Y, Siqueira WL, Proteome and peptidome of human acquired enamel pellicle on deciduous teeth, Int. J. Mol. Sci. 14 (2013) 920–934. doi: 10.3390/ijms14010920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Dorkhan M, Svensäter G, Davies JR, Salivary pellicles on titanium and their effect on metabolic activity in Streptococcus oralis., BMC Oral Health. 13 (2013) 32. doi: 10.1186/1472-6831-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Siqueira WL, Zhang W, Helmerhorst EJ, Gygi SP, Oppenheim FG, Identification of Protein Components in in vivo Human Acquired Enamel Pellicle Using LC−ESI−MS/MS, J. Proteome Res. 6 (2007) 2152–2160. doi: 10.1021/pr060580k. [DOI] [PubMed] [Google Scholar]
- [81].Deimling D, Hannig C, Hoth-Hannig W, Schmitz P, Schulte-Mönting J, Hannig M, Non-destructive visualisation of protective proteins in the in situ pellicle, Clin. Oral Investig. 11 (2007) 211–216. doi: 10.1007/s00784-007-0112-5. [DOI] [PubMed] [Google Scholar]
- [82].Hannig C, Ruggeri A, Al-Khayer B, Schmitz P, Spitzmüller B, Deimling D, Huber K, Hoth-Hannig W, Bowen WH, Hannig M, Electron microscopic detection and activity of glucosyltransferase B, C, and D in the in situ formed pellicle, Arch. Oral Biol. 53 (2008) 1003–1010. doi: 10.1016/j.archoralbio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- [83].Schüpbach P, Oppenheim FG, Lendenmann U, Lamkin MS, Yao Y, Guggenheim B, Electron-microscopic demonstration of proline-rich proteins, statherin, and histatins in acquired enamel pellicles in vitro., Eur. J. Oral Sci. 109 (2001) 60–68. doi: 10.1034/j.1600-0722.2001.00925.x. [DOI] [PubMed] [Google Scholar]
- [84].Cárdenas M, Elofsson U, Lindh L, Salivary mucin MUC5B could be an important component of in vitro pellicles of human saliva: An in situ ellipsometry and atomic force microscopy study, Biomacromolecules. 8 (2007) 1149–1156. doi: 10.1021/bm061055h. [DOI] [PubMed] [Google Scholar]
- [85].Milosevic A, The influence of surface finish and in-vitro pellicle on contact-angle measurement and surface morphology of three commercially available composite restoratives, J. Oral Rehabil. 19 (1992) 85–97. doi: 10.1111/j.1365-2842.1992.tb01593.x. [DOI] [PubMed] [Google Scholar]
- [86].Hannig M, Döbert A, Stigler R, Müller U, Prokhorova SA, Initial salivary pellicle formation on Solid substrates studied by AFM, J. Nanosci. Nanotechnol. 4 (2004) 532–538. doi: 10.1166/jnn.2004.082. [DOI] [PubMed] [Google Scholar]
- [87].Sotres J, Pettersson T, Lindh L, Arnebrant T, Nanowear of salivary films vs. substratum wettability, J. Dent. Res. 91 (2012) 973–978. doi: 10.1177/0022034512456704. [DOI] [PubMed] [Google Scholar]
- [88].Sotres J, Lindh L, Arnebrant T, Friction force spectroscopy as a tool to study the strength and structure of salivary films, Langmuir. 27 (2011) 13692–13700. doi: 10.1021/la202870c. [DOI] [PubMed] [Google Scholar]
- [89].Hertel S, Schulz A, Lang R, Hofmann T, König B, Hannig M, Hannig C, Activity and distribution pattern of enzymes in the in-situ pellicle of children, Arch. Oral Biol. 104 (2019) 24–32. doi: 10.1016/j.archoralbio.2019.05.017. [DOI] [PubMed] [Google Scholar]
- [90].Lima EMCX, Koo H, Vacca Smith AM, Rosalen PL, Del Bel Cury AA, Adsorption of salivary and serum proteins, and bacterial adherence on titanium and zirconia ceramic surfaces, Clin. Oral Implants Res. 19 (2008) 780–785. doi: 10.1111/j.1600-0501.2008.01524.x. [DOI] [PubMed] [Google Scholar]
- [91].Svendsen IE, Arnebrant T, Lindh L, Validation of mechanically-assisted sodium dodecyl-sulphate elution as a technique to remove pellicle protein components from human enamel, Biofouling. 24 (2008) 227–233. doi: 10.1080/08927010802018277. [DOI] [PubMed] [Google Scholar]
- [92].Dawes C, Salivary flow patterns and the health of hard and soft oral tissues, J. Am. Dent. Assoc. 139 (2008) 18S–24S. doi: 10.14219/jada.archive.2008.0351. [DOI] [PubMed] [Google Scholar]
- [93].Rudiger SG, Carlen A, Meurman JH, Kari K, Olsson J, Dental biofilms at healthy and inflamed gingival margins, J. Clin. Periodontol. 29 (2002) 524–530. doi:cpe290609 [pii]. [DOI] [PubMed] [Google Scholar]
- [94].Ferguson DB, Botchway CA, A comparison of circadian variation in the flow rate and composition of stimulated human parotid, submandibular and whole salivas from the same individuals, Arch. Oral Biol. 25 (1980) 559–568. doi: 10.1016/0003-9969(80)90068-0. [DOI] [PubMed] [Google Scholar]
- [95].Morge S, Adamczak E, Lindén LÅ, Variation in human salivary pellicle formation on biomaterials during the day, Arch. Oral Biol. 34 (1989) 669–674. doi: 10.1016/0003-9969(89)90023-X. [DOI] [PubMed] [Google Scholar]
- [96].Kensche A, Dürasch A, König B, Henle T, Hannig C, Hannig M, Characterization of the in situ pellicle ultrastructure formed under the influence of bovine milk and milk protein isolates, Arch. Oral Biol. 104 (2019) 133–140. doi: 10.1016/j.archoralbio.2019.05.021. [DOI] [PubMed] [Google Scholar]
- [97].Embery G, Heaney TG, Stanbury JB, Studies on the organic polyanionic constituents of human acquired dental pellicle, Arch. Oral Biol. 31 (1986) 623–625. doi: 10.1016/0003-9969(86)90087-7. [DOI] [PubMed] [Google Scholar]
- [98].Al-Hashimi I, Levine MJ, Characterization of in vivo salivary-derived enamel pellicle, Arch. Oral Biol. 34 (1989) 289–295. doi: 10.1016/0003-9969(89)90070-8. [DOI] [PubMed] [Google Scholar]
- [99].Siqueira WL, Oppenheim FG, Small molecular weight proteins/peptides present in the in vivo formed human acquired enamel pellicle, Arch. Oral Biol. 54 (2009) 437–444. doi: 10.1016/j.archoralbio.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Sönju T, Christensen TB, Kornstad L, Rölla G, Electron Microscopy, Carbohydrate Analyses and Biological Activities of the Proteins Adsorbed in Two Hours to Tooth Surfaces in vivo, Caries Res. 8 (1974) 113–122. doi: 10.1159/000260099. [DOI] [PubMed] [Google Scholar]
- [101].Li J, Helmerhorst E, Yao Y, Nunn M, Troxler R, Oppenheim F, Statherin is an in vivo pellicle constituent: identification and immuno-quantification, Arch. Oral Biol. 49 (2004) 379–385. doi: 10.1016/j.archoralbio.2004.01.002. [DOI] [PubMed] [Google Scholar]
- [102].Yao Y, Berg EA, Costello CE, Troxler RF, Oppenheim FG, Identification of protein components in human acquired enamel pellicle and whole saliva using novel proteomics approaches, J. Biol. Chem. 278 (2003) 5300–5308. doi: 10.1074/jbc.M206333200. [DOI] [PubMed] [Google Scholar]
- [103].Siqueira WL, Margolis HC, Helmerhorst EJ, Mendes FM, Oppenheim FG, Evidence of Intact Histatins in the in vivo Acquired Enamel Pellicle, J. Dent. Res. 89 (2010) 626–630. doi: 10.1177/0022034510363384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Armstrong WG, Amino-acid Composition of the Acquired Pellicle of Human Tooth Enamel, Nature. 210 (1966) 197–198. doi: 10.1038/210197a0. [DOI] [PubMed] [Google Scholar]
- [105].Sønju T, Rølla G, Chemical analysis of the acquired pellicle formed in two hours on cleaned human teeth in vivo. Rate of formation and amino acid analysis., Caries Res. 7 (1973) 30–8. http://www.ncbi.nlm.nih.gov/pubmed/4509045. [DOI] [PubMed] [Google Scholar]
- [106].Eggen KH, Rolla G, Gel filtration, ion exchange chromatography and chemical analysis of macromolecules present in acquired anamel pellicle (2-hour-pellicle), Eur. J. Oral Sci. 90 (1982) 182–188. doi: 10.1111/j.1600-0722.1982.tb00725.x. [DOI] [PubMed] [Google Scholar]
- [107].Rykke M, Sonju T, Rolla G, Interindividual and longitudinal studies of amino acid composition of pellicle collected in vivo, Scand J Dent Res. (1990) 29–34. [DOI] [PubMed] [Google Scholar]
- [108].Sønju Clasen AB, Hannig M, Skjørland K, Sønju T, Analytical and ultrastructural studies of pellicle on primary teeth., Acta Odontol. Scand. 55 (1997) 339–43. http://www.ncbi.nlm.nih.gov/pubmed/9477025. [DOI] [PubMed] [Google Scholar]
- [109].Li F, Weir MD, Fouad AF, Xu HHK, Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model, Dent. Mater. 30 (2014) 182–191. doi: 10.1016/j.dental.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Müller R, Eidt A, Hiller KA, Katzur V, Subat M, Schweikl H, Imazato S, Ruhl S, Schmalz G, Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers, Biomaterials. 30 (2009) 4921–4929. doi: 10.1016/j.biomaterials.2009.05.079. [DOI] [PubMed] [Google Scholar]
- [111].Zaltsman N, Ionescu AC, Weiss EI, Brambilla E, Beyth S, Beyth N, Surface-modified nanoparticles as anti-biofilm filler for dental polymers, PLoS One. 12 (2017) e0189397. doi: 10.1371/journal.pone.0189397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hannig M. s., Hannig C, The Pellicle and Erosion, Erosive Tooth Wear From Diagnosis to Ther. 25 (2012) 206–214. doi: 10.1159/000360376. [DOI] [Google Scholar]
- [113].Hannig M, The protective nature of the salivary pellicle, Int. Dent. J. 52 (2002) 417–423. doi: 10.1111/j.1875-595X.2002.tb00731.x. [DOI] [Google Scholar]
- [114].De Almeida PDV, Grégio AMT, Machado MÂN, De Lima AAS, Azevedo LR, Saliva composition and functions: A comprehensive review, J. Contemp. Dent. Pract. 9 (2008) 072–080. doi:1526–3711-497 [pii]. [PubMed] [Google Scholar]
- [115].Hahn Berg IC, Rutland MW, Arnebrant T, Lubricating Properties of the Initial Salivary Pellicle - An AFM Study, Biofouling. 19 (2003) 365–369. doi: 10.1080/08927010310001618571. [DOI] [PubMed] [Google Scholar]
- [116].Harvey NM, Yakubov GE, Stokes JR, Klein J, Lubrication and load-bearing properties of human salivary pellicles adsorbed ex vivo on molecularly smooth substrata, Biofouling. 28 (2012) 843–856. doi: 10.1080/08927014.2012.714777. [DOI] [PubMed] [Google Scholar]
- [117].Prinz JF, de Wijk RA, Huntjens L, Load dependency of the coefficient of friction of oral mucosa, Food Hydrocoll. 21 (2007) 402–408. doi: 10.1016/j.foodhyd.2006.05.005. [DOI] [Google Scholar]
- [118].Ranc H, Elkhyat A, Servais C, Mac-Mary S, Launay B, Humbert P, Friction coefficient and wettability of oral mucosal tissue: Changes induced by a salivary layer, Colloids Surfaces A Physicochem. Eng. Asp. 276 (2006) 155–161. doi: 10.1016/j.colsurfa.2005.10.033. [DOI] [Google Scholar]
- [119].Ramasubbu N, Thomas LM, Bhandary KK, Levine MJ, Structural characteristics of human salivary statherin: a model for boundary lubrication at the enamel surface., Crit. Rev. Oral Biol. Med. 4 (1993) 363–70. http://www.ncbi.nlm.nih.gov/pubmed/8373992. [DOI] [PubMed] [Google Scholar]
- [120].Turssi CP, Faraoni JJ, De Menezes M, Serra MC, Analysis of potential lubricants for in vitro wear testing, Dent. Mater. 22 (2006) 77–83. doi: 10.1016/j.dental.2005.02.010. [DOI] [PubMed] [Google Scholar]
- [121].Wu YQ, Arsecularatne JA, Hoffman M, Effect of acidity upon attrition-corrosion of human dental enamel, J. Mech. Behav. Biomed. Mater. 44 (2015) 23–34. doi: 10.1016/j.jmbbm.2014.12.016. [DOI] [PubMed] [Google Scholar]
- [122].Tsujimoto A, Barkmeier WW, Fischer NG, Nojiri K, Nagura Y, Takamizawa T, Latta MA, Miazaki M, Wear of resin composites: Current insights into underlying mechanisms, evaluation methods and influential factors., Jpn. Dent. Sci. Rev. 54 (2018) 76–87. doi: 10.1016/j.jdsr.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Schwender N, Huber K, Al Marrawi F, Hannig M, Ziegler C, Initial bioadhesion on surfaces in the oral cavity investigated by scanning force microscopy, in: Appl. Surf. Sci, 2005: pp. 117–122. doi: 10.1016/j.apsusc.2005.02.042. [DOI] [Google Scholar]
- [124].Bowen WR, Hilal N, Lovitt RW, Wright CJ, Direct Measurement of Interactions between Adsorbed Protein Layers Using an Atomic Force Microscope, J. Colloid Interface Sci. 352 (1998) 348–352. [DOI] [PubMed] [Google Scholar]
- [125].Barrantes A, Arnebrant T, Lindh L, Characteristics of saliva films adsorbed onto different dental materials studied by QCM-D, Colloids Surfaces A Physicochem. Eng. Asp. 442 (2014) 56–62. doi: 10.1016/j.colsurfa.2013.05.054. [DOI] [Google Scholar]
- [126].Shellis RP, Addy M, The Interactions between Attrition, Abrasion and Erosion in Tooth Wear, Erosive Tooth Wear From Diagnosis to Ther. 25 (2012) 32–45. doi: 10.1159/000359936. [DOI] [PubMed] [Google Scholar]
- [127].Soares LES, Lima LR, de Souza Vieira L, do Espírito Santo AM, Martin AA, Erosion effects on chemical composition and morphology of dental materials and root dentin, Microsc. Res. Tech. 75 (2012) 703–710. doi: 10.1002/jemt.21115. [DOI] [PubMed] [Google Scholar]
- [128].Yu H, Wegehaupt FJ, Wiegand A, Roos M, Attin T, Buchalla W, Erosion and abrasion of tooth-colored restorative materials and human enamel, J. Dent. 37 (2009) 913–922. doi: 10.1016/j.jdent.2009.07.006. [DOI] [PubMed] [Google Scholar]
- [129].Francisconi LF, Honório HM, Rios D, Magalhães AC, Machado MAAM, Buzalaf MAR, Effect of Erosive pH Cycling on Different Restorative Materials and on Enamel Restored with These Materials, Oper. Dent. 33 (2008) 203–208. doi: 10.2341/07-77. [DOI] [PubMed] [Google Scholar]
- [130].Sieber KR, Schmidt C, Baumann T, Lussi A, Carvalho TS, Acquired Enamel Pellicle Modification with Casein and Mucin in Different Concentrations and its Impact on Initial Dental Erosion, Caries Res. (2019) 1–10. doi: 10.1159/000499579. [DOI] [PubMed] [Google Scholar]
- [131].Leinonen J, Parkkila A, Salivary Carbonic Anhydrase Isoenzyme VI Is Located in the Human Enamel Pellicle, (1999) 185–190. [DOI] [PubMed]
- [132].Magalhães AC, Wiegand A, Rios D, Honório HM, Buzalaf MAR, Insights into preventive measures for dental erosion, J. Appl. Oral Sci. 17 (2009) 75–86. doi: 10.1590/S1678-77572009000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Proctor GB, Hamdan S, Carpenter GH, Wilde P, A statherin and calcium enriched layer at the air interface of human parotid saliva, Biochem. J. 389 (2005) 111–116. doi: 10.1042/BJ20042012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Delvar A, Lindh L, Arnebrant T, Sotres J, Interaction of Polyelectrolytes with Salivary Pellicles on Hydroxyapatite Surfaces under Erosive Acidic Conditions, ACS Appl. Mater. Interfaces. 7 (2015) 21610–21618. doi: 10.1021/acsami.5b07118. [DOI] [PubMed] [Google Scholar]
- [135].Kosoric J, Williams RAD, Hector MP, Anderson P, A synthetic peptide based on a natural salivary protein reduces demineralisation in model systems for dental caries and erosion, Int. J. Pept. Res. Ther. 13 (2007) 497–503. doi: 10.1007/s10989-007-9085-0. [DOI] [Google Scholar]
- [136].Mutahar M, O’Toole S, Carpenter G, Bartlett D, Andiappan M, Moazzez R, Reduced statherin in acquired enamel pellicle on eroded teeth compared to healthy teeth in the same subjects: An in-vivo study, PLoS One. 12 (2017) 1–11. doi: 10.1371/journal.pone.0183660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Tin-Oo MM, Saddki N, Hassan N, Factors influencing patient satisfaction with dental appearance and treatments they desire to improve aesthetics, BMC Oral Health. 11 (2011) 6. doi: 10.1186/1472-6831-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Jones G, Chlorhexidine: is it still the gold standard ?, Periodontol. 2000. 15 (2000) 55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- [139].Nathoo SA, Gaffar A, Studies on Dental Stains Induced by Antibacterial Agents and Rational Approaches for Bleaching Dental Stains, Adv. Dent. Res. 9 (1995) 462–470. doi: 10.1177/08959374950090041801. [DOI] [Google Scholar]
- [140].Ellingsen JE, Rolla G, Eriksen HM, Extrinsic dental stain caused by chlorhexidine and other denaturing agents, J. Clin. Periodontol. 9 (1982) 317–322. doi: 10.1111/j.1600-051X.1982.tb02098.x. [DOI] [PubMed] [Google Scholar]
- [141].Lee YK, Powers JM, Influence of salivary organic substances on the discoloration of esthetic dental materials - A review, J. Biomed. Mater. Res. - Part B Appl. Biomater. 76 (2006) 397–402. doi: 10.1002/jbm.b.30380. [DOI] [PubMed] [Google Scholar]
- [142].Joiner A, Elofsson UM, Arnebrant T, Adsorption of chlorhexidine and black tea onto in vitro salivary pellicles, as studied by ellipsometry, Eur. J. Oral Sci. 114 (2006) 337–342. doi: 10.1111/j.1600-0722.2006.00364.x. [DOI] [PubMed] [Google Scholar]
- [143].Lu Y, Bennick A, Interaction of tannin with human salivary proline-rich proteins, Arch. Oral Biol. 43 (1998) 717–728. doi: 10.1016/S0003-9969(98)00040-5. [DOI] [PubMed] [Google Scholar]
- [144].Versluis A, Tantbirojn D, Douglas WH, Why do shear bond tests pull out dentin?, J. Dent. Res. 76 (1997) 1298–1307. doi: 10.1177/00220345970760061001. [DOI] [PubMed] [Google Scholar]
- [145].Sheen S, Banfield N, Addy M, The effect of unstimulated and stimulated whole saliva on extrinsic staining in vitro - A developmental method, J. Dent. 30 (2002) 365–369. doi: 10.1016/S0300-5712(02)00053-2. [DOI] [PubMed] [Google Scholar]
- [146].Sheen S, Banfield N, Addy M, The propensity of individual saliva to cause extrinsic staining in vitro--a developmental method., J. Dent. 29 (2001) 99–102. doi: 10.1016/S0300-5712(00)00062-2. [DOI] [PubMed] [Google Scholar]
- [147].Jagger DC, Al-Akhazam L, Harrison A, Rees JS, The effectiveness of seven denture cleansers on tea stain removal from PMMA acrylic resin., Int. J. Prosthodont. 15 (2002) 549–552. [PubMed] [Google Scholar]
- [148].Carniello V, Peterson BW, van der Mei HC, Busscher HJ, Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth, Adv. Colloid Interface Sci. 261 (2018) 1–14. doi: 10.1016/j.cis.2018.10.005. [DOI] [PubMed] [Google Scholar]
- [149].Rogers JD, Palmer RJ, Kolenbrander PE, Scannapieco FA, Role of Streptococcus gordoniiAmylase-Binding Protein A in Adhesion to Hydroxyapatite, Starch Metabolism, and Biofilm Formation, Infect. Immun. 69 (2001) 7046–7056. doi: 10.1128/IAI.69.11.7046-7056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Nobbs AH, Jenkinson HF, Jakubovics NS, Stick to Your Gums, J. Dent. Res. 90 (2011) 1271–1278. doi: 10.1177/0022034511399096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Hannig C, Hannig M, The oral cavity - A key system to understand substratum-dependent bioadhesion on solid surfaces in man, Clin. Oral Investig. 13 (2009) 123–139. doi: 10.1007/s00784-008-0243-3. [DOI] [PubMed] [Google Scholar]
- [152].Ngo BKD, Grunlan MA, Protein Resistant Polymeric Biomaterials, ACS Macro Lett. 6 (2017) 992–1000. doi: 10.1021/acsmacrolett.7b00448. [DOI] [PubMed] [Google Scholar]
- [153].Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F, Eslick J, Camarda K, Katz JL, Adhesive/Dentin Interface: The Weak Link in the Composite Restoration, Ann. Biomed. Eng. 38 (2010) 1989–2003. doi: 10.1007/s10439-010-9969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Burke FJT, Wilson NHF, Cheung SW, Mjör IA, Influence of patient factors on age of restorations at failure and reasons for their placement and replacement, J. Dent. 29 (2001) 317–324. doi: 10.1016/S0300-5712(01)00022-7. [DOI] [PubMed] [Google Scholar]
- [155].Zhang N, Zhang K, Melo MAS, Weir MD, Xu DJ, Bai Y, Xu HHK, Effects of long-term water-aging on novel anti-biofilm and protein-repellent dental composite, Int. J. Mol. Sci. 18 (2017) 1–15. doi: 10.3390/ijms18010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Zhang N, Melo MAS, Bai Y, Xu HHK, Novel protein-repellent dental adhesive containing 2-methacryloyloxyethyl phosphorylcholine, J. Dent. 42 (2014) 1284–1291. doi: 10.1016/j.jdent.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Lee B-S, Chen Y-J, Wei T-C, Ma T-L, Chang C-C, Comparison of Antibacterial Adhesion When Salivary Pellicle Is Coated on Both Poly(2-hydroxyethyl-methacrylate)- and Polyethylene-glycol-methacrylate-grafted Poly(methyl methacrylate), Int. J. Mol. Sci. 19 (2018) 2764. doi: 10.3390/ijms19092764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Rupp F, Haupt M, Eichler M, Doering C, Klostermann H, Scheideler L, Lachmann S, Oehr C, Wendel HP, Decker E, Geis-Gerstorfer J, von Ohle C, Formation and Photocatalytic Decomposition of a Pellicle on Anatase Surfaces, J. Dent. Res. 91 (2012) 104–109. doi: 10.1177/0022034511424901. [DOI] [PubMed] [Google Scholar]
- [159].Horbett TA, Principles underlying the role of adsorbed plasma proteins in blood interactions with foreign materials, Cardiovasc. Pathol. 2 (1993) 137–148. doi: 10.1016/1054-8807(93)90054-6.25990608 [DOI] [Google Scholar]
- [160].Ge C, Du J, Zhao L, Wang L, Liu Y, Li D, Yang Y, Zhou R, Zhao Y, Chai Z, Chen C, Binding of blood proteins to carbon nanotubes reduces cytotoxicity, Proc. Natl. Acad. Sci. 108 (2011) 16968–16973. doi: 10.1073/pnas.1105270108. [DOI] [PMC free article] [PubMed] [Google Scholar]