Abstract
Objective:
This study explored motivators and challenges/barriers to sharing personal genetic test results (GTR) with family members (FM).
Methods:
Semi-structured, in-depth interviews were conducted with 62 women who had a pathogenic or likely pathogenic (P/LP) variant in a BRCA, PALB2, CHEK2, or ATM gene. Selective qualitative data analysis focused on eliciting motivators and challenges/barriers identified by participants when sharing their GTR with FM.
Results:
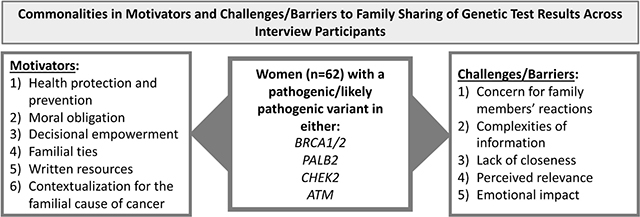
Motivators to sharing personal GTR with FM included: health protection and prevention; moral obligation; decisional empowerment; familial ties; written resources; and contextualization for a familial cause for cancer. Challenges/barriers to family sharing included: concern for FM reactions; complexities of information; lack of closeness; perceived relevance; and emotional impact.
Conclusions:
All motivators and challenges/barriers were identified across BRCA and non-BRCA carriers, demonstrating commonalities in family sharing of GTR among high- to moderate-penetrance hereditary BC (breast cancer) genes. Despite challenges/barriers, participants disclosed their GTR with most close FM, yet restrictions in communication and/or strain on the timing, manner of disclosing, and strategies used varied across certain FM.
Practice Implications:
These findings offer healthcare providers and researchers preliminary practical implications for broadly improving family sharing interventions across P/LP variants in BC risk genes by demonstrating important elements to include in family sharing letters.
Keywords: cancer genetic test results, family sharing, motivators, barriers, challenges
Graphical Abstract
1. Introduction
Between 5 and 10% of female breast cancer (BC) patients have an inherited predisposition, most often due to a pathogenic or likely pathogenic (P/LP) variant in the BRCA1 or BRCA2 (BRCA) genes [1]. The lifetime risk to develop BC for women with P/LP BRCA variants is 60–70% [2], and PALB2 variants is 53% [3], compared to a 12% lifetime risk for women in the general population [4]. P/LP variants in moderate penetrance genes such as CHEK2 and ATM generally confer lower lifetime cancer risks in the range of 25–30% [5, 6].
Identifying inherited cancer predisposition enables early detection, prevention, and cancer risk management strategies and may guide cancer treatment and help prevent second primary cancers for those already diagnosed [7–9]. Family members (FM) of a BRCA, PALB2, CHEK2, or ATM carrier may have up to a 50% chance of harboring the same P/LP variant [10, 11]. By undergoing genetic testing, FM may confirm their own cancer risks and determine optimal risk management strategies [12–14]. This can be especially important for FM who do not qualify for breast MRI screening based solely on family history of cancer [15].
Family sharing of genetic test results (GTR) is a complex yet critical step within the cancer control continuum [12, 16–19]. Currently due to restrictions on healthcare providers to disclose a patient’s test results, notification of cancer risks for at risk relatives falls to the individual tested [20, 21]. The most important reasons reported in prior literature for sharing GTR include the following: making FM aware of risk, suggesting FM undergo genetic testing, and fulfilling a perceived responsibility to inform [22–25]. Other motivators from prior literature include seeking emotional support and advice about management decisions [23, 26, 27]. Despite the importance of family sharing, rates of GTR disclosure indicate some at-risk FM remain uninformed [7, 12]. Even when results are shared, cascade testing among FM remains low (roughly 15–50%) [9, 28, 29].
The majority of prior research about FM sharing of inherited cancer GTR has focused almost exclusively on disclosure of BRCA results and highly penetrant colorectal cancer genes [12, 21, 23, 24, 26, 30–36]. Yet, a few recent studies report quantitative findings on family communication. Ricker and colleagues [9] assessed communication of GTR, which included a wide spectrum of high to moderate penetrance genes, in a diverse cohort three months after patients received their GTRs and found that 81% of patients with high-penetrance genes and 74% with moderate penetrance genes encouraged their FM to undergo genetic testing. Seeking to overcome identified barriers and facilitators to cascade testing, Caswell-Jin and colleagues [37] developed and evaluated an online intervention among at-risk relatives of individuals with at least one of 30 inherited cancer genes (including the genes relevant to this study) and found 88% of participants communicated GTR to at least one relative, most commonly with first-degree and female relatives. Finally, Cragun and colleagues [38] compared the proportions of FM with whom patients communicated GTR and found higher rates of family sharing among individuals with pathogenic variants in BRCA compared to those with pathogenic variants in more recently discovered BC genes (e.g., PALB2, CHEK2, ATM).
Given the scope of prior research, it remains unclear the potentially diverse types of motivators and barriers to family sharing of GTR that may exist across different inherited BC genes, including more recently discovered BC genes, such as PALB2, CHEK2, and ATM. Thus, the purpose of this exploratory, qualitative study was to first elicit motivators and challenges/barriers to family sharing of genetic risk information among four hereditary BC syndromes and then compare identified motivators and challenges/barriers across the four genes to potentially identify any differences. The following research question guided this analysis: What motivated or hindered participants’ abilities to share their GTR with FM and were there differences across carrier status?
2. Methods
2.1. Recruitment, participants, and data collection
This study was approved by the Vanderbilt University and University of South Florida Institutional Review Boards. Participants in this study were drawn from individuals who consented to recontact to participate in a related study conducted at Vanderbilt University Medical Center. Inclusion criteria for this study included women with a P/LP variant in a BRCA, PALB2, CHEK2, or ATM gene, who completed an online consent form and initial survey through a recruitment email invitation and agreed to complete an in-depth interview after being purposively sampled to assure diversity in cancer risk management and family sharing practices, with oversampling of minority women.
A total of 62 women participated in a phone interview (see Table 1). Of these participants, over 75% were non-Hispanic White and had private insurance. About half the participants were employed full-time and had an annual income of equal to or greater than $50,000. Nearly 75% of participants had a personal history of BC, and half of all participants self-reported as the first member of their family to receive genetic testing for inherited cancer.
Table 1.
Demographic and Clinical Characteristics of Study Participants Interviewed
| Overall N=62 | BRCA1/2 N=27 | PALB2 N=17 | CHEK2 N=13 | ATM N=5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Race/Ethnicity | - | - | - | - | - | - | - | - | - | - |
| Non-Hispanic White | 48 | 77% | 14 | 52% | 16 | 94% | 13 | 100% | 5 | 100% |
| Hispanic White | 7 | 11% | 7 | 26% | - | - | - | - | - | - |
| Black/African American | 5 | 8% | 4 | 15% | 1 | 6% | - | - | - | - |
| Other | 2 | 3% | 2 | 7% | - | - | - | - | - | - |
| Age ≥ 50 at time of interview | 39 | 63% | 14 | 52% | 12 | 71% | 10 | 77% | 3 | 60% |
| College graduate | 47 | 76% | 20 | 74% | 16 | 94% | 7 | 54% | 4 | 80% |
| Private insurance | 49 | 79% | 21 | 78% | 15 | 88% | 9 | 69% | 4 | 80% |
| Full-time employment | 33 | 53% | 16 | 59% | 11 | 65% | 6 | 46% | - | - |
| Income ≥ 50k | 40 | 65% | 14 | 52% | 12 | 71% | 12 | 92% | 2 | 40% |
| Age ≥ 50 at time of genetic testing | 36 | 58% | 13 | 48% | 12 | 71% | 8 | 62% | 3 | 60% |
| First relative to have genetic testing | 31 | 50% | 4 | 15% | 12 | 71% | 10 | 77% | 5 | 100% |
| Personal history of breast cancer | 45 | 73% | 19 | 70% | 13 | 76% | 8 | 62% | 5 | 100% |
| Family history of breast cancer † | 49 | 79% | 20 | 74% | 15 | 88% | 10 | 77% | 4 | 80% |
Family history represents a first- or second-degree relative with cancer diagnosis reported on available pedigree and/or questionnaire
The interview guide was developed by members of the study team trained in qualitative data collection and with backgrounds in public health, medical anthropology, and communication. Drawing on the Integrated Behavioral Model in order to reveal underlying challenges/barriers and motivators that influence behavior [39], questions were open-ended and included probes to guide the interviewer in capturing in-depth experiences. All interviews were scheduled at a time most convenient to the participant and were conducted over the phone by a member of the team trained in qualitative interviewing. Sample questions included: 1) how did you feel about the idea of sharing your GTR with FM; 2) describe the main issues or concerns you faced when deciding whether or not to tell your FM about your GTR; and 3) provide some examples of how FM reacted when you told them your GTR. Interviews were recorded and later transcribed using an online transcription software. Transcriptions were reviewed by two members of the team to ensure data accuracy. One interview had a failed recording, so detailed notes were used.
2.2. Data analysis
Preceding analysis, the authors conducted transcript data immersion. Data were analyzed using a “selective approach” to qualitative data analysis [40, 41]. The first step involved isolating sections of the interview transcripts that specifically addressed family sharing of GTR given the research question. Under guidance from the first author, trained in qualitative data analysis, the third author read interview transcripts to copy/paste relevant participant responses into four categories: (1) who the participant shared their GTR with, (2) how they shared their GTR, (3) what motivated their disclosures, and (4) what hindered or made their disclosures challenging. Once relevant text was selected, the first and second authors randomly checked 30% of the transcripts to ensure data extraction accuracy. The entire research team then met to discuss the selected data and identify any missing excerpts.
The second step involved utilizing the constant comparison method to identify motivators and challenges/barriers [42]. The first two authors re-reviewed specific motivators and challenge/barrier categories, then engaged in “open coding” to generate initial codes; after which they met to discuss and refine the codes [42], followed by employing “axial coding” to group relating codes [43]. To elicit motivators and challenges/barriers, Owen’s [44] criteria for thematic salience was utilized— recurrence (at least two mentions), repetition (repeated words/phrases), and forcefulness (vocalics). In keeping in line with the purpose of this study, the first two authors then compared all motivators and challenges/barriers across the genes in an attempt to identify potential differences. Last, the final list of themes—six motivators and five challenges/barriers to sharing GTR with FM—with exemplar quotes was shared and discussed with the entire research team, as well as whether differences across genes were prevalent.
3. Results
3.1. Motivators for sharing genetic test results with family members
Motivators to sharing GTR with FM were: 1) health protection and prevention; 2) moral obligation; 3) decisional empowerment; 4) familial ties; 5) written resources; and 6) contextualization for the familial cause of cancer. First, participants were motivated to share their GTR based on their personal experience with genetic testing that helped to protect their health. In turn, they wanted to offer FM the same personal health information. One participant explained, “Because I feel strongly that they [FM] need to know if they could potentially have this mutation to safeguard their health.” (59)BrC(56)PALB21 Another stated, “How much do you love your family? Do you love them enough to protect them, even if it might be hard for you to talk about?...I love them enough to let them know.” (68)BrC(65)CHEK2 Participants understood the connection between knowing one’s inherited cancer risk and the ability to engage in risk surveillance. One participant articulated, “I think it’s a good idea, because I just feel like sharing it would enable them to get better surveillance too.” (59)BrC(47)PALB2
For the second motivator, participants felt morally obligated to share their GTR with FM, as if it were a duty or responsibility. One participant shared, “I felt like it was my responsibility to share with my family…It was just a no-brainer. I would hope they would do the same if they were in my shoes…I think it really is a morality issue.” (47)BrC(37)BRCA A participant with no personal history of cancer explained, “I just think that having gone through this with my daughters, …, it’s morally the responsible thing to do.” (65)PALB2 Several participants stated they felt it was the “right thing” for their FM and would be unethical to withhold the information. One participant shared, “It’s not really a choice, it’s a responsibility…I just think that it is completely selfish not to divulge the information to people who it could affect.” (66)BrC(61)OvC(29)ATM
For the third motivator, participants were driven to share their GTR because they believed it would empower their FM to make informed healthcare decisions.
I just think that they [FM] need to know, and then they can decide for themselves. I’m not saying that they all have to get tested, but I just think it is knowledge that they need to have in order to make an educated decision as far as what they want to do about it. (57)BrC(49)PALB2
Similarly, another participant explained she wanted to share her GTR with FM in order to build “some awareness as well as [offer] information so that they would be informed and knowledgeable of the whole genetic BRCA, and so that they could make a decision on what they wanted to do.” (49)BC(36)BRCA A part of this motivator was the belief that knowing one’s inherited cancer risk would empower control over one’s future, which was evident across the gene groups: “Knowledge is power, basically. If you have the knowledge of what could happen, then you’d be prepared for it.” (68)BC(65)CHEK2
Regarding the fourth motivator, participants shared their GTR with FM whom they felt particularly close to. One participant said, “We are a pretty close bunch, so I had full access to them…There [are] open lines of communication.” (49)BrC(36)BRCA Another explained she did not have a problem sharing her GTR because, “We’re very open as far as our family goes. We all know just about everything about each other.” (68)BrC(65)CHEK2 Likewise, another participant shared, “I think our family [is] pretty open about things like that.” (48)BrC(43)PALB2
Our fifth motivator, while not as prominent, was exemplified by some participants who reported written resources from healthcare providers or testing labs (e.g., family sharing letters, genetic test reports, and general genetics information) assisted them in sharing their GTR. Several participants reported receiving a family sharing letter, inclusive of their GTR, cancer risk, and guidance for FM. “[It] definitely made me feel confident about what I was telling them [FM]. I had something to back it up, and I shared my actual records with them.” (59)BC(56) CHEK2. Another participant stated, “with the letter, more confident, because here’s what I got, here’s the results, here’s a copy. It helped, versus just telling someone because I think people believe, whether it’s right or wrong; if they have something in print and shows the research and shows the lab and shows whatever, I think they’re more likely to believe it.” F(59)BC(50)PALB2 Last, a different participant explained, “If I had to do it myself, it would be hard to explain it to them [FM], but because I got so much information from the testing company to forward, it was very easy.” (60)BrC(36)BRCA
The last motivator was also not as common, but, for some, sharing GTR with FM provided context for their own personal cancer diagnosis, helping them explain the striking presence of cancer in the family. One participant expressed,
We had such a long line of cancer that... It felt like a relief…I think just knowing that there was something, and it wasn’t just like a fluke or something like that, made it easier to say, ‘Hey, at least we have an answer now.’ (47)BrC(41)ATM
Having an answer was also expressed by another who said, “For me, [it] gave a justification. There was … why did I get cancer, and I did all these things healthy; well, you can get cancer if you’ve got some kind of a genetic issue.” (59)BrC(50)PALB2
3.2. Challenges/barriers for sharing genetic test results with family members
Although motivators assisted participants in sharing their GTR, challenges/barriers still existed, including: 1) concern for FM reactions; 2) complexities of information; 3) lack of closeness; 4) perceived relevance; and 5) emotional impact. For the first barrier, participants worried about how their FM would react to the GTR. One participant emphasized, “My sisters and I lived with a mother who was kind of a hypochondriac, so I kind of worried how is this going to affect them—Is it going make them worry about things that are not necessary that they need to worry about?” (51)BrC(50)PALB2 Similarly, another participant was concerned about her father’s reaction and explained, “I only tell him on a ‘need to know’ basis; he’s not a very emotional person, and he doesn’t speak about things; he kind of internalizes them.” (55)BrC(36)BRCA In addition to internalizing worry, participants were also concerned their FM would feel guilt for passing down the mutation. Another participant shared,
I didn’t want them [her parents] to feel bad because they did all they could, even had the test…and I think I didn’t want them to feel bad because they did say, ‘We feel bad that you have this,’ and I’m like, ‘Well it’s not your fault. That’s just how it is.’ (49)BrC(49)ATM
Participants’ assumptions of their FM emotional responses ultimately influenced whether or not, as well as when, to share their GTR. A different participant explained, “I chose not to tell them. I thought it might be too much information, but if any time that something came up with them, I would share the information with them.” (60)BrC(45)BRCA
For the second barrier, participants considered their ability to accurately relay genetic risk information specific to their result before deciding whether or not to share their GTR. A participant said, “I feel like I get myself mixed up… ‘Did I do that right? Did I say that right?’… I wasn’t necessarily completely sure about, like I thought I did, but until you talk about it, it’s like a totally different world.” (47)BrC(41)ATM Participants were also concerned about answering complex questions from FM. One participant with no personal cancer history stated, “I don’t have all the answers. There’s a lot of questions. I know they asked me why I didn’t just do testing, and why they didn’t just do mammograms and the percentage and the downside of this and that.” (63)BRCA Additionally, participants assessed their FM ability to comprehend. Another explained,
When you talk about this stuff, you have to assume your audience is fairly intelligent and understands kind of the basic premise of genetic testing. My cousin, who’s my only female cousin; she’s not that bright. She’s like, ‘I don’t understand.’ And then I’m just like, ‘Oh god, this isn’t my job.’ (54)BrC(49)PABL2
For the third barrier, participants reported they were hesitant to share their GTR with FM they were not close with either emotionally or physically. A participant said, “I didn’t feel comfortable really just calling them up and, ‘Oh, by the way, I have this gene.’” (57)BrC(49)PALB2 Similarly, a participant with no personal history of cancer shared, “I’m just not that close with them… We just don’t see each other that often, and we don’t really share information that personal.” (29)BRCA Regarding physical distance, a different participant explained, “Everybody lives out of state. All my cousins live in Ohio, Indiana. I have a few in Kentucky, but most of them is in Ohio and Indiana, and I don’t see them; I don’t talk to them.” (66)BrC(63)ATM
For the fourth barrier, participants often explained they did not share their GTR with FM who they believed the information was irrelevant, which was often assessed by their FM age. One participant stressed, “If it’s a cancer mutation, is it important to tell your 85-year-old parents? Probably, it’s not … why would you worry them?” (66)BrC(61)OvC(29)ATM Another participant believed her father would be distressed by the information. She articulated, “It’s just not something I felt necessary to talk with him about, because it wouldn’t affect his life. He’s 81…It’s pretty late in his life to do too much about it but feel guilty… He’s worried enough about me going through treatments, and whether I’ll survive long-term, and all of that.” (57)LungPancC(57)BrC(47)PALB2
For the fifth, less prominent barrier, some participants explained they delayed sharing their GTR while dealing with the negative, emotional impact of learning their own result. A participant expressed, “Initially, it was just hard to even talk about it without crying, and so that was the only way it was hard to share, because I was pretty emotional about it.” (59)BrC(56)PALB2 Participants described needing to come to terms with their own GTR before sharing it with FM: “It still hadn’t totally sunk in for me. So, I think it was just hard because it wasn’t completely real for me yet.” (36)BRCA
In sum, motivators and challenges/barriers to sharing GTR with FM were identified across women in our study with P/LP variants in BRCA genes as well as PALB2, CHEK2, and ATM genes, revealing commonalities in family sharing of GTR among high- to moderate- penetrance hereditary BC genes. In other words, what motivated and hindered women’s abilities to share their GTR with FM did not differ across carrier status within our population.
4. Discussion and Conclusions
4.1. Discussion
Utilizing an exploratory, qualitative study design [42], these findings offer rich, descriptive detail of family sharing motivating and challenging experiences of women with P/LP variants across BRCA, PALB2, CHEK2, and ATM, thus more broadly adding to the literature on family sharing among patients with P/LP variants in newer BC risk genes. As evident from the descriptive detail outlined in the results, this study also found both individuals with BRCA P/LP variants and those with P/LP variants in other BC risk genes experience substantially the same motivators and challenges/barriers to sharing GTR. This finding is important because it highlights the broad capacity for family sharing interventions to support and promote intrafamilial communication, regardless of the P/LP BC gene [7, 12, 20, 45]. Lastly, this study’s qualitative results provide researchers and healthcare providers with preliminary information to broadly improve family sharing interventions [46, 47], which has been called upon in recent literature [25, 38, 48, 49].
Consistent with extant literature, participants in this study reported sharing their GTR with FM to enable them to protect and prevent hereditary cancer [9, 23, 24, 29]; to empower decision making among FM [32, 50, 51]; sharing more with those they felt close to [8, 17, 21, 25, 33, 52, 53]; and because they felt responsible to share their GTR [22–25, 29, 54]. At the same time, the findings extend previous research, given participants stated additional motivators included written materials (e.g., family sharing letters1, information from genetic testing companies) and contextualization for familial cancer. To ensure patients accurately relay genetic risk information specific to their GTR, family sharing letters should convey the importance of knowing about the inherited P/LP variant, what cancer risks are (and are not) associated with it, and where the FM may receive genetic counseling. Additionally, given the ever-changing nature of guidelines due to new research and advancements in science, it may be helpful to include a disclaimer in the letter, indicating that information is subject to change, with a reference to the National Comprehensive Cancer Network. While the use of family sharing letters has become standard practice when heritable genetic risks are identified [55], unfortunately, healthcare providers do not consistently offer participants these letters; this is an opportunity lost especially for patients who have a P/LP variant in the newer, less commonly known genes (e.g., CHEK2, ATM). Thus, family sharing letters may be particularly helpful in communicating this genetic risk information given there is less information about these genes in comparison to BRCA. Downstream research on the usefulness of the letter content can target novel topics included.
Identified challenges/barriers to sharing GTR also confirm and extend prior research. Consistent with existing literature, participants in this study reported they were hesitant to share their GTR when they were concerned about how their FM would react [16, 26, 33, 34]; when they were emotionally or geographically distant [12, 21, 23, 24, 31, 35, 36, 52]; and when they felt the information was not relevant [32]. Other challenges/barriers identified—complexities of information and personal reactions—have not commonly been reported in the literature. Furthermore, despite high rates of family sharing, some challenges/barriers did restrict family communication among certain FM (e.g., extended FM and older FM), while others did not necessarily block family communication, but rather placed strain on the timing, manner of disclosing, and strategies behind whom to share their GTR. In short, identifying these challenges/barriers is a necessary first step in developing family-oriented interventions to better support patients in sharing complex genetic risk information [25, 38, 46, 47].
4.2. Conclusions
4.2.1. Limitations and Future Research
There are limitations of this study. First, participants were selected from a highly motivated population of women who participated in other inherited cancer research studies, which introduced a sampling bias despite attempts to purposively select those who did not share with all FM and those from underserved ethnic/racial groups. Second, participants self-reported their family sharing behaviors, and these were not verified with their FM. Third, although 62 interviews are larger than most interview studies, the sample of non-BRCA carriers is small (N=35), thus limiting the results’ generalizability for other BC risk genes. Future research should continue to explore non-BRCA carriers’ experiences.
Additional research is needed to evaluate FM reactions to results sharing, particularly among less studied genes such as PALB2, CHEK2, and ATM. Research efforts should also further examine disclosure behaviors in understudied, minority groups who may experience unique challenges/barriers and motivators that require tailored interventions sensitive to cultural nuances. Finally, future research should assess the role of healthcare providers and the utility of resources to develop effective interventions to improve rates and quality of family sharing, overcome age and gender discrepancies with sharing, and increase follow-up among at-risk FM.
4.3. Practice Implications
Last, given the exploratory nature of this study, preliminary practical implications are briefly discussed. As healthcare providers play an essential role in facilitating the family sharing process [7], these findings offer both providers, but also researchers, with initial information to broadly improve family sharing interventions across P/LP variants in BC risk genes [25, 46–48]. For example, helpful elements for family sharing letters may include the following: talking points of what and how to share GTR with FM; outlining the importance of sharing GTR with FM, including a brief discussion of possible challenges/barriers to family sharing; gene specific information (particularly more recently discovered BC genes) that breaks down the complexities of the information; and finally, contact information for genetics professionals [48, 49].
Research Highlights.
Regardless of genes, women experienced the same motivators and challenges/barriers.
New motivators included: written resources and contextualizing cancer in the family.
New challenges included: complexities of information and personal reactions.
Results provide preliminary information to improve family sharing interventions.
Acknowledgements:
Funding for this study was supported in part by the National Cancer Institute (NCI) VICC Specialized Program of Research Excellence (SPORE) in Breast Cancer (P50CA098131), the Ingram Professorship, the Kleberg Foundation, Vanderbilt Genetic Institute departmental funds, and a 2018 USF COPH Faculty Research Award. This project was also supported by CTSA (award number UL1 TR0002243) from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institute of Health.
I confirm all patient/personal identifiers have been removed or disguised so the patient/person(s) described are not identifiable and cannot be identified through the details of the story.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest exists.
This notation describes the participant’s characteristics: (Age when completed interview) Type of Cancer (age when diagnosed) P/LP variant in gene.
A family sharing letter contains basic information about the gene variant the individual (i.e., the patient) was identified with and the relevance of this information for family members and why they were prompted to share their genetic test result with that family member.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society, I. Breast Cancer Risk and Prevention: Breast Cancer Risk Factors You Cannot Change. 2020. [cited 2020 March 23]; Available from: https://www.cancer.org/cancer/breast-cancer/risk-and-prevention/breast-cancer-riskfactors-you-cannot-change.html.
- 2.Kuchenbaecker KB, et al. , Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA, 2017. 317(23): p. 2402–2416. [DOI] [PubMed] [Google Scholar]
- 3.Yang X, et al. , Cancer Risks Associated With Germline PALB2 Pathogenic Variants: An International Study of 524 Families. J Clin Oncol, 2020. 38(7): p. 674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Institute: Surveillance, E., and End Results Program. Cancer Stat Facts: Female Breast Cancer. 2016. [cited 2020 March 22]; Available from: https://seer.cancer.gov/statfacts/html/breast.html.
- 5.Marabelli M, Cheng SC, and Parmigiani G, Penetrance of ATM Gene Mutations in Breast Cancer: A Meta-Analysis of Different Measures of Risk. Genet Epidemiol, 2016. 40(5): p. 425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cybulski C, et al. , Risk of breast cancer in women with a CHEK2 mutation with and without a family history of breast cancer. J Clin Oncol, 2011. 29(28): p. 3747–52. [DOI] [PubMed] [Google Scholar]
- 7.Black L, et al. , Intrafamilial disclosure of risk for hereditary breast and ovarian cancer: points to consider. J Community Genet, 2013. 4(2): p. 203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katapodi MC, et al. , Individual and family characteristics associated with BRCA1/2 genetic testing in high-risk families. Psychooncology, 2013. 22(6): p. 1336–43. [DOI] [PubMed] [Google Scholar]
- 9.Ricker CN, et al. , Patient communication of cancer genetic test results in a diverse population. Transl Behav Med, 2018. 8(1): p. 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antoniou AC, et al. , Breast-cancer risk in families with mutations in PALB2. N Engl J Med, 2014. 371(6): p. 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung EL, et al. , Communication of BRCA results and family testing in 1,103 high-risk women. Cancer Epidemiol Biomarkers Prev, 2010. 19(9): p. 2211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daly MB, et al. , Communicating genetic test results within the family: Is it lost in translation? A survey of relatives in the randomized six-step study. Fam Cancer, 2016. 15(4): p. 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehniger J, et al. , Family communication of BRCA1/2 results and family uptake of BRCA1/2 testing in a diverse population of BRCA1/2 carriers. J Genet Couns, 2013. 22(5): p. 603–12. [DOI] [PubMed] [Google Scholar]
- 14.Katapodi MC, et al. , Cancer Predisposition Cascade Screening for Hereditary Breast/Ovarian Cancer and Lynch Syndromes in Switzerland: Study Protocol. JMIR Res Protoc, 2017. 6(9): p. e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weidner AE, et al. , Breast cancer screening implications of risk modeling among female relatives of ATM and CHEK2 carriers. Cancer, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derbez B, Is there a “right time” for bad news? Kairos in familial communication on hereditary breast and ovarian cancer risk. Soc Sci Med, 2018. 202: p. 13–19. [DOI] [PubMed] [Google Scholar]
- 17.Peters JA, et al. , Unpacking the blockers: understanding perceptions and social constraints of health communication in hereditary breast ovarian cancer (HBOC) susceptibility families. J Genet Couns, 2011. 20(5): p. 450–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chivers Seymour K, et al. , What facilitates or impedes family communication following genetic testing for cancer risk? A systematic review and meta-synthesis of primary qualitative research. J Genet Couns, 2010. 19(4): p. 330–42. [DOI] [PubMed] [Google Scholar]
- 19.Sciences, N.C.I.D.o.C.C.P. Cancer Control Continuum. 2019. [cited 2020 March 22]; Available from: https://cancercontrol.cancer.gov/od/continuum.html.
- 20.Daly MB, A Family-Centered Model for Sharing Genetic Risk. J Law Med Ethics, 2015. 43(3): p. 545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kardashian A, et al. , A Pilot study of the Sharing Risk Information Tool (ShaRIT) for Families with Hereditary Breast and Ovarian Cancer Syndrome. Hered Cancer Clin Pract, 2012. 10(1): p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCann S, et al. , Family communication, genetic testing and colonoscopy screening in hereditary non-polyposis colon cancer: a qualitative study. Psychooncology, 2009. 18(11): p. 1208–15. [DOI] [PubMed] [Google Scholar]
- 23.Hughes C, et al. , All in the family: evaluation of the process and content of sisters’ communication about BRCA1 and BRCA2 genetic test results. Am J Med Genet, 2002. 107(2): p. 143–50. [DOI] [PubMed] [Google Scholar]
- 24.McGivern B, et al. , Family communication about positive BRCA1 and BRCA2 genetic test results. Genet Med, 2004. 6(6): p. 503–9. [DOI] [PubMed] [Google Scholar]
- 25.Young AL, et al. , Talking across generations: Family communication about BRCA1 and BRCA2 genetic cancer risk. J Genet Couns, 2019. 28(3): p. 516–532. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton RJ, Bowers BJ, and Williams JK, Disclosing genetic test results to family members. Journal of Nursing Scholarship: An Official Publication of Sigma Theta Tau International Honor Society of Nursing, 2005. 1(37): p. 18–24. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg M and Smith RA, Support Seeking or Familial Obligation: An Investigation of Motives for Disclosing Genetic Test Results. Health Commun, 2016. 31(6): p. 668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blandy C, et al. , Testing participation in BRCA1/2-positive families: initiator role of index cases. Genet Test, 2003. 7(3): p. 225–33. [DOI] [PubMed] [Google Scholar]
- 29.Lieberman S, et al. , Familial communication and cascade testing among relatives of BRCA population screening participants. Genet Med, 2018. 20(11): p. 1446–1454. [DOI] [PubMed] [Google Scholar]
- 30.D’ Audiffret Van Haecke D. and de Montgolfier S, Genetic Test Results and Disclosure to Family Members: Qualitative Interviews of Healthcare Professionals’ Perceptions of Ethical and Professional Issues in France. J Genet Couns, 2016. 25(3): p. 483–94. [DOI] [PubMed] [Google Scholar]
- 31.Elrick A, et al. , Psychosocial and Clinical Factors Associated with Family Communication of Cancer Genetic Test Results among Women Diagnosed with Breast Cancer at a Young Age. J Genet Couns, 2017. 26(1): p. 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dean M and Rauscher EA, Men’s and Women’s Approaches to Disclosure About BRCA-Related Cancer Risks and Family Planning Decision-Making. Qual Health Res, 2018. 28(14): p. 2155–2168. [DOI] [PubMed] [Google Scholar]
- 33.Lafrenière D, et al. , Family communication following BRCA1/2 genetic testing: a close look at the process. J Genet Couns, 2013. 22(3): p. 323–35. [DOI] [PubMed] [Google Scholar]
- 34.Forrest K, et al. , To tell or not to tell: barriers and facilitators in family communication about genetic risk. Clin Genet, 2003. 64(4): p. 317–26. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald DJ, et al. , Selection of family members for communication of cancer risk and barriers to this communication before and after genetic cancer risk assessment. Genet Med, 2007. 9(5): p. 275–82. [DOI] [PubMed] [Google Scholar]
- 36.Healey E, et al. , Quantifying family dissemination and identifying barriers to communication of risk information in Australian BRCA families. Genet Med, 2017. 19(12): p. 1323–1331. [DOI] [PubMed] [Google Scholar]
- 37.Caswell-Jin JL, et al. , Cascade Genetic Testing of Relatives for Hereditary Cancer Risk: Results of an Online Initiative. J Natl Cancer Inst, 2019. 111(1): p. 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cragun D, et al. , Family communication of genetic test results among women with inherited breast cancer genes. J Genet Couns, 2020. [DOI] [PubMed] [Google Scholar]
- 39.Montano D and Kasprzyk D, Health behavior and health education: Theory, research, and practice.. Annals of Internal Medicine, 1992. 116(4). [Google Scholar]
- 40.van Manen M, Researching Lived Experience: Human Science for an Action Sensitive Pedagogy. 1990, Albany: Suny Press. [Google Scholar]
- 41.Bylund CL, Fisher CL, Brashers D, Edgerson S, Glogowski EA, Boyar SR, Kemel Y, Spencer S, and Kissane D, Sources of Uncertainty about daughters’ breast cancer risk that emerge during genetic counseling consultations. J of Genet Couns, 2012. 21(2): p. 292–3–4. [DOI] [PubMed] [Google Scholar]
- 42.Lindlof TR and Taylor BC, Qualitative Communication Research Methods. Third Edition ed. 2011, Thousand Oaks, CA: SAGE Publications, Inc. [Google Scholar]
- 43.Corbin J and Strauss A, Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Third Edition ed. 2007, Thousand Oaks, CA: SAGE. [Google Scholar]
- 44.Owen WF, Interpretive themes in relational communication. Quarterly Journal of Speech, 1984. 70(3): p. 274–287. [Google Scholar]
- 45.Bodurtha JN, et al. , The KinFact intervention - a randomized controlled trial to increase family communication about cancer history. J Womens Health (Larchmt), 2014. 23(10): p. 806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katapodi MC, et al. , Development of a Web-based Family Intervention for BRCA Carriers and Their Biological Relatives: Acceptability, Feasibility, and Usability Study. JMIR Cancer, 2018. 4(1): p. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikolaidis C, et al. , Challenges and Opportunities for Cancer Predisposition Cascade Screening for Hereditary Breast and Ovarian Cancer and Lynch Syndrome in Switzerland: Findings from an International Workshop. Public Health Genomics, 2018. 21(3–4): p. 121–132. [DOI] [PubMed] [Google Scholar]
- 48.Mendes Á, et al. , How communication of genetic information within the family is addressed in genetic counselling: a systematic review of research evidence. Eur J Hum Genet, 2016. 24(3): p. 315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rauscher EA, et al. , “How do we rally around the one who was positive?” Familial uncertainty management in the context of men managing BRCA-related cancer risks. Soc Sci Med, 2019. 242: p. 112592. [DOI] [PubMed] [Google Scholar]
- 50.Hesse-Biber S and An C, Within-Gender Differences in Medical Decision Making Among Male Carriers of the BRCA Genetic Mutation for Hereditary Breast Cancer. Am J Mens Health, 2017. 11(5): p. 1444–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rauscher EA and Dean M, “I’ve just never gotten around to doing it”: Men’s approaches to managing BRCA-related cancer risks. Patient Educ Couns, 2018. 101(2): p. 340–345. [DOI] [PubMed] [Google Scholar]
- 52.Etchegary H, et al. , Cultural differences in family communication about inherited cancer: implications for cancer genetics research. J Cult Divers, 2013. 20(4): p. 195–201. [PubMed] [Google Scholar]
- 53.Koehly LM, et al. , Characteristics of health information gatherers, disseminators, and blockers within families at risk of hereditary cancer: implications for family health communication interventions. Am J Public Health, 2009. 99(12): p. 2203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dancyger C, et al. , Comparing family members’ motivations and attitudes towards genetic testing for hereditary breast and ovarian cancer: a qualitative analysis. Eur J Hum Genet, 2010. 18(12): p. 1289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dheensa S, Lucassen A, and Fenwick A, Limitations and Pitfalls of Using Family Letters to Communicate Genetic Risk: a Qualitative Study with Patients and Healthcare Professionals. J Genet Couns, 2018. 27(3): p. 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]


