Abstract
Objectives:
To characterize dietary supplement usage among U.S. children, including product type, motivations, user characteristics, and trends over time with a primary focus on non-vitamin/non-mineral dietary supplements (NVNM).
Study design:
Overall, NVNM, and vitamin and/or mineral dietary supplement use; motivations for use, and trends in use over time were examined in children (≤19y) using the NHANES 1999–2016 data (n=42,510).
Results:
Between 1999 and 2016, overall DS and VM use among all children remained relatively stable at ~30%; yet, NVNM use increased from 2.9% to 6.4%, mainly due to increased use of omega-3 polyunsaturated fatty acids. NVNM use was higher in boys than in girls (3.9% vs 3.3%), and higher in older children than in younger children (ptrend<0.0001) – opposite of what was observed with VM use. Although both user groups shared two primary motivations, both motivations were reported by a significantly higher percent of VM users vs. NVNM users: to maintain health (38.7% vs. 23.1%) and to improve health (33.1% vs. 22.6%). NVNM users were much more likely to use DS for relaxation, stress, and sleep; for mental health; and for colon and bowel health.
Conclusions:
Although the prevalence of any DS and VM use among U.S. children have both remained stable, the prevalence of NVNM use has increased substantially over time. Yet, NVNM use remains relatively low overall. NVNM use exhibited different patterns by sex, age, and motivations when compared with VM use. Despite increasing NVNM use, high quality evidence supporting their use is lacking, especially in children.
Keywords: child, adolescent, NHANES, non-vitamin non-mineral supplement, botanical supplement, herbal supplement
More than one-half of U.S. adults and around one-third of infants, children, and adolescents (henceforth children) report using at least one dietary supplement (DS), and many report taking multiple products.1–6 The Dietary Supplement Health and Education Act of 1994, a legislation that defines and regulates DS, defines them as “any vitamin, mineral, herb or other botanical, or amino acid, or a concentrate, metabolite, constituent, extract, or a combination of these ingredients that seek to increase total intake”.7 Yet, it does not provide guidance on defining specific types of DS products.8
Previous research has characterized patterns of DS use overall – mainly cross-sectional reports on both micronutrient containing (i.e., vitamins and/or minerals, VM) and non-vitamin, non-mineral (NVNM) supplements – among U.S. adults 2, 5, 9 and children.3, 6, 10–12 However, among children, very little data has been published on DS use patterns over time. Therefore, the purpose of this analysis was to characterize types and trends of DS, especially NVNM products, commonly consumed by U.S. children along with motivations for their use using nationally representative, National Health and Nutrition Examination Survey (NHANES) data. NVNM within this analysis were inclusive of DS products that do not contain, or contain a very limited amount of VM. Patterns of DS usage were also examined by demographic, socioeconomic, and lifestyle factors.
Methods
This study presents data from the 1999–2016 NHANES, a nationally representative, continuous, cross-sectional survey of noninstitutionalized U.S. civilians, conducted by the Center for Disease Control and Prevention’s (CDC) National Center for Health Statistics (NCHS).13 The NHANES has a complex and stratified multistage probability sampling design, and uses analytic survey weights to account for nonresponse and oversampling of some groups. The NHANES protocol (and publicly released de-identified data) was approved by the Research Ethics Review Board and written informed consent was obtained for all participants or their proxies at the CDC/ NCHS. Nine survey cycles of data were combined to include all U.S. children aged ≤19y (n=42,469), inclusive of the years from 1999 to 2016. Children with missing data on DS use (n=42) were excluded, yielding a final analytic sample size of 42,510 children.
In NHANES, data are collected in two phases: an in-home interview where data on health information and demographics are first collected followed by a physical health examination in the Mobile Examination Center (MEC), a traveling clinic. Demographic, lifestyle, and socioeconomic characteristics are collected via the Computer-Assisted Personal Interview system during the in-home interview. For participants <16y of age or for those who are unable to provide their own information, a proxy is assigned.
The NHANES sampling age framework was applied to this analysis: 0–5y, 6–11y, and 12–19y of age. Prevalence of DS use among infants aged <1y was reported separately as these children had a distinct pattern of DS use compared with children 1–5y of age. In accordance with the NHANES protocol, race and Hispanic origin groupings were defined by NCHS as: non-Hispanic white, non-Hispanic black, Hispanic (including Mexican American), and other races, including multi-racial. Family income-to-poverty ratio (PIR) – the ratio of household income to the poverty threshold – was used as a proxy for family income, adjusted for family size and inflation. A PIR <130% is the cutoff to determine financial eligibility for the federal Supplemental Nutrition Assistance Program; therefore, consistent with previous studies, PIR was categorized as: <130%, 130–350%, and >350%.3, 14, 15 Health insurance was categorized as uninsured, private, or public at the time of survey collection. Those covered under both private and public insurance plans were included in the private health insurance category.
Screen time and body mass index (BMI) data was only available for participants ≥2y of age. Screen time was examined as a proxy for physical activity; it was calculated using the Physical Activity Questionnaire as the total number of hours per day spent looking at a screen (television, computer, or video game) and categorized as ≤1 hour/day, >1 to 2 hours/day, >2 to 4 hours/day, and >4 hours/day. BMI was calculated as kg/m2, using measurements of height (in meters) and weight (in kilograms) collected by trained technicians in the MEC as part of a physical examination. BMI percentiles were used to categorize each participant’s weight status as underweight (<5th percentile), healthy weight (5th to <85th percentile), overweight (85th to <95th percentile), or obese (≥95th percentile) according to the growth charts developed by the CDC for children 2–18y of age.16 For children >18y of age, CDC defined BMI cut-offs were used to categorize each participant’s weight status as underweight (<18.5 kg/m2), normal weight (18.5 to <25kg/m2), overweight (25.0 to <30 kg/m2) or obese (≥30 kg/m2).17
Dietary Supplement Information and Categorization
In NHANES, information on DS use over the past 30 days is collected through an in-home inventory and participant questionnaire known as the Dietary Supplement Questionnaire (DSQ), during the in-home interview (phase I). Participants are asked to show product containers for all DS taken in the past 30 days. Trained interviewers record the name and manufacturer from the product labels of each DS product reported. If DS product labels are not present (<12% of the time), a verbal report of these details are recorded. Trained nutritionists at the NCHS then review this data, obtain labels for supplements reported verbally, and compile this information into a product-level database. This database, known as the NHANES Dietary Supplement Database, and the DSQ participant reports for each cycle are all available online through the NHANES website.18 Additional details for the survey protocol can also be found on this site.18
Supplement use reported on the DSQ was defined as the use of any vitamins, minerals, or other DS use over the previous 30 days. Based on methods described in previous studies, all DS were classified into two primary and mutually exclusive categories – VM containing DS and NVNM containing DS.2, 3, 10, 19 If a supplement contained both VM and NVNM ingredients, a careful, product-specific review was conducted, and the supplement was classified according to its primary use and/or primary ingredient (Table 4; available at www.jpeds.com). For example, a DS containing mainly micronutrients with a small amount of omega-3 fatty acids, and primarily taken as a multivitamin, was classified as a VM. On the other hand, a DS containing cranberry concentrate with added vitamin C, and primarily taken for the antioxidant properties of cranberry, was classified as an NVNM. The majority of supplements that contained VM in combination with one or more NVNM product were primarily used for their VM content. These combinations usually contained lesser amounts of the NVNM ingredient than typically consumed when the NVNM was reported as an individual product or as part of a targeted blend. Consequently, unless they were the primary ingredient in such a combination, NVNM products were exclusive of VM. Based on previous literature, NVNM products were further classified into specific subcategories, including omega-3 polyunsaturated fatty acids (PUFAs), botanicals (inclusive of herbals), probiotics, and fiber and are further outlined in Table 4.2 For botanical supplements, we differentiated between DS containing single and multiple botanicals. The one exception was echinacea, which was not mutually exclusive of any other category due to low prevalence of its use. For this analysis, VM were aggregated into one category inclusive of multivitamin-mineral products and single nutrient-containing DS because the prevalence of single micronutrient supplement use is low.3 Although the inclusion of echinacea was data driven, overall VM and NVNM categories and all subcategories were based on previous NHANES studies in adults and children.2, 3, 5
Table 4 online.
Classification system used to create dietary supplement categories, NHANES 1999–2016
| Product Category | Definition | Examples |
|---|---|---|
| Vitamin-mineral/micronutrient (VM) | Any product containing one or more vitamins and/or one or more minerals as a primary ingredient (or for primary use); may contain small amounts of non-vitamin, non-mineral compounds | Sundown Kids multivitamin, Nature’s Plus high potency chewable iron with vitamin C and herbs |
| Non-vitamin, non-mineral (NVNM) | Any product containing botanicals/herbs, omega-3 PUFAs,a probiotics, fiber, joint, phosphatidyl choline, protein/amino acids, enzymes, melatonin, red yeast, brewer’s yeast, lipoic acid, carnitine, DHEA, CLA, bee pollen, gelatin, colostrum, creatine, cartilage, SAME, HTP, hyaluronic acid, collagen, malic acid, GABA, DMAE, betaine, caffeine, or kelp as a primary ingredient (and for primary use); may contain small amounts of vitamins and/or minerals | Puritan’s Pride turmeric, Dolphin Pals DHA gummies for kids, Spring Valley probiotic acidophilus |
Omega-3 PUFAs, polyunsaturated fatty acids; includes omega-3–6-9 PUFA containing supplements in which omega-3 was in higher quantity than the others. Also includes cod liver oil supplements that did not list “omega” or “fat” in the Dietary Supplement Database: Ingredient Information. These inclusions increased omega-3 PUFA DS by n=14 across all 18 years.
Additional analyses were completed to examine motivations for use of NVNM among children; however, these data were only available in NHANES 2007–2016 (n=785). Although the data available were generally consistent across these years, differential classification was used for some motivations, and is denoted in the tables.
Statistical Analyses
Data were analyzed using SAS (version 9.4; SAS Institute, Inc, Cary, NC, USA) and SAS-callable SUDAAN (version 11; RTI International, Research Triangle Park, NC, USA) software programs to properly account for survey weights and complex survey design, non-response, non-coverage, and planned oversampling of some population subgroups. All NHANES cycles covering 18 years (inclusive of 1999–2016) were used to describe prevalence of DS use, including NVNM use, VM use, and non-DS use by demographic, socioeconomic, and lifestyle characteristics. Prevalence of NVNM use in children was inclusive of those children taking only NVNM and children taking both VM and NVNM. Thus, VM only comprised those children who solely consumed VM. Descriptive statistics were generated as means or percentages, with a Taylor Series Linearization approach to approximate standard errors (SEs) for all estimates. These characteristics were compared by pairwise t-tests for categorical variables and orthogonal polynomial contrasts for ordinal variables as recommended by the NCHS (Table I).18 To evaluate trends in supplement use over time, P for trend across survey cycles was calculated using linear trend tests, taking into account the survey design (Table 2). Only those NVNM with a prevalence of ≥0.5% among all children at any time during the period (1999–2016) were analyzed. These trends were examined among all U.S. children and among all DS users. One-sided Rao-Scott Chi-square tests were used to test differences in NVNM use among DS users by age, sex, race/Hispanic origin, and family income over time (Figure 1), in motivations for use of NVNM and VM (Table 3), and in prevalence of each NVNM product type by age (Figure 2; available at www.jpeds.com). Motivations for use were also described for each NVNM product type (Table 5; available at www.jpeds.com). As recommended by the NCHS, estimates with a relative standard error ≥ 30% may be statistically unreliable, and these values were denoted in the tables;18 those with a relative standard error ≥40% were not reported.
Table 1.
Prevalence (% (SE))a of use and non-use of DS in the past 30 days by product type and demographic, socioeconomic, and lifestyle characteristics among all U.S. children (aged ≤19y), NHANES 1999–2016
| n | Prevalence (SE) of NVNM useb | n | Prevalence (SE) of VM only use | n | Prevalence (SE) of no DS use | |
|---|---|---|---|---|---|---|
| Percentage of all children (n=42510) | 1032 | 3.6 (0.2) | 9829 | 28.1 (0.5) | 31649 | 68.3 (0.6) |
| Sex | ||||||
| Males | 574 | 3.9 (0.2) 1 | 4822 | 27.3 (0.6) 1 | 16153 | 68.8 (0.7) |
| Females | 458 | 3.3 (0.3) 2 | 5007 | 28.9 (0.7) 2 | 15496 | 68.8 (0.7) |
| Age, yearsb | ||||||
| <1 | 26 | 1.1 (0.2) | 452 | 12.7 (0.7) | 3667 | 86.2 (0.8) |
| 1–5 | 212 | 2.5 (0.3) | 3567 | 38.4 (0.9) | 7439 | 59.1 (0.9) |
| 6–11 | 282 | 3.7 (0.4) | 2956 | 31.9 (0.8) | 7835 | 64.4 (0.9) |
| 12–19 | 512 | 4.5 (0.3)* | 2854 | 20.8 (0.6)* | 12708 | 74.7 (0.7)* |
| Race/ethnicityc | ||||||
| Non-Hispanic white | 479 | 4.6 (0.3) 1 | 3738 | 33.2 (0.8) 1 | 7682 | 62.2 (0.8) 1 |
| Non-Hispanic black | 108 | 1.1 (0.1) 2 | 1946 | 17.7 (0.6) 2 | 9243 | 81.2 (0.7) 2 |
| Hispanic | 285 | 2.2 (0.2) 3 | 2995 | 20.4 (0.6) 3 | 12405 | 77.4 (0.7) 3 |
| Family Incomed | ||||||
| Low | 311 | 2.5 (0.3) | 3664 | 20.0 (0.6) | 17434 | 77.5 (0.7) |
| Middle | 385 | 3.6 (0.3) | 3455 | 29.7 (0.8) | 9645 | 66.7 (0.8) |
| High | 336 | 5.0 (0.4)* | 2710 | 37.3 (0.9)* | 4570 | 57.6 (0.9)* |
| Household’s Education Level | ||||||
| Less than high school | 135 | 1.4 (0.2) | 1748 | 15.9 (0.7) | 11004 | 82.7 (0.8) |
| High school grad or equivalent | 193 | 3.0 (0.4) | 2119 | 24.7 (0.9) | 7470 | 72.2 (0.9) |
| Some college/ associate degree | 302 | 3.6 (0.3) | 2948 | 30.4 (0.9) | 7657 | 66.0 (0.9) |
| College degree or above | 349 | 6.0 (0.5)* | 2629 | 39.2 (1.1)* | 4183 | 54.9 (1.1)* |
| Health Insurance | ||||||
| Private | 461 | 5.2 (0.4) 1 | 3477 | 34.3 (0.8) 1 | 6882 | 60.5 (0.8) 1 |
| Public | 220 | 2.6 (0.3) 2 | 2285 | 20.3 (0.7) 2 | 10226 | 76.6 (1.6) 2 |
| None | 89 | 4.0 (0.8) 1,2 | 496 | 19.4 (1.3) 2 | 2366 | 74.7 (0.7) 2 |
| Weight Status (≥2 years) | ||||||
| Underweight | 33 | 3.3 (0.7) | 351 | 35.9 (2.1) | 698 | 60.7 (2.0) |
| Healthy | 606 | 4.2 (0.3) | 5657 | 31.4 (0.7) | 14770 | 64.5 (0.7) |
| Overweight | 141 | 3.6 (0.4) | 1160 | 26.7 (1.0) | 3935 | 69.7 (1.0) |
| Obese | 131 | 3.1 (0.3) | 1065 | 21.5 (0.8)* | 5031 | 75.4 (0.9)* |
| Screen Time (≥2 years) | ||||||
| ≤1 hour/day | 109 | 3.8 (0.6) | 1033 | 33.3 (1.6) | 2722 | 62.9 (1.5) |
| >1–2 hours/day | 187 | 4.8 (0.6) | 1482 | 34.1 (1.1) | 3338 | 61.2 (1.2) |
| >2–4 hours/day | 277 | 3.9 (0.3) | 2653 | 31.4 (0.8) | 6768 | 64.7 (0.8) |
| >4 hours/day | 228 | 3.2 (0.4) | 2627 | 27.7 (0.8)* | 8331 | 69.1 (0.8)* |
DS, dietary supplement; NHANES, National Health and Nutrition Examination Survey; NVNM, non-vitamin, non-mineral DS; VM, vitamin or mineral containing DS
Percentage (SE, standard error) adjusted for survey weights of NHANES; not all categories have the same sample sizes either due to missing data or because data were not collected in those <2 y of age.
Estimates with different numbered subscripts (i.e., 1, 2, or 3) were significantly different across subgroups within each category at p <0.0001 except NVNM use by sex and VM use by sex, for which p <0.05;
asterisk (*) indicates significant linear trend at p<0.0001, except VM use by screen time, for which p<0.001. Children <1y of age were not included in the linear trend analyses for age. All χ2 comparisons between user groups were statistically significant for all characteristics at p<0.0001, except sex for which p<0.01; none of the estimates had a relative SE ≥30%.
Hispanic includes those who identified as Mexican American. The “other” racial category is not presented herein but is represented in the overall prevalence estimates.
Poverty–income ratio (PIR) was used as a proxy for family income; Low = PIR <130%, Middle = 130%≤ PIR ≤ 350%, and High = PIR >350%.
Table 2.
Trends in prevalence (% (SE))a of all DS, NVNM, and VM use in the past 30 days among all U.S. children (≤19y of age) and of NVNM and VM use among children using any DS, NHANES 1999–2016a
| Among all children | Overall (n=42,517) | 1999–2000 (n=5076) | 2001–2002 (n=5621) | 2003–2004 (n=5072) | 2005–2006 (n=5367) | 2007–2008 (n=4213) | 2009–2010 (n=4316) | 2011–2012 (n=4195) | 2013–2014 (n=4405) | 2015–2016 (n=4252) | Ptrendb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All DSc | 31.7 (0.6) | 30.0 (1.0) | 33.0 (2.1) | 30.8 (2.1) | 31.3 (1.1) | 29.3 (1.0) | 32.0 (1.4) | 31.5 (1.8) | 33.1 (1.4) | 34.1 (2.6) | 0.20 |
| VM supplements | 28.1 (0.5) | 27.1 (0.9) | 30.6 (1.9) | 28.3 (1.9) | 28.6 (1.0) | 26.9 (1.9) | 28.4 (1.1) | 27.3 (1.6) | 27.9 (1.3) | 27.7 (1.9) | 0.54 |
| NVNM supplements | 3.6 (0.2) | 2.9 (0.6) | 2.4 (0.5) | 2.4 (0.4) | 2.8 (0.4) | 2.4 (0.5) | 3.6 (0.6) | 4.2 (0.6) | 5.1 (0.7) | 6.4 (1.1) | <0.0001 |
| Among all DS users | Overall (n=10,851) | 1999–2000 (n=1181) | 2001–2002 (n=1439) | 2003–2004 (n=1162) | 2005–2006 (n=1283) | 2007–2008 (n=947) | 2009–2010 (n=1170) | 2011–2012 (n=1188) | 2013–2014 (n=1265) | 2015–2016 (n=1226) | Ptrendb |
| VM supplements | 88.7 (0.6) | 90.4 (2.0) | 92.8 (1.3) | 92.2 (1.3) | 91.1 (1.0) | 91.7 (1.6) | 88.7 (1.6) | 86.7 (1.5) | 84.5 (2.0) | 81.2 (2.2) | <0.0001 |
| NVNM supplements | 11.3 (0.6) | 9.6 (2.0) | 7.2 (1.3) | 7.8 (1.3) | 8.9 (1.0) | 8.3 (1.6) | 11.3 (1.6) | 13.3 (1.5) | 15.5 (2.0) | 18.8 (2.2) | <0.0001 |
| Omega-3d | 1.2 (0.1) | - | - | - | 2.1 (0.6) | 3.2 (1.2)† | 5.6 (1.4) | 5.5 (1.1) | 6.0 (1.1) | 6.7 (1.5) | <0.0001 |
| Probiotics | 0.4 (0.1) | - | - | - | - | - | - | - | 1.5 (0.4) | 3.3 (0.8) | <0.001 |
| Fiber | 0.3 (0.0) | - | - | - | - | 2.2 (0.6)† | - | 1.0 (0.4)† | 2.1 (0.6) | 3.4 (0.5) | <0.0001 |
| Melatonin | 0.3 (0.0) | - | - | - | - | - | - | 2.2 (0.7)† | 2.7 (0.9)† | 3.3 (0.6) | <0.0001 |
| Botanicalse | 0.6 (0.1) | 2.9 (0.7) | 2.6 (0.5) | 2.7 (0.9)† | 1.8 (0.7)† | 1.3 (0.5)† | - | - | 2.7 (0.6) | 1.8 (0.6)† | 0.17 |
| Echinaceaf | 1.3 (0.2) | - | 1.6 (0.4) | 1.8 (0.6)† | - | - | 0.7 (0.2) | - | 1.3 (0.3) | - | 0.19 |
DS, dietary supplement; NVNM, non-vitamin, non-mineral DS; VM, vitamin or mineral containing DS; NHANES, National Health and Nutrition Examination Survey
Percentage (SE, standard error) adjusted for survey weights of NHANES
A Bonferroni-corrected p-value of 0.0056 was considered to be statistically significant
Including VM and NVNM
Omega-3 PUFAs (polyunsaturated fatty acids)
Main/only ingredient is a single plant-based supplement
Not mutually exclusive of VM or other NVNM
Relative SE ≥30%; estimates with relative SE ≥40% not reported
Figure 1.
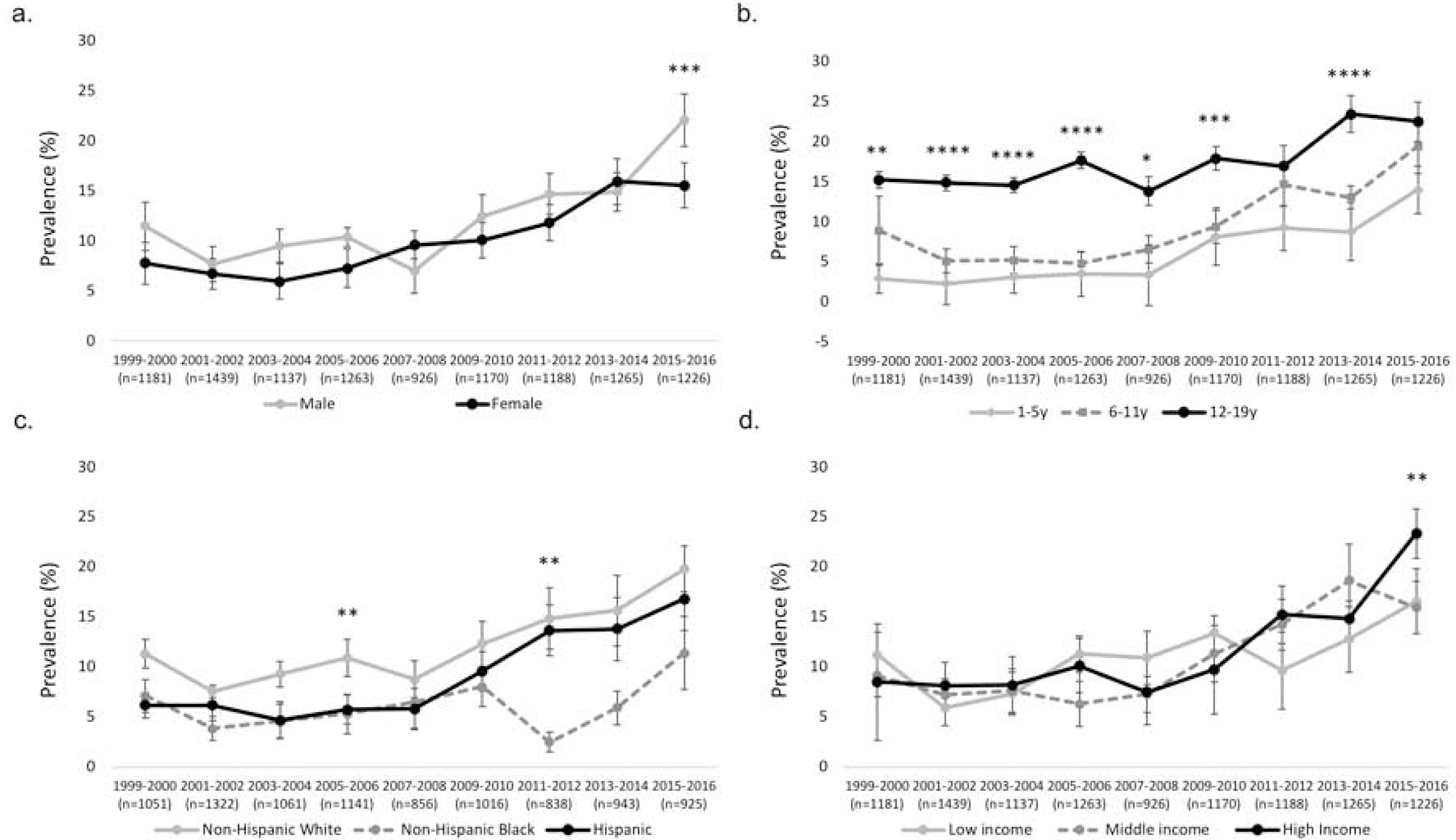
Trends in prevalence (% (SE)) of NVNM supplement use among U.S. children (≤19y of age) using any DS by sex, age, race/Hispanic origin, and family income, NHANES 1999–2016
NVNM, non-vitamin, non-mineral DS; DS, dietary supplement; NHANES, National Health and Nutrition Examination Survey; prevalence percentage (SE, standard error) adjusted for survey weights of NHANES
a. Prevalence of any NVNM use among DS users by sex; b. Prevalence of any NVNM use among DS users by age; prevalence among <1y not presented due to unreliable estimates; c. Prevalence of any NVNM use among DS users by race/Hispanic origin; d. Prevalence of any NVNM use among DS users by family income (poverty–income ratio (PIR) was used as a proxy for family income; Low = PIR<130%, Middle = 130%≤PIR≤350%, and High = PIR>350%); *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
Table 3.
Prevalence (% (SE))a of reported motivations for use of NVNM and VM in the past 30 days among U.S. children (≤19 y of age) using any DS, NHANES 2007–2016
| Motivation | NVNM users (n=785) | VM users (n=6,454) | p-valueb |
|---|---|---|---|
| To maintain health (stay healthy) | 23.1 (2.7) | 38.7 (1.3) | <0.0001 |
| To improve overall health | 22.6 (2.3) | 33.1 (1.4) | <0.0001 |
| To prevent health problems | 13.1 (1.9) | 14.4 (1.0) | 0.52 |
| For relaxation, stress, sleep | 12.7 (1.9) | 0.8 (0.3)† | <0.0001 |
| To boost immunity, prevent colds | 10.9 (1.6) | 16.5 (1.1) | 0.01 |
| To supplement the diet | 10.5 (2.1) | 20.6 (1.4) | <0.0001 |
| For mental health | 10.4 (1.8) | 1.3 (0.3) | <0.0001 |
| For bowel/colon health | 10.1 (1.7) | 0.7 (0.2) | <0.0001 |
| To improve digestion | 7.3 (1.3) | 1.2 (0.2) | <0.001 |
| For heart health, cholesterol | 5.4 (1.2) | 0.8 (0.2) | <0.0001 |
| For healthy skin, hair, and nailsc | 4.8 (0.8) | 3.2 (0.4) | 0.046 |
| To get more energy | 4.5 (0.9) | 5.3 (0.5) | 0.39 |
| For bone health | 2.7 (1.1) | 5.6 (0.8) | 0.01 |
| For weight loss | 2.6 (0.8)† | - | <0.0001 |
| Other reasons | 2.6 (0.6) | 1.5 (0.3) | 0.09 |
| For healthy joints, arthritis | 2.5 (0.7) | 1.0 (0.2) | <0.01 |
| To build muscle/weight gaind (n=700) | 2.3 (0.7)† | 1.4 (0.2) | 0.05 |
| For eye health | 1.0 (0.3)† | 1.0 (0.2) | 0.91 |
| For teeth, prevent cavities | - | 3.2 (0.5) | <0.0001 |
| For anemia, such as low iron | - | 2.4 (0.3) | <0.0001 |
| To maintain blood sugar, diabetes | - | 1.3 (0.2) | 0.35 |
| Kidney and bladder health | - | 0.4 (0.1) | 0.79 |
NVNM, non-vitamin, non-mineral DS; VM, vitamin or mineral containing DS; DS, dietary supplement; NHANES, National Health and Nutrition Examination Survey
Percentage (SE, standard error) adjusted for survey weights of NHANES; participants were able to select more than one motivation for each product
p-value based on Rao-Scott chi-square test
Hair and nails were in a separate category in 2007–2010 and combined in 2011–2016
Category not included in 2007–2008; combined in 2009–2010; included only weight gain in 2011–2012; and included as separate questions in 2013–2016 (but combined for this analysis)
Relative SE≥30%; estimates with relative SE≥40% not reported
Figure 2 online.
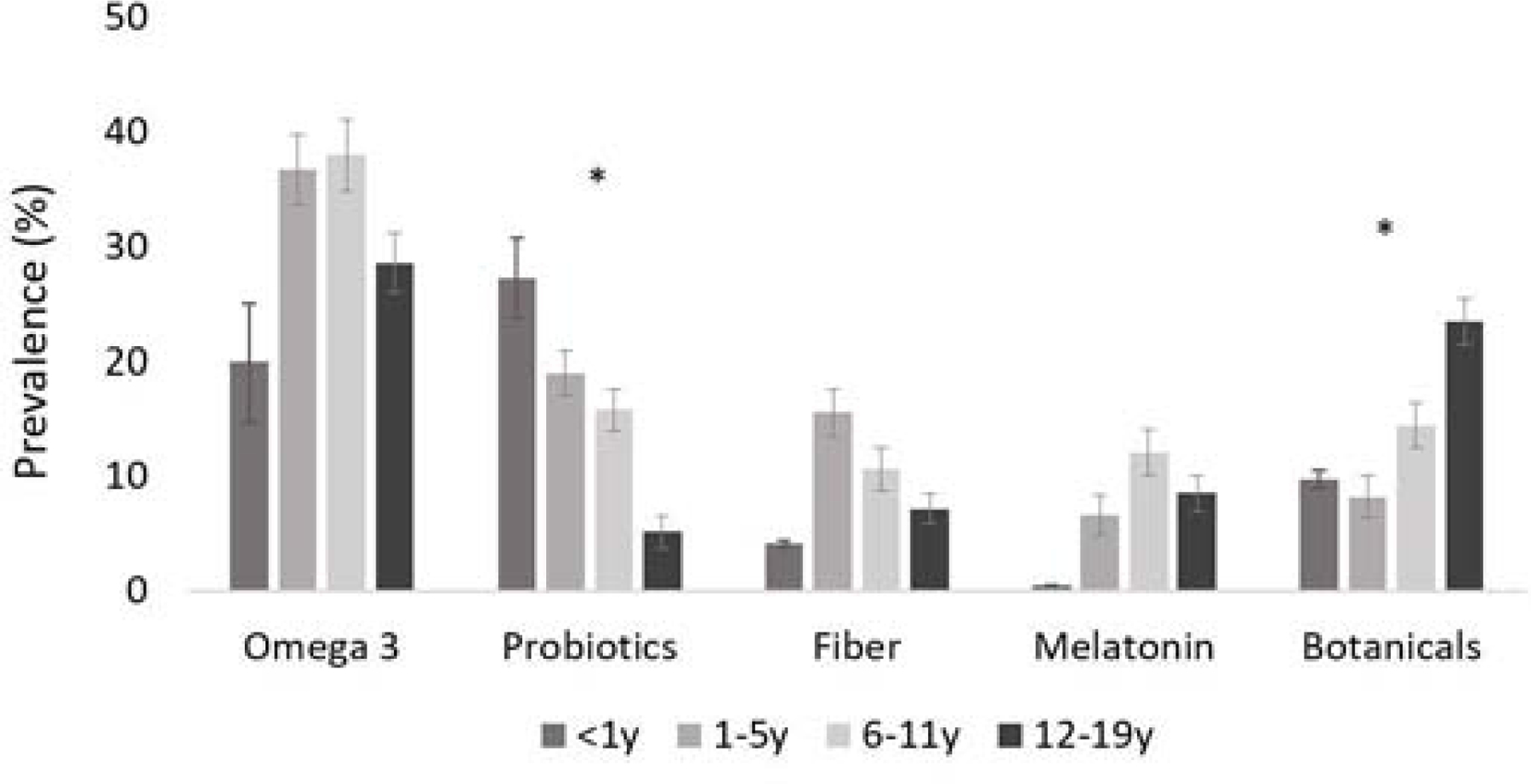
Prevalence (% (SE))a of use for each NVNM product type in the past 30 days among U.S. children (≤19y of age) using any NVNM DS by age, NHANES 1999–2016
NVNM, non-vitamin, non-mineral DS; DS, dietary supplement; NHANES, National Health and Nutrition Examination Survey
aPercentage (SE, standard error) adjusted for survey weights of NHANES; prevalence of echinacea too small to stratify
*Significant difference by age group at p<0.0001 using Rao-Scott chi-square test; the chi-square test for melatonin only included the last three age groups because the prevalence of melatonin among <1y was 0%
Table 5 online.
Prevalence (% (SE))a of specific NVNM products and their most frequently reported motivation for use in the past 30 days among U.S. children (≤19 y of age) using any NVNM DS, NHANES 2007–2016
| Prevalence (SE) among NVNM users (n=785) | Most commonly reported motivation | Prevalence (SE) of motivation | |
|---|---|---|---|
| Omega-3 PUFAs | 34.8 (2.5) | To maintain health (stay healthy) | 35.5 (3.8) |
| Probiotics | 10.9 (1.6) | For bowel/colon health | 41.8 (4.0) |
| Melatonin | 10.9 (1.6) | For relaxation, stress, sleep | 90.3 (2.0) |
| Botanicalsb | 10.8 (1.5) | To improve overall health | 38.5 (5.4) |
| Fiber | 9.7 (1.1) | For bowel/colon health | 49.4 (5.5) |
| Echinaceac | 2.6 (0.9)† | To boost immunity, prevent colds | 55.8 (14.9) |
NVNM, non-vitamin, non-mineral DS; DS, dietary supplement; NHANES, National Health and Nutrition Examination Survey; PUFA, polyunsaturated fatty acid
Percentage (SE, standard error) adjusted for survey weights of NHANES; participants were able to select more than one motivation for each product
Main/only ingredient is a single plant-based supplement
Not mutually exclusive of VM or other NVNM
Relative SE≥30%
Results
Descriptive Characteristics
As nationally representative of U.S. children, most (~68%) do not use any DS; about 4% of all children reported NVNM use whereas 28% reported VM use from 1999–2016 (Table 1). Prevalence of NVNM use was significantly higher in boys when compared with girls (3.9 vs 3.3%, p<0.05), and increased with age, regardless of sex; approximately 1% of infants reported DS use, and 4.5% of adolescents 12–19y reported doing so (ptrend<0.0001). NVNM use significantly differed by race/Hispanic origin groups; although only ~1% of non-Hispanic Black children reported taking an NVNM, use of NVNM products among Hispanic children was 2.2% and 4.6% among non-Hispanic White children (p<0.05). NVNM use was also linearly associated with family income (ptrend<0.0001) and household education (ptrend<0.0001). Children living in households with an education level of a college degree or higher had the highest use of NVNM (6.0%) whereas children living in households with an education level of less than high school had the lowest use (1.4%). NVNM use also tended to differ by type of health insurance; children with private health insurance (5.2%) were significantly more likely to use NVNM when compared with children who were insured under public health insurance (2.6%, P < .05). However, neither of these groups of children differed in prevalence of NVNM use when compared with those with no health insurance (4.0%). NVNM use did not follow a trend when examined by children’s weight status or amount of time spent in front of a screen. However, using pairwise comparisons, NVNM use was significantly lower among children with obese weight status compared with those with normal weight status (p<0.05, data not shown). Furthermore, NVNM use was significantly lower among children who spent >4 hours/day in front of a screen when compared with those who had >1–2 hours/day of screen time (p<0.05, data not shown).
Between 1999 and 2016, VM use was reported by 28% of all U.S. children and significantly differed by all descriptive characteristics (Table 1). Opposite the patterns observed with NVNM use, VM use was significantly higher among girls when compared with boys (28.9 vs 27.3%, p<0.05) and decreased by age (when infants <1y were excluded from analysis); the highest prevalence of reported VM use was among the youngest children (1–5y; 38.4%) (ptrend<0.0001). The pattern of VM use by race/Hispanic origin was similar to that for NVNM use (p<0.05). VM use also followed similar trends by family income and household education level when compared with NVNM use (ptrend<0.0001 for each). VM use was higher among children with private insurance when compared with children with public or no insurance, who did not differ. Prevalence of VM use decreased by weight status among children; nearly 36% of underweight children consumed VM, and only 21.5% of children with obesity did so (ptrend<0.0001). The prevalence of VM use also decreased by hours of screen time (ptrend<0.0001). Patterns of prevalence for no DS use for each of these characteristics were in the opposite direction as those for prevalence of VM use. However, there was no difference by sex.
Trends in Prevalence
Use of any DS and VM among all children remained relatively stable over time, with around 30% reporting use between 1999–2000 and 2015–2016 (Table 2). On the contrary, use of NVNM among all children increased by 120% during this period from 2.9% in 1999–2000 to 6.4% in 2015–2016 (ptrend<0.0001). NVNM use among children using any DS also increased by 96%, from 9.6% in 1999–2000 to 18.8% in 2015–2016 (ptrend<0.0001). Children using only VM made up the remaining proportion of DS users, and thus VM had a significant decreasing trend (ptrend<0.0001).
In the earlier survey years, with the exception of single botanicals and echinacea, NVNM use was low regardless of product type (Table 2). Omega-3 PUFA products increased by over 200%, from 2.2% in 2005–2006 to 6.8% in 2015–2016 (ptrend<0.0001). Prevalence of probiotic, fiber, and melatonin DS use was negligible in the previous decade, yet increasing trends in use were observed beginning in 2011 until 2016 (ptrend<0.0001 for all). From 2013–2016, use of probiotics increased by 120%, and use of fiber increased by 240%. Melatonin also increased, though not as rapidly as other product categories (22% increase). Use of single botanicals and echinacea did not show any discernable trend over this period. Other common NVNM that were used (but <0.5% of all children), included garlic, elderberry, and amino acids DS (data not shown).
Across the study period (1999–2016), there was no significant difference in NVNM among all DS users by sex until 2015–2016, when boys had higher prevalence of NVNM use compared with girls (p<0.001, Figure 1, A). Older children (12–19y) consistently had higher prevalence of NVNM use than younger children (Figure 1, B). However, the younger age groups (1–5y and 6–11y) exhibited a steeper increase about halfway through the study period, narrowing the gap by 2015–16. Among specific NVNM products, the prevalence of probiotic and botanical use differed by age of the child (Figure 2). No differences were found for specific NVNM product types by sex of the child (data not shown).
Non-Hispanic White children had the highest prevalence of NVNM use compared with Hispanic and non-Hispanic Black children (Figure 1, C). In general, marginal differences in the prevalence of NVNM use were observed between Hispanic and non-Hispanic Black children over time, with a notable divergence in 2007–2008. After a sharp decrease among non-Hispanic Black children during this time (p<0.01), their NVNM use slowly recovered after 2011–2012. Children living in high-income households tended to have higher NVNM use than those in low- and middle-income households, and this difference became significant in 2015–2016 (Figure 1, D).
Motivations
The two most common motivations for DS use were “to maintain health” and “to improve overall health”, though the percentages among VM were higher than those for NVNM (Table 3). For children under 2y of age, the top two motivations were “to improve digestion” and “to improve overall health” ( data not shown). More children living in low-income households reported “to improve overall health” compared with those in middle-income households (p<0.001) and in high-income households (p<0.05) (data not shown). Motivations for each type of DS product are reported in Table 5.
Less than 20% of participants reported taking NVNM at the recommendation of a physician or healthcare provider; however, this percentage did not differ for VM (data not shown). Notably, there was usage, albeit sparse, of some botanicals with safety concerns, including St. John’s wort, kava kava, and dong quai (data not shown).
Discussion
In this nationally representative analysis of U.S. children over the past two decades, there was an increase in the reported use of NVNM and overall DS use and VM use remained stable. Although DS use among U.S. adults trended upwards from 1999–2006,4 a more recent study showed overall DS and multivitamin multi-mineral use to be stable from 1999–2012, consistent with our findings in children; however, trends in NVNM use varied by product type.5 Our data also confirm previous papers with regard to patterns of VM use by demographic characteristics of DS users, including race/Hispanic origin, family income, and household educational attainment, as well as motivations for their use.3, 6, 11 However, our findings of higher prevalence of NVNM use among boys and of the opposite pattern for VM use is novel. No previous research has identified differential patterns of DS use by sex among children for VM,3, 6 or for NVNM intake.20, 21 We also found that NVNM use appears to be higher in adolescents than in younger children, which is contrary to the patterns observed for VM products. Our findings that NVNM use is highest among older children, while VM use is highest among younger children, is consistent with previous studies.3, 6, 11, 20. However, patterns of NVNM and VM use were more consistent by race/Hispanic origin; NVNM use was highest among non-Hispanic White children when compared with non-Hispanic Black and Hispanic children, a pattern that has been previously observed with VM supplements.22, 23 Similar to race/Hispanic origin, trends in VM use observed by weight status,3, 6, 24, 25 screen time,3, 6 and health insurance3, 6, 11 were also supported by previous literature evaluating NHANES data in children. NVNM use by type of health insurance also followed a previously reported pattern.11, 20 No studies were found reporting NVNM use by weight status or screen time.
The increase in use of NVNM across time appears to have been largely driven by the use of omega-3 PUFAs, probiotics, fiber, and melatonin. Our findings closely match that of a recent study that examined DS use and trends in children, including prevalence of omega-3 PUFAs, melatonin, and overall NVNM supplement use.26 However, where they used data from 2013–2014 for their main analyses, our study spans almost two decades, providing much more information and context.
In our study, omega-3 PUFAs use did not differ across age groups and were primarily reported to be used to maintain health. This is in line with previous reports of increasing omega-3 PUFA consumption among U.S. adults.2, 5 Though little is known about omega-3 supplementation patterns in children, some data suggest children (1–19y) have significantly lower energy-adjusted intakes of omega-3 PUFAs from the diet when compared with adults.27 Clinical trials with omega-3 supplementation in children generally focus on mental health and behavioral disorders; although the evidence is inconclusive, several such trials have reported therapeutic benefits.28–34 Omega-3 supplementation in early childhood has also been suggested to improve psychomotor and visual development,34 modify effects of indoor air pollution in children with asthma,35 and be effective in improving cardiovascular conditions in some children.36, 37 In adults, however, associations of omega-3 PUFA supplementation with most health outcomes have largely been null.38, 39
Our results also show a trend of increased use of probiotics and fiber, chiefly for bowel and colon health. Both product types were used more frequently in younger children than in adolescents. The prevalence of these products is still relatively small and could only be reliably estimated for the most recent survey cycles. This is in line with the trajectory of research on probiotics and prebiotic fibers, which has expanded in the previous decade.40 The gut microbiome has been shown to modulate the immune system as well as neurodevelopment via biochemical signaling.41 Studies have found beneficial effects of specific probiotic strains for gastrointestinal conditions, allergies, major depressive disorders, and autism spectrum disorders, among others.40–44 However, the evidence is not conclusive, and larger well-designed randomized control clinical trials are needed to validate these potential benefits.
Our findings also demonstrate an increase in the use of melatonin mainly for relaxation, stress, or as a sleep aid. Previous studies suggest melatonin is effective in treating children with insomnia.45–47 A meta-analysis found a significant increase in sleep time among children with neurocognitive disorders when given melatonin.48 Another meta-analysis reported that melatonin is overall well tolerated; however, long-term safety of the supplement has yet to be examined.49 U.S. sales of sleep supplements have been increasing steadily over the last three years as a whole; sales increased by 17.4% in 2019, and are projected to increase by over 30% in 2020, with a 17.1% increase in melatonin alone.50
Though there is still conflicting evidence of its benefits, echinacea has long been purported for enhancing immunomodulatory properties.20, 51 Recent meta-analyses of clinical trials have shown effectiveness of echinacea for treating upper respiratory tract infections in young children by itself52 and by enhancing the efficacy of vitamin C.53 However, not all of the trials in these meta-analyses showed a significant improvement, and some showed either no effect or adverse effects. Additional studies are warranted for examining the effects of echinacea alone and in conjunction with vitamin C.
The use of some DS among children and adolescents has been associated with adverse events,54, 55 and may have the potential to interact with prescription medications.56 At the same time, there have been observed benefits of NVNM in conjunction with medications, such as melatonin for insomnia treatment among children with ADHD using stimulant types of prescription medications.57
Strengths and Limitations
Our study leveraged almost two decades of data from NHANES, providing a large nationally representative sample to describe usage trends of DS among U.S. children. Trained researchers carefully classified all DS products into mutually exclusive categories, which have been consistently reported previously in the literature.2, 3, 10, 19 However, this was a manual process, and subjective decisions were made. As differential classifications may lead to significant variability in prevalence estimates of specific products, there is need for a standardized classification system for DS, especially NVNM.8 Another potential limitation was the use of proxies for children under 16 years of age as the reporter for DS used, as information from caregivers may be subject to bias, such as recall or social desirability bias. However, very little is known about measurement error in DS reporting.58 Furthermore, NHANES only began oversampling non-Hispanic Asians starting in 2011–2012; thus, we were not able to include this race/ Hispanic origin category in our analysis. Lastly, several NVNM prevalence estimates were too low to report, especially for earlier survey years, and many had unreliable standard errors. However, by 2015–16 most estimates were considered reliable.
Despite increases in the prevalence of use of NVNM products, high quality evidence supporting their use is lacking, especially in children. Our work presented here serves to provide nationally representative trend data on NVNM (and VM) and motivations for their use. More rigorous studies, such as randomized controlled trials, are needed to inform healthcare practitioners, children, and caregivers on the safety and effectiveness of NVNM.
Acknowledgements:
We thank Jaime Gahche, PhD (Office of Dietary Supplements, NIH) for her work and time spent on product classification and for her valuable input on the manuscript. We also thank Bridgette Kelleher, PhD (Psychological Sciences, Purdue University) for her assistance in the conceptualization of the manuscript and for her insightful comments.
Supported by the National Cancer Institute, National Institutes of Health (U01CA215834 [to R.B.]) R.B. has served as a consultant in the past to the NIH Office of Dietary Supplements, Nestle/Gerber, the General Mills Bell Institute, RTI International, and Nutrition Impact; is a trustee of the International Food Information Council and a board member of International Life Sciences Institute - North America; and has received travel support to present her research on dietary supplements.
Abbreviations:
- BMI
Body mass index
- CDC
Centers for Disease Control and Prevention
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- NVNM
Non-vitamin/non-mineral containing dietary supplements
- PIR
Income-to-poverty ratio
- PUFA
Polyunsaturated fatty acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors declare no conflicts of interest.
References:
- 1.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, et al. Dietary supplement use in the United States, 2003–2006. J Nutr 2011;141:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med 2013;173:355–61. [DOI] [PubMed] [Google Scholar]
- 3.Bailey RL, Gahche JJ, Thomas PR, Dwyer JT. Why US children use dietary supplements. Pediatr Res 2013;74:737–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gahche J, Bailey R, Burt V, Hughes J, Yetley E, Dwyer J, et al. Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994). NCHS Data Brief 2011:1–8. [PubMed] [Google Scholar]
- 5.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in Dietary Supplement Use Among US Adults From 1999–2012. Jama 2016;316:1464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jun S, Cowan AE, Tooze JA, Gahche JJ, Dwyer JT, Eicher-Miller HA, et al. Dietary Supplement Use among U.S. Children by Family Income, Food Security Level, and Nutrition Assistance Program Participation Status in 2011(−)2014. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Food and Drug Administration; Center for Food Safety and Applied Nutrition. Dietary Supplement Health and Education Act of 1994 Silver Spring, MD: Food and Drug Administration; Center for Food Safety and Applied Nutrition,; 1995. [Available from: https://health.gov/dietsupp/ch1.htm. [Google Scholar]
- 8.Bailey RL. Current regulatory guidelines and resources to support research of dietary supplements in the United States. Crit Rev Food Sci Nutr 2020;60:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowan AE, Jun S, Gahche JJ, Tooze JA, Dwyer JT, Eicher-Miller HA, et al. Dietary Supplement Use Differs by Socioeconomic and Health-Related Characteristics among U.S. Adults, NHANES 2011⁻2014. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gahche JJ, Herrick KA, Potischman N, Bailey RL, Ahluwalia N, Dwyer JT. Dietary Supplement Use among Infants and Toddlers Aged <24 Months in the United States, NHANES 2007–2014. J Nutr 2019;149:314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dwyer J, Nahin RL, Rogers GT, Barnes PM, Jacques PM, Sempos CT, et al. Prevalence and predictors of children’s dietary supplement use: the 2007 National Health Interview Survey. Am J Clin Nutr 2013;97:1331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey RL, Fulgoni VL 3rd, Keast DR, Lentino CV, Dwyer JT. Do dietary supplements improve micronutrient sufficiency in children and adolescents? J Pediatr 2012;161:837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Health and Nutrition Examination Survey [Internet]. National Center for Health and Statistics, Center for Disease Control and Prevention. Available from: https://www.cdc.gov/nchs/nhanes/index.htm. [Google Scholar]
- 14.Selhub J, Morris MS, Jacques PF. In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19995–20000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fakhouri TH, Hughes JP, Brody DJ, Kit BK, Ogden CL. Physical activity and screen-time viewing among elementary school-aged children in the United States from 2009 to 2010. JAMA Pediatr 2013;167:223–9. [DOI] [PubMed] [Google Scholar]
- 16.CDC. A SAS Program for the CDC Growth Charts
- 17.CDC. Defining Adult Overweight and Obesity
- 18.National Health and Nutrition Examination Survey: Analytic Guidelines [Internet]. National Center for Health Statistics. Available from: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx. [PubMed] [Google Scholar]
- 19.Cowan AE, Jun S, Tooze JA, Dodd KW, Gahche JJ, Eicher-Miller HA, et al. Comparison of 4 Methods to Assess the Prevalence of Use and Estimates of Nutrient Intakes from Dietary Supplements among US Adults. J Nutr 2020;150:884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu CH, Wang CC, Kennedy J. The prevalence of herb and dietary supplement use among children and adolescents in the United States: Results from the 2007 National Health Interview Survey. Complement Ther Med 2013;21:358–63. [DOI] [PubMed] [Google Scholar]
- 21.Black LI, Clarke TC, Barnes PM, Stussman BJ, Nahin RL. Use of complementary health approaches among children aged 4–17 years in the United States: National Health Interview Survey, 2007–2012. Natl Health Stat Report. 2015:1–19. [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmer MC, Rubio V, Kintziger KW, Barroso C. Racial/Ethnic Disparities in Dietary Intake of U.S. Children Participating in WIC. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace TC, Reider C, Fulgoni VL 3rd. Calcium and vitamin D disparities are related to gender, age, race, household income level, and weight classification but not vegetarian status in the United States: Analysis of the NHANES 2001–2008 data set. J Am Coll Nutr 2013;32:321–30. [DOI] [PubMed] [Google Scholar]
- 24.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hewawitharana SC, Thompson FE, Loria CM, Strauss W, Nagaraja J, Ritchie L, et al. Comparison of the NHANES dietary screener questionnaire to the Automated Self-Administered 24-Hour Recall for Children in the Healthy Communities Study. Nutr J 2018;17:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qato DM, Alexander GC, Guadamuz JS, Lindau ST. Prevalence of Dietary Supplement Use in US Children and Adolescents, 2003–2014. JAMA Pediatr 2018;172:780–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson M, Hein N, Hanson C, Smith LM, Anderson-Berry A, Richter CK, et al. Omega-3 Fatty Acid Intake by Age, Gender, and Pregnancy Status in the United States: National Health and Nutrition Examination Survey 2003–2014. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazahery H, Conlon CA, Beck KL, Mugridge O, Kruger MC, Stonehouse W, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol 2019;187:9–16. [DOI] [PubMed] [Google Scholar]
- 29.Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry 2006;163:1098–100. [DOI] [PubMed] [Google Scholar]
- 30.Reimers A, Ljung H. The emerging role of omega-3 fatty acids as a therapeutic option in neuropsychiatric disorders. Ther Adv Psychopharmacol 2019;9:2045125319858901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiNicolantonio JJ, O’Keefe JH. The Importance of Marine Omega-3s for Brain Development and the Prevention and Treatment of Behavior, Mood, and Other Brain Disorders. Nutrients. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martins BP, Bandarra NM, Figueiredo-Braga M. The role of marine omega-3 in human neurodevelopment, including Autism Spectrum Disorders and Attention-Deficit/Hyperactivity Disorder - a review. Crit Rev Food Sci Nutr 2020;60:1431–46. [DOI] [PubMed] [Google Scholar]
- 33.James S, Montgomery P, Williams K. Omega-3 fatty acids supplementation for autism spectrum disorders (ASD). Cochrane Database Syst Rev 2011:Cd007992. [DOI] [PubMed] [Google Scholar]
- 34.Shulkin M, Pimpin L, Bellinger D, Kranz S, Fawzi W, Duggan C, et al. n-3 Fatty Acid Supplementation in Mothers, Preterm Infants, and Term Infants and Childhood Psychomotor and Visual Development: A Systematic Review and Meta-Analysis. J Nutr 2018;148:409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brigham EP, Woo H, McCormack M, Rice J, Koehler K, Vulcain T, et al. Omega-3 and Omega-6 Intake Modifies Asthma Severity and Response to Indoor Air Pollution in Children. Am J Respir Crit Care Med 2019;199:1478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oner T, Ozdemir R, Doksöz O, Genc DB, Guven B, Demirpence S, et al. Cardiac function in children with premature ventricular contractions: the effect of omega-3 polyunsaturated fatty acid supplementation. Cardiol Young. 2018;28:949–54. [DOI] [PubMed] [Google Scholar]
- 37.Baumann C, Rakowski U, Buchhorn R. Omega-3 Fatty Acid Supplementation Improves Heart Rate Variability in Obese Children. Int J Pediatr 2018;2018:8789604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omega-3 Fatty Acids: National Institutes of Health Office of Dietary Supplements; 2019. [Available from: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/#h7.
- 39.Balk EM, Adams GP, Langberg V, Halladay C, Chung M, Lin L, et al. Omega-3 Fatty Acids and Cardiovascular Disease: An Updated Systematic Review. Evid Rep Technol Assess (Full Rep) 2016:1–1252. [DOI] [PubMed] [Google Scholar]
- 40.Wilkins T, Sequoia J. Probiotics for Gastrointestinal Conditions: A Summary of the Evidence. Am Fam Physician. 2017;96:170–8. [PubMed] [Google Scholar]
- 41.Ristori MV, Quagliariello A, Reddel S, Ianiro G, Vicari S, Gasbarrini A, et al. Autism, Gastrointestinal Symptoms and Modulation of Gut Microbiota by Nutritional Interventions. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 2017;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Akker CHP, van Goudoever JB, Szajewska H, Embleton ND, Hojsak I, Reid D, et al. Probiotics for Preterm Infants: A Strain-Specific Systematic Review and Network Meta-analysis. J Pediatr Gastroenterol Nutr 2018;67:103–22. [DOI] [PubMed] [Google Scholar]
- 44.Kaban RK, Wardhana, Hegar B, Rohsiswatmo R, Handryastuti S, Amelia N, et al. Lactobacillus reuteri DSM 17938 Improves Feeding Intolerance in Preterm Infants. Pediatr Gastroenterol Hepatol Nutr 2019;22:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Maanen A, Meijer AM, Smits MG, van der Heijden KB, Oort FJ. Effects of Melatonin and Bright Light Treatment in Childhood Chronic Sleep Onset Insomnia With Late Melatonin Onset: A Randomized Controlled Study. Sleep 2017;40. [DOI] [PubMed] [Google Scholar]
- 46.Janjua I, Goldman RD. Sleep-related melatonin use in healthy children. Can Fam Physician. 2016;62:315–7. [PMC free article] [PubMed] [Google Scholar]
- 47.Chang YS, Lin MH, Lee JH, Lee PL, Dai YS, Chu KH, et al. Melatonin Supplementation for Children With Atopic Dermatitis and Sleep Disturbance: A Randomized Clinical Trial. JAMA Pediatr 2016;170:35–42. [DOI] [PubMed] [Google Scholar]
- 48.Parker A, Beresford B, Dawson V, Elphick H, Fairhurst C, Hewitt C, et al. Oral melatonin for non-respiratory sleep disturbance in children with neurodisabilities: systematic review and meta-analyses. Dev Med Child Neurol 2019;61:880–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdelgadir IS, Gordon MA, Akobeng AK. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: a systematic review and meta-analysis. Arch Dis Child. 2018;103:1155–62. [DOI] [PubMed] [Google Scholar]
- 50.Reynolds C Sleep supplements expected to grow 30% in 2020: Nutrition Business Journal; 2020. [Available from: https://www.newhope.com/market-data-and-analysis/analysts-take-sleep-supplements-expected-grow-30-2020.
- 51.Sharifi-Rad M, Mnayer D, Morais-Braga MFB, Carneiro JNP, Bezerra CF, Coutinho HDM, et al. Echinacea plants as antioxidant and antibacterial agents: From traditional medicine to biotechnological applications. Phytother Res 2018;32:1653–63. [DOI] [PubMed] [Google Scholar]
- 52.Schapowal A, Klein P, Johnston SL. Echinacea reduces the risk of recurrent respiratory tract infections and complications: a meta-analysis of randomized controlled trials. Adv Ther 2015;32:187–200. [DOI] [PubMed] [Google Scholar]
- 53.Vorilhon P, Arpajou B, Vaillant Roussel H, Merlin É, Pereira B, Cabaillot A. Efficacy of vitamin C for the prevention and treatment of upper respiratory tract infection. A meta-analysis in children. Eur J Clin Pharmacol 2019;75:303–11. [DOI] [PubMed] [Google Scholar]
- 54.Or F, Kim Y, Simms J, Austin SB. Taking Stock of Dietary Supplements’ Harmful Effects on Children, Adolescents, and Young Adults. J Adolesc Health. 2019;65:455–61. [DOI] [PubMed] [Google Scholar]
- 55.Pomeranz JL, Barbosa G, Killian C, Austin SB. The Dangerous Mix of Adolescents and Dietary Supplements for Weight Loss and Muscle Building: Legal Strategies for State Action. J Public Health Manag Pract 2015;21:496–503. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi E, Sato Y, Nishijima C, Chiba T. Concomitant Use of Dietary Supplements and Medicines among Preschool and School-Aged Children in Japan. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masi G, Fantozzi P, Villafranca A, Tacchi A, Ricci F, Ruglioni L, et al. Effects of melatonin in children with attention-deficit/hyperactivity disorder with sleep disorders after methylphenidate treatment. Neuropsychiatr Dis Treat 2019;15:663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bailey RL, Dodd KW, Gahche JJ, Dwyer JT, Cowan AE, Jun S, et al. Best Practices for Dietary Supplement Assessment and Estimation of Total Usual Nutrient Intakes in Population-Level Research and Monitoring. J Nutr 2019;149:181–97. [DOI] [PMC free article] [PubMed] [Google Scholar]


