Abstract
Objective:
To compare pediatric overweight and obesity prevalence among non-Hispanic White, Mexican American, and non-Hispanic Black before and after adjusting body mass index (BMI) for pubertal status, assessed by Tanner stage, in US youth.
Study design:
We analyzed cross-sectional anthropometric and pubertal data from NHW, MA, and NHB youth in the National Health and Nutrition Examination Survey (NHANES) III. We developed specialized Tanner stage and chronological age-adjusted models to establish Tanner-stage adjusted BMI z-scores, which were then used to determine adjusted overweight/obesity prevalence. We compared pediatric overweight/obesity prevalence before and after pubertal status adjustment.
Results:
Among 3,206 youth aged 8 – 18 years (50% male; 26% NHW, 35% MA, 39% NHB), adjusting BMI for Tanner stage significantly reduced overweight (males 29% to 21%; females 29% to 17%) and obesity (male 14% to 7%; females 11% to 5%) prevalence across all races/ethnicities. The obesity prevalence reduction was more pronounced in MA (males 11% reduction; females 9% reduction) and NHB (males and females 10% reduction) compared with NHW (males 6% reduction; females 5% reduction). Similar patterns were seen in overweight prevalence.
Conclusions:
Adjusting for pubertal status reduced the prevalence of overweight/obesity in NHW, MA, and NHB youth. This suggests that adjusting for puberty incorporates changes otherwise not captured when only considering the age of a child. Adjusting BMI for pubertal status may be important when interpreting a youth’s weight status and consideration for obesity management, as well as when interpreting pediatric overweight/obesity prevalence data.
Keywords: Pediatric obesity, puberty, growth charts, body mass index, ethnic groups, health care surveys, epidemiologic methods
The Centers for Disease Control and Prevention (CDC) 2000 growth charts, based on cross-sectional national health examination surveys, are the main anthropometric assessment for US youth 2–20 years old.1 A limitation of these growth charts is that they only account for chronological age and, therefore, do not consider other factors that may affect normal growth timing and trajectory.
Evidence suggests that pubertal status may impact classification of anthropometric measures in youth, including height, weight, and body mass index (BMI).2–10 For example, in a cross-sectional UK study, Gillison et al found that early-maturing youth were five times more likely to be misclassified as overweight compared with “on-time” maturers.2 Further, studies from the US, Germany, and Denmark suggest that youth who are tall and/or undergo early maturity are more likely to have higher BMIs and/or be misclassified as obese.6–9 During puberty, there are sexually dimorphic increases in bone mineral content, lean body mass, and adiposity due to increases in gonadal sex steroids.11 “Early” maturers have increased lean mass and adiposity due to increased androgen and estrogen levels for age, respectively, which can increase BMI for age when compared with “on-time” maturers.12–14 Therefore, those experiencing earlier puberty may be more likely misclassified as overweight/obese.
Given the importance of properly categorizing weight status, we sought to test our hypothesis that incorporating pubertal status into a commonly used chronological age-only BMI metric decreases overweight/obesity prevalence among US youth. We first developed a BMI statistical model accounting for both chronological age (CA) and Tanner stage in 8–18 year old US youth in order to develop a Tanner-stage-age BMI (TSA-BMI) metric. We then examined overweight/obesity prevalence using TSA-BMI, and compared overweight/obesity prevalence as determined by TSA-BMI with that determined by the commonly used CDC 2000 chronological-age only BMI metric (CA-BMI). As pubertal timing is not congruent among races/ethnicities,5 we compared TSA-BMI with CA-BMI by race/ethnicity.
METHODS
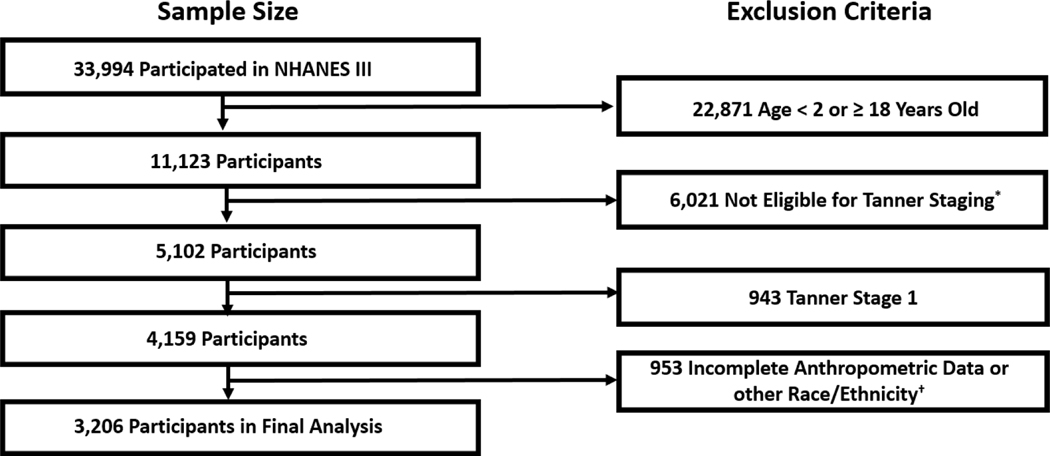
Our study population consisted of US children from the National Health and Nutritional Examinations Survey 1988–1994 (NHANES III). NHANES III was a complex cross-sectional survey of 39,695 individuals aged ≥2 months.15 CDC/National Center for Health Statistics institutional review board approval and documented consent was obtained from all participants. We used NHANES III because this was the last cycle to include pubertal assessment by Tanner staging.16 We only included participants aged 8–18 years because this was the group in which Tanner staging was performed. We excluded pre-pubertal children (Tanner stage 1) given our interest in pubertal status, and those with missing data from any of the following: age, weight, height, sex, and Tanner stage (Figure 1; available at www.jpeds.com).
Figure 1:
Study cohort flow diagram.
* Taner stage only perfomed in participants ages 2 through 18 years old
† Analysis limited to non-Hispanic black due to sample size limitations
Height and weight in NHANES III, used to determine BMI, were measured following standardized protocols.15 For race/ethnicity, participants were categorized as non-Hispanic White (NHW), Mexican American (MA), and non-Hispanic Black (NHB) based upon self-report per NHANES III groupings. Overall health was assessed by the question: “Would you say [your child’s] health is excellent, very good, good, fair, or poor?”
Pubertal status was determined by Marshall-Tanner criteria and evaluated by physicians who received standardized training.15,17–18 Tanner staging was based on inspection and comparison with standardized photos of the breast and pubic hair (for girls), and genitalia and pubic hair (for boys).15 For our analyses, we used breast (for girls) and genitalia (for boys) assessments because these are better markers of pubertal staging than pubic hair, which could falsely elevate pubertal status in children with premature adrenarche.19 We defined boys and girls as “early maturers” if their CA was less than US published national timing estimates for their sex-race/ethnicity population median age at entry into Tanner stage 2.20
We developed a specialized Tanner-stage BMI-for-age (TSA-BMI) metric incorporating both chronological age and pubertal stage using an extended function of the semi-parametric Lambda, Mu, Sigma (LMS) approach. We used the LMS method in a GAMLSS (Generalized Additive Models for Location, Scale, and Shape) technique of growth modeling to develop specialized age-conditioned growth functions within each Tanner stage.21–23 This technique ensures that each age and Tanner stage is incorporated into estimations of maturation-adjusted anthropometric normalized z-scores, and is similar to the approach used to develop the CDC and World Health Organization growth charts.1,23 Model diagnostics were followed to ascertain adequacy of fit per standard protocols.23 With each fitted function, TSA-BMI z-scores, analogous to US CDC 2000 CA z-scores, were calculated, as were corresponding TSA-BMI percentile scores. These z-scores were then used to derive indicators of weight status (overweight/obesity/severe obesity) to calculate prevalence within each category.
Overweight/obesity status for each participant was defined by a BMI-adjusted z-score ≥ +1.036 SD for overweight (equal to BMI ≥85th percentile; age and sex-adjusted), ≥ +1.645 SD for obesity (equal to BMI ≥95th percentile), and ≥ +1.975 SD for severe obesity (equal to BMI ≥1.2 times the 95th percentile24). We compared overweight/obesity/severe obesity prevalence obtained via CA-BMI with that obtained via TSA-BMI across race-ethnicity using Fieller’s theorem.25
Descriptive statistics are presented as means and percentages with standard errors (SE). To control for the three race/ethnicity groups, multiple comparisons of weight status indicators (overweight/obesity/severe obesity) were conducted at an α of 0.0167 (alpha/3). Confidence intervals (CI) were set a priori at 98.33% around each point estimate and derived from 5,000 resample bootstrap replications.25 For all other analyses, statistical significance was set at p<0.05 with complex survey design effects and weighting adjustments as appropriate. Analyses were conducted in R 3.6.0 (R Foundation, Vienna, Austria) and SAS 9.4 (SAS Institute, Cary NC, USA).
RESULTS
We included 3,206 participants aged 8–18 years, Tanner stage 2–5, with complete anthropometric data in our analysis. Primary descriptive characteristics of the study population are summarized in Table I. The mean age was 14.3 years and BMI was 21.3 kg/m2. MA youth had a higher BMI compared with NHW and NHB; however, there were no overall mean race/ethnicity differences (boys: P = .97; girls: p=0.08). Between 4–11% of participants were “early maturers,” with a higher prevalence in NHB compared with NHW and MA. The sample was largely in good health (<1% reported “poor health”).
Table I:
Population Descriptive Characteristics of a Cross-Sectional Cohort of 3,206 US Youth Ages 8 – 18 years
| Boys (n=1606) | Girls (n= 1600) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | All boys |
Non-Hispanic White (n= 402) |
Non-Hispanic Black (n= 638) |
Mexican American (n=566) |
p-value* | All girls |
Non-Hispanic White (n = 429) |
Non-Hispanic Black (n = 605) |
Mexican American (n = 566) |
p-value* |
| Mean(SE) | Mean(SE) | Mean(SE) | Mean(SE) | Mean(SE) | Mean(SE) | Mean(SE) | Mean(SE) | |||
| Age (years) | 14.3 (0.1) | 14.3 (0.2) | 14.1 (0.1) | 14.4 (0.1) | 0.47 | 14.4 (0.1) | 14.5 (0.2) | 14.0 (0.1) | 14.0 (0.2) | 0.04 |
| Height (cm) | 163.8 (0.7) | 164.6 (0.9) | 162.2 (0.7) | 160.3 (0.6) | 0.009 | 158.2 (0.5) | 158.8 (0.6) | 158.1 (0.5) | 154.2 (0.6) | 0.13 |
| Weight (kg) | 58.3 (1.0) | 58.8 (1.4) | 56.8 (1.0) | 57.7 (0.8) | 0.19 | 54.0 (0.7) | 53.9 (1.0) | 55.2 (0.7) | 52.9 (1.1) | 0.65 |
| BMI (kg/m2) | 21.2 (0.3) | 21.1 (0.3) | 21.0 (0.20) | 22.0 (0.2) | 0.97 | 21.4 (0.2) | 21.1 (0.3) | 21.8 (0.2) | 22.0 (0.3) | 0.08 |
| Health Rating† | % (SE) | % (SE) | % (SE) | % (SE) | p-value | % (SE) | % (SE) | % (SE) | % (SE) | p-value |
| Excellent | 43.8 (2.4) | 48.8 (2.9) | 34.2 (2.7) | 23.0 (2.5) | < 0.001 | 46.6 (2.4) | 52.0 (3.5) | 34.1 (3.0) | 32.2 (2.8) | < 0.001 |
| Very good | 30.3 (2.1) | 31.6 (2.5) | 27.6 (2.7) | 24.7 (2.0) | 27.2 (2.3) | 27.1 (3.1) | 31.3 (2.6) | 20.2 (2.5) | ||
| Good | 22.4 (2.2) | 18.2 (2.6) | 30.9 (3.1) | 39.1 (2.4) | 21.1 (2.1) | 18.1 (3.2) | 27.3 (2.8) | 30.4 (2.3) | ||
| Fair | 3.1 (0.6) | 1.1 (0.5) | 6.6 (1.2) | 11.9 (2.8) | 4.5 (0.8) | 2.3 (0.9) | 6.8 (1.7) | 15.0 (1.6) | ||
| Poor | 0.5 (0.3) | 0.3 (0.3) | 0.6 (0.4) | 1.3 (0.6) | 0.7 (0.4) | 0.5 (0.5) | 0.6 (0.4) | 2.2 (1.1) | ||
| “Early Maturers”‡ | 8.6 (1.2) | 8.1 (1.5) | 10.8 (1.3) | 7.7 (1.5) | 0.22 | 5.2 (0.7) | 4.0 (0.9) | 9.7 (1.3) | 5.0 (1.4) | < 0.001 |
BMI = body mass index
p-value set at 0.05 and accounted for complex survey design effects and race/ethnicity differences within gender
Determined from self/family-reported health rating question (NHANES III)
Chronological age less than US published national timing estimates for sex-race/ethnic population median age at entry into Tanner stage 2 (Sun et al.)
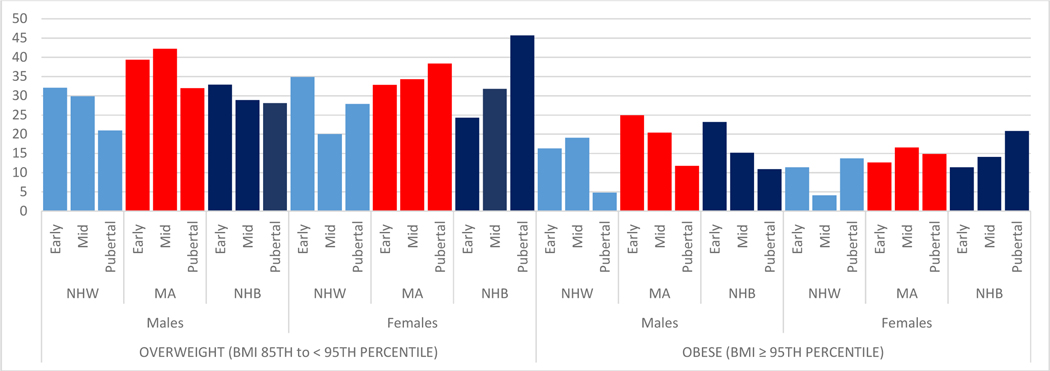
As shown in Figure 2, chronological age- and sex-adjusted (based upon the CDC 2000 growth curves per standard conventions26–27) overweight/obesity prevalence varied greatly across pubertal stage, race/ethnicity, and sex prior to Tanner-stage-age adjustments. For example, NHW and MA girls were more likely to be classified as overweight at early puberty (Tanner 2: 34.9% and 32.8%, respectively) compared with NHB girls (24.3%), and pubertal (Tanner 5) NHB girls had the highest overweight prevalence (45.7%). MA boys had higher overweight/obesity prevalence in early to mid-puberty (Tanner 2–4: 39.4–42.2% overweight, 20.4–25.0% obesity) compared with NHW or NHB boys.
Figure 2:
Numbers correspond to the percent prevalence in each category. The prevalence of overweight and obesity shown in this figure is chronological age- and sex-adjusted per the CDC 2000 growth charts per standard conventions.
BMI = body mass index; NHW = non-Hispanic White; MA = Mexican American; NHB = non-Hispanic Black
* Pubertal status categorized as early (Tanner stage 2), mid (Tanner stage 3–4), and pubertal (Tanner stage 5)
Table II summarizes overweight/obesity prevalence by race/ethnicity, comparing chronological age-only adjusted BMI (CA-BMI) with Tanner-stage-age adjusted BMI (TSA-BMI). Overall, using TSA-BMI significantly decreased overweight and obesity prevalence across all races/ethnicities for both sexes. For example, overweight prevalence decreased from 37.5% in MA boys and 35.8% in MA girls to 20.8% and 18.5%, respectively. Similarly, obesity prevalence decreased from 15.2% in NHB boys and 17.3% in NHB girls to 5.4% and 7.2%, respectively. There was no significant difference by race/ethnicity in severe obesity prevalence when comparing CA-BMI with TSA-BMI; however, sample sizes were small (zero–10 participants in each group).
Table II:
Overweight/Obesity Prevalence Before and After Adjustment for Pubertal Status (Tanner Stage) by Sex and Race/Ethnicity
| Boys | Girls | ||||||
|---|---|---|---|---|---|---|---|
| Overweight/Obesity Category | Race/Ethnicity | Non-Tanner Adjusted (CA-BMI) | Tanner Adjusted (TSA-BMI) | Non-Tanner Adjusted (CA-BMI) | Tanner Adjusted (TSA-BMI) | ||
| Prevalence (98.3% CI) | Prevalence (98.3% CI) | p-value | Prevalence (98.3% CI) | Prevalence (98.3% CI) | p-value | ||
|
Overweight (BMI 85th to < 95th percentile) |
NHW | 27.3 (22.0, 32.8) | 22.0 (17.2, 27.2) | < 0.001 | 25.7 (21.0, 30.9) | 17.0 (12.9, 21.3) | < 0.001 |
| MA | 37.5 (32.0, 42.9) | 20.8 (16.3, 25.6) | < 0.001 | 35.8 (30.5, 41.3) | 18.5 (14.2, 23.2) | < 0.001 | |
| NHB | 29.5 (25.2, 34.0) | 16.3 (13.1, 19.7) | < 0.001 | 38.1 (33.1, 43.1) | 15.6 (12.2, 19.5) | < 0.001 | |
| Overall | 28.6 (24.8, 32.6) | 20.8 (17.4, 24.7) | < 0.001 | 29.0 (25.4, 32.8) | 16.9 (13.9, 20.1) | < 0.001 | |
| Obese (BMI ≥ 95th percentile) | NHW | 13.4 (9.5, 17.7) | 7.0 (4.0, 10.6) | < 0.001 | 9.2 (6.2, 12.8) | 4.1 (2.4, 6.2) | < 0.001 |
| MA | 18.2 (14.3, 22.6) | 7.2 (4.6, 10.2) | < 0.001 | 15.0 (11.3, 19.3) | 5.9 (3.3, 9.2) | < 0.001 | |
| NHB | 15.2 (12.1, 18.5) | 5.4 (3.6, 7.4) | < 0.001 | 17.3 (13.5, 21.2) | 7.2 (4.6, 10.1) | < 0.001 | |
| Overall | 14.1 (11.3, 17.3) | 6.7 (4.5, 9.4) | < 0.001 | 11.3 (9.0, 14.0) | 4.9 (3.3, 6.5) | < 0.001 | |
CA-BMI = chronological age-only adjusted body mass index; TSA-BMI = Tanner stage and chronological age-adjusted body mass index; NHW = non-Hispanic White; NHB = non-Hispanic Black; MA = Mexican American
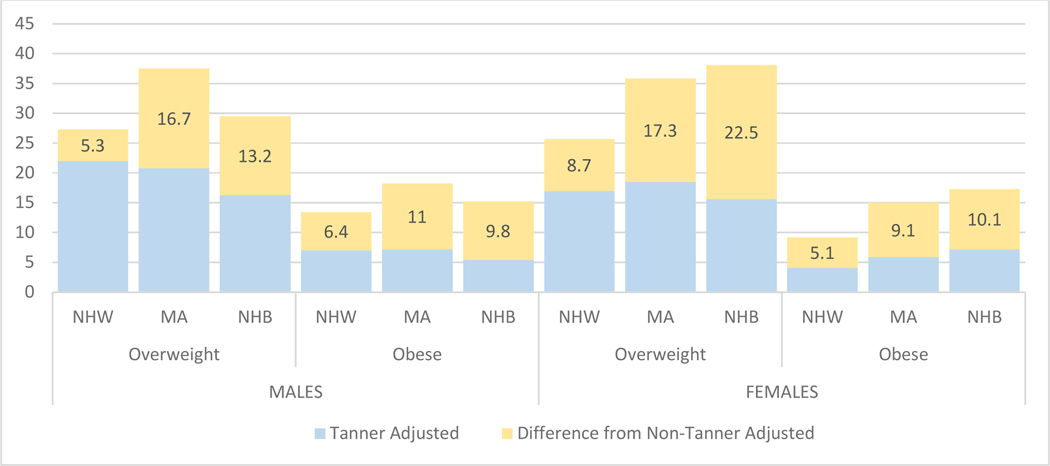
To quantify the magnitude of overweight/obesity misclassification by race/ethnicity, we calculated a percent prevalence-difference of overweight/obesity by subtracting the prevalence obtained by CA-BMI from that obtained by TSA-BMI (Figure 3). Overall, the prevalence that overweight/obesity decreased when comparing CA-BMI with TSA-BMI ranged from 5.1% (for NHW boys with obesity) to 22.5% (for NWB girls with overweight). The differences in overweight/obesity prevalence between CA-BMI and TSA-BMI were more pronounced in NHB and MA youth compared with NHW. For example, although obesity prevalence among NHW girls decreased by 5.1%, obesity prevalence in NHB and MA girls decreased by 10.1% and 9.1%, respectively.
Figure 3:
Numbers correspond to the percent prevalence difference that was calculated by substracting the prevalence obtained from chronological age-only adjusted BMI (CA-BMI) from that obtained by chronological and Tanner stage-adjusted BMI (TSA-BMI) in each category.
NHW = non-Hispanic White, MA = Mexican American; NHB = non-Hispanic Black
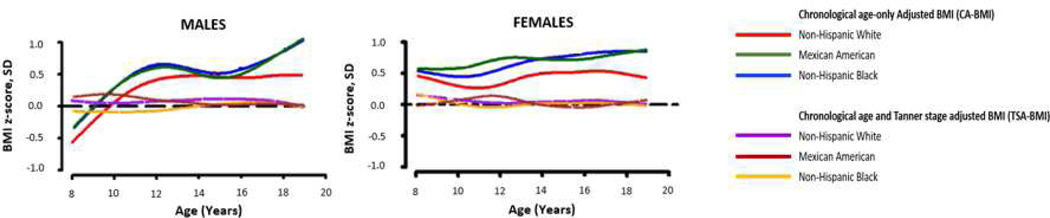
We found that, prior to Tanner adjustment the BMI curves were disparate among race/ethnicity, with overall higher BMI z-scores among NHB and MA youth compared with NHW youth at most ages. However, after Tanner adjustment the BMI curves overall condensed into similar curves (Figure 4; available at www.jpeds.com). This demonstrates that our adjustment corrects for differences that pubertal status has on BMI among race/ethnicity, indicating that the model performs as intended and, within our cohort, much of the variability in chronological age-only BMI reference data may due to maturation progression differences among races/ethnicities.
Figure 4:
This figure illustrates that, after adjusting for Tanner stage, BMI growth curves condense into similar curves. This suggests that adjusting BMI for Tanner stage corrects differences that pubertal status has on BMI among various races/ethnicities and much of the variability in current BMI-for-age reference data may be due to maturational progression differences across race/ethnicity.
BMI =body mass index; SD=standard deviation; NHW= non-Hispanic White, MA = Mexican American; NHB = non-Hispanic Black
To demonstrate the clinical utility of our model, Figure 5 shows sample TSA-BMI curves for Tanner 2 females superimposed on the CDC 2000 curves. This example demonstrates how the use of our model may avoid misclassifying an “earlier maturing” female as overweight or a “late maturing” female as underweight (BMI <5th percentile28), when both should have been classified as normal weight based upon their BMI after considering pubertal status.
Figure 5:
a) As an example of the clinical utility of our model, this figure depicts the TSA-BMI curves for Tanner stage II females (red dashed lines) superimposed on the CDC 2000 curves (black lines). Adjusting BMI by pubertal stage may allow a provider to avoid misclassifying an “early maturing” child as having a BMI in the overweight/obese category, or a “late maturing” child as having a BMI in the underweight category, when both actually have BMIs in the normal weight category after pubertal status is considered.
b) Patient 1 (symbol: blue dot) is an 8.25 year old “earlier maturing” Tanner stage II female. She would be considered overweight according to the CDC 2000 BMI-for-age charts with a sex- and age-adjusted BMI ≥85th percentile. However, after adjusting for pubertal stage (TSA-BMI), her BMI is in the normal range.
c) Patient 2 (symbol: green dot) is a 13.0 year old “late maturing” Tanner stage II female. She would be considered underweight according to the CDC 2000 BMI-for-age charts with a sex- and age-adjusted BMI <5th percentile. However, after adjusting for pubertal stage (TSA-BMI), her BMI is in the normal range.
BMI = body mass index; TSA-BMI = Tanner stage adjusted body mass index; y = years
DISCUSSION
In a multi-ethnic cross-sectional population of US youth, adjusting BMI for Tanner stage relative to chronological age resulted in reductions in pediatric overweight/obesity prevalence. Adjusting BMI for Tanner stage decreased overweight prevalence by 5.3–22.5% and obesity prevalence by 5.1–11.0%. Reductions in overweight/obesity prevalence were more pronounced in MA and NHB compared with NHW youth. Although adjusting for Tanner stage did not reduce severe obesity prevalence, our analysis was limited by small sample sizes in this category.
Our findings are consistent with prior studies and further elucidate the importance of considering puberty when determining weight status. Indeed, studies have shown that standard chronological age-only BMI z-scores may overestimate weight status prevalence if maturation is unaccounted.5–7 For example, Sorensen and Juul, in a cross-sectional study of Danish Caucasian youth, found that overweight/obesity prevalence was higher in early compared with late maturers despite similar body fat percentages.7 Gillison et al, in 9–11 year old UK children, found that adjusting weight for maturational status resulted in 32% of girls and 15% of boys with overweight being reclassified as normal weight, and 11% of boys and 8% of girls with obesity were reclassified as overweight.2 However, in this study maturational status was determined via the Khamis-Roche method, which calculates predicted adult height from a combination of a child’s height and weight with mid-parental height,29 and race/ethnicity was not considered due to under-representation.2
There are several biologic factors that may lead to children with earlier puberty having higher BMIs. Increased androgen production, which occurs at pubertal onset compared with pre-puberty, is associated with lower leptin levels and higher lean body mass.30–31 This may increase weight and, subsequently, BMI in early compared with on-time maturers. Moreover, increased estrogen, both directly and through aromatization of androgens during puberty,32 is associated with increased adiposity, which could also increase BMI in early maturers.13 Finally, studies have shown that overweight/obesity prevalence is higher in those who are relatively taller for age compared with those with average height or who are shorter. This may be due to relatively greater adiposity (if caloric intake is more than sufficient to achieve rapid linear growth, excess could be stored as subcutaneous fat) or lean mass in taller children.33–34 Such a scenario would occur in children undergoing earlier pubertal growth spurts compared with their peers, leading to a higher likelihood of overweight/obesity misclassification.
It is notable that overweight/obesity prevalence reductions after pubertal status adjustment were more pronounced in NHB compared with NHW youth. We hypothesize that this may stem from NHB being more likely to experience earlier puberty. Indeed, in our study the prevalence of “early maturers” was higher in NHB compared with NHW youth, a finding supported by previous literature. For example, among US girls, we previously showed that pubertal onset (defined by increases in luteinizing hormone and inhibin B) was 0.5 years delayed in NHB versus MA and NHW girls.35 Herman-Giddens et al found that US NHB boys reached Tanner stages 2–4 genital volume and pubic hair significantly earlier than NHW.36
We also found that reductions in overweight/obesity prevalence were more pronounced in MA compared with NHW youth, despite the prevalence of early pubertal onset being similar between these groups. This may be because MA youth are more likely to be misclassified as short, and tend to be shorter and heavier for their heights.2,37 In our previous study evaluating the effect of Tanner stage adjustment on short/tall stature prevalence, among “early maturers” MA were 45–60% more likely to be classified as short compared with NHW and NHB youth; however, after pubertal adjustment there were no significant differences between the groups in short stature prevalence.3 The fact that misclassification of short stature appears more pronounced in MA compared with NHB and NHW, in conjunction with how BMI is calculated (kg/m2), suggests that pubertal status adjustments could have a greater impact on overweight/obesity prevalence in MA youth compared with their counterparts.
It is also possible that MA, compared with NHW youth, had greater reductions in overweight/obesity prevalence despite similar timing of pubertal onset because of differences in the pattern of developing overweight/obesity by age between these populations.38 For example, in a study by Ogden et al examining obesity prevalence in US youth, MA youth had higher obesity prevalence in the 6–11 year old category (MA 25.0%, NHW 13.6%),38 during which time most youth are pre- or peri-pubertal. However, in the 12–19 year old category, during which time most youth are pubertal or post-pubertal, obesity prevalence between races/ethnicities was similar (MA 22.8%, NHW 19.6%).38 The fact that earlier development of obesity in MA youth during pre- and peri-puberty raises the obesity prevalence in these categories, in conjunction with earlier pubertal progression, may partially explain why, after adjusting for pubertal status MA youth had more pronounced reductions in obesity prevalence compared with NHW.
The diagnosis of pediatric obesity accompanies both medical and psychological sequelae, and is associated with increased healthcare utilization. Per the Endocrine Society, youth diagnosed with obesity should be prescribed intensive lifestyle interventions including diet modifications and increased physical activity.39 The American Diabetes Association recommends type 2 diabetes mellitus (T2DM) testing be considered in youth with overweight/obesity and one or more risk factors, including certain races/ethnicities (including MA and NHB).40 Further, medical providers often screen for additional obesity-related complications and co-morbidities in youth diagnosed with obesity, including non-alcoholic fatty liver disease, renal disease, and obstructive sleep apnea. These recommendations and practices all come with increased cost, both financially41–42 and in time. Further, a diagnosis of obesity may be associated with weight stigma and discrimination.43 On the contrary, if a child or adolescent is not diagnosed with obesity when they indeed have this, opportunities for earlier intervention and prevention of complications may be missed. Therefore, accurate overweight/obesity diagnoses are imperative.
Although our results suggest that, after adjusting for pubertal status overweight/obesity prevalence decreases, they do not suggest that US overweight/obesity prevalence is decreasing or otherwise not alarming. Prior investigations on US overweight/obesity prevalence did not apply our adjustment and, therefore, comparisons cannot be properly ascertained. Further, we do not know if or how adjusting for Tanner staging affects prevalence of other aspects of the metabolic syndrome, including hyperglycemia, hyperlipidemia, and hypertension. Indeed, T2DM prevalence among youth continues to increase.44
Our study has limitations. First, our results are based on NHANES III data, which occurred between 1988–1994. This was towards the beginning of the obesity epidemic, and largely pre-dated the significant rise in pediatric severe obesity.38 Therefore, we could not adequately assess if pubertal status reduces pediatric severe obesity prevalence due to sample size limitations. We utilized NHANES III because this was the last cycle to incorporate Tanner staging and more contemporaneous NHANES samples were not available.
It is unclear how these results apply to other races/ethnicities. Moreover, as our results were based on cross-sectional data, we could not determine temporality of relationships within individuals. Analyses of longitudinal data incorporating age, anthropometric variables, and pubertal status would allow for improved modeling than can be ascertained through cross-sectional studies. Future NHANES cycles and/or large-scale multi-ethnic longitudinal studies should include Tanner stage assessments. This is even more important in light of more recent NHANES cycles including body composition measures via techniques such as dual x-ray absorptiometry and bioelectrical impedance,16 which may differentially reflect metabolic status and cardiometabolic risk compared with BMI measures, and may become more useful in the clinical setting when such techniques become more widespread in clinical practice.45–46 Along these lines, including pubertal assessments into more primary care and weight management provider visits, where such body composition assessments may be adopted more often, could lead to the development of more robust longitudinal data registries to explore this.
Finally, it is important to note that creation of the CDC 2000 growth charts excluded weights from NHANES III participants ≥6 years old to avoid an upward shift in weight- and BMI-for-age curves due to rising overweight prevalence, thereby under-classifying overweight/obesity status.47 Because of this, our curves utilizing NHANES III participants may not align completely with CDC 2000 BMI curves and may be biased towards higher weight categories.
Adjusting for pubertal status appears to have a more profound impact on decreasing overweight/obesity prevalence among NHB and MA youth compared with NHW, likely due to differences in pubertal onset timing and patterns of weight gain between these race/ethnic groups. Pubertal adjustments may be important when interpreting overweight/obesity prevalence data. When considering an adolescent’s weight status in the clinical setting, it is also be important to account for pubertal status.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr Aaron Kelly for his review of this manuscript.
E.B. is currently funded through an NIH K23 Career Development Award, and is a site principal investigator for Novo Nordisk. B.M. is a consultant for Abbvie, Ascendis, BioMarin, Bluebird Bio, Endo Pharmaceuticals, Novo Nordisk, Pfizer, Sandoz, Sanofi Genzyme, Tolmar, and Vertice, and has received research support from Alexion, Abbvie, Amgen, Ascendis, BioMarin, Novo Nordisk, Opko, Protalix, Sandoz, Sangamo, Sanofi Genzyme, and Takeda. K.S. receives research support from the DHHS Federal Food and Drug Administration, NIH National Cancer Institute, National Science Foundation, Spruce Biosciences, Alexion and Neurocrine. O.A. declares no conflicts of interest. Research reported in this publication was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number K23DK125668. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- BMI
Body mass index
- CA-BMI
Chronological age adjusted body mass index
- CDC
Centers for Disease Control and Prevention
- CI
Confidence interval
- NHANES
National Health and Nutrition Examination Survey
- SD
Standard deviation
- SE
Standard error
- T2DM
Type 2 diabetes mellitus
- TSA-BMI
Tanner stage adjusted body mass index
Footnotes
Data Statement: Data used for this study is available from the National Health and Nutritional Examinations Survey 1988–1994 (NHANES III), which can be accessed at https://wwwn.cdc.gov/nchs/nhanes/nhanes3/DataFiles.aspx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Grummer-Strawn LM, Garza C, Johnson CL. Childhood growth charts. Pediatrics 2002;109(1):141–142. [DOI] [PubMed] [Google Scholar]
- 2.Gillison F, Cumming S, Standage M, Barnaby C, Katzmarzyk P. Assessing the impact of adjusting for maturity in weight status classification in a cross-sectional sample of UK children. BMJ Open 2017;7(6):e015769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Addo OY, Sarafoglou K, Miller BS. Effect of adjusting for tanner stage age on prevalence of short and tall stature of youths in the United States. J Pediatr 2018;201:93–99. [DOI] [PubMed] [Google Scholar]
- 4.Ramnitz MS, Lodish MB. Racial disparities in pubertal development. Semin Reprod Med 2013;31(5):333–339. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Adair L. How does maturity adjustment influence the estimates of overweight prevalence in adolescents from different countries using an international reference? Int J Obes Relat Metab Disord 2001;25(4):550–558. [DOI] [PubMed] [Google Scholar]
- 6.Himes JH. Maturation-related deviations and misclassification of stature and weight in adolescence. Am J Hum Biol 1999;11(4):499–504. [DOI] [PubMed] [Google Scholar]
- 7.Sorensen K, Juul A. BMI percentile-for-age overestimates adiposity in early compared with late maturing pubertal children. Eur J Endocrinol 2015;173(2):227–235. [DOI] [PubMed] [Google Scholar]
- 8.Bonthuis M, Jager KJ, Abu-Hanna A, Verrina E, Schaefer F, van Stralen KJ. Application of body mass index according to height-age in short and tall children. PLoS One 2013;8(8):e72068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stovitz SD, Demerath EW, Hannan PJ, Lytle LA, Himes JH. Growing into obesity: patterns of height growth in those who become normal weight, overweight, or obese as young adults. Am J Hum Biol 2011;23(5):635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller BS, Sarafoglou K, Addo OY. Development of tanner stage age adjusted CDC height curves for research and clinical applications. J Endocr Soc (2020). doi: 10.1210/jendso/bvaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soriano-Guillen L, Sarafoglou K, Argente J. Precocious puberty. In: Sarafoglou K, Hoffman GF, Roth KS, eds. Pediatric Endocrinology and Inborn Errors of Metabolism. 2nd ed. New York: McGraw Hill; 2017. p. 643–662. [Google Scholar]
- 12.Sarafoglou K, Forlenza GP, Addo OY Kyllo J, Lteif A, Hindmarsh PC, et al. Obesityin children with congenital adrenal hyperplasia in the Minnesota cohort: importance of adjusting body mass index for height-age. Clin Endocrinol 2017;86(5):708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grantham JP, Henneberg M. The estrogen hypothesis of obesity. PLOS One 2014;9(6):e99776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lizcano F, Guzman G. Estrogen deficiency and the origin of obesity during menopause. Biomed Res Int 2014;2014:757461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. National Center for Health Statistics. NHANES III (1988–1994) [updated 2020 Feb 21; cited 2020 Jul 27]. Available from: https://wwwn.cdc.gov/nchs/nhanes/nhanes3/Default.aspx.
- 16.NHANES: Historical summary of component content over time [updated 2009 May 7; cited 2020 Jul 27] Available from: https://wwwn.cdc.gov/nchs/data/nhanes/Historical_NHANES_component_matrix.pdf.
- 17.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45(239):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44(235):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunkel L, Sarafoglou K, Rey R, Lee PA. Variants of pubertal progression. In: Sarafoglou K, Hoffman GF, Roth KS, eds. Pediatric Endocrinology and Inborn Errors of Metabolism. 2nd ed. New York: McGraw Hill; 2017. p. 663–680. [Google Scholar]
- 20.Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, et al. , National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics 2002;110(5):911–919. [DOI] [PubMed] [Google Scholar]
- 21.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 1992;11(10):1305–19. [DOI] [PubMed] [Google Scholar]
- 22.Rigby R, Stasinopoulos D. Generalized additive models for location, scale and shape. J R Stat Soc C App 2005;54:507–554. [Google Scholar]
- 23.Cole TJ. The development of growth reference and growth charts. Ann Hum Biol2012;39(5):382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches. Circulation 2013;15(8):1689–1712. [DOI] [PubMed] [Google Scholar]
- 25.Fieller EC. Some problems in interval estimation. J Royal Statistics Soc Series B1954;16(2):175–185. [Google Scholar]
- 26.Han JC, Lawler DA, Kimm SYS. Childhood obesity – 2010: progress and challenges. Lancet 2010;375(9727):1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA 2018;319(16):1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maring B, Greenspan LC, Chandra M, Daniels SR, Sinaiko A, Prineas RJ, et al. Comparing U.S. pediatric and adult weight classification at the transition from late teenage to young adulthood. Pediatr Obes 2015;10:371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khamis HJ, Roche AF. Predicting adult stature without using skeletal age: the Khamis-Roche method. Pediatrics 1994;94:504–507. [PubMed] [Google Scholar]
- 30.Mason KA, Schoelwer MJ, Rogol AD. Androgens during infancy, childhood, and adolescence: physiology and use in clinical practice. Endocr Rev 2020;41(3):bnaa003. [DOI] [PubMed] [Google Scholar]
- 31.Luukkaa V, Pesonen U, Huhtaniemi I, Lehtonen A, Tilvis R, Tuomilehto J, et al. Inverse correlation between serum testosterone and leptin in men. J Clin Endocrinol Metab 1998;83(9):3243–3246. [DOI] [PubMed] [Google Scholar]
- 32.Biro FM, Pinney SM, Huang B, Baker ER, Walt Chandler D, Dorn LD. Hormone changes in peripubertal girls. J Clin Endocrinol Metab 2014;99(10):3829–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedman DS, Thornton JC, Mei Z, Wang J, Dietz WH, Pierson RN, et al. Height and adiposity among children. Obes Res 2004;12:846–853. [DOI] [PubMed] [Google Scholar]
- 34.Forbes GB. Relation of lean body mass to height in children and adolescents. Pediatr Res 1972;6:32–37. [DOI] [PubMed] [Google Scholar]
- 35.Addo OY, Miller BS, Lee PA, Hediger ML, Himes JH. Age at hormonal onset of puberty based on leutinizing hormone, inhibin B, and body composition in preadolescent US girls. Pediatr Res 2014;76(6):564–570. [DOI] [PubMed] [Google Scholar]
- 36.Herman-Giddens ME, Steffes J, Harris D, Slora E, Hussey M, Dowshen SA, et al. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics 2012;130(5):e1058–e1068. [DOI] [PubMed] [Google Scholar]
- 37.Martorell R, Mendoza FS, Castillo RO, Pawson IG, Budge CC. Short and plump physique of Mexican American children. Am J Phys Anthropol 1987;73:475–487. [DOI] [PubMed] [Google Scholar]
- 38.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA 2016;315(21):2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, Yanovksi JA. Pediatric obesity – assessment, treatment, and prevention: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2017;102(3):709–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and managemnt of youth-onset type 2 diabetes: a position statement by the American Diabetes Association. Diabetes Care 2018;41:2648–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tremmel M, Gerdtham U, Nilsson PM, Saha S. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health 2017;14:e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilden Tsai A, Williamson DF, Glick HA. Direc medical cost of overweight and obesity in the United States: a quantative systematic review. Obes Rev 2011;12(1):50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pont SJ, Puhl R, Cook SR, Slusser W. Stigma experienced by children and adolescents with obesity. Pediatrics 2017;140(6):e20173034. [DOI] [PubMed] [Google Scholar]
- 44.Mayer-Davies EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence trends of type 1 and type 2 diabetes among youth, 2002–2012. New Eng J Med 2017;376(15):1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanderwall C, Eickhoff J, Clark RR, Carrel AL. BMI z-score in obese children is a poor predictor of adiposity changes over time. BMC Pediatr 2018; 18:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly AS, Daniels SR. Rethinking the use of body mass index z-score in children and adolescents with severe obesity: time to kick it to the curb? J Pediatr. 2017;188:7–8. [DOI] [PubMed] [Google Scholar]
- 47.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11 2002;246:1–190. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.