Abstract
Objective:
To evaluate long-term transplant-free survival and causes of death in the Trisomy 21 (T21) population after surgery for congenital heart disease (CHD) in comparison with euploidic patients.
Study design:
This is a retrospective cohort study from the Pediatric Cardiac Care Consortium, enriched with prospectively collected data from the National Death Index and the Organ Procurement and Transplantation Network for patients with sufficient direct identifiers. Kaplan-Meier survival plots were generated and multivariable Cox proportional hazards models were used to examine risk factors for mortality between T21 and 1:1 matched euploidic patients with a comparable CHD.
Results:
Long-term survival analysis was completed for 3,376 patients with T21 (75,155 person-years) who met inclusion criteria. Thirty-year survival for patients with T21 ranged from 92.1% for ventricular septal defect to 65.3% for complex common atrioventricular canal. Of these, 2,185 patients with T21 were successfully matched to a euploidic patient. After a median follow-up of 22.86 years (IQR 19.45–27.14 years), 213 deaths occurred in the T21 group (9.7%), compared with 123 (5.6%) in the euploidic comparators. After adjustment for age, sex, era, congenital heart disease complexity and initial palliation, the hazard ratio of CHD-related mortality was 1.34 times higher in patients with T21 (95% CI 0.92–1.97, P = .127).
Conclusion:
CHD-related mortality for patients with T21 following cardiac surgical intervention is comparable with euploidic comparators. Children with T21 require lifelong surveillance for co-occurring conditions associated with their chromosomal abnormality.
Keywords: congenital heart surgery, genetics, outcomes, statistics (survival analysis)
Congenital heart disease (CHD) is present in 40–50% of children born with Trisomy 21 (T21)1 with several epidemiologic studies highlighting the impact CHD has on their survival.1–7 Despite the additional co-occurring conditions associated with T21, early operative mortality for some types of two-ventricle CHD has been reported to be similar in patients with T21 versus those without.8–12 However, long-term outcomes of children with T21 who survive CHD interventions are not well understood.13
The purpose of this retrospective cohort study was to describe in-hospital and long-term transplant-free survival, causes of death, and predictors of long-term mortality in children with T21 following surgical intervention for CHD with two-ventricle physiology. Additionally, we aimed to directly compare the long-term survival between children with T21 and euploidic children with equivalent CHD diagnoses and related surgeries.
METHODS
This study was approved by the Institutional Review Board at MemorialCare Health Services, the University of Minnesota, and Emory University with a waiver of consent.
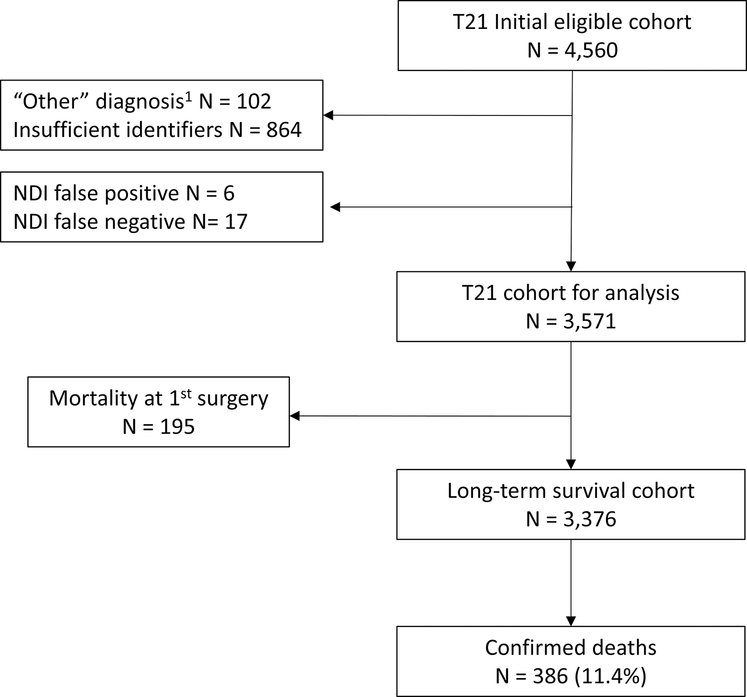
Data were obtained from the Pediatric Cardiac Care Consortium (PCCC), a large US-based registry formed in 1982, encompassing over 140,000 patient outcomes of CHD interventions through 2011.14 Patients with T21 who were enrolled in the PCCC and underwent their first CHD surgery in a US PCCC center before the age of 21 years and April 15, 2003 (implementation date of stricter Health Insurance Portability and Accountability Act [HIPAA] privacy rules) were eligible for inclusion. We included only patients who underwent their initial CHD surgery at a PCCC center to avoid introducing immortal person-time bias. A STROBE diagram is detailed in Figure 1 (available at www.jpeds.com). We excluded patients who underwent initial cardiac transplant, isolated patent ductus arteriosus ligation, a diagnosis of heterotaxy syndrome or its features (such as interrupted inferior vena cava, asplenia or polysplenia, situs ambiguous, or intestinal malrotation), single ventricle CHD, and patients who underwent a superior cavo-pulmonary anastomosis as part of a “one and a half” ventricle repair strategy. Long-term survival of children with T21 following single ventricle palliation was previously reported from our group.15
Figure 1,
online only. STROBE diagram of Trisomy 21 two-ventricle congenital heart surgery patients.
Data collection included age and weight at operation, year of operation, cardiac diagnoses, cardiac surgical interventions, and discharge outcome. The primary cardiac diagnosis was categorized as mild, moderate, or severe using a modified complexity classification proposed by the Canadian Consensus Conference on Adult Congenital Heart Disease in 1996.16–18 Based on this categorization, we identified the eight most common intracardiac diagnoses that could be matched with euploidic PCCC comparators from the same time period. These diagnostic categories included: 1) atrial septal defect (ASD), including ostium primum, secundum and sinus venosus type of defects; 2) incomplete atrioventricular canal, including partial or transitional atrioventricular canal (iAVC); 3) simple ventricular septal defect (VSD), including isolated perimembranous, supracristal or muscular defects; 4) complex VSD, with associated aortic valve stenosis, coarctation of the aorta (CoA), right ventricular outflow tract obstruction, or double outlet right ventricle (DORV with VSD physiology); 5) common atrioventricular canal (CAVC); 6) CAVC associated with tetralogy of Fallot (TOF) or DORV with TOF physiology with or without pulmonary atresia (CAVC-TOF); 7) CAVC with aortic arch obstruction including coarctation of the aorta (CoA) or interrupted aortic arch; and 8) Tetralogy of Fallot (all forms of TOF with and without pulmonary atresia). Other forms of CHD were excluded from the comparison analysis.
We collected data on subsequent procedures and reoperations performed within PCCC through 2011. Long-term survival of these patients with T21 was compared with PCCC euploidic comparators, matched 1:1 by cardiac diagnosis category. We categorized the treatment era as early (1982–1992), middle (1993–1997) and late (1998–2003) based on approximate tertiles of the date of first PCCC operation. Age groups were defined as neonatal (<28 days), infant (28 days to 364 days), child (1 to 5 years), and older child (6 to 20 years).
Death and transplant that occurred beyond the initial surgical hospitalization were obtained by subsequent PCCC records, linkage to the National Death Index (NDI) and the Organ Procurement and Transplantation Network (OPTN) respectively.19 Data registry forms were reviewed to obtain additional clinical details as needed. Linkage through December 31, 2019 was possible for the subset of patients who had direct identifiers available. The NDI is the “gold standard” of mortality and cause of death information in the United States (US).20 Sensitivity of the overall PCCC-NDI linkage reached 88.1% for death events among patients with adequate identifiers, and specificity exceeded 99%.19 Available PCCC identifiers were submitted to OPTN to determine transplant status from January 1988 (earliest available data from OPTN registry) through December 31, 2019. The OPTN data system includes data on all donor, wait-listed candidates and transplant recipients in the US, submitted by its members. The Health Resources and Services Administration, US Department of Health and Human Services, provides oversight to the activities of the OPTN contractor. Transplant status was defined as transplant listing or organ transplant identified through PCCC report or OPTN data. The sensitivity of the PCCC-OPTN linkage reached 89.7% and specificity exceeded 99%.19
Long-term mortality was assessed by death events reported in the NDI following discharge after the first PCCC cardiac surgery. Underlying and contributing causes of death were categorized using ICD-9/10 codes as reported by the NDI-PLUS, a supplementary NDI service collecting causes of death from death certificates. Underlying and contributing modes of death included CHD-related, cardiovascular disease (CVD)-related, and non-CHD, non-CVD-related deaths.
Demographic and clinical characteristics were summarized overall and by the comparator group using means and standard deviations, medians and ranges or counts and percentages, as appropriate. Group comparisons utilized Chi-square or Fisher exact test for categorical variables and independent sample t-test or Wilcoxon rank sum test for continuous variables. Kaplan-Meier survival estimates and log-rank tests were used to describe long-term transplant-free survival. Follow-up duration was determined from the date of first PCCC surgical hospital discharge through date of death, transplant or December 31, 2019 (date of latest NDI and OPTN update), whichever occurred first.
Risk factors associated with long-term survival were examined using univariable and multivariable Cox proportional hazard regression modeling and are presented as hazard ratios (HRs) with associated 95% confidence intervals. Univariable predictors with a significance < 0.20 were included in the multivariable model. The multivariable model for long-term survival included sex, age group, surgical era, initial palliation status, and CHD complexity. A similar multivariable model was fitted for patients with simple CAVC because this is the most common type of CHD in patients with T21. The Cox proportional hazard assumption was assessed graphically and statistically, and when violated, a Heaviside function was employed to address time-varying effects. Meaningful cut points for each time-varying variable were determined by graphical significance. Competing risk regression was used to compare CHD-related mortality between patients with T21 and euploidic controls, using non-CHD-related mortality or transplant as the competing risks. A two-sided p value < 0.05 was considered statistically significant. Statistical analysis was completed using STATA version 16.1 (StataCorp LLC, College Station, TX). The T21 cohort of eight most common diagnoses was matched 1:1 to PCCC patients without genetic conditions and with the same diagnosis category using simple random sampling.
RESULTS
A total of 4,560 patients with T21 underwent their first surgery for a two-ventricle CHD in the PCCC between 1982 and April 15, 2003 (Figure 1). The eight most common primary cardiac diagnoses were identified (n = 4,458), and the remaining 102 patients were excluded from further analysis. After exclusion of patients with insufficient identifiers or incorrect information, a total of 3,571 (80.1%) patients with T21 remained eligible for PCCC-NDI linkage (Figure 1). This accounts for 9.5% of the whole PCCC cohort meeting the same eligibility criteria and 69% of the patients with a chromosomal anomaly within this cohort.17 Within the T21 cohort, there were 247 deaths reported by PCCC records; of these, 230 had a matching NDI record of death, yielding a PCCC-NDI linkage sensitivity of 93.1% (95% CI 89.2–95.9%) for T21 in-hospital mortality. Characteristics of the T21 PCCC cohort by outcome are presented in Table I (available at www.jpeds.com). The most common CHD among patients with T21 was CAVC (44.0%) and therefore most fell under the moderate complexity category (59.5%). Patients with confirmed death following the first surgical hospitalization were more likely to have had initial surgery in the earliest surgical era, initial palliation, and more complex CHD than those not confirmed dead. Table 2 (available at www.jpeds.com) summarizes the initial and subsequent congenital heart surgeries during the same or subsequent hospitalization performed in the T21 cohort. Repair of CAVC (n = 1,445, 40.4%) and VSD closure (n = 948, 26.6%) were the most common initial operations, and the most common subsequent procedures were left atrioventricular valve repair/replacement (n = 188, 22.1%) or permanent pacemaker procedure (n = 167, 19.6%). In-hospital mortality for the first surgical procedure was 5.46% (195/3,571), and improved significantly from the earliest to the latest treatment era (from 8.1% to 3.8%, p < 0.001).
Table 1, online only.
Characteristics of PCCC T21 cohort
| Total | Death at 1st surg. hosp | Long-term survival | Confirmed death | P value | |
|---|---|---|---|---|---|
| (n = 3,571) | (n = 195) | (n = 2,990) | (n = 386) | ||
| Age 1st surg N (%) | <0.001 | ||||
| < 28 days | 134 (3.8) | 28 (14.4) | 79 (2.6) | 27 (7.0) | |
| 28–364 days | 2,587 (72.4) | 145 (74.4) | 2,173 (72.7) | 269 (69.7) | |
| 1–5 yr | 685 (19.2) | 17 (8.7) | 596 (19.9) | 72 (18.6) | |
| 6–20 yr | 165 (4.6) | 5 (2.6) | 142 (4.7) | 18 (4.7) | |
| Age 1st surgery (yrs) median, (IQR) | 0.51 (0.33–0.94) | 0.40 (0.20–0.65) | 0.52 (0.34–0.98) | 0.51 (0.28–0.90) | <0.001 |
| Sex N (%) | 0.326 | ||||
| Male | 1,725 (48.3) | 91 (46.7) | 1,434 (48.0) | 200 (51.8) | |
| Female | 1,846 (51.7) | 104 (53.3) | 1,556 (52.0) | 186 (48.2) | |
| Era 1st surg N (%) | <0.001 | ||||
| Early: 1982–1992 | 1,092 (30.6) | 89 (45.6) | 837 (28.0) | 166 (43.0) | |
| Middle: 1993–1997 | 1,103 (30.9) | 54 (27.7) | 937 (31.3) | 112 (29.0) | |
| Late: 1999–2003 | 1,376 (38.5) | 52 (26.7) | 1,216 (40.7) | 108 (28.0) | |
| Palliation N (%) | 313 (8.8) | 35 (17.9) | 182 (6.1) | 96 (24.9) | <0.001 |
| Complexity N (%) | <0.001 | ||||
| Mild | 1,191 (33.4) | 25 (12.8) | 1,085 (36.3) | 81 (21.0) | |
| Moderate | 2,124 (59.5) | 131 (67.2) | 1,749 (58.5) | 244 (63.2) | |
| Severe | 256 (7.2) | 39 (20.0) | 156 (5.2) | 61 (15.8) | |
| ECLS at 1st surgical hospitalization N (%) | 15 (0.4) | 10 (5.1) | 5 (0.2) | 0 | <0.001 |
| PPM at 1st surgical hospitalization N (%) | 75 (2.1) | 3 (1.5) | 57 (1.9) | 15 (3.9) | 0.050 |
| Cardiac Dx N (%) | <0.001 | ||||
| ASD | 239 (6.7) | 2 (1.0) | 219 (7.3) | 18 (4.7) | |
| Incomplete AVC | 324 (9.1) | 4 (2.0) | 289 (9.7) | 31 (8.0) | |
| VSD simple | 952 (26.7) | 23 (11.8) | 866 (29.0) | 63 (16.3) | |
| VSD complex | 60 (1.7) | 2 (1.0) | 54 (1.8) | 4 (1.0) | |
| CAVC | 1,570 (44.0) | 116 (59.5) | 1,268 (42.1) | 186 (48.2) | |
| CAVC+ arch obstr | 62 (1.7) | 13 (6.7) | 32 (1.1) | 17 (4.4) | |
| TOF | 205 (5.7) | 13 (6.7) | 162 (5.4) | 30 (7.8) | |
| CAVC + TOF | 159 (4.5) | 22 (11.3) | 100 (3.3) | 37 (9.6) | |
Given are number (N) and percent (%) by column for categorical variables, median and interquartile range (IQR) for continuous variable. P values represent differences between death at first surgical hospitalization, alive at follow-up, and confirmed death groups using Chi-square or Fisher’s exact test as appropriate for categorical variables, Kruskal-Wallis test for continuous variables. Surg.hosp = surgical hospitalization; ECLS = extracorporeal life support; PPM = permanent pacemaker; Dx = diagnosis; ASD = atrial septal defect; Incomplete AVC = partial or transitional atrioventricular canal; VSD = ventricular septal defect; CAVC = common atrioventricular canal; arch obstr = aortic arch obstruction including coarctation or interrupted aortic arch; TOF = tetralogy of Fallot.
Table 2, online only.
Initial and subsequent surgical procedures in patients with Trisomy 21a
| Procedure Name N (%) | Initial procedure (n = 3,571) | Procedure Name N (%) | Subsequent procedure (n =852) |
|---|---|---|---|
| CAVC repair | 1,445 (40.4) | LAVV repair or replacement | 188 (22.1) |
| VSD closure | 948 (26.6) | Permanent pacemaker | |
| initial | 115 (13.5) | ||
| replacement | 52 (6.1) | ||
| Incomplete AVC repair | 319 (8.9) | CAVC repair | 126 (14.8) |
| ASD repair | 238 (6.7) | VSD closure | 74 (8.7) |
| TOF repair | 152 (4.3) | Subaortic resection | 58 (6.8) |
| PA band | 135 (3.8) | TOF repair | 54 (6.3) |
| AP shunt | 132 (3.7) | TOF + CAVC repair | 39 (4.6) |
| TOF + CAVC repair | 75 (2.1) | AP shunt | 26 (3.0) |
| CoA/IAA repair | 54 (1.6) | RVOT procedure | 17 (2.0) |
| PDA ligation | 43 (1.2) | Supravalvar aortic stenosis repair | 14 (1.6) |
| Other1 | 30 (1.0) | Other2 | 89 (10.5) |
Given are frequency (n) and percent (%).
Note that diagnoses and procedures do not match completely; some patients underwent palliative procedures or reoperations.
CAVC = common atrioventricular canal; VSD = ventricular septal defect; Incomplete AVC = incomplete atrioventricular canal (partial and transitional); ASD = atrial septal defect; TOF = tetralogy of Fallot; PA = pulmonary artery; AP = aortopulmonary; CoA = coarctation of the aorta; IAA = interrupted aortic arch; PDA = patent ductus arteriosus; LAVV = left atrioventricular valve; RVOT = right ventricular outflow tract.
Other includes left ventricle to aorta tunnel (n= 13), CAVC + CoA (n = 8), RVOT procedure (n = 7), left atrioventricular (mitral) valve repair/replacement (n = 2).
Other2 includes right atrioventricular valve repair/replacement (n = 13), pulmonary valve replacement n= 12, ASD repair (n = 11), PA band (n = 10), pulmonary arterioplasty (n = 10), PDA ligation (n= 8), incomplete AVC repair (n= 7), CoA/IAA repair (n = 5), extracorporeal life support (n = 4), subpulmonary stenosis repair (n = 4), left ventricle to aorta tunnel (n = 2), atrial mass resection (n= 2) CAVC + CoA repair (n = 1).
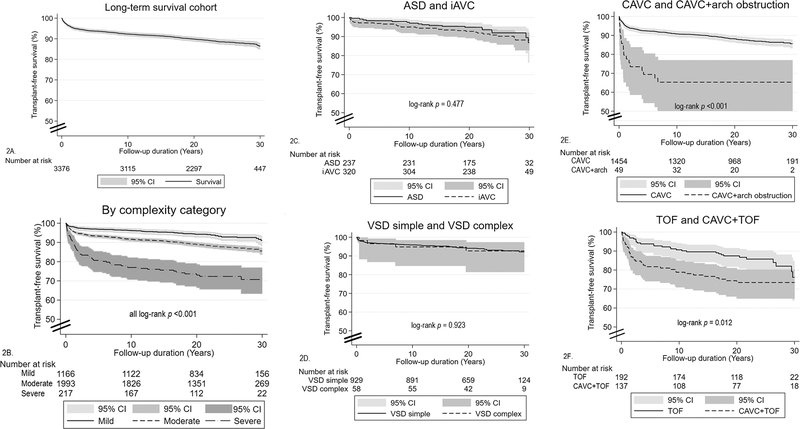
A total of 386 deaths (11.4%) occurred in the T21 long-term survival analysis group (n = 3,376) following discharge after the first cardiac surgery. Median follow-up duration was 22.6 years (IQR 19.0–27.0). Overall, transplant-free survival was 92.3% at 10 years, 89.8% at 20 years and 86.5% at 30 years (Figure 2, A). Survival varied significantly by the complexity of the underlying CHD (Figure 2, B) (all log-rank p < 0.001) and by individual lesion type (Figure 2, C–F). At the 30-year landmark, survival ranged from a high of 92.1% for simple VSD to low of 65.3% for CAVC with aortic arch obstruction; however, survival did not differ significantly between ASD, iAVC, simple VSD or complex VSD. Survival at 30 years was 85.6% for CAVC, the most common lesion among patients with T21.
Figure 2.
Kaplan-Meier transplant-free survival plots for patients with T21, conditioned on being discharged alive following first congenital heart surgery, by diagnosis group.
T21 and euploidic comparators matched 1:1 by cardiac diagnosis and who survived their first CHD surgery (T21 n = 2,185, euploidic comparators n = 2,185) formed the cohort for comparative survival analysis. Clinical characteristic comparison between them is presented in Table 3 (available at www.jpeds.com). There were no differences between the groups with regards to sex, surgical era, complexity, or need for initial palliative procedure. However, the T21 group was significantly younger than euploidic comparators at the time of both the initial and corrective cardiac surgeries (p < 0.001). Beyond the first cardiac surgical procedure, 30-year survival of patients with T21 was 88% (95% CI 86.0–89.7%) compared with 93.1% in comparators (95% CI 91.5–94.3%, log-rank p < 0.001) although the age at death was similar.
Table 3, online only.
Characteristics of T21 patients and matched euploidic comparators
| Euploidic comparators | T21 | p-value | |
|---|---|---|---|
| N = 2,185 | N = 2,185 | ||
| Males N (%) | 1,055 (48.3) | 1,064 (48.7) | 0.809 |
| Surgical era N (%) | 0.674 | ||
| Early 1982–1992 | 629 (28.8) | 632 (28.9) | |
| Mid 1993–1997 | 720 (32.9) | 694 (31.8) | |
| Late 1998–2003 | 836 (38.3) | 859 (39.3) | |
| Diagnosis category N (%) | NC | ||
| ASD | 237 (10.9) | 237 (10.9) | |
| Incomplete AVC | 320 (14.6) | 320 (14.6) | |
| VSD simple | 929 (42.5) | 929 (42.5) | |
| VSD complex | 58 (2.6) | 58 (2.6) | |
| CAVC | 391 (17.9) | 391 (17.9) | |
| CAVC+ aortic arch obstruction | 37 (1.7) | 37 (1.7) | |
| TOF | 192 (8.8) | 192 (8.8) | |
| CAVC + TOF | 21 (1.0) | 21 (1.0) | |
| CHD complexity N (%) | 0.457 | ||
| Mild | 1,166 (53.4) | 1,157 (52.9) | |
| Moderate | 921 (42.1) | 945 (43.2) | |
| Severe | 98 (4.5) | 83 (3.8) | |
| Staged repair N (%) | 164 (7.5) | 137 (6.3) | 0.120 |
| Age at 1st surgery (Yrs) median (IQR) | 0.96 (0.39–3.51) | 0.61 (0.37–1.38) | <0.001 |
| Age at corrective surgery (Yrs) median (IQR) | 1.13 (0.46–3.71) | 0.67 (0.40–1.56) | < 0.001 |
| ECLS at surgery N (%) | 4 (0.2) | 1 (<0.1) | 0.375 |
| First permanent pacemaker N (%) | 61 (2.8) | 66 (3.0) | 0.719 |
| Postop < 6 months SND or heart block | 53 (86.9) | 50 (75.8) | 0.115 |
| Other indication1 | 8 (13.1) | 16 (24.2) | |
| Heart or Lung Transplant N (%) | 4 (0.2) | 1 (< 0.1) | 0.375 |
| Any LAVV reop after iAVC N (%) | 24 (7.5) | 17 (5.3) | 0.333 |
| Any LAVV reop after CAVC N (%) | 61 (14.2) | 32 (7.4) | 0.001 |
| Any reoperation N (%) | 304 (13.9) | 316 (14.5) | 0.633 |
| 30-year survival % (95% CI) | 93.1 (91.5–94.3) | 88.0 (86.0–89.7) | <0.001 |
| Age at death (Yrs) median (IQR) | 7.07 (0.93–21.57) | 6.76 (1.27–18.53) | 0.876 |
| Follow-up duration (Yrs) median (IQR) | 23.14 (19.7–27.29) | 22.70 (19.17–27.00) | 0.020 |
| Cause of death N (%) | 0.013 | ||
| CHD-related | 50 (40.7) | 58 (27.2) | |
| Other cardiac-related | 16 (13.1) | 51 (23.9) | |
| Not cardiac-related | 57 (46.3) | 104 (48.8) | |
| # Contributing COD/death event | |||
| Mean (SD) | 2.82 (1.52) | 3.31 (1.54) | 0.005 |
Given are frequency (N) and percent (%) for categorical variables, median and interquartile range (IQR) or mean and standard deviation (SD) for continuous variables. 30-year survival is Kaplan-Meier survival estimate. P values represent Fisher’s exact test for categorical variables, log-rank test, t-test or Wilcoxon rank-sum test for continuous variables.
Other indication included late (>6 months postoperative) sinus node dysfunction or heart block in 14 T21 patients and 5 euploidic comparators, preoperative sinus node dysfunction or heart block in 4, biventricular pacing in 1.
NC= not calculated, two groups were matched 1:1 on diagnosis category; ASD = atrial septal defect; iAVC = incomplete atrioventricular canal (partial and transitional; VSD = ventricular septal defect; CAVC = common atrioventricular canal; TOF = tetralogy of Fallot; ECLS = extracorporeal life support; LAVV = left atrioventricular valve; reop = reoperation including repair or replacement; SND = sinus node dysfunction; CHD = congenital heart disease; COD = cause of death
Univariable predictors of late all-cause mortality (following discharge from the first cardiac surgery) with a significance less than 0.2 between patients with T21 and euploidic comparators included sex, age group at first surgery, surgical era, need for initial palliation, and CHD complexity. These variables were included in the Cox hazards model with application of a Heaviside function for predictors that violated the proportional hazards assumption. In the multivariable model, T21 was associated with a late all-cause mortality hazard ratio of 1.83 (95% CI 1.46–2.28, p < 0.001), compared with euploidic comparators. Earlier surgical era, need for initial palliation, and more complex CHD were also associated with increased hazard of late mortality, as was neonatal or infant age at first surgery in the first 2 years of follow-up (Table 4). Analysis of late all-cause mortality predictors specific among patients with CAVC identified T21 (HR 2.23, 95% CI: 1.39–3.60, p =0.001), earlier surgical era, and need for initial palliation as statistically significant predictors of mortality (Table 4). In the comparative survival cohort T21 was not associated with increased risk of CHD-related mortality in competing risk regression (SHR 1.34, 95% CI 0.92–1.97, p = 0.127) (Table 5).
Table 4.
Univariable and multivariable predictors of all-cause mortality following first cardiac surgery for T21 cohort and euploidic comparators
| Univariable | Multivariable | |||
| Risk Factor | HR (95% CI) | P value | HR (95% CI) | P value |
| T21 | 1.74 (1.39–2.17) | <0.001 | 1.83 (1.46–2.28) | <0.001 |
| Euploidic | Ref | Ref | ||
| Sex | ||||
| Male | 1.16 (0.94–1.44) | 0.159 | 1.15 (0.93–1.43) | 0.196 |
| Female | Ref | Ref | ||
| Age at first surgery | ||||
| < 28 days* | ||||
| ≤ 2 yrs follow-up | 7.31 (4.86–11.00) | <0.001 | 4.64 (2.26–9.53) | <0.001 |
| >2 yrs follow-up | 1.99 (1.08–3.66) | 0.044 | 0.57 (0.26–1.23) | 0.153 |
| 28–364 days* | ||||
| ≤ 2 yrs follow-up | 1.41 (1.00–1.99) | 0.048 | 2.69 (1.50–4.85) | 0.001 |
| > 2 yrs follow-up | 0.77 (0.58–1.02) | 0.071 | 0.74 (0.47–1.16) | 0.192 |
| 1–5 yrs | 0.78 (0.61–0.99) | 0.038 | 1.06 (0.68–1.65) | 0.786 |
| 6–20 yrs | Ref | Ref | ||
| Surgical Era | ||||
| Early 1982–1992 | Ref | Ref | ||
| Middle 1993–1997 | 0.78 (0.63–0.98) | 0.031 | 0.66 (0.50–0.84) | 0.001 |
| Late 1998–2003 | 0.76 (0.56–0.1.02) | 0.062 | 0.53 (0.37–0.76) | 0.001 |
| Initial palliation | 4.87 (3.80–6.24) | <0.001 | 3.09 (2.24–4.27) | <0.001 |
| Complexity Category | ||||
| Simple | Ref | Ref | ||
| Moderate | 1.41 (1.14–1.75) | 0.001 | 1.72 (1.36–2.18) | <0.001 |
| Severe | 4.69 (3.46–6.35) | <0.001 | 2.96 (1.96–4.47) | <0.001 |
| For CAVC | Univariable | Multivariable | ||
| Risk Factor | HR (95% CI) | P value | HR (95% CI) | P value |
| T21 | 1.89 (1.19–3.00) | 0.006 | 2.23 (1.39–3.60) | 0.001 |
| Euploidic | Ref | Ref | ||
| Sex | ||||
| Male | 1.21 (0.78–1.89) | 0.386 | 1.21 (0.77–1.90) | 0.412 |
| Female | Ref | Ref | ||
| Age at first surgery | ||||
| < 28 days | 3.65 (1.59–8.41) | 0.010 | 0.68 (0.15–3.05) | 0.620 |
| 28–264 days | 0.69 (0.42–1.14) | 0.574 | 0.57 (0.17–1.86) | 0.352 |
| 1–5 yrs | 1.03 (0.56–1.90) | 0.926 | 0.78 (0.22–2.79) | 0.706 |
| 6–20 yrs | Ref | Ref | ||
| Surgical Era | ||||
| Early 1982–1992 | Ref | Ref | ||
| Middle 1993–1997 | 0.72 (0.46–1.12) | 0.147 | 0.44 (0.29–0.75) | 0.002 |
| Late 1998–2003 | 0.46 (0.24–0.90) | 0.013 | 0.35 (0.16–0.76) | 0.008 |
| Initial palliation | 6.84 (4.23–11.07) | <0.001 | 7.07 (4.03–12.42) | <0.001 |
Reported are hazard ratio (HR) and 95% confidence intervals for multivariable Cox regression.
Proportional hazards assumption was violated and follow-up duration cut points were created based on survival curve.
Ref = reference category; T21 = Trisomy 21; CAVC = common atrioventricular canal; PPM = permanent pacemaker; LAVV = left atrioventricular valve
Table 5.
Competing risk univariable and multivariable regression for CHD-related mortality following discharge after initial cardiac surgery
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Risk Factor | SHR (95% CI) | P value | SHR (95% CI) | P value |
| Trisomy 21 | 1.16 (0.80–1.70) | 0.425 | 1.34 (0.92–1.97) | 0.127 |
| Euploidic | Ref | Ref | ||
| Sex | ||||
| Male | 1.28 (0.87–1.87) | 0.204 | 1.21 (0.81–1.79) | 0.346 |
| Female | Ref | Ref | ||
| Age at first surgery | ||||
| < 28 days | 9.08 (5.84–14.13) | <0.001 | 2.74 (0.78–9.60) | 0.114 |
| 28–364 days | 0.77 (0.52–1.12) | 0.172 | 1.77 (0.61–5.09) | 0.291 |
| 1–5 yrs | 0.68 (0.44–1.06) | 0.093 | 1.77 (0.63–5.03) | 0.280 |
| 6–20 yrs | Ref | Ref | ||
| Surgical Era | ||||
| Era 1982–1992 | Ref | Ref | ||
| Middle 1993–1997 | 0.65 (0.44–0.94) | 0.025 | 0.48 (0.31–0.74) | 0.001 |
| Late 1998–2003 | 0.58 (0.33–1.00) | 0.051 | 0.34 (0.18–0.65) | 0.001 |
| Initial palliation | 12.47 (8.55–18.20) | <0.001 | 6.16 (3.48–10.92) | <0.001 |
| Complexity Category | ||||
| Simple | Ref | Ref | ||
| Moderate | 1.55 (1.06–2.27) | 0.023 | 2.64 (1.60–4.37) | <0.001 |
| Severe | 8.80 (5.74–13.50) | <0.001 | 4.16 (1.99–8.68) | <0.001 |
Reported are subdistribution hazard ratio (SHR) and 95% confidence intervals. Competing risk was non-CHD related death or transplant. Ref = reference category
The distribution and comparison of underlying and contributing causes of death (COD) between patients with T21 and euploidic comparators are presented in Table 6. Euploidic patients were more likely to have CHD identified as the underlying COD (40.7% vs 27.2%, p = 0.016), but less likely to have other cardiovascular disease (CVD) identified as the underlying COD (13.0% vs 22.9%, p = 0.016). Among those with CVD-related underlying COD, patients with T21 were more likely to die from pulmonary hypertension (6.1% vs 0.8%, p = 0.021) or cerebrovascular disease (4.2% vs 0, p = 0.029). They were also more likely to die from leukemia and other cancers (8.9% vs 2.4%, p = 0.038), and less likely to die from external causes such as accidents or medical complications (5.6% vs 12.2%, p = 0.038).
Table 6.
Comparison of underlying and contributing causes of death between T21 and euploidic comparators following congenital heart surgery
| Underlying Cause of Death | Contributing Causes of Death | ||||||
|---|---|---|---|---|---|---|---|
| Cause | Euploidic N = 123 | T 21 N = 213 | P-value | Cause | Euploidic N = 123 | T 21 N = 213 | P-value |
| CHD N (%) | 50 (40.7) | 58 (27.2) | 0.016 | CHD | 62 (50.4) | 98 (46.0) | 0.496 |
| CVD N (%) | 16 (13.0) | 51 (22.9) | 0.016 | CVDa | 68 (55.3) | 127 (59.6) | 0.491 |
| Pulmonary HTN | 1 (0.8) | 13 (6.1) | 0.021 | Heart failure | 23 (18.7) | 42 (19.7) | 0.886 |
| Other CVD | 7 (5.7) | 6 (2.8) | 0.241 | Cardiac arrest | 9 (7.3) | 43 (20.2) | 0.002 |
| Cerebrovascular disease | 0 (0) | 9 (4.2) | 0.029 | Other CVD | 25 (20.3) | 32 (15.0) | 0.229 |
| Endocarditis | 4 (3.3) | 7 (3.3) | 0.986 | Pulmonary HTN | 3 (2.4) | 36 (16.9) | < 0.001 |
| Heart failure | 2 (1.6) | 8 (3.8) | 0.336 | Arrhythmia | 22 (17.9) | 15 (7.0) | 0.003 |
| Ischemic heart disease | 1 (0.8) | 6 (2.8) | 0.430 | Cerebrovascular disease | 4 (3.3) | 7 (3.3) | 0.986 |
| Cardiac arrest | 0 (0) | 2 (0.9) | 0.534 | Ischemic heart disease | 1 (0.8) | 5 (2.3) | 0.421 |
| Arrhythmia | 1 (0.8) | 0 (0) | 0.366 | Endocarditis | 4 (3.3) | 4 (1.9) | 0.471 |
| Non-CVD, non-CVD | 57 (46.3) | 104 (48.8) | 0.734 | Non-CHD, non CVDa | 88 (71.5) | 181 (85.0) | 0.004 |
| Trisomy 21 | 0 (0) | 29 (13.6) | < 0.001 | Other respiratory | 20 (16.3) | 62 (29.1) | 0.008 |
| External causes: Accidents, injuries, complications | 15 (12.2) | 12 (5.6) | 0.038 | Trisomy 21 | 0 (0) | 80 (37.6) | < 0.001 |
| Other respiratory | 8 (6.5) | 14 (6.6) | 0.980 | Unknown or other | 28 (22.8) | 35 (16.4) | 0.191 |
| Leukemia, other malignancy or hematologic | 3 (2.4) | 19 (8.9) | 0.022 | External causes: Accidents, injuries, complications | 23 (18.7) | 33 (15.5) | 0.451 |
| Unknown or other | 9 (7.3) | 11 (5.2) | 0.476 | Infection/sepsis | 13 (10.6) | 23 (10.8) | 0.948 |
| Gastrointestinal | 6 (4.9) | 7 (3.3) | 0.560 | Pneumonia/ARDS | 5 (4.1) | 28 (13.1) | 0.007 |
| Pneumonia/ARDS | 2 (1.6) | 12 (5.6) | 0.093 | Neurologic | 14 (11.4) | 18 (8.5) | 0.441 |
| Infection/sepsis | 9 (7.3) | 0 (0) | <0.001 | Leukemia, other malignancy or hematologic | 6 (4.9) | 24 (11.3) | 0.049 |
| Neurologic | 5 (4.1) | 0 (0) | 0.006 | Gastrointestinal | 7 (5.7) | 17 (8.0) | 0.514 |
Given are frequency and (percent) for categorical variables. P value from Fisher’s exact test. CHD = congenital heart disease; CVD = cardiovascular disease; HTN = hypertension; ARDS = adult respiratory distress syndrome.
Percentages do not equal 100% because most patients had more than one contributing cause of death.
Pulmonary hypertension was also more commonly identified as a CVD-related contributing COD in patients with T21 (17.1% vs 2.9%, p <0.001). Overall, patients with T21 had higher number of contributing CODs per death event than euploidic comparators (3.31 vs 2.82, p = 0.005) (Table 3). Patients with T21 were more likely to have contributing COD such as cardiac arrest, pneumonia, other respiratory causes, and leukemia or other cancer and the comparators were more likely to have arrhythmia. Trisomy 21 was identified as the underlying COD in 13.6% of deaths and a contributing COD in 37.6% of deaths in the T21 group.
DISCUSSION
Long-term outcome studies from the PCCC and other sources reported the increased risk for premature mortality among patients with operated CHD, compared with the general population.17,21,22 Additional risk for premature death was observed in the subgroup with chromosomal abnormalities compared with those without.17 However, limited data exist on the specific effect of T21 on long-term survival after congenital heart surgery.
Our study identified T21 as a significant predictor of 30-year all-cause mortality across each one of the diagnostic subgroups. However, our study did not identify T21 as a significant predictor of CHD-related mortality. This dichotomous finding is related to the serious life-threatening co-occurring conditions such as pulmonary hypertension, respiratory illnesses, higher incidence of leukemia and other cancers in patients with T21, which increase the risk of long-term mortality independently of their CHD.
Previous studies that examined the effect of T21 on short- or mid-term outcomes across the spectrum of congenital heart surgery did not find differences in mortality between patients with or without T21.8,9,23–28 In a longer-term study, Reller et al reported that survival up to 20 years following repair of CAVC was significantly shorter for children with T21, although there were no differences in long-term survival in other types of CHD. In the T21 group with CAVC, pulmonary hypertension was judged to have contributed to 55% of late deaths.11 Several other smaller studies failed to identify differences between T21 and euploidic patients.10,28–33
There may be several explanations for the discrepancies between our results and previous studies, such as smaller sample sizes, single center or single subset of CHD, which all can affect the generalizability of results.10,28–33 In addition, the potential inadvertent inclusion of patients with heterotaxy in comparison studies may have adversely affected both short- and long-term survival.34 Some previous studies that compared in-hospital or long-term outcomes between patients with or without T21 did not specifically report if patients with heterotaxy were excluded.9,26,32,35
The COD analysis in our cohort confirms the report by Reller et al that pulmonary hypertension is an important underlying and contributing COD in patients with T21.11 This finding underscores the need for ongoing assessment and treatment of pulmonary hypertension with phosphodiesterase-type 5 inhibitors or endothelin receptor antagonists as indicated.36–40 Indirect causes of pulmonary hypertension such as obstructive sleep apnea and sleep-disordered breathing are also common in patients with T21.41–43 Pneumonia and the increased incidence of respiratory infections in children with T21 throughout their life is well known, and likely due to a combination of immunological vulnerability, anatomical abnormalities, obstructive sleep apnea, gastroesophageal reflux and oropharyngeal aspiration.44 Pneumonia in patients with T21 is also associated with increased severity of the illness, leading to increased risk of hospitalization and need for mechanical ventilation.45 Health supervision guidelines for children with T21 have previously been established 46, and similar evidence-based guidelines for adults with T21 are now available.47 The role of the adult congenital cardiology clinic is important to provide ongoing monitoring and follow-up of sequelae of T21 with CHD.48 In addition, primary care provider and specialist coordination, as well as family education is imperative to mitigate the long-term health consequences in patients with T21. As seen in this study, co-occurring conditions related to T21 may be more influential in long-term mortality than repaired CHD, so a true medical home and coordinated primary and specialty care are crucial for ongoing health maintenance in children and adults with T21.
Trisomy 21 remains a significant risk factor for shortened long-term survival following congenital heart surgery, but not a predictor of CHD-related mortality. Co-occurring conditions of T21 such as pulmonary hypertension, pneumonia, and sleep-disordered breathing as well as hematological malignancies are important factors in long-term mortality. Patients with T21 would benefit from a multidisciplinary and cooperative approach in managing their lifelong co-occurring conditions.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the contributions of all the centers that participated in the Pediatric Cardiac Care Consortium.
Funded by The Larry and Helen Hoag Foundation (to S.S., J.P.). Additional support was provided from the National Heart, Lung, and Blood Institute (RO1-HL122392) and the Department of Defense (PR180683 [L.K.]). No funding sources had any role in design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The data reported here have been supplied by UNOS as the contractor for the Scientific Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government. The authors declare no conflicts of interest.
Abbreviations
- CHD
Congenital heart disease
- T21
Trisomy 21
- PCCC
Pediatric Cardiac Care Consortium
- HIPAA
Health Insurance Privacy and Portability Act
- ASD
Atrial septal defect
- iAVC
Incomplete atrioventricular canal (transitional and partial)
- VSD
Ventricular septal defect
- DORV
Double outlet right ventricle
- CAVC
Common atrioventricular canal
- TOF
Tetralogy of Fallot
- NDI
National Death Index
- OPTN
Organ Procurement and Transplantation Network
- US
United States
- CVD
Cardiovascular disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frid C, Drott P, Lundell B, Rasmussen F, Annerén G. Mortality in Down’s syndrome in relation to congenital malformations. J Intellect Disabil Res JIDR. 1999;43 :234–241. [DOI] [PubMed] [Google Scholar]
- 2.Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down’s syndrome in the USA from 1983 to 1997: a population-based study. Lancet Lond Engl. 2002;359:1019–1025. doi: 10.1016/s0140-6736(02)08092-3 [DOI] [PubMed] [Google Scholar]
- 3.Zhu JL, Hasle H, Correa A, Schendel D, Friedman JM, Olsen J, et al. Survival among people with Down syndrome: a nationwide population-based study in Denmark. Genet Med Off J Am Coll Med Genet. 2013;15:64–69. doi: 10.1038/gim.2012.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Leary L, Hughes-McCormack L, Dunn K, Cooper SA. Early death and causes of death of people with Down syndrome: A systematic review. J Appl Res Intellect Disabil JARID. 2018;31:687–708. doi: 10.1111/jar.12446 [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen SA, Wong L-Y, Correa A, Gambrell D, Friedman JM. Survival in infants with Down syndrome, Metropolitan Atlanta, 1979–1998. J Pediatr. 2006;148:806–812. doi: 10.1016/j.jpeds.2006.01.010 [DOI] [PubMed] [Google Scholar]
- 6.Bull C, Rigby ML, Shinebourne EA. Should management of complete atrioventricular canal defect be influenced by coexistent Down syndrome? Lancet Lond Engl. 1985;1:1147–1149. doi: 10.1016/s0140-6736(85)92444-4 [DOI] [PubMed] [Google Scholar]
- 7.Irving CA, Chaudhari MP. Cardiovascular abnormalities in Down’s syndrome: spectrum, management and survival over 22 years. Arch Dis Child. 2012;97:326–330. doi: 10.1136/adc.2010.210534 [DOI] [PubMed] [Google Scholar]
- 8.Evans JM, Dharmar M, Meierhenry E, Marcin JP, Raff GW. Association between Down syndrome and in-hospital death among children undergoing surgery for congenital heart disease: a US population-based study. Circ Cardiovasc Qual Outcomes. 2014;7:445–452. doi: 10.1161/CIRCOUTCOMES.113.000764 [DOI] [PubMed] [Google Scholar]
- 9.Fudge JC, Li S, Jaggers J, O’Brien SM, Peterson ED, Jacobs JP, et al. Congenital heart surgery outcomes in Down syndrome: analysis of a national clinical database. Pediatrics. 2010;126:315–322. doi: 10.1542/peds.2009-3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lange R, Guenther T, Busch R, Hess J, Schreiber C. The presence of Down syndrome is not a risk factor in complete atrioventricular septal defect repair. J Thorac Cardiovasc Surg. 2007;134:304–310. doi: 10.1016/j.jtcvs.2007.01.026 [DOI] [PubMed] [Google Scholar]
- 11.Reller MD, Morris CD. Is Down syndrome a risk factor for poor outcome after repair of congenital heart defects? J Pediatr. 1998;132:738–741. doi: 10.1016/s0022-3476(98)70372-5 [DOI] [PubMed] [Google Scholar]
- 12.Al-Hay AA, MacNeill SJ, Yacoub M, Shore DF, Shinebourne EA. Complete atrioventricular septal defect, Down syndrome, and surgical outcome: risk factors. Ann Thorac Surg. 2003;75:412–421. doi: 10.1016/s0003-4975(02)04026-2 [DOI] [PubMed] [Google Scholar]
- 13.Best KE, Rankin J. Long term survival of individuals born with congenital heart disease: A systematic review and meta analysis. J Am Heart Assoc Cardiovasc Cerebrovasc Dis. 2016;5:e002846. doi: 10.1161/JAHA.115.002846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moller JH. Using data to improve quality: the Pediatric Cardiac Care Consortium. Congenit Heart Dis. 2016;11:19–25. doi: 10.1111/chd.12297 [DOI] [PubMed] [Google Scholar]
- 15.Peterson JK, Setty SP, Knight JH, Thomas AS, Moller JH, Kochilas LK. Postoperative and long-term outcomes in children with Trisomy 21 and single ventricle palliation. Congenit Heart Dis. 2019;:854–863. doi: 10.1111/chd.12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JI, et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170–1175. [DOI] [PubMed] [Google Scholar]
- 17.Spector LG, Menk JS, Knight JH, McCracken C, Thomas AS, Vinocur JM, et al. Trends in long-term mortality after congenital heart surgery. J Am Coll Cardiol. 2018;71:2434–2446. doi: 10.1016/j.jacc.2018.03.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connelly MS, Webb GD, Somerville J, Warnes CA, Perloff JK, Liberthson RR, et al. Canadian Consensus Conference on adult congenital heart disease 1996. Can J Cardiol. 1998;14:395–452. [PubMed] [Google Scholar]
- 19.Spector LG, Menk JS, Vinocur JM, Oster ME, Harvey BA, St. Louis JD, et al. In hospital vital status and heart transplants after intervention for congenital heart disease in the Pediatric Cardiac Care Consortium: Completeness of ascertainment using the National Death Index and United Network for Organ Sharing Datasets. J Am Heart Assoc. 2016;5:e003783. doi: 10.1161/JAHA.116.003783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morales DLS, McClellan AJ, Jacobs JP. Empowering a database with national long-term data about mortality: The use of national death registries. Cardiol Young. 2008;18:188–195. doi: 10.1017/S1047951108002916 [DOI] [PubMed] [Google Scholar]
- 21.Nieminen HP, Jokinen EV, Sairanen HI. Late results of pediatric cardiac surgery in Finland. Circulation. 2001;104:570–575. doi: 10.1161/hc3101.093968 [DOI] [PubMed] [Google Scholar]
- 22.Raissadati A, Nieminen H, Haukka J, Sairanen H, Jokinen E. Late causes of death after pediatric cardiac surgery: A 60-year population-based study. J Am Coll Cardiol. 2016;68:487–498. doi: 10.1016/j.jacc.2016.05.038 [DOI] [PubMed] [Google Scholar]
- 23.Toth R, Szanto P, Prodan Z, Lex DJ, Sapi E, Szatmari A, et al. Down syndrome and postoperative complications after paediatric cardiac surgery: a propensity-matched analysis. Interact Cardiovasc Thorac Surg. 2013;17:691–697. doi: 10.1093/icvts/ivt267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoashi T, Miyata H, Murakami A, Hirata Y, Hirose K, Matsumura G, et al. The current trends of mortality following congenital heart surgery: the Japan Congenital Cardiovascular Surgery Database. Interact Cardiovasc Thorac Surg. 2015;21:151–156. doi: 10.1093/icvts/ivv109 [DOI] [PubMed] [Google Scholar]
- 25.Lal PS, Chavan B, Devendran VR, Varghese R, Murmu UC, Kumar RS. Surgical outcome of congenital heart disease in Down’s syndrome. Asian Cardiovasc Thorac Ann. 2013;21:166–169. doi: 10.1177/0218492312450701 [DOI] [PubMed] [Google Scholar]
- 26.Atz AM, Hawkins JA, Lu M, Cohen MS, Colan SD, Pediatric Heart Network Investigators et al. Surgical management of complete atrioventricular septal defect: associations with surgical technique, age, and trisomy 21. J Thorac Cardiovasc Surg. 2011;141:1371–1379. doi: 10.1016/j.jtcvs.2010.08.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.St Louis JD, Jodhka U, Jacobs JP, He X, Hill KD, Pasquali SK, et al. Contemporary outcomes of complete atrioventricular septal defect repair: Analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. J Thorac Cardiovasc Surg. 2014;148:2526–2531. doi: 10.1016/j.jtcvs.2014.05.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tumanyan MR, Filaretova OV, Chechneva VV, Gulasaryan RS, Butrim IV, Bockeria LA. Repair of complete atrioventricular septal defect in infants with down syndrome: Outcomes and long-term results. Pediatr Cardiol. 2015;36:71–75. doi: 10.1007/s00246-014-0966-7 [DOI] [PubMed] [Google Scholar]
- 29.Michielon G, Marino B, Oricchio G, Digilio MC, Iorio F, Filippelli S, et al. Impact of DEL22q11, trisomy 21, and other genetic syndromes on surgical outcome of conotruncal heart defects. J Thorac Cardiovasc Surg. 2009;138:565–570.e2. doi: 10.1016/j.jtcvs.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 30.Mathew P, Moodie D, Sterba R, Murphy D, Rosenkranz E, Homa A. Long term follow-up of children with Down syndrome with cardiac lesions: Clin Pediatr (Phila). 29:569–574. doi: 10.1177/000992289002901003 [DOI] [PubMed] [Google Scholar]
- 31.Masuda M, Kado H, Tanoue Y, Fukae K, Onzuka T, Shiokawa Y, et al. Does Down syndrome affect the long-term results of complete atrioventricular septal defect when the defect is repaired during the first year of life? Eur J Cardiothorac Surg. 2005;27:405–409. doi: 10.1016/j.ejcts.2004.11.027 [DOI] [PubMed] [Google Scholar]
- 32.Miller A, Siffel C, Lu C, Riehle-Colarusso T, Frías JL, Correa A. Long-term survival of infants with atrioventricular septal defects. J Pediatr. 2010;156:994–1000. doi: 10.1016/j.jpeds.2009.12.013 [DOI] [PubMed] [Google Scholar]
- 33.Ginde S, Lam J, Hill GD, Cohen S, Woods RK, Mitchell ME, et al. Long-term outcomes after surgical repair of complete atrioventricular septal defect. J Thorac Cardiovasc Surg. 2015;150:369–374. doi: 10.1016/j.jtcvs.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 34.Serraf A, Bensari N, Houyel L, Capderou A, Roussin R, Lebret E, et al. Surgical management of congenital heart defects associated with heterotaxy syndrome. Eur J Cardiothorac Surg. 2010;38:721–727. doi: 10.1016/j.ejcts.2010.02.044 [DOI] [PubMed] [Google Scholar]
- 35.Hoashi T, Hirahara N, Murakami A, Hirata Y, Ichikawa H, Kobayashi J, et al. Current surgical outcomes of congenital heart surgery for patients with Down syndrome in Japan. Circ J Off J Jpn Circ Soc. 2018;82:403–408. doi: 10.1253/circj.CJ-17-0483 [DOI] [PubMed] [Google Scholar]
- 36.Beghetti M, Rudzinski A, Zhang M. Efficacy and safety of oral sildenafil in children with Down syndrome and pulmonary hypertension. BMC Cardiovasc Disord. 2017;17. doi: 10.1186/s12872-017-0569-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010 [DOI] [PubMed] [Google Scholar]
- 38.Crepaz R, Romeo C, Montanaro D, De Santis S. Long-term results of treatment with bosentan in adult Eisenmenger’s syndrome patients with Down’s syndrome related to congenital heart disease. BMC Cardiovasc Disord. 2013;13. doi: 10.1186/1471-2261-13-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duffels MGJ, Vis JC, van Loon RLE, Nieuwkerk PT, van Dijk APJ, Hoendermis ES, et al. Effect of bosentan on exercise capacity and quality of life in adults with pulmonary arterial hypertension associated with congenital heart disease with and without Down’s syndrome. Am J Cardiol. 2009;103:1309–1315. doi: 10.1016/j.amjcard.2009.01.021 [DOI] [PubMed] [Google Scholar]
- 40.Vorhies EE, Ivy DD. Drug treatment of pulmonary hypertension in children. Pediatr Drugs. 2014;16:43–65. doi: 10.1007/s40272-013-0052-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Miguel-Díez J, Villa-Asensi JR, Alvarez-Sala JL. Prevalence of sleep-disordered breathing in children with Down syndrome: polygraphic findings in 108 children. Sleep. 2003;26:1006–1009. [DOI] [PubMed] [Google Scholar]
- 42.Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Prevalence of obstructive sleep apnea in children with Down syndrome. Sleep. 2016;39:699–704. doi: 10.5665/sleep.5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan H, Gozal D, Kheirandish-Gozal L. Obstructive sleep apnea in children: a critical update. Nat Sci Sleep. 2013;5:109–123. doi: 10.2147/NSS.S51907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ram G, Chinen J. Infections and immunodeficiency in Down syndrome. Clin Exp Immunol. 2011;164:9–16. doi: 10.1111/j.1365-2249.2011.04335.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santoro SL, Chicoine B, Jasien JM, Kim JL, Stephens M, Bulova P, et al. Pneumonia and respiratory infections in Down syndrome: A scoping review of the literature. Am J Med Genet Part A. 2020;1–14. doi: 10.1002/ajmg.a.61924 [DOI] [PubMed] [Google Scholar]
- 46.Bull MJ, the Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics. 2011;128:393–406. doi: 10.1542/peds.2011-1605 [DOI] [PubMed] [Google Scholar]
- 47.Tsou AY, Bulova P, Capone G, Chicone B, Gelaro B, Global Down Syndrome Foundation Medical Care Guidelines for Adults with Down Syndrome Workgroup. Medical care of adults with Down syndrome: A clinical guideline. JAMA. 2020;324:1543–1556. doi: 10.1001/jama.2020.17024 [DOI] [PubMed] [Google Scholar]
- 48.Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. 2018 AHA/ACC Guideline for the management of adults with congenital heart disease. Circulation. 2019;139:e698–e800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.