Abstract
Background and Aims:
Little is known about how weight trajectories among women during menopausal transition and beyond may be related to risk of Type 2 diabetes (T2DM). The aim of this study was to examine associations between body mass index (BMI) trajectories over 20 years, age of obesity onset, cumulative obese-years and incidence of T2DM among middle-age women.
Methods and Results:
12,302 women enrolled in the Australian Longitudinal Study on Women’s Health (ALSWH) were surveyed in 1996 (Survey 1, age 45–50), 1998 and then every three years to 2016. Self-reported weight and height were collected for up to eight time points. Incident diabetes was assessed via validated self-report of physician-diagnosed diabetes. Growth mixture models were used to identify distinct BMI trajectories. 1380 (11.2%) women newly developed T2DM over an average 16 years of follow-up. Seven distinct BMI trajectories were identified with differential risk of developing T2DM. Initial BMI was positively associated with T2DM risk. We also observed that risk of T2DM was positively associated with rapid weight increase, early age of obesity onset, and greater obese-years.
Conclusion:
Slowing down weight increases, delaying the onset of obesity, or reducing cumulative exposure to obesity may substantially lower the risk of developing T2DM.
Keywords: Diabetes, Weight, Trajectory, Body mass index, age of obesity onset, obese-years
INTRODUCTION
In Australia, almost two-thirds (63%) of adults were overweight or obese, and 28% were obese in 2017 [1]. Despite the widely acknowledged association of obesity with T2DM, less is known about how weight trajectories over the adult life course may be related to risk of the disease. Compared to one or two measurements, analysing trajectories based on repeated measurements will more accurately quantify dynamics of changes, and will be less sensitive to selection of time period and the impact of outliers. Studies have shown that using time-dependent exposures substantially improved the ability to predict mortality and cardiovascular disease [2]. A recent study using repeated measurements of BMI from childhood to adulthood observed that faster BMI increases between ages 10–19 years were positively associated with adult hyperglycaemia [3]. Rapid weight gain in early life was also associated with increased risk for CVD and T2DM in adults [4, 5]. However, it is unclear whether the rate of BMI increase during adult life is associated with risk of T2DM.
Two statistical techniques commonly used for modeling longitudinal data are: growth curve models and latent class growth analysis (LCGA), also known as group-based trajectory models. Growth mixture modeling (GMM) [6], is a generalized model of both growth curve models and LCGA models. The GMM allows for both subpopulation (different trajectories within different subpopulations) and random variation among individuals within a group [6].
To better understand relationships between weight trajectories and T2DM during adult life, we applied GMM to examine the associations between BMI trajectory patterns over 20-years of the adult life course, including age of obesity onset and cumulative exposure to obesity, and subsequent risk of T2DM. We applied the GMM model because it overcomes two extreme assumptions of “random individual variation” in the growth curve models and “zero within-group variance” in LCGA models [6].
METHODS
Australian Longitudinal Study on Women’s Health (ALSWH)
The ALSWH is a longitudinal population-based study of Australian women that commenced in 1996 with the recruitment of nationally representative samples of women in three age cohorts, born in 1921–1926 (aged 70–75 at baseline), 1946–1951 (aged 45–50) and 1973–1978 (aged 18–23). The study is designed to examine relationships between biological, psychological, social and lifestyle factors and women’s physical health, emotional well-being, and their use of and satisfaction with health care [7]. Details of the design, recruitment, implementation and progress of the ALSWH study are described elsewhere [7, 8]. The study was approved by the Universities of Newcastle and Queensland Ethic Review Committees. All participants provided written informed consent.
Study population
This study was based on data from the 1946–1951 birth cohort (n=13,714). The following participants were excluded from the analysis: women who reported a history of diabetes at enrolment or reported having diabetes more than two years ago at first follow-up (n=431); women who answered only the baseline survey (n=650); and women with missing values for major covariates at baseline, including physical activity, income and stress (n=218), or women with missing BMI during the follow-up (n=113). After exclusions, 12,302 women remained in the analytic cohort.
Follow-up
Participants were surveyed in 1996 (Survey 1, age 45–50), 1998, and then every three years to 2016 (Survey 8, age 65–70). Surveys were sent by mail, and an online option has been available since 2013. For the 1946–51 cohort, the retention rates were more than 80% of eligible at each survey point [9]. Details on eligible participants and loss to follow-up at each survey are provided in Supplemental Table 1.
Measurements
Outcome:
Incidence of T2DM during follow-up
Incident T2DM was defined as a positive self-report of a new diagnosis of diabetes during follow-up. The type of diabetes was distinguished by asking whether diabetes was insulin dependent (type 1) or non-insulin dependent (type 2) diabetes at baseline survey and first two follow-ups. In the later surveys, the question was changed to “in the past three years, have you been diagnosed or treated for diabetes (high blood sugar)?” We only considered non-insulin-dependent diabetes through the first two follow-ups, and assumed that diabetes first diagnosed later in life was T2DM. Self-reported diabetes in the ALSWH has been validated against administrative hospital data records with substantial agreement for diabetes (kappa>0.70) for the 1946–1951 cohort [10].
Exposure:
Self-reported weight and height collected at up to eight surveys.
Weight and height were reported at each survey. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Up to eight measures of BMI were included in the analysis. All measures of BMI after the date of diagnosis of T2DM were removed.
The accuracy of BMI estimated from self-reported height and weight has been previously validated in this cohort [11], with substantial agreement (84%) between BMI categories derived from self-reported and measured height and weight data, especially for healthy weight women (94%) [11].
Covariates:
Participants’ social-demographic characteristics (age, educational attainment, income adequacy), lifestyle factors (physical activity, smoking, alcohol use, and dietary intake), country of birth, and perceived stress were considered as potential confounders. All confounders were collected at baseline, except dietary intake was collected at survey 3. Detailed categorization for each covariate is presented in Table 1. Income adequacy was assessed by an item asking “how do you manage on the income you have available?” Physical activity was measured using items from Active Australia’s 1999 National Physical Activity Survey [12] by asking the amount of time spent in the last week on different activities expressed as metabolic equivalent (METs)/week (continuous). Stress score (continuous) was a summed score for perceived stress across a range of life domains. The score has been demonstrated to be reliable and valid [9]. Dietary intake was based on Food Frequency Questionnaire collected at survey 3; total energy intake was included as a potential confounder.
Table 1.
Comparison of baseline characteristics across BMI trajectories
| variable name | Overall N=12302 | NW-low Stable N=1003 | NW-mid Modest increase N=3169 | NW-high rapid increase N=1571 | OW-high rapid increase N=4245 | OW-high modest increase N=1390 | OB-low rapid increase N=782 | OB-high rapid increase N=142 | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Age | 47.6 ± 1.5 | 47.5 ± 1.5 | 47.5 ± 1.5 | 47.6 ± 1.4 | 47.6 ± 1.5 | 47.6 ± 1.5 | 47.5 ± 1.5 | 47.6 ± 1.5 | 0.8 |
| Physical activity (Mets) | 13.7 ± 12.5 | 17.2 ± 13.6 | 14.6 ± 12.5 | 13.7 ± 12.3 | 13.1 ± 12.3 | 12.3 ± 12.4 | 11.5 ± 11.2 | 10.8 ± 12.2 | <0.0001 |
| Mean stress score | 0.7 ± 0.5 | 0.6 ± 0.5 | 0.6 ± 0.5 | 0.6 ± 0.5 | 0.7 ± 0.5 | 0.8 ± 0.6 | 0.8 ± 0.6 | 0.8 ± 0.6 | <0.0001 |
| Total energy intake | 6655.6 ± | 6574.5 ± | 6552.3 ± | 6490.5 ± | 6680.0 ± | 6805.1 ± | 6943.9 ± | 7634.0 ± | |
| (KJ/day) | 2432.7 | 2166.4 | 2309.6 | 2231.7 | 2478.9 | 2637.2 | 2779.3 | 3116.5 | <0.0001 |
| Smoking | <0.0001 | ||||||||
| Never | 6385 (53.5%) | 588 (59.9%) | 1722 (56.1%) | 847 (55.5%) | 2115 (51.4%) | 665 (49.1%) | 377 (49.9%) | 71 (51.4%) | |
| Former | 3436 (28.8%) | 279 (28.4%) | 890 (29.0%) | 436 (28.6%) | 1177 (28.6%) | 396 (29.2%) | 218 (28.9%) | 40 (29.0%) | |
| Current: <10 cigs/day) | 386 (3.2%) | 34 (3.5%) | 104 (3.4%) | 55 (3.6%) | 117 (2.8%) | 43 (3.2%) | 31 (4.1%) | 2 (1.4%) | |
| Current: 10–19 cigs/day | 508 (4.3%) | 30 (3.1%) | 109 (3.5%) | 75 (4.9%) | 207 (5.0%) | 50 (3.7%) | 30 (4.0%) | 7 (5.1%) | |
| Current: ≥ 20 cigs/day | 1222 (10.2%) | 51 (5.2%) | 246 (8.0%) | 112 (7.3%) | 495 (12.0%) | 201 (14.8%) | 99 (13.1%) | 18 (13.0%) | |
| Alcohol intake | 0.06 | ||||||||
| Low risk, non or rarely drinker | 11569 (94.7%) | 957 (95.9%) | 2956 (94.2%) | 1483 (94.9%) | 3999 (94.8%) | 1303 (94.3%) | 735 (95.2%) | 136 (96.5%) | 0.06 |
| Risky drinker (3 to 4 drinks per day) | 538 (4.4%) | 40 (4.0%) | 157 (5.0%) | 66 (4.2%) | 184 (4.4%) | 63 (4.6%) | 25 (3.2%) | 3 (2.1%) | |
| High risk drinker (5 or more drinks per day) | 107 (0.9%) | 1 (0.1%) | 25 (0.8%) | 14 (0.9%) | 37 (0.9%) | 16 (1.2%) | 12 (1.6%) | 2 (1.4%) | |
| Education | <0.0001 | ||||||||
| High school or lower | 8134 (66.1%) | 560 (55.8%) | 1967 (62.1%) | 966 (61.5%) | 2999 (70.6%) | 966 (69.5%) | 570 (72.9%) | 106 (74.6%) | |
| Technical training | 2411 (19.6%) | 237 (23.6%) | 642 (20.3%) | 347 (22.1%) | 760 (17.9%) | 270 (19.4%) | 135 (17.3%) | 20 (14.1%) | |
| University degree or higher | 1757 (14.3%) | 206 (20.5%) | 560 (17.7%) | 258 (16.4%) | 486 (11.4%) | 154 (11.1%) | 77 (9.8%) | 16 (11.3%) | |
| Income adequacy | <0.0001 | ||||||||
| Difficult always | 1731 (14.1%) | 98 (9.8%) | 345 (10.9%) | 181 (11.5%) | 610 (14.4%) | 284 (20.4%) | 167 (21.4%) | 46 (32.4%) | |
| Difficult sometimes | 3520 (28.6%) | 228 (22.7%) | 842 (26.6%) | 427 (27.2%) | 1292 (30.4%) | 443 (31.9%) | 254 (32.5%) | 34 (23.9%) | |
| Not too bad | 5153 (41.9%) | 443 (44.2%) | 1407 (44.4%) | 699 (44.5%) | 1739 (41.0%) | 523 (37.6%) | 293 (37.5%) | 49 (34.5%) | |
| It is easy | 1898 (15.4%) | 234 (23.3%) | 575 (18.1%) | 264 (16.8%) | 604 (14.2%) | 140 (10.1%) | 68 (8.7%) | 13 (9.2%) | |
| Country of birth | <0.0001 | ||||||||
| Australian born | 9329 (76.7%) | 718 (72.2%) | 2356 (75.2%) | 1195 (76.6%) | 3218 (76.6%) | 1093 (80.1%) | 627 (81.1%) | 122 (87.1%) | |
| Other English | |||||||||
| speaking background | 1683 (13.8%) | 161 (16.2%) | 411 (13.1%) | 234 (15.0%) | 595 (14.2%) | 170 (12.5%) | 98 (12.7%) | 14 (10.0%) | |
| Europe | 754 (6.2%) | 67 (6.7%) | 209 (6.7%) | 87 (5.6%) | 274 (6.5%) | 82 (6.0%) | 34 (4.4%) | 1 (0.7%) | |
| Asia | 287 (2.4%) | 41 (4.1%) | 119 (3.8%) | 33 (2.1%) | 73 (1.7%) | 12 (0.9%) | 7 (0.9%) | 2 (1.4%) | |
| Other | 112 (0.9%) | 8 (0.8%) | 36 (1.1%) | 12 (0.8%) | 40 (1.0%) | 8 (0.6%) | 7 (0.9%) | 1 (0.7%) | |
NW – Normal BMI at baseline; OW – Overweight at baseline; OB – Obesity at baseline. Low- at low end of the category; mid – at middle of the category; high- at high end of the category. Slope: <1 – stable; 1-<2 - modest increase; 2 or higher – rapid increase.
Values expressed as n (%) for categorical variables, mean ± standard deviation for continuous variables
P-value comparisons across class categories are based on Chi-square test for categorical variables; p-values for continuous variables are based on ANOVA
Statistical analysis
Growth mixture models (GMM) were used to identify distinct BMI trajectories over the adult life course. Mplus 8.2 [13] was used to perform GMM. First, we determined whether linear or quadratic growth models fit the BMI data better. Z-score results indicated that the quadratic model fit the data better, so the quadratic model was used for further analyses. Second, we determined the number of distinct BMI trajectories needed to capture the population’s heterogeneity. The process began by specifying a 1-class GMM model, then increasing the number of classes and comparing the fit of each model. The optimal number of groups was selected using the Bayesian Information Criterion (BIC). A lower BIC indicates better fit to the data. Third, once the best-fitting model was identified, participants were assigned into the trajectory groups to which their posterior membership probability was largest. A new variable for BMI trajectory was created.
Cox proportional hazards regression models were used to evaluate the association of the new BMI trajectory variable from GMM with risk of T2DM. Survival was defined as time between baseline and the date of newly diagnosed diabetes, censoring due to death or loss to follow-up, or end of follow‐up at survey 8, whichever came first. In the multivariate-adjusted models, potential confounders were included as covariates. The Cox model was fitted using the PHREG procedure in SAS 9.4.
For a subset of women (n=10,174, 83% of study women) who were not obese at baseline, we further divided women into two groups according to whether they became obese or not (yes, no) during follow-up. For those who became obese, we derived age of obesity onset and duration from predicted BMI trajectories. Similar to pack-years for smoking, we further estimated obese-years as a cumulative exposure by calculating the area under the curve that was above 30 kg/m2 by trapezoid rule [14]. Age of onset of obesity and obese-years were treated as time-varying covariates in Cox models.
Finally, we calculated the hazard ratios that would be obtained from consideration only of baseline overweight and obesity status for comparison with HRs for trajectories. We also examined models after adjusting further for baseline BMI.
RESULTS
Among 12,302 women who were free of diabetes at baseline, 1,380 women (11.2%) developed T2DM over an average of 16 years of follow-up. Overall, the average BMI increased from 25.93 kg/m2 at baseline to 27.77 kg/m2 at survey 8. The obesity rate increased from 17.8% at baseline to 30.1% at survey 8.
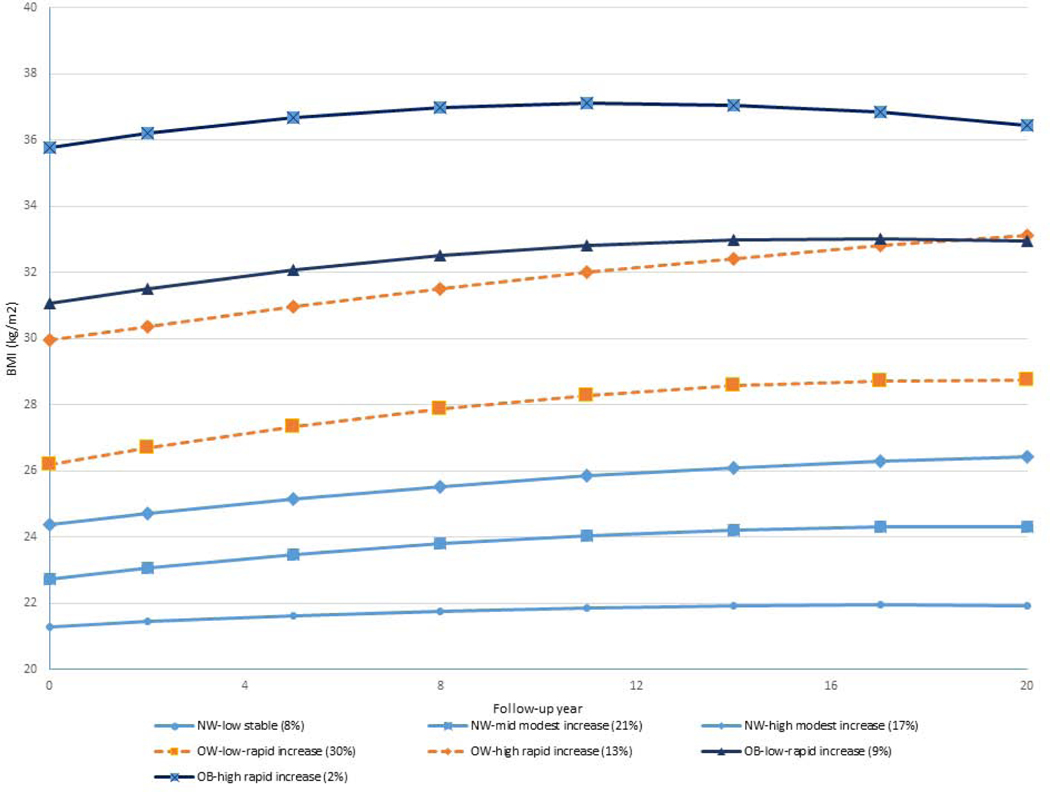
We identified a seven-class solution based on BIC (Supplemental Figure 1), ranked the seven classes based on their mean BMI at baseline from low to high, and then categorized them as normal weight (NW - BMI<25kg/m2), overweight (OW –BMI: 25-<30kg/m2) or obese (OB – BMI:≥30kg/m2) at baseline (Figure 1). Within each category of BMI at baseline, we classified as low, middle or high when three trajectories were identified, or as low or high when two trajectories were identified. We further categorized as stable, modest increase or rapid increase based on slope (<1- stable, 1–2 – modest increase, 2 or more – rapid increase). The largest proportion of women belonged to the “OW-low rapid increase” group (30%), with initial BMI around 26 kg/m2 and a slope of weight growth of 2.68 kg/m2 per 10 years throughout follow-up.
Figure 1. BMI trajectories over 20 years of follow-up.
(within each category of BMI at baseline (normal:<25, overweight: 25-<30, and obese: ≥30 kg/m2), we classified as low, middle or high based on the rank of their values when three trajectories were identified, or as low or high when two trajectories were identified. We further categorized as stable, modest increase or rapid increase based on slope (<1- stable, 1–2 – modest increase, 2 or more – rapid increase).
The next most frequent categories were “NW-mid modest increase” group (21%) and then NW high modest increase (17%). A small group of women belonged to the “OB high rapid increase” group (2%) with initial BMI around 36 kg/m2 and rapid weight gain thereafter (Figure 1).
Table 1 shows baseline characteristics across BMI trajectories. Based on the mean value of BMI at baseline in each class from low to high, compared with the NW-low stable class, women in the OB-high rapid increase classe were more likely to be physically inactive, have higher stress score, have higher total energy intake, smoke more, have lower education level, were more likely to have difficulty in managing on available income, and be Australian born. There was no difference in age at baseline and alcohol intake across classes (Table 1). Comparison of baseline characteristics by diabetes status is provided in Supplemental Table 2.
Estimated parameters for each class including intercept, slope and quadratic terms are listed in Table 2. Specific growth trajectories are presented in Figure 1. BMI change patterns during the late adult life-course were similar with a modest or rapid weight gain in the early period (positive slope) followed by a slight decrease in the late period (negative quadratic term). Compared with women in the “NW-low stable” class, women in the “NW-high modest increase” class had non-significant higher risk of T2DM (HR=1.32, 95% CI: 0.84–2.06), and women in all other classes had significantly higher risk of T2DM with HRs ranging from 2.06 (95% CI: 1.38–3.07) for “NW-mid modest increase” class to 8.35 (95% CI: 5.59–12.47) for “OB-Low rapid increase” class after adjustment for potential confounders. All three random parameters of the quadratic model were positively associated with risk of T2DM. That is, increased risk of T2DM was associated with increasing intercept (initial BMI), slope and quadratic term (Table 2).
Table 2.
Estimated means of intercept, slope and quadratic parameters for each class, and its hazard ratio (HR) for Type 2 diabetes mellitus(T2DM) incidence
| Classes 1 | Incident T2DM | Multivariable-adjusted model 2 HR (95%CI) | Intercept | Slope | Quadratic |
|---|---|---|---|---|---|
| NW-low stable (8%) | 28 | Reference | 21.29 | 0.79 | −0.24 |
| NW-mid modest increase (21%) | 172 | 2.06 (1.38–3.07) | 22.74 | 1.67 | −0.44 |
| NW-high modest increase (17%) |
61 | 1.32 (0.84–2.06) | 24.37 | 1.72 | −0.35 |
| OW-low rapid increase (30%) |
620 | 5.59 (3.82–8.17) | 26.18 | 2.68 | −0.70 |
| OW-high rapid increase (13%) |
293 | 8.06 (5.46–11.91) | 29.94 | 2.20 | −0.30 |
| OB-low rapid increase (9%) |
177 | 8.35 (5.59–12.47) | 31.07 | 2.39 | −0.73 |
| OB-high rapid increase (2%) |
29 | 7.62 (4.51–12.85) | 35.78 | 2.26* | −0.97* |
| Quadratic model parameters | |||||
| Intercept | 1.12 (1.11 1.13) | ||||
| Slope | 1.14 (1.10 1.17) | ||||
| Quadratic | 1.14 (1.05 1.24) |
- these estimates were not significant. All other estimates were significant (p<.05).
NW – Normal BMI at baseline; OW – Overweight at baseline; OB – Obesity at baseline Low- at low end of the category; mid – at middle of the category; high- at high end of the category Slope: <1 – stable; 1-<2 - modest increase; 2 or higher – rapid increase.
Model adjusted for age (in continuous), physical activity in Metabolic equivalent (METs)/week (in continuous), smoking (never, former, current smoker: <10 cigarettes/day, 10–19 cigarettes/day, ≥ 20 cigarettes/day), alcohol (low risk, non or rarely drinker, risky drinker, high risk drinker), and education (higher school or lower, technical training, university degree or higher), income adequacy (impossible, difficult always, difficult sometimes, not too bad, it is easy), stress score (in continuous), total energy intake, and country of birth (Australian born, other English speaking background, Europe, Asia, other).
Finally, we examined the association between age of obesity onset, and obese-years and risk of T2DM among a subset of women who were not obese at baseline but became obese during follow-up. We observed that age of obesity onset was negatively associated with risk of T2DM (HR=0.88, 95% CI: 0.86–0.91). Obese-years were positively associated with risk of T2DM in a dose-response fashion (Table 3). Looking at BMI at baseline only, women with baseline obesity (BMI>=30) had an HR of 4.90 (95% CI: 4.26 5.63), which was lower than the HR of 8.35 (OB-low rapid increase) or 7.72 (OB-high rapid increase) in Table 2 for women starting out obese and experiencing an increase in BMI over time. Similarly, women who were overweight at baseline had an HR of 2.32 (95% CI: 2.01 2.67), which was lower than for the two trajectories of OW at baseline in Table 2 (HR=5.59 or 8.06).
Table 3.
Associations between age at onset of obesity, obese-years, and risk of type 2 diabetes incidence among 10,174 (83%) women who were not obese at baseline
| Cases/Population | HR (95% CI) | |
|---|---|---|
| Become obese during follow-up | ||
| No | 596/8484 | Reference |
| Yes | 216/1680 | 2.71 (2.31 – 3.19) |
| Age at onset of obesity among women who became obese (per year) | 216/1680 | 0.88 (0.86 – 0.91)* |
| Obese –years | ||
| Not obese | 596/8494 | Reference |
| <10 | 139/913 | 2.58 (2.14 3.12) |
| 10-<30 | 55/511 | 2.98 (2.24 3.95) |
| 30 or more | 22/256 | 3.53 (2.29 5.45) |
| P for trend | 812/10174 | <0.0001 |
for a one year increment in age of obesity onset among women who became obese during follow-up.
When we added initial BMI simultaneously in the model of analysis for change patterns, we found that the magnitudes of HRs for weight change patterns were substantially reduced, however, the order and the significance of HRs for change patterns remained the same. For example, compared with women in the “NW-low stable” class, women in the “NW-high modest increase” class had non-significant risk of T2DM, and women in all other classes had significantly higher risk of T2DM with HRs ranging from 1.95 (95% CI: 1.31–2.92) for “NW-mid modest increase” class to 3.91 (95% CI: 2.56–5.98) for “OB-Low rapid increase” class after further adjustment for initial BMI.
DISCUSSION
Using data from a large population-based prospective cohort of Australian middle-aged women with 20 years of follow-up, we identified seven distinct BMI trajectories with differential risk of developing T2DM. Our data suggest that not only initial BMI, but also its pattern of change play important roles in the risk of developing T2DM. Specifically, we observed that both long-term weight-change slope and quadratic terms were positively associated with risk of T2DM independent of initial BMI. We also observed that cumulative exposure to obesity measured as obese-years was positively associated with risk of T2DM, and the younger women were at obesity onset, the higher the risk of T2DM.
Few previous studies have examined the associations between BMI trajectories and T2DM incidence [15–17], diabetes-related metabolic markers, or inflammation [18–21]. Most observed that steeper weight-gain trajectories were associated with greater risks for diabetes incidence, diabetes-related metabolic markers (levels of glucose, insulin and HOMA-IR), or elevated hs-CRP [15, 18–21], which is in line with our findings. A study conducted in Australia did not observe risk differences for diabetes incidence among three classes (high stable, steady increase or increase-decrease) compared with a low stable class for women age<50 years. However, that study included only 16 diabetes cases in the steady increase pattern and 5 cases in the increase-decrease pattern [17]. One case-control study from Japan reported that increasing BMI was not associated with the onset of T2DM [16]. This may be because the study used retrospectively collected weight data, which was more likely to have recall bias.
Our study revealed that baseline BMI level was a significant predictor for risk of developing T2DM. We noted that risk of T2DM for the “OB-high rapid increase” group was not the highest as expected, given this group had the highest mean initial BMI. This may be due to survival or selection bias. Studies have reported that elevated risk of diabetes may begin to plateau after 11–15 years of being obese [22].
Besides baseline BMI, our study indicated that both long-term weight-change slope and quadratic terms during late adulthood were significantly associated with elevated risk for T2DM. The faster the increase, the higher the risk of T2DM. Similar findings have been reported in other studies [3, 23]. One possible mechanism is that rapid weight gain may lead to altered adipose tissue distribution and result in higher insulin concentration. It is well-accepted that excess adiposity can have deleterious metabolic effects (such as increased insulin resistance or pro-inflammatory cytokines [24]), therefore increasing the risk of developing diabetes. Another explanation is that rapid weight gain may lead to an early onset of obesity that was also associated with higher risk of T2DM in our data.
The higher risk of T2DM among women who became obese at a younger age may be due to the long duration of obesity. Previous studies have shown that durations of obesity or abdominal obesity were associated with a significantly higher risk of diabetes, independent of the degree of BMI or abdominal adiposity [22]. Prolonged obesity duration may result in additional metabolic changes, leading to the development of hyperglycaemia and diabetes [25]. Similarly, our data show that obese-years, a cumulative effect of obesity, was associated with higher risk of T2DM in a dose-response fashion. This may be because obese-years reflect the cumulative damage of obesity on the body. A previous study compared different measures of obesity as predictors of diabetes risk and reported that obese-years was a better predictor for T2DM than duration of obesity or level of BMI alone [26]. Other studies have also shown that diabetes risk is particularly high in individuals who were obese as adolescents relative to those with adult-onset obesity [27]. Obesity during young age may be more deleterious for insulin resistance and diabetes than obesity during older age [27]. These data highlight the need for diabetes prevention efforts to begin early to slow down weight increases or delay the onset of obesity.
In addition, metabolism of women would be greatly changed after menopause. Studies have shown that the hormonal changes after menopause are associated with lower metabolic rate and increased total body fat, especially abdominal fat [28]. This may explain why we observed only increasing BMI trends for the population, and why the rapid increase pattern increases risk of diabetes. The mechanisms underlying obesity and type 2 diabetes remain unclear. Insulin resistance and pancreatic β-cell dysfunction are currently proposed as two main pathogenic mechanisms in the progression of obesity to diabetes [29]. Weight gain and obesity are associated with reduced insulin sensitivity of tissues [25]. β-cells compensate by increasing insulin secretion to maintain a normal blood glucose level and glucose tolerance; however, when the amount of insulin produced by β-cells is insufficient to compensate for the reduced insulin sensitivity of tissues, this may progressively lead to impaired glucose tolerance and then diabetes [25, 29].
Strengths of the study include a large prospective cohort with long term follow-up. We also applied GMM to identify distinct classes of BMI change over time. Like many other models, GMM may be susceptible to local solutions. We incorporated the use of random starting values to avoid local solutions. Several limitations deserve mention. First, data on weight in our study were self-reported. Although self-reported height and weight were found to be reliable [11], potential misclassification in trajectory assignment may be an issue. Also, because the exact timing of incidence of diabetes was unknown, it is possible that some weights that were reported close to diabetes diagnosis may be affected by undiagnosed diabetes. Second, women who experienced rapid weight gain and developed diabetes prior to the start of the study were excluded, with potential to underestimate the effects of adult weight and weight change on diabetes risk. Third, we did not have information on HbA1C. Although self-reported diabetes in the cohort has been demonstrated to be a reliable indicator of diagnosed diabetes [10], a certain degree of misclassification may still exist. However, this type of misclassification is likely to be non-differential, which may make our results conservative. Fourth, we assigned individuals into their most likely trajectory patterns and did not take into account the uncertainly of the classification. Fifth, although we were able to adjust for major confounders, other variables such as age of menopause and breastfeeding were unavailable, thus residual confounding cannot be ruled out. Finally, our results are based on middle age women, which may limit generalizability to other populations. More studies in large and diverse populations are needed to confirm our findings.
In conclusion, our data suggest that slowing down weight increases, delaying the onset of obesity, or reducing cumulative exposure to obesity may substantially lower the risk of developing diabetes in middle-aged women. Our findings offer more refined guidelines regarding diabetes prevention and control, showing, for example, that it is not too late for women to reduce weight to lower diabetes risk, and that slower rates of weight gain over time are better than rapid increases.
Supplementary Material
Highlights.
Type 2 diabetes risk was associated with rapid weight increase
Diabetes risk is higher with early versus later age of obesity onset
Diabetes risk is also higher with greater cumulative exposure to obesity
Maintaining normal weight or slowing down weight gain lowers diabetes risk.
Acknowledgements
The research on which this paper is based was conducted as part of the Australian Longitudinal Study on Women’s Health by the University of Queensland and the University of Newcastle. We are grateful to the Australian Government Department of Health for funding and to the women who provided the survey data. The authors also thank Professor Graham Giles of the Cancer Epidemiology Division, Cancer Council Victoria, for permission to use the Dietary Questionnaire for Epidemiological Studies (Version 2), Melbourne: Cancer Council Victoria, 1996. This study was supported by a Pilot and Feasibility Award within the CDMD NIH/NIDDK Grant Number P30 DK097512.
Footnotes
Conflict of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].A picture of overweight and obesity in Australia (https://www.aihw.gov.au/getmedia/172fba28-785e-4a08-ab37-2da3bbae40b8/aihw-phe-216.pdf.aspx?inline=true). Australian Institute of Health and Welfare; 2017. [Google Scholar]
- [2].Reinikainen J, Laatikainen T, Karvanen J, Tolonen H. Lifetime cumulative risk factors predict cardiovascular disease mortality in a 50-year follow-up study in Finland. Int J Epidemiol. 2015;44:108–16. [DOI] [PubMed] [Google Scholar]
- [3].Zhang T, Xu J, Li S, Bazzano LA, He J, Whelton PK, et al. Trajectories of childhood BMI and adult diabetes: the Bogalusa Heart Study. Diabetologia. 2019;62:70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. Jama. 2009;301:2234–42. [DOI] [PubMed] [Google Scholar]
- [5].Barker DJP, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. New Engl J Med. 2005;353:1802–9. [DOI] [PubMed] [Google Scholar]
- [6].Berlin KS, Parra GR, Williams NA. An Introduction to Latent Variable Mixture Modeling (Part 2): Longitudinal Latent Class Growth Analysis and Growth Mixture Models. J Pediatr Psychol. 2014;39:188–203. [DOI] [PubMed] [Google Scholar]
- [7].Brown WJ, Bryson L, Byles JE, Dobson AJ, Lee C, Mishra G, et al. Women’s Health Australia: recruitment for a national longitudinal cohort study. Women Health. 1998;28:23–40. [DOI] [PubMed] [Google Scholar]
- [8].Dobson AJ, Hockey R, Brown WJ, Byles JE, Loxton DJ, McLaughlin D, et al. Cohort Profile Update: Australian Longitudinal Study on Women’s Health. Int J Epidemiol. 2015;44:1547,a-f. [DOI] [PubMed] [Google Scholar]
- [9].ALSWH. Australian Longitudinal Study on Women’s Health website https://www.alswh.org.au/about/sample; 2019. [Google Scholar]
- [10].Navin Cristina TJ, Stewart Williams JA, Parkinson L, Sibbritt DW, Byles JE. Identification of diabetes, heart disease, hypertension and stroke in mid- and older-aged women: Comparing self-report and administrative hospital data records. Geriatrics & gerontology international. 2016;16:95–102. [DOI] [PubMed] [Google Scholar]
- [11].Burton NW, Brown W, Dobson A. Accuracy of body mass index estimated from self-reported height and weight in mid-aged Australian women. Aust N Z J Public Health. 2010;34:620–3. [DOI] [PubMed] [Google Scholar]
- [12].Armstrong T, Bauman A, Davies J. Physical activity patterns of Australian Adults: results of the 1999 National Physical Activity Survey Australian Institute of Health and Welfare, Canberra; 2000. [Google Scholar]
- [13].Muthen LK, Muthen BO. Mplus User’s Guide (Seventh Edition). Los Angeles, CA: Muthen & Muthen; 1998-2016. [Google Scholar]
- [14].Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes care. 1994;17:152–4. [DOI] [PubMed] [Google Scholar]
- [15].Fagherazzi G, Vilier A, Affret A, Balkau B, Bonnet F, Clavel-Chapelon F. The association of body shape trajectories over the life course with type 2 diabetes risk in adulthood: a group-based modeling approach. Ann Epidemiol. 2015;25:785–7. [DOI] [PubMed] [Google Scholar]
- [16].Mano Y, Yokomichi H, Suzuki K, Takahashi A, Yoda Y, Tsuji M, et al. Do body mass index trajectories affect the risk of type 2 diabetes? A case-control study. BMC Public Health. 2015;15:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Peter RS, Keller F, Klenk J, Concin H, Nagel G. Body mass trajectories, diabetes mellitus, and mortality in a large cohort of Austrian adults. Medicine. 2016;95:e5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gordon-Larsen P, Koehler E, Howard AG, Paynter L, Thompson AL, Adair LS, et al. Eighteen year weight trajectories and metabolic markers of diabetes in modernising China. Diabetologia. 2014;57:1820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thompson AL, Koehler E, Herring AH, Paynter L, Du S, Zhang B, et al. Weight Gain Trajectories Associated With Elevated C-Reactive Protein Levels in Chinese Adults. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Walsh EI, Shaw J, Cherbuin N. Trajectories of BMI change impact glucose and insulin metabolism. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2018;28:243–51. [DOI] [PubMed] [Google Scholar]
- [21].Nano J, Dhana K, Asllanaj E, Sijbrands E, Ikram MA, Dehghan A, et al. Trajectories of BMI Before Diagnosis of Type 2 Diabetes: The Rotterdam Study. Obesity (Silver Spring). 2020;28:1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Reis JP, Hankinson AL, Loria CM, Lewis CE, Powell-Wiley T, Wei GS, et al. Duration of abdominal obesity beginning in young adulthood and incident diabetes through middle age: the CARDIA study. Diabetes Care. 2013;36:1241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang Y, Yatsuya H, Li Y, Chiang C, Hirakawa Y, Kawazoe N, et al. Long-term weight-change slope, weight fluctuation and risk of type 2 diabetes mellitus in middle-aged Japanese men and women: findings of Aichi Workers’ Cohort Study. Nutrition & diabetes. 2017;7:e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hansen E, Hajri T, Abumrad NN. Is all fat the same? The role of fat in the pathogenesis of the metabolic syndrome and type 2 diabetes mellitus. Surgery. 2006;139:711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. [DOI] [PubMed] [Google Scholar]
- [26].Abdullah A, Wolfe R, Mannan H, Stoelwinder JU, Stevenson C, Peeters A. Epidemiologic merit of obese-years, the combination of degree and duration of obesity. Am J Epidemiol. 2012;176:99–107. [DOI] [PubMed] [Google Scholar]
- [27].The NS, Richardson AS, Gordon-Larsen P. Timing and duration of obesity in relation to diabetes: findings from an ethnically diverse, nationally representative sample. Diabetes care. 2013;36:865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, et al. Understanding weight gain at menopause. Climacteric. 2012;15:419–29. [DOI] [PubMed] [Google Scholar]
- [29].Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.