Abstract
Background:
Early hemorrhage control is essential to optimal trauma care. Hybrid operating rooms offer early, concomitant performance of advanced angiographic and operative hemostasis techniques, but their clinical impact is unclear. Herein, we present our initial experience with a dedicated, trauma hybrid operating room.
Study Design:
This retrospective cohort analysis of 292 adult trauma patients undergoing immediate surgery at a Level I trauma center compared patients managed after implementation of a dedicated, trauma hybrid operating room (n=186) with historic controls (n=106). The primary outcomes were time to hemorrhage control (systolic blood pressure ≥100 mmHg without ongoing vasopressor or transfusion requirements), early blood product administration, and complications.
Results:
Patient characteristics were similar between cohorts (age 41 years, 25% female, 38% penetrating trauma). The hybrid cohort had lower initial hemoglobin (10.2 vs. 11.1 g/dL, p=0.001) and a greater proportion of patients undergoing resuscitative endovascular balloon occlusion of the aorta (9% vs. 1%, p=0.007). Cohorts had similar case mixes and intraoperative consultations with cardiothoracic or vascular surgery (13%). Twenty-one percent of all hybrid cases included angiography. The interval between OR arrival and hemorrhage control was shorter in the hybrid cohort (49 vs. 60 minutes, p=0.005). From 4–24 hours after arrival, the hybrid cohort had fewer red cell (0.0 vs. 1.0, p=0.001) and plasma transfusions (0.0 vs. 1.0, p<0.001). The hybrid cohort had fewer infectious complications (15% vs. 27%, p=0.009) and ventilator days (2.0 vs. 3.0, p=0.011), and similar in-hospital mortality (13% vs. 10%, p=0.579).
Conclusion:
Implementation of a dedicated, trauma hybrid operating room was associated with earlier hemorrhage control and fewer early blood transfusions, infectious complications, and ventilator days.
Keywords: Hemorrhage, bleeding, injury, REBOA, resuscitation, transfusion
Introduction
Traumatic injury causes more than 5 million deaths annually, accounting for approximately 9% of all deaths worldwide.1 From 1999–2015 the United States, unintentional injury and homicide were responsible for 21.4% of all years of potential life lost prior to age 70 years, followed by malignant neoplasms (19.0%) and heart disease (13.9%).2 Hemorrhage accounts for approximately 40% of all trauma deaths, more than 80% of all trauma deaths in the operating room, and is the most common cause of potentially preventable (treatable) death from trauma.3–6 To facilitate early hemorrhage control, numerous regulatory and verifying bodies have mandated the immediate availability of an operating room for acutely injured patients.7
In-hospital patient triage patterns affect the timing and efficacy of early hemorrhage control procedures. Approximately half of all hypotensive trauma patients are transferred directly from the emergency department to an operating room for hemorrhage control.8 Transferring an unstable, bleeding trauma patient to a computed tomography (CT) scanner or Interventional Radiology suite can be time-consuming and dangerous, especially when it imposes steric hindrance on resuscitative efforts and delays operative hemorrhage control. Yet, certain injuries are optimally managed by angiographic techniques under fluoroscopy (e.g., zone 3 retroperitoneal hemorrhage secondary to pelvic fracture, solid organ injury hemorrhage from a pseudoaneurysm, and resuscitative endovascular balloon occlusion of the aorta (REBOA) for sub-diaphragmatic hemorrhage).9–15 Time-sensitive decisions for patient triage and management strategies are, by necessity made with limited information garnered from the primary survey; erroneous decisions have dire consequences.
Alternatively, hybrid operating rooms furnished with angiographic equipment offer opportunities to identify and control sources of hemorrhage with angiographic techniques while performing operative exploration to control surgically accessible hemorrhage.16 At Japanese and Canadian centers, implementation of a trauma hybrid operating room has been associated earlier initiation of hemorrhage control procedures and decreased mortality among patients with exsanguination or hemorrhagic shock.17,18 Despite successful adoption in other countries, hybrid operating rooms in the United States are rarely dedicated to managing acutely injured patients, and therefore, hybrid suites are usually occupied with an elective or urgent nontrauma case when a trauma patient presents with hemorrhagic shock.9,17,19–21 Additionally, hybrid operating rooms are not usually staffed for immediate access at night and during weekends. Among the few dedicated, trauma hybrid operating rooms in the North America, it is unclear whether they yield advantages in obtaining early hemorrhage control and improving clinical outcomes. The purpose of this study is to critically evaluate the use of a dedicated, trauma hybrid operating room at a Level I trauma center with the null hypothesis that hybrid operating room use would not be associated with differences in time to hemorrhage control, blood product administration, morbidity, or mortality compared with historic controls.
Methods
Study population
This retrospective cohort analysis included 292 consecutive adult trauma patients who underwent immediate operation (i.e., within four hours of arrival) at a Level I trauma center. The primary analysis compared patients managed after implementation of a dedicated, trauma hybrid operating room during a 42-month period ending April 2020 (n=186) with historic control patients managed during a 42-month period immediately prior to implementation of the hybrid trauma operating room (n=106). Derivation of the study population is illustrated in Figure 1. Immediate surgery was defined as occurring within four hours of arrival to maintain consistency with published literature.22 Exclusion criteria were age less than 18 years, initial operation for hemorrhage control at referring facility (n=18), patients who suffered blunt traumatic arrest (n=19) in the emergency department or pre-hospital setting, and patients who had immediate surgery for purposes other than hemorrhage control (e.g., isolated tracheostomy or cricothyroidotomy to secure an airway, neurosurgery for isolated traumatic brain injury, diagnostic laparoscopy, wound exploration and closure, n=41). These patients were excluded a priori because surgery at an outside facility or for purposes other than hemorrhage control would confound associations between time to hemorrhage control and outcomes, and survival following surgery for blunt traumatic arrest is rare, and would confound the analysis of complications and in-hospital mortality. This study was approved by the University of Florida Institutional Review Board (#202001256).
Figure 1:
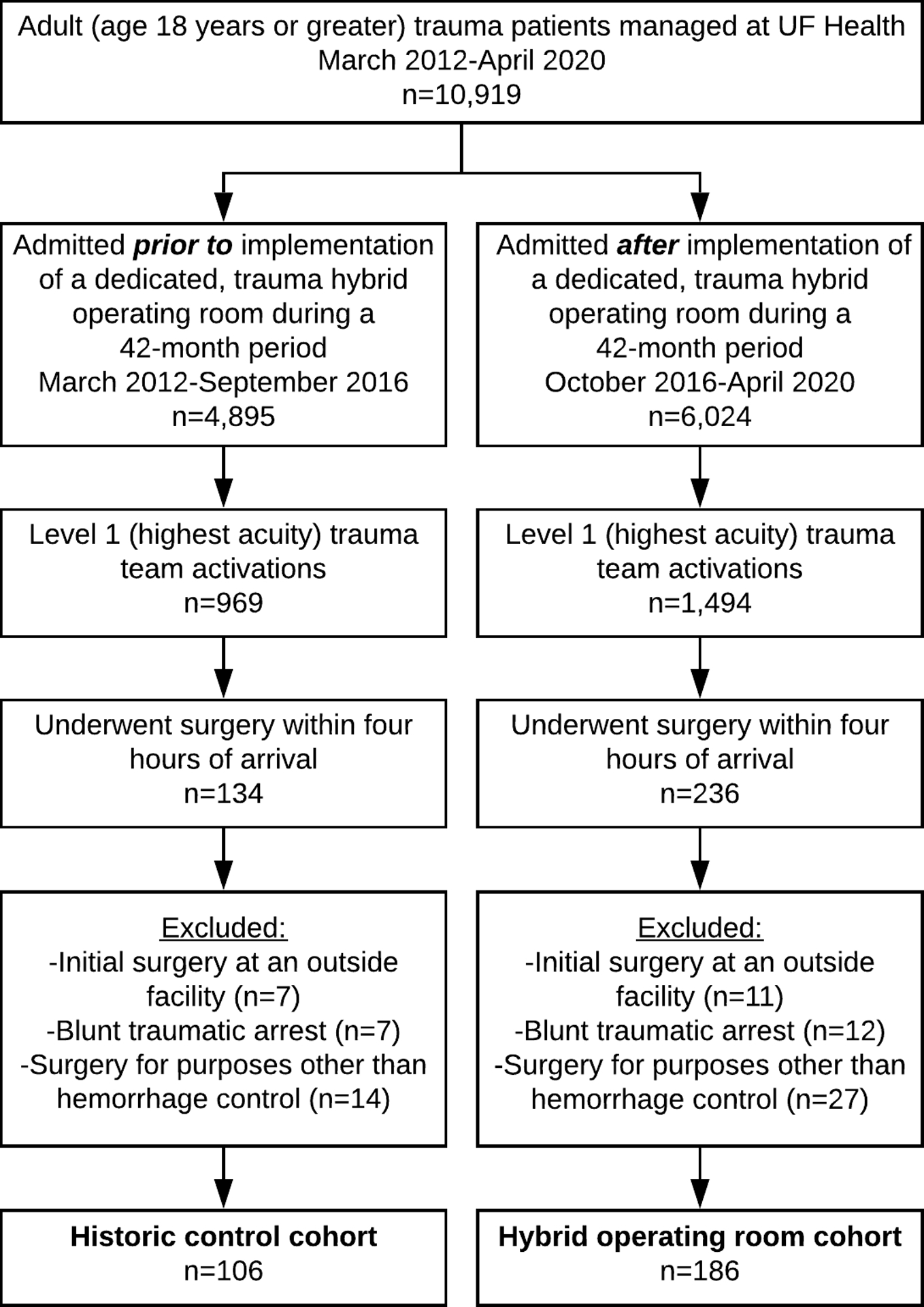
Derivation of the study population. UF: University of Florida.
Trauma hybrid operating room specifications, REBOA placement, and staff training
The trauma hybrid operating room was located in a repurposed and remodeled angiography suite located within an operating room complex located one floor above an emergency department containing six trauma resuscitation bays. The hybrid operating room contained a ceiling-mounted C-arm and a carbon fiber fluoroscopy-compatible table with Trendelenburg, reverse Trendelenburg, and lateral tilt capabilities, and was accompanied by a fluoroscopy control room behind lead-lined glass windows for radiation shielding (Philips AlluraClarity, Figure 2). The initial cost associated with transitioning an angiographic suite to the trauma hybrid room was in excess of $1.5 million. Additionally, the expense of maintaining the dedicated trauma hybrid room is significant.
Figure 2:
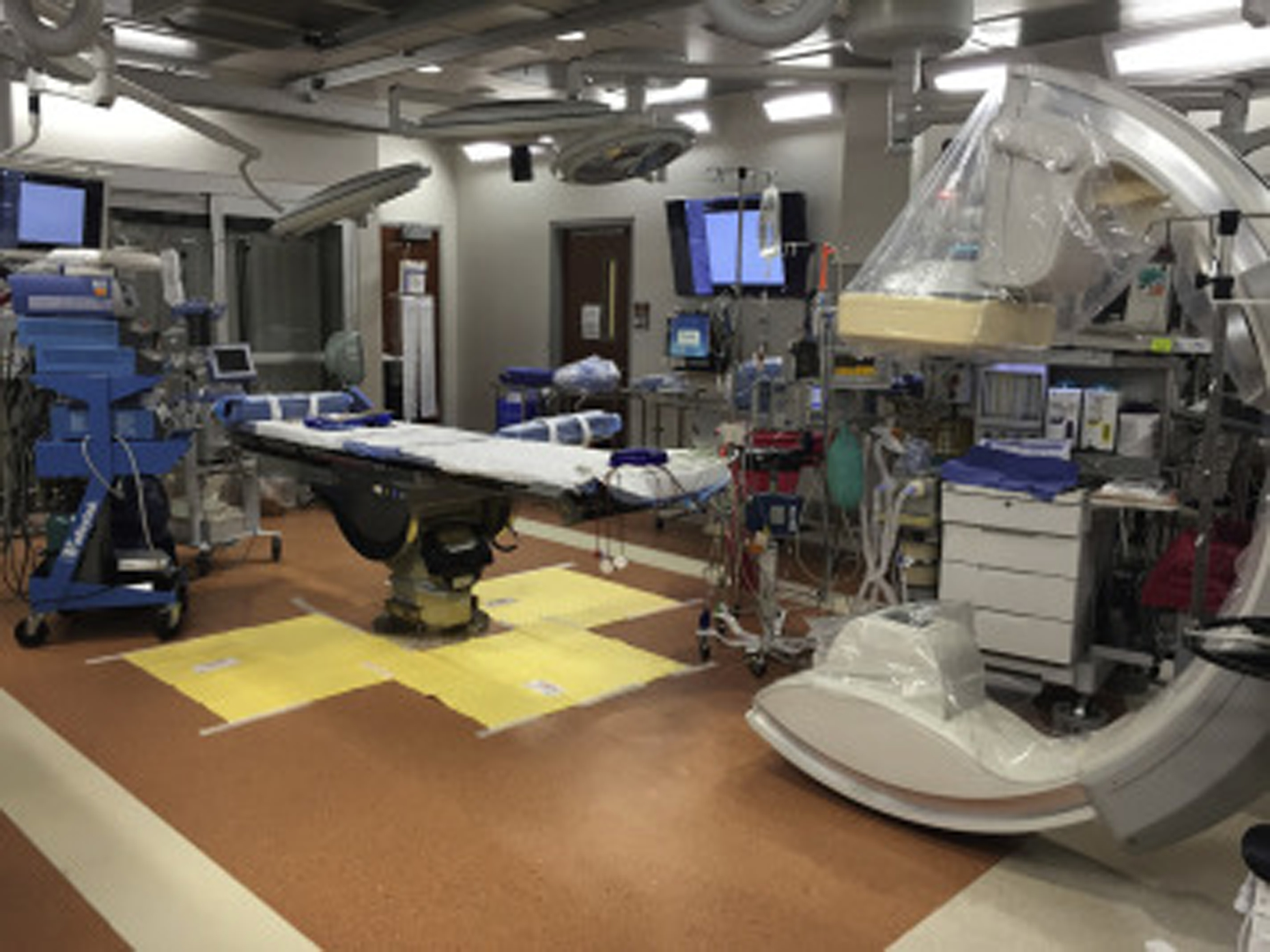
The dedicated, trauma hybrid operating room contains a ceiling-mounted C-arm and a carbon fiber fluoroscopy-compatible table, and is accompanied by a fluoroscopy control room behind lead-lined glass windows.
REBOA catheters were placed by trauma surgeons for patients with blunt or non-thoracic penetrating trauma, suspected sub-diaphragmatic hemorrhage, systolic blood pressure less than 90 mmHg, and a transient response or no response to volume resuscitation. Balloon inflation was initially performed in Zone 1 for all patients; subsequent decisions regarding balloon deflation and withdrawal to Zone 3 were at the discretion of the attending trauma surgeon and determined by hemodynamic response, completion of operative hemorrhage control techniques, and injury patterns (e.g., for a patient with a pelvic fracture, hemorrhagic shock, and a retroperitoneal hematoma in the pelvis, the balloon would be repositioned to Zone 3 as soon as it was confirmed that proximal sources of hemorrhage were controlled).9 Early in the study period, REBOA was performed with a 12 French introducer and aortic occlusion balloon (Cook Medical) with femoral arterial access obtained by direct cut-down or percutaneously at the discretion of the attending trauma surgeon. Subsequently, REBOA was performed using a 7 French introducer and aortic occlusion balloon (Prytime Medical) with femoral access obtained percutaneously. Both catheters had to be deflated to allow distal aortic blood flow, in contrast with the newer partial-REBOA philosophy that feature efforts to allow a controlled amount of distal aortic blood flow. The senior author trained trauma surgeons and senior residents in REBOA concepts and techniques with a series of 90-minute slide presentations and hands-on simulation sessions. In addition, several 30-minute REBOA orientation sessions were offered to emergency department, operating room, and ancillary staff. Operating Room staff received orientation and training regarding the design and intent of the hybrid trauma operating room. All other diagnostic and therapeutic angiographic hemorrhage control procedures (e.g., endovascular stent placement, balloon angioplasty, coil placement, and embolization) were performed by interventional radiologists and vascular surgeons who were called in consultation by the trauma team. These procedures were performed in the trauma hybrid operating room without the need to relocate the patient.
Data collection
Data regarding patient characteristics, hemorrhage control procedures, resuscitation parameters, and clinical outcomes were collected from a prospective, institutional trauma registry and supplemented by manual review of operative reports and intraoperative Anesthesia data flowsheets contained in electronic health records. Patient characteristics were represented by demographic variables, mechanism of injury, Injury Severity and Glasgow Coma Scale scores, endotracheal intubation in the field or emergency department, vital signs, laboratory values, and extended focused assessment with sonography for trauma (EFAST) exam findings.
Hemorrhage control procedural data included performance of REBOA, sternotomy or thoracotomy and associated maneuvers (including aortic cross clamping, pericardiotomy, cardiac laceration repair, and pulmonary resection or tractotomy), laparotomy and associated maneuvers (including solid organ resection or repair, hollow viscous resection, and diaphragm repair), preperitoneal pelvic packing, neck exploration, and operative management of named vessels (including vascular bypass, interposition graft placement, patch repair, endovascular stenting, balloon angioplasty, primary repair, and ligation), intraoperative consultation with non-trauma specialties, and the performance of angiographic procedures within 12 hours of arrival, including anatomic sites and therapeutic interventions (including endovascular stent placement, balloon angioplasty, coil placement, and embolization). Anesthesia data flowsheets were analyzed to identify operating room start and end times, vasopressor and blood product administration, and intraoperative hemodynamics. These variables were used to identify the time of hemorrhage control, defined as achieving a systolic blood pressure 100 mmHg or greater without ongoing vasopressor or blood product transfusion requirements or subsequent episodes of hypotension with systolic blood pressure less than 90 mmHg. These systolic blood pressure thresholds were selected to maintain consistency with consensus regarding the principles of damage control resuscitation.23 Resuscitation parameters included the administration of tranexamic acid within four hours of arrival as well as red blood cell and plasma transfusions within four hours of arrival and 4–24 hours after arrival. The initial four-hour window for tranexamic acid administration and earliest blood product administration was chosen to maintain consistency with the four-hour cutoff for immediate surgery, which was based on previous work regarding hemorrhage control procedures for trauma patients.22 Blood product administration more than 24 hours after arrival was not assessed because it is unlikely that late transfusions would be substantially affected by hemorrhage control procedures performed within four hours of arrival, but would be affected by other factors related to secondary procedures.
Clinical outcomes data included postoperative complications, which were classified as infectious or non-infectious. Infectious complications were further classified by type (i.e., bloodstream infection, pneumonia, surgical site infection, urinary tract infection, and others), as recorded in a prospective, institutional trauma registry. In addition, all complications were classified according to the Clavien-Dindo system that was adapted for trauma patients by Naumann et al.24 This system ranks complications from 1 (least severe) to 5 (most severe) with sub classifications as follows: 1) deviation from the initial management plan such as a bedside intervention that does not require anesthetic; 2) pharmacologic treatment or unexpected transfusion, 3A) unplanned procedural intervention without general anesthesia, 3B) unplanned procedural intervention with general anesthesia, 4A) complication requiring ICU admission or prolonged ICU admission without multi-organ failure or cardiopulmonary resuscitation, 4B) complication requiring ICU admission or prolonged ICU admission with multi-organ failure or cardiopulmonary resuscitation, 5A) in-hospital mortality after implementation of palliative care, and 5B) in-hospital mortality during active treatment. The scoring system also includes the suffix P to indicate permanent disability; the P suffix was omitted from this study because prospective, long-term follow-up would be necessary to accurately assess permanent disability. Other outcomes included lengths of stay in the hospital and in the ICU, days on mechanical ventilation, and discharge disposition.
Statistical analysis
The primary statistical objective was to assess differences in patient characteristics, hemorrhage control procedures, resuscitation parameters, and clinical outcomes between patients managed before and after implementation of a dedicated, trauma hybrid operating room. Therefore, variables representing each of those domains were directly compared between cohorts. Continuous variables were compared by the non-parametric Kruskal-Wallis test and reported as median values with interquartile ranges. Binary variables were compared by Fisher’s Exact test and reported as raw numbers with percentages. Time to hemorrhage control after arrival in the operating room for trauma patients has not been previously reported, which precludes the performance of a power analysis. Therefore, this study was performed as an exploratory analysis. Statistical analysis was performed using the open source Python (version 3.7.6) programming language with the Spyder (version 4.0.1) environment and SPSS (version 23, IBM, Armonk, NY). Significance was set at α=0.05.
Results
Approximately 15% of all triage level 1 patients underwent immediate surgery
There were 4,895 trauma team activations during the pre-hybrid era and 6,024 during the hybrid era. Pre-hybrid era activations had median injury severity score 9 [interquartile range 4–17] and 8.1% in-hospital mortality. The hybrid-era activations had median injury severity score 6 [1–14] and 6.4% in-hospital mortality. There were 969 triage level 1 (highest acuity) trauma team activations during the pre-hybrid era and 1,494 during the hybrid era. The pre-hybrid era level 1 activations had median injury severity score 13 [5–22] and 15.8% in-hospital mortality. The hybrid-era activations had median injury severity score 14 [4–26] and 22.1% in-hospital mortality.
After excluding patients who underwent initial surgery at an outside facility or for purposes other than hemorrhage control (e.g., isolated tracheostomy or cricothyroidotomy to secure an airway, neurosurgery for isolated traumatic brain injury, diagnostic laparoscopy, wound exploration and closure), or suffered blunt traumatic arrest, there were 106 patients who underwent immediate operation (i.e., within four hours) during the pre-hybrid era, representing 11% of all level 1 trauma team activations; these 106 patients composed the historic control cohort in the primary analysis. After implementation of the dedicated, trauma hybrid operating room, there were 186 patients who underwent immediate surgery, representing 12% of all level 1 trauma team activations; these 186 patients composed the hybrid operating room cohort in the primary analysis.
Patient characteristics were similar between hybrid operating room and historic control cohorts
Patient characteristics are listed in Table 1. Control and hybrid cohorts had similar demographics. Overall median age was 41 years. Twenty five percent of all patients were female. Penetrating trauma was the mechanism of injury for 38% of all cases, with similar proportions between cohorts. Median injury severity scores suggested severe injury in control and hybrid cases (18 and 22, respectively, p=0.187). In both cohorts, 35% of all patients were intubated in the field or emergency department; the other 65% were intubated in the operating room. Control and hybrid cases had similar initial systolic blood pressure (95 mmHg in both groups), pH (7.29 and 7.28, respectively), and lactic acid (3.1 and 3.4 mmol/L, respectively). Initial hemoglobin levels were significantly lower among hybrid cases (10.2 vs. 11.1 g/dL, p=0.001). The hybrid cohort also had a somewhat slower clotting reaction time on thromboelastography, though the difference was not statistically significant (3.7 vs. 3.2 minutes, p=0.072).
Table 1:
Characteristics of patients undergoing immediate surgery for hemorrhage control before and after implementation of a dedicated, trauma hybrid operating room (OR).
| Patient characteristics | Control cases (n=106) | Hybrid OR cases (n=186) | P |
|---|---|---|---|
| Age | 40 [26–52] | 41 [27–61] | 0.176 |
| Female | 22 (21%) | 52 (28%) | 0.208 |
| Injury Severity Score | 18 [13–27] | 22 [13–29] | 0.187 |
| Blunt injury | 63 (59%) | 119 (64%) | 0.454 |
| Penetrating injury | 43 (41%) | 67 (36%) | 0.454 |
| Traumatic arrest in field or EDa | 1 (1%) | 7 (4%) | 0.266 |
| Intubated in field or ED | 37 (35%) | 66 (35%) | >0.999 |
| Glasgow Coma Scale | 15 [3–15] | 15 [7–15] | 0.662 |
| Best eye opening response | 4 [1–4] | 4 [1–4] | 0.230 |
| Best verbal response | 5 [1–5] | 5 [1–5] | 0.689 |
| Best motor response | 6 [1–6] | 6 [4–6] | 0.474 |
| Heart rate | 107 [90–124] | 110 [93–128] | 0.464 |
| Respiratory Rate | 18 [15–22] | 20 [17–24] | 0.008 |
| Systolic blood pressure (mmHg) | 95 [84–111] | 95 [86–109] | 0.886 |
| Mean arterial pressure (mmHg) | 70 [61–82] | 71 [61–83] | 0.990 |
| FAST performed | 80 (75%) | 154 (83%) | 0.169 |
| FAST negative | 40 (38%) | 73 (39%) | 0.804 |
| FAST equivocal | 5 (5%) | 10 (5%) | >0.999 |
| FAST positive | 35 (33%) | 71 (38%) | 0.448 |
| Temperature (Celsius) | 36.3 [35.3–36.8] | 36.3 [35.7–36.7] | 0.513 |
| pH | 7.29 [7.21–7.35] | 7.28 [7.19–7.34] | 0.230 |
| Lactic acid (mmol/L) | 3.1 [2.0–4.7] | 3.4 [1.9–5.4] | 0.666 |
| Hemoglobin (g/dL) | 11.1 [10.0–13.0] | 10.2 [9.0–11.7] | 0.001 |
| International Normalized Ratio | 1.3 [1.1–1.4] | 1.2 [1.1–1.3] | 0.087 |
| Had a rapid TEG | 52 (49%) | 72 (39%) | 0.109 |
| Had a regular TEG | 56 (53%) | 128 (69%) | 0.008 |
| Reaction time (min) | 3.2 [2.6–4.4] | 3.7 [2.9–4.4] | 0.072 |
| Alpha angle (degrees) | 68.8 [60.1–73.2] | 68.5 [62.3–72.5] | 0.974 |
| K time (min) | 1.5 [1.2–2.5] | 1.6 [1.2–2.2] | 0.955 |
| Maximum amplitude (mm) | 59.2 [51.4–63.3] | 59.3 [52.3–63.0] | 0.884 |
| Clot stability (Kdynes/cm2) | 7.29 [5.74–8.33] | 7.29 [5.77–8.29] | >0.999 |
These cases are traumatic arrests following penetrating trauma, as blunt traumatic arrests were excluded. ED: emergency department, FAST: focused assessment with sonography for trauma, TEG: thromboelastograph. Data are presented as n (%) or median [interquartile range].
The hybrid operating room cohort had greater use of REBOA and intraoperative angiography
Hemorrhage control procedures and resuscitation parameters are listed in Table 2. After implementation of a dedicated, trauma hybrid operating room, a significantly greater proportion of patients underwent REBOA (8% vs. 1%, p=0.013). Cohorts had similar rates of sternotomy or thoracotomy, laparotomy, preperitoneal pelvic packing, neck exploration, and operative management of a named vessel. Among hybrid cases, there were slightly higher rates of aortic cross clamping (5% vs. 1%, p=0.062) and primary repair of a named vessel (11% vs. 5%, p=0.085), though the differences were not statistically significant. Intraoperative consultation with cardiothoracic or vascular surgery was obtained in 13% of all cases, with similar rates between cohorts. In the control cohort, 18% of all cases involved angiography within 12 hours of arrival, and 85% of these angiographic procedures were performed separately in an Interventional Radiology suite (p<0.001). In the hybrid cohort, 21% of all cases involved angiography, and all of these angiographic procedures were performed in the hybrid operating room. The incidence of therapeutic angiography was similar between control and hybrid cohorts (12% and 13%, respectively).
Table 2:
Hemorrhage control procedures and resuscitation parameters before and after implementation of a dedicated, trauma hybrid operating room (OR).
| Hemorrhage control and resuscitation | Control cases (n=106) | Hybrid OR cases (n=186) | p |
|---|---|---|---|
| Transferred from ED directly to OR | 59 (56%) | 119 (64%) | 0.172 |
| Underwent REBOA | 1 (1%) | 18 (8%) | 0.013 |
| Underwent sternotomy or thoracotomy | 7 (7%) | 24 (13%) | 0.115 |
| Aortic cross clamp placed | 4 (4%) | 13 (7%) | 0.309 |
| Pericardiotomy | 5 (5%) | 19 (10%) | 0.123 |
| Cardiac laceration repair | 1 (1%) | 6 (3%) | 0.428 |
| Pulmonary resection or tractotomy | 1 (1%) | 3 (2%) | >0.999 |
| Underwent laparotomy | 81 (76%) | 144 (77%) | 0.885 |
| Solid organ resection | 29 (27%) | 47 (25%) | 0.782 |
| Solid organ repair | 15 (14%) | 40 (22%) | 0.161 |
| Hollow viscous resection | 25 (24%) | 30 (16%) | 0.123 |
| Diaphragm repair | 10 (9%) | 16 (9%) | 0.833 |
| Underwent preperitoneal pelvic packing | 9 (8%) | 23 (12%) | 0.338 |
| Underwent neck exploration | 9 (8%) | 13 (7%) | 0.650 |
| Operative management of a named vessel | 28 (26%) | 58 (31%) | 0.425 |
| Bypass, interposition graft, or patch repair | 7 (7%) | 11 (6%) | 0.805 |
| Endovascular stent or balloon angioplasty | 2 (2%) | 2 (1%) | 0.623 |
| Primary repair | 5 (5%) | 20 (11%) | 0.085 |
| Ligation | 15 (14%) | 22 (12%) | 0.586 |
| CT or Vascular Surgery consultation | 14 (13%) | 23 (12%) | 0.856 |
| Underwent angiography | 19 (18%) | 39 (21%) | 0.647 |
| Angiography performed in IR suite | 16 (15%) | 0 (0%) | <0.001 |
| Central/aortogram | 5 (5%) | 8 (4%) | >0.999 |
| Peripheral/extremity angiography | 3 (3%) | 5 (3%) | >0.999 |
| Visceral angiography | 6 (6%) | 9 (5%) | 0.787 |
| Pelvic angiography | 7 (7%) | 22 (12%) | 0.221 |
| Therapeutic angiographya | 13 (12%) | 24 (13%) | >0.999 |
| Obtained hemorrhage controlb | 102 (96%) | 176 (95%) | 0.777 |
| Interval: OR start to hemorrhage control (min) | 60 [42–84] | 49 [34–69] | 0.005 |
| Total OR plus angiography time (min) | 133 [92–243] | 135 [91–188] | 0.971 |
| TXA administered 0–4 h after arrival | 20 (19%) | 33 (18%) | 0.875 |
| RBC transfusions 0–4 h after arrival | 3.0 [0.0–5.0] | 2.5 [1.0–5.0] | 0.730 |
| Plasma transfusions 0–4 h after arrival | 2.0 [0.0–4.0] | 1.5 [0.0–4.0] | 0.742 |
| RBC transfusions 4–24 h after arrival | 1.0 [0.0–3.0] | 0.0 [0.0–2.0] | 0.001 |
| Plasma transfusions 4–24 h after arrival | 1.0 [0.0–3.0] | 0.0 [0.0–1.0] | <0.001 |
Endovascular stent placement, balloon angioplasty, coil placement, or embolization.
Systolic blood pressure 100 mmHg or greater without ongoing vasopressor or blood product transfusion requirements. ED: emergency department, REBOA: resuscitative endovascular balloon occlusion of the aorta, CT: presented as n (%) or median [interquartile range]. Cardiothoracic, IR: Interventional Radiology, TXA: tranexamic acid, RBC: red blood cell. Data are presented as n (%) or median [interquartile range].
The hybrid operating room cohort achieved earlier hemorrhage control and received fewer early blood product transfusions
Tranexamic acid and blood product administration within four hours of arrival was similar between cohorts (Table 2). Hemorrhage control (i.e., systolic blood pressure 100 mmHg or greater without ongoing vasopressor or blood product transfusion requirements or subsequent episodes of hypotension with systolic blood pressure less than 90 mmHg) was obtained in 96% of all cases, with similar rates between cohorts. The interval between the operating room start time and hemorrhage control was significantly shorter in the hybrid cohort (49 vs. 60 minutes, p=0.005). Between 4 and 24 hours after arrival, hybrid cases had significantly lower median red blood cell transfusions (0.0 [0.0–2.0] vs. 1.0 [0.0–3.0], p=0.001) and plasma transfusions (0.0 [0.0–1.0] vs. 1.0 [0.0–3.0], p<0.001).
The hybrid operating room cohort had lower incidence of pneumonia and fewer days on mechanical ventilation
Clinical outcomes are listed in Table 3. The overall incidence of complications was high and similar between control and hybrid cohorts (55% and 48%, respectively). Infectious complications occurred in a greater proportion of control cases (27% vs. 15%, p=0.009). The difference in infectious complications between groups was primarily attributable to a lesser incidence of pneumonia in the hybrid cohort (4% vs. 12%, p=0.008). Applying a Clavien-Dindo complication classification scheme that was adapted for trauma patients by Naumann et al.,24 the control cases had a greater proportion of type 4B complications, i.e., requirement for ICU readmission, re-intubation, or prolonged ICU stay (14% vs. 6%, p=0.036). Although hospital length of stay and ICU length of stay were similar between cohorts, the control cohort had significantly more days on mechanical ventilation (3.0 vs. 2.0, p=0.011). Discharge dispositions were similar between control and hybrid cohorts, including in-hospital mortality (10% vs. 13%, p=0.579).
Table 3:
Clinical outcomes before and after implementation of a dedicated, trauma hybrid operating room (OR).
| Clinical outcomes | Control cases (n=106) | Hybrid OR cases (n=186) | p |
|---|---|---|---|
| Postoperative complications | |||
| Any complication | 58 (55%) | 90 (48%) | 0.331 |
| Any infectious complication | 29 (27%) | 27 (15%) | 0.009 |
| Pneumonia | 13 (12%) | 7 (4%) | 0.008 |
| Bloodstream infection | 9 (9%) | 8 (4%) | 0.163 |
| Surgical site infection | 6 (6%) | 9 (5%) | 0.787 |
| Urinary tract infection | 5 (5%) | 4 (2%) | 0.293 |
| Clostridium difficile infection | 0 (0%) | 1 (1%) | >0.999 |
| Graft infection | 0 (0%) | 1 (1%) | >0.999 |
| Clavien-Dindo classificationsa | |||
| Overall, median | 2.0 [0.0–4.0] | 0.0 [0.0–4.0] | 0.364 |
| Grade 1, n (%) | 3 (3%) | 6 (3%) | 0.331 |
| Grade 2, n (%) | 13 (12%) | 19 (10%) | 0.697 |
| Grade 3a, n (%) | 4 (4%) | 7 (4%) | >0.999 |
| Grade 3b, n (%) | 5 (5%) | 9 (5%) | >0.999 |
| Grade 4a, n (%) | 7 (7%) | 12 (6%) | >0.999 |
| Grade 4b, n (%) | 15 (14%) | 12 (6%) | 0.036 |
| Grade 5a, n (%) | 7 (7%) | 9 (5%) | 0.596 |
| Grade 5b, n (%) | 4 (4%) | 16 (9%) | 0.150 |
| Hospital length of stay (d) | 9.5 [5.0–23.3] | 9.0 [5.8–19.0] | 0.791 |
| ICU length of stay (d) | 6.0 [2.0–17.0] | 5.0 [2.0–13.0] | 0.636 |
| ICU-free hospital days | 4.0 [2.0–6.0] | 4.0 [1.0–7.3] | 0.615 |
| Days on mechanical ventilation | 3.0 [1.0–8.0] | 2.0 [1.0–5.3] | 0.011 |
| Ventilator-free ICU days | 2.0 [0.0–6.3] | 3.0 [1.0–7.0] | 0.144 |
| Discharge disposition | |||
| Home | 52 (49%) | 104 (56%) | 0.274 |
| Prison | 6 (6%) | 5 (3%) | 0.215 |
| Another hospital | 5 (5%) | 7 (4%) | 0.762 |
| Subacute/inpatient rehabilitation | 17 (16%) | 20 (11%) | 0.204 |
| Long-term acute care | 13 (12%) | 22 (12%) | >0.999 |
| Custodial care/nursing home | 2 (2%) | 1 (1%) | 0.299 |
| Hospice | 0 (0%) | 2 (1%) | 0.536 |
| In-hospital mortality | 11 (10%) | 25 (13%) | 0.579 |
| Non-home discharge | 54 (51%) | 82 (44%) | 0.274 |
Adapted for trauma by Naumann et al.18 ICU: intensive care unit. Data are presented as n (%) or median [interquartile range].
Discussion
Among trauma patients undergoing immediate surgery within four hours of arrival at a Level I trauma center, implementation of a dedicated, trauma hybrid operating room was associated with earlier hemorrhage control, fewer red cell and plasma transfusions 4–24 hours after arrival, lower incidence of pneumonia and overall infectious complications, and fewer days on mechanical ventilation. Blood product administration within four hours of arrival was not significantly lower in the hybrid operating room cohort, which may be partially attributable to significantly lower initial hemoglobin levels in the hybrid cohort. In addition, blood transfusion requirements within four hours of arrival are substantially affected by blood loss at the time of injury and during transportation to the emergency department. The observations that the hybrid cohort also had lower incidence of infectious complications and pneumonia is consistent with previous work demonstrating that blood product administration is associated with transfusion-related immunomodulation and increased risk for infection.25,26 It is unclear whether the control cohort had greater incidence of pneumonia due to more ventilator days, or vice versa. Previous work suggests that the association is bidirectional.27,28 Collectively, results from this study are supported by biologically plausible and evidence-based associations among the timing of hemorrhage control, blood product transfusion, infectious complications, and ventilator days. These observations suggest that implementation of a dedicated, trauma hybrid operating room may improve outcomes for trauma patients requiring immediate surgery for hemorrhage control.
Associations between time to hemorrhage control and clinical outcomes require further attention and investigation. Hybrid operating room use has been associated with shorter intervals between arrival and the start time of interventions for hemorrhage control.17,18 However, the authors are unaware of any prior reports of time to hemorrhage control and hemodynamic stability among trauma patients. There is a discussion of unpublished observations from the Pragmatic Randomized Optimal Platelet and Plasma Ratio (PROPPR) study, which states that time to hemostasis after arrival in the operating room was approximately 67 minutes, variable among sites involved in the study, and was independently associated with reduced 30-day mortality.29 Subsequent publications from the PROPPR group state that anatomic hemostasis was determined by the surgeon’s assessment that bleeding within the surgical field was controlled and no further hemostatic interventions were anticipated.30 This definition is different than the one used in the present study; therefore, it is difficult to compare with results from the present study, which featured somewhat shorter intervals between arrival in the operating room and hemorrhage control (60 minutes in the control cohort and 49 minutes in the hybrid operating room cohort). Other published work indirectly support the hypothesis that early hemorrhage control is associated with better outcomes. In an analysis of National Trauma Data Base patients with severe truncal hemorrhage, Alarhayem et al.31 demonstrated that mortality after severe truncal hemorrhage increases significantly within 30 minutes of injury and then decreases over time, questioning the veracity of the “golden hour” paradigm. In an analysis of five prospective studies including more than four thousand trauma patients, Fox et al. reported that the median interval between admission and death from hemorrhage is approximately two hours, suggesting that early hemorrhage control within this two-hour window has the potential to mitigate hemorrhagic death after trauma.32
Placement of the REBOA device was more straight-forward and accurate when performed in the trauma hybrid operating room due to the immediate availability of high quality fluoroscopic imaging and capable assistants. The potential for REBOA to improve outcomes after blunt and non-thoracic, penetrating trauma is probably underestimated by this study and others.9,10 During the study period, REBOA was performed using a 7 French ER-REBOA™ (Prytime Medical, Boerne, TX) catheter that must be deflated to allow distal aortic blood flow. This strategy is effective in temporizing non-compressible, sub-diaphragmatic arterial hemorrhage, but also induces profound distal ischemia.33–35 To mitigate the consequences of distal ischemia, the balloon may be intermittently deflated, but this often leads to substantial hemodynamic lability and ongoing hemorrhage when a high-pressure head is suddenly unleashed, violating the principles of damage control resuscitation.23 New partial occlusion catheters allow variable amounts of distal aortic blood flow with less hemodynamic lability, and have demonstrated efficacy in pre-clinical settings.36 Therefore, associations between REBOA and clinical outcomes among blunt and non-thoracic, penetrating trauma patients will require further investigation of partial aortic occlusion catheters in clinical settings. In addition, further research is needed to determine whether certain patients with major, hepatic venous injuries benefit from balloon occlusion of the vena cava and selective embolization of portal venous hemorrhage.15,37–39
This study is limited by its retrospective, single-institution design, which introduces selection bias, restricts the sample size, and limits the generalizability of its findings. Selection bias was minimized by including all consecutive cases meeting a priori inclusion and exclusion criteria. The restricted sample size increases the probability of false negative results, which are difficult to identify in the absence of a power analysis; in this study, a power analysis could not be performed because there are no previously published data establishing associations between time to hemorrhage control and clinical outcomes among trauma patients. Yet, there were several statistically significant associations in primary outcomes that are biologically plausible and consistent with known, evidence-based associations, suggesting that the study was adequately powered for several of the primary outcomes. Deeper understanding of associations between early hemorrhage control and clinical outcomes will require analysis of large datasets that contain the granularity necessary to pinpoint the time of hemorrhage control and hemodynamic stability. Unfortunately, “big” data and granular data rarely co-exist. Electronic health records may offer a viable solution.40 However, electronic health record data structures and coding practices vary across institutions. Therefore, collaborative efforts to standardize data across institutions are necessary. The authors suggest the Fast Healthcare Interoperability Resources (FHIR) data standard to achieve data interoperability, and seek collaborations with other trauma centers in building FHIR-compliant, multi-institutional electronic health record datasets.
Conclusions
Implementation of a dedicated, trauma hybrid operating room was associated with increased use of REBOA and other intraoperative angiographic procedures, earlier hemorrhage control, and fewer early blood transfusions, infectious complications, and ventilator days. Associations between time to hemorrhage control and clinical outcomes require further investigation, ideally using granular, standardized electronic health record data from multiple institutions.
Discussion.
Thomas R. Scalea, M.D.
Baltimore, Maryland
Initially, trauma care was delivered at the University of Florida, Gainesville in a standard manner, patients had surgery in operating rooms, angiography /embolization in the Interventional Radiology Suite, and post op care in the ICU. After implementation of a dedicated, trauma hybrid operating room, open surgery and endovascular intervention was performed in a single location. The patient populations were essentially the same. The authors nicely demonstrated that the time between OR arrival and hemostasis was shorter when using the hybrid operating room. In addition, patients treated in the hybrid operating room had fewer red cell and plasma transfusions postoperatively, as well as fewer infectious complications and fewer ventilator days.
We recently demonstrated that implementation of a dedicated Trauma Endovascular Trauma Service increased case volumes and shortened time to therapy. We also used a hybrid operating room. In your institution, who does the endovascular therapy? Was it really the location or the people that made the difference? Where is the Interventional Radiology suite at your institution? At Shock Trauma, it is located a city block away as opposed to the hybrid OR which is 50 feet away from the Resuscitation Unit. Migrating into the hybrid OR was an easy choice for us.
Did the authors perform every trauma hemostatic procedure in the hybrid operating room? Certainly, at our institution, the volume of operative hemostasis would never allow us to do all our cases in the hybrid suite. Even when we consider simply catheter hemostasis, as we have ramped up our endovascular volume, we simply do not always have an open hybrid OR available. We have reserved that special resource for either complex endovascular cases, like TEVAR, or cases that require combined open surgery and catheter therapy. We often embolize pelvic fracture bleeding in a regular OR with a radiolucent table and a vascular C-arm. How do they ensure availability of the room and/or practitioner? I would appreciate the authors thoughts on this. When they use the hybrid room, do they use other available techniques such as cone-beam CT?
It would seem that the authors utilized REBOA more in the hybrid era but that seems likely because they only performed REBOA in the OR. Why? We routinely place REBOA catheters in the Resuscitation Unit and confirm placement with a plain X-ray. We have had almost no problems with proper placement. Do the authors believe that this practice has altered their findings?
Our orthopedic surgeons do not like the fixed imaging systems. We often then move the patients to a room with a mobile C arm after the hybrid portions are completed. Have the authors encountered this? The authors demonstrated decreased time from OR arrival to achievement of hemostasis. This difference was statistically significant. However, the absolute difference was only 11 minutes. Do the authors really believe the salutary effects they observed on transfusions and postoperative complications can be explained by a difference of 11 minutes?
Finally, the authors demonstrated no survival advantage. In the end, this is the most important statistic. The numbers here are still relatively small so additional data may demonstrate survival advantage. The authors point out that the University of Florida was willing to provide over $1.5 million to outfit this resource. Ours was slightly more expensive. There are additional costs to maintaining it. Do the authors believe that their data can empower practitioners at other high-volume institutions to advocate for this special, but expensive resource? How do they envision us studying this? If not mortality, what other outcomes matter?
The authors come from a prestigious trauma center and the senior authors are exceptionally well known. They have innovated in the past and this is another example of them pushing the boundaries. We agree with their findings/sentiments and I applaud them on this work. Perhaps a small number of like-minded centers could band together and demonstrate this technology’s worth.
I appreciate the privilege of the virtual podium and thank the Southern Surgical Association for the honor of discussing this paper.
R. Stephen Smith, M.D.
Gainesville, Florida
We thank Dr. Scalea for his insightful comments. His experience and expertise in this area is unquestioned. The experience at the Maryland Shock Trauma unit can certainly serve as an example of how to develop hybrid operating room capability. I will attempt to answer Dr. Scalea’s questions.
The trauma hybrid operating room at the University of Florida is adjacent to a fully functioning angiography suite. So the advantage is not just the location or the personnel, but the combination. We believe the key is to have an easily accessible multifunction suite and to have the proper specialists in that location in a timely manner. We preferentially use the hybrid trauma operating room for hemodynamically unstable trauma patients. This capability requires commitment from several specialties. We also place REBOA in the trauma bays, but have found this procedure to proceed with greater efficiency in the hybrid OR due to the excellent imaging system and the skilled assistants present in the hybrid OR. We agree with Dr. Scalea in regards to orthopedic interventions. While an external fixator can be readily placed in the hybrid room, more extensive and definitive procedures are better performed in an orthopedic operating room. We have demonstrated that injured patients treated in the trauma hybrid operating room have quicker hemostasis, decreased transfusion requirements and have fewer complications, but we did not demonstrate improved survival in this small initial experience. We believe that larger studies, with greater statistical power, may demonstrate greater rates of survival. We wholeheartedly agree with Dr. Scalea’s suggestion that a multicenter trial is the best way to answer this question.
Acknowledgement
The authors thank Drs. Kevin Behrns and Gilbert Upchurch, Jr. for supporting the trauma hybrid operating room at the institutional level and Dr. Lawrence Lottenberg for his contributions to building a Level I trauma center at the University of Florida.
Footnotes
This work will be presented virtually at the 132nd Annual Meeting of The Southern Surgical Association.
The authors do not have disclosures.
References
- 1.Peden M, McGee K, Sharma G. The injury chart book: a graphical overview of the global burden of injuries. Geneva: World Health Organization. 2002;5. [Google Scholar]
- 2.Years of Potential Life Lost (YPLL) Reports, 1999 – 2015. Available at: https://webappa.cdc.gov/sasweb/ncipc/ypll10.html. Acessed June 9, 2020.
- 3.Kauvar DS, Wade CE. The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit Care. 2005;9 Suppl 5:S1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185–193. [DOI] [PubMed] [Google Scholar]
- 5.Hoyt DB, Bulger EM, Knudson MM, et al. Death in the operating room: an analysis of a multi-center experience. J Trauma. 1994;37(3):426–432. [PubMed] [Google Scholar]
- 6.Kalkwarf KJ, Drake SA, Yang Y, et al. Bleeding to Death in a Big City: An Analysis of All Trauma Deaths From Hemorrhage in a Metropolitan Area Over One Year. J Trauma Acute Care Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 7.Resources for optimal care of the injured patient: an update. Task Force of the Committee on Trauma, American College of Surgeons. Bull Am Coll Surg. 1990;75(9):20–29. [PubMed] [Google Scholar]
- 8.Bjerke HS, Petersen SR, Bokhari, Franklin G. Prehospital hypotension as a valid indicator of trauma team activation - Discussion. Journal of Trauma-Injury Infection and Critical Care. 2000;48(6):1037–1039. [DOI] [PubMed] [Google Scholar]
- 9.Darrabie MD, Croft CA, Brakenridge SC, et al. Resuscitative Endovascular Balloon Occlusion of the Aorta: Implementation and Preliminary Results at an Academic Level I Trauma Center. J Am Coll Surg. 2018;227(1):127–133. [DOI] [PubMed] [Google Scholar]
- 10.Brenner M, Inaba K, Aiolfi A, et al. Resuscitative Endovascular Balloon Occlusion of the Aorta and Resuscitative Thoracotomy in Select Patients with Hemorrhagic Shock: Early Results from the American Association for the Surgery of Trauma’s Aortic Occlusion in Resuscitation for Trauma and Acute Care Surgery Registry. J Am Coll Surg. 2018;226(5):730–740. [DOI] [PubMed] [Google Scholar]
- 11.Heetveld MJ, Harris I, Schlaphoff G, Balogh Z, D’Amours SK, Sugrue M. Hemodynamically unstable pelvic fractures: recent care and new guidelines. World J Surg. 2004;28(9):904–909. [DOI] [PubMed] [Google Scholar]
- 12.Agolini SF, Shah K, Jaffe J, Newcomb J, Rhodes M, Reed JF. Arterial embolization is a rapid and effective technique for controlling pelvic fracture hemorrhage. Journal of Trauma-Injury Infection and Critical Care. 1997;43(3):395–399. [DOI] [PubMed] [Google Scholar]
- 13.Ogura T, Lefor AT, Nakano M, Izawa Y, Morita H. Nonoperative management of hemodynamically unstable abdominal trauma patients with angioembolization and resuscitative endovascular balloon occlusion of the aorta. Journal of Trauma and Acute Care Surgery. 2015;78(1):132–135. [DOI] [PubMed] [Google Scholar]
- 14.Polanco PM, Brown JB, Puyana JC, Billiar TR, Peitzman AB, Sperry JL. The swinging pendulum: A national perspective of nonoperative management in severe blunt liver injury. Journal of Trauma and Acute Care Surgery. 2013;75(4):590–595. [DOI] [PubMed] [Google Scholar]
- 15.Belyayev L, Herrold JA, Ko A, et al. Endovascular Adjuncts for Hybrid Liver Surgery. J Trauma Acute Care Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 16.Scalea TM. The importance of fracture pattern in guiding therapeutic decision-making in patients with hemorrhagic shock and pelvic ring disruptions - Editorial comment. Journal of Trauma-Injury Infection and Critical Care. 2002;53(3):450–451. [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita T, Yamakawa K, Matsuda H, et al. The Survival Benefit of a Novel Trauma Workflow that Includes Immediate Whole-body Computed Tomography, Surgery, and Interventional Radiology, All in One Trauma Resuscitation Room A Retrospective Historical Control Study. Annals of Surgery. 2019;269(2):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carver D, Kirkpatrick AW, D’Amours S, Hameed SM, Beveridge J, Ball CG. A Prospective Evaluation of the Utility of a Hybrid Operating Suite for Severely Injured Patients: Overstated or Underutilized? Ann Surg. 2020;271(5):958–961. [DOI] [PubMed] [Google Scholar]
- 19.Kirkpatrick AW, Vis C, Dube M, et al. The evolution of a purpose designed hybrid trauma operating room from the trauma service perspective: The RAPTOR (resuscitation with angiography percutaneous treatments and operative resuscitations). Injury-International Journal of the Care of the Injured. 2014;45(9):1413–1421. [DOI] [PubMed] [Google Scholar]
- 20.Yamakawa K, Fujimi S, Watanabe A, et al. The hybrid emergency room system: a novel trauma evaluation and care system created in Japan. Acute Med Surg. 2019;6(3):247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tatum D, Duchesne J, Pereira B, et al. Time to Hemorrhage Control in a Hybrid ER System: Is it Time to Change? Shock. 2020. [DOI] [PubMed] [Google Scholar]
- 22.McIntyre LK, Schiff M, Jurkovich GJ. Failure of nonoperative management of splenic injuries: causes and consequences. Arch Surg. 2005;140(6):563–568; discussion 568–569. [DOI] [PubMed] [Google Scholar]
- 23.Woolley T, Thompson P, Kirkman E, et al. Trauma Hemostasis and Oxygenation Research Network position paper on the role of hypotensive resuscitation as part of remote damage control resuscitation. Journal of Trauma and Acute Care Surgery. 2018;84:S3–S13. [DOI] [PubMed] [Google Scholar]
- 24.Naumann DN, Vincent LE, Pearson N, et al. An adapted Clavien-Dindo scoring system in trauma as a clinically meaningful nonmortality endpoint. J Trauma Acute Care Surg. 2017;83(2):241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311(13):1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21(6):327–348. [DOI] [PubMed] [Google Scholar]
- 27.Kalanuria AA, Ziai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care. 2014;18(2):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867–903. [DOI] [PubMed] [Google Scholar]
- 29.Holcomb JB. Transport Time and Preoperating Room Hemostatic Interventions Are Important: Improving Outcomes After Severe Truncal Injury. Crit Care Med. 2018;46(3):447–453. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen M, Pirracchio R, Kornblith LZ, et al. Dynamic Impact of Transfusion Ratios on Outcomes in Severely Injured Patients: Targeted Machine Learning Analysis of the PROPPR Randomized Clinical Trial. J Trauma Acute Care Surg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alarhayem AQ, Myers JG, Dent D, et al. Time is the enemy: Mortality in trauma patients with hemorrhage from torso injury occurs long before the “golden hour”. American Journal of Surgery. 2016;212(6):1101–1105. [DOI] [PubMed] [Google Scholar]
- 32.Fox EE, Holcomb JB, Wade CE, Bulger EM, Tilley BC, Grp PS. Earlier Endpoints Are Required for Hemorrhagic Shock Trials among Severely Injured Patients. Shock. 2017;47(5):567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coccolini F, Ceresoli M, McGreevy DT, et al. Aortic balloon occlusion (REBOA) in pelvic ring injuries: preliminary results of the ABO Trauma Registry. Updates Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 34.Vella MA, Dumas RP, DuBose J, et al. Intraoperative REBOA: an analysis of the American Association for the Surgery of Trauma AORTA registry. Trauma Surg Acute Care Open. 2019;4(1):e000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto R, Cestero RF, Suzuki M, Funabiki T, Sasaki J. Resuscitative endovascular balloon occlusion of the aorta (REBOA) is associated with improved survival in severely injured patients: A propensity score matching analysis. American Journal of Surgery. 2019;218(6):1162–1168. [DOI] [PubMed] [Google Scholar]
- 36.Russo RM, Franklin CJ, Davidson AJ, et al. A new, pressure-regulated balloon catheter for partial resuscitative endovascular balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds CL, Celio AC, Bridges LC, et al. REBOA for the IVC? Resuscitative balloon occlusion of the inferior vena cava (REBOVC) to abate massive hemorrhage in retrohepatic vena cava injuries. J Trauma Acute Care Surg. 2017;83(6):1041–1046. [DOI] [PubMed] [Google Scholar]
- 38.Castelli P, Caronno R, Piffaretti G, Tozzi M. Emergency endovascular repair for traumatic injury of the inferior vena cava. Eur J Cardio-Thorac. 2005;28(6):906–908. [DOI] [PubMed] [Google Scholar]
- 39.Bui TD, Mills JL. Control of Inferior Vena Cava Injury Using Percutaneous Balloon Catheter Occlusion. Vasc Endovasc Surg. 2009;43(5):490–493. [DOI] [PubMed] [Google Scholar]
- 40.Stanford Medicine 2017 Health Trends Report: Harnessing the Power of Data in Health. Accessed 23 Feb 2019. Available at: http://med.stanford.edu/content/dam/sm/sm-news/documents/StanfordMedicineHealthTrendsWhitePaper2017.pdf.


