Abstract
Purpose
Previous research has demonstrated that lower health-related quality of life (HRQoL) is associated with higher morbidity and mortality, especially in-patient groups. The association of HRQoL with all-cause mortality in community samples requires further investigation. This study aimed to examine whether HRQoLf predicts all-cause mortality in older healthy community-dwelling people from Australia and the United States (U.S.) enrolled in the Aspirin in Reducing Events in the Elderly (ASPREE) trial. We also explored whether this association varies by gender or country.
Method
A prospective cohort of 19,106 individuals aged 65–98 years, who were without a dementia diagnosis or a known major life-limiting disease, and completed the 12-item short-form-HRQoL at recruitment (2010–2014). They were followed until June 2017. Cox proportional-hazard models were used to determine the association between the physical (PCS) and mental component scores (MCS) of HRQoL and all-cause mortality, adjusting for sociodemographic factors, health-related behaviours and clinical measures. Hazards ratios were estimated for every 10-unit increase in PCS or MCS.
Results
There were 1052 deaths over a median 4.7-years (interquartile range 3.6–5.7) of follow-up, with 11.9 events per 1000 person-years. Higher PCS was associated with lower all-cause mortality (HR 0.83, 95% CI 0.77, 0.89) in the entire sample, while higher MCS was associated with lower mortality among U.S. participants only (HR 0.78, 95% CI 0.63, 0.95). Gender differences in the association of either PCS or MCS with mortality were not observed.
Conclusion
Our large study provides evidence that HRQoL is inversely associated with all-cause mortality among initially healthy older people.
Keywords: Health-related quality of life, 12-item short form SF-12, Mortality, Predictor, Prospective cohort, Older people, Australia, United States
Introduction
Globally, the number of older people aged 65 years and over is increasing at a rate faster than other age groups [1]. The global aging population is expected to double in size from 900 million in 2015 to approximately two billion in 2050 [2]. Such a dramatic increase in the aging population has not come with a proportionate increase in healthy years lived for older individuals [3]. Therefore, identifying older individuals most at risk of developing more severe chronic diseases and premature deaths, and thus in greatest need of targeted preventative interventions is important to reduce the associated economic burden [1].
Health-related quality of life (HRQoL) is an individual’s self-perceived health status in relation to the social, cultural and environmental context [4–6]. HRQoL assesses the impact of health conditions on the daily lives of individuals and is useful in describing the life experiences of older adults as it relates to their health [4, 5]. For this reason, HRQoL has increasingly been used for assessing the quality of healthcare services, effectiveness of interventions, and in cost-utility analyses as a supplement to objective clinical measures [7]. Indeed, it has been shown that lower self-reported HRQoL is associated with higher morbidity and mortality in patients affected by chronic diseases such as cancer or heart failure [8, 9]. Additionally, a systematic review of 47 studies concluded that higher HRQoL as measured by the physical component score was associated with lower all-cause mortality risk among the general population [10] which also aligns with the finding of a recent study in middle-aged Swedish adults [11].
However, assessment of the association between HRQoL and all-cause mortality among initially healthy older males and females is still limited. Additionally, in that review, less than two thirds (28 studies) focused exclusively on older people aged 65 years and above [10]. Among the studies conducted in older people, only eight studies had a very large sample size (i.e. > 10,000 older people or national sample) [10] and the majority of these (n = 5) had a strong gender imbalance (i.e. < 4.2% representation of one gender) while others had a very short follow-up period from 1 to 2.5 years [10]. Gender difference plays an important role in the social consequences of illness and wellbeing including health-care seeking behaviours and the availability of support by their family and society [12]. Furthermore, given that mortality risk varies by gender [13] and women often have lower HRQoL than men [14], it is important to understand the association between HRQoL and mortality by gender. However, the previous systematic review did not evaluate whether the associations differed by gender [10]. Hence, the predictive power of HRQoL for adverse health outcomes has not yet been adequately determined in the context of healthy community-dwelling older people. Understanding such a relationship enables identification of an individual’s risk of adverse health outcomes beyond the broad biochemical functioning and morbidity assessed through traditional clinical measures. As such, this in turn could help to increase the efficacy of disease prevention strategies and healthcare resources for older people.
The aim of this study was to examine whether the physical (PCS) or mental component scores (MCS) of HRQoL predict all-cause mortality among community-dwelling older people and to determine whether these associations differ by gender. We used data from a five-year clinical trial—ASPREE (Aspirin in Reducing Events in the Elderly) which was conducted among relatively healthy older people in Australia and the United States (U.S.) [15]. Since demographic, social, cultural and healthcare differences exist between Australia and the U.S. [16], the current study also explored whether these associations differ between countries.
Methods
Study design and study population
The present study is a secondary data analysis of the prospective ASPREE cohort, which was the first multicentre, randomized, double blinded, placebo-controlled trial on the effects of low-dose aspirin for primary prevention of disability and dementia in older people [15]. The recruitment process was undertaken from March 2010 through December 2014. In Australia, the recruitment was carried out mostly through general practice, and the U.S. recruited through clinical trial and academic centres [15]. The ASPREE inclusion criteria were initially healthy individuals aged ≥ 70 years, or aged ≥ 65 years for U.S. minority (i.e. African American and Hispanic) groups [15]. Detailed descriptions of the exclusion criteria have been reported previously [15]. In briefly, individuals were excluded if they had: (a) a history of a diagnosed cardiovascular disease (CVD) (i.e. myocardial infarction, heart failure, angina pectoris, or stroke, etc.), (b) a clinical diagnosis of atrial fibrillation, (c) a systolic blood pressure ≥ 180 mmHg and/or a diastolic blood pressure ≥ 105 mmHg, (d) a condition with high risk of major bleeding, (e) anaemia, (f) a diagnosis of dementia or a Modified Mini-Mental State Examination (3MS) score ≤ 77, (g) a serious illness likely to be fatal within the next 5 years (as perceived by the recruiting general practitioner), or (h) if they were unable to perform any one of the 6 Katz activities of daily activities (ADLS), or (i) were current use of aspirin or other antiplatelet medication [15]. The trial included 19,114 relatively healthy participants aged 65–98 years, who were free of overt cardiovascular diseases (CVD), atrial fibrillation, dementia, major physical disability, anaemia, high risk of major bleeding, or serious illness likely to be fatal within the next 5 years [17]. Of the total ASPREE sample, 16,703 (87%) were from Australia and 2411 (13%) were from the U.S. [17]. ASPREE was approved by multiple Institutional Review Boards in Australia and the U.S.
Determinant: health-related quality of life
HRQoL was assessed using the validated Medical Outcomes Study 12-item short form (SF-12) (version-2) questionnaire [18]. The SF-12 is a shorter alternative to the 36-item short form (SF-36) and it consists of 12 questions [19]. Therefore, the SF-12 only takes a few minutes to complete and is commonly used in many epidemiological studies [19]. The 12 items of the SF-12 include eight domains: physical functioning, role limitation because of physical health problems, bodily pain, general health, vitality (energy/fatigue), social functioning, role limitation due to emotional problems, and mental health [19].
In this study we considered PCS and MCS, the two core subdomains of SF-12 as the determinants. The PCS and MCS were generated using norm-based methods with a mean of 50 and standard deviation of 10 [19]. The PCS was calculated using a heavier weighting on physical functioning, role-physical, bodily pain and general health domains whereas MCS was derived using a heavier weighting on vitality, social functioning, role-emotional and mental health domains [19]. Higher values of PCS and MCS indicate a better physical and mental HRQoL respectively [19].
Outcome: mortality
All-cause mortality was defined as any cause of death that occurred in trial participants after their enrolment in the study but before June 12, 2017, confirmed by two independent sources. All-cause mortality included death due to cancer, CVD, haemorrhage (hemorrhage), or other reasons. Death was identified during the course of trial regular activity (i.e. phone calls to the participants’ residence quarterly and face-to-face visit annually), or notified by the next of kin or a close contact during the trial. All deaths were confirmed by two independent sources, such as family members, primary care physician or public death notice. The lists of all Australian and U.S. participants who were lost to follow-up or withdrew were verified with the relevant National Death Index in each country at the end of the trial [20]. Additionally, in Australia, the vital status was checked and verified on a weekly basis through the Ryerson Index, a community-maintained on-line index of Australian death notices [21].
Covariates
Baseline variables were considered as covariates based on previous studies [10, 22, 23] and whether they were associated with both HRQoL (either PCS or MCS) and mortality in the study cohort. The baseline sociodemographic information, collected during face-to-face interviews included age, gender, years of education, country of residence, living situation (i.e. living at home alone or living with others) and race/ethnicity. The health-related behaviours were smoking status, alcohol consumption and daily walking time. Participants were queried as to their current smoking status and coded as (a) never, (b) former, or (c) current. Alcohol intake in any day was analysed as (a) never/former, (b) current with 1–2 standard drinks, or (c) current with 3 + standard drinks. Physical capacity was the longest amount of time walking outside home without any rest in the last 2 weeks was assessed and defined as (a) none, (b) ≤ 15 min, (c) 16–30 min, or (d) > 30 min). The clinical measures collected using interviews and/or clinical examination included personal cancer history (yes/no); hypertension (yes/no based on whether the participants were using treatment for high blood pressure or whether the average of three blood pressure measurements was above the normal range i.e. systolic blood pressure was ≥ 140 mmHg or diastolic blood pressure was ≥ 90 mmHg); diabetes mellitus (yes/no, based on the self-report of diabetes, fasting glucose ≥ 126 mg/dL or on treatment for diabetes), and a measure of global cognition, the Modified Mini-Mental State Examination (3MS) (continuous measure). Additionally, body mass index (BMI) was calculated by dividing weight in kilograms by height in meters squared and then analysed as (a) underweight (BMI: less than 18.5), (b) normal weight (BMI: 18.5 to 24.9), (c) overweight (BMI: 25 to 29.9), or (d) obese (BMI: 30 or more) [24].
Statistical analysis
Chi-square test, t-test and Wilcoxon rank-sum test were used to identify PCS, MCS, sociodemographic, health-related behaviours and clinical measures differences by survival status. From the lists of the sociodemographic factors, health-related behaviours and clinical measures, it was assessed whether each variable was associated with both health-related quality of life (HRQoL) (either PCS or MCS) and all-cause mortality, in order to include as confounders in the adjusted models.
Baseline PCS and MCS components of SF-12 were considered as a predictor of all-cause mortality using Cox proportional-hazards regression. The hazards ratios were estimated for every 10-unit increase in PCS/MCS.
The multivariable models adjusted for age, gender, education, living situation, smoking, alcohol, longest amount of time walking outside home without any rest, cancer history, hypertension, diabetes mellitus, BMI, and 3MS. The interaction terms between PCS/MCS and gender (men and women) or country (Australia and the U.S.) were fitted in the multivariable Cox models to test the heterogeneity of these associations (p-value for interaction). Then, the multivariable Cox models were repeated with analyses stratified by gender and country. In addition, for the purpose of comparison with previous studies, PCS/MCS were also treated as categorical variables using four quartiles, with the lowest quartile (worst HRQoL) as the referent category.
In addition, the sensitivity analyses were undertaken using the final models. First, to assess the reverse causality, we repeated main analyses after excluding individuals who died or censored in the first year of the study follow-up to ensure these early end points were not affecting the observed associations. Secondly, we repeated main analyses with PCS and MCS which were derived using Australian HRQoL population norms [25–27], as the exposures. Thirdly, we repeated the main analyses with SF-6D index score as the exposure, using preference-based algorithm provided by the University of Sheffield (under license) [28].
For Cox proportional-hazards models, using the stset and stcox STATA commands, we specified HRQoL administration at baseline as the enter (specifies the date when a subject first comes under observation, meaning that any failures, were they to occur, would be recorded in the data from this time point), mortality as the failure (specifies that mortality is the outcome and at that date the participant stops being observed), and end of follow-up (June 12, 2017) as the censored date. Analyses were performed in STATA statistical software version 15.0 (StataCorpLP, College Station, Texas, the U.S.). A p-value of < 0.05 was used to determine statistical significance.
Results
A total of 19,106 participants (99.96% of individuals enrolled in ASPREE) completed the baseline SF-12. Over the 4.7-year median follow-up period (interquartile range 3.6–5.7), there were 1052 deaths and the risk of death from any cause was 11.9 events per 1000-person years. Of the total 1052 deaths, it included 522 (49.6%) cancer deaths, 203 (19.3%) CVD deaths, 53 (5.0%) haemorrhage (hemorrhage) deaths, and 274 (26%) other causes of death. Those who died during follow-up had lower PCS scores, and were more likely to be older, male, living alone, have less education, be a current or former smoker and have more comorbidities (Table 1).
Table 1.
Characteristics of participants
| Total (N = 19,106) | Died (N = 1052) | Alive (N = 18,054) | p-valuea | |
|---|---|---|---|---|
| Health-related quality of life | ||||
| Physical component scores | ||||
| Mean (standard deviation) | 48.3 (8.8) | 45.8 (9.5) | 48.5 (8.7) | < 0.001b |
| Mental component scores | ||||
| Mean (standard deviation) | 55.7 (7.1) | 55.4 (7.5) | 55.7 (7.1) | 0.24b |
| Total (N = 19,106) n (%) |
Died (N = 1052) n (%) |
Alive (N = 18,054) n (%) |
p-valuea | |
| Sociodemographic factors | ||||
| Age | ||||
| Median (interquartile range) | 74 (71.6–77.7) | 77.6 (73.4–82.2) | 73.9 (71.6–77.4) | < 0.001b |
| Gender | ||||
| Male | 8329 (43.6%) | 583 (55.4%) | 7746 (42.9%) | < 0.001 |
| Female | 10,777 (56.4%) | 469 (44.6%) | 10,308 (57.1%) | |
| Country | ||||
| Australia | 16,696 (87.4%) | 912 (86.7%) | 15,784 (87.4%) | 0.49 |
| United States | 2410 (12.6%) | 140 (13.3%) | 2270 (12.6%) | |
| Years of education | ||||
| < 9 years | 3001 (15.7%) | 212 (20.2%) | 2789 (15.5%) | < 0.001 |
| 9–11 years | 5633 (29.5%) | 307 (29.2%) | 5326 (29.5%) | |
| 12–15 years | 5570 (29.2%) | 316 (30.0%) | 5254 (29.1%) | |
| 16–21 years | 4901 (25.7%) | 217 (20.6%) | 4684 (26.0%) | |
| Living situation | ||||
| At home alone | 6249 (32.7%) | 431 (41.0%) | 5818 (32.2%) | < 0.001 |
| With others | 12,857 (67.3%) | 621 (59.0%) | 12,236 (67.8%) | |
| Race/ethnicity | ||||
| White | 17,443 (91.3%) | 960 (91.3%) | 16,483 (91.3%) | 0.66 |
| Black/African-American | 900 (4.7%) | 54 (5.1%) | 846 (4.7%) | |
| Hispanic/Asiatic/other | 763 (4.0%) | 38 (3.6%) | 725 (4.0%) | |
| Health-related behaviors | ||||
| Smoking status | ||||
| Never | 10,575 (55.4%) | 468 (44.5%) | 10,107 (56.0%) | < 0.001 |
| Former | 7796 (40.8%) | 489 (46.5%) | 7307 (40.5%) | |
| Current | 735 (3.9%) | 95 (9.0%) | 640 (3.5%) | |
| Alcohol consumption | ||||
| Never/former | 4468 (23.4%) | 282 (26.8%) | 4186 (23.2%) | 0.002 |
| Current with 1–2 drinks | 11,981 (62.7%) | 606 (57.6%) | 11,375 (63.0%) | |
| Current with 3 + drinks | 2657 (13.9%) | 164 (15.6%) | 2493 (13.8%) | |
| Average longest amount of time walking outside home without any rest (last 2 weeks) | ||||
| No walking | 839 (4.4%) | 68 (6.5%) | 771 (4.3%) | |
| < = 15 min | 2341 (12.3%) | 194 (18.5%) | 2147 (11.9%) | < 0.001 |
| 16–30 min | 4144 (21.7%) | 272 (26.0%) | 3872 (21.5%) | |
| > 30 min | 11,741 (61.6%) | 514 (49.1%) | 11,227 (62.3%) | |
| Clinical measures | ||||
| Personal cancer history | ||||
| Yes | 3658 (19.2%) | 296 (28.3%) | 3362 (18.7%) | < 0.001 |
| No | 15,371 (80.8%) | 751 (71.7%) | 14,620 (81.3%) | |
| Hypertension | ||||
| Yes | 14,191 (74.3%) | 818 (77.8%) | 13,373 (74.1%) | 0.01 |
| No | 4915 (25.7%) | 234 (22.2%) | 4681 (25.9%) | |
| Diabetes mellitus | ||||
| Yes | 2044 (10.7%) | 155 (14.7%) | 1889 (10.5%) | < 0.001 |
| No | 17,062 (89.3%) | 897 (85.3%) | 16,165 (89.5%) | |
| Body mass index | ||||
| < 18.5 | 103 (0.5%) | 26 (2.5%) | 77 (0.4%) | < 0.001 |
| 18.5–24.9 | 4859 (25.6%) | 321 (30.7%) | 4538 (25.3%) | |
| 25.0–29.9 | 8449 (44.4%) | 441 (42.2%) | 8008 (44.6%) | |
| > = 30.0 | 5608 (29.5%) | 258 (24.7%) | 5350 (29.8%) | |
| General cognition (Modified Mini-Mental State examination) | ||||
| Mean (standard deviation) | 93.4 (4.6) | 92.1 (5.0) | 93.5 (4.6) | < 0.001b |
Age distribution (median and interquartile range) by gender: males = 73.8 years (71.6–77.3 years) and females = 74.1 years (71.7–78.0 years). Hypertension = systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or on treatment for high blood pressure, diabetes mellitus = self-report of diabetes or fasting glucose ≥ 126 mg/dL or on treatment for diabetes
MCS mental component score; PCS physical component score
p-value from Chi-square test comparison of participants alive versus those who died
p-value from t-test/Wilcoxon rank-sum test comparison of participants alive versus those who died
Physical HRQoL and mortality
A 10-unit increase in PCS was associated with a 17% decreased risk of all-cause mortality (Table 2). The all-cause mortality rate for individuals in the highest PCS quartile group was 35% less than that of lowest quartile group (HR 0.65, 95% CI 0.53 to 0.78) (Supplementary Table S1). There was an inverse dose–response association between PCS quartiles and death, with higher PCS associated with lower mortality risk (Fig. 1). There was no heterogeneity of the effects in the association between PCS and all-cause mortality according to country or gender (p-values for interaction 0.38 and 0.13, respectively) (Table 2 and Supplementary Table S3).
Table 2.
Crude and adjusted hazard ratios for all-cause mortality by 10-unit increase in SF-12 physical component scores (PCS) according to country of recruitment
| Total (N = 19,106) | Country (N = 19,106) | p-value for interactionc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Australia (N = 16,696) | U.S. (N = 2410) | |||||||||
| N | No. of deaths | Hazard ratiob (95% CI) | N | No. of deaths | Hazard ratiob (95% CI) | N | No. of deaths | Hazard ratiob (95% CI) | ||
| All-cause mortalitya | ||||||||||
| PCS | 19,106 | 1052 | 0.73 (0.68–0.78) | 16,696 | 912 | 0.73 (0.68–0.78) | 2410 | 140 | 0.74 (0.63–0.88) | 0.87 |
| PCS adjustedd | 19,105 | 1052 | 0.78 (0.73–0.84) | 16,695 | 912 | 0.79 (0.73–0.84) | 2410 | 140 | 0.78 (0.65–0.94) | 0.75 |
| PCS adjustede | 18,901 | 1037 | 0.83 (0.77–0.89) | 16,521 | 898 | 0.83 (0.77–0.89) | 2380 | 139 | 0.86 (0.70–1.04) | 0.38 |
PCS physical component score, U.S. United States of America
All-cause mortality censored at June 12, 2017, providing 88,345.0 person-years of observation (the average length of observation was 4.6 ± SD1.3 years, the median length of observation was 4.7 years, with participants ranging between 0 and 7.3 years of observation)
For every 10-unit increase in PCS
p-value for interaction between PCS and country
Adjusted for age at randomization, gender, education, and living situation
Adjusted for age at randomization, gender, education, living situation, smoking, alcohol, average longest amount of time walking outside home without any rest (last 2 weeks), cancer history, hypertension, diabetes, body mass index, and modified mini-mental state examination score
Fig. 1.
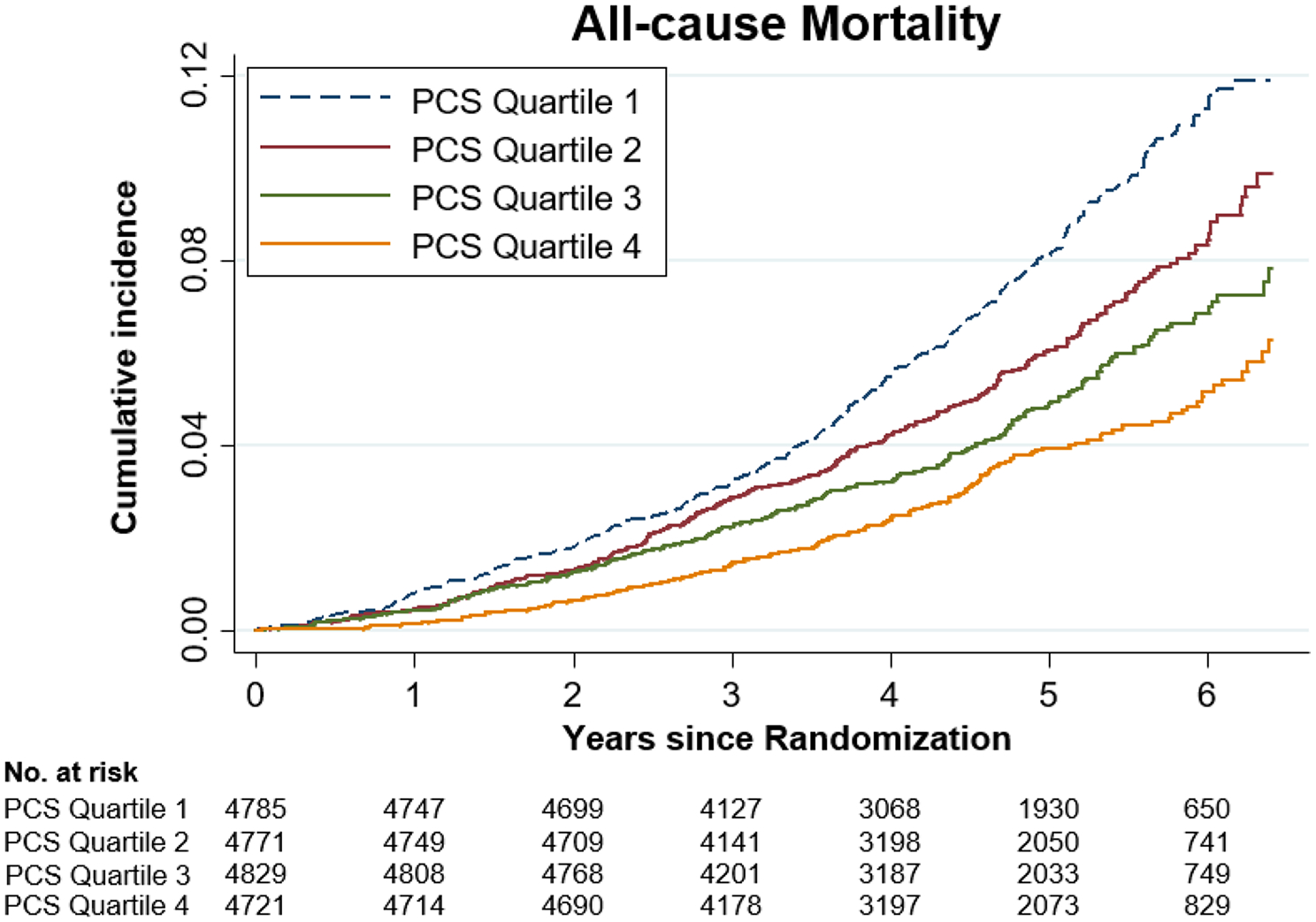
Crude Cumulative incidence of death related to any cause according to the physical component scores (PCS) quartiles. PCS physical component scores, Quartile 1 = lowest, Quartile 4 = highest
Mental HRQoL and mortality
No association was observed between MCS and all-cause mortality in the entire sample (Table 3 and Fig. 2) and no evidence that the association varied by gender (p-value for interaction 0.44; Supplementary Table S4). However, country appeared to modify the association (p-value for interaction 0.06; Table 3). Among the U.S. participants, a 10-unit increase in MCS was associated with a 22% decreased risk of all-cause mortality (Table 3 and Supplementary Fig. S1), and the all-cause mortality rate for individuals in the highest MCS quartile was 47% less than that of lowest quartile group (HR 0.53, 95% CI 0.33 to 0.85) (Supplementary Table S2). Among Australian participants, there was no evidence of an association between MCS and all-cause mortality (Table 3, Supplementary Table S2 and Supplementary Fig. S2).
Table 3.
Crude and adjusted hazard ratios for all-cause mortality by 10-unit increase in SF-12 mental component scores (MCS) according to country of recruitment
| Total (N = 19,106) | Country (N = 19,106) | p-value for interactionc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Australia (N = 16,696) | U.S. (N = 2410) | |||||||||
| N | No. of deaths | Hazard ratiob (95% CI) | N | No. of deaths | Hazard ratiob (95% CI) | N | No. of deaths | Hazard ratiob (95% CI) | ||
| All-cause mortalitya | ||||||||||
| MCS | 19,106 | 1052 | 0.94 (0.86–1.02) | 16,696 | 912 | 0.98 (0.89–1.07) | 2410 | 140 | 0.77 (0.64–0.93) | 0.03 |
| MCS adjustedd | 19,105 | 1052 | 0.91 (0.84–0.99) | 16,695 | 912 | 0.95 (0.87–1.04) | 2410 | 140 | 0.76 (0.63–0.92) | 0.03 |
| MCS adjustede | 18,901 | 1037 | 0.95 (0.87–1.03) | 16,521 | 898 | 0.98 (0.90–1.08) | 2380 | 139 | 0.78 (0.63–0.95) | 0.06 |
MCS mental component score, U.S. United States of America
All-cause mortality censored at June 12, 2017, providing 88,345.0 person-years of observation (the average length of observation was 4.6 ± SD1.3 years, the median length of observation was 4.7 years, with participants ranging between 0 and 7.3 years of observation)
For every 10-unit increase in MCS
p-value for interaction between MCS and country
Adjusted for age at randomization, gender, education, and living situation
Adjusted for age at randomization, gender, education, living situation, smoking, alcohol, average longest amount of time walking outside home without any rest (last 2 weeks), cancer history, hypertension, diabetes, body mass index, and modified mini-mental state examination score
Fig. 2.
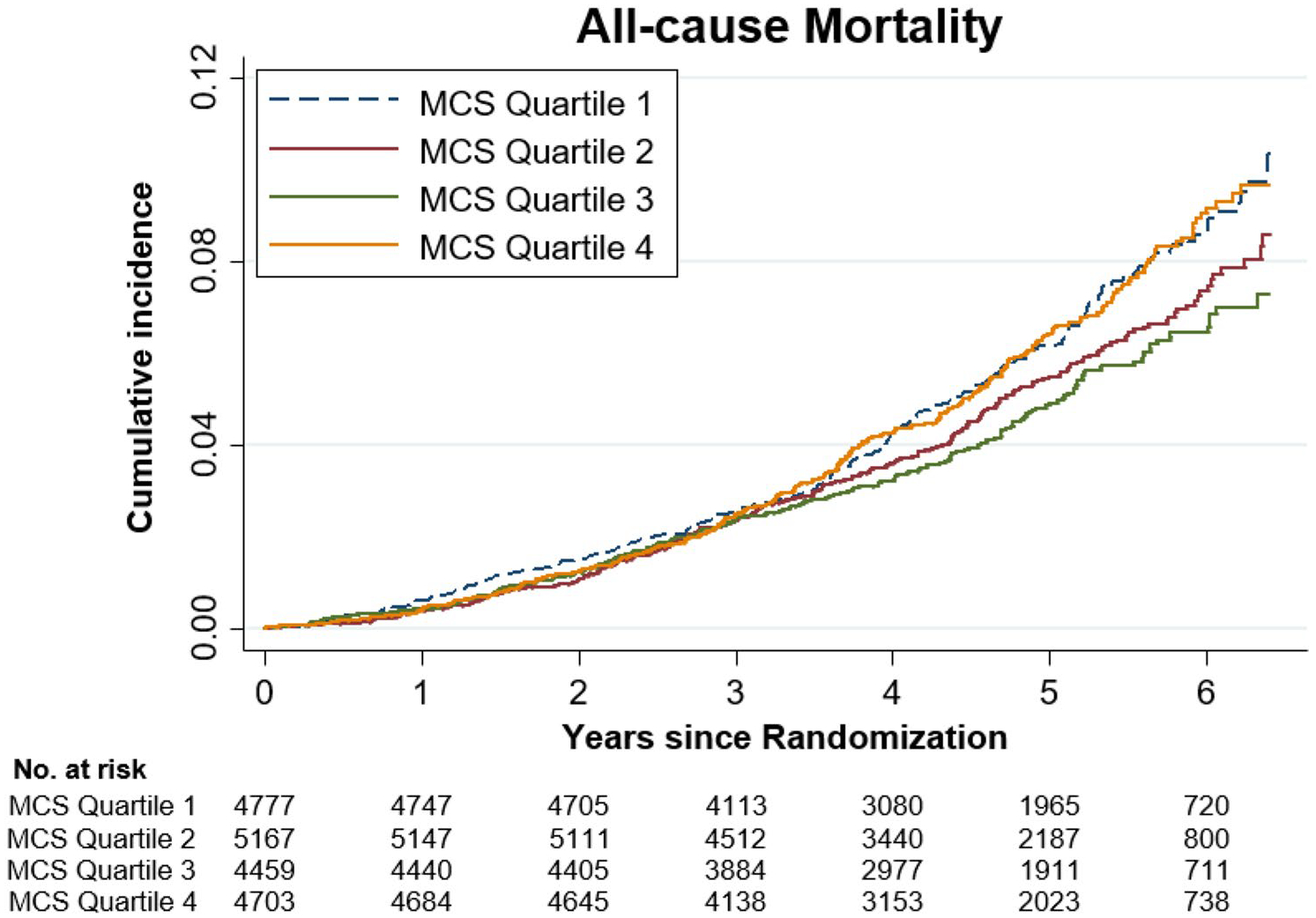
Crude Cumulative incidence of death related to any cause according to the mental component scores (MCS) quartiles. MCS mental component scores, Quartile 1 = lowest, Quartile 4 = highest
Our PCS and MCS finding did not change when we only adjusted for age, gender, education and living status (Tables 2 and 3 and Supplementary Table S3 and S4).
The proportional hazards assumption was satisfied for our Cox models (p-values > 0.05). Sensitivity analyses assessing reverse causality, Australian HRQoL population norms and SF-6D were also consistent with our main findings (Supplementary Table S5–S7). In the fully adjusted model, age, gender, education, living situation, smoking, alcohol, average longest amount of time walking outside home without any rest (last 2 weeks), cancer history, diabetes, BMI, and 3MS score were also associated with all-cause mortality (Supplementary Table S8).
Discussion
The present study conducted in a large cohort of initially healthy, community-dwelling older people reported an inverse dose–response association between HRQoL and all-cause mortality. Over the 4.7-year median follow-up, higher PCS was associated with lower all-cause mortality (17% decrease per 10-unit PCS increase), which did not differ by gender and was similar within Australia and the U.S. Increasing MCS scores were associated with reduced all-cause mortality only among the U.S. participants (22% decrease per 10-unit MCS increase). The association between MCS and all-cause mortality did not vary by gender.
The present finding substantiates the evidence of a recent systematic review demonstrating that higher HRQoL was inversely associated with all-cause mortality [10]. Furthermore, our PCS finding is aligned with previous studies reporting that higher PCS is a predictor of reduced all-cause mortality in German, Spanish and Scottish population-based samples aged 20 years and over [29–31]. Among 4261 German community-dwelling adults aged 20–79 years, the all-cause mortality rate for the highest PCS quartile group was 44% less than that of lowest quartile group over an average follow-up of 9.7 years [29]. Similarly, among Spanish and Scottish adults that spanned a broad age range of 20–65 years and above, the all-cause mortality rate of the highest tertile or quintile group was 42–64% less than that of lowest group over an average 6.3-year and to 7.6-year follow-up respectively [30, 31].
Our MCS finding of U.S. individuals also supports the conclusions of previous studies conducted in the U.S. and other countries. Among 2166 U.S. adults aged 65 years or more, the highest quartile of SF-12 MCS had 60% lower mortality compared to the lowest quartile over a 2.3-year follow-up [32]. Similarly, among the 7702 and 40,508 U.S. older predominantly males veterans, every 10-unit increase in SF-36 MCS was associated with 17–28% decreased risk of all-cause mortality over 1-year follow-up [33, 34]. Moreover, among the 17,777 UK and 4424 Taiwanese community-dwelling people aged 40 years and older, a 10-unit increase in MCS was associated with 12% and 14% decreased risk of all-cause mortality over an average 6.5-year and 3-year follow-up respectively [35, 36]. Noticeably, the magnitude of association from the present study is similar to those of prior studies despite the different groups studied in terms of size, eligibility criteria and follow-up period.
On the other hand, our finding among the Australian sample of no association between MCS and all-cause mortality is in accordance with the conclusion of two previous Australian studies, which were undertaken among 10,721 older women and 9979 adults aged 25 years and above with 15-year and 7.4-year follow-up, respectively [37, 38]. Previous studies in other countries have also observed no association, for example a study of 2373 Spanish older people with 6-years follow-up [39]. The findings of our study, together with previous evidence, suggest that PCS may be predictive of all-cause mortality across different countries, while the association between MCS and all-cause mortality might be related to specific sociodemographic and cultural factors, which may thus be country-dependent. Of note, recruitment in Australia was aimed to be the general population, while recruitment in the U.S. was aimed to be representative of minority ethnic groups [15]. Additionally, our Australian sample was recruited mainly through general practice whereas the recruitment in the U.S. was undertaken through clinical trial and academic centres [15]. To include sufficient numbers from the U.S. minority ethnic group in ASPREE, the minimum age cut-off of recruitment was lowered from 70 to 65 years [15]. Therefore, approximately one fourth of our U.S. sample were individuals aged 65–69 years while our Australian sample was aged 70 years and above [23]. In addition, our U.S. sample had a more ethnic mix with larger proportions of specific ethnic groups, and had a higher education level than our Australian sample [23]. These differences in ethnic groups, age and recruitment approach likely contributes to the differences in MCS finding of two countries. However, the reason for the association between MCS and all-cause mortality differing between the two countries (i.e. Australia and the U.S.) in our study is unclear.
The concept of HRQoL focuses only on the health aspects of quality of life (QoL) [6]. QoL is generally referred to a measure of wellbeing which reflects the positive aspects of an individual’s life including life satisfaction, good health and a sense of meaning which cannot be identified by laboratory tests [5, 6]. Therefore, higher HRQoL is likely associated with a positive attitude towards adopting a healthy lifestyle and seeking preventive measures. In contrast, low HRQoL may reflect a negative attitude towards regular screening and improving health which in turn may lead to the occurrences of chronic diseases conditions and premature death [22]. In addition, the self-reported HRQoL reflects an individual’s health perception in relation to health risks, health conditions, functional wellness and social support [6]. Therefore, our finding of individuals with poor HRQoL at baseline being at a greater risk of death over a median 4.7 years showed that HRQoL can be an indicator of future adverse health outcomes and it could possibly help identify older individuals at high risk prior to onset of clinical symptoms. Additional research is required to explore the potential mechanisms by which HRQoL is associated with adverse health outcomes. In this present study, we highlighted that HRQoL could subjectively inform clinicians about physical limitations which may usually not be reported by the patient to the doctor or may not be considered as relevant during the clinical assessment even if reported.
Limitations and strengths
The main limitation of this study is the unavailability of adjustment for dietary patterns, which is known to be associated with quality of life [40] and mortality risk [41]. Furthermore, although our study participants were initially healthy and free of known life-limiting illnesses, it is common to have health conditions in older age, which might impact our present findings. While we adjusted for several clinical measures (cancer history, hypertension, diabetes mellitus, BMI and 3MS), we did not have complete information on other relevant health conditions [2] such as osteoarthritis, asthma, Parkinson’s disease, pneumonia. Moreover, the analysis reported in this manuscript were based on data collected during the ASPREE trial which ended after a median 4.7 years of follow-up. However, our findings support the evidence of prior studies with 6–9 years follow-ups [29–31]. In addition, since women and men are different in genetic, social selection, healthcare seeking behaviours and social consequences of illness [12, 42], women tend to live longer and suffer more morbidities in later life compared to men [42]. Therefore, it is more likely for women, than men, to survive to 65 years and have more opportunities to be included in research involving older individuals. There was no strong imbalance in the total sample (56% women), but was more imbalanced in the U.S. sample (67% women). The SF-12 used in our study is a self-reported subjective measure. Thus, the subjective nature of reporting is inherent in the instrument itself. However, the SF-12 is a validated tool, and commonly used for accessing HRQoL in many population-based studies [19].
Other strengths include a prospective cohort study design, a large sample of older initially relatively healthy individuals aged 65–98 years with a median follow-up of 4.7 years and a well-balanced sampling strategy in terms of age and gender. Hence, to the best of our knowledge, this study is the largest and longest to investigate the association between either PCS or MCS derived from SF-12 and all-cause mortality risk in community-dwelling older people [10]. Moreover, our baseline HRQoL was assessed completely independently to the outcome of all-cause mortality (i.e. baseline HRQoL assessment was completed before the outcome), which was verified through a systematic verification procedure. Therefore, our study is less likely to have differential measurement error which can lead to biased findings. Additionally, in ASPREE, the data collection processes were undertaken by well-trained and skilled staff. Therefore, accounting for the reliable and valid data of sociodemographic factors, health-related behaviours and some clinical measures based on medical records rather than self-reporting in our adjusted Cox-proportional hazards models was another strength of this study.
Clinical implications
Our sample of community-dwelling older people initially free of known major life-limiting diseases is unique as it represents relatively healthy older individuals who will be increasingly seen in primary health settings with an aging population. Our unique study identified that even among relatively healthy older adults, higher physical HRQoL predicts lower mortality risk. Therefore, incorporating the SF-12 HRQoL questionnaire into annual health checklist by health practitioners or routine public health data collection would be helpful to identify older individuals most at risk for premature death. In particular, our results are consistent with the decision of the Australian Commission on Safety and Quality in Health Care to incorporate the SF-12 into the routine collection of Patients Reported Outcome Measures (PROMs) as a policy goal for the Australian health system [43]. Increasing mortality risk prediction among older people could permit greater screening and clinical follow-up and potentially targeted interventions to reduce risk. This in turn would reduce the economic burden associated with ill health, especially given the rapid growth in the aging population.
Conclusion
The present finding from a prospective cohort study with a large sample of older people adds to the evidence that higher HRQoL is associated with a decreased risk of all-cause mortality. The predictive ability of MCS for all-cause mortality likely depends on sociodemographic and cultural differences. Our results provide additional evidence to the capacity of SF-12 for mortality risk prediction in the aging population. Further research is recommended to better understand the mechanisms whereby self-reported HRQoL predicts mortality risk, and potential mediating and moderating factors. Further investigations in this context will provide valuable guidance for the clinical management of an aging population.
Supplementary Material
Acknowledgements
We would like to thank the ASPREE participants who volunteered for this study, the general practitioners and staff of the medical clinics who support the study participants, and the trial staff and management team of the ASPREE study in Australia and the United States, and the ASPREE Investigator Group listed on www.aspree.org.
Funding The ASPREE (ASPirin in Reducing Events in the Elderly) trial was mainly supported by grants from the National Institute on Aging and the National Cancer Institute at the National Institutes of Health (Grant Number U01AG029824); the National Health and Medical Research Council of Australia (Grant Numbers 334047 and 1127060); Monash University (Australia) and the Victorian Cancer Agency (Australia). Other funding resources and collaborating organizations of the ASPREE study are listed on http://www.aspree.org. AZZP is supported by Monash International Tuition Scholarship (Medicine, Nursing, and Health Sciences) and Monash Graduate Scholarship (30072360). RFP is supported by a National Heart Foundation of Australia Postdoctoral Fellowship (101927). JR and CMR are supported by a National Health and Medical Research Council Dementia Research Leader Fellowship (APP 1135727) and Principal Research Fellowship (APP1136372) respectively. Funders had no role in the design and conduct of the study, in the collection, analysis, and interpretation of data and in the writing, review and submission of the manuscript.
Footnotes
ASPREE Investigator Group listed on www.aspree.org.
Supplementary information The online version of this article (https://doi.org/10.1007/s11136-020-02723-y) contains supplementary material, which is available to authorized users.
Data availability All individual participant data (re-identifiable) that underlie the results reported in this Manuscript, are available upon request to qualified researchers without limit of time, subject to approval of the analyses by the Principal Investigators and a standard data sharing agreement. Details regarding requests to access the data will be available through the web site (www.ASPREE.org). The data will then be made available through a web-based data portal safe haven at Monash University, Australia.
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval ASPREE (ASPirin in Reducing Events in the Elderly) trial is being conducted in accordance with the Declaration of Helsinki 1964 as revised in 2008, the NHMRC Guidelines on Human Experimentation, the federal patient privacy (HIPAA) law and ICH-GCP guidelines and the International Conference of Harmonization Guidelines for Good Clinical Practice. ASPREE also follows the Code of Federal Regulations as it relates to areas of clinical research. The overall management and conduct of the ASPREE clinical trial is the responsibility of the ASPREE Steering Committee. The data of the present secondary data-analysis study was from a five-year ASPREE clinical trial (Trial Registration: International Standard Randomized Controlled Trial Number Register (ISRCTN 83772183) and clinicaltrials.gov (NCT 01038583)). The current secondary data analysis has been approved by the Monash University Human Research Ethics Committee (project ID: 21714). The ASPREE trial has been approved by multiple Institutional Review Boards in Australia and the U.S. (www.aspree.org).
Consent to participate This study used data from a five-year ASPREE (ASPirin in Reducing Events in the Elderly) clinical trial (Trial Registration: International Standard Randomized Controlled Trial Number Register (ISRCTN 83772183) and clinicaltrials.gov (NCT 01038583)). All individual participants of ASPREE clinical trial signed informed consent on participation.
Consent for publication All authors gave their approval for submission.
References
- 1.World Health Organization. (2011). Global Health and Aging. Retrieved 9 September, 2019, from https://www.who.int/ageing/publications/global_health.pdf.
- 2.World Health Organization. (2020). Aging and Health. Retrieved 5 January, 2020, from https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
- 3.Salomon JA, Wang H, Freeman MK, Vos T, Flaxman AD, Lopez AD, et al. (2012). Healthy life expectancy for 187 countries, 1990–2010: A systematic analysis for the Global Burden Disease Study 2010. The Lancet, 380(9859), 2144–2162. [DOI] [PubMed] [Google Scholar]
- 4.Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. (1998). Cross-validation of item selection and scoring for the SF-12 Health Survey In Nine Countries: Results from the IQOLA project. Journal of Clinical Epidemiology, 51(11), 1171–1178. [DOI] [PubMed] [Google Scholar]
- 5.Ferrans CE (2005). Definitions and conceptual models quality of life. In Lipscomb J, Gotay CC, & Snyder C (Eds.), Outcomes assessment in cancer: Measures, methods, and applications (pp. 14–30). Cambridge: Cambridge University Press. [Google Scholar]
- 6.Karimi M, & Brazier J (2016). Health, health-related quality of life, and quality of life: What is the difference? Pharmaco-Economics, 34(7), 645–649. [DOI] [PubMed] [Google Scholar]
- 7.Gill TM, & Feinstein AR (1994). A critical appraisal of the quality of quality-of-life measurements. JAMA, 272(8), 619–626. [PubMed] [Google Scholar]
- 8.Mehanna HM, & Morton RP (2006). Does quality of life predict long-term survival in patients with head and neck cancer? Archives of Otolaryngology: Head & Neck Surgery, 132(1), 27–31. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Artalejo F, Guallar-Castillón P, Pascual CR, Otero CM, Montes AO, García AN, et al. (2005). Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Archives of Internal Medicine, 165(11), 1274–1279. [DOI] [PubMed] [Google Scholar]
- 10.Phyo AZZ, Freak-Poli R, Craig H, Gasevic D, Stocks N, Gonzalez-Chica DA, et al. (2020). Quality of Life and mortality in the general population: A systematic review and meta-analysis. BMC Public Health, 20, 1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nilsson E, Festin K, Lowén M, & Kristenson M (2019). SF-36 predicts 13-year CHD incidence in a middle-aged Swedish general population. Quality of Life Research, 29(4), 971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlassoff C (2007). Gender differences in determinants and consequences of health and illness. Journal of Health, Population, and Nutrition, 25(1), 47. [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. (2019). Global Health Observatory (GHO) data: World Health Statistics 2019: Monitoring health for the SDGs. Retrieved 28 January, 2020, from https://www.who.int/gho/publications/world_health_statistics/2019/en/.
- 14.Cherepanov D, Palta M, Fryback DG, & Robert SA (2010). Gender differences in health-related quality-of-life are partly explained by sociodemographic and socioeconomic variation between adult men and women in the US: Evidence from four US nationally representative data sets. Quality of Life Research, 19(8), 1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimm R, Mcneil JJ, Applegate W, Beilin L, Espinoza S, Johnston CI, et al. (2013). Study design of ASPirin in reducing events in the elderly (ASPREE): A randomized, controlled trial. Contemporary Clinical Trials, 36(2), 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Index Mundi - Country Facts. (2020). United States vs. Australia. Retrieved October 2020, from https://www.indexmundi.com/factbook/compare/united-states.australia.
- 17.Mcneil JJ, Woods RL, Nelson MR, Murray AM, Reid CM, Kirpach B, et al. (2017). Baseline characteristics of participants in the ASPREE (ASPirin in reducing events in the elderly) study. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 72(11), 1586–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware JE Jr., Kosinski M, & Keller SD (1996). A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Medical Care, 34(3), 220–233. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE Jr., Kosinski M, Turner-Bowker DM, & Gandek B (2002). User’s manual for the SF-12v2® health survey with a supplement documenting SF-12® health survey). Lincoln, RI: QualityMetric Incorporated. [Google Scholar]
- 20.Mcneil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, et al. (2018). Effect of aspirin on all-cause mortality in the healthy elderly. The New England Journal of Medicine, 379(16), 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryerson Index Inc. The Ryerson Index home page. Retrieved 11 November, 2019, from http://www.ryersonindex.org/.
- 22.Idler E, & Amini Y (1997). Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior, 38(1), 21–37. [PubMed] [Google Scholar]
- 23.Stocks N, González-Chica D, Woods R, Lockery J, Wolfe R, Murray A, et al. (2019). Quality of Life for 19,114 participants in the ASPREE (ASPirin in Reducing Events in the Elderly) study and their association with sociodemographic and modifiable lifestyle risk factors. Quality of Life Research, 28(4), 935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. (2000). Obesity: Preventing and managing the global epidemic. Report of a WHO Consultation on Obesity. Retrieved 20 February, 2020, from https://www.who.int/gho/ncd/risk_factors/bmi_text/en/. [PubMed]
- 25.Hawthorne G, Osborne R, Taylor A, & Sansoni J (2007). The SF36 Version 2: Critical analyses of population weights, scoring algorithms and population norms. An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 16(4), 661–673. [DOI] [PubMed] [Google Scholar]
- 26.Mancuso S (2017). SF-12 v2 Scoring using Australian Population Weights (Sam Mancuso in Scoring Algorithm, Self-Report Measures). Retrieved 5 October, 2020, from https://sammancusocom/2017/11/08/sf-12-v2-scoring-using-australia-population-weights/. [Google Scholar]
- 27.Mancuso S Personal communication with Rosanne Freak-Poli and Aung Zaw Zaw Phyo. 29th September 2020 to 4th October 2020.
- 28.Brazier EJ, & Roberts EJ (2004). The estimation of a preference-based measure of health from the SF-12. Medical Care, 42(9), 851–859. [DOI] [PubMed] [Google Scholar]
- 29.Haring R, Feng Y-S, Moock J, Völzke H, Dörr M, Nauck M, et al. (2011). Self-perceived quality of life predicts mortality risk better than a multi-biomarker panel, but the combination of both does best. BMC Medical Research Methodology, 11(1), 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munoz MA, Subirana I, Elosua R, Covas MI, Baena-Diez JM, Ramos R, et al. (2011). Utility of a short quality of life questionnaire to predict cardiovascular events. International Journal of Cardiology, 151(3), 392–394. [DOI] [PubMed] [Google Scholar]
- 31.Ul-Haq Z, Mackay DF, & Pell JP (2014). Association between physical and mental health-related quality of life and adverse outcomes: A retrospective cohort study of 5,272 Scottish adults. BMC Public Health, 14, 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorr DA, Jones SS, Burns L, Donnelly SM, Brunker CP, Wilcox A, et al. (2006). Use of health-related, quality-of-life metrics to predict mortality and hospitalizations in community-dwelling seniors. Journal of the American Geriatrics Society, 54(4), 667–673. [DOI] [PubMed] [Google Scholar]
- 33.Fan VS, Au DH, Mcdonell MB, & Fihn SD (2004). Intraindividual change in SF-36 in ambulatory clinic primary care patients predicted mortality and hospitalizations. Journal of Clinical Epidemiology, 57(3), 277–283. [DOI] [PubMed] [Google Scholar]
- 34.Singh JA, Borowsky SJ, Nugent S, Murdoch M, Zhao Y, Nelson DB, et al. (2005). Health-related quality of life, functional impairment, and healthcare utilization by veterans: Veterans’ quality of life study. Journal of the American Geriatrics Society, 53(1), 108–113. [DOI] [PubMed] [Google Scholar]
- 35.Myint PK, Luben RN, Surtees PG, Wainwright NWJ, Welch AA, Bingham SA, et al. (2007). Self-reported mental health-related quality of life and mortality in men and women in the European Prospective Investigation into Cancer (EPIC-Nor-folk): A prospective population study. Psychosomatic Medicine, 69(5), 410–414. [DOI] [PubMed] [Google Scholar]
- 36.Tsai SY, Chi LY, Lee CH, & Chou P (2007). Health-related quality of life as a predictor of mortality among community-dwelling older persons. European Journal of Epidemiology, 22(1), 19–26. [DOI] [PubMed] [Google Scholar]
- 37.Leigh L, Hudson IL, & Byles JE (2015). Sleeping difficulty, disease and mortality in older women: A latent class analysis and distal survival analysis. Journal of Sleep Research, 24(6), 648–657. [DOI] [PubMed] [Google Scholar]
- 38.Williams ED, Rawal L, Oldenburg BF, Renwick C, Shaw JE, & Tapp RJ (2012). Risk of cardiovascular and all-cause mortality: Impact of impaired health-related functioning and diabetes—The Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetes Care, 35(5), 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otero-Rodriguez A, Leon-Munoz LM, Balboa-Castillo T, Banegas JR, Rodriguez-Artalejo F, & Guallar-Castillon P (2010). Change in health-related quality of life as a predictor of mortality in the older adults. Quality of Life Research, 19(1), 15–23. [DOI] [PubMed] [Google Scholar]
- 40.Govindaraju T, Sahle BW, Mccaffrey TA, Mcneil JJ, & Owen AJ (2018). Dietary patterns and quality of life in older adults: A systematic review. Nutrients. 10.3390/nu10080971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Afshin A, Sur PJ, Fay KA, Cornaby L, Ferrara G, Salama JS, et al. (2019). Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet, 393(10184), 1958–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Austad SN, & Fischer KE (2016). Sex differences in lifespan. Cell Metabolism, 23(6), 1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams K, Sansoni J, Morris D, Grootemaat P, & Thompson C (2016). Patient-reported outcome measures: Literature review. Sydney: Australian Commission on Safety and Quality in Health Care (ACSQHC). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


