Abstract
PURPOSE
This investigation characterized sexual activity and sexual function in hematopoietic cell transplantation (HCT) survivors, compared them to norms, and examined factors associated with sexual dysfunction with a goal of identifying targets for intervention to improve sexual health.
PATIENTS AND METHODS
Surviving adults from a large transplant center were asked to complete an annual survey with a core of health questions and a module on sexual activity and function. Participants completed the Sexual Function Questionnaire (SFQ), Cancer and Treatment Distress (CTXD), and Revised Dyadic Adjustment Scale (RDAS). Clinical data were collected from the transplant medical database. Multivariate logistic regressions identified factors associated with sexual activity and function.
RESULTS
Participating survivors (N=1742) were a mean of 11.9 years (range 0.4–43.1) after HCT, mean age 57.6 and 53% male. Women were more likely than men to report not being sexually active in the past year (39% vs 27%) and low sexual function (64% vs 32%) for those sexually active. Male and female survivors reported lower rates of sexual activity and function than comparison norms (all P<.01). In regressions, factors associated with not being sexually active included older age, having less than 4 years of college education, low performance status, and not being in a committed relationship. Additional factors for men included non-myeloablative conditioning and not being employed or in school. Low sexual functioning for men and women was associated with low performance status, and, for women, a committed relationship of lower quality, while for men the association was with older age.
CONCLUSIONS
Sexual dysfunction is common in both men and women after HCT, regardless of time since treatment. Survivors need routine evaluation and access to multimodal interventions.
Keywords: hematopoietic stem cell transplantation, sexual functioning, survivorship
Introduction
Patients who undergo hematopoietic cell transplantation (HCT) may experience a decline in sexual health as one of their many survivorship issues.1–4 Sexual health can be divided into being ‘sexually active’ and ‘sexual function’, which refers to qualities of sexual well-being including sexual problems.5 Sexual issues after HCT are common with survivors reporting an inability to have sex (36%), derive pleasure from sex (31%), or have interest in sex (28%).6 Sexual dysfunction is associated with patients’ medical status as well as their perceived health status and psychosocial profile.7,8
To provide appropriate counseling on sexual health before and after HCT, health care professionals need to understand the epidemiology of sexual dysfunction and associated risk factors. The life stage at which the disease began (whether it was before or after becoming sexually active), physical symptoms (such as pain and fatigue), mental symptoms (such as depression or body image), and the health of a couple’s relationship, among other factors, can influence success in recovering sexual functioning after transplantation.6 Shifting social and familial roles also can impact intimacy or body image and thereby affect sexual functioning.9 Evidence indicates that informing patients and their partners about the effect of their treatment on sexual health increases the chances of achieving satisfactory function following cancer treatment.10 Yet healthcare providers prefer for patients to take the initiative in addressing this topic and consequently information on how treatments affect a patient’s sexual activity and well-being is infrequently discussed with HCT patients.11–14
Assessment of sexual health is an integral part of survivorship evaluation after HCT.1 Physical problems can affect sexual function, with both men and women experiencing an increased number of sexual problems from pretransplant to one year after HCT. For women these problems continue unresolved for at least five years.2,3 Men’s sexual health is more affected by erectile dysfunction and lack of libido, while women also experience lack of libido along with lack of sexual satisfaction, vaginal dryness and dyspareunia.2,3,15 These findings support the need to study men and women separately. In addition, most studies report the experience of small samples of patients evaluated early after HCT rather than long-term survivors.
The broad purpose of this investigation was to characterize sexual activity and function of adult male and female long-term HCT survivors, to compare this to the expected norms and to understand the factors associated with sexual activity and functioning after HCT to identify targets for intervention to improve sexual health. Based on previous research in patients earlier after HCT, we hypothesized that even long-term survivors would report lower sexual activity and sexual function than a general population and that lower sexual activity and sexual function would be associated with modifiable factors that would differ for male and female survivors.
Materials and Methods
Participants
The Fred Hutchinson Cancer Research Center (Fred Hutch) Institutional Review Board approved the patient surveys, procedures, and data sources. All surviving adult patients transplanted at Fred Hutch in Seattle, Washington who consent to long-term follow-up received an annual survey along with a self-addressed, stamped return envelope. Non-responders were mailed one reminder letter if a response was not received. As is routine, the annual survey consisted of a core set of questions along with the sexual function module that was distributed for one year. Each year an advisory group lead by the senior author (SJL) selects a focus for a module added to the medically focused core survey for a one-year time frame based on research gaps and priorities at that time. The research module analyzed for this report was mailed to a total of 4,484 adults between July 1, 2014-June 30, 2015. Clinical data were available from the transplant medical database.
Survey Measures
The core survey collected overall health, work/school status, Karnofsky performance status, medical comorbidities, chronic graft-versus-host disease (cGVHD), and medications.16–19 It contained four items with high reliability (alpha =0.90), scored as mean for perceived “post-transplant recovery,” with items such as “I have recovered from my transplant or treatment” rated from 1=not at all to 5=very much. Based on the sample distribution, scores were categorized into low (≤3), moderate (3–4) and high recovery (>4). The annual module included 4 validated scales: Sexual Functioning Questionnaire – Short Form (SFQ), Cancer and Treatment Distress (CTXD), Revised Dyadic Adjustment Scale (RDAS), and Patient Health Questionnaire (PHQ)-4.
The SFQ short form is a 12-item brief version of the 22-item SFQ measure, developed as a clinical research tool to assess sexual activity and the phases of sexual response and sexual problems. The first 3 items are descriptive, defining whether a person has “been sexually active in the past year (alone or with a partner)?” and in the past month, with the third question asking reasons if not sexually active. The remaining 9 items provide an SFQ total mean score for sexual function ranging from 0 to 5 with a higher score indicating better sexual function, and with subscales of interest, satisfaction, sexual activity, and problems. Only the problems subscale differs for males and females, both with four items including one common problem “lack of sexual interest or desire.” The SFQ is designed to allow comparisons between males and females and has been tested in clinical and non-clinical populations, with strong validity and reliability (alpha =0.85 for the SFQ short form with this cohort).2,20–25 The short form and longer version are strongly correlated (r=0.95).22 Receiver operating characteristic analysis with general population normative data indicates that a cutpoint of <2.6, denoting poorer sexual function, results in specificity of 0.87 and sensitivity of 0.87.26
The CTXD is a 23-item measure developed with HCT recipients to assess distress or worry.27,28 It consists of six subscales including uncertainty, health burden, family strain, identity, finances, and medical demands. Questions are rated on a 4-point Likert scale (0=none, 3=severe) with the items averaged for a mean score. The CTXD has demonstrated strong validity and reliability (alpha =0.95) in long-term survivors and other cancer patients.29,30 The established cutpoint indicating elevated distress in survivors is 0.90 or higher. Of note, the CTXD, including each of its subscales, has demonstrated strong associations with measures of post-traumatic stress symptoms (p<0.0001), indicating that it also measures those at risk for PTSD.19
The 14-item RDAS is a valid and reliable measure of relationship quality and is only completed by those in a marriage or cohabiting partnership.31 Response formats vary across 0–4 and 0–5 scales. Summary scores range from 0 to 69, with higher values indicating greater adjustment. Per established cut-offs, scores less than 48 are categorized as lower in relationship quality.32 Internal consistency for the sample was alpha =0.90.31
The Patient Health Questionnaire (PHQ) is a widely used, reliable and validated measure for assessing anxiety and depression symptoms. The first two mood items for the anxiety and depression scales are acceptable as the PHQ-4. A score of 3 or greater on the 2 anxiety or 2 depression symptoms indicates a positive screen for anxious or depressed mood and is associated with functional impairment, disability days, and healthcare use.33
Copies of the surveys are available by contacting the corresponding author at LTFU@fredhutch.org.
The comparison group data was collected with previous studies of the SFQ in HCT survivors, from siblings or friends known to the survivor since before transplant and matched to those survivors on age (within 5 years), sex, and race/ethnicity.26
Statistical Analyses
Descriptive and inferential analyses were performed using IBM SPSS Statistics version 26 and SAS version 9.4. Incomplete surveys were analyzed for the data provided, with no imputation for missing or incomplete data. Univariate analyses were conducted with chi square or independent samples t-tests separately by sex, except for direct comparisons between men and women for rates of not being sexually active. For those who reported being sexually active in the previous year, we analyzed differences in characteristics between those who reported high levels of sexual function compared to those reporting low levels. We also analyzed sexual activity and sexual function in survivors compared to an adult general population that was comparable in race, ethnicity, education and marital status.25,26 Because older age was associated with likelihood of not being sexually active and with lower sexual function in both survivors and comparisons, and because age distribution differed in the samples, we used logistic regression controlling for age to compare the samples. After checking for multicollinearity, with no variance inflation factor above 3, we conducted multivariate logistic regressions within each sex. Multivariate models included variables with P<.05 in the univariate tests, to identify factors that were independently associated with not being sexually active in the past year or with low levels of sexual function at two-sided P<.05. With our large sample size and the number of variables and regressions, we selected P<.05 rather than the commonly used P<.10 as the cut off for inclusion in regressions to reduce the risk of Type I error.
Results
Sexual Function Survey Respondents vs Non-Respondents and Comparisons
A total of 1742 patients, 41% of 4272 eligible and approached, answered the sexual function module and are included in analyses. Respondents were a mean of 11.9 years (range: 0.4–43.1, SD 9.5) post-HCT and 57.6 years of age (range: 18.2–87.2, SD 12.6), with 53% male (Table 1).
Table 1.
Characteristics of HCT survivors who have been versus have not been sexually active in the past year
| Men | Women | ||||||
|---|---|---|---|---|---|---|---|
| Whole group (N=1742) | Sexually inactive (N=252) | Sexually active (N=674) | P value | Sexually inactive (N=321) | Sexually active (N=495) | P value | |
| BASELINE CHARACTERISTICS | |||||||
| Age at transplant, n (%) | <.001 | <.001 | |||||
| <18 | 100 (6) | 13 (5) | 39 (6) | 12 (3) | 36 (7) | ||
| 18–39 | 494 (28) | 42 (17) | 194 (29) | 68 (21) | 190 (38) | ||
| 40–64 | 966 (55) | 141 (56) | 391 (58) | 194 (61) | 240 (49) | ||
| ≥65 | 182 (11) | 56 (22) | 50 (7) | 47 (15) | 29 (6) | ||
| Age Mean (SD) Range | 45.6 (15.7) | 51.7 (16.0) | 44.9 (15.1) | 49.2 (14.3) | 41.2 (15.8) | ||
| <1–79 | 2–79 | 1–74 | 4–73 | <1–72 | |||
| Ethnicity, n (%) | .27 | .80 | |||||
| Hispanic | 47 (3) | 6 (2) | 27 (4) | 8 (2) | 11 (2) | ||
| Non-Hispanic | 1658 (95) | 231 (92) | 631 (93) | 302 (94) | 469 (95) | ||
| Missing | 37 (2) | 15 (6) | 17 (3) | 12 (4) | 12 (3) | ||
| Race, n (%) | .17 | .25 | |||||
| White | 1515 (87) | 207 (82) | 591 (88) | 278 (87) | 439 (89) | ||
| Non-white | 147 (8) | 27 (11) | 55 (8) | 30 (9) | 35 (7) | ||
| Missing | 80 (5) | 18 (7) | 28 (4) | 12 (4) | 21 (4) | ||
| Education, n (%) | <.001 | <.001 | |||||
| < 4 years college | 757 (43) | 136 (54) | 242 (36) | 180 (56) | 200 (40) | ||
| 4 years college or more | 957 (55) | 106 (42) | 424 (63) | 139 (43) | 289 (58) | ||
| Missing | 28 (2) | 10 (4) | 9 (1) | 3 (1) | 6 (2) | ||
| Diagnosis, n (%) | .11 | .03 | |||||
| Acute Leukemia | 434 (25) | 62 (25) | 149 (22) | 80 (25) | 143 (29) | ||
| Chromic Leukemia | 333 (19) | 41 (16) | 147 (22) | 51 (16) | 94 (19) | ||
| Lymphoma | 373 (21) | 72 (29) | 159 (24) | 53 (17) | 89 (18) | ||
| Myeloma | 260 (15) | 42 (17) | 93 (14) | 57 (17) | 68 (14) | ||
| Other Malignancy | 247 (14) | 27 (11) | 90 (13) | 65 (20) | 65 (13) | ||
| Nonmalignant Disease | 95 (6) | 8 (3) | 36 (5) | 15 (5) | 36 (7) | ||
| TRANSPLANT FACTORS | |||||||
| Type of transplant, n (%) | .046 | .44 | |||||
| Autologous | 560 (32) | 97 (38) | 202 (30) | 111 (35) | 150 (30) | ||
| Allogeneic related | 642 (37) | 83 (33) | 247 (37) | 118 (37) | 194 (39) | ||
| Allogeneic unrelated | 540 (31) | 72 (29) | 225 (33) | 92 (29) | 151 (31) | ||
| Conditioning regimen, n (%) | <.001 | .007 | |||||
| Myeloablative | 1586 (91) | 209 (83) | 618 (92) | 289 (90) | 470 (95) | ||
| Non-Myeloablative | 156 (9) | 43 (17) | 56 (8) | 32 (10) | 25 (5) | ||
| Cell source, n (%) | .03 | .05 | |||||
| Bone marrow | 616 (35) | 73 (29) | 242 (36) | 103 (32) | 198 (40) | ||
| Peripheral blood | 1094 (63) | 172 (68) | 425 (63) | 212 (66) | 285 (58) | ||
| Cord | 32 (2) | 7 (3) | 7 (1) | 6 (2) | 12 (2) | ||
| TBI ≥ 10 Gy, n (%) | .58 | <.001 | |||||
| Yes | 539 (31) | 81 (32) | 204 (30) | 73 (23) | 181 (37) | ||
| No | 1203 (69) | 171 (68) | 470 (70) | 248 (77) | 314 (63) | ||
| CURRENT CLINICAL STATUS | |||||||
| Years Post-Transplant, M (SD) | 11.9 (9.5) | 10.6 (9.2) | 11.6 (9.3) | .15 | 11.7 (9.6) | 13.2 (9.9) | .03 |
| Current age, n (%)) | <.001 | <.001 | |||||
| 18–39 | 177 (10) | 17 (7) | 69 (10) | 19 6() | 72 (15) | ||
| 40–64 | 1022 (59) | 114 (45) | 422 (63) | 166 (52) | 320 (65) | ||
| ≥65 | 543 (31) | 121 (48) | 183 (27) | 136 (42) | 103 (21) | ||
| Age Mean (SD) Range | (57.6, 12.6) | 62.4 (12.7) | 56.6 (12) | 60.9, (11.9) | 54.5 (12.3) | ||
| 18–87 | 18–87 | 21–83 | 18–84 | 20–80 | |||
| Relapse After Transplant, n (%) | .06 | .36 | |||||
| Yes | 155 (9) | 32 (13) | 58 (9) | 29 (9) | 36 (7) | ||
| No | 1587 (91) | 220 (87) | 616 (91) | 292 (91) | 459 (93) | ||
| Current cGVHD,* n (%) | .06 | .27 | |||||
| None or mild | 1060 (90) | 134 (86) | 432 (92) | 183 (87) | 311 (90) | ||
| Moderate/severe | 122 (10) | 21 (14) | 40 (8) | 27 (13) | 34 (10) | ||
| Genital cGVHD,b n (%) | .29 | .49 | |||||
| Yes | 41 (2) | 0 (0) | 3 (0.4) | 17 (5) | 21 (4) | ||
| No | 1701 (98) | 252 (100) | 671 (99.6) | 304 (95) | 474 (96) | ||
| CURRENT SELF-REPORTED CHARACTERISTICS | |||||||
| Employment status, n (%) | <.001 | <.001 | |||||
| Employed or school full or part time | 892 (52) | 70 (28) | 381 (57) | 138 (43) | 303 (61) | ||
| Homemaker/retired | 545 (31) | 119 (47) | 185 (27) | 114 (36) | 127 (26) | ||
| Unemployed/disabled | 292 (16) | 60 (24) | 102 (15) | 65 (20) | 65 (13) | ||
| Missing | 13 (1) | 3 (1) | 6 (1) | 4 (1) | 0 | ||
| Relationship Status, n (%) | <.001 | <.001 | |||||
| Married/living with partner | 1361 (78) | 187 (74) | 585 (87) | 169 (53) | 420 (85) | ||
| Single/separated/divorced/widowed | 351 (20) | 56 (23) | 78 (12) | 148 (46) | 69 (14) | ||
| Missing | 30 (2) | 9 (3) | 11 (1) | 4 (1) | 6 (1) | ||
| Medication use, n (%) | |||||||
| Immunosuppressants | 257 (15) | 46 (18) | 99 (15) | .18 | 52 (16) | 60 (12) | .10 |
| Thyroid hormone | 662 (38) | 82 (33) | 251 (37) | .12 | 117 (36) | 212 (43) | .06 |
| Hormonal therapy | 553 (32) | 72 (29) | 242 (36) | .06 | 79 (25) | 160 (32) | .007 |
| Anti-diabetics | 560 (32) | 86 (34) | 249 (37) | .23 | 77 (24) | 148 (30) | .19 |
| Anti-hypertensive | 749 (43) | 106 (42) | 293 (43) | .91 | 145 (45) | 205 (41) | .05 |
| Psychotropic medication | 731 (42) | 96 (38) | 277 (41) | .34 | 139 (43) | 219 (44) | .97 |
| Prescription medication for pain | 622 (36) | 102 (40) | 259 (38) | .57 | 102 (32) | 159 (32) | .46 |
| Karnofsky Performance Status, n (%) | <.001 | <.001 | |||||
| 100% | 736 (42) | 67 (26) | 323 (48) | 106 (33) | 240 (48) | ||
| 90% | 557 (31) | 82 (33) | 201 (29) | 110 (34) | 164 (33) | ||
| 80% or lower | 439 (25) | 102 (40) | 149 (22) | 99 (31) | 89 (18) | ||
| Missing | 10 (1) | 1 (1) | 1 (1) | 6 (2) | 2 (1) | ||
| Post-Transplant Recovery, M (SD) | 3.97 (0.99) | 3.62 (1.07) | 4.07 (0.93) | <.001 | 3.78 (1.06) | 4.13 (0.92) | <.001 |
| Post-Transplant Recovery, n (%) | <.001 | <.001 | |||||
| Low recovery, <3.0 | 263 (15) | 66 (26) | 83 (12) | 60 (18) | 54 (11) | ||
| Moderate recovery | 394 (23) | 62 (25) | 149 (22) | 83 (26) | 100 (20) | ||
| High recovery ≥4.0 | 1069 (61) | 122 (48) | 436 (65) | 174 (55) | 338 (68) | ||
| Missing | 16 (1) | 2 (1) | 7 (1) | 4 (1) | 3 (1) | ||
| CTXD Distress score, M (SD) | 0.64 (0.48) | 0.77 (0.58) | 0.58 (0.54) | <.001 | 0.74 (0.60) | 0.57 (0.60) | <.001 |
| CTXD Elevated distress >0.90, n (%) | 493 (28) | 92 (37) | 173 (27) | <.001 | 116 (36) | 112 (23) | <.001 |
| PHQ 2-item Anxiety & Depression, M (SD) | |||||||
| Anxiety | 0.84 (1.38) | 0.79 (1.4) | 0.71 (1.3) | .39 | 1.1 (1.5) | 0.91 (1.4) | .12 |
| Depression | 0.67(1.20) | 0.80 (1.3) | 0.57 (1.1) | .01 | 0.89 (1.3) | 0.59 (1.1) | .001 |
| PHQ 2-item Anxiety & Depression, n (%) | |||||||
| Elevated anxiety (3+) | 164 (9) | 23 (9) | 54 (8) | .57 | 38 (12) | 49 (10) | .37 |
| Elevated depression (3+) | 123 (7) | 26 (10) | 42 (6) | .03 | 33 (10) | 22 (4) | .001 |
| Revised Dyadic Adjustment Scale (RDAS), M (SD) | 53.1 (8.1) | 50.9 (9.8) | 53.6 (7.4) | <.001 | 51.4 (8.7) | 53.7 (7.8) | .003 |
| Low RDAS <48, n (%) | 272 (16) | 51 (20) | 105 (16) | .003 | 44 (14) | 74 (15) | .01 |
TBI: total body irradiation; cGVHD, chronic graft versus host disease; M: mean score; SD: standard deviation; CTXD, Cancer and Treatment Distress; PHQ, Patient Health Questionnaire
Allogeneic transplants only
Male and female respondents were more likely than non-respondents to be older at transplantation and to have received a transplant for malignancy (Supplemental Table S1). Male respondents had more factors that distinguished respondents from non-respondents including multiple myeloma, having received peripheral blood grafts, a conditioning regimen without high dose total body irradiation (TBI), and fewer years since transplant.
Adult comparison non-cancer norms (N=179) were comparable in sociodemographic factors with the main exception that the comparison sample was younger (Table S2), with the age difference resulting from a larger number of the HCT respondents being over age 65 (P<.001). The female comparison sample was somewhat more likely to be white and to have a lower education level.
Sexual Activity
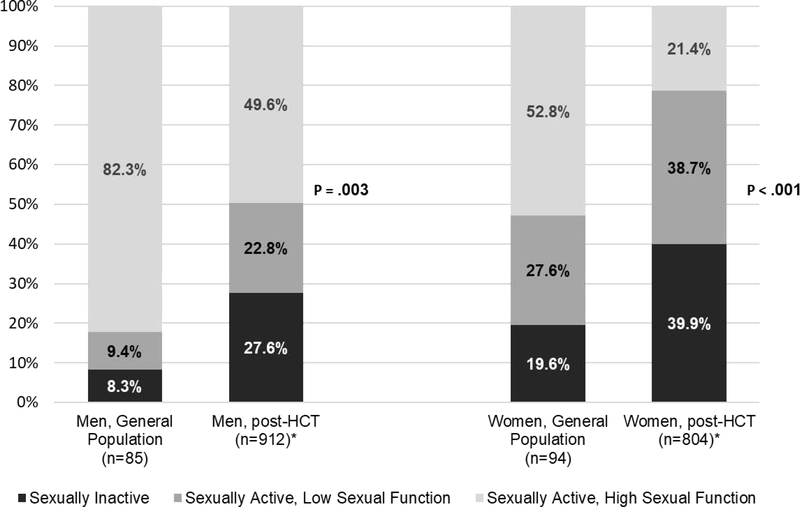
Women were more likely than men to report not being sexually active in the past year (39.3% vs 27.2%, P<.001; Figure 1). For both sexes these rates were higher than comparisons norms (comparison women: 19.6% not sexually active, Odds Ratio (OR) 2.14, Confidence Interval (CI) 1.24, 3.70, P=.006; comparison men: 8.3%, OR 2.99, CI 1.35, 6.63, P=.007). In univariate analyses, among other factors, survivors of both sexes were less likely to be sexually active if they were older at HCT and when completing the survey, if they reported worse performance status and post-transplant recovery, received a non-myeloablative transplant, did not have college degrees, were not working or in school, were not married or partnered, more cancer-related distress and depressive symptoms and lower relationship quality (Table 1). Women also were less likely to be sexually active if less time had elapsed since transplantation, and if they were not taking hormonal therapy.
Figure 1.
Comparison of men and women after HCT compared with general population norms for rates of those sexually inactive, sexually active with low sexual function and sexually active with high sexual function. In logistic regression controlling for age, P value compares rates between survivors and general population within sexes. *Note that 1.5% each of sexually active men and sexually active women had unknown sexual function data and were excluded from the figure.
In multivariate logistic regression final models, characteristics of men and women who were not sexually active included older age, not having 4 years of college, having low performance status, and not being in a committed relationship (Table 3). Men additionally were less likely to be sexually active if they received non-myeloablative conditioning or were not employed or in school.
Table 3.
Multivariate analysis of sexual activity and sexual function in men and women survivors
| Factors in Final Regression Models: | Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value |
|---|---|---|---|---|
| SEXUALLY INACTIVE IN THE PAST YEAR: | ||||
| MALES (n=847) | FEMALES (n=704) | |||
| Current Age | <.0001 | <.0001 | ||
| 18–39 | 1.0 | 1.0 | ||
| 40–64 | 1.6 (0.8–3.1) | .21 | 3.9 (1.9–7.7) | .0001 |
| ≥65 | 4.0 (1.9–8.5) | .0003 | 10.6 (5.1–22.0) | <.0001 |
| Education | <.0001 | .006 | ||
| 4 years college or more | 1.0 | 1.0 | ||
| <4 years college | 2.2 (1.6–3.2) | 1.6 (1.2–2.3) | ||
| Conditioning Regimen | .02 | |||
| Myeloablative | 1.0 | NA | ||
| Non-Myeloablative | 1.8 (1.1–2.9) | |||
| Employment Status | .02 | |||
| Employed or school full or part time | 1.0 | NA | ||
| Homemaker/retired | 1.8 (1.2–2.7) | .005 | ||
| Unemployed/disabled | 1.5 (0.9–2.5) | .08 | ||
| Karnofsky Performance Status | <.0001 | .0004 | ||
| 100% | 1.0 | 1.0 | ||
| 90% | 1.8 (1.1–2.7) | .009 | 1.3 (0.8–1.9) | .25 |
| 80% or lower | 2.6 (1.7–4.0) | <.0001 | 2.4 (1.6–3.8) | <.0001 |
| Relationship Status | <.0001 | <.0001 | ||
| In a committed relationship, RDAS high quality | 1.0 | 1.0 | ||
| In a committed relationship, RDAS low quality | 1.9 (1.2–2.9) | .006 | 1.6 (1.0–2.6) | .06 |
| Not in a committed relationship | 3.6 (2.2–5.8) | <.0001 | 7.0 (4.6–10.7) | <.0001 |
| LOW SEXUAL FUNCTIONING: | ||||
| MALES (n=621) | FEMALES (n=465) | |||
| Current Age | <.0001 | |||
| 18–39 | 1.0 | NA | ||
| 40–64 | 3.1 (1.4–7.2) | .007 | ||
| ≥65 | 7.2 (3.1–17.1) | <.001 | ||
| Karnofsky Performance Status | <.0001 | .002 | ||
| 100% | 1.0 | 1.0 | ||
| 90% | 2.8 (1.9–4.3) | <0.001 | 1.9 (1.2–3.0) | .004 |
| 80% or lower | 4.0 (2.5–6.4) | <0.001 | 2.3 (1.3–4.0) | .004 |
| Relationship Status | .02 | |||
| In a committed relationship, RDAS high quality | NA | 1.0 | ||
| In a committed relationship, RDAS low quality | 2.3 (1.2–4.4) | .001 | ||
| Not in a committed relationship | 0.8 (0.5–1.4) | .41 | ||
RDAS, Revised Dyadic Adjustment Scale
Reasons for sexual inactivity varied between sexes (Table 4). The most endorsed reason for men was physical issues (39%) whereas for women it was the lack of a partner (37%). In both sexes, the second most common issue was a lack of interest in sexual activity, reported by 29% of inactive men and 34% of inactive women.
Table 4.
Reasons for sexual inactivity in past year in HCT survivorsa
| Reason for lack of sexual activity | Men who are not sexually active (n=252) n (%) | Women who are not sexually active (n=321) n (%) | P valueb |
|---|---|---|---|
| Never been active | 11 (4) | 16 (5) | .73 |
| Issue linked to self: | |||
| Too Tired | 24 (10) | 24 (8) | .38 |
| Not Interested | 73 (29) | 108 (34) | .23 |
| Physical problem | 99 (39) | 63 (20) | <.001 |
| Issue linked to partner: | |||
| Partner is not interested | 55 (22) | 42 (13) | .006 |
| Partner is too tired | 6 (2) | 9 (3) | .75 |
| Partner has a physical problem | 23 (9) | 43 (13) | .11 |
| No Partner | 38 (15) | 118 (37) | <.001 |
May report more than one reason
P value comparing frequency in men vs women among those not sexually active in past year.
Sexual Function
Among those who were sexually active, more women (n=311, 64.4%) than men (n=208, 31.5%) reported low sexual functioning (P<.001; Table 2). Both female and male survivors were more likely to report low sexual functioning than comparison norms (OR 3.42, CI 2.03, 5.75, P<.001 for women; OR 3.13, CI 1.46, 6.70, P=.003 for men; Figure 1).
Table 2.
Characteristics of HCT survivors with high or low sexual function among those who are sexually active
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Low sexual function a (N=208) | High sexual function a (N=452) | P value | Low sexual function a (n=311) | High sexual function a (N=172) | P value | |
| BASELINE STATUS | ||||||
| Age at transplant, n (%) | <.001 | .53 | ||||
| <18 | 0 | 39 (8) | 21 (4) | 14 (8) | ||
| 18–39 | 43 (21) | 148 (33) | 115 (37) | 71 (40) | ||
| 40–64 | 145 (69) | 237 (53) | 159 (51) | 76 (45) | ||
| ≥65 | 20 (10) | 28 (6) | 16 (8) | 11 (7) | ||
| Ethnicity, n (%) | .13 | .55 | ||||
| Hispanic | 5 (3) | 22 (5) | 8 (3) | 3 (2) | ||
| Non-Hispanic | 200 (96) | 420 (93) | 294 (94) | 165 (96) | ||
| Missing | 1 (1) | 10(2) | 9 (3) | 4 (2) | ||
| Race, n (%) | .47 | .20 | ||||
| White | 186 (90) | 395 (86) | 276 (88) | 157 (92) | ||
| Non-white | 15 (7) | 40 (9) | 24 (8) | 8 (4) | ||
| Missing | 7 (3) | 17 (5) | 11 (4) | 7 (4) | ||
| Education, n (%) | .82 | .33 | ||||
| < 4 years college | 76 (37) | 163 (36) | 133 (42) | 65 (38) | ||
| 4 years college or more | 128 (62) | 286 (63) | 176 (57) | 104 (60) | ||
| Missing | 4 (1) | 3 (2) | 2 (1) | 3 (2) | ||
| Diagnosis, n (%) | .06 | .49 | ||||
| Acute Leukemia | 46 (22) | 101 (22) | 88 (28) | 54 (31) | ||
| Chromic Leukemia | 39 (19) | 104 (23) | 53 (17) | 38 (22) | ||
| Lymphoma | 56 (27) | 101 (22) | 58 (19) | 29 (17) | ||
| Myeloma | 38 (18) | 53 (12) | 49 (16) | 18 (11) | ||
| Other Malignancy | 22 (11) | 64 (14) | 40 (13) | 22 (13) | ||
| Nonmalignant Disease | 7 (3) | 29 (6) | 23 (7) | 11 (6) | ||
| TRANSPLANT VARIABLES | ||||||
| Type of transplant, n (%) | .13 | .05 | ||||
| Autologous | 73 (35) | 125 (28) | 107 (34) | 41 (24) | ||
| Allogeneic related | 69 (33) | 177 (39) | 114 (37) | 73 (42) | ||
| Allogeneic unrelated | 66 (32) | 150 (33) | 90 (29) | 58 (34) | ||
| Conditioning regimen, n (%) | .07 | .20 | ||||
| Myeloablative | 185 (89) | 421 (93) | 294 (95) | 167 (97) | ||
| Non-Myeloablative | 23 (11) | 31 (7) | 17 (6) | 5 (3) | ||
| Cell source, n (%) | .20 | .55 | ||||
| Bone marrow | 60 (29) | 177 (39) | 118 (38) | 94 (55) | ||
| Peripheral blood | 147 (71) | 269 (60) | 185 (60) | 74 (43) | ||
| Cord | 1 (1) | 6 (1) | 8 (3) | 4 (2) | ||
| TBI ≥10 Gy, n (%) | .50 | .90 | ||||
| Yes | 59 (28) | 140 (31) | 114 (37) | 64 (37) | ||
| No | 149 (72) | 312 (69) | 197 (63) | 108 (63) | ||
| CURRENT CLINICAL STATUS | ||||||
| Years Post-Transplant M (SD) | 10.6 (8.7) | 12.1 (9.5) | .05 | 12.7 (9.8) | 14.0 (9.7) | .14 |
| Current age M (SD) | 60.9 (9.1) | 54.4 (12.9) | <.001 | 54.3 (12.3) | 54.7 (12.5) | .77 |
| Relapse after Transplant, n (%) | .16 | .06 | ||||
| Yes | 23 (11) | 35 (8) | 27 (9) | 7 (4) | ||
| No | 185 (89) | 417 (92) | 284 (91) | 165 (96) | ||
| Current cGVHD, b n (%) | 0.34 | .05 | ||||
| None or mild | 121 (90) | 302 (92) | 178 (87) | 123 (94) | ||
| Moderate/severe | 14 (10) | 25 (8) | 26 (13) | 8 (6) | ||
| Genital cGVHD,b n (%) | .19 | .49 | ||||
| Yes | 2 (1) | 1 (0.2) | 15 (5) | 6 (4) | ||
| No | 206 (99) | 451 (99.8) | 296 (95) | 166 (97) | ||
| CURRENT SELF-REPORTED CHARACTERISTICS | ||||||
| Employment status, n (%) | .001 | .30 | ||||
| Employed or school full or part time | 95 (47) | 281 (61) | 185 (60) | 112 (65) | ||
| Homemaker/retired | 69 (33) | 108 (24) | 79 (25) | 48 (25) | ||
| Unemployed/disabled | 40 (18) | 61 (14) | 47 (15) | 18 (10) | ||
| Missing | 4 (2) | 2 (1) | 0 | 0 | ||
| Relationship status, n (%) | .87 | .42 | ||||
| Married/living with partner | 181 (88) | 393 (86) | 268 (86) | 142 (82) | ||
| Single/separated/divorced/widowed | 25 (11) | 52 (12) | 41 (13) | 27 (16) | ||
| Missing | 2 (1) | 7 (2) | 2 (1) | 4 (2) | ||
| Medication use, n (%) | ||||||
| Immunosuppressants | 37 (18) | 59 (13) | .11 | 40 (13) | 19 (11) | .56 |
| Low thyroid | 80 (39) | 164 (36) | 0.66 | 133 (40) | 75 (44) | .79 |
| Hormonal therapy | 69 (34) | 167 (36). | 0.36 | 98 (32) | 61 (35) | .36 |
| Anti-diabetics | 75 (37) | 168 (36) | 0.58 | 97 (31) | 49 (29) | .59 |
| Anti-hypertensive | 85 (41) | 202 (45) | 0.21 | 131 (43) | 72 (40) | .99 |
| Psychotropic medication | 87 (43) | 184 (40) | 0.98 | 142 (45) | 73 (43) | .53 |
| Pain | 85 (42) | 168 (37) | 0.57 | 104 (34) | 51 (30) | .42 |
| Karnofsky Performance Status, n (%) | <.001 | .004 | ||||
| 100% | 61 (29) | 256 (56) | 133 (44) | 99 (58) | ||
| 90% | 77 (36) | 122 (27) | 112 (35) | 49 (28) | ||
| 80% or lower | 70 (35) | 73 (16) | 66 (21) | 22 (13) | ||
| Missing | 0 | 1 (1) | 0 | 2 (1) | ||
| Post-Transplant Recovery, M (SD) | 3.76 (1.00) | 4.21 (0.86) | <.001 | 4.01 (0.93) | 4.33 (0.83) | <.001 |
| Post-Transplant Recovery, n (%) | <.001 | .006 | ||||
| Low recovery, <3.0 | 41 (19) | 39 (8) | 42 (13) | 11 (6) | ||
| Moderate recovery | 62 (30) | 84 (19) | 70 (22) | 28 (16) | ||
| High recovery >4.0 | 104 (50) | 324 (72) | 197 (64) | 132 (77) | ||
| Missing | 1 (1) | 5 (1) | 2 (1) | 1 (1) | ||
| CTXD** Distress score, M (SD) | 0.74 (0.60) | 0.50 (0.50) | <.001 | 0.64 (0.58) | 0.48 (0.47) | .002 |
| CTXD** Elevated distress >0.90, n (%) | 72 (35) | 94 (21) | <.001 | 78 (25) | 33 (19) | .13 |
| PHQ 2-item Anxiety, Depression, M (SD) | ||||||
| Anxiety | 0.81 (1.40) | 0.66 (1.42) | .14 | 0.94 (1.43) | 0.88 (1.31) | .66 |
| Depression | 0.76 (1.25) | 0.48 (1.08) | .003 | 0.74 (1.18) | 0.36 (0.82) | <.001 |
| PHQ 2-item Anxiety, Depression, n (%) | ||||||
| Elevated anxiety (3+) | 23 (11) | 28 (6) | .03 | 34 (11) | 15 (9) | .47 |
| Elevated depression (3+) | 20 (10) | 19 (4) | .006 | 20 (6) | 2 (1) | .008 |
| Revised Dyadic Adjustment Scale (RDAS), M (SD) | 52.5 (7.6) | 54.1 (7.3) | .01 | 52.7 (7.7) | 53.3 (7.8) | .001 |
| Low RDAS <48, n (%) | 38 (21) | 66 (17) | .24 | 56 (21) | 16 (11) | .02 |
TBI: total body irradiation; cGVHD, chronic graft versus host disease; M: mean score; SD: standard deviation; CTXD, Cancer and Treatment Distress; PHQ, Patient Health Questionnaire
Sexual Function Low = Sexual Function Questionnaire (SFQ) Mean score <2.6, Sexual Function High = SFQ Mean score ≥2.6
Allogeneic transplants only
In univariate analyses, for both sexes low sexual functioning was associated with worse performance status and post-transplant recovery, more cancer-associated distress and depressive symptoms, and low relationship quality for those in a committed relationship, among other factors (Table 2). In multivariate analyses (Table 3), low sexual function in men was associated with age 40 and older and worse performance status. In women, low sexual function was also associated with low performance status, and being in a lower quality, more distressed relationship as opposed to either having no partner or being in a high quality, low distress committed relationship.
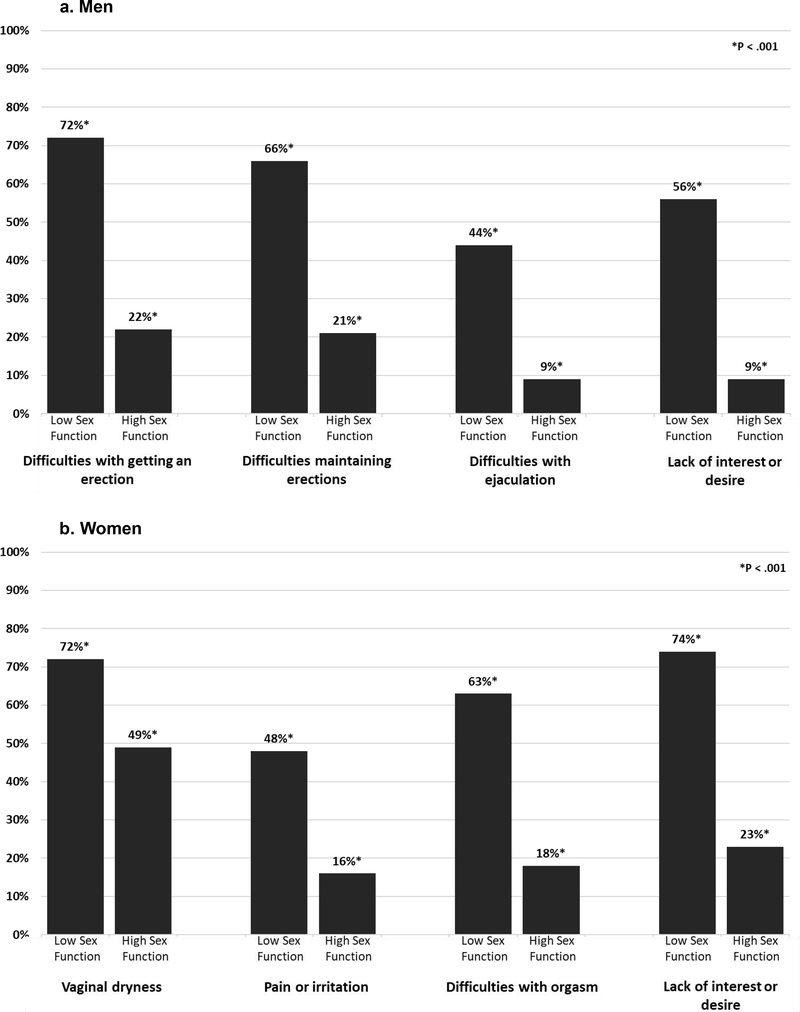
Sexual problems were more common for both men and women who had low sexual function (all P<.001; Figure 2). Among survivors who were sexually active, the most frequently reported problem for men was erectile dysfunction (37.5%) and for women vaginal dryness (63.1%). Lack of sexual interest or desire was a problem for 55.2% of women and 23.5% of men who were sexually active.
Figure 2.
Rates of sexual functioning problems occurring at least half the time during sexual activity after HCT among survivors who were sexually active in the past year comparing those with low sexual function vs high sexual function (all P<.001) for men (2a) and women (2b).
Discussion
Long-term HCT survivors report less sexual activity and lower sexual function compared to non-cancer, general population norms. Lower sexual functioning was broadly associated with worse physical, emotional and relationship health. For this diverse cohort of survivors, ranging in age from 18–87 and in time post-treatment from 4 months to 43 years, nearly 40% of women and 27% of men had not been sexually active in the previous year while 64% of women and 32% of men who were sexually active reported low sexual function. Although aging was associated with less sexual activity and function, time since treatment was not, suggesting that for long-term survivors sexual problems after HCT do not improve over time.3,34 Problems with sexual function occurred across all symptoms measured and were far more common in survivors who also reported poor performance status.
Several non-modifiable factors (including age, education, transplant procedures, and the availability of a partner) were identified as strongly associated with lower post-transplant sexual activity and functioning. However, modifiable symptoms such as erectile dysfunction, vaginal dryness and dyspareunia, as well as relationship quality, treatment-related distress, and depression can be addressed medically or behaviorally. Low libido was an issue for both sexes and could be improved through sexuality counseling after potentially related medical conditions are evaluated and treated. Although we found that active cGVHD was not associated with sexual activity or function in contrast to other studies,2,6,35 the proportion with self-reported moderate to severe cGVHD was very low as would be expected in this long-term survivor population with a mean time since HCT of 11 years. However, considering the association between genital cGVHD and sexual function in both men and women as suggested by several case-series, experts recommend performing a full genital exam and gonadal function evaluation in HCT survivors starting from 3–6 months post-HCT, and then yearly.36–40
Understanding why individuals are not sexually active will help to define tailored, multimodal interventions to improve both sexual activity and satisfaction. We found that the second most common reason for sexual inactivity for both sexes was a lack of interest, reported by about one third of participants who were not sexually active and as a problem for well over half of survivors who were sexually active but had low sexual function. While some survivors may consider their lack of sexual activity as not problematic, for others lack of sexual interest or desire is a reason to seek treatment, particularly if they are in a committed relationship where their partners wish to engage in sexual activity. A recent clinical trial in HCT survivors showed strong efficacy in improving sexual interest and function, suggesting that if lack of interest is distressing, it is treatable.41
Notable in our results, low sexual function in women was associated with being in a relationship of low quality, more so than being unpartnered. The fact that relationship quality was associated with sexual functioning among sexually active women but not among sexually active men is commensurate with prior research on sex differences in sexuality. For women more so than for men, sexuality is strongly linked to relationship context, in particular a close, committed relationship.28,29 These findings underscore intimacy-based models of female sexuality,30 and suggest that women in particular may benefit from couple-based approaches to enhance feelings of closeness and bolster relationship quality. We note that both male and female survivors, in univariate analyses, were more likely to be sexually active if they had a committed partner and if they had high relationship quality. Thus, while relationships may be more central issues for women, relationships are also relevant to men’s sexual function.
Physical problems were a major reason for not being sexually active and performance status was the one consistent risk factor across men and women in models of sexual inactivity and sexual dysfunction. Chronic disease and sex have a complicated relationship, and sexual health after HCT can be impacted by numerous medical conditions42 including genital changes, cGVHD, hormone function, cardiovascular disease and other chronic conditions. Chronic illnesses are usually described incompletely, and chronically ill patients are often excluded from studies of sexual health, limiting our knowledge of strategies that may improve sexual function in cancer survivors who commonly have more comorbidities than general populations.7
Our study had some limitations. This was a cross-sectional survey with a response rate of 41%, in predominantly White adult survivors. The survey was conducted in English, thus potentially limiting the reach to survivors comfortable answering questions in English. Translating the survey to other languages could increase participation. Community-based participatory research could also be an approach through which to identify methods to improve participation from groups who have enrolled at lower rates. Respondents differed from non-respondents in some clinical characteristics. Nevertheless, our results are consistent with smaller studies of shorter times after HCT and represent a large number of long-term survivors.15 Furthermore, the response rate is higher than or consistent with other studies using this methodology and population.18,19,43 Questions in the study were restricted to limit responder burden. Future studies could investigate sexual function in relation to body image, factors that disrupt sexual responsiveness, and sexual orientation. We did not identify sexual minorities, and research on sexual and racial minorities in HCT survivorship is lacking.44 Questions related to sexual orientation should also be included when assessing sexual function. Sexual minorities may have different predictors of sexual function and satisfaction and should be studied. We only surveyed patients and work is needed to understand long-term impacts on spouses/partners of HCT recipients. Although we cannot be certain, the responses may underestimate the extent of sexual health problems in HCT survivors in that non-responders may be biased toward those with more sexual difficulties. Alternatively, it is possible that those with problems were more motivated to respond and our results could overestimate the level of sexual problems. Comparing these results to other studies in this population, we believe that responses are generally consistent with what has been found in other HCT survivor studies though their time frames of assessment are usually much closer after HCT.
These results provide valuable evidence for intervention development. They highlight the importance of informing patients about the high prevalence of low sexual function after HCT, screening for patients who require help, and offering them multimodal interventions to address potential clinical and psychological issues linked to their sexual issues as needed. These steps are advocated by a recent American Society of Clinical Oncology (ASCO) survivorship expert panel and the National Comprehensive Cancer Network (NCCN) Survivorship Guideline.44–46 In Table 5 we provide a summary of these guidelines supplemented by recommendations specific to HCT and cGVHD.13,36,38,47–49
Table 5:
Summary of sexual health recommendations for multimodal interventions in adult HCT survivors*
| Screening (All Adult Males and Females) |
Screening Questions: (from the NCCN Survivorship Guideline)46 1. Do you have any concerns regarding your sexual function, sexual activity, sexual relationship or sex life? Yes/No 2. Are these concerns causing you distress or would you like to discuss them? Yes/No Evaluation: if yes to both screening questions, provide a more complete evaluation (see NCCN Survivorship Guideline for details). Screening Time Points: prior to HCT, at discharge or around 100 days, at 6 months, 12 months and when providing long-term follow-up. |
|
| Education (All Adult Males and Females) |
Use specific printed education material such as a fact-sheet.13,47,55 Initiate discussion with the patient, and if possible, the partner, about sexual health and intimacy issues, making a distinction between the ability to engage in intercourse and sexual satisfaction. Discussions ideally take place before HCT and are repeated at screening time points during the survivorship process, taking into account the patient’s priorities, literacy level, cultural/religious perspective and sexual orientation. Patients can also be directed to websites with reliable information such as www.cancer.org; www.cancer.gov; www.macmillan.org.uk; www.cancersexnetwork.org; www.isswsh.org; www.menopause.org and www.aasect.org. If a specialized sex therapist is not available to lead discussions, another therapist with sexual health education and training can help normalize the experience and offer support and guidance. Referral to sexuality specialists should be available at all transplant centers. |
|
| If evaluation indicates Physical Sexual or Genital Issues (All Adult Males and Females) |
Physical assessment is recommended with a full genital exam, screening for genital GVHD, sexually transmitted infections (including human papilloma virus), hypogonadism and a full medication review. Survivors with chronic GVHD should receive physical assessments annually or more often if genital symptoms change. |
|
| MALES | FEMALES | |
| Management of physical or genital issues |
Erectile dysfunction, Consider: - Phosphodiesterase type 5 inhibitor medications (contraindicated in case of the use of nitrates in any form) - Pelvic physical therapy - Vacuum erectile device, medicated urethral system for erection, or intracavernous injection - Surgical interventions Genital GVHD,36,48 Consider: - Low threshold for referral to dermatology or urology - Topical high-potency corticosteroids (0.05%) or topical calcineurin inhibitors (0.1%) - Circumcision (in case of phimosis or insufficient response to topical treatment) - Surgical intervention in case of Peyronie’s disease and/or meatal stenosis Lack of Libido, Consider: - Hormone therapy - Referral for couples or individual counseling with a sexual function trained therapist |
Irritation or Sensitivity, Consider: - Avoid chemical irritants such as soaps and feminine wash products and use preferentially warm water only for hygiene. Early Menopause, Consider: - Hormone therapy initiated as soon after HCT as possible, as topical and/or systemic treatment (consider the patient’s age and risk/benefit ratio based on breast cancer and thromboembolic family and personal history). Note that intravaginal dehydroepiandrosterone is also a potential topical option for post-menopausal women with contra-indications for hormone therapy. Genital GVHD,38,48,49,56–58 Consider: - Low threshold for referral to dermatology or gynecologist - Avoidance of chemical and mechanical irritants - Use of emollients (e.g. bacteriostatic gels, petroleum jelly or lanolin cream) - Use of oral medications (e.g. diphenhydramine, hydroxyzine or doxepin) in case of pruritus - Topical high-potency corticosteroids (0.05%), hydrocortisone, or calcineurin inhibitors ointments (0.1%) - Vaginal dilators or low-dose estradiol vaginal rings in case of stenosis - Manual or surgical lysis of vaginal adhesions Vaginal Pain, Vaginismus and/or Vaginal Stenosis, Consider: - Vaginal lubricants/moisturizers for all sexual activity or touch - Pain relief medication (e.g. local 4% aqueous lidocaine, selective estrogen receptor modulator such as Ospemifene in post-menopausal women) - Pelvic floor physiotherapy and/or Kegel exercises - Vaginal dilators or low-dose estradiol vaginal rings Lack of Libido, Consider: - Flibanserin - Vaginal vibrators - Referral for couples or individual counseling with a sexual function trained the rapist Vasomotor symptoms, Consider: - Medication (e.g.: HRT, paroxetine, venlafaxine, gabapentin or clonidine) - Cognitive behavioral therapy - Behavioral or Integrative medicine options (slow-breathing techniques, relaxation, self-hypnosis) |
| Management of psychological issues | - Discuss body image (including weight change, scarring, hair loss, disfigurement, attractiveness) - Consider individual or couples counseling or therapy; depending on issues may be a sex therapist or a cancer survivorship therapist |
|
| Management of relationship issues | - Consider couple’s counseling with a therapist knowledgeable about sex therapy | |
Interventions for other cancer survivors could be relevant and adaptable for HCT survivors.50,51 A single-arm study testing a multimodal intervention which included assessment, medical evaluation and treatment, education, and tailored intervention in allogeneic recipients was efficacious in improving sexual interest, activity, function and satisfaction.41 This intervention was delivered at varying times following HCT (ranging from 3–73 months) so the optimal time point for intervention remains unclear. Additionally, interventions that involve partners have yielded stronger effects relative to patient-only approaches.44 Given our findings regarding relationship quality and sexual health, we recommend involving partners and including content designed to enhance partner communication and intimacy. In general, cancer-related sexual intervention research is characterized by a great deal of variability with respect to content, delivery modality, dose and outcome measures, and is dominated by small, one-arm feasibility trials rather than fully powered randomized controlled trials.51–53 Even so, an evidence-based guideline for cancer survivors provides a foundation for further development and testing of interventions for HCT survivors.50 Online delivery of interventions may help to reduce barriers to participation.52
In summary, our study showed that sexual activity and function is lower in long-term HCT survivors than the general population. This lower sexual health is associated with a number of physical health and psychosocial characteristics calling for a multimodal, integrated care approach as advocated by a number of recent guideline initiatives.1,54 Studies of tailored interventions need to be conducted to improve sexual health in long-term survivors as early as possible after transplantation.
Supplementary Material
Highlights.
After HCT 39% of women and 27% of men report no sexual activity in the past year.
Of those sexually active, 64% of women and 32% of men report low sexual function.
Rates of sexual dysfunction do not differ across the years after HCT.
Function declines with aging, low performance status & lack of stable relationship.
Vaginal dryness (63%), erectile dysfunction (38%) & low libido are most prevalent.
Acknowledgments
Acknowledgement of Research Support: Funded in part by grants from the National Cancer Institute CA215134, CA201179, CA018029, CA15704
Footnotes
Disclaimers: The authors have no potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bevans M, El-Jawahri A, Tierney DK, et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: The Patient-Centered Outcomes Working Group Report. Biol Blood Marrow Transplant. 2017;23(4):538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noerskov KH, Schjodt I, Syrjala KL, Jarden M. Sexual function 1-year after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;51(6):833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syrjala KL, Kurland BF, Abrams JR, Sanders JE, Heiman JR. Sexual function changes during the 5 years after high-dose treatment and hematopoietic cell transplantation for malignancy, with case-matched controls at 5 years. Blood. 2008;111(3):989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syrjala KL, Langer SL, Abrams JR, Storer BE, Martin PJ. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol 2005;23(27):6596–6606. [DOI] [PubMed] [Google Scholar]
- 5.Lammerink EA, de Bock GH, Pras E, Reyners AK, Mourits MJ. Sexual functioning of cervical cancer survivors: a review with a female perspective. Maturitas. 2012;72(4):296–304. [DOI] [PubMed] [Google Scholar]
- 6.Thygesen KH, Schjodt I, Jarden M. The impact of hematopoietic stem cell transplantation on sexuality: a systematic review of the literature. Bone Marrow Transplant. 2012;47(5):716–724. [DOI] [PubMed] [Google Scholar]
- 7.Verschuren JE, Enzlin P, Dijkstra PU, Geertzen JH, Dekker R. Chronic disease and sexuality: a generic conceptual framework. J Sex Res. 2010;47(2):153–170. [DOI] [PubMed] [Google Scholar]
- 8.Yi JC, Syrjala KL. Sexuality after hematopoietic stem cell transplantation. Cancer J. 2009;15(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norskov KH, Schmidt M, Jarden M. Patients’ experience of sexuality 1-year after allogeneic Haematopoietic Stem Cell Transplantation. Eur J Oncol Nurs. 2015;19(4):419–426. [DOI] [PubMed] [Google Scholar]
- 10.Kim IR, Jang SY, Shin HS, et al. Association between sexuality knowledge and sexual dysfunction in hematopoietic stem cell transplantation patients and their partners. Patient Educ Couns. 2020. [DOI] [PubMed] [Google Scholar]
- 11.Claessens JJ, Beerendonk CC, Schattenberg AV. Quality of life, reproduction and sexuality after stem cell transplantation with partially T-cell-depleted grafts and after conditioning with a regimen including total body irradiation. Bone Marrow Transplant. 2006;37(9):831–836. [DOI] [PubMed] [Google Scholar]
- 12.Humphreys CT, Tallman B, Altmaier EM, Barnette V. Sexual functioning in patients undergoing bone marrow transplantation: a longitudinal study. Bone Marrow Transplant. 2007;39(8):491–496. [DOI] [PubMed] [Google Scholar]
- 13.Wendt C Perception and Assessment of Verbal and Written Information on Sex and Relationships after Hematopoietic Stem Cell Transplantation. J Cancer Educ. 2017;32(4):681–689. [DOI] [PubMed] [Google Scholar]
- 14.Eeltink CM, Witte BI, Stringer J, et al. Health-care professionals’ perspective on discussing sexual issues in adult patients after haematopoietic cell transplantation. Bone Marrow Transplant. 2018;53(3):235–245. [DOI] [PubMed] [Google Scholar]
- 15.Dyer G, Gilroy N, Bradford J, et al. A survey of fertility and sexual health following allogeneic haematopoietic stem cell transplantation in New South Wales, Australia. Br J Haematol. 2016;172(4):592–601. [DOI] [PubMed] [Google Scholar]
- 16.Georges GE, Bar M, Onstad L, et al. Survivorship after Autologous Hematopoietic Cell Transplantation for Lymphoma and Multiple Myeloma: Late Effects and Quality of Life. Biol Blood Marrow Transplant. 2020;26(2):407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SJ, Onstad L, Chow EJ, et al. Patient-reported outcomes and health status associated with chronic graft-versus-host disease. Haematologica. 2018;103(9):1535–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw BE, Syrjala KL, Onstad LE, et al. PROMIS measures can be used to assess symptoms and function in long-term hematopoietic cell transplantation survivors. Cancer. 2018;124(4):841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang J, Lee SJ, Storer BE, et al. Rates and Risk Factors for Post-Traumatic Stress Disorder Symptomatology among Adult Hematopoietic Cell Transplant Recipients and Their Informal Caregivers. Biol Blood Marrow Transplant. 2019;25(1):145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford JS, Kawashima T, Whitton J, et al. Psychosexual functioning among adult female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32(28):3126–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhat GS, Nelivigi G, Barude V, Shastry A. Sexuality in Surgically Treated Carcinoma Penis Patients and Their Partners. Indian J Surg. 2018;80(1):19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostbye T, Kolotkin RL, He H, et al. Sexual functioning in obese adults enrolling in a weight loss study. J Sex Marital Ther. 2011;37(3):224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampton AJ, Walker LM, Beck A, Robinson JW. A brief couples’ workshop for improving sexual experiences after prostate cancer treatment: a feasibility study. Support Care Cancer. 2013;21(12):3403–3409. [DOI] [PubMed] [Google Scholar]
- 24.Daleboudt GM, Broadbent E, McQueen F, Kaptein AA. The impact of illness perceptions on sexual functioning in patients with systemic lupus erythematosus. J Psychosom Res. 2013;74(3):260–264. [DOI] [PubMed] [Google Scholar]
- 25.Syrjala KL ST, Abrams JR, Atkins TZ, Brown WS, Sanders JE et al. Sexual function measurement and outcomes in cancer survivors and matched controls. Journal of Sex Research. 2000;Volume 37(3):213–225. [Google Scholar]
- 26.Syrjala K Sexual Functioning Questionnaire (SFQ) Scoring Manual. Unpublished manual available upon request. 2017.
- 27.Syrjala KL, Yi JC, Langer SL. Psychometric properties of the Cancer and Treatment Distress (CTXD) measure in hematopoietic cell transplantation patients. Psychooncology. 2016;25(5):529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Syrjala KL, Chapko ME. Evidence for a biopsychosocial model of cancer treatment-related pain. Pain. 1995;61(1):69–79. [DOI] [PubMed] [Google Scholar]
- 29.Han CJ, Gigic B, Schneider M, et al. Risk factors for cancer-related distress in colorectal cancer survivors: one year post surgery. J Cancer Surviv. 2020;14(3):305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syrjala KL, Sutton SK, Jim HS, et al. Cancer and treatment distress psychometric evaluation over time: A BMT CTN 0902 secondary analysis. Cancer. 2017;123(8):1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busby DM CC, Crane DR, Larson JH. A revision of the dyadic adjustment scale for use with distressed and nondistressed couples: construct hierarchy and multidimensional scales. Journal of Marital and Family Therapy. 1995;21(3):298–308. [Google Scholar]
- 32.Crane DR MK, Bean RA. Establishing criterion scores for the Kansas Marital Satisfaction Scale and the Revised Dyadic Adjustment Scale. The American Journal of Family Therapy. 2000;28(1):7. [Google Scholar]
- 33.Kroenke K, Spitzer RL, Williams JB, Lowe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613–621. [DOI] [PubMed] [Google Scholar]
- 34.Syrjala KL, Roth-Roemer SL, Abrams JR, et al. Prevalence and predictors of sexual dysfunction in long-term survivors of marrow transplantation. J Clin Oncol. 1998;16(9):3148–3157. [DOI] [PubMed] [Google Scholar]
- 35.Wong FL, Francisco L, Togawa K, et al. Longitudinal trajectory of sexual functioning after hematopoietic cell transplantation: impact of chronic graft-versus-host disease and total body irradiation. Blood. 2013;122(24):3973–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller SM, Haeusermann P, Rovo A, et al. Genital chronic GVHD in men after hematopoietic stem cell transplantation: a single-center cross-sectional analysis of 155 patients. Biol Blood Marrow Transplant 2013;19(11):1574–1580. [DOI] [PubMed] [Google Scholar]
- 37.Lara LA, De Andrade JM, Mauad LM, et al. Genital manifestation of graft-vs.-host disease: a series of case reports. J Sex Med. 2010;7(9):3216–3225. [DOI] [PubMed] [Google Scholar]
- 38.Murphy J, McKenna M, Abdelazim S, Battiwalla M, Stratton P. A Practical Guide to Gynecologic and Reproductive Health in Women Undergoing Hematopoietic Stem Cell Transplant. Biol Blood Marrow Transplant. 2019;25(11):e331–e343. [DOI] [PubMed] [Google Scholar]
- 39.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21(3):389–401 e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(3):348–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Jawahri A, Fishman SR, Vanderklish J, et al. Pilot study of a multimodal intervention to enhance sexual function in survivors of hematopoietic stem cell transplantation. Cancer. 2018;124(11):2438–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z, Mewawalla P, Stratton P, et al. Sexual health in hematopoietic stem cell transplant recipients. Cancer. 2015;121(23):4124–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenberg AR, Syrjala KL, Martin PJ, et al. Resilience, health, and quality of life among long-term survivors of hematopoietic cell transplantation. Cancer. 2015;121(23):4250–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brotto LA, Yule M, Breckon E. Psychological interventions for the sexual sequelae of cancer: a review of the literature. J Cancer Surviv. 2010;4(4):346–360. [DOI] [PubMed] [Google Scholar]
- 45.Carter J, Lacchetti C, Andersen BL, et al. Interventions to Address Sexual Problems in People With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Adaptation of Cancer Care Ontario Guideline. J Clin Oncol. 2018;36(5):492–511. [DOI] [PubMed] [Google Scholar]
- 46.Sanft T, Denlinger CS, Armenian S, et al. NCCN Guidelines Insights: Survivorship, Version 2.2019. J Natl Compr Canc Netw. 2019;17(7):784–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eeltink CM, Incrocci L, Leeuw IMV, Zweegman S. Recommended patient information sheet on the impact of haematopoietic cell transplantation on sexual functioning and sexuality. Ecancermedicalscience. 2019;13:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamilton BK, Goje O, Savani BN, Majhail NS, Stratton P. Clinical management of genital chronic GvHD. Bone Marrow Transplant. 2017;52(6):803–810. [DOI] [PubMed] [Google Scholar]
- 49.Kornik RI, Rustagi AS. Vulvovaginal Graft-Versus-Host Disease. Obstet Gynecol Clin North Am. 2017;44(3):475–492. [DOI] [PubMed] [Google Scholar]
- 50.Barbera L, Zwaal C, Elterman D, et al. Interventions to address sexual problems in people with cancer. Curr Oncol. 2017;24(3):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arthur EK, Wills CE, Menon U. A Systematic Review of Interventions for Sexual Well-Being in Women With Gynecologic, Anal, or Rectal Cancer. Oncol Nurs Forum. 2018;45(4):469–482. [DOI] [PubMed] [Google Scholar]
- 52.Kang HS, Kim HK, Park SM, Kim JH. Online-based interventions for sexual health among individuals with cancer: a systematic review. BMC Health Serv Res. 2018;18(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langer SL, Yi JC, Storer BE, Syrjala KL. Marital adjustment, satisfaction and dissolution among hematopoietic stem cell transplant patients and spouses: a prospective, five-year longitudinal investigation. Psychooncology. 2010;19(2):190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burns LJ, Abbetti B, Arnold SD, et al. Engaging Patients in Setting a Patient-Centered Outcomes Research Agenda in Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2018;24(6):1111–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eeltink CM, Incrocci L, Leeuw IMV-d, Zweegman S. Recommended patient information sheet on the impact of haematopoietic cell transplantation on sexual functioning and sexuality. Ecancermedicalscience. 2019;13:987–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kornik RI, Rustagi AS. Vulvovaginal Graft-Versus-Host Disease . Obstetrics and Gynecology Clinics of North America. 2017;44(3):475–492. [DOI] [PubMed] [Google Scholar]
- 57.Murphy J, McKenna M, Abdelazim S, Battiwalla M, Stratton P. A Practical Guide to Gynecologic and Reproductive Health in Women Undergoing Hematopoietic Stem Cell Transplant. Biology of Blood and Marrow Transplantation. 2019;25(11):e331–e343. [DOI] [PubMed] [Google Scholar]
- 58.Hirsch P, Leclerc M, Rybojad M, et al. Female genital chronic graft-versus-host disease: importance of early diagnosis to avoid severe complications. Transplantation. 2012;93(12):1265–1269. [DOI] [PubMed] [Google Scholar]
- 59.Carter J, Lacchetti C, Andersen BL, et al. Interventions to Address Sexual Problems in People With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Adaptation of Cancer Care Ontario Guideline. Journal of Clinical Oncology. 2017;36(5):492–511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.