Abstract
In this meta-analysis, 17 rumen epithelial 16S rRNA gene Illumina MiSeq amplicon sequencing data sets were analyzed to identify a core rumen epithelial microbiota and core rumen epithelial OTUs shared between the different studies included. Sequences were quality-filtered and screened for chimeric sequences before performing closed-reference 97% OTU clustering, and de novo 97% OTU clustering. Closed-reference OTU clustering identified the core rumen epithelial OTUs, defined as any OTU present in ≥ 80% of the samples, while the de novo data was randomly subsampled to 10,000 reads per sample to generate phylum- and genus-level distributions and beta diversity metrics. 57 core rumen epithelial OTUs were identified including metabolically important taxa such as Ruminococcus, Butyrivibrio, and other Lachnospiraceae, as well as sulfate-reducing bacteria Desulfobulbus and Desulfovibrio. Two Betaproteobacteria OTUs (Neisseriaceae and Burkholderiaceae) were core rumen epithelial OTUs, in contrast to rumen content where previous literature indicates they are rarely found. Two core OTUs were identified as the methanogenic archaea Methanobrevibacter and Methanomethylophilaceae. These core OTUs are consistently present across the many variables between studies which include different host species, geographic region, diet, age, farm management practice, time of year, hypervariable region sequenced, and more. When considering only cattle samples, the number of core rumen epithelial OTUs expands to 147, highlighting the increased similarity within host species despite geographical location and other variables. De novo OTU clustering revealed highly similar rumen epithelial communities, predominated by Firmicutes, Bacteroidetes, and Proteobacteria at the phylum level which comprised 79.7% of subsampled sequences. The 15 most abundant genera represented an average of 54.5% of sequences in each individual study. These abundant taxa broadly overlap with the core rumen epithelial OTUs, with the exception of Prevotellaceae which were abundant, but not identified within the core OTUs. Our results describe the core and abundant bacteria found in the rumen epithelial environment and will serve as a basis to better understand the composition and function of rumen epithelial communities.
Keywords: rumen wall, rumen epithelium, epimural, microbiota, ruminant
Introduction
Domesticated ruminants such as cattle (Bos taurus), sheep (Ovis aries), goats (Capra aegagrus), and yaks (Bos grunniens) form an important segment of agriculture around the world with over 3.5 billion domesticated ruminants providing a source of high-quality animal protein in the form of meat and dairy products (Cammack et al., 2018). Non-domesticated ruminants also fill essential ecological niches as primary consumers. Ruminants are distinct from monogastric animals in their ability to degrade cellulose and hemicellulose during feed digestion in the rumen, the first of four digestive chambers in the ruminant gastrointestinal tract system (Paz et al., 2018). Digestion of plant material in the rumen is primarily conducted by a complex and diverse community of bacteria, fungi, protozoa, and archaea, and understanding these communities may provide insight into the metabolic processes essential for host animal health, resilience under stress conditions, and feed efficiency.
The rumen microbiota has been classified into three major groups: microorganisms attached to the solid plant material fraction of the rumen contents, microorganisms free-floating in the liquid fraction of the rumen contents, and microorganisms attached to the rumen wall, which is also called rumen epithelium. The rumen wall bacteria are also referred to as epimural bacteria (Mead and Jones, 1981). Many studies have contributed to identifying the solid and liquid fractions of the rumen content microbiota, as well as functional characterization of rumen content microbiomes (Henderson et al., 2015; Mizrahi and Jami, 2018). However, substantially fewer studies have targeted the rumen epithelial microbiota. It has been known for decades that almost the entire rumen epithelium is covered by microorganisms (McCowan et al., 1978, 1980; Cheng et al., 1979; Wallace et al., 1979; Dinsdale et al., 1980; Mead and Jones, 1981; Hill, 1982; Mueller et al., 1984; Rieu et al., 1990). Previous work suggests that these communities differ in composition from the solid and liquid associated microbial communities of the rumen content (Wallace et al., 1979; Sadet et al., 2007; Mao et al., 2015; Holman and Gzyl, 2019). A common concept in microbial ecology is the core microbiome or microbiota, generally defined as OTUs or taxa which are present in the majority of samples from a given environment. These core microbiota taxa are hypothesized to fill important niches and functions within the microbiome (Shade and Handelsman, 2012; Astudillo-García et al., 2017). Previous analyses based on rumen content samples from cattle (Henderson et al., 2015; Holman and Gzyl, 2019) have contributed to establishing a core rumen content microbiota. This study provides the first step to identify the core rumen epithelial microbiota.
A number of studies have been conducted over the last 10 years to investigate the composition of the rumen epithelial microbiota (Sadet-Bourgeteau et al., 2010; Chen et al., 2011; Li M. et al., 2012; Li R. W. et al., 2012; Petri et al., 2013; Liu et al., 2015, 2016; Wetzels et al., 2015; McCann et al., 2016; De Mulder et al., 2017; Schären et al., 2017; Sbardellati et al., 2020) leading to a considerable increase of our knowledge about the composition of rumen epithelial microbial communities in different ruminants. However, the knowledge about functional properties of rumen epithelial microbial communities is still highly limited, with a few notable exceptions (Wallace et al., 1979; Jin et al., 2016; Mann et al., 2018). Because rumen epithelial microorganisms are directly associated with host tissue, it is likely that they play an integral role in ruminant metabolism and host-microbe interactions, including nutrient exchange across the rumen epithelium (Cheng et al., 1979).
Nowadays, amplicon sequencing using the Illumina MiSeq sequencing platform is a commonly used method to analyze microbial community composition by amplifying and sequencing selected hypervariable regions of the 16S rRNA gene (Goodrich et al., 2014; Knight et al., 2018). For analysis of amplicon sequencing data from different studies, closed-reference OTU clustering and de novo OTU clustering approaches can be used, and each provides distinct advantages and disadvantages. De novo OTU clustering allows for identification of novel diversity because it does not reject sequences with low similarity to sequences in the reference database. However, it will not be able to cluster reads across multiple hypervariable regions of the 16S rRNA gene into the same OTU, and is therefore unable to meaningfully compare between studies at the OTU level of specificity across a varied dataset such as the one presented in this analysis (Goodrich et al., 2014) and is primarily used in our study at the phylum and genus levels. Closed-reference OTU clustering can cluster sequences from multiple hypervariable regions of the 16S rRNA gene into the same OTU if each sequence maps to the same full-length reference sequence. This allows for meaningful comparison between OTUs across studies which sequenced different hypervariable regions of the 16S rRNA gene as long as the same reference database is used (Callahan et al., 2017). However, novel sequences may be excluded from closed-reference clustering datasets. This study performed both closed-reference and de novo OTU clustering, and provides results from each for different analyses, mitigating the disadvantages while maintaining the advantages of each clustering approach. We aimed to identify a core rumen epithelial microbiota by creating a unified data set of publicly available 16S rRNA gene amplicon sequencing data generated using Illumina MiSeq sequencing technology of rumen epithelial samples from a broad set of studies. In total, 17 datasets that met our inclusion criteria regarding read depth and quality were downloaded from the NCBI SRA and the ENA repositories. Here, we provide the first in-depth meta-analysis of the rumen epithelial bacterial microbiota providing evidence for the presence of abundant rumen epithelial microbial phylotypes independent of the differences between the studies included.
Materials and Methods
Data Collection
We performed a literature search for studies with published 16S rRNA gene amplicon sequencing data from the epithelial fraction of the rumen as of November 2019. We identified 17 studies comprising 342 rumen epithelial samples that met our criteria for inclusion (Illumina Miseq sequencing, and a minimum read depth of 10,000 sequences after quality control on a per-sample basis) available through NCBI SRA and the European Nucleotide Archive and downloaded these samples via the NCBI sra-toolkit. A list of all studies and samples included in this analysis is available in Supplementary Table 1.
16S rRNA Gene Amplicon Sequence Preprocessing and Quality Control
After data collection, all samples were pooled by file type (assembled contigs or separated paired-end reads). Contigs were stringently quality filtered (Q average of 35 or greater over the full sequence) using mothur v1.43.0 (Schloss et al., 2009). Paired-end reads were merged and quality filtered using the “make.contigs” command in mothur (Schloss et al., 2009). Subsequently, all samples were pooled and filtered using a minimum read length of 250 bp, a zero ambiguities threshold, and a maximum homopolymer length of eight bases. Possible chimeric sequences were removed using the “chimera.vsearch” command in mothur using the SILVA.gold reference database provided by the mothur website. This initial quality filtered data set of 19.3 million high quality sequence reads was aligned against the SILVA SSU database release 132 (Quast et al., 2012) before closed-reference and de novo OTU clustering using a 97% similarity threshold in QIIME2 (Bolyen et al., 2019) and mothur (Schloss et al., 2009), respectively.
Closed-Reference OTU Clustering and Generation of Shared OTU Tables
Closed-reference OTU clustering was performed to enable clustering of sequences deriving from different hypervariable regions of the 16S rRNA gene into the same OTU by clustering to the same full-length 16S rRNA gene sequence in the reference database. Datasets included in this study targeted different hypervariable regions of the 16S rRNA, including V3-V5, V3-V4, V4, and V2-V3, thus necessitating this technique for identifying OTUs shared between datasets. More information about the hypervariable regions used in each dataset is available in Supplementary Table 1. Closed-reference OTU clustering was performed with a 97% similarity threshold to the reference sequences in the SILVA SSU 132 release reference database using QIIME2’s (Bolyen et al., 2019) implementation of VSEARCH (Rognes et al., 2016). OTU information and representative sequences were then exported to mothur for taxonomic classification with the SILVA SSU 132 release. This was done to ensure that the classification process was uniform between the closed-reference and de novo OTU clustering-based analyses.
Closed-reference clustered OTU data was exported for analysis with Phyloseq v1.32 (McMurdie and Holmes, 2013) in R 3.6.2 (R Core Team, 2020). Beta diversity between samples was visualized using principle coordinates analysis (PCoA) based on Bray-Curtis distances. Samples were not subsampled to a uniform sequencing depth due to the presence of multiple samples with low read depth after closed-reference clustering (fewer than 5,000 reads).
The percentage of samples in which each OTU was detected was then calculated, resulting in a list of 57 OTUs that were found in ≥ 80% of samples, which we considered to be core rumen epithelial OTUs. An additional 90 OTUs were present in ≥ 80% of cattle samples. The threshold of 80% was chosen to account for the high degree of variability in our dataset, allowing us to capture more biologically relevant signal. A higher threshold such as the 90% threshold used in the meta-analysis in Holman and Gzyl (2019) would result in substantially fewer core rumen epithelial OTUs but might exclude potentially important taxa. Further information is available in Supplementary Tables 2, 3. A heat map was generated for visualization of these percentages using the ggplot2 v3.2.1 package (Wickham, 2016) in R using a color palette from the viridis package v0.5.1 (Garnier, 2018).
De novo OTU Clustering and Generation of Community Diversity and Abundance Metrics
De novo OTU clustering, in contrast to closed reference OTU clustering, allows for inclusion of sequences with less than 97% similarity to sequences in the reference database, allowing novel diversity to be maintained within the dataset. This is particularly important when working with samples derived from environments containing taxa without high-quality reference sequences. For this reason, de novo OTU clustering was also included in this study to generate beta diversity metrics and other whole community measures. Each sample was randomly subsampled to 10,000 reads after quality control using the “sub.sample” command in mothur to reduce bias toward studies with higher sequencing depths. De novo OTUs were clustered at 97% similarity using the mothur v1.43.0 “cluster.split” command. OTUs were taxonomically classified using the SILVA SSU 132 release as a reference in mothur. Representative sequences from the 50 most abundant OTUs were queried with NCBI BLAST against the NCBI rRNA/ITS database. The representative sequences were also compared to our rumen genome collection (RGC) a curated database of publicly available rumen derived genomes from whole genome sequencing of organisms in the Hungate 1000 collection (Seshadri et al., 2018) and high quality metagenome assembled genomes (MAGs) from Svartström et al. (2017) and Stewart et al. (2019) using BLAST + (Camacho et al., 2009). A threshold of ≥ 90% nucleotide identity and E value cutoff of < 10–3 was used in both cases. The results of these comparisons are presented in Supplementary Table 4.
OTU data was exported to Phyloseq v1.32 in R v3.6.2. Beta diversity between samples was visualized using PCoA based on Bray-Curtis distances. Bray-Curtis distances were also used to quantitatively compare communities by study and by animal type using permutational multivariate analysis of variance (PERMANOVA) and permutational multivariate analysis of dispersion (PERMDISP2) with the “adonis” and “betadisper” commands of the R package vegan v2.5.6 (Jari Oksanen et al., 2019), the results of which are available in Supplementary Table 5. The tax_glom function of Phyloseq was used to aggregate abundance data by phyla and by genera to generate relative abundance plots of the 10 most abundant phyla and 15 most abundant genera across all samples, which were plotted by averaging abundances by study and by animal. All figures were generated with the ggplot2 package v3.2.1 (Wickham, 2016) using color palettes from the viridis package v0.5.1 (Garnier, 2018).
Results
Closed-Reference OTU Clustering
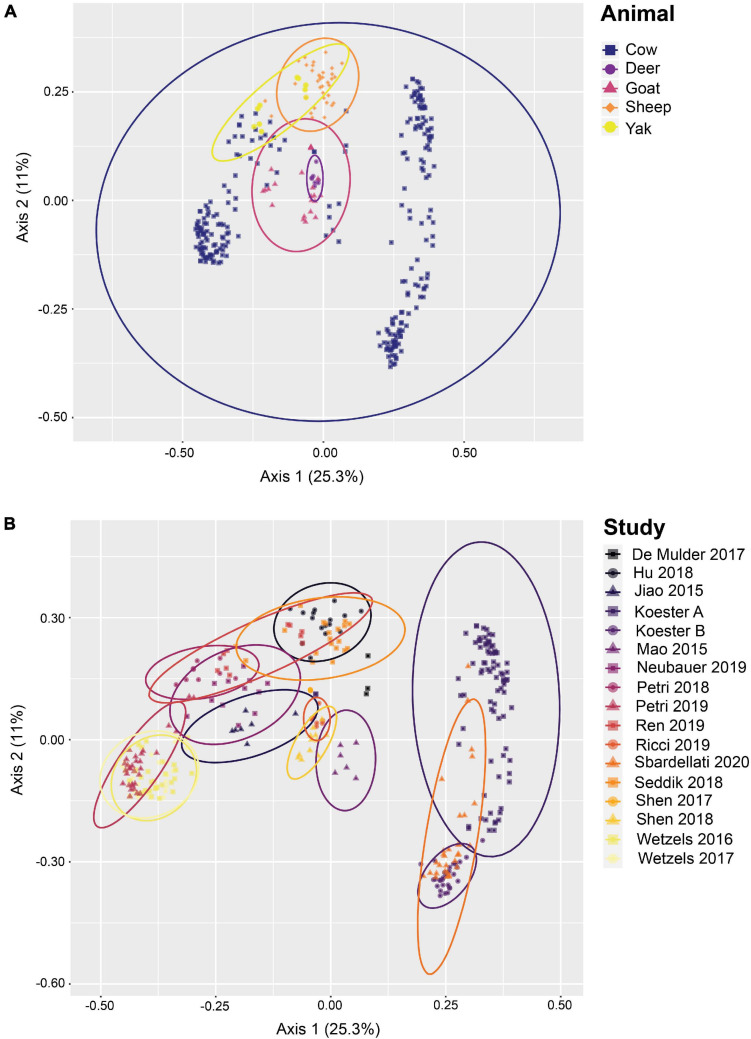
The 342 samples from 17 different studies contained 19,254,761 high-quality sequences after removal of low quality and potentially chimeric sequences. After closed-reference OTU clustering, 21,850 OTUs representing 14,563,483 sequences were used for downstream analysis. The average number of high-quality sequences remaining per sample was 42,583 sequences, with a standard deviation of 31,732 sequences. 97.7% of all sequence reads were classified as bacterial, 2.1% of the sequence reads as archaeal, and 0.2% as unknown. The 21,508 closed-reference OTUs were classified into 53 phyla, with Firmicutes, Proteobacteria, and Bacteroidetes as the three most abundant phyla at 41.8, 20.0, and 19.6% relative abundance, respectively, across the entire data set. Altogether, these three phyla accounted for 81.4% of all sequences. The most abundant genera were Campylobacter (8.4%), Prevotella (6.0%), Christensenellaceae R-7 group (5.8%), Butyrivibrio (5.4%), and an uncultured genus of the Neisseriaceae (3.7%). PCoA of all samples (Figure 1) provides evidence of rumen epithelial community clustering by study as well as animal.
FIGURE 1.
Beta diversity of rumen epithelial microbial communities revealed by principle coordinates analysis based on Bray-Curtis distances after closed-reference OTU clustering. (A) Samples highlighted by animal, (B) shows samples highlighted by study. See Supplementary Table 1 for more details on the studies.
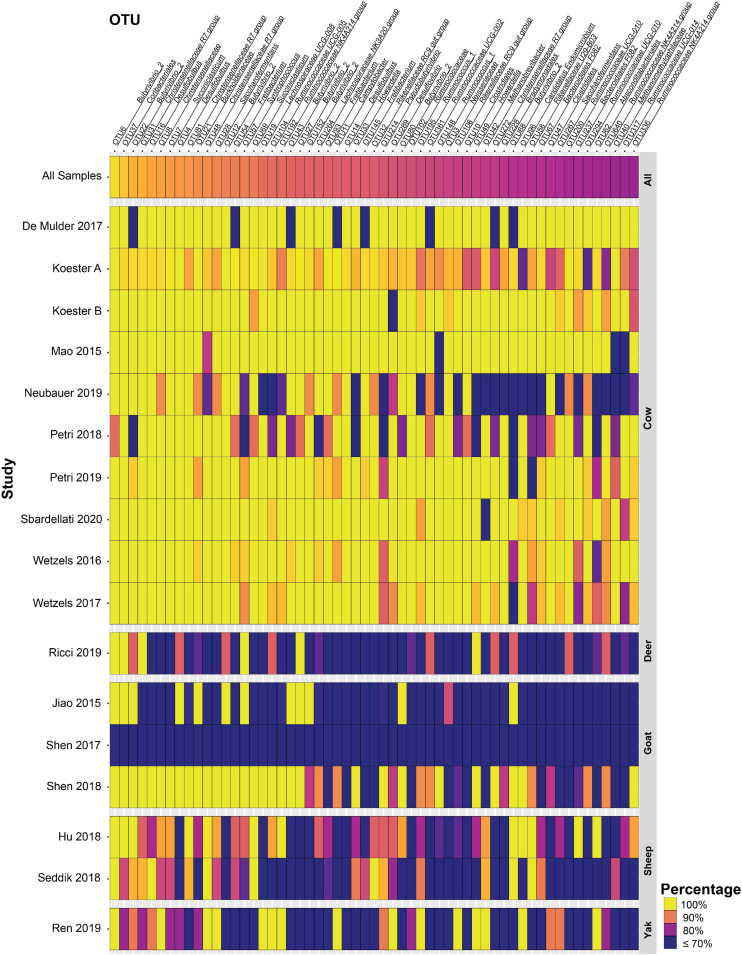
In total, 57 and 16 OTUs were found in 80 or 90% or more of all samples after closed-reference clustering, representing the core rumen epithelial OTUs which are shared between the different studies. These core rumen epithelial OTUs are shown as a heatmap in Figure 2. OTUs 6 and 22 (both classified as Butyrivibrio) were present in 98.0 and 94.7% of the samples, respectively. Six other Butyrivibrio OTUs (OTUs 12, 152, 27, 43, 80, and 68) were also present in greater the 80% of all samples. Additional core rumen epithelial OTUs in the Lachnospiraceae family include Pseudobutyrivibrio (OTU 214), Syntrophococcus (OTU 57), Howardella (OTUs 33 and 49), Lachnospiraceae NK3A20 group (OTU 264), and Lachnospiraceae UCG-008 group (OTU 19). Other members of the core rumen epithelial OTUs included OTUs classified as Christensenellaceae R-7 group (OTUs 31, 48, 81, and 272), Christensenellaceae unclassified (OTU 8), and the order Coriobacteriales (OTU37). Several core OTUs were identified as Ruminococcaceae, including Ruminococcus (OTUs 148 and 195), Ruminococcaceae NK4A214 group (OTUs 192, 640, and 336), UCG-002 group (OTU 361), UCG-005 group (OTU 104), UCG-010 group (OTUs 200 and 294), and UCG-014 group (OTU 317), Ruminococcaceae unclassified (OTU 102), and Saccharofermentans (OTUs 28 and 267). Additional core OTUs were identified as Burkholderiaceae (OTU 21), Neisseriaceae (OTU 3), Rikenellaceae RC9 gut group (OTUs 32 and 106), Desulfobulbus (OTUs 4, 14, and 16), Desulfovibrio (OTU 289), Succiniclasticum (OTUs 7 and 69), Campylobacter (OTU 11), Fretibacterium (OTUs 54, 93, and 145), Bradymondales (OTU 228), and Endomicrobiaceae (OTU 96). Two core OTUs were identified as archaea, Methanobrevibacter (OTU 42) and Methanomethylophilaceae (OTU 40). When considering only samples from cattle, a total of 147 OTUs were found in ≥ 80% of samples, and 63 OTUs were present in ≥ 90% of samples (Supplementary Table 3). Of these 147 OTUs, the 90 that were unique to the cattle only core OTUs are presented as a heat map in Figure 3.
FIGURE 2.
Heat map of 57 core rumen epithelial OTUs (OTUs present in ≥ 80% of samples after closed-reference clustering) showing OTUs shared between different studies. The color represents the percentage of samples in a study that contain the respective OTU. The percentage across all samples is represented at the top, and studies are sorted by animal. Values less than 70% have been truncated to 70% for greater resolution of high values. See Supplementary Table 1 for more details on the studies.
FIGURE 3.
Heat map of the 90 additional core rumen epithelial OTUs identified considering only cattle samples. Only OTUs present in ≥ 80% of cattle samples after closed-reference clustering and not included in the 57 core OTUs shared among all studies presented in Figure 2 are shown. The color represents the percentage of samples in a cattle study that contain the respective OTU. The percentage across all cattle samples is represented at the top. Values less than 70% have been truncated to 70% for greater resolution of the high values. See Supplementary Table 3 for more information about the cattle core rumen epithelial OTUs.
De novo OTU Clustering
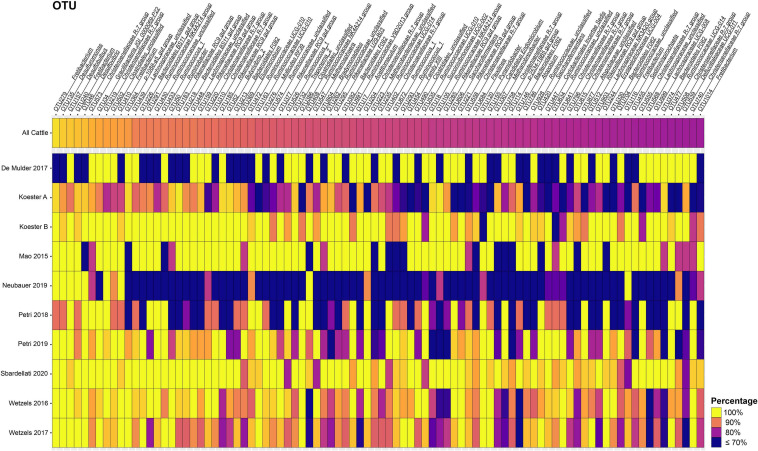
De novo OTU clustering allows the inclusion of novel diversity and is therefore better suited to generating alpha and beta diversity metrics than closed-reference OTU clustering, as well as measuring the relative abundance of taxa across samples from multiple studies. Thus, in addition to closed-reference clustering, de novo OTU clustering of the high-quality reads using a 97% similarity threshold was performed after randomly subsampling each sample to a read depth of 10,000, resulting in a total of 71,929 OTUs comprising 3,420,000 reads. 97.4% of all reads were bacterial, 1.9%, of the reads were archaeal, and 0.7% of the reads were classified as unknown. PCoA plots (Figure 4) based on Bray-Curtis distances provided evidence of community clustering by study as well as animal species, as seen after closed-reference OTU clustering. Additionally, the PCoA plots reveal a geographical split between cattle centroids.
FIGURE 4.
Beta diversity of rumen epithelial microbial communities revealed by principle coordinates analysis based on Bray-Curtis distances after de novo OTU clustering. (A) Samples highlighted by animal, (B) shows samples highlighted by study. See Supplementary Table 1 for more details on the studies.
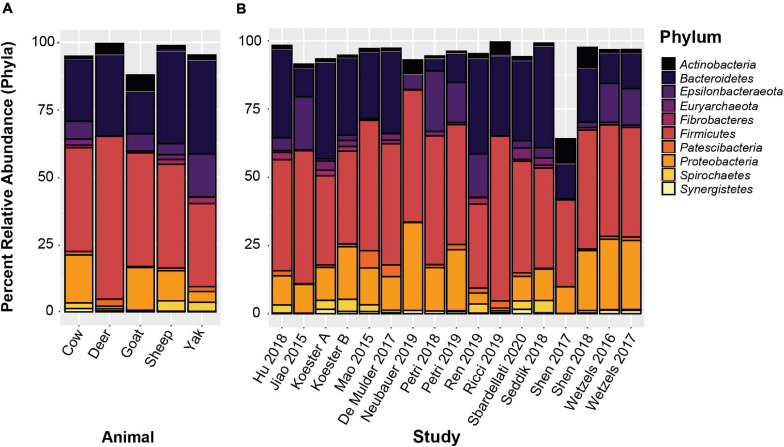
De novo clustered OTUs were classified into 53 phyla similar to the closed-reference clustered OTUs. The three most abundant phyla were Firmicutes, Bacteroidetes, and Proteobacteria at 39.1, 24.5, and 16.14% relative abundance across the entire data set, respectively. These three phyla represented 80.3% of all reads, which was highly similar to the results obtained from the closed-reference OTU clustering. Less abundant phyla with a relative abundance greater than 1% were Epsilonbacteraeota (6.4%), Spirochaetes (2.2%), Euryarchaeota (1.9%), Actinobacteria (1.4%), Patescibacteria (1.2%), Fibrobacteres (1.0%), and Synergistetes (1.0%). The 10 phyla with the highest relative abundance across all samples are displayed averaged by animal and by study in Figure 5. While variation between relative abundances is present across both animal species and study; overall, the composition of the rumen epithelial microbiota is largely similar, with Firmicutes, Bacteroidetes and Proteobacteria remaining the predominant phyla across most studies. Neubauer et al. (2019), a cattle dataset, had a lower abundance of Bacteroidetes, while Ren et al. (2019) and Ricci et al. (2019), the sole yak and deer datasets, respectively, display a lower abundance of Proteobacteria.
FIGURE 5.
The 10 most abundant phyla based on de novo OTU clustering. (A) Displays the relative abundances averaged by host animal, (B) displays relative abundances averaged by study.
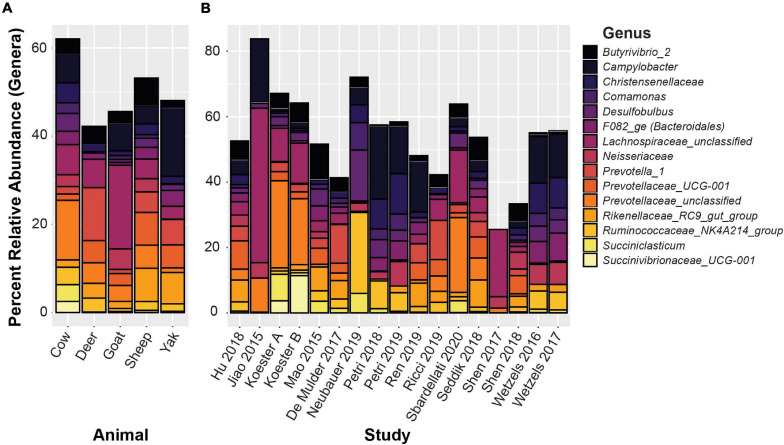
De novo clustered OTUs were classified into 1,297 unique genera. The five most abundant genus-level classifications were Prevotellaceae (unclassified) (11.4%), Lachnospiraceae (unclassified) (7.3%), Campylobacter (6.4%), Christensenellaceae R-7 group (3.9%), and Butyrvibrio_2 (3.6%). With the exception of unclassified Lachnospiraceae, these genera were also highly abundant in the closed-reference OTU clustering. The 15 genera with the highest relative abundance are displayed in Figure 6, representing 59.0% of the total relative abundance across all samples. In addition to the genera mentioned above, these include Desulfobulbus (3.4%), Ruminococcaceae NK4A214 group (3.4%), Succiniclasticum (3.0%), Bacteroidales family F082 (2.8%) Neisseriaceae (2.7%), Rikenellaceae RC9 gut group (2.5%), Comamonas (2.0%), and Succinivibrionaceae UCG-001 (1.9%). Two additional Prevotella and Prevotellaceae phylotypes were also present, and the total relative abundance of Prevotellaceae within the top 15 genera was 16.0%. The 50 most abundant OTUs with taxonomic information from NCBI Blast and the RGC appended are displayed in Supplementary Table 4. Not surprisingly, more variability was found in the genus-level data compared to the phylum-level analyses. Nevertheless, these 15 most abundant genera represented between 25.5 and 83.8% of all sequences in each of the studies.
FIGURE 6.
The 15 most abundant genera based on de novo OTU clustering. (A) Displays relative abundances averaged by host animal, (B) displays relative abundance averaged by study.
Discussion
Core Rumen Epithelial OTUs Revealed by Closed-Reference OTU Clustering
Here we perform the first large-scale meta-analysis of rumen epithelial bacterial communities. Our results provide deep insights into common and abundant bacterial phylotypes of the rumen epithelium that are commonly found in spite of differences between individual studies. Closed-reference OTU clustering enabled the creation of a set of 57 core rumen epithelial OTUs found in ≥ 80% of all 342 samples. Given the broad range of host species, diets, farm management practices, geographical locations, hypervariable regions sequenced, and other variables between studies, these core OTUs can be inferred to be remarkably consistent members of the rumen epithelial microbiota. Sixteen core rumen epithelial OTUs were present in ≥ 90% of samples, the threshold used in Holman and Gzyl (2019). Additional information can be found in Supplementary Table 2. When considering only cattle samples a total of 147 OTUs are present in ≥ 80% of samples and 63 OTUs are present in ≥ 90% of samples (Figure 3 and Supplementary Table 3), which suggests that the core rumen epithelial microbiome is larger when considering an individual host species. The previous meta-analysis of the bovine gastrointestinal tract microbiota presented in Holman and Gzyl (2019) likewise found that certain taxa were consistently found across multiple studies and different GI tract locations. Several of the groups identified as core rumen epithelial OTUs have also been identified in their previous meta-analysis of rumen contents. The analysis by Holman and Gzyl (2019) identified four bacterial genera present in ≥ 90% of all 2,662 rumen content samples included in their study, including Prevotella, Ruminococcus, Lachnospiraceae UCG-008, and Eubacterium coprostanoligenes group. Several Lachnospiraceae core rumen epithelial OTUs were identified in our study, including one Lachnospiraceae UCG-008 OTU shared by 84.9% of samples. We also identified several Ruminococcaceae core OTUs and two Ruminococcus OTUs shared by 81.8 and 80.7% of samples. No core OTUs were identified as either Prevotella or Eubacterium coprostanoligenes. This suggests that, while there are differences in the core rumen content and core rumen epithelial microbiota, there is also overlap between them.
Eight core rumen epithelial OTUs, including the two OTUs found in the highest number of samples (OTUs 6 and 22), were classified as Butyrivibrio, a genus of Lachnospiraceae known to produce butyrate, which aids the host in forming tight junctions in the epithelial layer (Peng et al., 2009), as well as providing an energy source. Butyrivibrio rumen isolates have been shown to encode a large number of carbohydrate-active enzymes and to be able to degrade and grow on complex plant carbohydrates such as hemicellulose, pectins, mannans, and starch, suggesting they play a role in fiber degradation in the rumen environment (Palevich et al., 2019). Several additional core rumen epithelial OTUs were classified to Lachnospiraceae including to the genera Syntrophococcus (OTU 57), Howardella (OTUs 33 and 49), Pseudobutyrivibrio (OTU 214), Lachnospiraceae_UCG-008 (OTU 19), and Lachnospiraceae NK3A20 group (OTU 264). A quarter of all core rumen epithelial OTUs identified in this study were in the family Lachnospiraceae, highlighting their ubiquity in the rumen epithelial community. While Lachnospiraceae are commonly found in the GI tract of many animals (including humans) (Sorbara et al., 2020), and in many GI tract segments, they are notable as one of few bacterial taxa in which cellulases are commonly found in the rumen (Moraïs and Mizrahi, 2019). Twelve core rumen epithelial OTUs were classified as Ruminococcaceae, including OTUs 192, 336, and 640 which was classified as Ruminococcaceae NK4A214 group, a phylotype that has been previously identified to be present in 50% of all bovine GI tract samples (Holman and Gzyl, 2019). Functional characterization of this group in the future may aid in a better understanding of its role in ruminant metabolism as core rumen epithelial and core bovine GI tract taxa. Two Ruminococcaceae core OTUs (OTUs 148 and 195) were classified as Ruminococcus, members of which have exhibited cellulose-degrading capabilities alongside utilization of many other polysaccharide substrates. Some Ruminococcus are capable of crystalline cellulose deconstruction and express GH48 family exoglucanases which were identified as the most highly expressed cellulase (Moraïs and Mizrahi, 2019). Five core rumen epithelial OTUs (OTUs 8, 31, 48, 81, and 272) were classified as members of the Christensenellaceae family, with OTUs 31, 48, 81, and 272 further classified as Christensenellaceae R-7 group. This group was found to be associated with bovine rumen samples more frequently than fecal samples in the previous bovine GI tract meta-analysis (Holman and Gzyl, 2019). The type strain for this group is Christensenella minuta, isolated from human fecal samples which demonstrated butyrate and acetate production (Morotomi et al., 2012). One core rumen epithelial OTU (OTU 37) was classified as a member of the Coriobacteriales, an order of commensal bacteria with broad saccharolytic capabilities which produce lactic, and acetic acid as well as ethanol and hydrogen (Gupta et al., 2013, 2017).
Three core rumen epithelial OTUs (OTUs 4, 14, and 16) were classified as Desulfobulbus, a genus capable of reducing sulfate to hydrogen sulfide, which can cause inflammation in the GI tract. Expression of the dsrAB genes responsible for sulfate reduction has been shown for Desulfobulbus in a recent metatranscriptome sequencing study of rumen epithelial samples (Mann et al., 2018), suggesting that sulfate-reducing bacteria actively reduce sulfate at the rumen epithelium. The related sulfate-reducer Desulfovibrio has been isolated from the rumen of sheep (Howard and Hungate, 1976). Two core rumen epithelial OTUs (OTUs 3 and 21) were classified as taxa of the Betaproteobacteria, Neisseriaceae, and Burkholderiaceae, respectively. Little is known about the role these Betaproteobacteria play in the rumen. Burkholderiales and unclassified Betaproteobacteria have previously been found to have a positive correlation with Isoflavone-enriched feed in Holstein cows (Kasparovska et al., 2016), while Neisseria was positively associated with urea supplementation in the absence of urea hydrolysis inhibitors in a simulated rumen fermentation environment (Jin et al., 2016). However, in both cases, the mechanism which drives the association is unknown. The genus Burkholderia contains B. pseudomallei and B. mallei which are zoonotic pathogens that cause meliodosis and glanders/farcy, respectively. Meliodosis has been confirmed in ruminants (Limmathurotsakul et al., 2012). Neisseriaceae-like phylotypes were found to be highly abundant and active on the rumen epithelium in a metatranscriptome sequencing study, which revealed high expression of key genes involved in nitrogen metabolism (Mann et al., 2018). One core rumen epithelial OTU (OTU 11) was classified as Campylobacter, a genus which can cause infertility and abortion in cattle (Hoffer, 1981). However, it is unclear whether its presence in the rumen can lead to disease, as it is ubiquitously present in the rumen epithelial community. Rumen epithelial Campylobacter phylotypes have been shown to display high expression levels of genes in involved in nitrogen metabolism and in oxidative stress response (Mann et al., 2018). Two core rumen epithelial OTUs (OTUs 32 and 106) were classified as Rikenellaceae RC9 gut group. Little is known about this group and their role in ruminant metabolism, but two genera of the Rikenellaceae family, Rikenella and Alistipes, are known to produce VFAs, including acetate, propionate, and succinate (Graf, 2014). OTU 228 is a core rumen epithelial OTU identified as a member of the Bradymonadales, an order of bacteria about which relatively little is known, save for the fact that they prey on other bacteria and appear to preferentially prey on Bacteroidetes and Proteobacteria (Mu et al., 2020), two of the most abundant phyla in the rumen epithelial environment. OTU 96 is a core rumen epithelial OTU identified as a member of the Endomicrobiaceae family, a clade which has a tight association as intracellular symbionts of flagellate and ciliate protozoa in termite and cockroach gut environments as well as the rumen microbiota. The protozoa are associated with cellulose degradation in termites, but the function of Endomicrobiaceae in the rumen is not well characterized (Zheng et al., 2016; Levy and Jami, 2018). OTUs 42 and 40 are archaeal core rumen epithelial OTUs identified as Methanobrevibacter and Methanomethylophilaceae, respectively. Methanobrevibacter is known to contribute to methane production and is both free living and an endosymbiont of protozoa in the rumen (Levy and Jami, 2018). Little is known about the Methanomethylophilaceae family at present, but methylotrophic members have been associated with reduced methane emissions from the rumen (Poulsen et al., 2013). The occurrence of an additional 90 cattle-only core rumen epithelial OTUs (Figure 3) highlights the increased similarity between samples derived from specific species as compared to the broader rumen epithelial community surveyed here. The occurrence of a species-specific epithelial core microbiota may have important implications for understanding each host environment, and as additional data becomes available studies focusing on the core rumen epithelial microbiota of a single host species will be highly valuable in addition to broader approaches.
Average Relative Abundance of Taxa in Rumen Epithelial Communities Revealed by de novo OTU Clustering
In addition to establishing a set of core rumen epithelial OTUs, the other goal of this study was to characterize the most abundant taxa observed in rumen epithelial communities across all studies. The data included in this study reflects the available published rumen epithelial 16S rRNA amplicon sequencing datasets in the literature, which are primarily from cattle. Beta diversity of samples using Bray-Curtis distances (displayed as PCoA in Figure 4) suggests that while samples cluster strongly by study, as seen in previous meta-analyses (Henderson et al., 2015; Holman et al., 2017; Holman and Gzyl, 2019), clustering by animal is also a factor. PERMANOVA and PERMADISP2 results testing these variables are presented in Supplementary Table 5. Two distinct centroids are apparent when examining the distribution of cattle samples, which appear separated in large part by geographic difference. The cluster on the left consists of samples from the United States while the cluster on the right consists of samples from Austria, suggesting that geography may be a factor that contributes the composition of rumen epithelial microbial communities (see Supplementary Table 1). Differences that appear to follow geographic patterns may be driven by climate factors, diet, host lineage, and farm management practices which differ between countries and individual farms, as noted in a previous large-scale analysis of microbial communities in rumen content samples (Henderson et al., 2015).
However, the results of our analysis indicate that, at the phylum level, community makeup between cattle, goats, and sheep are highly similar, and dominated by Bacteroidetes, Firmicutes, and Proteobacteria, which together made up 79.7% of subsampled sequence reads from all included studies. Proteobacteria were substantially less abundant in yak and deer. However, each were represented here by a single dataset only. Epsilonbacteraeota (6.4% of all reads) showed extremely low abundance in deer samples at 0.001% relative abundance (Figure 5). Diversity in community composition between studies increases when observing communities at the genus (or lowest available taxonomic) level (Figure 6). However, the top 15 genera represent 59.0% of all subsampled sequence reads and averaged 54.5% of sequences in each individual study. This is a remarkable level of similarity between rumen epithelial communities across this highly varied dataset, and these abundant taxa overlap substantially with the core rumen epithelial OTUs identified by closed reference clustering. This overlap includes the taxa Campylobacter, Christensenellaceae R7 group, Neisseriaceae, Butyrivibrio, Desulfobulbus, Ruminococcaceae NK4A214 group, Succiniclasticum, and Rikenellaceae RC9 gut group, as well as an OTU labeled Lachnospiraceae unclassified. Prevotellaceae are highly abundant in the de novo clustered OTUs, but no Prevotellaceae OTUs were identified as core OTUs in the analysis of the closed-reference clustered OTUs.
Limitations and Future Directions
Meta-analyses of livestock microbiota data has been shown to provide valuable insights into common and shared members of microbiomes as highlighted by two recent studies on pigs and cattle (Holman et al., 2017; Holman and Gzyl, 2019). Nevertheless, there are also several limitations to the dataset analyzed in this study which present challenges when attempting to make inferences about differences in community structure between individual datasets. The host animals in each study received different diets, were maintained with different farm management practices, were sampled at different stages of life, and derived from different geographic regions. Differences in sampling technique, location within the rumen, and washing of the rumen epithelia after sampling may also introduce biases between studies. Deer and yak were represented by a single dataset each, lowering the comparability between cattle and less represented ruminants, since study has been shown in previous meta-analyses to be a major contributing factor to variation in animal microbiome data (Holman et al., 2017; Holman and Gzyl, 2019). We chose to exclude studies that used sequencing platforms other than the Illumina MiSeq platform, particularly pyrosequencing studies, to increase the read depth of the samples, improve overall sequence quality and increase homogeneity of read length distribution. However, the variation in read count between studies remained high. After removal of low quality and chimeric sequences, samples ranged from 10,083 reads to 174,668 reads. To avoid biasing our de novo OTU clustering toward OTUs from studies with higher read depth, random subsampling to 10,000 reads per sample was employed. In the closed-reference OTU clustering analysis, sequences with less than 97% similarity to reference sequences were lost, exacerbating the range of sequence depths per sample. Sequence loss during closed-reference clustering should also be considered non-random since it is dependent on the reference database used. In addition to variation in sequencing depth, studies varied by hypervariable region sequenced, primers used, and DNA extraction methods. Hypervariable regions differ in their ability to differentiate and classify taxa (Kim et al., 2011; Yang et al., 2016) while primer choice and DNA extraction methods can introduce their own biases. An alternative to OTUs known as Amplicon Sequence Variants (ASVs) uses exact sequences rather than clustering, which can increase comparability between studies compared to OTU clustering which is contextual (Callahan et al., 2017). However, ASVs do not resolve the difficulty of comparing across different hypervariable regions and were not suitable for this meta-analysis.
Nevertheless, in spite of the limitations mentioned above, our dataset reveals the presence of shared and abundant members of the rumen epithelial microbiota among different ruminants and conditions. Therefore, our results provide a high degree of confidence in establishing core rumen bacterial phylotypes that are present in rumen epithelial communities around the world, across varied diets, management practices, and in different ruminants. Many of the core and abundant rumen epithelial phylotypes have also been found to be abundant in pyrosequencing-based rumen epithelial microbiome studies performed in various ruminants as well as studies using cloning approaches (Cho et al., 2006; Sadet-Bourgeteau et al., 2010; Li M. et al., 2012; Petri et al., 2013; Malmuthuge et al., 2014), which were not included in our analysis. The abundance of these core rumen epithelial phylotypes in pyrosequencing data thus provides additional evidence for the abundance and importance of the core rumen epithelial microbiota identified here. Additionally, several members of the core rumen epithelial microbiota are taxa not commonly found in rumen content, such as Campylobacter, Burkholderiaceae, Comamonas, Desulfobulbus, and Neisseriaceae (Henderson et al., 2015; Holman and Gzyl, 2019). It is thus highly likely that these members of the core rumen epithelial microbiota have key functions at the rumen epithelium or are better suited to the epithelial environment than the fluid and particulate rumen fractions. It is important to note that most of the members of the rumen epithelial core microbiota have yet to be functionally examined in detail. This is particularly important given the critical role of epithelial and mucosal surfaces for maintaining gut integrity (Aschenbach et al., 2019). The availability of metagenome-assembled rumen genomes, sequencing of functional genes, and rumen epithelial metatranscriptome sequencing have provided first functional insights into the rumen epithelial microbiota (Jin et al., 2017; Mann et al., 2018; Stewart et al., 2019). Given their high prevalence, further functional characterization of these core rumen epithelial microbes through genomic, meta-transcriptomic, and culture-based approaches will be a valuable contribution to our understanding of the rumen epithelial environment, ruminant metabolism, and the impact of these microbes on the host.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the animal study because this meta-analysis study used only already available sequencing datasets from different animal trials. No animal trial was performed in this study. Thus, no animal ethics committee approval was required.
Author Contributions
SS-E and CA conceived and designed the study. CA analyzed the sequencing data. LK provided sequencing datasets. CA, LK, and SS-E wrote the manuscript. All authors have reviewed and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. CA was supported by the SMART Scholarship funded through USD/R&E (The Under Secretary of Defense-Research and Engineering), the National Defense Education Program (NDEP)/BA-1, Basic Research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.625400/full#supplementary-material
References
- Aschenbach J. R., Zebeli Q., Patra A. K., Greco G., Amasheh S., Penner G. B. (2019). Symposium review: the importance of the ruminal epithelial barrier for a healthy and productive cow. J. Dairy Sci. 102 1866–1882. 10.3168/jds.2018-15243 [DOI] [PubMed] [Google Scholar]
- Astudillo-García C., Bell J. J., Webster N. S., Glasl B., Jompa J., Montoya J. M., et al. (2017). Evaluating the core microbiota in complex communities: a systematic investigation. Environ. Microbiol. 19 1450–1462. 10.1111/1462-2920.13647 [DOI] [PubMed] [Google Scholar]
- Bolyen E., Rideout J. R., Dillon M. R., Bokulich N. A., Abnet C. C., Al-Ghalith G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan B. J., McMurdie P. J., Holmes S. P. (2017). Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 11 2639–2643. 10.1038/ismej.2017.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., et al. (2009). BLAST+: architecture and applications. BMC Bioinformatics 10:421. 10.1186/1471-2105-10-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack K. M., Austin K. J., Lamberson W. R., Conant G. C., Cunningham H. C. (2018). Ruminnat nutrition symposium: tiny but mighty: the role of the rumen microbes in livestock production1. J. Anim. Sci. 96 752–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Penner G. B., Li M., Oba M., Guan L. L. (2011). Changes in bacterial diversity associated with epithelial tissue in the beef cow rumen during the transition to a high-grain diet. Appl. Environ. Microbiol. 77 5770–5781. 10.1128/aem.00375-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., McCowan R. P., Costerton J. W. (1979). Adherent epithelial bacteria in ruminants and their roles in digestive tract function. Am. J. Clin. Nutr. 32 139–148. 10.1093/ajcn/32.1.139 [DOI] [PubMed] [Google Scholar]
- Cho S. J., Cho K. M., Shin E. C., Lim W. J., Hong S. Y., Choi B. R., et al. (2006). 16S rDNA analysis of bacterial diversity in three fractions of cow rumen. J. Microbiol. Biotechnol. 16 92–101. [Google Scholar]
- De Mulder T., Goossens K., Peiren N., Vandaele L., Haegeman A., De Tender C., et al. (2017). Exploring the methanogen and bacterial communities of rumen environments: solid adherent, fluid and epimural. FEMS Microbiol. Ecol. 93. 10.1093/femsec/fiw251 [DOI] [PubMed] [Google Scholar]
- Dinsdale D., Cheng K. J., Wallace R. J., Goodlad R. A. (1980). Digestion of epithelial tissue of the rumen wall by adherent bacteria in infused and conventionally fed sheep. Appl. Environ. Microbiol. 39 1059–1066. 10.1128/aem.39.5.1059-1066.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier S. (2018). Viridis: Default color Maps from ‘Matplotlib’. R package version 0.5.1. Available online at: https://CRAN.R-project.org/package=viridis [Google Scholar]
- Goodrich J. K., Di Rienzi S. C., Poole A. C., Koren O., Walters W. A., Caporaso J. G., et al. (2014). Conducting a microbiome study. Cell 158 250–262. 10.1016/j.cell.2014.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J. (2014). “The family rikenellaceae,” in The Prokaryotes: Other Major Lineages of Bacteria and The Archaea, eds Rosenberg E., DeLong E. F., Lory S., Stackebrandt E., Thompson F. (Berlin: Springer; ), 857–859. 10.1007/978-3-642-38954-2_134 [DOI] [Google Scholar]
- Gupta R. S., Chen W. J., Adeolu M., Chai Y. (2013). Molecular signatures for the class Coriobacteriia and its different clades; proposal for division of the class Coriobacteriia into the emended order Coriobacteriales, containing the emended family Coriobacteriaceae and Atopobiaceae fam. nov., and Eggerthellales ord. nov., containing the family Eggerthellaceae fam. nov. Int. J. Syst. Evol. Microbiol. 63(Pt 9), 3379–3397. 10.1099/ijs.0.048371-0 [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Nanda A., Khadka B. (2017). Novel molecular, structural and evolutionary characteristics of the phosphoketolases from bifidobacteria and Coriobacteriales. PLoS One 12:e0172176. 10.1371/journal.pone.0172176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G., Cox F., Ganesh S., Jonker A., Young W., Abecia L., et al. (2015). Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 5:14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. H. (1982). Effect of dietary extremes on Impala (Aepyceros melampus) rumen epimural flora. Appl. Environ. Microbiol. 44 198–202. 10.1128/aem.44.1.198-202.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer M. A. (1981). Bovine campylobacteriosis: a review. Can. Vet. J. 22 327–330. [PMC free article] [PubMed] [Google Scholar]
- Holman D. B., Gzyl K. E. (2019). A meta-analysis of the bovine gastrointestinal tract microbiota. FEMS Microbiol. Ecol. 95:fiz072. [DOI] [PubMed] [Google Scholar]
- Holman D. B., Brunelle B. W., Trachsel J., Allen H. K. (2017). Meta-analysis to define a core microbiota in the swine gut. mStystem 2:e00004–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. H., Hungate R. E. (1976). Desulfovibrio of the sheep rumen. Appl. Environ. Microbiol. 32 598–602. 10.1128/aem.32.4.598-602.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jari Oksanen F., Blanchet G., Friendly M., Kindt R., Legendre P. (2019). vegan: Community Ecology Package. R package version 2.5-7. Available online at: https://CRAN.R-project.org/package=vegan [Google Scholar]
- Jin D., Zhao S., Wang P., Zheng N., Bu D., Beckers Y., et al. (2016). Insights into abundant rumen ureolytic bacterial community using rumen simulation system. Front. Microbiol. 7:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D., Zhao S., Zheng N., Bu D., Beckers Y., Denman S. E., et al. (2017). Differences in ureolytic bacterial composition between the rumen digesta and rumen wall based on urec gene classification. Front. Microbiol. 8:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasparovska J., Pecinkova M., Dadakova K., Krizova L., Hadrova S., Lexa M., et al. (2016). Effects of isoflavone-enriched feed on the rumen microbiota in dairy cows. PLoS One 11:e0154642. 10.1371/journal.pone.0154642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Morrison M., Yu Z. (2011). Evaluation of different partial 16S rRNA gene sequence regions for phylogenetic analysis of microbiomes. J. Microbiol. Methods 84 81–87. 10.1016/j.mimet.2010.10.020 [DOI] [PubMed] [Google Scholar]
- Knight R., Vrbanac A., Taylor B. C., Aksenov A., Callewaert C., Debelius J., et al. (2018). Best practices for analysing microbiomes. Nat. Rev. Microbiol. 16 410–422. [DOI] [PubMed] [Google Scholar]
- Levy B., Jami E. (2018). Exploring the prokaryotic community associated with the rumen ciliate protozoa population. Front. Microbiol. 9:2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Zhou M., Adamowicz E., Basarab J. A., Guan L. L. (2012). Characterization of bovine ruminal epithelial bacterial communities using 16S rRNA sequencing. PCR-DGGE, and qRT-PCR analysis. Vet. Microbiol. 155 72–80. 10.1016/j.vetmic.2011.08.007 [DOI] [PubMed] [Google Scholar]
- Li R. W., Connor E. E., Li C., Baldwin, Vi R. L., Sparks M. E. (2012). Characterization of the rumen microbiota of pre-ruminant calves using metagenomic tools. Environ. Microbiol. 14 129–139. 10.1111/j.1462-2920.2011.02543.x [DOI] [PubMed] [Google Scholar]
- Limmathurotsakul D., Thammasart S., Warrasuth N., Thapanagulsak P., Jatapai A., Pengreungrojanachai V., et al. (2012). Melioidosis in animals. Thailand, 2006-2010. Emerg. Infect. Dis. 18 325–327. 10.3201/eid1802.111347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. H., Bian G. R., Zhu W. Y., Mao S. Y. (2015). High-grain feeding causes strong shifts in ruminal epithelial bacterial community and expression of toll-like receptor genes in goats. Front. Microbiol. 6:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. H., Zhang M. L., Zhang R. Y., Zhu W. Y., Mao S. Y. (2016). Comparative studies of the composition of bacterial microbiota associated with the ruminal content, ruminal epithelium and in the faeces of lactating dairy cows. Microb. Biotechnol. 9 257–268. 10.1111/1751-7915.12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmuthuge N., Griebel P. J., Guan le L. (2014). Taxonomic identification of commensal bacteria associated with the mucosa and digesta throughout the gastrointestinal tracts of preweaned calves. Appl. Environ. Microbiol. 80 2021–2028. 10.1128/aem.03864-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann E., Wetzels S. U., Wagner M., Zebeli Q., Schmitz-Esser S. (2018). Metatranscriptome sequencing reveals insights into the gene expression and functional potential of rumen wall bacteria. Front. Microbiol. 9: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S., Zhang M., Liu J., Zhu W. (2015). Characterising the bacterial microbiota across the gastrointestinal tracts of dairy cattle: membership and potential function. Sci. Rep. 5:16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J. C., Luan S., Cardoso F. C., Derakhshani H., Khafipour E., Loor J. J. (2016). Induction of subacute ruminal acidosis affects the ruminal microbiome and epithelium. Front. Microbiol. 7:701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowan R. P., Cheng K. J., Costerton J. W. (1980). Adherent bacterial populations on the bovine rumen wall: distribution patterns of adherent bacteria. Appl. Environ. Microbiol. 39 233–241. 10.1128/aem.39.1.233-241.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowan R. P., Cheng K. J., Bailey C. B., Costerton J. W. (1978). Adhesion of bacteria to epithelial cell surfaces within the reticulo-rumen of cattle. Appl. Environ. Microbiol. 35 149–155. 10.1128/aem.35.1.149-155.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie P. J., Holmes S. (2013). phyloseq: an r package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:e61217. 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead L. J., Jones G. A. (1981). Isolation and presumptive identification of adherent epithelial bacteria (“epimural” bacteria) from the ovine rumen wall. Appl. Environ. Microbiol. 41 1020–1028. 10.1128/aem.41.4.1020-1028.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi I., Jami E. (2018). Review: the compositional variation of the rumen microbiome and its effect on host performance and methane emission. Animal 12 S220–S232. [DOI] [PubMed] [Google Scholar]
- Moraïs S., Mizrahi I. (2019). Islands in the stream: from individual to communal fiber degradation in the rumen ecosystem. FEMS Microbiol. Rev. 43 362–379. 10.1093/femsre/fuz007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morotomi M., Nagai F., Watanabe Y. (2012). Description of christensenella minuta gen. nov., sp. nov., isolated from human faeces, which forms a distinct branch in the order Clostridiales, and proposal of Christensenellaceae fam. nov. Int. J. Syst. Evol. Microbiol. 62 144–149. 10.1099/ijs.0.026989-0 [DOI] [PubMed] [Google Scholar]
- Mu D.-S., Wang S., Liang Q.-Y., Du Z.-Z., Tian R., Ouyang Y., et al. (2020). Bradymonabacteria, a novel bacterial predator group with versatile survival strategies in saline environments. Microbiome 8:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller R. E., Asplund J. M., Iannotti E. L. (1984). Successive changes in the epimural bacterial community of young lambs as revealed by scanning electron microscopy. Appl. Environ. Microbiol. 47 715–723. 10.1128/aem.47.4.715-723.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer V., Humer E., Mann E., Kroger I., Reisinger N., Wagner M., et al. (2019). Effects of clay mineral supplementation on particle-associated and epimural microbiota, and gene expression in the rumen of cows fed high-concentrate diet. Anaerobe 59 38–48. 10.1016/j.anaerobe.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Palevich N., Kelly W. J., Leahy S. C., Denman S., Altermann E., Rakonjac J., et al. (2019). Comparative genomics of rumen butyrivibrio spp. uncovers a continuum of polysaccharide-degrading capabilities. Appl. Environ. Microbiol. 86 e1993–e1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz H. A., Hales K. E., Wells J. E., Kuehn L. A., Freetly H. C., Berry E. D., et al. (2018). Rumen bacterial community structure impacts feed efficiency in beef cattle. J. Anim. Sci. 96 1045–1058. 10.1093/jas/skx081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Li Z.-R., Green R. S., Holzman I. R., Lin J. (2009). Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 139 1619–1625. 10.3945/jn.109.104638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri R. M., Schwaiger T., Penner G. B., Beauchemin K. A., Forster R. J., McKinnon J. J., et al. (2013). Changes in the rumen epimural bacterial diversity of beef cattle as affected by diet and induced ruminal acidosis. Appl. Environ. Microbiol. 79:3744. 10.1128/aem.03983-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen M., Schwab C., Borg Jensen B., Engberg R. M., Spang A., Canibe N., et al. (2013). Methylotrophic methanogenic thermoplasmata implicated in reduced methane emissions from bovine rumen. Nat. Commun. 4:1428. [DOI] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. (2012). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41 D590–D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2020). R: a Language and Environment for Statistical Computing. Vienna: R Foundation for Statitical Computing. [Google Scholar]
- Ren Q., Si H., Yan X., Liu C., Ding L., Long R., et al. (2019). Bacterial communities in the solid, liquid, dorsal, and ventral epithelium fractions of yak (Bos grunniens) rumen. Microbiologyopen 9:e963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci S., Sandfort R., Pinior B., Mann E., Wetzels S. U., Stalder G. (2019). Impact of supplemental winter feeding on ruminal microbiota of roe deer Capreolus capreolus. Wildlife Biol. 1-11:11. [Google Scholar]
- Rieu F., Fonty G., Gaillard B., Gouet P. (1990). Electron microscopy study of the bacteria adherent to the rumen wall in young conventional lambs. Can. J. Microbiol. 36 140–144. 10.1139/m90-025 [DOI] [PubMed] [Google Scholar]
- Rognes T., Flouri T., Nichols B., Quince C., Mahe F. (2016). VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. 10.7717/peerj.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadet S., Martin C., Meunier B., Morgavi D. P. (2007). PCR-DGGE analysis reveals a distinct diversity in the bacterial population attached to the rumen epithelium. Animal 1 939–944. 10.1017/s1751731107000304 [DOI] [PubMed] [Google Scholar]
- Sadet-Bourgeteau S., Martin C., Morgavi D. P. (2010). Bacterial diversity dynamics in rumen epithelium of wethers fed forage and mixed concentrate forage diets. Vet. Microbiol. 146 98–104. 10.1016/j.vetmic.2010.04.029 [DOI] [PubMed] [Google Scholar]
- Sbardellati D. L., Fischer A., Cox M. S., Li W., Kalscheur K. F., Suen G. (2020). The bovine epimural microbiota displays compositional and structural heterogeneity across different ruminal locations. J. Dairy Sci. 103 3636–3647. 10.3168/jds.2019-17649 [DOI] [PubMed] [Google Scholar]
- Schären M., Kiri K., Riede S., Gardener M., Meyer U., Hummel J., et al. (2017). Alterations in the rumen liquid-, particle- and epithelium-associated microbiota of dairy cows during the transition from a silage- and concentrate-based ration to pasture in Spring. Front. Microbiol. 8:744. 10.3389/fmicb.2017.00744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75 7537–7541. 10.1128/aem.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri R., Leahy S. C., Attwood G. T., Teh K. H., Lambie S. C., Cookson A. L., et al. (2018). Cultivation and sequencing of rumen microbiome members from the Hungate1000 collection. Nat. Biotechnol. 36 359–367. 10.1038/nbt.4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shade A., Handelsman J. (2012). Beyond the venn diagram: the hunt for a core microbiome. Environ. Microbiol. 14 4–12. 10.1111/j.1462-2920.2011.02585.x [DOI] [PubMed] [Google Scholar]
- Sorbara M. T., Littmann E. R., Fontana E., Moody T. U., Kohout C. E., Gjonbalaj M., et al. (2020). Functional and genomic variation between human-derived isolates of lachnospiraceae reveals inter- and intra-species diversity. Cell Host Microbe 28 134–146.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R. D., Auffret M. D., Warr A., Walker A. W., Roehe R., Watson M. (2019). Compendium of 4,941 rumen metagenome-assembled genomes for rumen microbiome biology and enzyme discovery. Nat. Biotechnol. 37 953–961. 10.1038/s41587-019-0202-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svartström O., Alneberg J., Terrapon N., Lombard V., de Bruijn I., Malmsten J., et al. (2017). Ninety-nine de novo assembled genomes from the moose (Alces alces) rumen microbiome provide new insights into microbial plant biomass degradation. ISME J. 11 2538–2551. 10.1038/ismej.2017.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J., Cheng K. J., Dinsdale D., ØRskov E. R. (1979). An independent microbial flora of the epithelium and its role in the ecomicrobiology of the rumen. Nature 279 424–426. 10.1038/279424a0 [DOI] [PubMed] [Google Scholar]
- Wetzels S. U., Mann E., Metzler-Zebeli B. U., Wagner M., Klevenhusen F., Zebeli Q., et al. (2015). Pyrosequencing reveals shifts in the bacterial epimural community relative to dietary concentrate amount in goats. J. Dairy Sci. 98 5572–5587. 10.3168/jds.2014-9166 [DOI] [PubMed] [Google Scholar]
- Wickham H. (2016). ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag. [Google Scholar]
- Yang B., Wang Y., Qian P.-Y. (2016). Sensitivity and correlation of hypervariable regions in 16S rRNA genes in phylogenetic analysis. BMC Bioinformatics 17:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Dietrich C., Radek R., Brune A. (2016). Endomicrobium proavitum, the first isolate of Endomicrobia class. nov. (phylum Elusimicrobia)–an ultramicrobacterium with an unusual cell cycle that fixes nitrogen with a Group IV nitrogenase. Environ. Microbiol. 18 191–204. 10.1111/1462-2920.12960 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.