Abstract
Alzheimer's disease (AD) is an irrevocable neurodegenerative condition characterized by the presence of senile plaques comprising amassed β-amyloid peptides (Aβ) and neurofibrillary tangles mainly comprising extremely phosphorylated Tau proteins. Recent studies have emphasized the role of microRNAs (miRNAs) in the development of AD. A number of miRNAs, namely, miR-200a-3p, miR-195, miR-338-5p, miR-34a-5p, miR-125b-5p, miR-132, miR-384, miR-339-5p, miR-135b, miR-425-5p, and miR-339-5p, have been shown to participate in the development of AD through interacting with BACE1. Other miRNAs might affect the inflammatory responses in the course of AD. Aberrant expression of several miRNAs in the plasma samples of AD subjects has been shown to have the aptitude for differentiation of AD subjects from healthy subjects. Finally, a number of AD-modifying agents affect miRNA profile in cell cultures or animal models. We have performed a comprehensive search and summarized the obtained data about the function of miRNAs in AD in the current review article.
Keywords: Alzheimer's disease, miRNA, marker, expression, polymorphism
Introduction
Alzheimer's disease (AD) is an irrevocable neurodegenerative condition with a progressive course, and it is the chief reason for dementia in the elderly (Prince et al., 2013). AD is characterized by pervasive cognitive defects and memory deficits, leading to the dependence of the majority of AD patients on others for their routine activities. From a pathological point of view, AD is defined by the presence of senile plaques comprising amassed β-amyloid peptides (Aβ) and neurofibrillary tangles mainly comprising extremely phosphorylated Tau proteins (Ballard et al., 2011). The most accepted hypotheses for the development of AD are based on these two main pathological events [i.e., Aβ accumulation and Tau accumulation (Wang et al., 2019a)]. The amyloid precursor protein is converted to Aβ through consecutive enzymatic reactions catalyzed by β-secretase (BACE1) and γ-secretase (containing presenilin 1 and presenilin 2) (Querfurth and LaFerla, 2010). Recent studies have emphasized the role of microRNAs (miRNAs) in the development of AD (Wang et al., 2019a). These ~22 nucleotide transcripts post-transcriptionally regulate the expression of several target genes through binding with 3' UTR and destructing the target transcript or reducing its translation (O'Brien et al., 2018). Sequence complementarity mainly regulates the miRNA/mRNA interactions leading to the ability of one miRNA to target several genes and the possible regulation of one gene by multiple miRNAs. Therefore, miRNAs are potential means for investigating multifactorial disorders such as AD (Iqbal and Grundke-Iqbal, 2010). A leading investigation in this regard has examined the number of brain-associated miRNAs expressed in the human hippocampus specimens obtained from fetal, adult, and AD patients, revealing misregulation of certain miRNAs in the AD brain and their possible contribution to the pathological processes of this disorder (Lukiw, 2007). Dysregulation of other miRNAs has also been verified in multiple studies, and the underlying mechanisms of their contribution in AD have been identified in some cases. We have performed a comprehensive search and summarized the obtained data about the function of miRNAs in AD in this review article.
Dysregulated miRNAs in AD
Dysregulation of miRNAs has been demonstrated in human AD subjects or animal models of AD. Moreover, several researchers have induced or suppressed the expression of some miRNAs in the cell/animal models of AD to appraise their function in the progression of AD. In a rat model of AD caused by the administration of Aβ25−35 into the brain, downregulation of SOX6 and over-expression of miR-129-5p have shortened the dormant escape period and enhanced the time of crossing platforms, repairing the pathological damage, blocking neuronal apoptosis, and decreasing inflammation. Based on the protective effects of miR-129-5p against nerve damage and inflammation, miR-129-5p has been suggested as a candidate for therapeutic options against AD, as it acts to suppress SOX6 (Zeng et al., 2019). Expression of miR-200a-3p has been shown to be repressed in animal and cell models of AD. miR-200a-3p can suppress cell apoptosis, inactivate Bax/caspase-3 axis, and decrease Aβ1−42 and tau phosphorylation in cell experiments. Mechanistically, these effects are mediated through the modulation of translocation of BACE1 and PRKACB. Taken together, the neuroprotective impact of miR-200a-3p is accomplished by inhibition of BACE1 expression and subsequent suppression of Aβ production as well as reduction of PKA expression and Tau phosphorylation (Wang et al., 2019b). miR-455-3p has been shown to bind with 3' UTR of APP gene to decrease its expression and reduce expression of mitochondrial fission proteins (Kumar et al., 2019). Mutant APP cells that show expression of miR-455-3p exhibit upregulation of synaptic genes. Over-expression of miR-455-3p in mutant APP cells reduces the number of mitochondria and increases the size of the mitochondria. Taken together, miR-455-3p controls APP processing and protects against mutant APP-associated mitochondrial dysfunction and synaptic anomalies in AD (Kumar et al., 2019). Expression of miR-455-3p has been shown to be increased in postmortem brain samples, fibroblasts, and plasma samples of patients with AD compared with controls (Kumar et al., 2017; Kumar and Reddy, 2018). As a primary event, expression of miR-409-5p has been decreased in an APP/PS1 double transgenic mice model of AD. Over-expression experiments have shown that this miRNA has a harmful impact on neurite outgrowth, reduces neuron survival, and quickens the progression of Aβ1−42-associated pathologic events (Guo et al., 2019). In line with the observed downregulation of miR-409-5p in APP/PS1 AD model, Aβ1−42 peptide has been shown to downregulate miR-409-5p levels. A luciferase study has shown that Plek is a target of miR-409-5p (Guo et al., 2019). Ectopic expression of miR-409-5p has induced neurotoxic effects and interferes with neuron survival and differentiation, while Plek upregulation could partly protect the neurite outgrowth from these toxic effects. Taken together, reduction of miR-409-5p expression in the early stages of AD might be a self-protective response to lessen the synaptic injury induced by Aβ (Guo et al., 2019). miR-132 is another downregulated miRNA in AD. Experiments in a rat model of AD have shown upregulation of AChE, iNOS, ROS, MDA, MAPK1, and p-MAPK1 and downregulation of SOD, GSH-Px, and miR-132. Over-expression of miR-132 has reversed these markers demonstrating the role of this miRNA in the suppression of hippocampal iNOS expression and oxidative stress through reduction of MAPK1 levels (Deng et al., 2020). However, expression of this miRNA has been demonstrated to be reduced in neurally-originated plasma exosomes of AD subjects (Cha et al., 2019). Table 1 shows the summary of studies that reported decreased levels of miRNAs in AD.
Table 1.
Downregulated miRNAs in AD subject, animal models of AD, and related cell lines and their functions in progression of AD.
| microRNA | Samples | Assessed cell line | Gene/protein interaction | Signaling pathway | Function | References |
|---|---|---|---|---|---|---|
| miR-129-5p | 90 male-specific pathogen-free (SPF) Sprague-Dawley (SD) rats | Hippocampal neuron cells of rat | SOX6 | - | Its upregulation represses apoptosis and inflammatory reactions and attenuates neural injury by targeting SOX6. | Zeng et al., 2019 |
| miR-200a-3p | Plasma samples from 7 patients with AD and 5 age-matched healthy individual, APP/PS1 mice, SAMP8, and SAMR1 mice | NB-1 | BACE1, PRKACB | - | Has neuroprotective effects, suppresses apoptosis, and decreases Aβ production through regulating expression of BACE1 and PRKACB | Wang et al., 2019b |
| miR-326 | APPswe/PS1d E9 double transgenic mouse | - | VAV1 | JNK signaling pathway | Its overexpression decreased neuronal apoptosis and Aβ accumulation and elevated viability of neuron cells by targeting VAV1. | He et al., 2020 |
| miR-98 | 70 Kunming mice | Hippocampal neuronal cells | HEY2 | Notch signaling pathway | Represses apoptosis of hippocampal neurons and shows enhanced survival of these cells by targeting HEY2 and inactivating the Notch signaling pathway | Chen et al., 2019 |
| miR-196a | 60 male Sprague-Dawley mice | HEK-293T | LRIG3 | PI3/Akt pathway | Its upregulation ameliorated cognitive decline, inhibited apoptosis, and increased survival of neurons by targeting LRIG3. | Yang et al., 2019a |
| miR-195 | Postmortem human brain tissues and CSF samples from AD patients and MCI subjects, Human ApoE4+/+ or ApoE3+/+ knock-in (KI) mice | Mouse primary neuron | synj1 | - | Its overexpression alleviated cognitive impairment and decreased Aβ deposition and tau hyper-phosphorylation. | Cao et al., 2020 |
| miR-195 | SAMP8 and SAMR1 mice | HEK293, N2a | BACE1 | - | Its overexpression reduced Aβ production through targeting BACE1. | Zhu et al., 2012 |
| miR-338-5p | Hippocampal tissue samples from patients with AD and normal subjects, 5XFAD transgenic (TG) mice | - | BACE1 | NF-κB signaling pathway | Its overexpression prevented Aβ formation, neuroinflammation, cognitive deficit and impaired learning ability by targeting BACE1. | Qian et al., 2019 |
| miR-338-5p | Male C57BL/6 mice and male APP/PS1 transgenic mice | Primary hippocampal neurons | BCL2L11 | - | Its overexpression prevented Aβ deposition, cognitive decline, and reduced apoptosis rate of neurons by targeting BCL2L11. | Li et al., 2020a |
| miR-133b | Serum samples from 105 AD patients and 98 control individuals | SH-SY5Y | EGFR | - | Its overexpression reduced apoptosis rate and improved cell viability. | Wang et al., 2019c |
| miR-124 | Male APP/PS1 transgenic mice | - | C1ql3 | - | Its overexpression increased angiogenesis and lowered the accumulation of Aβ and prevented memory decline and learning impairment. | Zhang et al., 2019 |
| miR-124-3p | - | N2a/APP695swe cells | Caveolin-1 | PI3K/Akt/GSK3β pathway | Its upregulation abated Tau hyperphosphorylation and cellular apoptosis by targeting Caveolin-1 and modulation of PI3K/Akt/GSK3β pathway. | Kang et al., 2017 |
| miR-101a | Plasma samples from 46 AD patients 60 healthy individuals, APPswe/ PS1ΔE9 transgenic mice | SH-SY5Y | MAPK1 | MAPK pathway | Regulates autophagy through targeting MAPK1 and modulating the MAPK pathway | Xiao et al., 2019 |
| miR-22 | Serum samples from 33 patients with AD and 30 healthy volunteers, APP/PS1 double transgenic mice | MG cells | GSDMD | - | Its overexpression suppressed secretion of inflammatory factors and pyroptosis also decreased GSDMD expression. | Han et al., 2020 |
| miR-34a | - | SH-SY5Y | Caspase-2 | - | Its upregulation suppressed neurotoxicity induced by Aβ through targeting Caspase-2. | Wang et al., 2019c |
| miR-34a | APP/PS1 transgenic mice | SH-SY5Y, primary cortical neuronal cells | cyclin D1 | - | Regulates apoptosis rate and neuronal cell cycle by targeting cyclin D1 | Modi et al., 2016 |
| miR-34a-5p miR-125b-5p |
Serum samples from 27 AD patients and 27 age-matched control individuals | N2a, MCN | BACE1 | - | Their overexpression ameliorated oxidative stress and apoptosis induced by Aβ through targeting BACE1. | Li et al., 2020b |
| miR-181a | APP/PS1 transgenic mice and male wild-type C67BL/6J mice | Murine brain pericytes | FOXO1 | - | Its overexpression alleviated cognitive decline, reduced accumulation of Aβ, and slowed pericyte loss by targeting FOXO1. | Wu et al., 2019 |
| miR-31 | Female AD triple-transgenic mice | HT-22, HEK293, SH-SY5Y | APP | - | Its overexpression reduced Aβ accumulation and alleviated neuropathology of AD and memory impairment. | Barros-Viegas et al., 2020 |
| miR-409-5p | APPswe/PS1ΔE9 double transgenic mice | PC12, Neuro2A, HEK293T | Plek | - | Its overexpression expression aggravated cell survival and differentiation and impaired neurite outgrowth by targeting Plek. | Guo et al., 2019 |
| miR-107 | CSF samples from 22 AD patients and 10 healthy controls | SH-SY5Y | FGF7 | FGFR2/PI3K/Akt pathway | Its upregulation reduced apoptosis and inflammation also elevated proliferation of SH-SY5Y through regulation of FGF7/FGFR2/PI3K/Akt Pathway induced by Aβ. | Chen et al., 2020a |
| miR-107 | - | hCMEC/D3, NHA, HBVP | Endophilin-1 | - | Its overexpression inhibited disruption of the blood–brain barrier induced by Aβ and alleviated impaired function of endothelial cells by targeting Endophilin-1. | Liu et al., 2016a |
| miR-107 miR-103 |
Post-mortem brain tissues from 12 AD patients and 12 age- and gender-matched control individuals | SK-N-BE, HEK-293 | CDK5R1 | - | Can be implicated in AD pathogenesis through regulation of CDK5R1 expression and consequently influencing p53 levels | Moncini et al., 2017 |
| miR-212 | Plasma sample from 31 AD patients and 31 control subjects | SH-SY5Y, IMR-32 | PDCD4 | PI3K/AKT signaling pathway | Reduces neurotoxicity of Aβ by targeting PDCD4 regulation of PI3K/AKT signaling pathway | Chang, 2020 |
| miR-433 | Serum samples from 118 AD patients and 62 healthy controls | SH-SY5Y, SK-N-SH | JAK2 | - | Its overexpression improved the viability of neurons by targeting JAK2. Its expression is associated with MMSE scores. | Wang and Zhang, 2020 |
| miR-132 | 70 SPF Sprague-Dawley rats | HEK 293T | MAPK1 | MAPK1 signal pathway | Suppresses oxidative stress and alleviated cognitive performance by targeting MAPK1 | Deng et al., 2020 |
| miR-132 | P301S Tau transgenic mice | Primary cortical and hippocampal neuron cultures | Rbfox1, GSK3β, EP300, Calpain 2 | - | Has neuroprotective effects including reduces neurotoxicity of Aβ and improves elongation of neurite and decreases neuronal death through targeting Rbfox1, GSK3β, EP300, and Calpain 2 | El Fatimy et al., 2018 |
| miR-132 miR-212 |
Human post-mortem brain tissues from 10 AD patients and 6 control subjects | Primary human neurons, SH-SY5Y | NOS1 | - | Low expression of miR-132 and miR-212 disrupted the balance of S-nitrosylation through modulation of NOS1 expression. | Wang et al., 2017 |
| miR-132 miR-212 |
Brain tissues from 29 AD patients and 16 controls | PC12, primary neurons | PTEN, FOXO3a, P300 | AKT signaling pathway | Regulates survival and apoptosis of neuronal cells through targeting PTEN, FOXO3a, and P300. | Wong et al., 2013 |
| miR-132 | Post-mortem brain tissues from AD patients, 3xTg-AD mice lacking the miR-132/212 cluster | Neuro2a, Neuro2a APPSwe/Δ9, HEK293T, HEK293-APPSwe | Sirt1 | - | Its deletion was associated with increased Aβ production and the establishment of amyloid plaque. | Hernandez-Rapp et al., 2016 |
| miR-132 | Brain tissues from AD patients and normal controls, APPPS1 mice | HEK293-APPswe | ITPKB | - | Regulates Aβ formation and TAU phosphorylation through targeting ITPKB and modulation of ERK1/2 and BACE1 activity. | Salta et al., 2016 |
| miR-9-5p | - | HT22 | GSK-3β | Nrf2/Keap1 signaling | Its overexpression caused a reduction in the apoptosis rate, oxidative stress, and prevention of mitochondrial malfunction by targeting GSK-3β. | Liu et al., 2020 |
| miR-377 | - | SH-SY5Y | CDH13 | - | Its upregulation enhanced cell proliferation and prevented occurrence apoptosis by targeting CDH13. | Liu et al., 2018 |
| miR-221 | Blood samples from 21 AD patients and 17 controls | SH-SY5Y | ADAM10 | - | Can be implicated in AD pathogenesis through regulation of ADAM10 expression | Manzine et al., 2018 |
| miR-186 | 72 male Sprague–Dawley (SD) rats | Hippocampal neuronal cells | IL2 | JAK-STAT signaling pathway | Its upregulation inhibited apoptosis and enhanced cell proliferation through targeting IL2 and regulation of the JAK-STAT signaling pathway. | Wu et al., 2018a |
| miR-330 | 14 C57 mice | Primary neuron cells obtained from mice | VAV1 | MAPK signaling pathway | Its overexpression reduced oxidative stress, ameliorated mitochondrial dysfunction, and decreased the generation of Aβ by targeting VAV1. | Han et al., 2018 |
| let-7f-5p | C57BL/6J-TgN (APP/PS1) ZLFILAS mice | Bone marrow mesenchymal stem cells | Caspase-3 | - | Its overexpression inhibited apoptosis induced by Aβ through targeting caspase-3. It also increased the survival rate of MSCs in mouse brain. | Shu et al., 2018 |
| miR-107 | 60 male C57 mice | - | - | - | Its overexpression alleviated spatial memory dysfunction, hippocampal long-term potentiation and prevented the elimination of pyramidal neurons induced resulted from neurotoxicity of Aβ. | Shu et al., 2018 |
| miRNA-140-5p | Post mortem brain tissues from 21 AD patients and 22 normal subjects | SHSY5Y, CHP212 | ADAM10, SOX2 | - | Is implicated in AD pathogenesis through targeting ADAM10 and its transcription factor SOX2 | Akhter et al., 2018 |
| miR-384 | Serum and CSF samples from 32 MCI patients, 45 AD patients, and 50 control individuals | SH-SY5Y, HEK293 | BACE-1, APP | - | Its overexpression decreased the expression of BACE-1 and APP so it can contribute to AD pathogenesis. | Liu et al., 2014b |
| miR-188-5p | Brain tissues from 5 AD patients and 3 controls, 5XFAD mice | Primary hippocampal neuron cells | - | - | Its overexpression alleviated cognitive dysfunction and memory loss also restored synaptic activity. | Lee et al., 2016 |
| miR-193b | Plasma and CSF samples from AD patients, MCI patients and control subjects, APP/PS1 double-transgenic | SH-SY5Y, HEK293 | APP | - | Its upregulation downregulated APP expression so it can be implicated in AD pathogenesis. | Liu et al., 2014a |
| miR-153 | APPswe/PSΔE9 mice | SH-SY5Y, HEK-293T, M17 | APP, APLP2 | - | Its overexpression downregulated expression APP and APLP2 so can be an important factor in the pathogenesis of AD. | Liang et al., 2012 |
| miR-153 | Brain tissues from 15 AD patients and 5 normal controls | HeLa, primary human fetal brain cultures | APP | - | Can be implicated in AD pathogenesis through targeting APP and reducing APP expression | Long et al., 2012 |
| miR-16 | SAMP8 mice, SAMR1 mice, and BALb/c mice | Neuroblastoma2a and NIH3T3 | APP | - | Its upregulation downregulated the expression of APP and consequently prevented APP accumulation. | Liu et al., 2012 |
| miR-339-5p | Frozen brain tissues from 20 AD patients and 5 controls | HeLa, U373 MG, human primary brain cultures | BACE1 | - | Can contribute to AD pathogenesis through targeting BACE1 | Long et al., 2014 |
| miR-214-3p | CSF samples from eight patients with sporadic AD and 8 age-matched healthy volunteers, SAMR1 and SAMP8 mice | Primary neurons obtained from SAMP8 mice, SH-SY5Y | Atg12 | - | Its upregulation decreased autophagy and apoptosis rate in neuronal cells and improved cognitive function through targeting Atg12. | Zhang et al., 2016a |
| miR-222 | APPswe/PSΔE9 mice | SH-SY5Y, HEK-293T | p27Kip1 | - | Regulates cell cycle by targeting p27Kip1 so can be involved in AD pathogenesis | Wang et al., 2015a |
| miR-29c | CSF samples from 30 AD patients and 30 age-matched controls | Primary hippocampal neurons | DNMT3 | - | Regulates neuronal proliferation by targeting DNMT3 and regulation of BDNF expression. | Yang et al., 2015 |
| miR-101 | - | Primary hippocampal neurons | APP | - | Its overexpression lead to decreased accumulation of Aβ through targeting APP. | Vilardo et al., 2010 |
| miR-181c | SAMP8 and SAMR1 mice | HT-22, HEK293A | crmp2 | - | Can be implicated in the pathogenesis of AD by targeting crmp2 and downregulation of crmp2 expression | Zhou et al., 2016 |
| miR-135b | Blood samples from 25 AD patients and 25 age-matched healthy individuals, | Primary hippocampal cells derived from SAMR1 mice | BACE1 | - | Its overexpression elevated cell proliferation and improved memory function and learning capacity by targeting BACE1. | Zhang et al., 2016b |
Although several studies have reported downregulation of miR-132 in AD (Wong et al., 2013; El Fatimy et al., 2018; Cha et al., 2019; Deng et al., 2020), Liu et al. have reported high levels of miR-132 in patients with mild cognitive impairment and AD vs. normal individuals. They have shown the impact of miR-132 upregulation in the induction of apoptosis in neurons through increasing Bax/Bcl-2 ratio (Liu and Zhang, 2019). Moreover, they have reported that miR-132 increases Tau phosphorylation and expression levels of Rb, Histone H1, and CDK-5. Collectively, they have suggested that miR-132 participates in AD by controlling cell apoptosis and the GTDC-1/CDK-5/Tau phosphorylation axis (Liu and Zhang, 2019). In addition to GTDC-1, miR-132 is also known to regulate the expression of synaptic proteins via complement C1q (Xu et al., 2019). Similarly, expression of miR-132 has been shown to be decreased in AD-derived plasma exosomes (Cha et al., 2019). miR-128 has also been over-expressed in the brain samples of AD patients (Liu et al., 2019). Experiments in AD mice have demonstrated parallel upregulation of miR-128 and downregulation of PPARγ in the cerebral cortex. The interaction between these two transcripts has been validated through functional assays. miR-128 silencing has suppressed AD-like features, amyloid plaque creation, Aβ production, and inflammation in AD mice through upregulating PPARγ (Liu et al., 2019). miR-425-5p is another upregulated miRNA in patients with AD and the cellular model of AD. Upregulation of miR-425-5p has induced cell apoptosis, stimulated expression of GSK-3β, and enhanced tau phosphorylation through targeting HSPB8 (Yuan et al., 2020). miR-146a is also upregulated in AD and participates in the pathogenesis of this condition via targeting Lrp2 and inhibiting the Akt signaling pathway, modulating ROCK1 expression and decreasing Tau phosphorylation, and influencing inflammatory responses via modulation of IRAK-1 (Cui et al., 2010; Wang et al., 2016). Insulin and liver X receptor (LXR) activators have been shown to increase the miR-7-1 levels. Expression of this miRNA has changed within the brains of diet-induced obese animals as well as AD patients, which is in parallel with the downregulation of its target genes IRS-2 and IDE. Upregulation of miR-7 has enhanced extracellular Aβ levels in neurons and interfered with the eradication of Aβ by microglia. Collectively, insulin can act via the HNRNPK-miR-7 cascade to post-transcriptionally affect metabolic pathways in AD (Fernández-de Frutos et al., 2019). Table 2 lists upregulated miRNAs in AD.
Table 2.
Upregulated miRNAs in AD subject, animal models of AD or related cell lines and their functions in progression of AD.
| microRNA | Samples | Assessed cell line | Gene/protein interaction | Signaling pathway | Function | References |
|---|---|---|---|---|---|---|
| miR-132 | Frozen human postmortem brain specimens from 10 patients with mild cognitive impairment, 10 patients with AD, and 10 controls | Human cortical neuron culture | GTDC-1 | - | Enhances neuronal apoptosis and Tau phosphorylation by targeting GTDC-1 | Liu and Zhang, 2019 |
| miR-30b | Human hippocampal tissues, C57BL/6J mice, and 5XFAD APP transgenic mice | NG108–15, HEK 293/293T | ephB2, sirt1, GluA2 | NF-κB signaling pathway | Disrupts cognitive and synaptic functions and its knockdown reverses this effect by targeting ephB2, sirt1, and GluA2 | Song et al., 2019 |
| miR-128 | APP/PSA/Tau triple transgenic mice and C57BL/6 mice | N2a cells | PPARγ | - | Its knockout suppressed AD development, Aβ production, and inflammatory reactions by targeting PPARγ. | Liu et al., 2019 |
| miR-128 | Plasma samples from 20 patients with AD and age and education-matched normal subjects | MCN, Neuro2a | PPAR-γ | - | Its inhibition abated neurotoxicity of Aβ through regulation of PPAR-γ and deactivated NF-κB. | Geng et al., 2018 |
| miR-7 | Postmortem human brains from AD patients and individuals without severe neurological and psychological disorders male C57BL/6 mice | N2a cell, BV-2 | INSR, IRS-2, IDE | Insulin signaling | Enhances extracellular Aβ and suppresses its clearance by regulating Insulin signaling through targeting INSR, IRS-2, and IDE | Fernández-de Frutos et al., 2019 |
| miR-592 | 54 Sprague-Dawley (SD) male rats established as an AD model | Astrocyte culture | KIAA0319 | Keap1/Nrf2/ARE signaling pathway | Its downregulation attenuated oxidative stress and enhanced cell survival through upregulation of KIAA0319. | Huang et al., 2020 |
| miR-425-5p | Postmortem brain tissue samples from | HEK293/tau | HSPB8 | - | Elevates apoptosis and tau phosphorylation through downregulation of HSPB8 | Yuan et al., 2020 |
| miR-425-5p miR-339-5p |
Blood samples (for PBMC) from 45 AD patients and 41 age- and gender-matched healthy controls | N2a/APPswe | BACE1 | - | Can be implicated in AD pathogenesis through modulating expression of BACE1 | Ren et al., 2016 |
| miR-25 | 30 male Kunming mice | Hippocampal neuronal cells | KLF2 | Nrf2 signaling pathway | Represses proliferation of hippocampal neuron cells and induced apoptosis in these cells by targeting KLF2 | Duan and Si, 2019 |
| miR-138 | - | SH-SY5Y | DEK | - | Increases apoptosis rate in SH-SY5Y cells by targeting DEK and downregulation of its expression | Miao et al., 2020 |
| miR-138q | - | N2a/APP, HEK293/tau | RARA | - | Can be implicated in the pathogenesis of AD through the promotion of tau phosphorylation by targeting RARA | Wang et al., 2015b |
| miR-149-5p | Plasma samples from 30 AD patients and 30 healthy controls | 293/APPsw | KAT8 | - | Can be implicated in AD pathology by targeting KAT8 to negatively regulate H4K16ac | Zhou et al., 2020 |
| miR-125b | Cerebral tissues from nine AD patients, eight patients with MCI, and 10 normal individuals | Neuronal cells obtained from human fatal cortical tissues | FOXQ1 | - | Promotes phosphorylation of Tau and apoptosis of neuronal cells by targeting FOXQ1 | Ma et al., 2017 |
| miR-125b | CSF samples from 24 AD patients and 24 healthy controls | Neuro2a APPSwe/Δ9 | - | - | Promotes cellular apoptosis, oxidative stress, and expression of inflammatory factors and suppressed cell proliferation by regulating SphK1 | Jin et al., 2018 |
| miR-125b | Brain tissue specimens 10 AD patients and 5 healthy controls, C57BL/6 wild-type mice | Primary hippocampal and cortical neuron obtained from embryonic day 19 rat | Bcl-W, DUSP6, PPP1CA | MAPK signaling | Its high expression resulted in increased tau phosphorylation through targeting Bcl-W, DUSP6 and PPP1CA. also its overexpression led to perished associative learning in mice. | Banzhaf-Strathmann et al., 2014 |
| miR-200b miR-200c |
Wild-type C57BL/6J mice and Tg2576 mice | PMNCs, SH-SY5Y | - | - | Transfection with miR-200b/miR-200c alleviated memory impairment and improved spatial learning through regulation of S6K1-mediated insulin signaling. | Higaki et al., 2018 |
| miR-200c | Plasma samples from 14 AD patients and 13 normal controls, APPswe/PS1ΔE9 double-transgenic mice | PC12 | PTEN | - | Its overexpression improved neuronal survival and neurite outgrowth by targeting PTEN. | Wu et al., 2016 |
| miR-10a | 50 male Sprague-Dawley (SD) rats | - | BDNF | BDNF-TrkB signaling pathway | Promotes apoptosis and cell growth arrest by targeting BDNF and inhibition of BDNF-TrkB signaling pathway | Wu et al., 2018b |
| miR-1908 | Blood samples from 20 AD patients and 20 age-matched control individuals | THP-1, U87 | ApoE | - | Disrupts clearance of Aβ by ApoE through downregulation of its expression | Wang et al., 2018 |
| miR-139 | SAMR1 and SAMP8 mice | Primary hippocampal cell | CB2 | - | Its overexpression improved memory function and learning ability by targeting CB2. | Tang et al., 2017 |
| miR-146a | - | SH-SY5Y | Lrp2 | Akt signaling pathway | Raised the rate of cellular apoptosis through targeting Lrp2 and inhibition of Akt signaling pathway | Wang et al., 2016 |
| miR-146a | Brain tissues from 17 AD patients, 5xFAD mice | SH-SY5Y | ROCK1 | ROCK1/PTEN pathway | Its inhibition decreased phosphorylation of tau proteins and improved memory function by modulating ROCK1 expression. | Wang et al., 2016 |
| miR-146a | Brain tissues from 36 AD patients and 30 control subjects | Primary human astroglial (HAG) cells, primary HNG | IRAK-1 | - | Targets IRAK-1 and downregulated its expression so caused a sustained inflammatory response | Cui et al., 2010 |
| miR-33 | APPsw/PSEN1Δ9 (APP/PS1) transgenic mice | N2a, N2a-APPsw, H4-APPsw | ABCA1 | - | Downregulates expression of ABCA1 and consequently impaired Aβ clearance | Kim et al., 2015 |
| miR-34c | C57 mice | Primary hippocampal neurons, N2a | VAMP2 | - | Its downregulation alleviated learning and memory dysfunction and synaptic impairment through targeting VAMP2. | Hu et al., 2015 |
| miR-26b | APP/PS1 double-transgenic mice | N2a, HEK293 | IGF-1 | - | Augments production of Aβ by targeting IGF-1 and its inhibition reversed these effects | Liu et al., 2016b |
| miR-98 | APP/PS1 mice | HEK293, N2a | IGF-1 | - | Its inhibition suppressed Aβ generation and tau phosphorylation by regulating the expression of IGF-1. | Hu et al., 2013 |
| miR-206 | Blood samples from 30 AD patients and 30 healthy controls | BV-2 | IGF-1 | - | Elevates inflammatory responses induced by LPS and promoted the release of Aβ from microglia cell through targeting IGF-1 | Xing et al., 2016 |
| miR-574 | APP/PS1 double transgenic mice and wild type mice | Primary hippocampal neurons obtained from mice | Nrn1 | - | Is involved in the regulation of synaptic activity and cognitive function through targeting Nrn1 | Li et al., 2015 |
| miR-26b | Postmortem brain tissues from 10 patients with MCI, 10 patients with AD, and eight controls | Primary cortical neurons obtained from Sprague Dawley rat | Rb1 | - | Promotes tau phosphorylation and cell cycle entry and consequently lead apoptosis by targeting Rb1 | Absalon et al., 2013 |
| miR-922 | - | SH-SY5Y, HEK-293T | UCHL1 | - | Enhances phosphorylation of tau proteins by targeting UCHL1 so contributed to AD pathogenesis | Zhao et al., 2014 |
| miR-485-3p | Serum samples from 89 AD patients and 62 healthy controls | SH-SY5Y, BV2 | AKT3 | - | Its knockdown promoted cell proliferation, inhibited apoptosis and neuroinflammation partly by targeting AKT3. Its expression has been associated with MMSE score, inflammatory response. | Yu et al., 2020 |
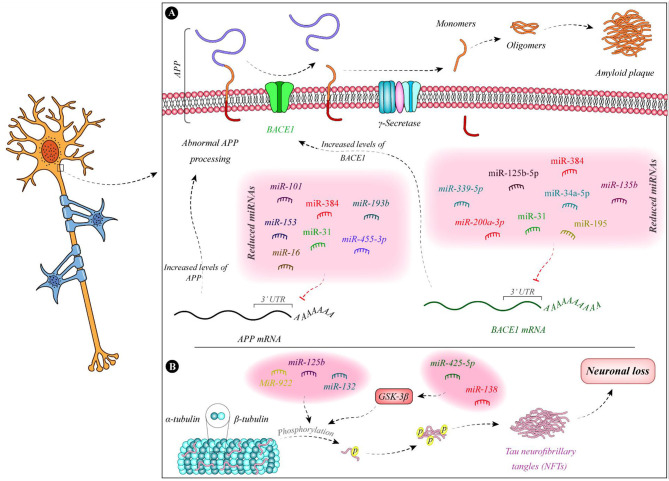
Figure 1 demonstrates the function of a number of miRNAs in the pathogenesis of AD.
Figure 1.
Summary of the function of miRNAs in the pathogenesis of AD. (A) Expressions of miR-135b (Zhang et al., 2016b), miR-195 (Zhu et al., 2012), miR-34a-5p (Liang et al., 2020), miR-384 (Liu et al., 2014b), miR-125b-5p (Liang et al., 2020), miR-31 (Barros-Viegas et al., 2020), miR-200a-3p (Pan et al., 2019), and miR-339-5p (Long et al., 2014) are decreased in patients with Alzheimer's disease. These miRNAs bind with the 3' UTR of BACE1 and decrease its expression. Therefore, the downregulation of these miRNAs leads to the upregulation of BACE1. In addition, expression levels of some APP-binding miRNAs namely miR-101 (Vilardo et al., 2010), miR-153 (Liang et al., 2012), miR-16 (Liu et al., 2012), miR-384 (Liu et al., 2014b), miR-31 (Barros-Viegas et al., 2020), miR-193b (Liu et al., 2014a), and miR-455-3p (Kumar et al., 2019) is decreased in patients with Alzheimer's disease. (B) Tau phosphorylation leads to defects in microtubules and induction of neurofibrillary tangles which result in neuron death. miR-138 and miR-425-5p are increased in Alzheimer's disease. These miRNAs regulate the expression of GSK-3β and enhance Tau phosphorylation (Wang et al., 2015b; Yuan et al., 2020). In addition, downregulation of miR-132 and upregulation of miR-125b and miR-922 leads to Tau hyperphosphorylation (Zhao et al., 2014; Salta et al., 2016; Ma et al., 2017).
Prognostic and Diagnostic Role of miRNAs in AD
The prognostic role of miRNAs in AD has been assessed in a single study. Xie et al. have evaluated serum levels of miR-206, miR-132, BDNF, and SIRT1 in a cohort of patients with amnestic mild cognitive impairment at baseline and after 5-year follow-up. Their results have shown higher levels of miR-206 in patients who converted to AD both at the baseline and after 5-year follow-up. However, miR-132 levels have been statistically similar between the conversion and non-conversion groups at both time points. Based on the Kaplan-Meier analysis, AD conversion has been correlated with over-expression of miR-206. In addition, multivariate Cox regression analysis has shown the suitability of serum miR-206 and its target BDNF as indicators of AD conversion (Xie et al., 2017). The diagnostic role of several miRNAs has been appraised in AD. For instance, downregulation of miR-433 and miR-133b in serum samples could precisely differentiate between AD subjects and normal persons (Yang et al., 2019b; Wang and Zhang, 2020). Moreover, the expression profile of the former miRNA in the cerebrospinal fluid (CSF) has appropriate diagnostic power for distinguishing AD patients (Wang and Zhang, 2020). The most astonishing results have been obtained for miR-34c. Expression of miR-34c has been increased in both cellular and plasma constituents of blood specimens of AD patients. The area under the receiver operating characteristic curve has been estimated to be 0.99. Moreover, expression of miR-34c has been inversely correlated with mental performance, as described by the mini-mental state examination. miR-34c has also been shown to affect the expression of numerous genes being involved in neuron survival and oxidative processes (Bhatnagar et al., 2014). Expression levels of miR-132 and miR-212 in neural-derived extracellular vesicles have been demonstrated to differentiate patients with AD from healthy subjects, yet their aptitude in identifying both AD and mild cognitive impairment as different from a healthy status has not been suitable (Cha et al., 2019). Table 3 summarizes the outlines of various studies that have reported on the diagnostic value of miRNAs in AD.
Table 3.
Diagnostic role of miRNAs in AD.
| microRNA | Expression pattern | Samples | ROC curve analysis | References | ||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | AUC | ||||
| miR-133b | Downregulated | Serum samples from 105 AD patients and 98 control individuals | 90.8% | 74.3% | 0.907 | Yang et al., 2019b |
| miRNA-101a | Downregulated | Plasma samples from 46 AD patients 60 healthy individuals | 0.913 | 0.733 | 0.8725 | Xiao et al., 2019 |
| miR-433 | Downregulated | Serum samples from 118 AD patients and 62 healthy controls | 78.8% | 80.6% | 0.827 | Wang and Zhang, 2020 |
| miR-433 | Downregulated | CSF samples from 32 AD patients and 12 controls | 84.4% | 91.7% | 0.952 | |
| hsa-miR-21-5p | Downregulated (in AD patients compared with DLB patients) | Plasma extracellular vesicles from 18 patients with dementia with Lewy bodies (DLB), 10 AD patients and 15 age- and sex-matched healthy controls | - | - | 0.93 | Gámez-Valero et al., 2019 |
| hsa-miR-451a | Downregulated (in AD patients compared with DLB patients) | - | - | 0.95 | ||
| miR-103 | Downregulated (in AD patients compared with PD patients and controls) | Plasma samples from 120 AD patients, 120 patients with Parkinson's disease (PD) and 120 healthy subjects | 80.0% | 84.2% | 0.891 | Wang et al., 2020 |
| miR-103 | Downregulated (in AD patients compared with PD patients and controls) | 86.7% | 55.0% | 0.775 | ||
| miR-107 | Downregulated (in AD patients compared with controls) | 77.5% | 59.2% | 0.739 | ||
| miR-132 | Downregulated | Blood samples (for neurally derived plasma exosomes) from 16 AD patients, 16 patients with mild cognitive impairment (MCI), and 31 controls | - | - | 0.58 (distinguishing AD and MCI patients from controls) | Cha et al., 2019 |
| miR-132 | Downregulated | - | - | 0.77 (distinguishing AD patients from controls) | ||
| miR-212 | Downregulated | - | - | 0.68 (distinguishing AD and MCI patients from controls) | ||
| miR-212 | Downregulated | - | - | 0.84 (distinguishing AD patients from controls) | ||
| has-miR-346 has-miR-345-5p has-miR-122-3p has-miR-208b-3p has-miR-1291 hsa-miR-640 has-miR-499a-5p has-miR-650 has-miR-1285-3p has-miR-1299 has-miR-1267 has-miR-206 |
Upregulated Upregulated Upregulated Downregulated Upregulated Upregulated Downregulated Upregulated Upregulated Upregulated Upregulated Downregulated |
Serum samples from 51 controls and 32 AD patients | 90.0% | 66.7% | - | Zhao et al., 2020 |
| miR-106b | Downregulated | Serum samples from 56 AD patients and 50 healthy volunteers | 94% | 62% | 0.80. | Madadi et al., 2020 |
| miR-16-5p | Downregulated | CSF samples from 17 Young-onset AD (YOAD), 13 Late-onset AD (LOAD) and 12 healthy controls | - | - | 0.760 | McKeever et al., 2018 |
| miR-451a | Downregulated | - | - | 0.951 | ||
| miR-605-5p | Downregulated | - | - | 0.706 | ||
| miR-125b-5p | Upregulated | - | - | 0.723 | ||
| miR-451a | Downregulated | - | - | 0.847 | ||
| miR-605-5p | Downregulated | - | - | 0.765 | ||
| miR-125b-5p | Upregulated | - | - | 0.785 | ||
| miR-501-3p | Downregulated | Serum samples from 36 patients with AD and 22 age-matched control volunteers | 53% | 100%, | 0.82 | Hara et al., 2017 |
| hsa-miR-26a-5p hsa-miR-181c-3p hsa-miR-126-5p hsa-miR-22-3p hsa-miR-148b-5p hsa-miR-106b-3p hsa-miR-6119-5p hsa-miR-1246 hsa-miR-660-5p |
Downregulated Downregulated Downregulated Downregulated Downregulated Upregulated Upregulated Upregulated Upregulated |
Serum samples 121 patients with AD and 86 healthy controls | - | - | 0.987 | Guo et al., 2017 |
| hsa-miR-106a-5p | Downregulated | Blood samples from 172 AD patients and 109 healthy controls | 68% | 93% | - | Yilmaz et al., 2016 |
| miR-31 miR-93 miR-143 miR-146a |
Downregulated Downregulated Downregulated Downregulated |
Serum samples 79 AD patients and 75 controls | - | - | 0.709 | Li et al., 2015 |
| miR-342-3p | Downregulated | Serum samples from 208 patients with AD and 205 age- and sex-matched healthy volunteers | 81.5% | 70.1% | - | Tan et al., 2014 |
| miR-125a-5p | Upregulated | CSF samples from 48 patients with behavioral variant of frontotemporal dementia (bvFTD), 48 patients with AD and 44 healthy controls | 74% | 82% | 0.75 | Denk et al., 2018 |
| miR-30a-5p | Upregulated | 78% | 68% | 0.73 | ||
| miR-20a-5p | Upregulated | Serum samples from 48 patients with bvFTD, 47 patients with AD, and 38 healthy controls | - | 92% | 0.85 | |
| miR-29b-3p | Upregulated | 93% | - | 0.83 | ||
| miR-26b-5p | Upregulated | 89% | 89% | 0.97 | ||
| miR-320a | Downregulated | 83% | 90% | 0.90 | ||
| miR-483-5p | Upregulated | Plasma samples from 20 AD patients, 15 MCI-AD patients and 15 non-demented controls (CTR) | - | - | 0.99 (AD vs. CTR) | Nagaraj et al., 2017 |
| miR-483-5p | - | - | 0.95 (MCI-AD vs. CTR) | |||
| miR-502-3p | Upregulated | - | - | 0.94 (AD vs. CTR) | ||
| miR-502-3p | - | - | 0.86 (MCI-AD vs. CTR) | |||
| miR-485-3p | Upregulated | Serum samples from 89 AD patients and 62 healthy controls | 84.3% | 96.8% | 0.933 | Yu et al., 2020 |
| miR-425 | Upregulated | Blood samples (for PBMC) from 45 AD patients and 41 age- and gender-matched healthy controls | - | - | 0.868 | Ren et al., 2016 |
| miR-339 | Upregulated | - | - | 0.761 | ||
| miR-206 | Upregulated (in aMCI-AD group compared with aMCI-aMCI group) | Serum sample from 458 amnestic mild cognitive impairment (aMCI) | 95.5% | 77.8% | 0.95 | Xie et al., 2017 |
| miR-455-3p | Upregulated | Postmortem brain samples from 27 AD patients and 15 controls | - | - | 0.792 | Kumar and Reddy, 2018 |
| miR-455-3p | Upregulated | Skin fibroblast cell from 4 patients with familial AD, 6 patients with sporadic AD, and eight healthy control | - | - | 0.861 | |
| miR-455-3p | Upregulated | Serum samples from 10 AD patients, 20 MCI patients and 18 healthy controls | - | - | 0.79 | Kumar et al., 2017 |
| miR-455-3p | Upregulated | Postmortem brain tissues from 16 AD patients and 5 controls | - | - | 0.86 | |
| miR-34c | Upregulated | Plasma samples from 110 AD patients and 123 control subjects | 0.92 | 0.96 | 0.99 | Bhatnagar et al., 2014 |
| miR-29a | Upregulated | CSF samples from 18 patients with AD and 20 healthy volunteers | 89% | 70% | 0.87 | Müller et al., 2016 |
miRNA Polymorphisms and Risk of AD
Boscher et al. have screened a larger cohort of early-onset AD (EOAD) patients who did not have autosomal dominant mutations for the presence of genetic polymorphisms. They have recognized 86 copy number variants (CNVs) in miRNA-coding genes, 31 of them being only present in EOAD cases. Duplication of the MIR138-2 locus has been one of these CNVs. Based on the role of miR-138 in Aβ production and tau phosphorylation, this CNV might be implicated in the risk of EOAD (Boscher et al., 2019). Functionally, miR-138 upregulation enhances Aβ synthesis and tau phosphorylation through modulation of GSK-3β and FERMT2 (Boscher et al., 2019). Other studies have demonstrated the role of rs2910164 of pri-miR-146a, rs57095329 of miR-146a, and rs2291418 of miR-1229 precursor in conferring risk of AD (Table 4). Zhang et al. have scanned the coding region of pri-miR-146a in AD patients. Among the four single nucleotide polymorphisms (SNPs) located in this genomic region, rs2910164 has been identified as a risk locus for AD as the C allele of this SNP has enhanced risk of AD.
Table 4.
miRNA polymorphisms and risk of AD.
| microRNA | Polymorphism | Samples | Population | Assay method | Function | References |
|---|---|---|---|---|---|---|
| miR-138 | Copy number variant (CNV) | Whole exome sequencing data of 546 unrelated patients with early-onset Alzheimer's disease (EOAD) and 597 controls subjects | French | QMPSF | Its duplication was observed in EOAD patients and functional studies showed that miR-138 upregulation caused increased production of Aβ and higher phosphorylation of tau. So miR-138 gene dosage can be a potential risk factor for EOAD. |
Boscher et al., 2019 |
| Pri-miR-146a | SNP (rs2910164) | Blood samples from 103 AD patients and 206 healthy controls | Han Chinese | Sequencing | Rare C allele of this SNP was correlated AD and low expression of mature miR-146a-5p. | Zhang et al., 2015 |
| miR-146a | SNP (rs57095329) | Blood samples from 292 AD patients 300 healthy volunteers | Chinese | ABI PRISM SNapShot method | AA genotype of rs57095329 was correlated with an elevated predisposition to AD and was associated with high expression of miR-146a. | Cui et al., 2014 |
| miR-1229 precursor | SNP (rs2291418) | Analysis of GWAS data on late-onset AD | - | - | rs2291418 was associated with AD risk. An allele of rs2291418 was correlated with an increased miR-1229-3p expression that targets an AD-related gene, SORL1, so can have an important role in AD. | Ghanbari et al., 2016 |
Notably, this variant has been shown to reduce the expression of mature miR-146a-5p, releasing TLR2 from its inhibitory effects. Moreover, cell line studies have shown the impact of the C allele on upregulation of expression of TNF-α after induction with β-amyloid. Therefore, this SNP might predispose patients to AD by disturbing the production of mature miRNA and influencing the activity and expression level of TLR2 (Zhang et al., 2015). Cui et al. have analyzed the genotype and allele frequencies of rs2910464 and rs57095329 of miR-146a and have reported that the AA genotype of the former SNP increases susceptibility to AD and results in cognitive reduction in the affected individuals. Contrary to the previously mentioned study by Zhang et al., the risk genotype has been associated with higher levels of miR-146a in the PBMCs of control subjects and has exerted more robust effects on IL-6 and IL-1β synthesis following stimulation with LPS (Cui et al., 2014). Finally, in a genome-wide association study, Ghanbari et al. have detected an association between rs2291418 in the miR-1229 precursor and risk of AD. The risk allele of this SNP has been shown to increase the expression of miR-1229-3p, thus decreasing the expression of SORL1, an AD-associated gene. In addition, among more than 42,000 variants in miRNA-binding regions, 10 variants in the 3' UTR of nine genes have been associated with this disorder; among them has been rs6857, which enhances the miR-320e-mediated modulation of PVRL2 expression (Ghanbari et al., 2016).
Effects of Herbal/Chemical Agents on the Expression of miRNAs in the Context of AD
Osthole, the active component of the fruits of the genus Cnidium moonnieri (L.) Cussion has been shown to affect the AD course via modulation of miRNAs expression. Lin et al. have shown miR-101a-3p as the main affected miRNA by osthole. APP has been identified as the target of miR-101a-3p. Osthole has enhanced the learning and memory aptitude in an animal model of AD, and it has inhibited APP levels by promoting the expression of miR-101a-3p (Lin et al., 2019). Other studies have verified the effects of Osthole on the expression of miR-9 (Li et al., 2016, 2017). Functionally, osthole enhances the viability of neurons, decreases apoptosis of these cells, and reverses the decline of synaptic proteins in APP-expressing cells by affecting miR-9 expression and consequently decreasing CAMKK2 and p-AMPKα levels (Li et al., 2016). Additionally, osthole has pro-survival effects in APP-expressing neural stem cells through suppression of the Notch pathway (Li et al., 2017). Moreover, Berberine has been shown to enhance proliferation and attenuate neuron apoptosis via regulation of miR-188/NOS1 molecular cascade (Chen et al., 2020b). Treatment of Aβ-treated murine microglia and neuroblastoma cells with this substance or upregulation of miR-188 in these cells has accelerated cell proliferation and suppressed caspase-3 activity and apoptosis (Chen et al., 2020b). Finally, exmedetomidine has been demonstrated to accomplish neuroprotective effects and enhance cognitive function in an animal model of AD by modulating the miR-129/YAP1/JAG1 cascade (Sun et al., 2020). Table 5 shows the effects of different AD-modifying compounds on the expression of miRNAs.
Table 5.
Effect of different compounds on microRNAs.
| microRNA | Compound | Cell line | Animal model | Gene/protein interaction | Results | References |
|---|---|---|---|---|---|---|
| miR-101a-3p | Osthole | SH-SY5Y | APP/PS1 mice | APP | miR-101a-3p was upregulated by Osthole and its upregulation led to improved memory function and learning capacity and prevented Aβ formation through targeting APP | Lin et al., 2019 |
| miR-9 | Osthole | Neural stem cells obtained from newborn C57BL/6 mice | APP/PS1 double transgenic mice | - | miR-9 was upregulated by Osthole and this caused improved cell survival, reduced cell death, alleviated cognitive deficit. | Li et al., 2017 |
| miR-9 | Osthole | SH-SY5Y, primary cortical neurons obtained from C57BL/6 mice | - | - | Osthole improved cell survival and suppressed apoptosis through upregulation of miR-9 expression. | Li et al., 2016 |
| miR-34a | Tiaoxin Recipe | - | APPswe/PS1ΔE9 mice | - | Tiaoxin Recipe downregulated expression of miR-34a and ameliorated memory dysfunction, Aβ formation | Boscher et al., 2019 |
| miR-188 | Berberine | BV2, N2a | - | NOS1 | Berberine enhanced proliferation and inhibited apoptosis partly through regulation of the miR-188/NOS1 axis | Chen et al., 2020b |
| miR-129 | Dexmedetomidine | Primary hippocampal neurons | Male NIH Swiss mice | YAP1 | miR-129 was upregulated by Dexmedetomidine and its upregulation led to decreased apoptosis rate and alleviated cognitive decline through targeting YAP1 and prevention of YAP1 interaction with JAG1 | Sun et al., 2020 |
Discussion
Numerous studies have demonstrated abnormal expression of miRNAs in AD subjects or cell/animal models of AD. However, each miRNA has been the subject of expression and functional assays in few independent studies. miR-146 has been among the miRNAs most assessed in the context of AD, as its expression levels, functions, and polymorphisms have been assessed in association with AD. miR-9 is another AD-associated miRNA whose expression has been altered following treatment of APP-expressing cells with anti-AD substances. In some cases, altered expression of a certain miRNA is regarded as a part of a self-protective process. For instance, the reduction of miR-409-5p expression in the early stages of AD might be associated with lower Aβ-induced synaptic injury (Guo et al., 2019). Similarly, upregulation of miR-200b and miR-200c has protective effects against AD-associated neurotoxicity (Higaki et al., 2018). However, in most cases, an aberrant miRNA signature directly participates in the pathogenesis of AD. miRNAs partake in the pathobiology of AD through various mechanisms, including the regulation of BACE1 activity. miR-200a-3p, miR-195, miR-338-5p, miR-34a-5p, miR-125b-5p, miR-132, miR-384, miR-339-5p, miR-135b, miR-425-5p, and miR-339-5p are among the miRNAs whose interaction with BACE1 has been verified in different investigation. Other miRNAs, such as miR-129-5p, miR-22, and miR-206, might affect the inflammatory responses in the course of AD. Moreover, a number of miRNAs, such as miR-326, miR-338-5p, miR-124-3p, miR-34a, miR-326, and miR-98, modulate apoptotic pathways in neurons, thereby affecting the AD course via this route. Tau phosphorylation can be modulated by some miRNAs, namely, miR-200a-3p, miR-326, miR-124-3p, miR-146a, miR-425-5p, and miR-132. Expression of miR-132 has been assessed by several investigations with most of them reporting its downregulation in AD (Wong et al., 2013; El Fatimy et al., 2018; Cha et al., 2019; Deng et al., 2020). Yet, Liu et al. have reported over-expression of miR-132 in patients with mild cognitive impairment and AD vs. normal individuals (Liu and Zhang, 2019).
Abnormal levels of miRNAs in serum or CSF samples have been shown to distinguish AD subjects from normal subjects, indicating their suitability as disease biomarkers. However, these studies have not been validated in independent cohorts. miR-103, miR-126, miR-93, miR-29, miR-424, and miR-181 are among AD-associated miRNAs with biomarker potential whose application as disease biomarkers has been validated in other disorders (So et al., 2020).
Animal studies have shown promising results regarding the impact of miRNA modifications on the course of AD. However, based on the unavailability of brain tissue for therapeutic interventions in human subjects, identification of appropriate transport mechanisms for delivery of anta-/ago-miRs to this tissue is an important issue.
The anti-AD effects of Osthole, Tiaoxin Recipe, Berberine, and Dexmedetomidine have been shown to be exerted through modulation of a number miRNAs, such as miR-101a-3p, miR-9, miR-34a, miR-188, and miR-129, emphasizing further the impact of miRNAs in the progression of AD. However, these results should be verified in human subjects as well.
Few studies have shown the association between miRNA CNVs/ SNPs and the risk of AD. However, these results have not been verified in different ethnic groups. Re-assessment of the results of genome-wide association studies with a focus on non-coding regions might lead to the identification of further risk loci for this multifactorial condition.
Finally, a limitation of several functional investigations in this field is that they have assessed miRNA functions in cell lines such as HEK293 and SH-SY5Y, which are not true models of AD.
Author Contributions
MT and SG-F wrote the draft and revised it. MS, MH, and MG collected the data, designed the tables, and figures. All authors contributed to the article approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- AD
Alzheimer's disease
- microRNA
miRNA
- Aβ
β-amyloid peptides
- BACE1
β-secretase
- EOAD
early-onset Alzheimer's disease
- CNV
copy number variant
- SNP
single nucleotide polymorphism
- AChE
Acetylcholinesterase
- iNOS
Inducible nitric oxide synthase
- ROS
reactive oxygen species
- MDA
Malondialdehyde
- MAPK
mitogen-activated protein kinase
- SOD
Superoxide dismutase
- GSH-Px
glutathione peroxidase.
References
- Absalon S., Kochanek D. M., Raghavan V., Krichevsky A. M. (2013). MiR-26b, upregulated in Alzheimer's disease, activates cell cycle entry, tau-phosphorylation, and apoptosis in postmitotic neurons. J. Neurosci. 33, 14645–14659. 10.1523/JNEUROSCI.1327-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter R., Shao Y., Shaw M., Formica S., Khrestian M., Leverenz J. B., et al. (2018). Regulation of ADAM10 by miR-140-5p and potential relevance for Alzheimer's disease. Neurobiol. Aging. 63, 110–119. 10.1016/j.neurobiolaging.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C., Gauthier S., Corbett A., Brayne C., Aarsland D., Jones E. (2011). Alzheimer's disease. Lancet 377, 1019–1031. 10.1016/S0140-6736(10)61349-9 [DOI] [PubMed] [Google Scholar]
- Banzhaf-Strathmann J., Benito E., May S., Arzberger T., Tahirovic S., Kretzschmar H., et al. (2014). Micro RNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer's disease. EMBO J. 33, 1667–1680. 10.15252/embj.201387576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros-Viegas A. T., Carmona V., Ferreiro E., Guedes J., Cardoso A. M., Cunha P., et al. (2020). MiRNA-31 improves cognition and abolishes amyloid-β pathology by targeting APP and BACE1 in an animal model of Alzheimer's disease. Mol. Therapy Nucl. Acids 19, 1219–1236. 10.1016/j.omtn.2020.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S., Chertkow H., Schipper H. M., Shetty V., Yuan Z., Jones T., et al. (2014). Increased microRNA-34c abundance in Alzheimer's disease circulating blood plasma. Front. Mol. Neurosci. 7:2. 10.3389/fnmol.2014.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscher E., Husson T., Quenez O., Laquerrière A., Marguet F., Cassinari K., et al. (2019). Copy number variants in miR-138 as a potential risk factor for early-onset Alzheimer's disease. J. Alzheimer's Dis. 68, 1243–1255. 10.3233/JAD-180940 [DOI] [PubMed] [Google Scholar]
- Cao J., Huang M., Guo L., Zhu L., Hou J., Zhang L., et al. (2020). MicroRNA-195 rescues ApoE4-induced cognitive deficits and lysosomal defects in Alzheimer's disease pathogenesis. Mol. Psychiatry. 2020, 1–15. 10.1038/s41380-020-0824-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha D. J., Mengel D., Mustapic M., Liu W., Selkoe D. J., Kapogiannis D., et al. (2019). miR-212 and miR-132 are downregulated in neurally derived plasma exosomes of Alzheimer's patients. Front. Neurosci. 13:1208. 10.3389/fnins.2019.01208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. W. Q. (2020). MicroRNA miR-212 regulates PDCD4 to attenuate Ab25–35-induced neurotoxicity via PI3K/AKT signaling pathway in Alzheimer's disease. Biotechnol. Lett. 42, 1789–1797. 10.1007/s10529-020-02915-z [DOI] [PubMed] [Google Scholar]
- Chen F. Z., Zhao Y., Chen H. Z. (2019). MicroRNA-98 reduces amyloid β-protein production and improves oxidative stress and mitochondrial dysfunction through the Notch signaling pathway via HEY2 in Alzheimer's disease mice. Int. J. Mol. Med. 43, 91–102. 10.3892/ijmm.2018.3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Li L., Liu C., Song L. (2020b). Berberine attenuates Aβ-induced neuronal damage through regulating miR-188/NOS1 in Alzheimer's disease. Mol. Cell. Biochem. 474, 285–294. 10.1007/s11010-020-03852-1 [DOI] [PubMed] [Google Scholar]
- Chen W., Wu L., Hu Y., Jiang L., Liang N., Chen J., et al. (2020a). MicroRNA-107 ameliorates damage in a cell model of Alzheimer's disease by mediating the FGF7/FGFR2/PI3K/Akt pathway. J. Mol. Neurosci. 70, 1589–1597. 10.1007/s12031-020-01600-0 [DOI] [PubMed] [Google Scholar]
- Cui J. G., Li Y. Y., Zhao Y., Bhattacharjee S., Lukiw W. J. (2010). Differential regulation of interleukin-1 receptor-associated kinase-1 (IRAK-1) and IRAK-2 by microRNA-146a and NF-κB in stressed human astroglial cells and in Alzheimer disease. J. Biol. Chem. 285, 38951–38960. 10.1074/jbc.M110.178848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Li Y., Ma G., Wang Y., Cai Y., Liu S., et al. (2014). A functional polymorphism in the promoter region of microRNA-146a is associated with the risk of Alzheimer disease and the rate of cognitive decline in patients. PLoS ONE 9:e89019. 10.1371/journal.pone.0089019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Zhang J., Sun X., Ma G., Luo G., Miao Z., et al. (2020). miR-132 improves the cognitive function of rats with Alzheimer's disease by inhibiting the MAPK1 signal pathway. Exp. Therapeutic Med. 20:1. 10.3892/etm.2020.9288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk J., Oberhauser F., Kornhuber J., Wiltfang J., Fassbender K., Schroeter M. L., et al. (2018). Specific serum and CSF microRNA profiles distinguish sporadic behavioural variant of frontotemporal dementia compared with Alzheimer patients and cognitively healthy controls. PLoS ONE 13:e0197329. 10.1371/journal.pone.0197329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q., Si E. (2019). MicroRNA-25 aggravates Aβ1-42-induced hippocampal neuron injury in Alzheimer's disease by downregulating KLF2 via the Nrf2 signaling pathway in a mouse model. J. Cell. Biochem. 120, 15891–15905. 10.1002/jcb.28861 [DOI] [PubMed] [Google Scholar]
- El Fatimy R., Li S., Chen Z., Mushannen T., Gongala S., Wei Z., et al. (2018). MicroRNA-132 provides neuroprotection for tauopathies via multiple signaling pathways. Acta Neuropathol. 136, 537–555. 10.1007/s00401-018-1880-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-de Frutos M., Galán-Chilet I., Goedeke L., Kim B., Pardo-Marqués V., Pérez-García A., et al. (2019). MicroRNA 7 impairs insulin signaling and regulates Aβ levels through posttranscriptional regulation of the insulin receptor substrate 2, insulin receptor, insulin-degrading enzyme, and liver X receptor pathway. Mol. Cell. Biol. 39, e00170–e00119. 10.1128/MCB.00170-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gámez-Valero A., Campdelacreu J., Vilas D., Ispierto L., Reñé R Álvarez, R., et al. (2019). Exploratory study on microRNA profiles from plasma-derived extracellular vesicles in Alzheimer's disease and dementia with Lewy bodies. Transl. Neurodegeneration 8:31. 10.1186/s40035-019-0169-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L., Zhang T., Liu W., Chen Y. (2018). Inhibition of miR-128 abates Aβ-mediated cytotoxicity by targeting PPAR-γ via NF-κB inactivation in primary mouse cortical neurons and Neuro2a cells. Yonsei Med. J. 59, 1096–1106. 10.3349/ymj.2018.59.9.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari M., Ikram M. A., De Looper H. W., Hofman A., Erkeland S. J., Franco O. H., et al. (2016). Genome-wide identification of microRNA-related variants associated with risk of Alzheimer's disease. Sci. Rep. 6:28387. 10.1038/srep28387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Cai Y., Ye X., Ma N., Wang Y., Yu B., et al. (2019). MiR-409-5p as a regulator of neurite growth is down regulated in APP/PS1 murine model of Alzheimer's disease. Front. Neurosci. 13:1264. 10.3389/fnins.2019.01264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R., Fan G., Zhang J., Wu C., Du Y., Ye H., et al. (2017). A 9-microRNA signature in serum serves as a noninvasive biomarker in early diagnosis of Alzheimer's disease. J. Alzheimer's Dis. 60, 1365–1377. 10.3233/JAD-170343 [DOI] [PubMed] [Google Scholar]
- Han C., Guo L., Yang Y., Guan Q., Shen H., Sheng Y., et al. (2020). Mechanism of microRNA-22 in regulating neuroinflammation in Alzheimer's disease. Brain Behav. 2020:e01627. 10.1002/brb3.1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Zhou Y., Zhang R., Wu K., Lu Y., Li Y., et al. (2018). MicroRNA Let-7f-5p promotes bone marrow mesenchymal stem cells survival by targeting caspase-3 in alzheimer disease model. Front. Neurosci. 12:333. 10.3389/fnins.2018.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara N., Kikuchi M., Miyashita A., Hatsuta H., Saito Y., Kasuga K., et al. (2017). Serum microRNA miR-501-3p as a potential biomarker related to the progression of Alzheimer's disease. Acta Neuropathol. Commun. 5:10. 10.1186/s40478-017-0414-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Chen W., Zeng J., Tong W., Zheng P. (2020). MicroRNA-326 decreases tau phosphorylation and neuron apoptosis through inhibition of the JNK signaling pathway by targeting VAV1 in Alzheimer's disease. J. Cell. Physiol. 235, 480–493. 10.1002/jcp.28988 [DOI] [PubMed] [Google Scholar]
- Hernandez-Rapp J., Rainone S., Goupil C., Dorval V., Smith P. Y., Saint-Pierre M., et al. (2016). microRNA-132/212 deficiency enhances Aβ production and senile plaque deposition in Alzheimer's disease triple transgenic mice. Sci. Rep. 6:30953. 10.1038/srep30953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki S., Muramatsu M., Matsuda A., Matsumoto K., Satoh J-i, Michikawa M., et al. (2018). Defensive effect of microRNA-200b/c against amyloid-beta peptide-induced toxicity in Alzheimer's disease models. PLoS ONE 13:e0196929. 10.1371/journal.pone.0196929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Wang H., Chen K., Cheng P., Gao S., Liu J., et al. (2015). MicroRNA-34c downregulation ameliorates amyloid-β-induced synaptic failure and memory deficits by targeting VAMP2. J. Alzheimer's Dis. 48, 673–686. 10.3233/JAD-150432 [DOI] [PubMed] [Google Scholar]
- Hu Y-K., Wang X., Li L., Du Y-H., Ye H-T., Li C-Y. (2013). MicroRNA-98 induces an Alzheimer's disease-like disturbance by targeting insulin-like growth factor 1. Neurosci. Bulletin 29, 745–751. 10.1007/s12264-013-1348-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Wu X., Xue Y., Zhou Y., Xiang H., Yang W., et al. (2020). MicroRNA-3614 regulates inflammatory response via targeting TRAF6-mediated MAPKs and NF-κB signaling in the epicardial adipose tissue with coronary artery disease. Int. J. Cardiol. 2020:45. 10.1016/j.ijcard.2020.09.045 [DOI] [PubMed] [Google Scholar]
- Iqbal K., Grundke-Iqbal I. (2010). Alzheimer's disease, a multifactorial disorder seeking multitherapies. Alzheimers Dement. 6, 420–424. 10.1016/j.jalz.2010.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Tu Q., Liu M. (2018). MicroRNA-125b regulates Alzheimer's disease through SphK1 regulation. Mol. Med. Rep. 18, 2373–2380. 10.3892/mmr.2018.9156 [DOI] [PubMed] [Google Scholar]
- Kang Q., Xiang Y., Li D., Liang J., Zhang X., Zhou F., et al. (2017). MiR-124-3p attenuates hyperphosphorylation of Tau protein-induced apoptosis via caveolin-1-PI3K/Akt/GSK3β pathway in N2a/APP695swe cells. Oncotarget 8:24314. 10.18632/oncotarget.15149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Yoon H., Horie T., Burchett J. M., Restivo J. L., Rotllan N., et al. (2015). microRNA-33 regulates ApoE lipidation and amyloid-β metabolism in the brain. J. Neurosci. 35, 14717–14726. 10.1523/JNEUROSCI.2053-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Reddy A. P., Yin X., Reddy P. H. (2019). Novel MicroRNA-455-3p and its protective effects against abnormal APP processing and amyloid beta toxicity in Alzheimer's disease. Biochim. Biophys. Acta 1865, 2428–2440. 10.1016/j.bbadis.2019.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Reddy P. H. (2018). MicroRNA-455-3p as a potential biomarker for Alzheimer's disease: an update. Front. Aging Neurosci. 10:41. 10.3389/fnagi.2018.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Vijayan M., Reddy P. H. (2017). MicroRNA-455-3p as a potential peripheral biomarker for Alzheimer's disease. Hum. Mol. Genet. 26, 3808–3822. 10.1093/hmg/ddx267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Kim H., An K., Kwon O-B, Park S., Cha J. H., et al. (2016). Replenishment of microRNA-188-5p restores the synaptic and cognitive deficits in 5XFAD mouse model of Alzheimer's disease. Sci Rep. 6, 1–14. 10.1038/srep34433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Wei G., Bai Y., Li Y., Huang F., Lin J., et al. (2015). MicroRNA-574 is involved in cognitive impairment in 5-month-old APP/PS1 mice through regulation of neuritin. Brain Res. 1627, 177–188. 10.1016/j.brainres.2015.09.022 [DOI] [PubMed] [Google Scholar]
- Li J., Li D., Zhou H., Wu G., He Z., Liao W., et al. (2020a). MicroRNA-338-5p alleviates neuronal apoptosis via directly targeting BCL2L11 in APP/PS1 mice. Aging 12:10.18632. 10.18632/aging.104005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Xu Y., Wang B., Huang J., Li Q. (2020b). miR-34a-5p and miR-125b-5p attenuate Aβ-induced neurotoxicity through targeting BACE1. J. Neurol. Sci. 2020:116793. 10.1016/j.jns.2020.116793 [DOI] [PubMed] [Google Scholar]
- Li S., Yan Y., Jiao Y., Gao Z., Xia Y., Kong L., et al. (2016). Neuroprotective effect of osthole on neuron synapses in an Alzheimer's disease cell model via upregulation of MicroRNA-9. J. Mol. Neurosci. 60, 71–81. 10.1007/s12031-016-0793-9 [DOI] [PubMed] [Google Scholar]
- Li S-H., Gao P., Wang L-T., Yan Y-H., Xia Y., Song J., et al. (2017). Osthole stimulated neural stem cells differentiation into neurons in an Alzheimer's disease cell model via upregulation of microRNA-9 and rescued the functional impairment of hippocampal neurons in APP/PS1 transgenic mice. Front. Neurosci. 11:340. 10.3389/fnins.2017.00340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Zhu H., Xu Y., Huang L., Ma C., Deng W., et al. (2012). MicroRNA-153 negatively regulates the expression of amyloid precursor protein and amyloid precursor-like protein 2. Brain Res. 1455, 103–113. 10.1016/j.brainres.2011.10.051 [DOI] [PubMed] [Google Scholar]
- Liang X., Wang L., Wang M., Liu Z., Liu X., Zhang B., et al. (2020). MicroRNA-124 inhibits macrophage cell apoptosis via targeting p38/MAPK signaling pathway in atherosclerosis development. Aging 12:13005. 10.18632/aging.103387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Liang X., Yao Y., Xiao H., Shi Y., Yang J. (2019). Osthole attenuates APP-induced Alzheimer's disease through upregulating miRNA-101a-3p. Life Sci. 225, 117–131. 10.1016/j.lfs.2019.04.004 [DOI] [PubMed] [Google Scholar]
- Liu C-G., Song J., Zhang Y-Q., Wang P-C. (2014a). MicroRNA-193b is a regulator of amyloid precursor protein in the blood and cerebrospinal fluid derived exosomal microRNA-193b is a biomarker of Alzheimer's disease. Mol. Med. Rep. 10, 2395–2400. 10.3892/mmr.2014.2484 [DOI] [PubMed] [Google Scholar]
- Liu C-G., Wang J-L., Li L., Wang P-C. (2014b). MicroRNA-384 regulates both amyloid precursor protein and β-secretase expression and is a potential biomarker for Alzheimer's disease. Int. J. Mol. Med. 34, 160–166. 10.3892/ijmm.2014.1780 [DOI] [PubMed] [Google Scholar]
- Liu D. Y., Zhang L. (2019). MicroRNA-132 promotes neurons cell apoptosis and activates Tau phosphorylation by targeting GTDC-1 in Alzheimer's disease. Eur. Rev. Med. Pharmacol. Sci. 23, 8523–32. 10.26355/eurrev_201910_19166 [DOI] [PubMed] [Google Scholar]
- Liu F., Zhang Z., Chen W., Gu H., Yan Q. (2018). Regulatory mechanism of microRNA-377 on CDH13 expression in the cell model of Alzheimer's disease. Eur. Rev. Med. Pharmacol. Sci. 22, 2801–8. 10.26355/eurrev_201805_14979 [DOI] [PubMed] [Google Scholar]
- Liu H., Chu W., Gong L., Gao X., Wang W. (2016b). MicroRNA-26b is upregulated in a double transgenic mouse model of Alzheimer's disease and promotes the expression of amyloid-β by targeting insulin-like growth factor 1. Mol. Med. Rep. 13, 2809–2814. 10.3892/mmr.2016.4860 [DOI] [PubMed] [Google Scholar]
- Liu J., Zuo X., Han J., Dai Q., Xu H., Liu Y., et al. (2020). MiR-9-5p inhibits mitochondrial damage and oxidative stress in AD cell models by targeting GSK-3β. Biosci. Biotechnol. Biochem. 84, 2273–2280. 10.1080/09168451.2020.1797469 [DOI] [PubMed] [Google Scholar]
- Liu W., Cai H., Lin M., Zhu L., Gao L., Zhong R., et al. (2016a). MicroRNA-107 prevents amyloid-beta induced blood-brain barrier disruption and endothelial cell dysfunction by targeting Endophilin-1. Exp. Cell Res. 343, 248–257. 10.1016/j.yexcr.2016.03.026 [DOI] [PubMed] [Google Scholar]
- Liu W., Liu C., Zhu J., Shu P., Yin B., Gong Y., et al. (2012). MicroRNA-16 targets amyloid precursor protein to potentially modulate Alzheimer's-associated pathogenesis in SAMP8 mice. Neurobiol. Aging 33, 522–534. 10.1016/j.neurobiolaging.2010.04.034 [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang Y., Liu P., Bai H., Li X., Xiao J., et al. (2019). MicroRNA-128 knockout inhibits the development of Alzheimer's disease by targeting PPARγ in mouse models. Eur. J. Pharmacol. 843, 134–144. 10.1016/j.ejphar.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Long J. M., Ray B., Lahiri D. K. (2012). MicroRNA-153 physiologically inhibits expression of amyloid-β precursor protein in cultured human fetal brain cells and is dysregulated in a subset of Alzheimer disease patients. J. Biol. Chem. 287, 31298–31310. 10.1074/jbc.M112.366336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J. M., Ray B., Lahiri D. K. (2014). MicroRNA-339-5p downregulates protein expression of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) in human primary brain cultures and is reduced in brain tissue specimens of Alzheimer disease subjects. J. Biol. Chem. 289, 5184–5198. 10.1074/jbc.M113.518241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw W. J. (2007). Micro-RNA speciation in fetal, adult and Alzheimer's disease hippocampus. Neuroreport 18, 297–300. 10.1097/WNR.0b013e3280148e8b [DOI] [PubMed] [Google Scholar]
- Ma X., Liu L., Meng J. (2017). MicroRNA-125b promotes neurons cell apoptosis and Tau phosphorylation in Alzheimer's disease. Neurosci. Lett.661, 57–62. 10.1016/j.neulet.2017.09.043 [DOI] [PubMed] [Google Scholar]
- Madadi S., Saidijam M., Yavari B., Soleimani M. (2020). Downregulation of serum miR-106b: a potential biomarker for Alzheimer disease. Archiv. Physiol. Biochem. 2020, 1–5. 10.1080/13813455.2020.1734842 [DOI] [PubMed] [Google Scholar]
- Manzine P. R., Pelucchi S., Horst M. A., Vale F. A., Pavarini S. C., Audano M., et al. (2018). microRNA 221 Targets ADAM10 mRNA and is Downregulated in Alzheimer's Disease. J. Alzheimer's Dis. 61, 113–123. 10.3233/JAD-170592 [DOI] [PubMed] [Google Scholar]
- McKeever P. M., Schneider R., Taghdiri F., Weichert A., Multani N., Brown R. A., et al. (2018). MicroRNA expression levels are altered in the cerebrospinal fluid of patients with young-onset Alzheimer's disease. Mol. Neurobiol. 55, 8826–8841. 10.1007/s12035-018-1032-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J., Jing J., Shao Y., Sun H. (2020). MicroRNA-138 promotes neuroblastoma SH-SY5Y cell apoptosis by directly targeting DEK in Alzheimer's disease cell model. BMC Neurosci. 21, 1–8. 10.1186/s12868-020-00579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi P. K., Jaiswal S., Sharma P. (2016). Regulation of neuronal cell cycle and apoptosis by microRNA 34a. Mol. Cell. Biol. 36, 84–94. 10.1128/MCB.00589-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncini S., Lunghi M., Valmadre A., Grasso M., Del Vescovo V., Riva P., et al. (2017). The miR-15/107 family of microRNA genes regulates CDK5R1/p35 with implications for Alzheimer's disease pathogenesis. Mol. Neurobiol. 54, 4329–4342. 10.1007/s12035-016-0002-4 [DOI] [PubMed] [Google Scholar]
- Müller M., Jäkel L., Bruinsma I. B., Claassen J. A., Kuiperij H. B., Verbeek M. M. (2016). MicroRNA-29a is a candidate biomarker for Alzheimer's disease in cell-free cerebrospinal fluid. Mol. Neurobiol. 53, 2894–2899. 10.1007/s12035-015-9156-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj S., Laskowska-Kaszub K., Debski K. J., Wojsiat J., Dabrowski M., Gabryelewicz T., et al. (2017). Profile of 6 microRNA in blood plasma distinguish early stage Alzheimer's disease patients from non-demented subjects. Oncotarget 8:16122. 10.18632/oncotarget.15109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J., Hayder H., Zayed Y., Peng C. (2018). Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 9:402. 10.3389/fendo.2018.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Zhang D., Zhang J., Qin P., Wang J. (2019). LncRNA RMRP silence curbs neonatal neuroblastoma progression by regulating microRNA-206/tachykinin-1 receptor axis via inactivating extracellular signal-regulated kinases. Cancer Biol. Therapy 20, 653–665. 10.1080/15384047.2018.1550568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C. P. (2013). The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer's Dement. 9, 63–75 e2. 10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Qian Q., Zhang J., He F-P, Bao W-X, Zheng T-T, Zhou D-M, et al. (2019). Downregulated expression of microRNA-338-5p contributes to neuropathology in Alzheimer's disease. FASEB J. 33, 4404–44017. 10.1096/fj.201801846R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth H. W., LaFerla F. M. (2010). Alzheimer's disease. N. Engl. J. Med. 362, 329–344. 10.1056/NEJMra0909142 [DOI] [PubMed] [Google Scholar]
- Ren R-J., Zhang Y-F., Dammer E. B., Zhou Y., Wang L-l., Liu X-H., et al. (2016). Peripheral blood MicroRNA expression profiles in Alzheimer's disease: screening, validation, association with clinical phenotype and implications for molecular mechanism. Mol. Neurobiol. 53, 5772–5781. 10.1007/s12035-015-9484-8 [DOI] [PubMed] [Google Scholar]
- Salta E., Sierksma A., Vanden Eynden E., De Strooper B. (2016). miR-132 loss de-represses ITPKB and aggravates amyloid and TAU pathology in Alzheimer's brain. EMBO Mol. Med. 8, 1005–1018. 10.15252/emmm.201606520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu B., Zhang X., Du G., Fu Q., Huang L. (2018). MicroRNA-107 prevents amyloid-β-induced neurotoxicity and memory impairment in mice. Int. J. Mol. Med. 41, 1665–1672. 10.3892/ijmm.2017.3339 [DOI] [PubMed] [Google Scholar]
- So J. B. Y., Kapoor R., Zhu F., Koh C., Zhou L., Zou R., et al. (2020). Development and validation of a serum microRNA biomarker panel for detecting gastric cancer in a high-risk population. Gut 2020:322065. 10.1136/gutjnl-2020-322065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Hu M., Zhang J., Teng Z-q, Chen C. (2019). A novel mechanism of synaptic and cognitive impairments mediated via microRNA-30b in Alzheimer's disease. EBioMedicine 39, 409–421. 10.1016/j.ebiom.2018.11.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Zhao J., Li C. (2020). Dexmedetomidine provides protection against hippocampal neuron apoptosis and cognitive impairment in mice with Alzheimer's disease by mediating the miR-129/YAP1/JAG1 axis. Mol. Neurobiol. 57, 5044–5055. 10.1007/s12035-020-02069-z [DOI] [PubMed] [Google Scholar]
- Tan L., Yu J-T., Tan M-S., Liu Q-Y., Wang H-F., Zhang W., et al. (2014). Genome-wide serum microRNA expression profiling identifies serum biomarkers for Alzheimer's disease. J. Alzheimer's Dis. 40, 1017–1027. 10.3233/JAD-132144 [DOI] [PubMed] [Google Scholar]
- Tang Y., Bao J., Su J., Huang W. (2017). MicroRNA-139 modulates Alzheimer's-associated pathogenesis in SAMP8 mice by targeting cannabinoid receptor type 2. Genet. Mol. Res. 16:19166. 10.4238/gmr16019166 [DOI] [PubMed] [Google Scholar]
- Vilardo E., Barbato C., Ciotti M., Cogoni C., Ruberti F. (2010). MicroRNA-101 regulates amyloid precursor protein expression in hippocampal neurons. J. Biol. Chem. 285, 18344–18351. 10.1074/jbc.M110.112664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Huang Y., Wang L-L, Zhang Y-F, Xu J., Zhou Y., et al. (2016). MicroRNA-146a suppresses ROCK1 allowing hyperphosphorylation of tau in Alzheimer's disease. Sci. Rep. 6:26697. 10.1038/srep26697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chen C., Zhang Y. (2020). An investigation of microRNA-103 and microRNA-107 as potential blood-based biomarkers for disease risk and progression of Alzheimer's disease. J. Clin. Lab. Anal. 34:e23006. 10.1002/jcla.23006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Liu J., Wang Q., Jiang H., Zeng L., Li Z., et al. (2019b). MicroRNA-200a-3p mediates neuroprotection in Alzheimer-related deficits and attenuates amyloid-beta overproduction and tau hyperphosphorylation via coregulating BACE1 and PRKACB. Front. Pharmacol. 10:806. 10.3389/fphar.2019.00806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Qin L., Tang B. (2019a). MicroRNAs in Alzheimer's disease. Front. Genet. 10:153. 10.3389/fgene.2019.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Zhang J. (2020). Clinical significance of miR-433 in the diagnosis of Alzheimer's disease and its effect on Aβ-induced neurotoxicity by regulating JAK2. Exp. Gerontol. 141:111080. 10.1016/j.exger.2020.111080 [DOI] [PubMed] [Google Scholar]
- Wang X., Tan L., Lu Y., Peng J., Zhu Y., Zhang Y., et al. (2015b). MicroRNA-138 promotes tau phosphorylation by targeting retinoic acid receptor alpha. FEBS Lett. 589, 726–729. 10.1016/j.febslet.2015.02.001 [DOI] [PubMed] [Google Scholar]
- Wang X., Xu Y., Zhu H., Ma C., Dai X., Qin C. (2015a). Downregulated microRNA-222 is correlated with increased p27Kip1 expression in a double transgenic mouse model of Alzheimer's disease. Mol. Med. Rep. 12, 7687–7692. 10.3892/mmr.2015.4339 [DOI] [PubMed] [Google Scholar]
- Wang Y., Luo X., Liu Y., Han G., Sun D. (2019c). Long noncoding RNA RMRP promotes proliferation and invasion via targeting miR-1-3p in non–small-cell lung cancer. J. Cell. Biochem. 120, 15170–15181. 10.1002/jcb.28779 [DOI] [PubMed] [Google Scholar]
- Wang Y., Veremeyko T., Wong AH-K, El Fatimy R., Wei Z., Cai W., et al. (2017). Downregulation of miR-132/212 impairs S-nitrosylation balance and induces tau phosphorylation in Alzheimer's disease. Neurobiol. Aging 51, 156–166. 10.1016/j.neurobiolaging.2016.12.015 [DOI] [PubMed] [Google Scholar]
- Wang Z., Qin W., Wei C., Tang Y., Zhao L., Jin H., et al. (2018). The microRNA-1908 up-regulation in the peripheral blood cells impairs amyloid clearance by targeting ApoE. Int. J. Geriatric Psychiatry 33, 980–986. 10.1002/gps.4881 [DOI] [PubMed] [Google Scholar]
- Wong H-KA., Veremeyko T., Patel N., Lemere C. A., Walsh D. M., Esau C., et al. (2013). De-repression of FOXO3a death axis by microRNA-132 and-212 causes neuronal apoptosis in Alzheimer's disease. Hum. Mol. Genet. 22, 3077–3092. 10.1093/hmg/ddt164 [DOI] [PubMed] [Google Scholar]
- Wu B. W., Wu M. S., Guo J. D. (2018b). Effects of microRNA-10a on synapse remodeling in hippocampal neurons and neuronal cell proliferation and apoptosis through the BDNF-TrkB signaling pathway in a rat model of Alzheimer's disease. J. Cell. Physiol. 233, 5281–5292. 10.1002/jcp.26328 [DOI] [PubMed] [Google Scholar]
- Wu D. M., Wen X., Wang Y. J., Han X. R., Wang S., Shen M., et al. (2018a). Effect of microRNA-186 on oxidative stress injury of neuron by targeting interleukin 2 through the janus kinase-signal transducer and activator of transcription pathway in a rat model of Alzheimer's disease. J. Cell. Physiol. 233, 9488–9502. 10.1002/jcp.26843 [DOI] [PubMed] [Google Scholar]
- Wu Q., Ye X., Xiong Y., Zhu H., Miao J., Zhang W., et al. (2016). The protective role of microRNA-200c in Alzheimer's disease pathologies is induced by beta amyloid-triggered endoplasmic reticulum stress. Front. Mol. Neurosci. 9:140. 10.3389/fnmol.2016.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Yuan X., Bai J., Han R., Li Z., Zhang H., et al. (2019). MicroRNA-181a protects against pericyte apoptosis via directly targeting FOXO1: implication for ameliorated cognitive deficits in APP/PS1 mice. Aging 11:6120. 10.18632/aging.102171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Gu Y., Wang G., Chen S. (2019). c-Myc, RMRP, and miR-34a-5p form a positive-feedback loop to regulate cell proliferation and apoptosis in multiple myeloma. Int. J. Biol. Macromol. 122, 526–537. 10.1016/j.ijbiomac.2018.10.207 [DOI] [PubMed] [Google Scholar]
- Xie B., Liu Z., Jiang L., Liu W., Song M., Zhang Q., et al. (2017). Increased serum miR-206 level predicts conversion from amnestic mild cognitive impairment to Alzheimer's disease: a 5-year follow-up study. J. Alzheimer's Dis. 55, 509–520. 10.3233/JAD-160468 [DOI] [PubMed] [Google Scholar]
- Xing H., Guo S., Zhang Y., Zheng Z., Wang H. (2016). Upregulation of microRNA-206 enhances lipopolysaccharide-induced inflammation and release of amyloid-β by targeting insulin-like growth factor 1 in microglia. Mol. Med. Rep. 14, 1357–1364. 10.3892/mmr.2016.5369 [DOI] [PubMed] [Google Scholar]
- Xu N., Li A-D, Ji L-L, Ye Y., Wang Z-Y, Tong L. (2019). miR-132 regulates the expression of synaptic proteins in APP/PS1 transgenic mice through C1q. Eur. J. Histochem. 63:3008. 10.4081/ejh.2019.3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Song Y., Zhou X., Deng Y., Liu T., Weng G., et al. (2015). DNA methyltransferase 3, a target of microRNA-29c, contributes to neuronal proliferation by regulating the expression of brain-derived neurotrophic factor. Mol. Med. Rep. 12, 1435–1442. 10.3892/mmr.2015.3531 [DOI] [PubMed] [Google Scholar]
- Yang K., Feng S., Ren J., Zhou W. (2019a). Upregulation of microRNA-196a improves cognitive impairment and alleviates neuronal damage in hippocampus tissues of Alzheimer's disease through downregulating LRIG3 expression. J. Cell. Biochem. 120, 17811–17821. 10.1002/jcb.29047 [DOI] [PubMed] [Google Scholar]
- Yang Q., Zhao Q., Yin Y. (2019b). miR-133b is a potential diagnostic biomarker for Alzheimer's disease and has a neuroprotective role. Exp. Therapeutic Med. 18, 2711–2718. 10.3892/etm.2019.7855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz S. G., Erdal M. E., Özge A. A., Sungur M. A. (2016). Can peripheral MicroRNA expression data serve as epigenomic (upstream) biomarkers of Alzheimer's disease? Omics 20, 456–461. 10.1089/omi.2016.0099 [DOI] [PubMed] [Google Scholar]
- Yu L., Li H., Liu W., Zhang L., Tian Q., Li H., et al. (2020). MiR-485-3p serves as a biomarker and therapeutic target of Alzheimer's disease via regulating neuronal cell viability and neuroinflammation by targeting AKT3. Mol. Genet. Genom. Med. 2020:e1548. 10.1002/mgg3.1548 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yuan J., Wu Y., Li L., Liu C. (2020). MicroRNA-425-5p promotes tau phosphorylation and cell apoptosis in Alzheimer's disease by targeting heat shock protein B8. J. Neural Transmission. 2020, 1–8. 10.1007/s00702-019-02134-5 [DOI] [PubMed] [Google Scholar]
- Zeng Z., Liu Y., Zheng W., Liu L., Yin H., Zhang S., et al. (2019). MicroRNA-129-5p alleviates nerve injury and inflammatory response of Alzheimer's disease via downregulating SOX6. Cell Cycle 18, 3095–3110. 10.1080/15384101.2019.1669388 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang B., Wang A., Xia C., Lin Q., Chen C. (2015). A single nucleotide polymorphism in primary-microRNA-146a reduces the expression of mature microRNA-146a in patients with Alzheimer's disease and is associated with the pathogenesis of Alzheimer's disease. Mol. Med. Rep. 12, 4037–4042. 10.3892/mmr.2015.3968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. Y., Huang S. H., Gao S. X., Wang Y. B., Jin P., Lu F. J. (2019). Upregulation of lncRNA RMRP promotes the activation of cardiac fibroblasts by regulating miR-613. Mol. Med. Rep. 20, 3849–3857. 10.3892/mmr.2019.10634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li Q., Liu C., Gao S., Ping H., Wang J., et al. (2016a). MiR-214-3p attenuates cognition defects via the inhibition of autophagy in SAMP8 mouse model of sporadic Alzheimer's disease. Neurotoxicology 56, 139–149. 10.1016/j.neuro.2016.07.004 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xing H., Guo S., Zheng Z., Wang H., Xu D. (2016b). MicroRNA-135b has a neuroprotective role via targeting of β-site APP-cleaving enzyme 1. Exp. Therapeut. Med. 12, 809–814. 10.3892/etm.2016.3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Kang J., Svetnik V., Warden D., Wilcock G., David Smith A., et al. (2020). A machine learning approach to identify a circulating MicroRNA signature for Alzheimer disease. J. Appl. Lab. Med. 5, 15–28. 10.1373/jalm.2019.029595 [DOI] [PubMed] [Google Scholar]
- Zhao Z-B., Wu L., Xiong R., Wang L-L, Zhang B., Wang C., et al. (2014). MicroRNA-922 promotes tau phosphorylation by downregulating ubiquitin carboxy-terminal hydrolase L1 (UCHL1) expression in the pathogenesis of Alzheimer's disease. Neuroscience 275, 232–237. 10.1016/j.neuroscience.2014.06.013 [DOI] [PubMed] [Google Scholar]
- Zhou H., Zhang R., Lu K., Yu W., Xie B., Cui D., et al. (2016). Deregulation of miRNA-181c potentially contributes to the pathogenesis of AD by targeting collapsin response mediator protein 2 in mice. J. Neurol. Sci. 367, 3–10. 10.1016/j.jns.2016.05.038 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Chen Z., Chen A., Ma J., Qian J., Ge J. (2020). Elevated serum miR-133a predicts patients at risk of periprocedural myocardial injury after elective percutaneous coronary intervention. Cardiol. J. 2020:34. 10.5603/CJ.a2020.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H-C., Wang L-M., Wang M., Song B., Tan S., Teng J-F., et al. (2012). MicroRNA-195 downregulates Alzheimer's disease amyloid-β production by targeting BACE1. Brain Res. Bulletin 88, 596–601. 10.1016/j.brainresbull.2012.05.018 [DOI] [PubMed] [Google Scholar]