Abstract
The data contained in this publication refers to a new approach to design composite pervaporation membranes that could be useful in water treatment. The work is based on the rational prediction of the membrane mass transfer coefficient using the resistance in series model and the corresponding experimental membranes were tested with several aqueous solutions comparatively to a commercially available porous distillation membrane (PVDF). All the related data, i.e. permeation water fluxes and conductivity of the permeate, were collected for hours, in the range 3 to 7 h. The strategy was to develop pervaporation membranes by coating a porous PVDF support (122µm) with various dense layers (hydrophobic polymers: Teflon™ AF2400, PMP, PTMSP). The objective was to avoid definitely the wetting problem observed in membrane distillation while keeping approximately the permeance than the porous support. The data reported here are related to the surface property of the membranes (contact angles), to the mechanical resistance of the membranes, to the wetting phenomena observed directly and recorded by observing the variation of water flux through the membranes and to the conductivity of the water condensed at the permeate side.
Keywords: Hydrophobic composite membranes, Contact angles, Water permeance, Conductivity, Desalination
Specifications Table
| Subject | Chemical Engineering |
| Specific subject area | desalination, pervaporation, membrane distillation |
| Type of data | Table, Image, Figure |
| How data were acquired | The porous and composites membranes were tested in direct contact configuration cell with an active surface of 40 cm2. The centrifuge pumps, temperature, and flux sensors are provided from RS Components, the bench conductivity meter is a Jenway 4520 and the balance is from Sartorius (0.01 g). The data related to mass transfer were recorded using LabVIEW software, the images were recorded in 12 megapixels size with the camera of a smartphone (Samsung 7). The contact angles were measured with the apparatus Dataphysics Instruments GmbH (DI) Germany equipped with a goniometer OCA-PSA Drop 8 using the software SCA20. |
| Data format | Raw and Analyzed |
| Parameters for data collection | The porous support and hydrophobic composite membranes were prepared and characterized by several solutions (Distillated water, salt solutions with or without surfactant, i.e. SDS) |
| Description of data collection | The operating conditions are as follows:
|
| For each experiment, the feed solution, initially pure water on the feed side, is circulated during about 2 hours to reach steady state conditions linked to the stabilization of temperatures on both side of the membrane. The warm and cold temperatures are recorded at the inputs and outputs of the membrane cell.The water vapor which permeates through the membrane is condensed on the downstream side in the flowing cold liquid. The excess of liquid is directly collected and weighted. In these conditions of steady state, the mass increase is linear versus time | |
| Data source location | Université de Lorraine, CNRS, LRGP, F-54000 Nancy, France |
| Data accessibility | With the article as Excel file |
| Related research article | Tarik Eljaddi, Deisy Lizeth Mejia Mendez, Eric Favre, Denis Roizard, Development of new pervaporation composite membranes for desalination: theoretical and experimental investigations, https://doi.org/10.1016/j.desal.2021.115006[1]. |
Value of the Data
-
•
The data of the permeation experiments show that significant water fluxes can be obtained with the lab-made pervaporation membranes using operating conditions similar to membrane distillation.
-
•
These data will be a useful reference for the researchers who will try to develop improved PV membranes for desalination. Indeed these data provide valuable information to understand the wetting problem with porous membrane by direct observation and by recording the variation parameters like flux and conductivity.
-
•
Calculation of the mass transfer coefficients was made accordingly to the resistance in series model. The permeation data will be useful to test further models of pervaporation mass transfer.
1. Data Description
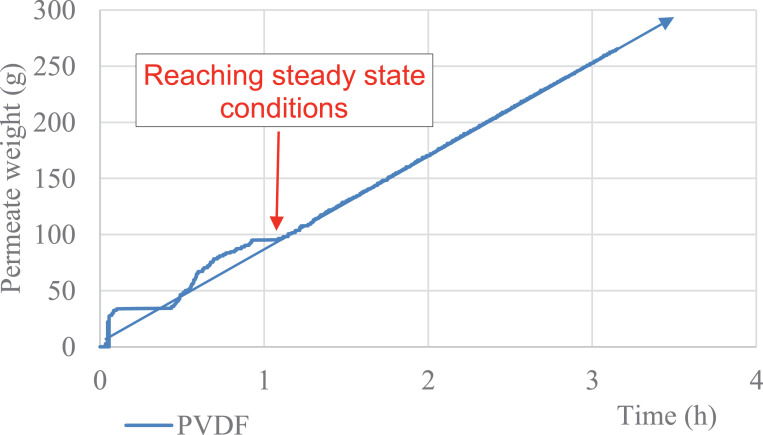
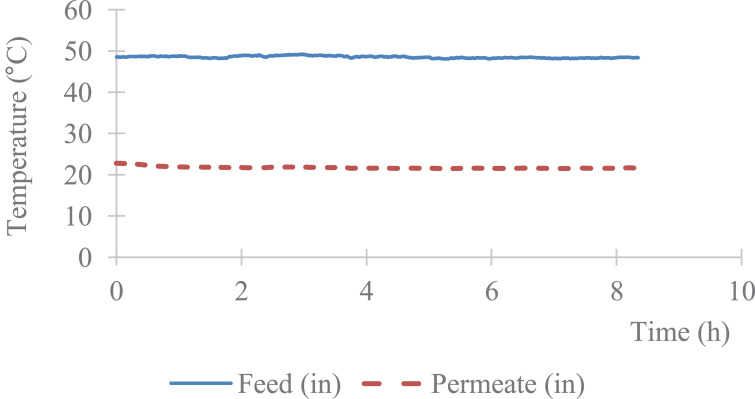
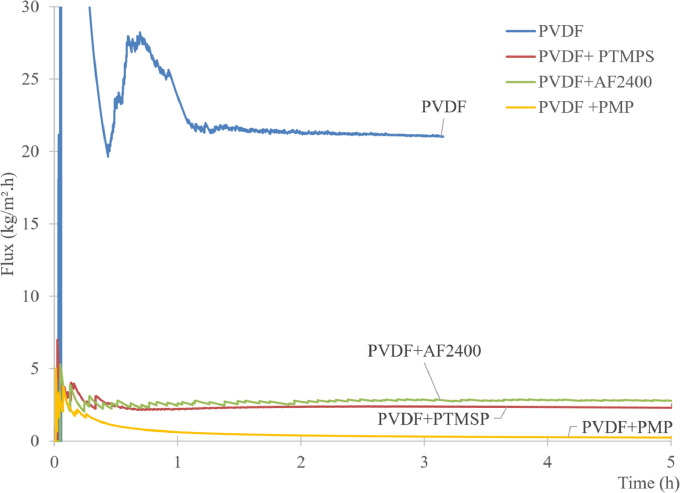
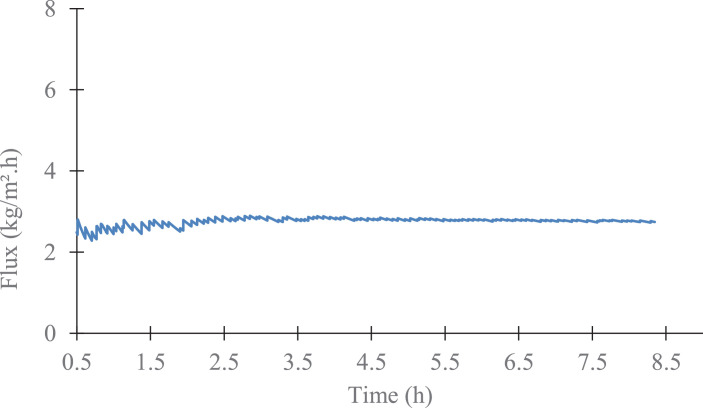
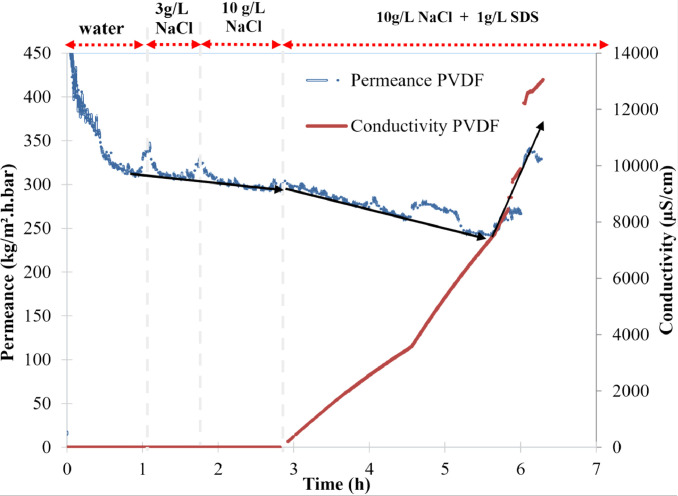
The data describe the direct contact membrane distillation configuration used to carry out the experiment and show the hydrophobicity property of porous PVDF (Fig. 1-a, b), the automatic recording of the permeate weight and the steady state reached after 1 h (Fig. 2). The Fig. 3 shows an example of temperature stability registered at the feed and at the permeate sides. The recorded water fluxes of the pristine PVDF and of the hydrophobic composite membranes are presented in Fig. 4 and an example of the water flux of PVDF+AF2400 for more than 8 h are presented in Fig. 5. The Fig. 6 presents the variation of water permeance through pristine PVDF with different feeds: distilled water, several salt feed solutions with and without surfactant. The increase of the permeate conductivity can be related to the wetting phenomenon. The transparent cell allows to see the wetting steps: it starts on the feed side, at the aqueous solution inlet, by partial wetting, leading after sometimes to total wetting (Fig. 7 a-e). Finally, the Table 1 gathers the value of mass transfer and permeability corresponding with the variation of thickness, Table 2 and Table 3 represent respectively the contact angle data and pull-off test data of pristine PVDF and composites membranes.
Fig. 1.
a) View of the flat cell used for the desalination experiments: total area 40cm2, direct contact membrane distillation configuration (DCMD). The downstream side is shown here with a piece of a PVDF membrane in place. The cell was home-made using a transparent glassy material (PMMA) to help the visualization of the tangential flow and of the wetting phenomenon.
b) A drop a water was deposed on the membrane: the spherical shape of the drop is due to the hydrophobic property of the membrane surface (contact angle: 125°).
Fig. 2.
Automatic recording of the permeate weight: steady state is reached after about 1h. The main governing parameter is the temperature regulation. The stabilized slope is 85.6 g/h for pristine PVDF.
Fig. 3.
Example of the stability of feed and permeate temperatures versus time. Case of PVDF and Teflon™ AF2400 experiments with pure water.
Fig. 4.
Example of flux stability versus time. Case of PVDF and of the composite membranes with pure water.
Fig. 5.
Example of flux stability versus time. Case of PVDF coated with Teflon™ AF 2400, long lasting experiment with pure water.
Fig. 6.
Investigation of the wetting resistance of the membranes versus feeds containing successively NaCl (up to 10g/L) and NaCl with a surfactant (SDS 1g/L). The addition of the surfactant induces first the partial the wetting of the membrane (time≈3h) and then a liquid convective flux through all pores (time≈5.5h).
Fig. 7.
Visualization of the wetting phenomena with a porous PVDF membrane, from the feed inlet to the feed outlet. Feed: NaCl 10g/L + 1g/L SDS à 50°C. On the Fig. 7_b) and Fig. 7_c) the dark strips indicate some partial wetting.
Table 1.
Permeability of glassy polymers for water (P, Barrer) and related mass transfer coefficient versus thickness of the active layer [2,3].
| PVDF | PTMSP | PMP | Teflon AF2400 | |
|---|---|---|---|---|
| Porous | Dense layer | Dense layer | Dense layer | |
| Permeability (Barrer ±10%) | NA | 11 200 | 1 000 | 4000 |
Table 2.
Contact angle measurements of pristine PVDF and composite membranes.
Table 3.
Pull-Off adhesion test measurements of pristine PVDF and composite membranes.
| Experiment | PVDF pristine | PMP | PTMSP | Teflon AF2400 |
|---|---|---|---|---|
| 1 | 0.26 | 1.63 | 1.44 | 3.34 |
| 2 | 0.2 | 1.45 | 1.48 | 3.27 |
| 3 | 0.24 | 1.51 | 1.75 | 3.24 |
| Average (MPa) | 0.2 | 1.5 | 1.6 | 3.3 |
| Deviation | 0.02 | 0.07 | 0.13 | 0.04 |
2. Experimental Design, Materials and Methods
2.1. Membrane modification
The first step was the preparation of the polymer solution, with the dissolution of the polymer mass in cyclohexane (2wt% PMP, 2wt% PTMSP) or in Fluorinert FC 75TM (1wt% Teflon™ AF2400) to obtain homogenous solutions. The polymer was added as a powder in the solvent and stirred overnight to get clear solutions. All remaining particles were filtered before used. In the second step, the air bubbles of the solutions were eliminated with an ultrasonic bath for 5mn and then the applicator film (Elcometer 4340 Motorized Film Applicator) was used to cast the polymer solutions as wet thin films (about 50 micrometers) on the surface of the PVDF support. After the evaporation of the solvent, composite membranes were obtained and dried carefully for several hours in an oven (50 °C) before characterization and utilization.
2.2. Permeance determination
The transmembrane water flux of the membranes was measured with a direct contact setup (Fig. 1). Flat samples of membranes of 40cm² were used. The centrifuge pumps, temperature, and flux sensors were provided from RS Components. The conductivity of the feed solutions and of the permeate were measured with a bench conductivity meter, brand Jenway 4520 [9] measured with a balance, brand Sartorius, accuracy 0.01 g. During the experiments, both the conductivity and the mass variation were acquired continuously online.
The operating conditions were as follows:
-
•
Both the feed and the permeate solutions circulate in close loops of about 1.5L each.
-
•
Cross flow is ensured on each side of the membrane in a counter current way.
-
•
Pump flow rates: respectively 1L/min on the feed side and 0.8L/min on the permeate side - the water vapor is condensed on the cold side and is continuously weighted by the balance as the water in excess coming through the membrane.
-
•
The conductivity of water is continuously measured and recorded in microsiemens (µS/cm) - the initial conductivity of the 3g/L and 10g/L feed solutions as respectively 6.5 and 18.9 mS/cm at room temperature.
2.3. Contact angle measurements
Samples of 2 cm × 2 cm were prepared for each membrane and fixed horizontally in the contact angles DI apparatus. Water drop of 7 µl was deposited on membrane surface using a perpendicular syringe. The picture of the water drop, and membrane were taking using a specific camera, and with a software, the angle contact was calculated. For each sample the measurement was repeated several times using several drops deposited at different places (Table 2) till the mean deviation was below 4°.
2.4. Pull-off test measurements
The adhesion between porous support PVDF and dense hydrophobic dense layer of AF2400, PMP and PTSMP was mechanically checked using the device Elcometer 510. The membrane sample was fixed between a metallic support and sample support (diameter of 20 mm) by Locite Snellijmen Glue. After certain time when the glue was dried, the automatic gauge was fixed on the sample support and it was pulled off automatically by the machine with a rate of 0.10 MPa/s. the same measurement was repeated three times with new samples. The mean deviation is small (Table 3).
Ethics Statement
This work reports original scientific data recorded at LRGP Laboratory. No extra partner has been involved. The contribution of all authors is well mentioned. This work does not involved the use of human subjects neither involved animal experiments.
None data collected from social media platforms were used.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships which have, or could be perceived to have, influenced the work reported in this article.
Acknowledgments
David Brunello (david.brunello@univ-lorraine.fr) and Charly Koenig (charly.koenig@univ-lorraine.fr) and his team are gratefully acknowledged for their skillful contributions respectively to the online acquisition system and to the conception and fabrication of the experimental direct contact cell. The authors are very grateful to Région GrandEst and to Europe, programme ERASMUS (project EU-METALIC), for their financial support and grant for Dr. T. Eljaddi.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.dib.2021.106943.
Appendix. Supplementary materials
References
- 1.Eljaddi T., Deisy M., Favre E., Roizard D. Rational and experimental approaches to develop composite membranes for desalination by pervaporation. Desalination. 2020 [Google Scholar]
- 2.Mejia Mendez D.L., Castel C., Lemaitre C., Favre E. Membrane distillation (MD) processes for water desalination applications. Can dense self-standing membranes compete with microporous hydrophobic materials? Chem. Eng. Sci. 2018;188:84–96. doi: 10.1016/j.ces.2018.05.025. [DOI] [Google Scholar]
- 3.2020. Polymethylpentene Film - Online Catalog Source - Supplier of Research Materials in Small Quantities - Goodfellow.http://www.goodfellow.com/A/PolymethylpenteneFilm.html. Accessed November 26, 2020. [Google Scholar]
- 4.Al-Anezi A.A.-H., Sharif A.O., Sanduk M.I., Khan A.R. Experimental investigation of heat and mass transfer in tubular membrane distillation module for desalination. ISRN Chem. Eng. 2012;2012:1–8. doi: 10.5402/2012/738731. [DOI] [Google Scholar]
- 5.Gomez J., Fraser-reid A.M., Cristobal Lopez B. Springer; 2010. Fluorous Chemistry. [Google Scholar]
- 6.Shao L., Samseth J., Hagg M.B. Effect of plasma treatment on the gas permeability of poly(4-methyl-2-pentyne) membranes. Plasma Process. Polym. 2007;4:823–831. doi: 10.1002/ppap.200600219. [DOI] [Google Scholar]
- 7.Volkov A.V., Tsarkov S.E., Gilman A.B., Khotimsky V.S., Roldughin V.I., Volkov V.V. Surface modification of PTMSP membranes by plasma treatment: Asymmetry of transport in organic solvent nanofiltration. Adv. Colloid Interface Sci. 2015;222:716–727. doi: 10.1016/j.cis.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y., Tian M., Li X., Wang Y., An A.K., Fang J., He T. Anti-wetting behavior of negatively charged superhydrophobic PVDF membranes in direct contact membrane distillation of emulsified wastewaters. J. Memb. Sci. 2017 doi: 10.1016/j.memsci.2017.04.040. [DOI] [Google Scholar]
- 9.http://www.jenway.com/product.asp?dsl=248, visited 27 may 2020, (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.