Abstract
Non-coding ribonucleic acids (ncRNAs) are a diverse group of RNA molecules that are mostly not translated into proteins after transcription, including long non-coding RNAs (lncRNAs) with longer than 200 nucleotides non-coding transcripts and microRNAs (miRNAs) which are only 18–22 nucleotides. As families of evolutionarily conserved ncRNAs, lncRNAs activate and repress genes via a variety of mechanisms at both transcriptional and translational levels, whereas miRNAs regulate protein-coding gene expression mainly through mRNA silencing. ncRNAs are widely involved in biological functions, such as proliferation, differentiation, migration, angiogenesis, and apoptosis. Idiopathic pulmonary fibrosis (IPF) is a progressive lung disease with a poor prognosis. The etiology of IPF is still unclear. Increasing evidence shows the close correlations between the development of IPF and aberrant expressions of ncRNAs than thought previously. In this study, we provide an overview of ncRNAs participated in pathobiology of IPF, seeking the early diagnosis biomarker and aiming for potential therapeutic applications for IPF.
Keywords: ncRNAs, miRNAs, lncRNAs, IPF, pathogenesis
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive type of interstitial pneumonia and is currently incurable. IPF is characterized by progressive lung scarring with constantly decreasing the function of the lungs. The prevalence of IPF shows that almost 5 million individuals suffer from this disease worldwide in the United States, and the 3 years and 5 years mortality rates of IPF are at approximately 50 and 80%, respectively [1]. Therefore, there is an urgent unmet need for deep understanding of the IPF biology, with a view to creating new possibilities of more effective targeted therapies. In the past decades, increasing studies reported that non-coding ribonucleic acids (ncRNAs) played important roles both in basic understanding of the molecular pathogenesis of IPF and for their use in novel therapeutic approaches.
ncRNAs, including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), are transcribed from human genome such as message RNAs (mRNAs) but are not translated into proteins after transcription. These ncRNAs directly or indirectly participate in the regulation of multiple functions of intracellular pathobiological processes and affect gene expression by regulating the function of mRNA, DNA methylation, and histone modification and participate in the occurrence and development of lung diseases, thus providing new ideas for the diagnosis and drug treatment targets of diseases [2,3,4].
2. Methods
The non-coding RNAs (ncRNAs), microRNAs (miRNAs), long non-coding RNAs (lncRNAs), idiopathic pulmonary fibrosis (IPF) were used as keywords searched in PubMed and web of science databases. The papers about reviews, meta-analysis, systematic reviews, books, and documents were excluded. Then, about 85,076 articles from PubMed and web of science databases in the past 5 years (2015–2019) (Figure 1) related to ncRNAs and IPF were screened and reviewed.
Figure 1.
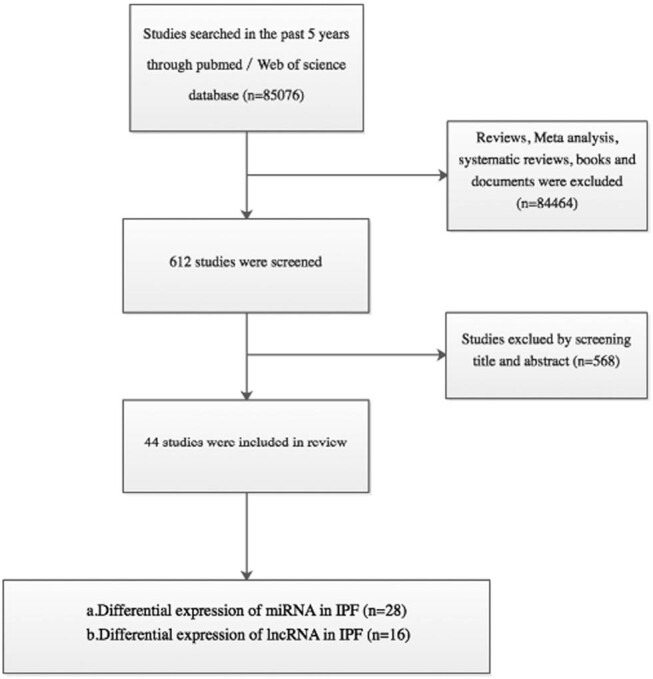
Flow chart for study selection.
3. Result
The articles of ncRNAs and IPF are related in the following three areas: (1) the biogenesis and functions of miRNAs in IPF; (2) the biogenesis and functions of lncRNAs in IPF; and (3) the interaction networks among lncRNAs, miRNAs, and mRNA in IPF.
3.1. Expression changes and functions of miRNAs in IPF
3.1.1. Biogenesis and biological functions of miRNAs
miRNAs are short single-stranded non-coding RNA molecules found in eukaryotic cells with a length of about 22nt, whose biological processes involve a series of complex steps. The precursor transcripts (pri-miRNAs) are a capped and polyadenylation transcript produced by RNA polymerase II [5]. Then, under the action of enzymes such as RNase III endonuclease and Drosha, the pri-miRNAs form precursor miRNAs (pre-miRNAs) with stem-looped structures and transport from the nucleus into the cytoplasm via the exportin-5 complex and the nuclear protein Ran-GTP [6,7]. In cytoplasm, the pre-miRNAs are further processed by Dicer into RNA duplex structure. Subsequently, the miRNA strand forms an RNA-induced silencing complex with the Argonaute protein and pairs with the corresponding target mRNA [8]. As the bases of the pairing sites are completely or incompletely complementary, the miRNA will degrade or block the translation of the mRNA.
miRNAs are involved in many biological processes such as biological development, cell proliferation and differentiation, tissue damage repair, and organ fibrosis and exert a wide range of physiological regulation [9,10,11]. In addition, a miRNA can affect the expression of multiple genes. In the meantime, a gene with multiple partial complementary binding sites can be targeted by multiple miRNAs [12]. Although several studies [13,14,15] have shown that there is a certain relationship between miRNA and the pathogenesis of IPF, regrettably, the specific mechanism remains unclear and needs further exploration.
3.1.2. Diagnostic role of miRNAs in IPF
IPF is one of the most serious fibrotic pulmonary diseases, which commonly afflict the elderly. The main pathological manifestations are the alternation of fibrotic areas with mild or normal pulmonary parenchymal areas which simultaneously occur scar formation and honeycomb-like changes [16]. In short, the IPF is characterized by pulmonary interstitial fibrosis and respiratory function deterioration. miRNA has been found to be stably present in various body fluids such as blood, bronchoalveolar lavage fluid, and sputum and has the advantages of disease-specific expression and easy to detect and quantify [2]. It has become a good diagnostic biomarker for the pulmonary disease. Indeed, several studies have suggested that there are some significant differential expressions of miRNAs in the lung of IPF patients and play a vital role in the occurrence and development of IPF. Therefore, detecting the expression of these miRNAs in the body fluids of patients can predict IPF in advance or perform molecular targeted therapy to improve the prognosis of patients, which is a good biological indicator for the diagnosis and prognosis of IPF [17,18,19].
To explore the potential of miRNAs as biomarkers of IPF, Li et al. [17] analyzed the differential expression of serum miRNAs between IPF patients and healthy people by microarray analysis and biological functions analysis. Subsequently, the results were verified by quantitative reverse transcription-polymerase chain reaction (qRT-PCR). The biological function analysis results showed that there was a significant differential expression of 60 miRNAs in the serum of patients with IPF, including eight upregulated and 52 downregulated miRNAs. The gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of differential miRNAs expression pattern showed that the miRNAs might be involved in the biological process of IPF at different cellular levels. Furthermore, the qRT-PCR results combined with the microarray analysis results revealed that the expression level of miRNA-21, miRNA-155, and miRNA-101-3p in serum was significantly changed and were associated with decreased forced vital capacity (FVC) and lung injury levels in IPF patients. The above results indicate that miRNAs are involved in different biological processes in the development of IPF, and the expression level of miRNA can be used as an indicator for the diagnosis and prognosis of patients with IPF.
In a recent study, Njock et al. [18] found that three miRNAs were significantly abnormal in IPF patients by analyzing sputum exosome miRNA used miRNA quantitative PCR array. Further, they found a significant negative correlation between miR-142-3p and the ratio of pulmonary carbon monoxide diffusion volume to alveolar ventilation volume. This interesting study indicates that sputum exosomes miRNA may be a good marker for the diagnosis and pathological analysis of IPF.
Yang et al. [19] analyzed the human serum miRNAs among healthy subjects and rapid-progressing and slow-progressing IPF patients by TaqMan microRNA assay. The results showed that 47 signature miRNAs were expressed in IPF patients, including 21 upregulated and 26 downregulated miRNAs. Subsequently, these differentially expressed miRNAs were processed by bioinformatics analysis and verified six miRNAs (miR-21, miR-199a-5p, 32 miR-200c, miR-31, let-7a, and let-7d) by RT-PCR in three groups. KEGG signaling pathway enrichment analysis showed that differentially expressed serum miRNAs were significantly enriched in 53 signaling pathways, especially in TGF- β, MAPK, PI3K-Akt, and WNT signaling pathways, suggesting that these biological pathways were involved in the pathogenesis of IPF. Meanwhile, the results of RT-PCR suggested that patients with IPF had significantly higher expression of miR-21, miR-199a-5p, and miR-200c compared with healthy subjects, while miR-31, let-7a, and let-7d were significantly lower. The above results indicate the value of analyzing serum miRNAs to diagnose and monitor the prognosis of IPF.
3.1.3. Therapeutic value of miRNA in IPF
IPF is because of the uncontrolled production of extracellular matrix by fibroblasts in lung injury, which leads to progressive exacerbations and irreversible damages [20]. Numerous studies [20,21,22,23] have shown that the abnormal expression of miRNA is closely related to the pathogenesis of IPF, therefore determining that the role of specific miRNA in the pathogenesis of IPF may provide a new direction for new therapeutic application. Ge et al. [22] used the bleomycin (BML)-mediated mouse lung fibrosis model to demonstrate the inhibited pulmonary fibrosis in mice by upregulating miR-323a-3p expression. Further studies have found that miR-323a-3p can target critical mediators in the pulmonary fibrosis pathways such as TGF-α, TGF-β, and apoptosis and attenuate these pathway signals, thereby reducing cell apoptosis. Therefore, miR-323a-3p can simultaneously target multiple fibrosis signaling pathways and can be further developed as an anti-IPF therapeutic agent.
At present, the mature IPF model is BML-induced pulmonary fibrosis model in mice. Zhang et al. [24] found that BML induced epithelial–mesenchymal transition (EMT), which promoted the migration of pleural mesothelial cells and subpleural pulmonary fibrosis. The downregulation of miR-18a-5p played a key role in the pathogenesis of pulmonary fibrosis. Furthermore, they found that miR-18a-5p binds to the transforming growth factor β receptor II (TGF-β-RII) mRNA, thereby reducing TGF-β-RII expression and downregulating the TGF-β/Smad2/3 signaling pathway. Then, they demonstrated that overexpression of miR-18a-5p reduced BML-induced EMT and reduced lung pleural fibrosis in mice. In addition, Liu et al. [25] demonstrated the important role of miR-21 in the pathogenesis of IPF. The miR-21 can regulate the expression of inhibitory Smad/Smad7 signaling pathway via TGF-β1, promote the activation of fibroblasts, and lead to pulmonary fibrosis. In summary, regulation of specific miRNA expression may be a new approach to the treatment of IPF.
The core pathogenesis of IPF is an abnormal epithelial–mesenchymal crosstalk, which hinders the normal repair process after alveolar damage [26,27,28]. Li et al. [29] also determined miRNA expression profiles in IPF patients and normal lungs by Affymetrix microarray. By analyzing the miRNA expression profiles by bioinformatics and pathway enrichment, they found that seven miRNAs were significantly reduced in the lung tissue of patients with IPF. Among them, miR-130b-3p was the most obvious reduction, and it is used as a research target miRNA. Subsequently, the target gene of miR-130b-3p was determined to be insulin-like growth factor (IGF-1) by fluorescein assay and ELISA reagent. To verify that miR-130b-3p and its target gene IGF-1 cause IPF through the disorder of epithelial–mesenchymal crosstalk, they first established a co-culture system of lung epithelial cells and fibroblasts to mimic the environment of epithelial–interstitial crosstalk. Then, they inhibited the expression of miR-130b-3p in epithelial cells, and the results showed that fibroblasts significantly increased the expression of collagen I, cell proliferation, and migration in the co-culture system. These fibrosis-promoting phenomena could be blocked by IGF-1 antibodies. Thus, the downregulation of miR-130b-3p leads to the secretion of IGF-1 by lung epithelial cells, which promotes the activation of fibroblast and the disorder of epithelial–interstitial crosstalk, and miR-130b-3p may play a key regulatory role in IPF therapy.
3.2. Expression changes and functions of lncRNAs in IPF
3.2.1. Biogenesis and biological functions of lncRNAs
lncRNAs are ncRNAs greater than 200 nucleotides in length. lncRNAs lack coding open reading frames and cannot encode proteins, but they can exert biological functions through interaction with other genetic materials [30]. lncRNAs can be broadly classified into five categories according to the positional relationship with protein-coding genes, including sense lncRNAs, antisense lncRNAs, intronic lncRNAs, intergenic lncRNAs, and bidirectional lncRNAs [31]. Initially, lncRNA was considered to be a junk RNA because of its lack of biological function, only as a by-product of RNA polymerase II transcription and an interference factor for gene transcription. However, recent studies [32,33,34] have shown that lncRNAs have a variety of biological functions: (1) they participate in many important regulatory processes such as chromosome modification, transcriptional activation, and nuclear transport, (2) they protect protein-coding genes, and (3) they play a role in cis-regulation and trans-regulation. lncRNAs have multiple modes of action. For instance, lncRNAs can form complementary duplexes with transcripts-encoding protein genes to regulate gene expression levels [31]. Further, they can bind to specific proteins to form nucleic acid–protein complexes that regulate the protein activity or alter the protein localization in the cytoplasm [35,36].
Although lncRNAs are different from other nucleotide sequences and do not have high expression abundance, they are expressed at specific stages of biological development with high tissue-cell specificity and may become biomarkers of various diseases [37].
3.2.2. Functions of lncRNAs in IPF
The above has shown that lncRNAs are involved in a variety of biological processes, and substantial evidence suggests that mutations and disorders of lncRNAs may be closely related to the pathogenesis of IPF.
Hao et al. [38] extracted venous blood RNA from IPF patients and healthy subjects for transcriptome sequencing and bioinformatics analysis. They found a total of 1,816 differentially expressed lncRNAs, including 440 upregulated lncRNAs and 1,376 downregulated lncRNAs. GO enrichment analysis and KEGG pathway enrichment analysis revealed that differential lncRNA was primarily involved in chromosome segregation and regulation and enriched in the mTOR signaling pathway associated with IPF. Studies have shown that the expression of mTOR pathway is associated with collagen production and pulmonary edema levels in IPF patients, while RPS6KB2 is an important gene in mTOR pathway. Therefore, RPS6KB2 can be activated by growth factors and regulate mTOR signaling pathway. Subsequently, RT-qPCR confirmed that lncRNA AP003419.16 and its adjacent gene RPS6KB2 were highly expressed in IPF patients but low expression in healthy people. TGF-β1 is a key pro-fibrotic factor in the pathogenesis of IPF. They found that AP003419.16 and RPS6KB were highly expressed in TGF-β1-treated A549 cells, which was consistent with the RT-qPCR results. Thus, the expression of lncRNA AP003419.16 can predict the risk of IPF and becomes a new target for IPF molecular therapy.
Song et al. [39] first discovered that lncRNA-ITPF (lncITPF) was upregulated in IPF. Various experimental techniques such as RNA fluorescence in situ hybridization, nuclear isolation, and RNA sequencing have confirmed that lncITPF is located in the nucleus and regulates its host gene ITGBL1 to promote fibrosis by affecting the acetylation of H3 and H4 histones in its promoter region. Then, they studied the upstream and downstream mechanisms of lncITPF in pulmonary fibrosis. The upstream mechanism of lncITPF is mediated by the TGF-β1/Smad2/3 signaling pathway, and the downstream mechanism regulates the acetylation of H3 and H4 histones of ITGBL1 gene by interacting with heterogeneous nuclear ribonucleoprotein L. To assess the potential of lncITPF as a therapeutic target, interfered sequence of lncITPF (sh-lncITPF) was applied to an animal model of pulmonary fibrosis, and the expression of lncITPF in clinical IPF patients was detected. The results showed that the fibrosis indexes of α-sma, collagen, and vimentin in the sh-lncITPF group were lower than those in the control group. The expression level of lncITPF in clinical IPF patients was significantly higher than that in healthy subjects, and the Pearson’s correlation analysis showed that lncITPF expression level was correlated with FVC% predicted. Therefore, lncITPF may become one of the new targets for clinical IPF treatment.
In addition, Gao et al. [40] first discovered that lncRNA telomeric repeat-containing RNA (TERRA) can regulate telomere and mitochondrial function in the pathogenesis of IPF. They found that TERRA was highly expressed in peripheral blood mononuclear cells of IPF patients, and the TERRA expression levels were inversely correlated with FVC%. Telomerase can prolong telomeres and slow down cell senescence [41]. The progression of IPF is closely related to apoptosis, and mitochondria are the control center for apoptosis and oxidative stress. The study results have shown that TERRA inhibits telomerase activity and promotes the progression of IPF. RNA interferes with TERRA expression can regulate mitochondrial function and improves the function of oxidative stress-related genes (reactive oxygen species, superoxide dismutase, and catalase) and apoptosis-related genes (cytochrome-c, caspase-9, and caspase-3). Therefore, they believe that regulation of TERRA expression may become one of the IPF treatment pathways.
3.3. Interactions between lncRNAs and miRNAs and their regulatory networks in IPF
Currently, high-throughput sequencing technology was used to analyze the differentially expressed miRNAs in normal lung tissues and lung tissues of IPF patients, and then GO annotation and KEGG pathway enrichment analysis were used to screen the molecular targets most related to the diagnosis and prognosis of IPF, which laid the foundation for the follow-up clinical transformation therapy. We believe that identifying the diverging interaction network of lncRNAs and miRNAs orchestra and genes will provide important clues for understanding the mechanism of IPF and developing novel diagnostic and therapeutic strategies. At the same time, most of the latest progress of understanding of miRNA and lncRNA regulation in pulmonary fibrosis in the past 3 years are summarized and presented in Tables 1 and 2, respectively.
Table 1.
Differential expression of miRNA in IPF
| No. | Region | Sample | Target miRNA | Subjects (n) | Function | Ref. |
|---|---|---|---|---|---|---|
| 1 | Germany | Human lung tissues | Lethal 7d (MIRLET7D) | Control = 5 | Treatment | [47] |
| IPF = 10 | ||||||
| 2 | Belgium | Human lung tissues | ME1-16 | Control = 108 | Treatment | [48] |
| IPF = 160 | ||||||
| 3 | China | BAL | microRNA‑30a | Control = 16 | Treatment | [49] |
| IPF = 30 | ||||||
| 4 | China | Human plasma | miR‑324‑5p miR‑630 | Control = 10 | Treatment | [17] |
| IPF = 10 | ||||||
| 5 | Belgium | Human sputum | miR-142-3p | Control = 14 | Diagnosis | [18] |
| IPF = 16 | ||||||
| 6 | China | Human lung tissues | miR-409-5p has-miR-376c | Control = 25 | Treatment | [50] |
| IPF = 23 | Diagnosis | |||||
| 7 | China | Human blood | miR-708-3p | Control = 78 | Treatment | [15] |
| IPF = 78 | ||||||
| 8 | Mexico | Fibrotic primary fibroblast cells | miRNA-21 | Control = 6 | Treatment | [51] |
| IPF = 6 | Diagnosis | |||||
| 9 | Greece | BAL | miR-185 miR-29a | IPF = 57 | Treatment | [21] |
| LC = 32 | ||||||
| 10 | China | Rat lung tissues | miR-541-5p | Rat | Treatment | [23] |
| 11 | California | Mouse lung tissues | miRNA-29c | Control = 4 | Treatment | [14] |
| Human lungs tissues | IPF = 7 | |||||
| 12 | China | Human blood | miR-30a | Control = 46 | Treatment | [52] |
| IPF = 46 | ||||||
| 13 | Texas | Alveolar epithelial cells | miR-34a | Cells | Treatment | [53] |
| 14 | China | Rat pleural mesothelial cells | miR-18a-5p | Cells | Treatment | [24] |
| 15 | China | Lung resident mesenchymal stem cells | miR-497-5p | Cells | Treatment | [54] |
| 16 | Birmingham | Mice lung tissues | miR-34a | Mice | Treatment | [55,56] |
| 17 | USA | Bronchial epithelia | miR-323a-3p | Lung transplant patients = 11 | Treatment | [22] |
| Lung transplant patients with BOS = 7 | ||||||
| 18 | China | Plasma | miR-25-3p let-7d-5p | AE-IPF = 3 | Diagnosis | [57] |
| S-IPF = 3 | ||||||
| Control = 3 | ||||||
| 19 | China | Mice lung tissues | miR-29b | Mice | Treatment | [58] |
| 20 | Japan | Human serum | miR-21-5p | Control = 21 | Prognosis | [59] |
| IPF = 41 | ||||||
| 21 | Italy | Human lung tissues | miR-200 | Control = 34 | Diagnosis | [60] |
| IPF = 34 | ||||||
| 22 | China | Human lung tissues | miR‑221 | Control = 10 | Treatment | [61] |
| IPF = 10 | ||||||
| 23 | Japan | Human lung tissues | miR-29a | Control = 17 | Treatment | [62] |
| LC with IPF = 8 | Diagnosis | |||||
| 24 | China | Lung resident mesenchymal stem cells | miR-877-3p | Cells | Treatment | [20] |
| 25 | Indiana | Human lung tissues | miR-185 miR-186 | Control = 15 | Treatment | [63] |
| IPF = 15 | ||||||
| 26 | China | Human lung tissues | miR-130b-3p | Control = 3 | Treatment | [29] |
| IPF = 4 | ||||||
| 27 | China | Human lung tissues | miR-26a | NM | Treatment | [64] |
| 28 | Birmingham | Human lung fibroblasts | miR-27a-3p | Cells | Treatment | [13] |
LC: lung cancer; BOS: bronchiolitis obliterans syndrome; AE-IPF: acute exacerbation of idiopathic pulmonary fibrosis; S-IPF: stable of idiopathic pulmonary fibrosis; NM: not mentioned.
Table 2.
Differential expression of lncRNA in IPF
| No. | Region | Sample | Target lncRNA | Regulated miRNA/genes | Subjects (n) | Function | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | China | Mice lung fibrosis tissues | H19 | miR-196a/COL1A1 | Mice | Treatment | [4] |
| 2 | China | Human peripheral blood | TERRA | Telomeres; mitochondria; associated genes; components associated with telomeres; mitochondria-associated cyclin E gene | Control = 24 | Treatment | [40] |
| IPF = 24 | |||||||
| 3 | China | Sprague Dawley (SD) rats | lnc-PCF | miR-344a-5p/map3k11 | Rats | Treatment | [43] |
| 4 | China | The primary lung fibroblasts | PFAR | miR-15a/YAP1-Twist1 | Cells | Treatment | [65] |
| 5 | China | Human lung tissues | H19 | miR-140/Smad3 | Control = 15 | Treatment | [3] |
| IPF = 15 | Diagnosis | ||||||
| 6 | China | Human lung tissues | ZEB1-AS1 | miR-141-3p/ZEB1 | NM | Treatment | [66] |
| 7 | China | Blood | lncITPF | ITGBL1 | Control = 76 | Treatment | [39] |
| IPF = 76 | Diagnosis | ||||||
| 8 | China | Mice fibrotic hearts | PFAR | miR-138/YAP1 | Mice | Treatment | [42,67] |
| 9 | China | Mouse lung fibroblasts | PFRL | miR-26a/Smad2 | Cells | Treatment | [68] |
| 10 | China | Mice lung tissues | lncRNAPCAT29 | miRNA-221/N4bp2; Plxna | Mice | Treatment | [69] |
| 11 | China | Mice lung tissues | lncRNA-ATB | miR-200c/ZEB1 | Mice | Treatment | [70] |
| 12 | China | Blood | AP003419.16 | RPS6KB2 | Control = 4 | Treatment | [38] |
| IPF = 4 | |||||||
| 13 | China | Mice lung tissues | MALAT1 | miR-503/PI3K p85 | Mice | Treatment | [71] |
| 14 | China | Mouse fibroblast cells | H19 | miR-29b/COL1A1; Acta2 | Cells | Treatment | [58] |
| 15 | China | Mice lung tissues | CHRF | miR-489/MyD88; Smad3 | Mice | Treatment | [72] |
| 16 | China | Mouse fibroblast cells | lincRNA-p21 | Thy-1 | Cells | Treatment | [73] |
| Diagnosis |
NM: not mentioned.
It has been found that lncRNAs with target sequences similar to miRNAs can regulate the abundance of RNA by isolating miRNAs, or that lncRNAs can directly bind to miRNAs and regulate their functions. Although miRNAs and lncRNAs play an important role in the occurrence and progression of IPF, their regulatory networks in IPF remain unclear. Therefore, numerous studies [4,42,43] have been carried out on the intrinsic regulatory networks of lncRNAs and miRNAs in the pathogenesis of IPF.
A recent study [44] showed that lncRNA NONMMUT021928 (PFAL) competitively binds to miR-18a and promotes pulmonary fibrosis through CTGF gene. Li et al. [44] first found that PFAL was significantly upregulated in pulmonary fibrosis mice and TGF-β1-induced fibrotic pulmonary fibroblasts. The in vitro and in vivo experiments further demonstrated that overexpression of PFAL promoted the proliferation, migration, myofibroblastic transformation, extracellular matrix deposition, and fibrosis of lung fibroblasts by modulating miR-18a. On the contrary, the above fibrosis indexes were all decreased after the PFAL was knocked out. Further, they studied the intrinsic mechanism and showed that PFAL played the role of an endogenous RNA, competitively binds to miR-18a, and inhibits its expression and activity, thereby promoting lung fibroblast activation and fibrosis. In addition, they found that knock-out of miR-18a resulted in fibrosis of lung fibroblasts, whereas its overexpression reduced TGF-β1-induced pulmonary fibrosis. At the same time, the expression of miR-18a was decreased in patients with IPF by RT-PCR. Therefore, they believe that by knocking out PFAL and then regulating the expression of miR-18a, it may become a new treatment for pulmonary fibrosis.
Liu et al. [43] found that a novel lncRNA BC158825 (lnc-PCF) can target miR-344a-5p, thereby regulating its target gene map3k11 to promote pulmonary fibrosis. They first performed microarray analysis of fibrotic lung tissue and normal lung tissue, and the results showed that lnc-PCF was significantly expressed compared to other transcripts. Subsequently, the epithelial cells’ knocked-out and knocked-in lnc-PCF were detected by real-time cell analysis system, flow cytometry, and western blot analysis. The results showed that lnc-PCF promoted TGF-β1-induced epithelial cell proliferation. lncRNA can be targeted to regulate the function of miRNAs. Therefore, they based on TargetScan, miRanda data, and miRbase and used bioinformatics to predict the target miRNA of lnc-PCF. The predictive miRNAs were verified by qRT-PCR in fibrotic lung tissue and fibrotic cells, and ultimately determined that microRNAs-344a-5p were potential targets of lnc-PCF. miRNAs function by interacting with target genes. Consequently, they predicted the target gene of miR-344a-5p by the same method and determined that map3k11 as an important target gene of miR-344a-5p. To evaluate the therapeutic effect of interfering with lnc-PCF, adenovirus encapsulated with interfered sequence of lnc-PCF (sh-lnc-PCF) was injected into lung tissue of rats with pulmonary fibrosis. The results showed that the indexes of pulmonary fibrosis in sh-lnc-PCF group were significantly lower than those in control group, which confirmed that lnc-PCF was one of the potential targets for IPF treatment.
In addition, Lu et al. [4] took lncRNA H19 as a research target for the pathogenesis of IPF. They found that the expression of H19 was significantly increased in TGF-β-induced fibroblasts and BML-induced pulmonary fibrosis. Then, the target miRNAs of H19 were predicted by exploring Starbase v2.0 (http://starbase.sysu.edu.cn) and verified by dual-luciferase reporter assay. The results showed that miR-196a was a target of H19 in IPF. COL1A1 is a known target gene of miR-196a. To explore the relationship between H19, miR-196a, and COL1A1, they found that the expression of COL1A1 decreased with the decrease in H9, which could be reversed by microRNA-196a inhibitors. Therefore, they believe that H19 may competitively bind to microRNA-196a to regulate COL1A1 expression. Finally, in vitro and in vivo results have shown that downregulation of H19 attenuates lung fibroblast activation and pulmonary fibrosis, the phenomenon that can be reversed by miR-196a inhibitors. This confirmed once again that lncRNA H19 could promote the expression of COL1A1 through the competitive binding of microRNA-196a and accelerate the process of pulmonary fibrosis. Therefore, they believe that H19 plays an important role in the pathogenesis of IPF, and H19 is one of the directions of IPF gene-targeted therapy.
4. Conclusions
In most studies, total RNA was extracted from lung tissue or blood samples of IPF patients and normal people, and transcriptome sequencing (including microRNAs and lncRNAs sequencing) was performed [19,45]. The sequencing results were subjected to bioinformatics analysis and enrichment analysis to classify differentially expressed miRNAs or lncRNAs, and one or more important miRNAs (or lncRNAs) were selected as research objects. Further, target miRNAs (or genes) were identified by various experimental techniques such as exploring database, RNA fluorescence in situ, and ELISA. Finally, the in vivo or in vitro experiments were conducted to verify the role of target miRNAs, target lncRNAs, and target genes in the pathogenesis of IPF by qRT-PCR, western blot, and transfection. The research model is reasonable, but there are also certain limitations. One miRNA may regulate the expression of multiple genes, and the same gene may be regulated by multiple miRNAs. This phenomenon also applies to lncRNAs. Therefore, this research model can clearly explain one of the modes of action but failed to link the multiple modes of IPF.
Further need to demonstrate the correlation between gene expression and disease occurrence. In brief, the safety of gene therapy. For instance, Du et al. [46] found that the expression of lncRNA CDKN2B-AS1 and adjacent gene CDKN2A in peripheral blood of patients with IPF was significantly downregulated and activated p53 signaling pathway to promote the formation of lung cancer. Therefore, the follow-up study needs to further explore gene target therapy for IPF patients while avoiding the occurrence of cancer or fatal diseases.
In addition, there is currently no uniform method for miRNA or lncRNA isolation, and the specimens used to extract total RNA mainly come from lung tissue or blood specimens of IPF patients. The extraction methods of these specimens belong to invasive examinations. Moreover, research on biological samples such as saliva and sputum in IPF patients is still less; therefore, the detection of miRNA expression in these samples to diagnose IPF needs further investigation. At present, there is no treatment for IPF etiology or reversal of IPF. The role of ncRNAs in the pathogenesis of IPF requires a lot of research to explore, which provides a new scheme for molecular targeted therapy of IPF.
Acknowledgments
The authors thank the team members for helpful discussions.
Footnotes
Funding: This work was supported in part by the grants from the Science and Technology Planning Project of Inner Mongolia (No. 20130414), Natural Science Foundation of Inner Mongolia (No. 2015MS0809), and the Science Foundation of Baotou Medical College (No. BYJJ-YF 201732).
Conflict of interest statement: Authors state no conflict of interest.
Data availability statements: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Min Qiu, Email: yzqmyzx@163.com.
Zheng Yang, Email: yzqmyzx@163.com.
References
- [1].Nho RS. Alteration of aging-dependent microRNAs in idiopathic pulmonary fibrosis. Drug Dev Res. 2015;76:343–53. [DOI] [PMC free article] [PubMed]; Nho RS. Alteration of aging-dependent microRNAs in idiopathic pulmonary fibrosis. Drug Dev Res. 2015;76:343–53. doi: 10.1002/ddr.21272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pattarayan D, Thimmulappa RK, Ravikumar V, Rajasekaran S. Diagnostic potential of extracellular microRNA in respiratory diseases. Clin Rev Allergy Immunol. 2018;54:480–92. [DOI] [PubMed]; Pattarayan D, Thimmulappa RK, Ravikumar V, Rajasekaran S. Diagnostic potential of extracellular microRNA in respiratory diseases. Clin Rev Allergy Immunol. 2018;54:480–92. doi: 10.1007/s12016-016-8589-9. [DOI] [PubMed] [Google Scholar]
- [3].Wang X, Cheng Z, Dai L, Jiang T, Jia L, Jing X, et al. Knockdown of long noncoding RNA H19 represses the progress of pulmonary fibrosis through the transforming growth factor beta/Smad3 pathway by regulating microRNA 140. Mol Cell Biol. 2019;39(12):e00143-19. [DOI] [PMC free article] [PubMed]; Wang X, Cheng Z, Dai L, Jiang T, Jia L, Jing X. et al. Knockdown of long noncoding RNA H19 represses the progress of pulmonary fibrosis through the transforming growth factor beta/Smad3 pathway by regulating microRNA 140. Mol Cell Biol. 2019;39(12):e00143–19. doi: 10.1128/MCB.00143-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lu Q, Guo Z, Xie W, Jin W, Zhu D, Chen S, et al. The lncRNA H19 mediates pulmonary fibrosis by regulating the miR-196a/COL1A1 axis. Inflammation. 2018;41:896–903. [DOI] [PubMed]; Lu Q, Guo Z, Xie W, Jin W, Zhu D, Chen S. et al. The lncRNA H19 mediates pulmonary fibrosis by regulating the miR-196a/COL1A1 axis. Inflammation. 2018;41:896–903. doi: 10.1007/s10753-018-0744-4. [DOI] [PubMed] [Google Scholar]
- [5].Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development (Cambridge, England). 2005;132:4645–52. [DOI] [PubMed]; Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development (Cambridge, England) 2005;132:4645–52. doi: 10.1242/dev.02070. [DOI] [PubMed] [Google Scholar]
- [6].Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the microprocessor complex. Nature. 2004;432:231–5. [DOI] [PubMed]; Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the microprocessor complex. Nature. 2004;432:231–5. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- [7].Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18:3016–27. [DOI] [PMC free article] [PubMed]; Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18:3016–27. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. [DOI] [PMC free article] [PubMed]; Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Foshay KM, Gallicano GI. Small RNAs, big potential: the role of microRNAs in stem cell function. Curr Stem Cell Res Ther. 2007;2:264–71. [DOI] [PubMed]; Foshay KM, Gallicano GI. Small RNAs, big potential: the role of microRNAs in stem cell function. Curr Stem Cell Res Ther. 2007;2:264–71. doi: 10.2174/157488807782793781. [DOI] [PubMed] [Google Scholar]
- [10].Bueno MJ, Perez de Castro I, Malumbres M. Control of cell proliferation pathways by microRNAs. Cell Cycle (Georgetown, Tex). 2008;7:3143–8. [DOI] [PubMed]; Bueno MJ, Perez de Castro I, Malumbres M. Control of cell proliferation pathways by microRNAs. Cell Cycle (Georgetown, Tex) 2008;7:3143–8. doi: 10.4161/cc.7.20.6833. [DOI] [PubMed] [Google Scholar]
- [11].Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252–63. [DOI] [PubMed]; Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252–63. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- [12].Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. [DOI] [PubMed]; Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- [13].Cui H, Banerjee S, Xie N, Ge J, Liu RM, Matalon S, et al. MicroRNA-27a-3p is a negative regulator of lung fibrosis by targeting myofibroblast differentiation. Am J Respir Cell Mol Biol. 2016;54:843–52. [DOI] [PMC free article] [PubMed]; Cui H, Banerjee S, Xie N, Ge J, Liu RM, Matalon S. et al. MicroRNA-27a-3p is a negative regulator of lung fibrosis by targeting myofibroblast differentiation. Am J Respir Cell Mol Biol. 2016;54:843–52. doi: 10.1165/rcmb.2015-0205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xie T, Liang J, Geng Y, Liu N, Kurkciyan A, Kulur V, et al. MicroRNA-29c prevents pulmonary fibrosis by regulating epithelial cell renewal and apoptosis. Am J Respir Cell Mol Biol. 2017;57:721–32. [DOI] [PMC free article] [PubMed]; Xie T, Liang J, Geng Y, Liu N, Kurkciyan A, Kulur V. et al. MicroRNA-29c prevents pulmonary fibrosis by regulating epithelial cell renewal and apoptosis. Am J Respir Cell Mol Biol. 2017;57:721–32. doi: 10.1165/rcmb.2017-0133OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu B, Li R, Zhang J, Meng C, Zhang J, Song X, et al. MicroRNA-708-3p as a potential therapeutic target via the ADAM17-GATA/STAT3 axis in idiopathic pulmonary fibrosis. Exp Mol Med. 2018;50:e465. [DOI] [PMC free article] [PubMed]; Liu B, Li R, Zhang J, Meng C, Zhang J, Song X. et al. MicroRNA-708-3p as a potential therapeutic target via the ADAM17-GATA/STAT3 axis in idiopathic pulmonary fibrosis. Exp Mol Med. 2018;50:e465. doi: 10.1038/emm.2017.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Balderas-Martinez YI, Rinaldi F, Contreras G, Solano-Lira H, Sanchez-Perez M, Collado-Vides J, et al. Improving biocuration of microRNAs in diseases: a case study in idiopathic pulmonary fibrosis. Database J Biol Database Curation. 2017;2017:bax030. [DOI] [PMC free article] [PubMed]; Balderas-Martinez YI, Rinaldi F, Contreras G, Solano-Lira H, Sanchez-Perez M, Collado-Vides J. et al. Improving biocuration of microRNAs in diseases: a case study in idiopathic pulmonary fibrosis. Database J Biol Database Curation. 2017;2017:bax030. doi: 10.1093/database/bax030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li R, Wang Y, Song X, Sun W, Zhang J, Liu Y, et al. Potential regulatory role of circular RNA in idiopathic pulmonary fibrosis. Int J Mol Med. 2018;42:3256–68. [DOI] [PMC free article] [PubMed]; Li R, Wang Y, Song X, Sun W, Zhang J, Liu Y. et al. Potential regulatory role of circular RNA in idiopathic pulmonary fibrosis. Int J Mol Med. 2018;42:3256–68. doi: 10.3892/ijmm.2018.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Njock MS, Guiot J, Henket MA, Nivelles O, Thiry M, Dequiedt F, et al. Sputum exosomes: promising biomarkers for idiopathic pulmonary fibrosis. Thorax. 2019;74:309–12. [DOI] [PMC free article] [PubMed]; Njock MS, Guiot J, Henket MA, Nivelles O, Thiry M, Dequiedt F. et al. Sputum exosomes: promising biomarkers for idiopathic pulmonary fibrosis. Thorax. 2019;74:309–12. doi: 10.1136/thoraxjnl-2018-211897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang G, Yang L, Wang W, Wang J, Wang J, Xu Z. Discovery and validation of extracellular/circulating microRNAs during idiopathic pulmonary fibrosis disease progression. Gene. 2015;562:138–44. [DOI] [PubMed]; Yang G, Yang L, Wang W, Wang J, Wang J, Xu Z. Discovery and validation of extracellular/circulating microRNAs during idiopathic pulmonary fibrosis disease progression. Gene. 2015;562:138–44. doi: 10.1016/j.gene.2015.02.065. [DOI] [PubMed] [Google Scholar]
- [20].Wang C, Gu S, Cao H, Li Z, Xiang Z, Hu K, et al. miR-877-3p targets Smad7 and is associated with myofibroblast differentiation and bleomycin-induced lung fibrosis. Sci Rep. 2016;6:30122. [DOI] [PMC free article] [PubMed]; Wang C, Gu S, Cao H, Li Z, Xiang Z, Hu K. et al. miR-877-3p targets Smad7 and is associated with myofibroblast differentiation and bleomycin-induced lung fibrosis. Sci Rep. 2016;6:30122. doi: 10.1038/srep30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bibaki E, Tsitoura E, Vasarmidi E, Margaritopoulos G, Trachalaki A, Koutoulaki C, et al. miR-185 and miR-29a are similarly expressed in the bronchoalveolar lavage cells in IPF and lung cancer but common targets DNMT1 and COL1A1 show disease specific patterns. Mol Med Rep. 2018;17:7105–12. [DOI] [PMC free article] [PubMed]; Bibaki E, Tsitoura E, Vasarmidi E, Margaritopoulos G, Trachalaki A, Koutoulaki C. et al. miR-185 and miR-29a are similarly expressed in the bronchoalveolar lavage cells in IPF and lung cancer but common targets DNMT1 and COL1A1 show disease specific patterns. Mol Med Rep. 2018;17:7105–12. doi: 10.3892/mmr.2018.8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ge L, Habiel DM, Hansbro PM, Kim RY, Gharib SA, Edelman JD, et al. miR-323a-3p regulates lung fibrosis by targeting multiple profibrotic pathways. JCI Insight. 2016;1:e90301. [DOI] [PMC free article] [PubMed]; Ge L, Habiel DM, Hansbro PM, Kim RY, Gharib SA, Edelman JD. et al. miR-323a-3p regulates lung fibrosis by targeting multiple profibrotic pathways. JCI Insight. 2016;1:e90301. doi: 10.1172/jci.insight.90301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ren L, Yang C, Dou Y, Zhan R, Sun Y, Yu Y. MiR-541-5p regulates lung fibrosis by targeting cyclic nucleotide phosphodiesterase 1A. Exp Lung Res. 2017;43:249–58. [DOI] [PubMed]; Ren L, Yang C, Dou Y, Zhan R, Sun Y, Yu Y. MiR-541-5p regulates lung fibrosis by targeting cyclic nucleotide phosphodiesterase 1A. Exp Lung Res. 2017;43:249–58. doi: 10.1080/01902148.2017.1349210. [DOI] [PubMed] [Google Scholar]
- [24].Zhang Q, Ye H, Xiang F, Song LJ, Zhou LL, Cai PC, et al. miR-18a-5p inhibits sub-pleural pulmonary fibrosis by targeting TGF-beta receptor II. Mol Ther J Am Soc Gene Ther. 2017;25:728–38. [DOI] [PMC free article] [PubMed]; Zhang Q, Ye H, Xiang F, Song LJ, Zhou LL, Cai PC. et al. miR-18a-5p inhibits sub-pleural pulmonary fibrosis by targeting TGF-beta receptor II. Mol Ther J Am Soc Gene Ther. 2017;25:728–38. doi: 10.1016/j.ymthe.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–97. [DOI] [PMC free article] [PubMed]; Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ. et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–97. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Noble PW, Homer RJ. Back to the future: historical perspective on the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2005;33:113–20. [DOI] [PubMed]; Noble PW, Homer RJ. Back to the future: historical perspective on the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2005;33:113–20. doi: 10.1165/rcmb.F301. [DOI] [PubMed] [Google Scholar]
- [27].Prasad S, Hogaboam CM, Jarai G. Deficient repair response of IPF fibroblasts in a co-culture model of epithelial injury and repair. Fibrog Tissue Repair. 2014;7:7. [DOI] [PMC free article] [PubMed]; Prasad S, Hogaboam CM, Jarai G. Deficient repair response of IPF fibroblasts in a co-culture model of epithelial injury and repair. Fibrog Tissue Repair. 2014;7:7. doi: 10.1186/1755-1536-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet (London, England). 2011;378:1949–61. [DOI] [PubMed]; King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet (London, England) 2011;378:1949–61. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- [29].Li S, Geng J, Xu X, Huang X, Leng D, Jiang D, et al. miR-130b-3p modulates epithelial-mesenchymal crosstalk in lung fibrosis by targeting IGF-1. PLoS One. 2016;11:e0150418. [DOI] [PMC free article] [PubMed]; Li S, Geng J, Xu X, Huang X, Leng D, Jiang D. et al. miR-130b-3p modulates epithelial-mesenchymal crosstalk in lung fibrosis by targeting IGF-1. PLoS One. 2016;11:e0150418. doi: 10.1371/journal.pone.0150418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Amort T, Souliere MF, Wille A, Jia XY, Fiegl H, Worle H, et al. Long non-coding RNAs as targets for cytosine methylation. RNA Biol. 2013;10:1003–8. [DOI] [PMC free article] [PubMed]; Amort T, Souliere MF, Wille A, Jia XY, Fiegl H, Worle H. et al. Long non-coding RNAs as targets for cytosine methylation. RNA Biol. 2013;10:1003–8. doi: 10.4161/rna.24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huang G, Zhu H, Wu S, Cui M, Xu T. Long noncoding RNA can be a probable mechanism and a novel target for diagnosis and therapy in fragile X syndrome. Front Genet. 2019;10:446. [DOI] [PMC free article] [PubMed]; Huang G, Zhu H, Wu S, Cui M, Xu T. Long noncoding RNA can be a probable mechanism and a novel target for diagnosis and therapy in fragile X syndrome. Front Genet. 2019;10:446. doi: 10.3389/fgene.2019.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–66. [DOI] [PMC free article] [PubMed]; Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–66. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Clark MB, Mattick JS. Long noncoding RNAs in cell biology. Semin Cell Dev Biol. 2011;22:366–76. [DOI] [PubMed]; Clark MB, Mattick JS. Long noncoding RNAs in cell biology. Semin Cell Dev Biol. 2011;22:366–76. doi: 10.1016/j.semcdb.2011.01.001. [DOI] [PubMed] [Google Scholar]
- [34].Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–8. [DOI] [PubMed]; Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A. et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–8. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- [35].Kinney SR, Pradhan S. Ten eleven translocation enzymes and 5-hydroxymethylation in mammalian development and cancer. Adv Exp Med Biol. 2013;754:57–79. [DOI] [PubMed]; Kinney SR, Pradhan S. Ten eleven translocation enzymes and 5-hydroxymethylation in mammalian development and cancer. Adv Exp Med Biol. 2013;754:57–79. doi: 10.1007/978-1-4419-9967-2_3. [DOI] [PubMed] [Google Scholar]
- [36].Sanchez Calle A, Kawamura Y, Yamamoto Y, Takeshita F, Ochiya T. Emerging roles of long non-coding RNA in cancer. Cancer Sci. 2018;109:2093–2100. [DOI] [PMC free article] [PubMed]; Sanchez Calle A, Kawamura Y, Yamamoto Y, Takeshita F, Ochiya T. Emerging roles of long non-coding RNA in cancer. Cancer Sci. 2018;109:2093–2100. doi: 10.1111/cas.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Guo J, Liu Z, Gong R. Long noncoding RNA: an emerging player in diabetes and diabetic kidney disease. Clin Sci (London, England: 1979). 2019;133:1321–39. [DOI] [PubMed]; Guo J, Liu Z, Gong R. Long noncoding RNA: an emerging player in diabetes and diabetic kidney disease. Clin Sci (London, England: 1979) 2019;133:1321–39. doi: 10.1042/CS20190372. [DOI] [PubMed] [Google Scholar]
- [38].Hao X, Du Y, Qian L, Li D, Liu X. Upregulation of long noncoding RNA AP003419.16 predicts high risk of aging associated idiopathic pulmonary fibrosis. Mol Med Rep. 2017;16:8085–91. [DOI] [PMC free article] [PubMed]; Hao X, Du Y, Qian L, Li D, Liu X. Upregulation of long noncoding RNA AP003419.16 predicts high risk of aging associated idiopathic pulmonary fibrosis. Mol Med Rep. 2017;16:8085–91. doi: 10.3892/mmr.2017.7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Song X, Xu P, Meng C, Song C, Blackwell TS, Li R, et al. lncITPF promotes pulmonary fibrosis by targeting hnRNP-L depending on its host gene ITGBL1. Mol Ther J Am Soc Gene Ther. 2019;27:380–93. [DOI] [PMC free article] [PubMed]; Song X, Xu P, Meng C, Song C, Blackwell TS, Li R. et al. lncITPF promotes pulmonary fibrosis by targeting hnRNP-L depending on its host gene ITGBL1. Mol Ther J Am Soc Gene Ther. 2019;27:380–93. doi: 10.1016/j.ymthe.2018.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gao Y, Zhang J, Liu Y, Zhang S, Wang Y, Liu B, et al. Regulation of TERRA on telomeric and mitochondrial functions in IPF pathogenesis. BMC Pulm Med. 2017;17:163. [DOI] [PMC free article] [PubMed]; Gao Y, Zhang J, Liu Y, Zhang S, Wang Y, Liu B. et al. Regulation of TERRA on telomeric and mitochondrial functions in IPF pathogenesis. BMC Pulm Med. 2017;17:163. doi: 10.1186/s12890-017-0516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yu TY, Kao YW, Lin JJ. Telomeric transcripts stimulate telomere recombination to suppress senescence in cells lacking telomerase. Proc Natl Acad Sci USA. 2014;111:3377–82. [DOI] [PMC free article] [PubMed]; Yu TY, Kao YW, Lin JJ. Telomeric transcripts stimulate telomere recombination to suppress senescence in cells lacking telomerase. Proc Natl Acad Sci USA. 2014;111:3377–82. doi: 10.1073/pnas.1307415111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhao X, Sun J, Chen Y, Su W, Shan H, Li Y, et al. lncRNA PFAR promotes lung fibroblast activation and fibrosis by targeting miR-138 to regulate the YAP1-twist axis. Mol Ther J Am Soc Gene Ther. 2018;26:2206–17. [DOI] [PMC free article] [PubMed]; Zhao X, Sun J, Chen Y, Su W, Shan H, Li Y. et al. lncRNA PFAR promotes lung fibroblast activation and fibrosis by targeting miR-138 to regulate the YAP1-twist axis. Mol Ther J Am Soc Gene Ther. 2018;26:2206–17. doi: 10.1016/j.ymthe.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liu H, Wang B, Zhang J, Zhang S, Wang Y, Zhang J, et al. A novel lnc-PCF promotes the proliferation of TGF-beta1-activated epithelial cells by targeting miR-344a-5p to regulate map3k11 in pulmonary fibrosis. Cell Death Dis. 2017;8:e3137. [DOI] [PMC free article] [PubMed]; Liu H, Wang B, Zhang J, Zhang S, Wang Y, Zhang J. et al. A novel lnc-PCF promotes the proliferation of TGF-beta1-activated epithelial cells by targeting miR-344a-5p to regulate map3k11 in pulmonary fibrosis. Cell Death Dis. 2017;8:e3137. doi: 10.1038/cddis.2017.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li X, Yu T, Shan H, Jiang H, Sun J, Zhao X, et al. lncRNA PFAL promotes lung fibrosis through CTGF by competitively binding miR-18a. FASEB J Off Publ Fed Am Soc Exp Biol. 2018;32:5285–97. [DOI] [PubMed]; Li X, Yu T, Shan H, Jiang H, Sun J, Zhao X. et al. lncRNA PFAL promotes lung fibrosis through CTGF by competitively binding miR-18a. FASEB J Off Publ Fed Am Soc Exp Biol. 2018;32:5285–97. doi: 10.1096/fj.201800055R. [DOI] [PubMed] [Google Scholar]
- [45].Liang H, Gu Y, Li T, Zhang Y, Huangfu L, Hu M, et al. Integrated analyses identify the involvement of microRNA-26a in epithelial-mesenchymal transition during idiopathic pulmonary fibrosis. Cell Death Dis. 2014;5:e1238. [DOI] [PMC free article] [PubMed]; Liang H, Gu Y, Li T, Zhang Y, Huangfu L, Hu M. et al. Integrated analyses identify the involvement of microRNA-26a in epithelial-mesenchymal transition during idiopathic pulmonary fibrosis. Cell Death Dis. 2014;5:e1238. doi: 10.1038/cddis.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Du Y, Hao X, Liu X. Low expression of long noncoding RNA CDKN2B-AS1 in patients with idiopathic pulmonary fibrosis predicts lung cancer by regulating the p53-signaling pathway. Oncol Lett. 2018;15:4912–8. [DOI] [PMC free article] [PubMed]; Du Y, Hao X, Liu X. Low expression of long noncoding RNA CDKN2B-AS1 in patients with idiopathic pulmonary fibrosis predicts lung cancer by regulating the p53-signaling pathway. Oncol Lett. 2018;15:4912–8. doi: 10.3892/ol.2018.7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rubio K, Singh I, Dobersch S, Sarvari P, Gunther S, Cordero J, et al. Inactivation of nuclear histone deacetylases by EP300 disrupts the MiCEE complex in idiopathic pulmonary fibrosis. Nat Commun. 2019;10:2229. [DOI] [PMC free article] [PubMed]; Rubio K, Singh I, Dobersch S, Sarvari P, Gunther S, Cordero J. et al. Inactivation of nuclear histone deacetylases by EP300 disrupts the MiCEE complex in idiopathic pulmonary fibrosis. Nat Commun. 2019;10:2229. doi: 10.1038/s41467-019-10066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].McDonough JE, Kaminski N, Thienpont B, Hogg JC, Vanaudenaerde BM, Wuyts WA. Gene correlation network analysis to identify regulatory factors in idiopathic pulmonary fibrosis. Thorax. 2019;74:132–40. [DOI] [PMC free article] [PubMed]; McDonough JE, Kaminski N, Thienpont B, Hogg JC, Vanaudenaerde BM, Wuyts WA. Gene correlation network analysis to identify regulatory factors in idiopathic pulmonary fibrosis. Thorax. 2019;74:132–40. doi: 10.1136/thoraxjnl-2018-211929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Liu B, Jiang T, Hu X, Liu Z, Zhao L, Liu H, et al. Downregulation of microRNA30a in bronchoalveolar lavage fluid from idiopathic pulmonary fibrosis patients. Mol Med Rep. 2018;18:5799–806. [DOI] [PubMed]; Liu B, Jiang T, Hu X, Liu Z, Zhao L, Liu H. et al. Downregulation of microRNA30a in bronchoalveolar lavage fluid from idiopathic pulmonary fibrosis patients. Mol Med Rep. 2018;18:5799–806. doi: 10.3892/mmr.2018.9565. [DOI] [PubMed] [Google Scholar]
- [50].Wang L, Huang W, Zhang L, Chen Q, Zhao H. Molecular pathogenesis involved in human idiopathic pulmonary fibrosis based on an integrated microRNA mRNA interaction network. Mol Med Rep. 2018;18:4365–73. [DOI] [PMC free article] [PubMed]; Wang L, Huang W, Zhang L, Chen Q, Zhao H. Molecular pathogenesis involved in human idiopathic pulmonary fibrosis based on an integrated microRNA mRNA interaction network. Mol Med Rep. 2018;18:4365–73. doi: 10.3892/mmr.2018.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Diaz-Pina G, Ordonez-Razo RM, Montes E, Paramo I, Becerril C, Salgado A, et al. The role of ADAR1 and ADAR2 in the regulation of miRNA-21 in idiopathic pulmonary fibrosis. Lung. 2018;196:393–400. [DOI] [PubMed]; Diaz-Pina G, Ordonez-Razo RM, Montes E, Paramo I, Becerril C, Salgado A. et al. The role of ADAR1 and ADAR2 in the regulation of miRNA-21 in idiopathic pulmonary fibrosis. Lung. 2018;196:393–400. doi: 10.1007/s00408-018-0115-9. [DOI] [PubMed] [Google Scholar]
- [52].Zhang S, Liu H, Liu Y, Zhang J, Li H, Liu W, et al. miR-30a as potential therapeutics by targeting TET1 through regulation of Drp-1 promoter hydroxymethylation in idiopathic pulmonary fibrosis. Int J Mol Sci. 2017;18(3):633. [DOI] [PMC free article] [PubMed]; Zhang S, Liu H, Liu Y, Zhang J, Li H, Liu W. et al. miR-30a as potential therapeutics by targeting TET1 through regulation of Drp-1 promoter hydroxymethylation in idiopathic pulmonary fibrosis. Int J Mol Sci. 2017;18(3):633. doi: 10.3390/ijms18030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shetty SK, Tiwari N, Marudamuthu AS, Puthusseri B, Bhandary YP, Fu J, et al. p53 and miR-34a feedback promotes lung epithelial injury and pulmonary fibrosis. Am J Pathol. 2017;187:1016–34. [DOI] [PMC free article] [PubMed]; Shetty SK, Tiwari N, Marudamuthu AS, Puthusseri B, Bhandary YP, Fu J. et al. p53 and miR-34a feedback promotes lung epithelial injury and pulmonary fibrosis. Am J Pathol. 2017;187:1016–34. doi: 10.1016/j.ajpath.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chen X, Shi C, Wang C, Liu W, Chu Y, Xiang Z, et al. The role of miR-497-5p in myofibroblast differentiation of LR-MSCs and pulmonary fibrogenesis. Sci Rep. 2017;7:40958. [DOI] [PMC free article] [PubMed]; Chen X, Shi C, Wang C, Liu W, Chu Y, Xiang Z. et al. The role of miR-497-5p in myofibroblast differentiation of LR-MSCs and pulmonary fibrogenesis. Sci Rep. 2017;7:40958. doi: 10.1038/srep40958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cui H, Ge J, Xie N, Banerjee S, Zhou Y, Liu RM, et al. miR-34a promotes fibrosis in aged lungs by inducing alveolar epithelial dysfunctions. Am J Physiol Lung Cell Mol Physiol. 2017;312:L415–24. [DOI] [PMC free article] [PubMed]; Cui H, Ge J, Xie N, Banerjee S, Zhou Y, Liu RM. et al. miR-34a promotes fibrosis in aged lungs by inducing alveolar epithelial dysfunctions. Am J Physiol Lung Cell Mol Physiol. 2017;312:L415–24. doi: 10.1152/ajplung.00335.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cui H, Ge J, Xie N, Banerjee S, Zhou Y, Antony VB, et al. miR-34a inhibits lung fibrosis by inducing lung fibroblast senescence. Am J Respir Cell Mol Biol. 2017;56:168–78. [DOI] [PMC free article] [PubMed]; Cui H, Ge J, Xie N, Banerjee S, Zhou Y, Antony VB. et al. miR-34a inhibits lung fibrosis by inducing lung fibroblast senescence. Am J Respir Cell Mol Biol. 2017;56:168–78. doi: 10.1165/rcmb.2016-0163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Min H, Fan S, Song S, Zhuang Y, Li H, Wu Y, et al. Plasma microRNAs are associated with acute exacerbation in idiopathic pulmonary fibrosis. Diagn Pathol. 2016;11:135. [DOI] [PMC free article] [PubMed]; Min H, Fan S, Song S, Zhuang Y, Li H, Wu Y. et al. Plasma microRNAs are associated with acute exacerbation in idiopathic pulmonary fibrosis. Diagn Pathol. 2016;11:135. doi: 10.1186/s13000-016-0583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tang Y, He R, An J, Deng P, Huang L, Yang W. The effect of H19-miR-29b interaction on bleomycin-induced mouse model of idiopathic pulmonary fibrosis. Biochem Biophys Res Commun. 2016;479:417–23. [DOI] [PubMed]; Tang Y, He R, An J, Deng P, Huang L, Yang W. The effect of H19-miR-29b interaction on bleomycin-induced mouse model of idiopathic pulmonary fibrosis. Biochem Biophys Res Commun. 2016;479:417–23. doi: 10.1016/j.bbrc.2016.09.028. [DOI] [PubMed] [Google Scholar]
- [59].Makiguchi T, Yamada M, Yoshioka Y, Sugiura H, Koarai A, Chiba S, et al. Serum extracellular vesicular miR-21-5p is a predictor of the prognosis in idiopathic pulmonary fibrosis. Respir Res. 2016;17:110. [DOI] [PMC free article] [PubMed]; Makiguchi T, Yamada M, Yoshioka Y, Sugiura H, Koarai A, Chiba S. et al. Serum extracellular vesicular miR-21-5p is a predictor of the prognosis in idiopathic pulmonary fibrosis. Respir Res. 2016;17:110. doi: 10.1186/s12931-016-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chilosi M, Calio A, Rossi A, Gilioli E, Pedica F, Montagna L, et al. Epithelial to mesenchymal transition-related proteins ZEB1, beta-catenin, and beta-tubulin-III in idiopathic pulmonary fibrosis. Mod Pathol Off J US Can Acad Pathol Inc. 2017;30:26–38. [DOI] [PubMed]; Chilosi M, Calio A, Rossi A, Gilioli E, Pedica F, Montagna L. et al. Epithelial to mesenchymal transition-related proteins ZEB1, beta-catenin, and beta-tubulin-III in idiopathic pulmonary fibrosis. Mod Pathol Off J US Can Acad Pathol Inc. 2017;30:26–38. doi: 10.1038/modpathol.2016.147. [DOI] [PubMed] [Google Scholar]
- [61].Wang YC, Liu JS, Tang HK, Nie J, Zhu JX, Wen LL, et al. miR221 targets HMGA2 to inhibit bleomycin induced pulmonary fibrosis by regulating TGFbeta1/Smad3-induced EMT. Int J Mol Med. 2016;38:1208–16. [DOI] [PubMed]; Wang YC, Liu JS, Tang HK, Nie J, Zhu JX, Wen LL. et al. miR221 targets HMGA2 to inhibit bleomycin induced pulmonary fibrosis by regulating TGFbeta1/Smad3-induced EMT. Int J Mol Med. 2016;38:1208–16. doi: 10.3892/ijmm.2016.2705. [DOI] [PubMed] [Google Scholar]
- [62].Kamikawaji K, Seki N, Watanabe M, Mataki H, Kumamoto T, Takagi K, et al. Regulation of LOXL2 and SERPINH1 by antitumor microRNA-29a in lung cancer with idiopathic pulmonary fibrosis. J Hum Genet. 2016;61:985–93. [DOI] [PubMed]; Kamikawaji K, Seki N, Watanabe M, Mataki H, Kumamoto T, Takagi K. et al. Regulation of LOXL2 and SERPINH1 by antitumor microRNA-29a in lung cancer with idiopathic pulmonary fibrosis. J Hum Genet. 2016;61:985–93. doi: 10.1038/jhg.2016.99. [DOI] [PubMed] [Google Scholar]
- [63].Lei GS, Kline HL, Lee CH, Wilkes DS, Zhang C. Regulation of collagen V expression and epithelial-mesenchymal transition by miR-185 and miR-186 during idiopathic pulmonary fibrosis. Am J Pathol. 2016;186:2310–6. [DOI] [PMC free article] [PubMed]; Lei GS, Kline HL, Lee CH, Wilkes DS, Zhang C. Regulation of collagen V expression and epithelial-mesenchymal transition by miR-185 and miR-186 during idiopathic pulmonary fibrosis. Am J Pathol. 2016;186:2310–6. doi: 10.1016/j.ajpath.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Liang H, Liu S, Chen Y, Bai X, Liu L, Dong Y, et al. miR-26a suppresses EMT by disrupting the Lin28B/let-7d axis: potential cross-talks among miRNAs in IPF. J Mol Med (Berlin, Germany). 2016;94:655–65. [DOI] [PubMed]; Liang H, Liu S, Chen Y, Bai X, Liu L, Dong Y. et al. miR-26a suppresses EMT by disrupting the Lin28B/let-7d axis: potential cross-talks among miRNAs in IPF. J Mol Med (Berlin, Germany) 2016;94:655–65. doi: 10.1007/s00109-016-1381-8. [DOI] [PubMed] [Google Scholar]
- [65].Sun J, Su W, Zhao X, Shan T, Jin T, Guo Y, et al. lncRNA PFAR contributes to fibrogenesis in lung fibroblasts through competitively binding to miR-15a. Biosci Rep. 2019;39:BSR20190280. [DOI] [PMC free article] [PubMed]; Sun J, Su W, Zhao X, Shan T, Jin T, Guo Y. et al. lncRNA PFAR contributes to fibrogenesis in lung fibroblasts through competitively binding to miR-15a. Biosci Rep. 2019;39:BSR20190280. doi: 10.1042/BSR20190280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Qian W, Cai X, Qian Q, Peng W, Yu J, Zhang X, et al. lncRNA ZEB1-AS1 promotes pulmonary fibrosis through ZEB1-mediated epithelial-mesenchymal transition by competitively binding miR-141-3p. Cell Death Dis. 2019;10:129. [DOI] [PMC free article] [PubMed]; Qian W, Cai X, Qian Q, Peng W, Yu J, Zhang X. et al. lncRNA ZEB1-AS1 promotes pulmonary fibrosis through ZEB1-mediated epithelial-mesenchymal transition by competitively binding miR-141-3p. Cell Death Dis. 2019;10:129. doi: 10.1038/s41419-019-1339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Qu X, Song X, Yuan W, Shu Y, Wang Y, Zhao X, et al. Expression signature of lncRNAs and their potential roles in cardiac fibrosis of post-infarct mice. Biosci Rep. 2016;36:e00337. [DOI] [PMC free article] [PubMed]; Qu X, Song X, Yuan W, Shu Y, Wang Y, Zhao X. et al. Expression signature of lncRNAs and their potential roles in cardiac fibrosis of post-infarct mice. Biosci Rep. 2016;36:e00337. doi: 10.1042/BSR20150278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jiang H, Chen Y, Yu T, Zhao X, Shan H, Sun J, et al. Inhibition of lncRNA PFRL prevents pulmonary fibrosis by disrupting the miR-26a/Smad2 loop. Am J Physiol Lung Cell Mol Physiol. 2018;315:L563–75. [DOI] [PubMed]; Jiang H, Chen Y, Yu T, Zhao X, Shan H, Sun J. et al. Inhibition of lncRNA PFRL prevents pulmonary fibrosis by disrupting the miR-26a/Smad2 loop. Am J Physiol Lung Cell Mol Physiol. 2018;315:L563–75. doi: 10.1152/ajplung.00434.2017. [DOI] [PubMed] [Google Scholar]
- [69].Liu X, Gao S, Xu H. lncRNAPCAT29 inhibits pulmonary fibrosis via the TGFbeta1regulated RASAL1/ERK1/2 signal pathway. Mol Med Rep. 2018;17:7781–8. [DOI] [PubMed]; Liu X, Gao S, Xu H. lncRNAPCAT29 inhibits pulmonary fibrosis via the TGFbeta1regulated RASAL1/ERK1/2 signal pathway. Mol Med Rep. 2018;17:7781–8. doi: 10.3892/mmr.2018.8807. [DOI] [PubMed] [Google Scholar]
- [70].Liu Y, Li Y, Xu Q, Yao W, Wu Q, Yuan J, et al. Long non-coding RNA-ATB promotes EMT during silica-induced pulmonary fibrosis by competitively binding miR-200c. Biochim Biophys Acta Mol Basis Dis. 2018;1864:420–31. [DOI] [PubMed]; Liu Y, Li Y, Xu Q, Yao W, Wu Q, Yuan J. et al. Long non-coding RNA-ATB promotes EMT during silica-induced pulmonary fibrosis by competitively binding miR-200c. Biochim Biophys Acta Mol Basis Dis. 2018;1864:420–31. doi: 10.1016/j.bbadis.2017.11.003. [DOI] [PubMed] [Google Scholar]
- [71].Yan W, Wu Q, Yao W, Li Y, Liu Y, Yuan J, et al. MiR-503 modulates epithelial-mesenchymal transition in silica-induced pulmonary fibrosis by targeting PI3K p85 and is sponged by lncRNA MALAT1. Sci Rep. 2017;7:11313. [DOI] [PMC free article] [PubMed]; Yan W, Wu Q, Yao W, Li Y, Liu Y, Yuan J. et al. MiR-503 modulates epithelial-mesenchymal transition in silica-induced pulmonary fibrosis by targeting PI3K p85 and is sponged by lncRNA MALAT1. Sci Rep. 2017;7:11313. doi: 10.1038/s41598-017-11904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wu Q, Han L, Yan W, Ji X, Han R, Yang J, et al. miR-489 inhibits silica-induced pulmonary fibrosis by targeting MyD88 and Smad3 and is negatively regulated by lncRNA CHRF. Sci Rep. 2016;6:30921. [DOI] [PMC free article] [PubMed]; Wu Q, Han L, Yan W, Ji X, Han R, Yang J. et al. miR-489 inhibits silica-induced pulmonary fibrosis by targeting MyD88 and Smad3 and is negatively regulated by lncRNA CHRF. Sci Rep. 2016;6:30921. doi: 10.1038/srep30921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhou WQ, Wang P, Shao QP, Wang J. Lipopolysaccharide promotes pulmonary fibrosis in acute respiratory distress syndrome (ARDS) via lincRNA-p21 induced inhibition of Thy-1 expression. Mol Cell Biochem. 2016;419:19–28. [DOI] [PubMed]; Zhou WQ, Wang P, Shao QP, Wang J. Lipopolysaccharide promotes pulmonary fibrosis in acute respiratory distress syndrome (ARDS) via lincRNA-p21 induced inhibition of Thy-1 expression. Mol Cell Biochem. 2016;419:19–28. doi: 10.1007/s11010-016-2745-7. [DOI] [PubMed] [Google Scholar]


