Abstract
A mechanism of postmortem tenderization by adenosine 5′-monophosphate (AMP) on spent hen meat was investigated. Breast meat samples were made into a rectangular size of 7.5 × 5 × 2 cm and grouped into 5 different treatments, followed by immersion for 24 h at 4 ± 2°C in AMP marinade solutions of 0, 15, 30, 45, and 60 mmol/L that dissolved in 0.9% (w/v) saline solution. To investigate the enzymatic changes and tenderness-related traits, samples were stored until day 5 at a temperature of 2 ± 2°C. Result showed that each increase of 15 mmol/L AMP within marinade solution remarkably improved the myofibril fragmentation index and texture properties. The upregulation of tenderness-related enzymes was found for caspase-3 at 1 to 20.4 fold and 1 to 1.2 fold higher for the cathepsin-B, while a slight effect on calpains enzyme was observed. When compared with day 0 as a reference sample, the activity of the caspase-3 enzyme was more stable, as was cathepsin-B on the ultimate storage day, while the calpains enzyme showed a declining activity even after treatment. The flavor enhancement of 2.16- to 5.10-fold seemed to be a consequence of the AMP conversion into IMP that was responsible for the intensification of the umami-like flavor. No adverse effect was observed for instrumental surface color during the postmortem period. Therefore, this study suggested that the synergistic results after AMP treatment strongly contributed to postmortem tenderization mainly through cathepsin-B and caspase-3 enzyme upregulation, which led to more myofibrillar fragmentation and structural alteration of myofibrillar protein.
Key words: spent hen meat, AMP, texture propertiy, proteolytic enzyme, tenderization
Introduction
Among protein sources, spent layer hens are underutilized after reaching peak performance and entering the last cycle of egg laying (21–25 mo). Considering the rapid replacement of old laying hens with new pullets during the same period, old laying hens are commonly sold at a relatively low price for food and feed usage (Souza et al., 2011). The low price corresponds to poor meat quality, the existence of residual bone, and tough meat due to increased age, which leads to the increased formation of heat-stable collagen (Kersey and Waldroup, 1998). Even worse, the disposal of unutilized spent hens, which involves labor and transport costs, is an economic burden for the poultry industry (Freeman et al., 2009).
Spent hen meat is a potential functional food. Apart from its potential as an affordable protein source, numerous studies reported that spent hen-derived peptides possess essential physiological functions as anticancer, antihypertensive, antioxidant, immunomodulatory, and anti-inflammatory (Korhonen, 2009; Udenigwe, 2014; Li-Chan, 2015). These activities are likely due to the angiotensin-converting enzyme inhibitory peptides within spent hen meat that serve to inhibit the expression of proinflammatory interleukin 6 (Yu et al., 2018a), suppress the presence of antigen-presenting cells, and induce the increased activity of macrophages, dendritic cells, and anti-inflammatory interleukin 10 (Yu et al., 2018b). However, because meat tenderness is one of the most crucial factors for eating satisfaction (Wang et al., 2014), which determines the repurchasing intention of most consumers, the inferior texture of spent meat is apparently a factor that limits its continued consumption (Wattanachant et al., 2005).
Severe muscle is a product of cross-linkage between actin and myosin filaments, a myofibrillar protein, which together form a stiff muscle called actomyosin (Wang et al., 2015). In addition, increased age also leads to the increased formation of heat-stable collagen, another factor for tough texture (Kersey and Waldroup, 1998). Generally, after an animal is slaughtered, muscle is converted into meat during the postrigor period, where both collagen and actomyosin muscle formation remain unchanged (Steen et al., 1997). Alternatively, studies have proven that the application of physical and chemical treatments possibly contributes to the improvement of meat tenderness (Freeman et al., 2009; Souza et al., 2011; Wang et al., 2013). Particular factors for postmortem tenderness improvement are proposed by the complex interaction among tenderness-related enzymes (Lana and Zolla, 2016) that ultimately weaken the cross-linkage bonds between actin and myosin (Taylor et al., 1995), the liberation of actin from myofibrillar protein (Okitani et al., 2009), myofibril fragmentation, and the dissociation of actomyosin (Wang et al., 2013). Meanwhile, the challenges are mainly the consistent effect of tenderization and cost-effective treatment for continued utilization of the proposed method for meat tenderization in the poultry industry.
Adenosine 5′-monophosphate (AMP) which composed of sugar ribose, phosphate group, and the nucleobase adenine, is an essential component for the synthesis of RNA and DNA in all biological matter (Wang et al., 2015). During high energy expenditure in animal life, AMP involves in energy metabolism through the activation of 5′-adenosine monophosphate protein kinase (AMPK) (Richter and Ruderman, 2009) by binding to the specific γ regulatory subunit of AMPK. Its activation releases energy to regenerate adenosine triphosphate (ATP) from broken-down molecules (Krishan et al., 2015). In meat, AMP is generated from a complex reaction called adenylate kinase that occurs when ATP degradation happen during muscle glycogen depletions. The utilization of AMP is mainly proposed to enrich flavor and as additives for specific nutritions in food industry (Koguchi et al., 2003; Wang et al., 2013). Recent findings revealed that AMP could also contribute to a mechanism of postmortem tenderization through the upregulation of cell apoptosis and structural changes of muscle proteins (Wang et al., 2015; Gao et al., 2020). Chen et al. (2015) mentioned that the binding of AMP to the specific γ regulatory subunit of AMPK could maintains the continued activation of AMPK and consequently intensify the activity of tenderness-related cysteine-aspartic acid protease (caspase-3). In addition, a report by Wang et al. (2015) mentioned that the role of AMP in meat tenderization is through the ability of this nucleotide to enhance actomyosin dissociation, myofibril fragmentation, and the liberation of actin from myofibrillar protein. Meanwhile, the inosine monophosphate (IMP) is a result from the deamination process of AMP by adenosine monophosphate deaminase. It is known to positively contribute for the umami taste through the enhancement of glutamic acid (Zhang et al., 2013).
As the improvement in meat tenderness is a result of a complex interaction among variables, it is also affected by a series of enzymatic interactions during postmortem periods (Lana and Zolla, 2016). However, the enzymatic role in tenderness may vary among species. In particular, caspase-3 activity, an enzyme responsible for cell apoptosis, plays a significant role during postmortem tenderization by exhibiting more myofibril fragmentation in chickens (Chen et al., 2011). However, this enzyme did not play a meaningful function in postmortem tenderization in duck breast meat (He et al., 2019) or in bovine sirloin (Kemp et al., 2010). Instead, the calpain system and cathepsin-B enzyme activity play a major function in meat tenderization in most species (Lana and Zolla, 2016). With regard to understanding these unclear mechanisms in meat tenderization as affected by exogenous treatment, this study was performed with the objectives of 1) investigating the effect of spent hen meat treated with AMP treatment on postmortem tenderization and 2) revealing the mechanism of postmortem tenderization of spent hen meat after AMP treatment. This study is expected to provide useful information on postmortem tenderization for the poultry industry and increase the utilization of spent hen meat.
Materials and methods
Sample Preparation
This study utilized a total of 96 skinless pectoralis major spent hen muscles obtained from a local slaughterhouse 24 h postmortem. The sample preparation was conducted in a chilling room with a temperature of 4 ± 2°C. After visible fat was removed, the spent hen breast meat was cut into a similar rectangular size of 7.5 × 5 × 2 cm. Samples were divided into 5 different groups and subjected to immersed marination for 24 h at 2 ± 2°C in different concentrations of AMP solution at 0, 15, 30, 45, and 60 mmol/L that previously dissolved in 0.9% (w/v) saline solution. Subsequently, to investigate the enzymatic changes and quality properties, samples were stored until day 5 at a temperature of 2 ± 2°C. Samples were taken at day 0, day 1, day 3, and day 5. Data for day 0 were used as the untreated control.
pH
Ground breast meat samples in the amount of 5 g were homogenized with 45 mL of distilled water using a homogenizer (PH91; SMT Co., Ltd., Tokyo, Japan) for 1 min at a constant speed of 13,000 rpm. The pH value of homogenate was determined by pH meter probe (Seven Easy pH; Mettler-Toledo GmbH, Schwerzenbach, Switzerland) in triplicate.
Shear Force Value
After subjected to marination, the sample was put into a plastic bag and subjected to boiling in a water bath at a temperature 75°C for 35 min or until the final core temperature reached 71°C. The degree of meat tenderness was expressed as shear force value, where a lower value indicates a more tender meat. The measurement of shear force value was performed by using a TA-XT2i Plus (Stable Micro Systems, Surrey, UK). Briefly, the boiled sample was formed into a rectangular size with a size of 1.5 cm × 1.5 cm. Each sample was placed under the V blade and cut with a constant speed (assay parameters were: pretest speed: 2.0 mm/s; test speed: 1.0 mm/s; posttest speed: 10 mm/s). Each sample was repeated 5 times.
Myofibrillar Fragmentation Index
The determination of myofibrillar fragmentation index (MFI) was referring to a method by Culler et al. (1978) with slight modifications. Each of the marinated samples was prepared in triplicate. To ensure the elimination of visible fat and connective tissue, the sample was minced into a smaller size and fat was removed. Minced sample in the amount of 2 g was homogenized for 20 s in 10 vol (v/w) of precooled isolating buffer (0.01 mmol/L EDTA, 20 mmol/L K3PO4, 100 mmol/L KCl, and 1.0 mmol/L CaCl2; pH was adjusted to 7.0). Centrifugation was performed at 1,500 rpm for 15 min followed by a removal of supernatant. Precipitate was subjected to homogenization in 10 vol (v/w) of isolating buffer, centrifuged at same the condition. The supernatant was removed and precipitate was next resuspended in 2.5 vol (v/w) of separating buffer. Filtration was conducted by using Whatman paper no 1, while the additional of 2.5 vol (v/w) isolating buffer was used to rinse the precipitate. The absorbance was measured at at 540 nm was by using spectrophotometer. The optical density was multiplied with 200 to obtain the MFI.
Instrumental Surface Color
The instrumental surface color was recorded by measuring International Commission on Illumination's system for lightness (CIE L∗), redness (CIE a∗), and yellowness (CIE b∗) using a chroma meter (CR-400, Konica Minolta Inc., Tokyo, Japan). The light source of illuminant C (2° observer) with 8 mm aperture and attached-closed cone was calibrated using a white plate (Y = 93.6, X = 0.3134, y = 0.3194). Whiteness was calculated using the following formula: 100 – [(100 – CIE L∗)2 + (CIE a∗2 × CIE b∗2)]1/2.
Adenosine 5′-Monophosphate and Inosine Monophosphate
The method for analyzing 5′-nucleotide contents (adenosine monophosphate, IMP, and guanine monophosphate) was modified from the method of Jayasena et al. (2014). The determination of 5′-nucleoside was performed on 5 g of breast meat samples by HPLC (Shimadzu Nexera X2 HPLC, Kyoto, Japan) equipped with an SPD-M20A diode array detector at a wavelength of 254 nm. 5′-Nucleoside concentrations were expressed as mg of compound per 100 g of cooked matter (mg/100 g).
Activities of Calpains and Cathepsin-B
The determination of calpain and cathepsin-b enzyme was in accordance with a method by He et al. (2019). About 0.5 g of homogenated samples in 1.0 mL precooled lysate (25 mmol/L Tris-HCl, 150 mmol/L NaCl, 50 mmol/L EDTA, 1.0 mmol/L DTT, and 1.0% Triton-100; pH = 7.6) were centrifuged at 4°C, 12,000 g for 40 min. The protein concentration was measured by Biuret method. The supernatant of the samples were mixed with reaction buffer (115 mmol/L NaCl, 1.0 mmol/L KH2PO4, 5.0 mmol/L KCl, 2.0 mmol/L CaCl2, 1.2 mmol/L MgSO4, 25 mmol/L HEPES, and 0.6 mmol/L substrate (Suc-LY-AMC for calpains and ARR-AFC for cathepsin-B, respectively); pH = 7.4) at the ratio 1:1, and incubated at 37°C for 2 h. In control, the supernatant was replaced by ddH2O. The absorbance value of the mixture was detected at the wavelength of 380 nm/460 nm (excitation/emission) for calpains, and 400 nm/505 nm (excitation/emission) for cathepsin-B. The enzyme activity was shown as the relative absorbance value per min, per mg to control.
Activity of Caspase-3
Caspase-3 enzyme activities were determined in accordance with a method by He et al. (2019). About 0.5 g of homogenated samples in 1.0 mL lysate (100 mmol/L HEPES, 20% glycerol, 0.5 mmol/L EDTA, 5.0 mmol/L DTT, and 0.2% SDS; pH = 7.5) were centrifuged at the speed of 12,000 rpm for 20 min. The protein concentration of the supernatant was determined using the Biuret method. The supernatant was then mixed with the reaction buffer (100 mmol/L HEPES, 20% glycerol, 0.5 mmol/L EDTA, and 5.0 mmol/L DTT; pH = 7.5) at the ratio of 1:1. In control, the supernatant was replaced by ddH2O. After incubation at 37°C for 10 min, 1.0 mmol/L Ac-DEVD-pNA (dissolved in DMSO) was added in to react at 37°C for 1 h. The mixture was rapidly put in ice to terminate the reaction and the absorbance value of the mixture was detected at a wavelength of 405 nm. The enzyme activity was shown as the relative absorbance value per min, per mg to control.
Statistical Analysis
The data analyses in this study performing two-way multivariate analysis of variance using R-version 3.6.1 (The R-foundation for Statistical Computing, Vienna, Austria) with a respect to treatments and storage day. The significant mean value of each group was continuously analyzed by using the Duncan's multiple range test, with a consideration as significant for P-values lower than 0.05.
Results and discussion
pH Value
The alterations in pH value after treatment with AMP during storage time are shown in Table 1. Spent hen meat samples treated with AMP, regardless of the concentration, possessed a significantly higher pH value than the control group throughout the storage day (P < 0.05). In a fresh state, as recorded on day 0, the initial pH value was 5.68, which is within the normal range of 5.67 to 6.22 (Sulcerova et al., 2014). During storage, the ultimate pH value at day 5 in all meat samples was lower than that of day 1 (P < 0.001) but slightly higher than day 0, except for the control group (P > 0.05). A similar trend was reported by Wang et al. (2015), who reported an increasing pH value in duck breast meat after treatment with AMP.
Table 1.
pH value of spent hen breast meat after treated with adenosine monophosphate (AMP) during cold storage at 2 ± 2°C.
| Storage (day) | Treatments (mmol/L) |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | Sample | Storage | Storage × sample | ||
| 0 | 5.68x | 5.68z | 5.68z | 5.68z | 5.68z | 0.00 | <0.001 | <0.001 | 0.15 |
| 1 | 5.70c,x | 5.90b,x | 6.19a,w | 6.23a,x | 6.29a,w | 0.05 | |||
| 3 | 5.61b,xy | 5.81a,y | 5.83a,y | 5.85a,y | 5.83a,y | 0.02 | |||
| 5 | 5.59c,y | 5.75b,yz | 5.93a,x | 5.86ab,y | 5.97a,x | 0.04 | |||
a-cMeans within the same row are significantly different among treatment (P < 0.05).
x-zMeans within the same column are significantly different during storage day (P < 0.05).
The pH value indicates the biochemical reactions within the meat muscle (Juncher et al., 2001; Huff-Lonergan and Lonergan, 2005). It correlates with other meat quality traits, such as the ability of a muscle to retain water (Barbut et al., 2005), surface color, and physiological and inner muscle environments (Honikel et al., 1986). In general, after the animal is euthanized, there is a conversion of muscle into meat, led by the activation of glycolytic enzymes that break down glycogen into lactic acid and pyruvic acid as the final products (Lana and Zolla, 2016). A continuous decline in pH value is indeed a threat to meat quality, where an abnormally low pH value is responsible for the denaturation of sarcoplasmic protein (Huff-Lonergan and Lonergan, 2005), causing the inability of a muscle to retain water and eventually lowering the cooking yield and eating satisfaction (Shao et al., 2016). Treatment with AMP increased the pH value, which seemed to be caused by the ionic strength of AMP that improves the binding ability into meat protein, changes the surface environment (Song et al., 2020) and improves its capacity to retain immobilized water (Shao et al., 2016).
Shear Force Value
After treatment with AMP at various concentrations, the shear force value of spent hen meat can be seen in Table 2. Its value was significantly lower (P < 0.001) in all treatment groups, with the lowest value obtained in the 60 mmol/L group, signifying great tenderness. A gap of 15 mmol/L was found to generate an improvement in meat tenderness (P < 0.05). Storage day was also found to increase the tenderness of spent hen meat, where the ultimate storage day had a significantly lower value than that of day 0 (P < 0.001). Along with taste and nutritional content, meat tenderness is recognized as an important factor that determines eating satisfaction (Nadzirah et al., 2016). Generally, chicken breast meat is defined as acceptable with a shear force value ranging from 1.21 to 1.65 kgf (Zhuang et al., 2013). Therefore, the inferior texture of spent meat is apparently a factor that limits continuous consumption (Nowsad et al., 2000).
Table 2.
Shear force value (kgf) of spent hen breast meat after treated with adenosine monophosphate (AMP) during cold storage at 2 ± 2°C.
| Storage (day) | Treatments (mmol/L) |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | Sample | Storage | Storage × sample | ||
| 0 | 3.28x | 3.28x | 3.28x | 3.28x | 3.28x | 0.01 | <0.001 | <0.001 | 0.99 |
| 1 | 3.22a,x | 2.49b,y | 2.25b,c,x | 1.97c,y | 1.22d,y | 0.13 | |||
| 3 | 3.15a,x | 2.42b,y,z | 2.17b,c,x | 1.89c,y | 1.13d,y,z | 0.13 | |||
| 5 | 2.93a,x | 2.18b,z | 1.91b,c,y | 1.62c,z | 0.92d,z | 0.13 | |||
a-dMeans within the same row are significantly different among treatment (P < 0.05).
x-zMeans within the same column are significantly different during storage day (P < 0.05).
It is widely understood that actomyosin dissociation and the breakdown of myofibrillar structure during postmortem aging at specific temperatures are directly associated with meat tenderness (Culler et al., 1978). Recently, to improve meat tenderness practically, without requiring spacious storage and costly operations, marination has mostly been used (Arshad et al., 2016). As revealed by this study, in addition to its function as a flavor enhancer, marination with AMP improved the tenderness of spent hen meat, thus overcoming its inferior texture. This might be attributed to the synergistic effect of its phosphate chain to weaken cross-linkage bonds between actin and myosin, dissociate more actomyosin (Barbut, 1993; Spudich, 2001), and release free actin (Okitani et al., 2009). The ionic strength of AMP leads to the alteration of the meat surface environment, thus affecting its stronger capacity to retain water (Shao et al., 2016) and the creation of more space within myofibrils to avoid intracellular release of water (Honikel et al., 1986). These significant findings corresponded to previous studies on the semimembranosus muscle of cattle (Zou et al., 2019) and duck breast meat (Wang et al., 2015).
Myofibrillar Fragmentation Index
The degree of MFI was significantly higher in AMP-treated samples than in samples not subjected to any treatment (P < 0.001). As seen in Table 3, the highest MFI was found in spent hen meat marinated with 60 mmol/L. The increased gap of 15 mmol/L tended to exhibit a significantly higher MFI (P < 0.05). Storage day was also associated with a significantly higher MFI (P < 0.05), with day 5 showing a significantly higher value than day 0. The cycle of AMP production is determined by the concentration of Ca2+ ions (Harary et al., 1976) that exhibit a high ionic strength capacity. This ion caused a calcium-specific effect that maintains the interaction between actin and myosin (Lawrie et al., 1991) that is eventually responsible for the architectural changes of myofibril protein (Nurmahmudi and Sams, 1997) as a result of extracted protein and liberated actin (Culler et al., 1978). The higher MFI value indicates a significant change in the structure of meat myofibril. Generally, an MFI value in chicken breast meat of more than 75 could positively contribute for the enhancement of meat tenderness (Kim et al., 2016). In addition, the high MFI value for AMP-treated samples, as explained by Chen et al. (2020), might be attributed to the ability of the AMP phosphate chain to dissociate the sarcomere protein that ultimately modifies muscle cell integrity. This result was coincidental with a previous study by Wang et al. (2015), who revealed that curing with AMP possibly increases the MFI value through alterations of meat microstructural composition, especially the I-band and Z-line in duck breast meat. The MFI is an essential index that is directly correlated with meat tenderness (Takahasi et al., 1967). The quantification of MFI is based on the amount of extracted myofibrillar proteins, the background of postmortem meat tenderization (Gordon and Barbut, 1989).
Table 3.
Myofibrillar fragmentation index (MFI) of spent hen breast meat after treated with adenosine monophosphate (AMP) during cold storage at 2 ± 2°C.
| Storage (day) | Treatments (mmol/L) |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | Sample | Storage | Storage × sample | ||
| 0 | 34.57z | 34.57z | 34.57z | 34.57z | 34.57z | 0.00 | <0.001 | <0.001 | 0.61 |
| 1 | 36.76e,z | 80.96d,y | 121.53c,y | 121.53b,y | 161.16a,y | 8.35 | |||
| 3 | 86.36c,y | 206.80b,x | 210.30b,x | 213.11b,x | 230.27a,x | 9.73 | |||
| 5 | 154.17c,x | 199.90b,c,x | 200.73b,c,x | 256.83a,b,w | 272.97a,w | 12.04 | |||
a-eMeans within the same row are significantly different among treatment (P < 0.05).
w-zMeans within the same column are significantly different during storage day (P < 0.05).
Instrumental Surface Color
The surface color of spent hen meat is shown in Table 4. A significantly lower L∗ value was recorded in the treatment groups than in the control group on storage days 1 and 5 (P < 0.001). By contrast, the redness value was found to be significantly higher after treatment with AMP throughout storage than the control group (P < 0.001). A similar trend was also observed for the yellowness, where treated samples displayed a higher value than the control (P < 0.001). The lower lightness of the meat surface is considered to be correlated with the higher water content within muscle myofibrils. This condition is regarded as profitable for economic traits because it affects a higher water holding capacity (Barbut, 1993). This value is within the normal range for chicken breast meat, which is 48-53 (Qiao et al., 2001). In addition, the more red color on the meat surface is likely visually preferable for consumers. It could be used as a parameter to determine the freshness and quality of meat (Barido et al., 2020).
Table 4.
Instrumental surface color of spent hen breast meat after treated with adenosine monophosphate (AMP) during cold storage at 2 ± 2°C.
| Variable | Storage (day) | Treatments (mmol/L) |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | Sample | Storage | Storage × sample | |||
| 0 | 65.74x | 65.74x | 65.74x | 65.74x | 65.74x | 0.00 | ||||
| CIE L∗ | 1 | 66.91a,x | 63.65a,b,x | 62.24b,x,y | 56.75c,z | 61.26b,y | 0.85 | <0.001 | <0.001 | <0.05 |
| 3 | 62.65a,x | 62.28a,x | 59.26a,y,z | 60.49a,y | 62.12a,x,y | 0.52 | ||||
| 5 | 62.77b,x | 65.31a,x | 57.69c,z | 59.18c,y | 61.97b,y | 0.61 | ||||
| 0 | 0.35x | 0.35y | 0.35y | 0.35z | 0.35z | 0.00 | ||||
| CIE a∗ | 1 | 0.42d,x | 0.76c,d,x | 1.34b,x | 2.47a,x | 1.95a,b,x | 0.16 | <0.001 | <0.001 | <0.05 |
| 3 | 0.34b,x | 0.93a,x | 0.49b,y | 1.15a,y | 0.85a,y | 0.08 | ||||
| 5 | 0.24c,x | 0.72b,x | 0.31c,y | 1.32a,y | 1.19a,y | 0.17 | ||||
| 0 | 2.89x | 2.89y | 2.89x,y | 2.89y | 2.89y | 0.00 | ||||
| CIE b∗ | 1 | 2.85a,x | 2.47a,b,y | 1.76a,b,y | 0.83b,z | 1.51a,b,z | 0.26 | <0.001 | <0.001 | <0.05 |
| 3 | 2.58b,x | 5.01a,x | 3.84a,b,x | 5.53a,x | 4.97b,x | 0.39 | ||||
| 5 | 2.14b,y | 4.79a,x | 2.73b,x,y | 4.79a,x | 4.99a,x | 0.28 | ||||
a-cMeans within the same row are significantly different among treatment (P < 0.05).
x-zMeans within the same column are significantly different during storage day (P < 0.05).
Adenosine 5′-Monophosphate and Inosine Monophosphate
Table 5 shows the concentration of AMP in spent hen meat samples on day 1 after marination with exogenous AMP. The results showed that the concentration of AMP remained unchanged even after treatment with the highest concentration of 60 mmol/L (P > 0.05). Adenosine 5′-monophosphate is responsible for an umami-like taste. It is a nucleotide compound commonly found in all living organisms, including chickens (Wang et al., 2015). Adenosine 5′-monophosphate is involved in energy metabolism for the production of ATP and ADP (Xiao et al., 2011). However, after being subjected to the storage and processing stage, the existence of AMP in the meat may be broken or converted into another form (Takakura et al., 2014; Zhang et al., 2013). Therefore, the exogenous addition of AMP as a compound is addressed as a flavor enhancer in the food industry. The insignificant difference in AMP concentration found in this study after treatment might be caused by the conversion of AMP into IMP and ammonia by the AMP deaminase enzyme (Yang et al., 2020). This was similar to our previous study on the effect of chicken soup cooked with the addition of high AMP content mushrooms (Barido et al., 2020).
Table 5.
Adenosine monophosphate (AMP) and inosine monophosphate (IMP) profile of spent hen breast meat after treated with AMP.
| Variable | Treatments (mmol/L) |
SEM | P Value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | |||
| AMP (mg/g) | 0.051 | 0.048 | 0.049 | 0.050 | 0.055 | 0.008 | 0.06 |
| IMP (mg/g) | 1.03e | 2.23d | 3.36c | 4.31b | 5.27a | 0.289 | <0.01 |
a-e Means within the same row are significantly different among treatment (P < 0.05).
Accordingly, as also shown in Table 5, the concentration of IMP, which is another factor affecting flavor profile, was shown to be elevated by the higher concentration of AMP, with a different range of 15 mmol/L significantly increasing the concentration of IMP (P < 0.05). This study, as explained by Wang et al. (2015), showed that thoroughly permeating AMP into duck breast meat would result in biochemical conversion into inosinic acid and ammonia. Inosinic acid is widely used as a flavor enhancer in the food industry because it provides a raw umami-like taste and is considered a highly effective salt (Voet and Voet 2010). Although chicken meat also comprises IMP, because it is the derivative product of post–rigor mortis (Yang et al., 2020), the addition of processed meat with IMP aimed to give a richer taste after a possible loss by processing stage (Jayasena et al., 2014). The rich umami-like flavor chicken meat enriched with the addition of either pure or plant origin flavor enhancer is preferable (Barido et al., 2020). Therefore, the marination of spent hen breast meat with AMP could potentially improve flavor variations.
Calpain System
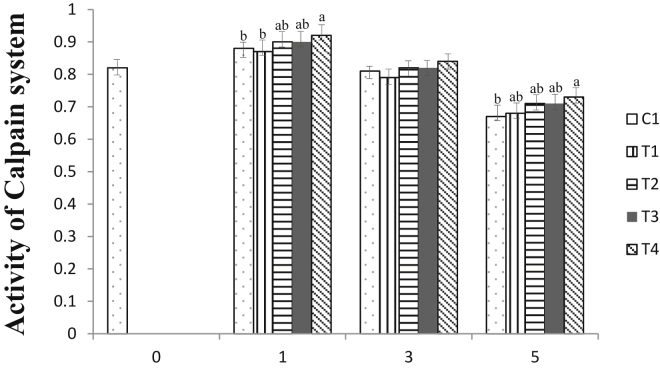
The involvement of the calpain system in meat tenderization is species-dependent (Zhao et al., 2017) and has been polemicized (Herrera-Mendez et al., 2006). Although its activity is mentioned to have a specific effect on duck breast meat (Chang and Chou, 2012), pork meat (Bee et al., 2007), and chicken breast meat (Zhao et al., 2017), tenderization during postmortem storage has a low effect on cattle meat (Veiseth et al., 2001). However, in this study, as seen in Figure 1, a slightly higher activity of calpain enzyme was only detected for the meat sample treated with the highest concentration of AMP at 60 mmol/L. There was no significant difference observed between the control and remaining treatment groups (P > 0.05), signifying that this calpain activity was well activated without the addition of AMP. The activation of the postmortem calpain enzyme, as mentioned by Melloni et al. (1996), is regulated through a complex interaction among muscle environments, especially the concentration of Ca2+ ions and phospholipid binding. Therefore, although the need for free Ca2+ ions for the activation of calpain enzymes is not as high as in mammals, marination with exogenous AMP apparently did not provide sufficient free Ca2+ ions for greater activation of the calpain system. In addition, as explained by Kaur et al. (2020), although this enzyme system is considered to play a major role during postmortem tenderization, the activity of the calpain system seemed to be less stable. The same trend was observed in duck breast meat; during postmortem tenderization, the contribution of calpain enzymes was lower than that of cathepsin-B and caspase-3 enzymes (He et al., 2019). This indicates that the calpain enzyme in spent hen breast meat did not significantly play a major role as a single enzyme for meat tenderization during the postmortem period.
Figure 1.
Calpain enzyme activities expressed in relative absorbance value per min, per mg to control of spent hen breast meat after treated with adenosine monophosphate (AMP) during cold storage at 2 ± 2°C. C1 (control), T1 (15 mmol/L AMP), T2 (30 mmol/L AMP), T3 (45 mmol/L AMP), and T4 (60 mmol/L AMP) stand for different range of AMP treatment.
Cathepsin-B Enzyme
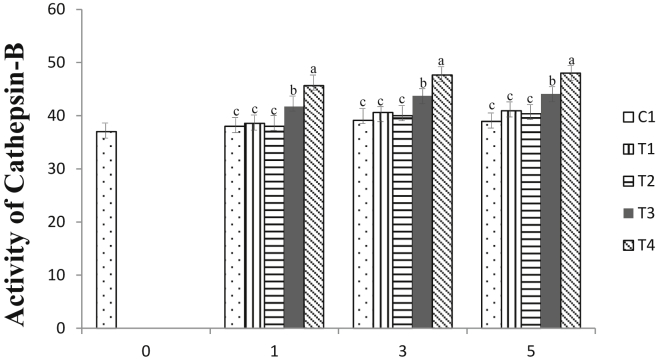
The activity of the cathepsin-B enzyme after AMP treatment is shown in Figure 2. Spent hen meat treated with AMP at a concentration of more than 45 mmol/L exhibited better activity than the control and remaining treatment groups (P < 0.05) throughout storage. No differences were recorded between the control and treatment groups of 15 and 30 mmol/L (P > 0.05) at the beginning of storage; however, all AMP-treated samples maintained a higher activity of cathepsin-B enzyme. Moreover, all AMP-treated samples showed stable and significantly higher activities at the final storage day compared with that of day 0. After rigor mortis, the pH decline affects the destruction of the sarcoplasmic reticulum (Zhao et al., 2017), depletion of endogenous Ca2+ from cells, and eventually interferes with the enzymatic mechanism (Quali, 1992). Rapid degradation of calpain activity is another consequence of endogenous ionic loss. Therefore, cathepsin enzymes, especially cathepsin-B, are considered to play a more significant effect on spent hen meat tenderization considering their stability (Kaur et al., 2020). This study was in accordance with a previous study (He et al., 2019) in duck breast meat, where the cathepsin-B activity was well maintained until storage day 4 with or without additional treatment, and another study (Lee et al., 2008) that revealed the greater effect of cathepsin-B enzyme after the calpain system was degraded. The upregulation mechanism of the cathepsin-B enzyme by AMP is not well understood; however, it is apparently caused by the increased liberation of cathepsin enzymes from lysosomes (Bowker et al., 2010; Lana and Zolla, 2016) as the effect of cell extraction by the strong ionic capacity of AMP.
Figure 2.
Cathepsin-B enzyme activities expressed in relative absorbance value per min, per mg to control of spent hen breast meat after treated with adenosine monophosphate (AMP) during cold storage at 2 ± 2°C. C1 (control), T1 (15 mmol/L AMP), T2 (30 mmol/L AMP), T3 (45 mmol/L AMP), and T4 (60 mmol/L AMP) stand for different range of AMP treatment.
Caspase-3
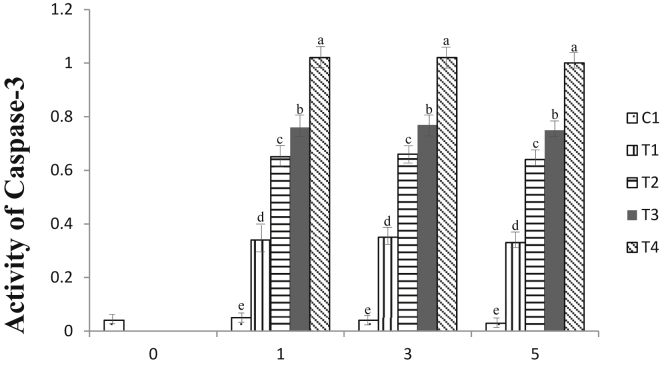
Treatment with AMP greatly upregulated the activity of the caspase-3 enzyme, the enzyme responsible for apoptosis (Kemp and Parr, 2008). The activity of the caspase-3 enzyme was significantly higher in the AMP-treated groups than in the control group (P < 0.05), as shown in Figure 3. The increased concentration of 15 mmol/L AMP led to the higher activity of caspase-3 in spent layer breast meat (P < 0.05), as seen with a significant difference for each treatment group. Compared with the initial storage day, the activity of caspase-3 enzyme in day 5 for all samples was significantly higher, except for the control. The effort to tenderize meat mainly focuses on the weakening bond between actin and myosin (Sentandreu et al., 2002) and the alteration of the architectural structure of myofibrillar proteins; namely, troponin-T, titin, desmin, and nebulin (Huff-Lonergan and Lonergan, 2005; Bowker et al., 2010). For many years, it has been believed that the calpain system and cathepsin enzymes are the major determinants of postmortem tenderization (Kohmarie et al., 1991). However, in hypoxic-ischemic conditions, after the oxygen supply by blood is stopped, the cells are destroyed and considered dead, therefore altering the muscle environment (Ouali et al., 2006). This apoptosis condition should be taken into account as part of the factors responsible for postmortem meat tenderization (Lana and Zolla, 2016), especially the caspase-3 enzyme that induces the occurrence of apoptosis (Chen et al., 2011). This study found that AMP was able to upregulate apoptosis-related enzymes. The reason might be that in addition to its ability to modulate the proliferation of various cells, including muscle cells (Jacobson et al., 1999), the upregulation of caspase-3 enzyme is attributed to a specific effect of AMP to bind onto specific γ-subregulatory sites of adenosine monophosphate kinase (AMPK), thus activating AMPK to induce a greater occurrence of apoptosis (Yang et al., 2020) and eventually contributing to postmortem meat tenderization. A similar trend was also observed in chicken meat (Nurmahmudi and Sams, 1997; Chen et al., 2011) and duck breast meat (He et al., 2019).
Figure 3.
Caspase-3 enzyme activities expressed in relative absorbance value per min, per mg to control of spent hen breast meat after treated with adenosine monophosphate (AMP) during cold storage at 2 ± 2°C. C1 (control), T1 (15 mmol/L AMP), T2 (30 mmol/L AMP), T3 (45 mmol/L AMP), and T4 (60 mmol/L AMP) stand for different range of AMP treatment.
Conclusion
Adenosine monophosphate could be used as a curing agent to improve texture properties of the spent hen meat. Marination of spent hen meat with AMP led to the upregulation of tenderness-related enzymes, especially the cathepsin-B and caspase-3 enzymes, with a slight effect on calpains enzyme. It also induced more degradation of myofibrillar protein, which ultimately improved its tenderness during the postmortem period. The flavor enhancement was also a consequence of the AMP conversion to IMP, which is responsible for the umami-like flavor. This study suggested that the upregulation of proteolytic enzymes by AMP could be part of the mechanism for postmortem tenderization in spent hen breast meat.
Acknowledgments
This study was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry through the Export Promotion Technology Development Program (617074054HD220).
Disclosures
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this article.
References
- Arshad M.A., Kwon J.H., Imran M., Sohaib M., Aslam A., Nawaz I., Amjad Z., Khan U., Javed M. Plant and bacterial proteases: a key towards improving meat tenderization, a mini review. Cogent Food Agric. 2016;2:1–10. [Google Scholar]
- Barbut S. Colour measurement for evaluating the pale soft exudative (PSE) occurrence in Turkey meat. Food Res. Int. 1993;26:39–43. [Google Scholar]
- Barbut S., Zhang L., Marcone M. Effects of pale, normal and dark chicken breast meat on microstructure, extractable proteins, and cooking of marinated fillets. Poult. Sci. 2005;84:797–802. doi: 10.1093/ps/84.5.797. [DOI] [PubMed] [Google Scholar]
- Barido F.H., Jang A., Pak J.I., Kim D.Y., Lee S.K. Investigation of taste-related compounds and antioxidative profiles of retorted samgyetang made from fresh and dried cordyceps militaris mushrooms. Food Sci. Anim. Resour. 2020;40:772–784. doi: 10.5851/kosfa.2020.e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barido F.H., Utama D.T., Jeong H.S., Kim J.T., Lee C.W., Park Y.S., Lee S.K. The effect of finishing diet supplemented with methionine/lysine and methionine/α-tocopherol on performance, carcass traits and meat quality of Hanwoo steers. Asian-Australas. J. Anim. Sci. 2020;33:69–78. doi: 10.5713/ajas.19.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee G., Anderson A.L., Lonergan S.M., Huff-Lonergan E. Rate and extent of pH decline affect proteolysis of cytoskeletal proteins and water-holding capacity in pork. Meat Sci. 2007;76:359–365. doi: 10.1016/j.meatsci.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Bowker B.C., Eastridge J.S., Paroczay E.W., Callahan J.A., Solomon M.B. Aging/tenderization mechanisms. In: Toldrá F., editor. Handbook of Meat Processing. Blackwell; Oxford, IA: 2010. [Google Scholar]
- Chang Y.S., Chou R.G.R. Postmortem role of calpains in Pekin duck skeletal muscles. J. Sci. Food Agric. 2012;92:1620–1626. doi: 10.1002/jsfa.4747. [DOI] [PubMed] [Google Scholar]
- Chen L., Feng X.C., Lu F., Xu X.L., Zhou G.H., Li Q.Y., Guo X.Y. Effects of camptothecin, etoposide and Ca2+ on caspase-3 activity and myofibrillar disruption of chicken during post-mortem ageing. Meat Sci. 2011;87:165–174. doi: 10.1016/j.meatsci.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Chen J., Ren Y.X., Zhang K.S., Xiong Y.L., Wang Q., Shang K., Zhang D. Site-specific incorporation of sodium tripolyphosphate into myofibrillar protein from mantis shrimp (Oratosquilla oratoria) promotes protein crosslinking and gel network formation. Food Chem. 2020;312:1–9. doi: 10.1016/j.foodchem.2019.126113. [DOI] [PubMed] [Google Scholar]
- Chen S., Zhou N., Zhang Z., Li W., Zhu W. Resveratrol induces cell apoptosis in adipocytes via ampk activation. Biochem. Biophys. Res. Commun. 2015;457:608–613. doi: 10.1016/j.bbrc.2015.01.034. [DOI] [PubMed] [Google Scholar]
- Culler R.D., Parrish F.C., Smith G.C., Cross H.R. Relationship of myofibril fragmentation index to certain chemical, physical and sensory characteristics of Bovine longissimus muscle. J. Food Sci. 1978;43:1177–1180. [Google Scholar]
- De Souza K.M.R., Araujo R.B., Dos Santos A.L., Rodrigues C.E.C., De Faria D.E., Trindade M.A. Adding value to the meat of spent laying hens manufacturing sausages with a healthy appeal. Rev. Bras. Cienc. Avic. 2011;13:57–63. [Google Scholar]
- Freeman S.R., Poore M.H., Middleton T.F., Ferket P.R. Alternative methods for disposal of spent laying hens: Evaluation of the efficacy of grinding, mechanical deboning, and of keratinase in the rendering process. Bioresour. Technol. 2009;100:4515–4520. doi: 10.1016/j.biortech.2009.01.077. [DOI] [PubMed] [Google Scholar]
- Gao, Zhang Y.J.Y., He L., Shi X.X., Han L., Yu Q., Yang Y., Song R., Han M.S., Zhao S. Associations among adenosine monophosphate-activated protein kinase, glycolysis, muscle characteristics, and apoptosis in post-mortem bovines longissimus muscle. Eur. Food Res. Technol. 2020;246:971–985. [Google Scholar]
- Gordon A., Barbut S. The effect of chloride salts on the texture, microstructure and stability of meat batters. Food Struct. 1989;8:271–283. [Google Scholar]
- Harrary I., Renaud J.F., Sato E., Wallace G.A. Calcium ions regulate cyclic AMP and beating in cultured heart cell. Nature. 1976;261:60–61. doi: 10.1038/261060a0. [DOI] [PubMed] [Google Scholar]
- He J., Fang D., Daodong P., Xiaoqun Z. Investigation of the relationships between different enzymes and post-mortem duck muscle tenderization. Poult. Sci. 2019;98:6125–6130. doi: 10.3382/ps/pez301. [DOI] [PubMed] [Google Scholar]
- Herrera-Mendez C.H., Becila S., Boudjellal A., Ouali A. Meat ageing: Reconsideration of the current concept. Trends Food Sci. Technol. 2006;17:394–405. [Google Scholar]
- Honikel K.O., Kim C.J., Hamm R., Roncales P. Sarcomere shortening of prerigor muscles and its influence on drip loss. Meat Sci. 1986;16:267–282. doi: 10.1016/0309-1740(86)90038-0. [DOI] [PubMed] [Google Scholar]
- Huff-Lonergan E., Lonergan S.M. Mechanisms of water holding capacity of meat: the role of postmortembiochemical and structural changes. Meat Sci. 2005;71:194–204. doi: 10.1016/j.meatsci.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Jacobson K.A., Hoffmann C., Cattabeni F., Abbracchio M.P. Adenosine-induced cell death: evidence for receptor-mediated signaling. Apoptosis. 1999;4:197–211. doi: 10.1023/a:1009666707307. [DOI] [PubMed] [Google Scholar]
- Jayasena D.D., Jung S., Kim H.J., Alahakoon A.U., Nam K.C., Jo C. Effect of sex on flavor-related and functional compounds in freeze-dried broth made from Korean native chicken. Food Sci. Anim. Resour. 2014;34:448–456. doi: 10.5851/kosfa.2014.34.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncher D., Ronn B., Mortensen E.T., Henckel P., A Karlsson A., Skibsted L.H. Effect of pre-slaughter physiological conditions on the oxidative stability of color and lipid oxidation during chill storage of pork. Meat Sci. 2001;58:347–357. doi: 10.1016/s0309-1740(00)00156-x. [DOI] [PubMed] [Google Scholar]
- Kaur L., Hui S.X., Boland M. Changes in cathepsin activity during low temperature storage and sous vide processing of beef brisket. Food Sci. Anim. Resour. 2020;40:415–425. doi: 10.5851/kosfa.2020.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp C.M., Parr T. The effect of recombinant caspase-3 on myofibrillar proteins in porcine skeletal muscle. Animal. 2008;2:1254–1264. doi: 10.1017/S1751731108002310. [DOI] [PubMed] [Google Scholar]
- Kemp C.M., Sensky P.L., Bardsley R.G., Buttery P.J., Parr T. Tenderness − an enzymatic view. Meat Sci. 2010;84:248–256. doi: 10.1016/j.meatsci.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Kersey J.H., Waldroup P.W. Utilization of spent hen meal in diets for broiler chickens. Poult. Sci. 1998;77:1377–1387. doi: 10.1093/ps/77.9.1377. [DOI] [PubMed] [Google Scholar]
- Kim H.W., Yan F.F., Hu J.Y., Cheng H.W., Kim H.B. Effects of probiotics feeding on meat quality of chicken breast during postmortem storage. Poult. Sci. 2016;95:1457–1464. doi: 10.3382/ps/pew055. [DOI] [PubMed] [Google Scholar]
- Koguchi T., Nakajima H., Koguchi H., Wada M., Yamamoto Y., Innami S. Suppressive effect of viscous dietary fiber on elevations of uric acid in serum and urine induced by dietary RNA in rats is associated with strength of viscosity. Int. J. Vitam. Nutr. Res. 2003;73:369–376. doi: 10.1024/0300-9831.73.5.369. [DOI] [PubMed] [Google Scholar]
- Koohmaraie M., Whipple G., Kretchmar D.H., Crouse J.D., Mersmann H.J. Postmortem proteolysis in Longissimus muscle from beef, lamb and pork carcasses. J. Anim. Sci. 1991;69:617–624. doi: 10.2527/1991.692617x. [DOI] [PubMed] [Google Scholar]
- Korhonen H. Milk-derived bioactive peptides: From science to applications. J. Funct. Foods. 2009;1:177–187. [Google Scholar]
- Krishan S., Richardson D.R., Sahni S. Adenosine monophosphate-activated kinase and its key role in catabolism: structure, regulation, biological activity, and pharmacological activation. Mol. Pharmacol. 2015;87:363–377. doi: 10.1124/mol.114.095810. [DOI] [PubMed] [Google Scholar]
- Lana A., Zolla L. Proteolysis in meat tenderization from the point of view of each single protein: a proteomic perspective. J. Proteomics. 2016;147:85–97. doi: 10.1016/j.jprot.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Lawrie R.A. 5th ed. Pergamon Press; NY: 1991. Meat Sci. [Google Scholar]
- Lee H.L., Sante-Lhoutellier V., Vigouroux S., Briand Y., Briand M. Role of calpains in post-mortem proteolysis in chicken muscle. Poult. Sci. 2008;87:2126–2132. doi: 10.3382/ps.2007-00296. [DOI] [PubMed] [Google Scholar]
- Li Chan E.C. Bioactive peptides and protein hydrolysates: research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015;1:28–37. [Google Scholar]
- Melloni E., Michetti M., Salamino F., Minafra R., Pontremoli S. Modulation of the calpain autoproteolysis by calpastatin and phospholipids. Biochem. Biophys. Res. Commun. 1996;229:193–197. doi: 10.1006/bbrc.1996.1779. [DOI] [PubMed] [Google Scholar]
- Nadzirah K., Zainal S., Noriham A., Normah I. Application of bromelain powder produced from pineapple crowns in tenderizing beef round cuts. Int. Food Res. J. 2016;23:1590–1599. [Google Scholar]
- Nowsad A.A.K.M., Kanoh S., Niwa E. Thermal gelation characteristics of breast and thigh muscles of spent hen and broiler and their surimi. Meat Sci. 2000;54:169–175. doi: 10.1016/s0309-1740(99)00091-1. [DOI] [PubMed] [Google Scholar]
- Nurmahmudi, Sams A.R. Tenderizing spent fowl meat with calcium chloride: biochemical characteristics of tenderized breast meat. Poult. Sci. 1997;76:543–547. doi: 10.1093/ps/76.3.543. [DOI] [PubMed] [Google Scholar]
- Okitani A., Ichinose N., Itoh J., Tsuji Y., Oneda Y., Hatae K., Migita K., Matsuishi M. Liberation of actin from actomyosin in meats heated to 65°C. Meat Sci. 2009;81:446–450. doi: 10.1016/j.meatsci.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Ouali A., Herrera-Mendez C.H., Coulis G., Becila S., Boudjellal A., Aubry L., Sentandreu M.A. Revisiting the conversion of muscle into meat and the underlying mechanisms. Meat Sci. 2006;74:44–58. doi: 10.1016/j.meatsci.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Qiao M., Fletcher D.L., Smith D.P., Northcutt J.K. The effect of broiler breast meat color on pH, moisture, water holding capacity, and emulsification capacity. Poult. Sci. 2001;80:676–680. doi: 10.1093/ps/80.5.676. [DOI] [PubMed] [Google Scholar]
- Quali A. Proteolytic and physicocohemical mechanisms involved in meat texture development. Biochimie. 1992;74:251–265. [PubMed] [Google Scholar]
- Richter E.A., Ruderman N.B. Adenosine monophosphate protein kinase (AMPK) and the biochemistry of exercise: implications for human health and disease. Biochem. J. 2009;418:261–275. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentandreu M.A., Coulis G., Ouali A. Role of muscle endopeptidases and their inhibitors in meat tenderness. Trends Food Sci. Technol. 2002;13:400–421. [Google Scholar]
- Shao J., Deng Y., Jia N N., Li R.R., Cao J.X., Liu D.Y., Li J.R. Low-field NMR determination of water distribution in meat batters with NaCl and polyphosphate addition. Food Chem. 2016;200:308–314. doi: 10.1016/j.foodchem.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Song D.H., Ham Y.K., Ha J.H., Kim Y.R., Chin K.B., Kim H.W. Impacts of pre-rigor salting with KCl on technological properties of ground chicken breast. Poult. Sci. 2020;99:597–603. doi: 10.3382/ps/pez527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J.A. The myosin swinging cross-bridge model. Nat. Rev. Mol. Cell. Biol. 2001;2:387–392. doi: 10.1038/35073086. [DOI] [PubMed] [Google Scholar]
- Steen D., Claeys E., Uytterhaegen L., De Smet S., Demeyer D. Early post-mortem conditions and the calpain/calpastatin system in relation to tenderness of double-muscled beef. Meat Sci. 1997;45:307–319. doi: 10.1016/s0309-1740(96)00124-6. [DOI] [PubMed] [Google Scholar]
- Šulcerová H., Mihok M., Jůzl M., Haščík P. Effect of addition of pollen and propolis to feeding mixtures during the production of broiler chickens ROSS 308 to the colour of thigh and breast muscle and pH determination. Acta Univ. Agric. Silvic. Mendel. Brun. 2014;59:359–366. [Google Scholar]
- Takahashi K., Fukazawa T., Yasui T. Formation of myofibrillar fragments and reversible contraction of sarcomeres in chicken pectoral muscle. J. Food Sci. 1967;32:409–413. [Google Scholar]
- Takakura Y., Mizushima M., Hayashi K., Masuzawa T., Nishimura T. Characterization of the key aroma compounds in chicken soup stock using aroma extract dilution analysis. Food Sci. Technol. Res. 2014;20:109–113. [Google Scholar]
- Taylor R.G., Geesink G.H., Thompson V.F., Koohmaraie M., Goll D.E. Is Z-disk degradation responsible for post- mortem tenderisation. J. Anim. Sci. 1995;73:1351–1367. doi: 10.2527/1995.7351351x. [DOI] [PubMed] [Google Scholar]
- Udenigwe C.C. Bioinformatics approaches, prospects and challenges of food bioactive peptide research. Trends Food Sci. Technol. 2014;36:137–143. [Google Scholar]
- Veiseth E., Shackelford S., Wheeler T., Koohmaraie M. Effect of post-mortem storage on μ-calpain and m-calpain in ovine skeletal muscle. J. Anim. Sci. 2001;79:1502–1508. doi: 10.2527/2001.7961502x. [DOI] [PubMed] [Google Scholar]
- Voet D., Voet J.G. 4th ed. John wiley and sons; Hoboken, NJ: 2010. Biochemistry. [Google Scholar]
- Wang D.Y., Deng S., Zhang M.H., Geng Z.M., Sun C., Bian H., Xu W., Zhu Y.Z., Liu F., Wu H.H. The effect of adenosine 5′-monophosphate (AMP) on tenderness, microstructure and chemical–physical index of duck breast meat. J. Sci. Food Agric. 2015;96:1467–1473. doi: 10.1002/jsfa.7243. [DOI] [PubMed] [Google Scholar]
- Wang D.Y., Dong H., Zhang M.H., Liu F., Bian H., Zhu Y.Z., Xu W. Changes in actomyosin dissociation and endogenous enzyme activities during heating and their relationship with duck meat tenderness. Food Chem. 2013;141:675–679. doi: 10.1016/j.foodchem.2013.04.034. [DOI] [PubMed] [Google Scholar]
- Wang D.Y., Zhang M.H., Bian H., Dong H., Xu W.M., Xu X.L., Zhu Y.Z., Liu F., Geng Z.M., Zhou G.H., Wang P. Proteolysis and cathepsin activities in the processing of dry-cured duck. Poult. Sci. 2014;93:687–694. doi: 10.3382/ps.2013-03335. [DOI] [PubMed] [Google Scholar]
- Wattanachant S., Benjakul S., Ledward D.A. Effect of heat treatment on changes in texture, structure and properties of Thai indigenous chicken muscle. Food Chem. 2005;93:337–348. [Google Scholar]
- Xiao B., Sanders M.J., Underwood E., Heath R., Mayer F.V., Carmena D., Jing C., Walker P.A., Eccleston J.F., Haire L.F., Saiu P., Howell S.A., Aasland R., Martin S.R., Carling D., Gamblin S.J. Structure of mammalian adenosine monophosphate protein kinase and its regulation by adenosine diphosphate. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Han L., Yu Q., Go Y., Song R. Study of the AMP-activated protein kinase role in energy metabolism changes during the postmortem aging of Yak Longissimus dorsal. Animals. 2020;10:427–442. doi: 10.3390/ani10030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Catherine J., Jianping W. Purification and identification of anti-inflammatory peptides from spent hen muscle proteins hydrolysate. Food Chem. 2018;253:101–107. doi: 10.1016/j.foodchem.2018.01.093. [DOI] [PubMed] [Google Scholar]
- Yu W., Catherine J., Jianping W. Spent hen muscle protein hydrolysate: a potential IL-10 stimulator 1 in a murine model. Food Func. 2018;19:4714–4719. doi: 10.1039/c8fo00589c. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Venkitasamy C., Pan Z., Wang W. Recent developments on umami ingredients of edible mushrooms: a review. Trends Food Sci. Technol. 2013;33:78–92. [Google Scholar]
- Zhao L., Xing T., Huang J., Qiao Y., Chen Y., Huang M. Involvement of l/m-calpain in the proteolysis and meat quality changes during post-mortem storage of chicken breast muscle. Anim. Sci. J. 2017;89:423–431. doi: 10.1111/asj.12921. [DOI] [PubMed] [Google Scholar]
- Zhuang H., Savage E.M. Comparison of cook loss, shear force, and sensory descriptive profiles of boneless skinless white meat cooked from a frozen or thawed state. Poul. Sci. 2013;92:3003–3009. doi: 10.3382/ps.2012-02801. [DOI] [PubMed] [Google Scholar]
- Zou Y., Yang H., Zhang M.H., Zhang X.X., Xu W.M., Wang D.Y. The influence of ultrasound and adenosine 5’-monophosphate marination on tenderness and structure of myofibrillar proteins of beef. Asian-Australas. J. Anim. Sci. 2019;32:1611–1620. doi: 10.5713/ajas.18.0780. [DOI] [PMC free article] [PubMed] [Google Scholar]