Abstract
Necrotic enteritis (NE) caused by Clostridium perfringens is among the most important enteric diseases in poultry production. This study examined the effects of 2 probiotics (Prob) and a synbiotic (Synb) during a naturally occurring NE challenge. On the day of hatch, 1200 Cobb male broilers were randomly allocated to 5 groups (8 pens/treatment, 30 birds/pen) including 1) negative control (NC): corn-soybean meal diet; 2) positive control (PC): NC + 453 g Stafac20/907 kg feed; 3) Prob 1: NC + 453 g Prob 1/907 kg feed; 4) Prob 2: NC + 453 g Prob 2/907 kg feed; and 5) Synb: NC + 453 g Synb/907 kg feed. One day after placement, birds were challenged by a coccidia vaccine to induce NE. Feed intake and body weights were measured on day 8 (NE onset) and end of starter (day 14) and grower (28) periods. On day 8, the small intestines of 3 birds/pen were examined for NE lesions. Ileal mucosal scrapings from one bird/pen were collected on day 8 and day 28 to profile the microbiota using 16S rRNA sequencing. Data were analyzed in JMP or QIIME 2 and significance between treatments identified by LSD or linear discriminant analysis effect size (P < 0.05). The Synb group significantly lowered NE lesion scores on day 8 and reduced day 0-14 mortality by 50% compared with NC. FCR was significantly better in all the groups, whereas ADG was higher in PC, Synb, and Prob 2 groups compared with NC from day 0 to day 28. Lower lesion scores in the Synb group were accompanied by lower relative abundance of Alistipes, ASF356, Faecalibaculum, Lachnospiraceae UCG-001, Muribaculum, Oscillibacter, Parabacteroides, Rikenellaceae RC9 gut group, Ruminococcaceae UCG-014, and Ruminiclostridium 9 compared with NC on day 8. On day 28, relative abundance of Lactobacillus was lower, whereas abundance of Bacteroides, Barnesiella, Butyricicoccus, CHKCI001, Eisenbergiella, Eubacterium hallii group, Helicobacter, Ruminococcaceae UCG-005, Ruminococcus torques group, and Sellimonas was significantly higher in the NC birds than in the Synb and Prob 2 groups. Collectively, these data indicate that during a subclinical naturally occurring NE, supplementation of specific additives could be effective in reducing intestinal lesions and mortality, and improving performance potentially through developing a signature microbial profile in the intestinal mucosal layer.
Key words: broilers, necrotic enteritis, lesion scores, microbiota, probiotic, synbiotic
Introduction
Necrotic enteritis (NE) caused by Clostridium perfringens is among the most important enteric diseases in poultry production (Smith, 2011; Gaucher et al., 2015). Compared with clinical forms, the negative effects on birds' performance and flock productivity manifested by subclinical NE are more prevalent (Shojadoost et al., 2012; Antonissen et al., 2016). Changes in microbial diversity and population could make the gut environment suitable for proliferation or pathogenesis of bacteria such as C. perfringens. Predisposing factors such as Eimeria and fishmeal would lead to disturbances in the microbiota providing a favorable ecological environment or nutrients that allows the proliferation of C. perfringens (Shojadoost et al., 2012; Antonissen et al., 2016). The use of antibiotic growth promoters (AGPs) has been an effective tool in maintaining gut health and modifying gut microbiota, thus improving bird performance and reducing incidence of enteric diseases (Danzeisen et al., 2011; Yadav and Jha, 2019). However, AGP removal has led to an increase in NE occurrence, especially the subclinical form (Smith, 2011; Gaucher et al., 2015).
The interaction between gut microbiota and the host is critical for the development and health of the gut (Gerritsen et al., 2011; Kau et al., 2011); thus, one of the additive groups that has been tested for their efficacy as alternatives to AGP are probiotics (Caly et al., 2015). Supplementation of probiotics promotes gut health and performance by reducing the number of pathogens in the gastrointestinal tract through competitive exclusion or production of antimicrobial peptides, thus improving intestinal maturation and integrity, as well as modifying immune responses (Lan et al., 2005; M'Sadeq et al., 2015). Supplementation of a probiotic (Lactobacillus johnsonii BS15) alleviated the negative impact of subclinical NE and increased the expression of interleukin (IL)-8, while decreased that of interferon (IFN)-γ and IL-10 in the ileum with no impact on IL-2 (Wang et al., 2017). A Lactobacillus acidophilus–based probiotic improved intestinal health by decreasing the relative abundance of Escherichia-Shigella in the ileum (Li et al., 2017). The probiotic bacteria Bacillus subtilis DSM 32315 increased average body weight, ADG, and the abundance of Firmicutes, while reduced the abundance of Bacteroidetes in the ceca of broiler chickens (Ma et al., 2018). Furthermore, this probiotic reduced the abundance of potentially harmful bacteria including Vampirovibrio, Escherichia-Shigella, and Parabacteroides (Ma et al., 2018). During a subclinical NE trial, supplementation of a probiotic (L. johnsonii BS15) improved FCR and body weight gain compared with the challenged control group (Wang et al., 2017).
Despite several reports, there has not been much emphasis on multicomponent additives or comparison of these additives with probiotics as alternatives to AGP, particularly during NE challenge. In a recent study, supplementation of a multicomponent additive had no effect on NE lesions in the small intestine of broiler chickens, but improved FCR during the starter and grower periods (Calik et al., 2019). This study was conducted to evaluate and compare the effects of a synbiotic (Synb) with 2 probiotics (Prob) on performance, pathology, and ileal mucosa microbial profiles during a naturally occurring NE challenge model.
Materials and methods
Birds, Housing, and Diets
A total of 1,200 day-old Cobb male broiler chickens were acquired from a local hatchery. Before placement, birds were weighed in groups of 30 chicks and allocated to one of 40 floor pens with dimensions 1.2 × 1.2 m for the first 14 days and 1.2 × 2.4 m from day 15 to day 28. Birds were assigned to the 5 dietary treatments (8 pens/treatment) as follows:
-
1)
Negative control (NC): corn-soybean meal basal diet;
-
2)
Positive control (PC): NC + 20 g virginiamycin (453 g Stafac20)/907 kg feed;
-
3)
Prob 1: NC + 453 g Prob 1/907 kg feed;
-
4)
Prob 2: NC + 453 g Prob 2/907 kg feed;
-
5)
Synb: NC + 453 g Synb/907 kg feed.
Prob 1 mainly consisted of B. subtilis DSM17299, whereas Prob 2 consisted of B. subtilis C-3102. Synb consisted of dried fermentation product of Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacillus reuteri, and fructooligosaccharides as a prebiotic source. All animal protocols were approved and conducted under the guidelines of the Virginia Tech Institutional Animal Care and Use Committee (IACUC # 18-136).
The composition of the basal diet is detailed in Table 1. All the diets were prepared at the Virginia Tech feed mill and subsequently crumbled (starter period) or pelleted (grower period). Each pen was equipped with a bucket-type feeder and a nipple drinker line with fresh wood shavings as litter (7.5 cm deep). Birds had ad libitum access to water and feed from placement (day 0) until the end of the study (day 28). Lighting schedule was 24 h light for the first 3 days, reduced to 23 h light:1 h dark for day 4 to 7, and reduced further to 18 h light:6 h dark thereafter. An automatic ventilation system was used to control the environment, and temperature was maintained as follows: 32°C for the first 3 days, then gradually reduced approximately 3°C each week until it reached 23°C at the start of week 4 where it remained constant until day 28.
Table 1.
Composition of basal diets (as fed basis, %)1.
| Ingredients (%) | Period (Days) |
|
|---|---|---|
| Starter (0–14) Crumble | Grower (15–28) Pellet | |
| Corn (7.81% CP) | 59.53 | 64.12 |
| Soybean meal (48% CP) | 33.5 | 28.80 |
| Soybean oil (9,000 kcal/kg) | 2.18 | 2.60 |
| Dicalcium phosphate (18.5% P, 22% Ca) | 2.05 | 1.92 |
| Calcium carbonate (37% Calcium) | 1.11 | 1.00 |
| Sodium chloride | 0.30 | 0.30 |
| Sodium bicarbonate | 0.07 | 0.07 |
| DL-Methionine (990 g/kg)2 | 0.38 | 0.34 |
| L-Lysine hydrochloride (788g L-Lysine/kg)3 | 0.37 | 0.35 |
| L-Threonine (985 g/kg)4 | 0.15 | 0.14 |
| Vitamin/trace mineral premix5 | 0.36 | 0.36 |
| Calculated analysis (% unless specified) | ||
| ME (kCal/kg) | 3,007 | 3,087 |
| Crude protein | 21.81 | 19.90 |
| Total phosphorus | 0.76 | 0.71 |
| Available phosphorus | 0.45 | 0.42 |
| Calcium | 0.90 | 0.84 |
| Chlorine | 0.33 | 0.33 |
| Sodium | 0.16 | 0.16 |
| Potassium | 0.85 | 0.77 |
| Methionine | 0.67 | 0.61 |
| Methionine + cysteine | 0.98 | 0.89 |
| Lysine | 1.32 | 1.19 |
| Threonine | 0.86 | 0.78 |
| Linoleic acid | 1.44 | 1.52 |
| Dietary cation-anion balance | 194 | 174 |
Treatments include negative control (NC): corn-soybean meal basal diet; positive control (PC): NC + 20 g virginiamycin (453 g Stafac20)/907 kg diet; probiotic 1 (Prob 1): NC + 453 g Bacillus subtilis DSM17299/907 kg diet; probiotic 2 (Prob 2): NC + 453 g Bacillus subtilis C-3102/907 kg diet; synbiotic (Synb): NC + 453 g dried fermentation product of Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacillus reuteri, and fructooligosaccharides/907 kg diet.
Rhodimet NP9, ADISSEO, GA, USA.
L-Lysine HCl, AJINOMOTO HEARTLAND, INC. Eddyville, IA, USA.
FENCHEM Ingredient Technology, Nanjing, China.
Vitamins supplied per kg diet: retinol 3.33 mg, cholecalciferol 0.1 mg, α-tocopherol acetate 23.4 mg, vitamin K3 1.2 mg, vitamin B1 1.6 mg, vitamin B2 9.5 mg, niacin 40 mg, pantothenic acid 9.5 mg, vitamin B6 2 mg, folic acid 1 mg, vitamin B12 0.016 mg, biotin 0.05 mg, choline 556 mg. Minerals supplied per kg diet: Mn 144 mg, Fe 72 mg, Zn 144 mg, Cu 16.2 mg, I 2.1 mg, Se 0.22 mg.
Necrotic Enteritis Challenge
To simulate field conditions, a unique, naturally occurring model developed by our laboratory was applied to induce subclinical NE (Calik et al., 2019; Emami et al., 2019, 2020) using a concentrated dose of a commercial coccidiosis vaccine. Briefly, this model consists of spraying a high dose (10 X) of commercial coccidiosis vaccine 24 h after bird placement, which in conjunction with the presence of C. perfringens spores in the barn environment leads to the development of a NE outbreak around 1 wk after vaccine application. For this trial, the Coccivac-B52 vaccine (containing live oocysts of Eimeria acervulina, Eimeria maxima, E. maxima MFP, Eimeria mivati, and Eimeria tenella; Merck Animal Health) was prepared at the proper concentration in the laboratory, kept on ice, and applied at the farm.
Mortality
Starting at placement, birds were monitored twice daily. For each dead bird, date, body weight, and cause of death were recorded. This procedure continued throughout the study (up to day 28) to record mortality/treatment for each period thus allowing for adjusting performance parameters for daily mortality.
Lesion Scores
On day 8, 3 birds were selected based on average body weight of each pen (24/treatment), euthanized by cervical dislocation, and the small intestines were examined for NE lesions and scored based on a 0–4 scale system (Prescott et al., 1978). Each section of the small intestine, that is, the duodenum, jejunum, and ileum, were scored separately by personnel blinded to the treatments. The lesion scoring criteria used were as follows:
0 = No gross lesions,
1 = Thin-walled or friable,
2 = Focal necrosis or ulceration,
3 = Multifocal coalescing areas (large patches) of necrosis,
4 = Severe extensive necrosis.
Performance
On arrival (day 0), birds were weighed in groups of 30 and assigned to each pen. Subsequently, birds were weighed on day 8 (7 days after the coccidiosis challenge, which was also the peak mortality) and at the end of starter (day 14) and grower (day 28) periods. In addition, feed consumption was also recorded on a per pen basis on day 8, day 14, and day 28. Finally, adjusted ADG, ADFI, and FCR were calculated for each period.
16S rRNA Gene Sequence, Data Processing, and Analysis
On day 8 and day 28, one bird/pen was euthanized, the ileum excised, mucosal scrapings collected, snap-frozen in liquid nitrogen, and then stored at −80°C until further analysis. DNA was extracted from mucosal scrapings using the Qiagen QIAamp PowerFecal DNA kit (Qiagen GmbH, Hilden, Germany) based on the supplier's instructions. Quality and quantity of extracted DNA were measured using gel electrophoresis and spectrophotometer. All DNA samples were diluted to 300 ng/μL and 50 μL of each sample were sent to the Virginia Tech Biocomplexity Institute for sequencing. The V4 region of the 16S rRNA gene was amplified using the Illumina MiSeq kit V3 (600-cycle format, 25 million reads, read length 2 × 300). Earth Microbiome Project primer sets 515F (Parada et al., 2016) and 806R (Apprill et al., 2015) generating amplicons in the 390 bp size were used.
The 16S sequencing data were analyzed as previously described (Emami et al., 2020). Briefly, data were analyzed using quantitative insight into microbial ecology (QIIME) software package 2 (Bolyen et al., 2019) and samples for day 8 and day 28 were rarefied to 10,891 and 36,075 sequences, respectively. α-Diversity and β-diversity were measured using Shannon index and Bray-Curtis, respectively. The differences in microbial community structure were identified by pairwise permutational multivariate analysis of variance (PERMANOVA). Linear discriminant analysis effect size was performed to identify the differential taxa between the treatment groups. Functions of microbiota were predicted using phylogenetic investigation of communities by reconstruction of unobserved states, Kyoto encyclopedia of genes and genomes (KEGG), and statistical analysis of the taxonomic and functional profiles (Parks et al., 2014; Weber et al., 2017).
Statistical Analyses
Statistical analysis for all data (except for the microbiota described earlier) was performed using the ANOVA procedure of JMP software (2014) and significance between treatments (P < 0.05) determined by the LSD test. The statistical model for data analysis is outlined below:
Yij = measured value for each observation (data),
μ = grand mean,
Ai = treatment effect,
eij = experimental error.
Results
Mortality
Mortality was significantly reduced in all the groups during the day 9 to 14 period compared with NC (Table 2). None of the treatments significantly reduced mortality during the overall (day 0 to 28) experimental period. However, compared with NC (5.83%), both PC (2.91%) and Synb (2.91%) reduced day 0 to 28 mortality by 50% (although not statistically different due to variability among replicate pens).
Table 2.
Effect of dietary probiotics (Prob) or synbiotic (Synb) supplementation on mortality (%) of broiler chickens under a naturally occurring subclinical necrotic enteritis challenge.
| Treatments1 | Time period (days) |
||||
|---|---|---|---|---|---|
| 0–8 | 9–14 | 0–14 | 15–28 | 0–28 | |
| NC | 2.91 | 2.40a | 5.00 | 1.00 | 5.83 |
| PC | 1.66 | 0.00b | 1.66 | 1.44 | 2.91 |
| Prob 1 | 4.58 | 0.48b | 5.41 | 1.10 | 6.25 |
| Prob 2 | 2.08 | 0.50b | 2.50 | 1.56 | 3.75 |
| Synb | 1.66 | 0.00b | 1.66 | 1.48 | 2.91 |
| SEM | 1.17 | 0.44 | 1.39 | 0.94 | 1.70 |
| P-value | 0.372 | 0.002 | 0.171 | 0.990 | 0.481 |
a,bIn each column, means with different letters are significantly different (P < 0.05).
Treatments include negative control (NC): corn-soybean meal basal diet; positive control (PC): NC+ 20 g virginiamycin (453 g Stafac20)/907 kg diet; probiotic 1 (Prob 1): NC + 453 g Bacillus subtilis DSM17299/907 kg diet; probiotic 2 (Prob 2): NC + 453 g Bacillus subtilis C-3102/907 kg diet; synbiotic (Synb): NC + 453 g dried fermentation product of Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacillus reuteri, and fructooligosaccharides/907 kg diet.
Necrotic Enteritis Lesion Scores
Supplementation of Synb significantly reduced lesion scores in the duodenum compared with NC (Table 3). There was no significant difference in NE lesion scores in the duodenum among the other treatments. In addition, there was no difference in NE lesion scores in the jejunum and ileum among all treatments.
Table 3.
Effect of dietary probiotics (Prob) or synbiotic (Synb) supplementation on intestinal lesion scores of broiler chickens under a naturally occurring subclinical necrotic enteritis challenge.1
| Treatments2 | Small intestine section |
||
|---|---|---|---|
| Duodenum | Jejunum | Ileum | |
| NC | 1.20a | 0.58 | 0.00 |
| PC | 1.05a,b | 0.38 | 0.02 |
| Prob 1 | 1.23a | 0.72 | 0.04 |
| Prob 2 | 0.95a,b | 0.45 | 0.00 |
| Synb | 0.75b | 0.37 | 0.00 |
| SEM | 0.11 | 0.09 | 0.01 |
| P-value | 0.038 | 0.073 | 0.231 |
a,bIn each column, means with different letters are significantly different (P < 0.05).
Data represent the mean value of 8 replicate pens of 3 birds/pen.
Treatments include negative control (NC): corn-soybean meal basal diet; positive control (PC): NC+ 20 g virginiamycin (453 g Stafac20)/907 kg diet; probiotic 1 (Prob 1): NC + 453 g Bacillus subtilis DSM17299/907 kg diet; probiotic 2 (Prob 2): NC + 453 g Bacillus subtilis C-3102/907 kg diet; synbiotic (Synb): NC + 453 g dried fermentation product of Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacillus reuteri, and fructooligosaccharides/907 kg diet.
Performance
Performance results are presented in Table 4. ADG was significantly higher for PC and Prob 2 during the grower period (day 15–28) than for NC. Cumulatively (day 0–28), Synb, Prob 2, and PC had significantly higher ADG than NC. There was no difference in ADFI during any of the experimental periods. During day 0 to 8, the Synb group had the best FCR, which was significantly lower than that of the NC birds. However, during day 9 to 14, FCR was significantly better for PC and Prob 2 groups than for NC. During the grower and overall experimental periods, FCR was significantly better in all the groups than in NC.
Table 4.
Effect of dietary probiotics (Prob) or synbiotic (Synb) supplementation on broiler performance under a naturally occurring subclinical necrotic enteritis model.1
| Item | Dietary treatments2 |
Statistics |
|||||
|---|---|---|---|---|---|---|---|
| NC | PC | Prob 1 | Prob 2 | Synb | SEM | P-value | |
| Day 0–8 | |||||||
| ADFI, g | 26.87 | 26.46 | 25.34 | 25.98 | 26.56 | 0.40 | 0.091 |
| ADG, g | 21.46 | 21.71 | 20.82 | 21.33 | 22.20 | 0.35 | 0.112 |
| FCR, g/g | 1.25a | 1.22a,b | 1.21a,b | 1.22a,b | 1.20b | 0.01 | 0.049 |
| Day 9–14 | |||||||
| ADFI, g | 61.63 | 62.80 | 61.61 | 62.92 | 63.68 | 1.12 | 0.642 |
| ADG, g | 41.26 | 44.34 | 42.22 | 45.10 | 44.31 | 1.03 | 0.064 |
| FCR | 1.49a | 1.42b,c | 1.47a,b | 1.40c | 1.44a,b,c | 0.02 | 0.050 |
| Day 0–14 | |||||||
| ADFI, g | 41.53 | 41.85 | 40.48 | 41.61 | 42.28 | 0.65 | 0.406 |
| ADG, g | 30.19 | 31.52 | 30.28 | 31.58 | 31.82 | 0.561 | 0.129 |
| FCR | 1.37a | 1.33b | 1.34a,b | 1.32b | 1.33b | 0.01 | 0.050 |
| Day 15–28 | |||||||
| ADFI, g | 121.99 | 125.80 | 118.88 | 125.47 | 123.27 | 1.95 | 0.101 |
| ADG, g | 77.35b | 85.18a | 79.05b | 84.22a | 82.06a,b | 1.79 | 0.017 |
| FCR | 1.58a | 1.48b | 1.50b | 1.49b | 1.50b | 0.02 | 0.035 |
| Day 0–28 | |||||||
| ADFI, g | 78.63 | 81.02 | 76.74 | 80.17 | 80.11 | 1.16 | 0.103 |
| ADG, g | 53.69c | 58.64a | 54.69b,c | 57.86a | 57.30a,b | 1.03 | 0.006 |
| FCR | 1.47a | 1.39b | 1.41b | 1.39b | 1.40b | 0.01 | <0.001 |
a-cIn each column, means with different letters are significantly different (P < 0.05).
Data represent the mean value of 8 replicate pens of 30 birds/pen.
Treatments include negative control (NC): corn-soybean meal basal diet; positive control (PC): NC+ 20 g virginiamycin (453 g Stafac20)/907 kg diet; probiotic 1 (Prob 1): NC + 453 g Bacillus subtilis DSM17299/907 kg diet; probiotic 2 (Prob 2): NC + 453 g Bacillus subtilis C-3102/907 kg diet; synbiotic (Synb): NC + 453 g dried fermentation product of Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacillus reuteri and fructooligosaccharides/907 kg diet.
Diversity and Composition of Ileal Microbiota
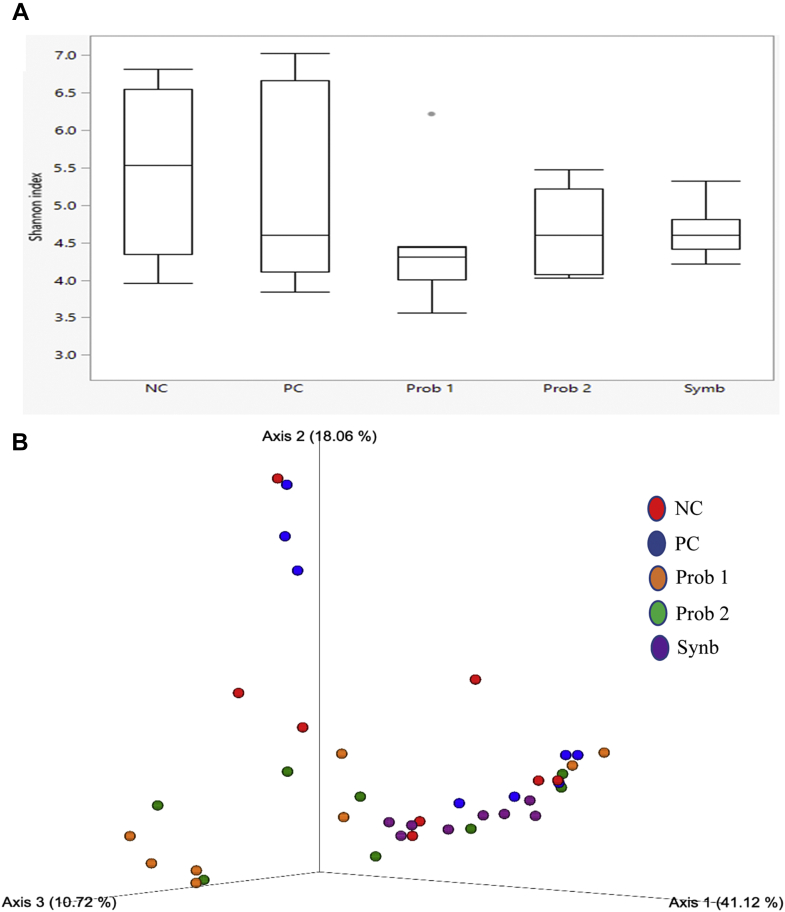
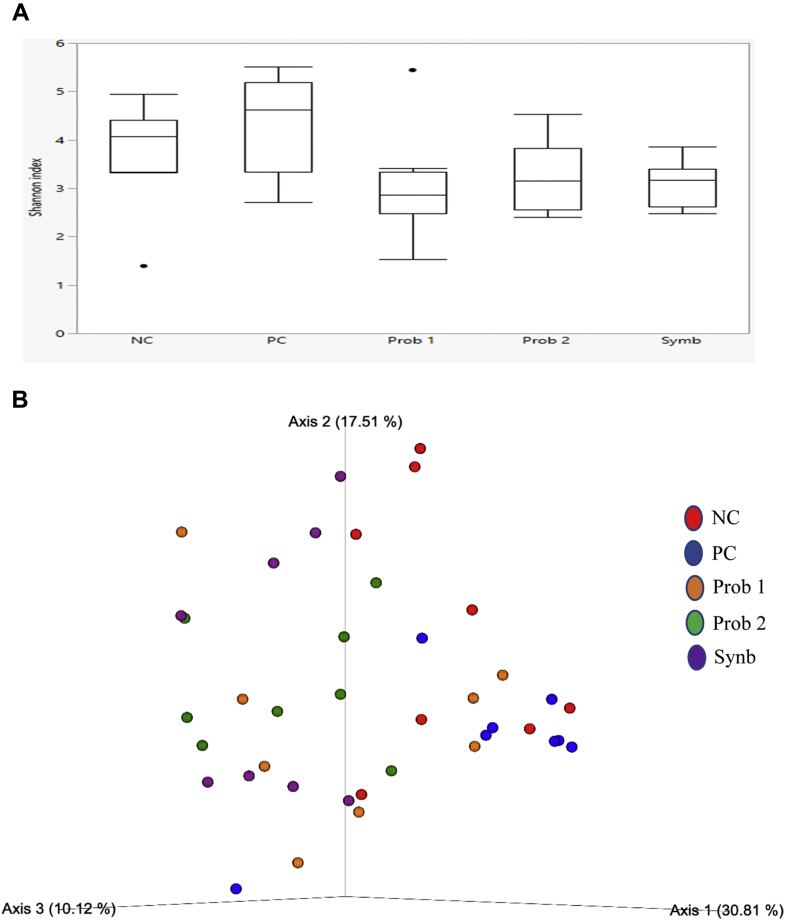
On day 8, microbial α-diversity significantly decreased in Prob 1 compared with the NC and Synb groups (Table 5; Figure 1A). On day 28, α-diversity was significantly higher in NC than in Synb, whereas it was significantly higher in PC than in the Prob 2 and Synb groups (Table 5; Figure 2A). β-Diversity PCoA plots showed the Synb group having the most compositional homogeneity compared with the other groups on day 8 (Figure 1B). The NC and Prob 1 birds had lower compositional homogeneity than the Synb, Prob 2, and PC birds on day 28 (Figure 2B). α-Diversity refers to the variance within the samples of a particular group, whereas β-diversity refers to the variance among groups. Both α- and β-diversity metrics consider 2 aspects of the community: the number of different organisms in a sample (richness) and the range of abundance for each one (evenness).
Table 5.
Pairwise Kruskal-Wallis test for phylogenetic diversity of treatment groups using Shannon index analysis on day 8 and 28.1
| Sampling day | Group 1 | Group 2 | H | P-value | q-value |
|---|---|---|---|---|---|
| Day 8 | NC | PC | 0.099 | 0.752 | 0.916 |
| Prob 1 | 3.981 | 0.045 | 0.229 | ||
| Prob 2 | 1.863 | 0.172 | 0.415 | ||
| Synb | 1.588 | 0.207 | 0.415 | ||
| PC | Prob 1 | 2.481 | 0.115 | 0.383 | |
| Prob 2 | 0.099 | 0.752 | 0.916 | ||
| Synb | 0.011 | 0.916 | 0.916 | ||
| Prob 1 | Prob 2 | 1.102 | 0.293 | 0.489 | |
| Synb | 3.981 | 0.045 | 0.229 | ||
| Prob 2 | Synb | 0.044 | 0.833 | 0.916 | |
| Day 28 | NC | PC | 1.588 | 0.207 | 0.296 |
| Prob 1 | 2.481 | 0.115 | 0.230 | ||
| Prob 2 | 2.161 | 0.141 | 0.235 | ||
| Synb | 4.411 | 0.035 | 0.118 | ||
| PC | Prob 1 | 3.573 | 0.058 | 0.146 | |
| Prob 2 | 4.411 | 0.035 | 0.118 | ||
| Synb | 4.863 | 0.027 | 0.118 | ||
| Prob 1 | Prob 2 | 0.705 | 0.400 | 0.445 | |
| Synb | 0.705 | 0.400 | 0.445 | ||
| Prob 2 | Synb | 0.000 | 1.000 | 1.000 |
Treatments include negative control (NC): corn-soybean meal basal diet; positive control (PC): NC+ 20 g virginiamycin (453 g Stafac20)/907 kg diet; probiotic 1 (Prob 1): NC + 453 g Bacillus subtilis DSM17299/907 kg diet; probiotic 2 (Prob 2): NC + 453 g Bacillus subtilis C-3102/907 kg diet; synbiotic (Synb): NC + 453 g dried fermentation product of Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacillus reuteri, and fructooligosaccharides/907 kg diet.
Figure 1.
Comparison of microbial population in broilers fed various dietary supplements on day 8. (A) Shannon's diversity index of each treatment group. The boxplots show the quartiles, median, and extremities of the values. (B) 3D PCoA plots based on weighted UniFrac distance matrix (Bray-Curtis). Each sphere represents a sample. Treatments include negative control (NC): corn-soybean meal basal diet; positive control (PC): NC + 20 g virginiamycin (453 g Stafac20)/907 kg feed; probiotic 1 (Prob 1): NC + 453 g Bacillus subtilis DSM17299/907 kg feed; probiotic 2 (Prob 2): NC + 453 g B. subtilis C-3102/907 kg feed; synbiotic (Synb): NC + 453 g dried fermentation product of Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacillus reuteri, and fructooligosaccharides/907 kg feed.
Figure 2.
Comparison of microbial populations in broilers fed various dietary supplements on day 28. (A) Shannon's diversity index of each treatment group. The boxplots show the quartiles, median, and extremities of the values. (B) 3D PCoA plots based on weighted UniFrac distance matrix (Bray-Curtis). Each sphere represents a sample. Treatments include negative control (NC): corn-soybean meal basal diet; positive control (PC): NC + 20 g virginiamycin (453 g Stafac20)/907 kg feed; probiotic 1 (Prob 1): NC + 453 g Bacillus subtilis DSM17299/907 kg feed; probiotic 2 (Prob 2): NC + 453 g B. subtilis C-3102/907 kg feed; synbiotic (Synb): NC + 453 g dried fermentation product of Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacillus reuteri, and fructooligosaccharides/907 kg feed.
PERMANOVA analyses on day 8 and day 28 revealed that the composition of microbial communities in the NC was different from than in the Prob 1, Prob 2, and Synb groups (Table 6). The relative abundance of amplicon sequence variants of ileal scrapings microbiota was analyzed at different ranking levels from phylum to genus. The dominant phyla across the groups were Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Tenericutes, and Epsilonbacteraeota together contributing greater than 99 and 75% of the whole phyla on day 8 and day 28, respectively (Table 7). Birds supplemented with additives had a greater abundance of Firmicutes and a lesser abundance of Bacteroidetes than the NC and PC on day 8. However, differences in relative abundance of the dominant bacterial phyla among the treatments decreased with age (Table 7).
Table 6.
Pairwise PERMANOVA statistic based on unweighted UniFrac distance matrix on day 8 and day 28.1
| Sampling day | Group 1 | Group 2 | Pseudo-F | p-value2 | q-value |
|---|---|---|---|---|---|
| Day 8 | NC | PC | 0.654 | 0.717 | 0.717 |
| Prob 1 | 1.925 | 0.040 | 0.156 | ||
| Prob 2 | 2.069 | 0.048 | 0.156 | ||
| Synb | 1.896 | 0.056 | 0.156 | ||
| PC | Prob 1 | 1.559 | 0.071 | 0.156 | |
| Prob 2 | 1.933 | 0.078 | 0.156 | ||
| Synb | 1.641 | 0.117 | 0.167 | ||
| Prob 1 | Prob 2 | 1.758 | 0.096 | 0.160 | |
| Synb | 0.937 | 0.519 | 0.576 | ||
| Prob 2 | Synb | 1.596 | 0.149 | 0.186 | |
| Day 28 | NC | PC | 1.079 | 0.376 | 0.481 |
| Prob 1 | 1.621 | 0.026 | 0.130 | ||
| Prob 2 | 1.710 | 0.054 | 0.180 | ||
| Synb | 1.947 | 0.008 | 0.080 | ||
| PC | Prob 1 | 1.161 | 0.273 | 0.481 | |
| Prob 2 | 0.967 | 0.433 | 0.481 | ||
| Synb | 1.03 | 0.314 | 0.481 | ||
| Prob 1 | Prob 2 | 1.282 | 0.148 | 0.370 | |
| Synb | 0.993 | 0.387 | 0.481 | ||
| Prob 2 | Synb | 0.809 | 0.688 | 0.688 |
Treatments include: negative control (NC): corn-soybean meal basal diet; positive control (PC): NC+ 20 g virginiamycin (453 g Stafac20)/907 kg diet; probiotic 1 (Prob 1): NC + 453 g Bacillus subtilis DSM17299/907 kg diet; probiotic 2 (Prob 2): NC + 453 g Bacillus subtilis C-3102/907 kg diet; synbiotic (Synb): NC + 453 g dried fermentation product of Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacillus reuteri, and fructooligosaccharides/907 kg diet.
P-values were calculated based on 999 permutation tests.
Table 7.
Comparison of microbial population in broiler chickens fed various dietary supplements on day 8 and 28 using 16S rRNA sequencing.
| Relative abundance (%) | Dietary treatments2 |
||||
|---|---|---|---|---|---|
| NC | PC | Prob 1 | Prob 2 | Synb | |
| Day 8 | |||||
| Actinobacteria | 1.95 | 2.72 | 0.09 | 0.15 | 0.33 |
| Bacteroidetes | 14.58 | 14.60 | 1.65 | 1.22 | 1.78 |
| Epsilonbacteraeota | 0.08 | 0.02 | 0.00 | 0.00 | 0.00 |
| Firmicutes | 81.82 | 76.35 | 96.75 | 97.43 | 96.94 |
| Proteobacteria | 1.39 | 5.99 | 1.09 | 0.90 | 0.80 |
| Tenericutes | 0.04 | 0.15 | 0.33 | 0.00 | 0.05 |
| Day 28 | |||||
| Actinobacteria | 0.35 | 1.73 | 0.17 | 0.27 | 0.30 |
| Bacteroidetes | 3.00 | 6.79 | 0.77 | 0.63 | 0.80 |
| Epsilonbacteraeota | 0.73 | 0.49 | 0.55 | 0.74 | 0.15 |
| Firmicutes | 75.40 | 60.18 | 80.80 | 83.95 | 85.95 |
| Proteobacteria | 2.27 | 5.22 | 1.48 | 1.23 | 1.13 |
| Tenericutes | 0.09 | 0.17 | 0.02 | 0.08 | 0.01 |
Taxonomic diversity table shows the relative abundance (%) of taxa at the phylum level in each group. The relative abundance of each sample was calculated based on the amplicon sequence variants table rarefied to 10,891 and 37,633 reads per sample for day 8 and day 28, respectively.1
Data represent the mean value of 1 bird/pen (8 birds/treatment).
Treatments include negative control (NC): corn-soybean meal basal diet; positive control (PC): NC+ 20 g virginiamycin (453 g Stafac20)/907 kg diet; probiotic 1 (Prob 1): NC + 453 g Bacillus subtilis DSM17299/907 kg diet; probiotic 2 (Prob 2): NC + 453 g Bacillus subtilis C-3102/907 kg diet; synbiotic (Synb): NC + 453 g dried fermentation product of Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacillus reuteri, and fructooligosaccharides/907 kg diet.
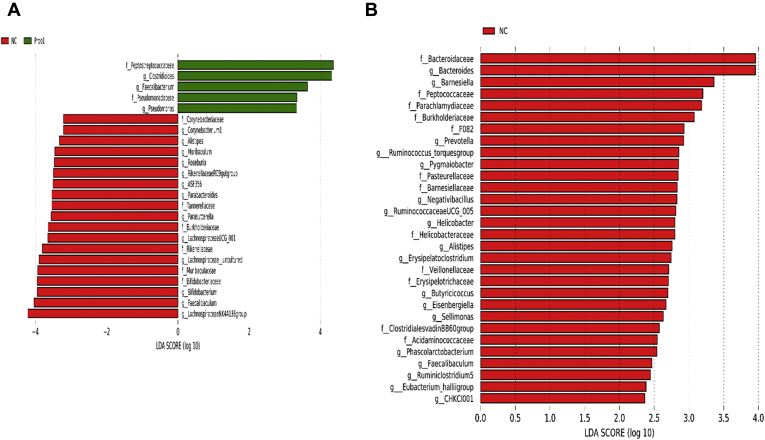
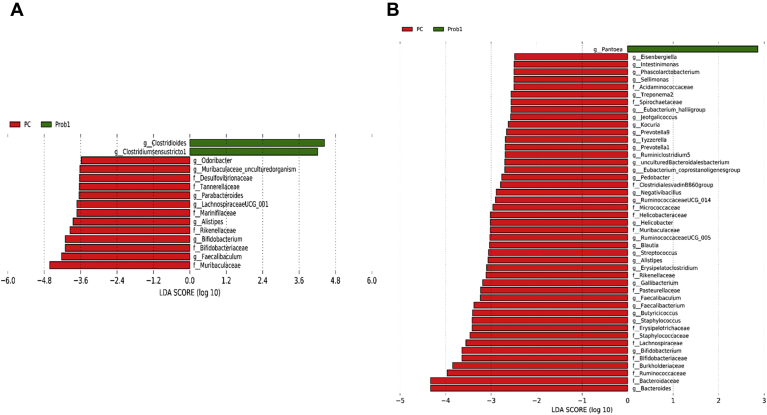
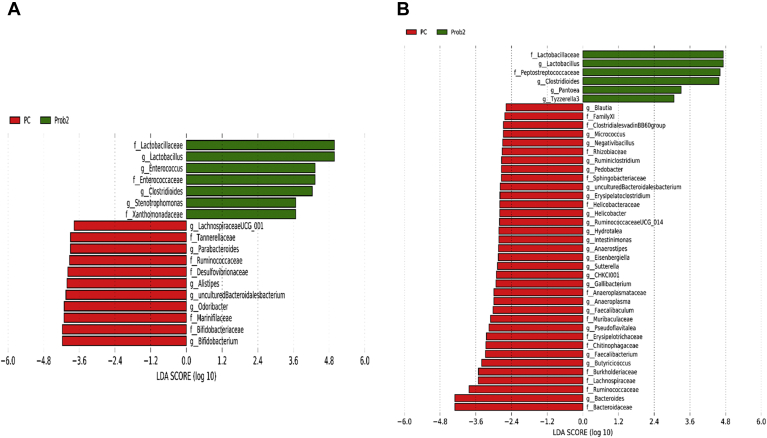
At the genus level, relative abundance of Clostridioides, Faecalibacterium, and Pseudomonas was significantly higher, whereas abundance of Alistipes, ASF356, Bifidobacterium, Faecalibaculum, Lachnospiraceae NK4A136 group, Lachnospiraceae UCG-001, Muribaculum, Parabacteroides, Rikenellaceae RC9 gut group, and Roseburia was significantly lower in Prob 1 group than in the NC on day 8 (Figure 3A). Furthermore, on day 28 as compared with Prob 1, NC birds had higher relative abundance of Alistipes, Bacteroides, Butyricicoccus, Erysipelatoclostridium, Faecalibaculum, Helicobacter, Negativibacillus, Phascolarctobacterium, Prevotella, Ruminococcaceae UCG-005, Ruminiclostridium 5, and Ruminococcus torques group (Figure 3B). Relative abundance of Clostridium sensu stricto 1 and Clostridioides was significantly higher, whereas abundance of Alistipes, Bifidobacterium, Faecalibaculum, Lachnospiraceae UCG-001, Parabacteroides, and Odoribacter was significantly lower in Prob 1 compared with PC on day 8 (Figure 4A). The Prob 1 group had lower relative abundance of Alistipes, Bacteroides, Blautia, Bifidobacterium, Butyricicoccus, Erysipelatoclostridium, Faecalibacterium, Faecalibaculum, Gallibacterium, Negativibacillus, Phascolarctobacterium, Prevotella 9, Ruminococcaceae UCG-005, Ruminococcaceae UCG-014, Ruminiclostridium 5, Staphylococcus, Staphylococcus, and Subdoligranulum were higher than the PC group on day 28 (Figure 4B).
Figure 3.
Taxonomic biomarkers highlighted by linear discriminant analysis effect size (LEfSe) (P ≤ 0.05 and LDA cutoff > 2.0) in ileal mucosa microbiota of broiler chickens challenged with a subclinical naturally occurring necrotic enteritis (n = 8/treatment). LEfSe uses relative abundances to determine the biomarkers (operational taxonomic units) to explain differences between groups. (A) and (B) Taxonomic biomarkers in NC vs. Prob 1 group on day 8 and day 28, respectively. NC (negative control): corn-soybean meal basal diet; Prob 1: NC + 453 g Bacillus subtilis DSM17299/907 kg feed. Abbreviation: LDA, linear discriminant analysis.
Figure 4.
Taxonomic biomarkers highlighted by linear discriminant analysis effect size (LEfSe) (P ≤ 0.05 and LDA cutoff > 2.0) in ileal mucosa microbiota of broiler chickens challenged with a subclinical naturally occurring necrotic enteritis (n = 8/treatment). LEfSe uses relative abundances to determine the biomarkers (operational taxonomic units) to explain differences between groups. (A) and (B) Taxonomic biomarkers in PC vs. Prob 1 group on day 8 and day 28, respectively. PC (positive control): basal diet + 20 g virginiamycin (453 g Stafac20)/907 kg feed; Prob 1: basal diet + 453 g Bacillus subtilis DSM17299/907 kg feed. Abbreviations: LDA, linear discriminant analysis.
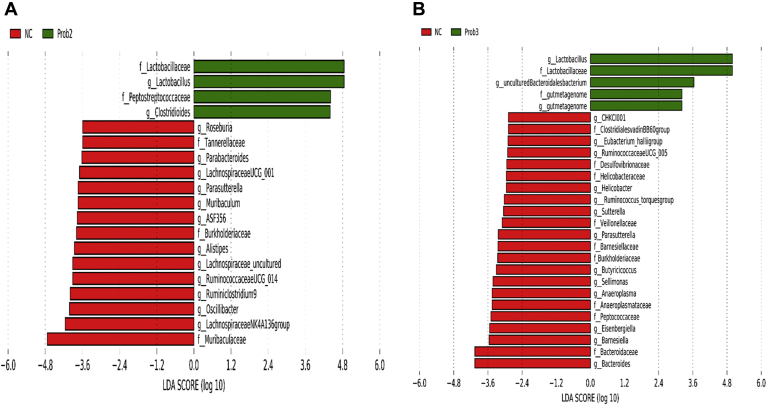
Relative abundance of Clostridioides and Lactobacillus was significantly higher, whereas those of Alistipes, ASF356, Lachnospiraceae NK4A136 group, Lachnospiraceae UCG-001, Muribaculum, Oscillibacter, Parabacteroides, Roseburia, and Ruminococcaceae UCG-014 were significantly lower in Prob 2 than in NC on day 8 (Figure 5A). On day 28, relative abundance of Lactobacillus was lower, whereas relative abundance of Bacteroides, Barnesiella, Butyricicoccus, CHKCI001, Eisenbergiella, Eubacterium hallii group, Helicobacter, Ruminococcaceae UCG-005, R. torques group, Sellimonas, and Sutterella were higher in the NC than in the Prob 2 group (Figure 5B). Relative abundance of Clostridioides, Enterococcus, and Lactobacillus was significantly higher, whereas abundance of Alistipes, Bifidobacterium, Lachnospiraceae UCG-001, Odoribacter, and Parabacteroides was significantly lower in the Prob 2 group than in PC on day 8 (Figure 6A). On day 28, relative abundance of Enterococcus, Clostridioides, Lactobacillus, and Tyzzerella 3 were higher, whereas relative abundance of Anaerostipes, Bacteroides, Blautia, Butyricicoccus, CHKCI001, Eisenbergiella, Erysipelatoclostridium, Faecalibacterium, Faecalibaculum, Gallibacterium, Helicobacter, Hydrotalea, Intestinimonas, Jeotgalicoccus, Kocuria, Micrococcus, Negativibacillus, Pedobacter, Pseudoflavitalea, Ruminococcaceae UCG-014, Ruminiclostridium, and Sutterella were lower in Prob 2 than in PC group (Figure 6A).
Figure 5.
Taxonomic biomarkers highlighted by linear discriminant analysis effect size (LEfSe) (P ≤ 0.05 and LDA cutoff > 2.0) in ileal mucosa microbiota of broiler chickens challenged with a subclinical naturally occurring necrotic enteritis (n = 8/treatment). LEfSe uses relative abundances to determine the biomarkers (operational taxonomic units) to explain differences between groups. (A) and (B) Taxonomic biomarkers in NC vs. Prob 2 group on day 8 and day 28, respectively. NC (negative control): corn-soybean meal basal feed; Prob 2: NC + 453 g Bacillus subtilis C-3102/907 kg feed. Abbreviation: LDA, linear discriminant analysis.
Figure 6.
Taxonomic biomarkers highlighted by linear discriminant analysis effect size (LEfSe) (P ≤ 0.05 and LDA cutoff > 2.0) in ileal mucosa microbiota of broiler chickens challenged with a subclinical naturally occurring necrotic enteritis (n = 8/treatment). LEfSe uses relative abundances to determine the biomarkers (operational taxonomic units) to explain differences between groups. (A) and (B) Taxonomic biomarkers in PC vs. Prob 2 group on day 8 and day 28, respectively. PC (positive control): basal diet + 20 g virginiamycin (453 g Stafac20)/907 kg feed; Prob 2: basal feed + 453 g Bacillus subtilis C-3102/907 kg feed. Abbreviation: LDA, linear discriminant analysis.
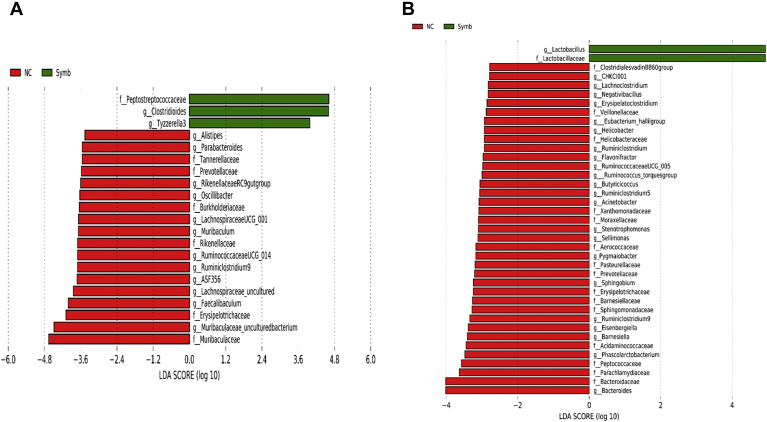
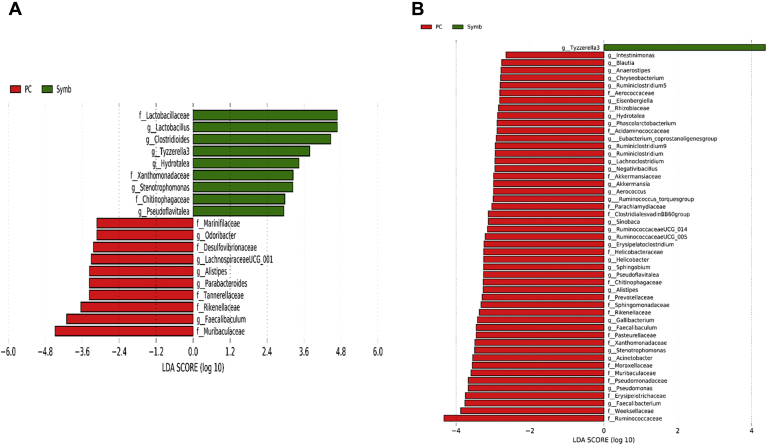
At the genus level, relative abundance of Clostridioides and Tyzzerella 3 was significantly higher, whereas relative abundance of Alistipes, ASF356, Faecalibaculum, Lachnospiraceae UCG-001, Muribaculum, Oscillibacter, Parabacteroides, Rikenellaceae RC9 gut group, Ruminococcaceae UCG-014, and Ruminiclostridium 9 was significantly lower in Synb than in NC on day 8 (Figure 7A). On day 28, relative abundance of Lactobacillus and Tyzzerella 3 was lower, whereas relative abundance of Acinetobacter, Bacteroides, Barnesiella, Butyricicoccus, CHKCI001, Eisenbergiella, Erysipelaroclostridium, Flavonifractor, E. hallii group, Helicobacter, Lachnoclostridium, Negativibacillus, Phascolarctobacterium, Ruminococcaceae UCG-005, Ruminiclostridium, Ruminiclostridium 5, Ruminiclostridium 9, R. torques group, Sellimonas, and Sphingobium were higher in NC compared with the Synb group (Figure 7B). Relative abundance of Clostridioides, Hydrotalea, Lactobacillus, Pseudoflavitalea, and Tyzzerella 3 was significantly higher, whereas abundance of Alistipes, Faecalibaculum, Lachnospiraceae UCG-001, Odoribacter, and Parabacteroides was significantly lower in Synb compared with PC on day 8 (Figure 8A). Compared with the Synb group on day 28, PC birds had less abundant Clostridioides and Tyzzerella 3 populations, whereas relative abundance of Acinetobacter, Aerococcus, Akkermansia, Alistipes, Anaerostipes, Bacteroides, Blautia, Butyricicoccus, Chryseobacterium, Eisenbergiella, Erysipelaroclostridium, Faecalibacterium, Faecalibaculum, Gallibacterium, Helicobacter, Hydrotalea, Intestinimonas, Jeotgalicoccus, Lachnoclostridium, Negativibacillus, Phascolarctobacterium, Pseudoflavitalea, Pseudomonas, Ruminococcaceae UCG-005, Ruminococcaceae UCG-005, Ruminiclostridium, Ruminiclostridium 9, R. torques group, Sinobaca, and Sphingobium was higher (Figure 8B).
Figure 7.
Taxonomic biomarkers highlighted by linear discriminant analysis effect size (LEfSe) (P ≤ 0.05 and LDA cutoff > 2.0) in ileal mucosa microbiota of broiler chickens challenged with a subclinical naturally occurring necrotic enteritis (n = 8/treatment). LEfSe uses relative abundances to determine the biomarkers (operational taxonomic units) to explain differences between groups. (A) and (B) Taxonomic biomarkers in NC vs. Synb group on day 8 and day 28, respectively. NC (negative control): corn-soybean meal basal diet; Synb: NC + 453 g dried fermentation product of Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacillus reuteri, and fructooligosaccharides/907 kg feed. Abbreviation: LDA, linear discriminant analysis.
Figure 8.
Taxonomic biomarkers highlighted by linear discriminant analysis effect size (LEfSe) (P ≤ 0.05 and LDA cutoff > 2.0) in ileal mucosa microbiota of broiler chickens challenged with a subclinical naturally occurring necrotic enteritis (n = 8/treatment). LEfSe uses relative abundances to determine the biomarkers (operational taxonomic units) to explain differences between groups. (A) and (B) Taxonomic biomarkers in PC vs. Synb group on day 8 and day 28, respectively. PC (positive control): basal diet + 20 g virginiamycin (453 g Stafac20)/907 kg feed; Synb: NC + 453 g dried fermentation product of Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacillus reuteri, and fructooligosaccharides/907 kg feed. Abbreviation: LDA, linear discriminant analysis.
Predicted Functions of Ileal Microbiota
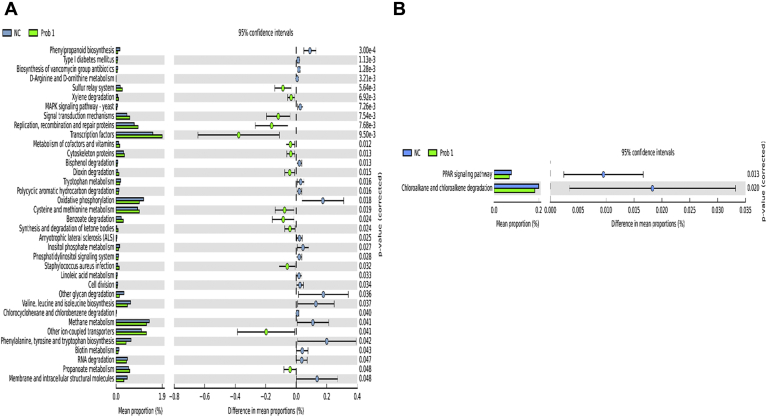
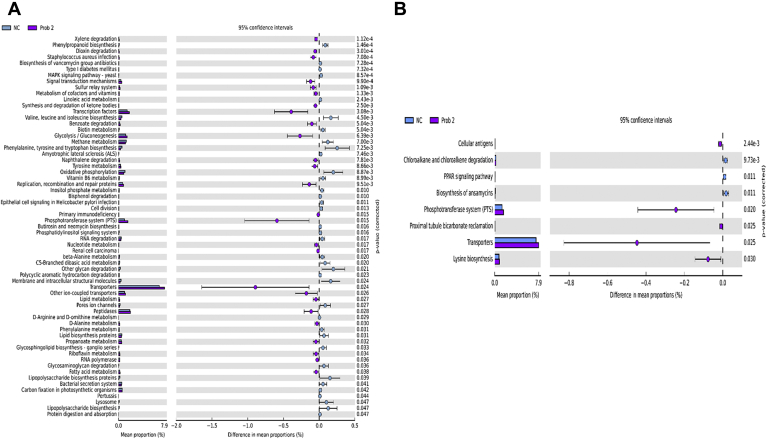
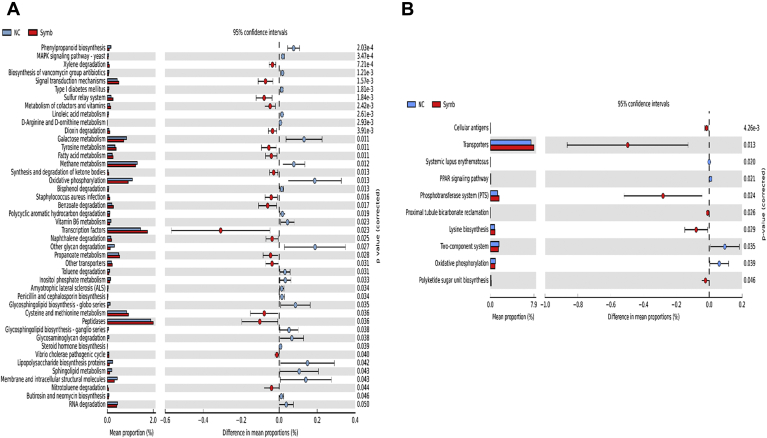
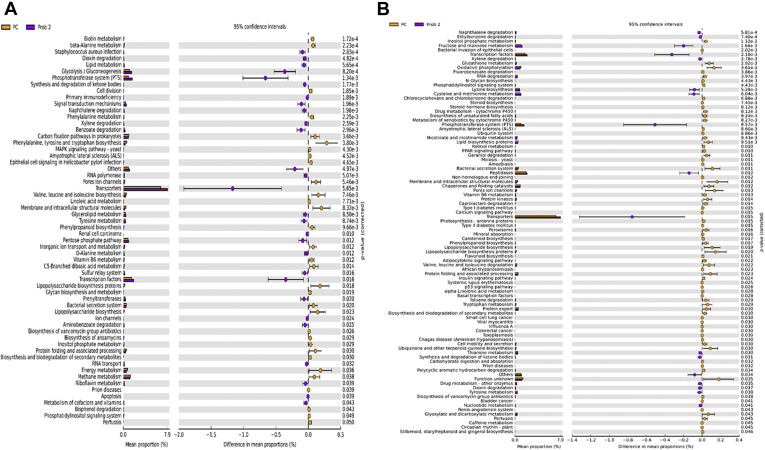
Prediction of microbial functions showed distinctive patterns among the treatment groups. There were 36 and 2 pathways at KEGG level 3 with distinctive enrichment between the NC and Prob 1 groups on day 8 and day 28, respectively (Figures 9A and 9B). Among these, the NC microbiota had greater numbers of functional genes involved in LPS biosynthesis, LPS biosynthesis proteins, C5 branched dibasic acid metabolism, and biosynthesis of vancomycin group antibiotics on day 8. Figures 10A and 10B indicate 63 and 8 pathways at KEGG level 3 with distinctive enrichment between NC and Prob 2 birds on day 8 and day 28, respectively. There were 47 and 14 pathways at KEGG level 3 with distinctive enrichment between the NC and Synb group on day 8 and day 28, respectively (Figures 11A and 11B). Among these, the Prob 2 and Synb groups had greater numbers of functional genes involved in propanoate metabolism, whereas LPS biosynthesis, LPS biosynthesis proteins, bacterial secretion system, and biosynthesis of vancomycin group antibiotics were enriched in the NC compared with the Prob 2 and Synb groups on day 8. On day 28, lysine biosynthesis, cellular antigens, and transporters proteasome, peroxisome, antigen processing, and presentation were enriched in both the Synb and Prob 2 birds compared with NC. Furthermore, polyketide sugar unit biosynthesis and phosphotransferase system were enriched in Synb compared with NC.
Figure 9.
Predicted functions of ileal mucosa microbiota in broiler chickens challenged with a subclinical naturally occurring necrotic enteritis at KEGG levels 3 (n = 8/treatment). (A) and (B) Differentially regulated metabolic pathways in NC vs. Prob 1 group on day 8 and day 28, respectively. NC (negative control): corn-soybean meal basal diet; Prob 1: NC + 453 g Bacillus subtilis DSM17299/907 kg feed. Abbreviation: KEGG, Kyoto encyclopedia of genes and genomes.
Figure 10.
Predicted functions of ileal mucosa microbiota in broiler chickens challenged with a subclinical naturally occurring necrotic enteritis at KEGG levels 3 (n = 8/treatment). (A) and (B) Differentially regulated metabolic pathways in NC vs. Prob 2 group on day 8 and day 28, respectively. NC (negative control): corn-soybean meal basal diet; Prob 2: NC + 453 g Bacillus subtilis C-3102/907 kg feed. Abbreviation: KEGG, Kyoto encyclopedia of genes and genomes.
Figure 11.
Predicted functions of ileal mucosa microbiota in broiler chickens challenged with a subclinical naturally occurring necrotic enteritis at KEGG levels 3 (n = 8/treatment). (A) and (B) Differentially regulated metabolic pathways in NC vs. Synb group on day 8 and day 28, respectively. NC (negative control): corn-soybean meal basal diet; Synb: NC + 453 g dried fermentation product of Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacillus reuteri and fructooligosaccharides/907 kg feed. Abbreviation: KEGG, Kyoto encyclopedia of genes and genomes.
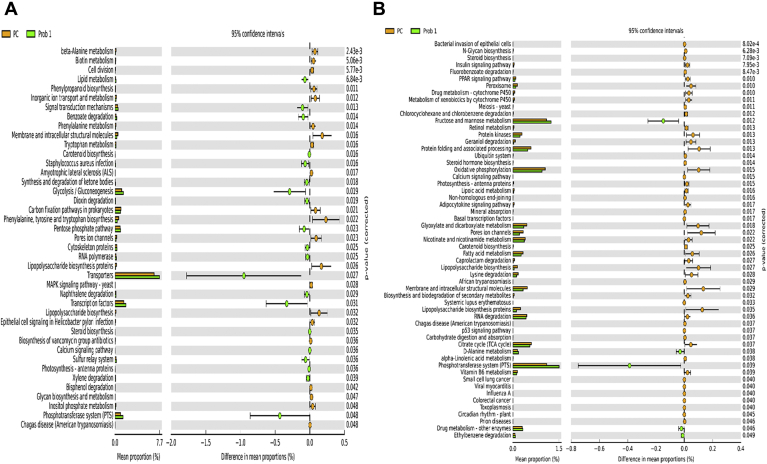
Figures 12A and 12B illustrate 41 and 57 pathways at KEGG level 3 with distinctive enrichment between PC and Prob 1 group on day 8 and day 28, respectively. Comparison of PC and Prob 2 group indicated 60 and 92 pathways at KEGG level 3 with distinctive enrichment on day 8 and day 28, respectively (Figures 13A and 13B). There were 34 and 80 pathways at KEGG level 3 with distinctive enrichment between PC and Synb group on day 8 and day 28, respectively (Figures 14A and 14B). The Prob 2 and Synb groups had greater numbers of functional genes involved in glycolysis/gluconeogenesis, phosphotransferase system and signal transduction mechanisms, whereas LPS biosynthesis, LPS biosynthesis proteins, and bacterial secretion system were enriched in PC compared with the Prob 2 and Synb groups on day 8. In addition, apoptosis was enriched in the Prob 2 group, whereas cell division and metabolism and biosynthesis of some amino acids were enriched in PC. On day 28, fructose and mannose metabolism, synthesis, and degradation of ketone bodies, phosphotransferase system, cysteine and methionine metabolism, lysine biosynthesis, transcription factors, dioxin degradation, Staphylococcus aureus infection, peptidases, and transporters were enriched in both the Synb and Prob 2 groups than in PC. Furthermore, bacterial invasion of epithelial cells, leucine and isoleucine degradation, calcium signaling pathway, PPAR signaling pathway, mineral absorption, LPS biosynthesis, adipocyte signaling pathway, lipid biosynthesis, proteins and vitamin B6 metabolism were enriched in PC compared with the Synb and Prob 2 groups.
Figure 12.
Predicted functions of ileal mucosa microbiota in broiler chickens challenged with a subclinical naturally occurring necrotic enteritis at KEGG levels 3 (n = 8/treatment). (A) and (B) Differentially regulated metabolic pathways in PC vs. Prob 1 group on day 8 and day 28, respectively. PC (positive control): basal diet + 20 g virginiamycin (453 g Stafac20)/907 kg feed; Prob 1: basal diet + 453 g Bacillus subtilis DSM17299/907 kg feed. Abbreviation: KEGG, Kyoto encyclopedia of genes and genomes.
Figure 13.
Predicted functions of ileal mucosa microbiota in broiler chickens challenged with a subclinical naturally occurring necrotic enteritis at KEGG levels 3 (n = 8/treatment). (A) and (B) Differentially regulated metabolic pathways in PC vs. Prob 2 group on day 8 and day 28, respectively. PC (positive control): basal diet + 20 g virginiamycin (453 g Stafac20)/907 kg feed; Prob 2: basal diet + 453 g Bacillus subtilis C-3102/907 kg feed. Abbreviation: KEGG, Kyoto encyclopedia of genes and genomes.
Figure 14.
Predicted functions of ileal mucosa microbiota in broiler chickens challenged with a subclinical naturally occurring necrotic enteritis at KEGG levels 3 (n = 8/treatment). (A) and (B) Differentially regulated metabolic pathways in PC vs. Synb group on day 8 and day 28, respectively. PC (positive control): basal diet + 20 g virginiamycin (453 g Stafac20)/907 kg feed; Synb: NC + 453 g dried fermentation product of Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacillus reuteri, and fructooligosaccharides/907 kg feed. Abbreviation: KEGG, Kyoto encyclopedia of genes and genomes.
Discussion
Synb was the only group with better FCR during day 0 to 8 and lower NE lesion scores on day 8 compared with NC. These improvements were associated with the modified ileal microbiota in the Synb group compared with NC on day 8. Microbial balance and macro- and micro-structural integrity of the gut are pivotal for gut health, which if compromised would negatively affect digestion, absorption, and metabolism of nutrients and may lead to the onset of enteric disease (Yegani and Korver, 2008; Ritzi et al., 2014). Changes in microbial diversity and population could make the gut environment suitable for proliferation or pathogenesis of bacteria such as C. perfringens, either directly or via promoting growth of other bacterial species that may competitively exclude Clostridia. Relative abundance of Bacteroides in the ileal digesta of broiler chickens increases with NE challenge (Bortoluzzi et al., 2019). Bacteroides and Prevotellaceae can degrade mucus oligosaccharides, which results in the disruption of intestinal mucosal barrier, thus causing intestinal inflammation (Rho et al., 2005; Berry et al., 2015). Supplementation of Synb reduced the relative abundance of Bacteroides compared with NC on day 8. Dietary supplementation of a Bacillus-based probiotic partially restored relative abundance of Bacteroides in the ileal digesta of NE-challenged birds to the level of the nonchallenged group (Bortoluzzi et al., 2019).
Predisposing factors such as Eimeria would lead to disturbances in the microbiota by impacting the gastrointestinal environment, therefore allowing the proliferation of C. perfringens by providing a favorable ecological environment and/or nutrients (Shojadoost et al., 2012; Antonissen et al., 2016). Eimeria infection had a marked effect on the microbiota composition in the ceca of infected birds (Stanley et al., 2014) and a coccidiosis challenge reduced the Ruminococcaceae family while 3 unknown Clostridium species were increased (Wu et al., 2014). Overgrowth of Clostridium sensu stricto 1 and reduction in the number of Lactobacillus were associated with NE (Antonissen et al., 2016; Fasina et al., 2016; Li et al., 2017). Mixed C. perfringens and Eimeria challenge led to a significant increase in Clostridium sensu stricto 1 and a reduction in Lactobacillus with the concurrent increase in NE lesions (Yang et al., 2019). The NC and Prob 1 groups had higher NE lesion scores as well as higher relative abundance of Clostridium sensu stricto 1 on day 8. By contrast, Synb and Prob 2 supplementation reduced lesion scores and relative abundance of Clostridium sensu stricto 1 and ASF356 (a Clostridium species), while increased the abundance of Lactobacillus compared with NC. Lactobacillus is among the predominant bacterial genera in the gastrointestinal tract of broiler chickens (Wei et al., 2013). These bacteria have direct and indirect beneficial effects. Direct effects include immunomodulation via attachment and interaction with enterocytes, antagonistic activity against pathogens by production of lactate thus lowering pH and making the gastrointestinal tract environment unsuitable for acid-sensitive pathogens, and the production of bacteriostatic and bactericidal substances (Servin, 2004; Belenguer et al., 2007; Pan and Yu, 2014). Furthermore, probiotic bacteria such as Lactobacillus prevent adherence of pathogens to the intestinal mucosa by competitive exclusion and displacing pathogenic bacteria (Servin and Coconnier, 2003; Collado et al., 2005). Supplementation of Synb and Prob 1 increased the relative abundance of Lactobacillus in the ileal mucosa, which may illustrate a positive effect on gut health and performance of the birds. Likewise, supplementation of a probiotic to the diet of broiler chickens challenged with NE increased abundance of Lactobacillaceae and Clostridiaceae families in the cecal digesta compared with the challenged control group (Whelan et al., 2019).
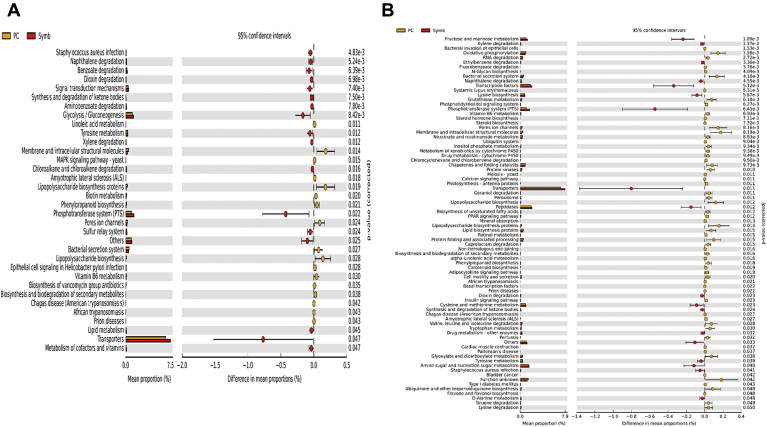
All the additives improved FCR significantly during day 0 to 28 compared with NC. In addition, ADG significantly increased in the PC, Prob 2, and Synb groups compared with NC for the same period. Supplementation of B. subtilis to the diet of broiler chickens challenged with NE significantly decreased lesion scores and improved FCR compared with challenged control (Bortoluzzi et al., 2019; Sokale et al., 2019; Whelan et al., 2019). Higher abundance of Lactobacillus was correlated with feed intake which results in poor FCR, whereas Faecalibacterium was correlated with improved FCR (Stanley et al., 2016). Despite similar FCR during day 0 to 28, relative abundance of Faecalibacterium was higher and that of Lactobacillus was lower in PC birds than in the Prob 1, Prob 2, and Synb groups on day 28. This may imply that a specific genus of bacteria might not be a good indicator of bird's performance and health; instead, considering the whole microbial profile of the gastrointestinal tract would be a better approach. Relative abundance of Bacteroides, Barnesiella, Butyricicoccus, CHKCI001, Eisenbergiella, E. hallii group, Helicobacter, Ruminococcaceae UCG-005, R. torques group, and Sellimonas was lower in the Synb and Prob 2 groups (with better FCR) than in NC on day 28. Previous reports indicate that manipulation of the gut microbiota during a NE challenge might prevent/alleviate its negative effects. Fructooligosaccharides constitute one component of Synb. In vitro studies have shown high levels of propionate production from fructooligosaccharides fermentation (Asano et al., 2003). Absorption of propionate by the chicken cecal mucosa could improve host energy metabolism and improve production (Pineda-Quiroga et al., 2019). Dietary supplementation of a prebiotic (but not probiotic and synbiotic) to laying hens enriched cecal microbial genes involved in propanoate metabolism (Pineda-Quiroga et al., 2019). Furthermore, production of short chain fatty acids reduces the cecum pH, thus inhibiting the growth of Enterobacteriaceae (which includes Escherichia-Shigella) (van Der Wielen et al., 2000). A L. acidophilus–based probiotic improved intestinal health by decreasing the relative abundance of Escherichia-Shigella in the ileum (Li et al., 2017). The probiotic bacteria B. subtilis DSM 32315 increased the abundance of Firmicutes while reduced the abundance of Bacteroidetes in the ceca of broiler chickens (Ma et al., 2018). Furthermore, this probiotic decreased the abundance of potentially harmful bacteria including Vampirovibrio, Escherichia-Shigella, and Parabacteroides (Ma et al., 2018). Relative abundance of Lachnospiraceae, Ruminococcaceae, and unclassified family members belonging to the Clostridiales order were decreased in the cecal digesta of NE-challenged broiler chickens fed a diet supplemented with Bacillus-based probiotic compared with the challenged control. However, the abundance of Lactobacillaceae and Clostridiaceae families was significantly increased in probiotic fed birds (Whelan et al., 2019). Lower relative abundance of Escherichia-Shigella (belonging to Enterobacteriaceae) in the Synb and Prob 2 groups than PC was associated with the reduction in LPS biosynthesis, LPS biosynthesis proteins and bacterial secretion system in these birds compared with those of NC and PC as indicated by the KEGG pathway. Lipopolysaccharides induce mucosal immune responses (Lucke et al., 2018); therefore, mucosal immune response to the overgrowth of opportunistic bacteria due to the dysbiosis caused by the NE challenge might have been attenuated by the supplementation of Synb and Prob 2.
Based on the findings presented herein, we can conclude that under a naturally occurring NE challenge model, dietary supplementation of Synb to broilers could significantly reduce day 8 lesion scores and improve FCR during the peak challenge period (day 0–8). These changes might be due to modified microbiota as influenced by supplementation of the Synb additive. In addition, birds fed diets supplemented with Synb and Prob 1 had significantly better FCR and higher ADG during day 0 to 28 than NC. Better performance was associated with the modified microbiota in the Synb and Prob 1 groups on day 28, and might partially explain the protective effect of these additives against a naturally occurring subclinical NE.
Acknowledgments
This work was supported, in part, by USDA-NIFA Hatch funds to the Virginia Agricultural Experiment Station, Virginia Tech.
Disclosures
The authors declare no conflict of interest.
References
- Antonissen G., Eeckhaut V., Van Driessche K., Onrust L., Haesebrouck F., Ducatelle R., Moore R.J., Van Immerseel F. Microbial shifts associated with necrotic enteritis. Avian Pathol. 2016;45:308–312. doi: 10.1080/03079457.2016.1152625. [DOI] [PubMed] [Google Scholar]
- Apprill A., McNally S., Parsons R., Weber L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 2015;75:129–137. [Google Scholar]
- Asano I., Hamaguchi K., Fujii S., Iino H. In vitro digestibility and fermentation of mannooligosaccharides from coffee mannan. Food Sci. Technol. Res. 2003;9:62–66. [Google Scholar]
- Belenguer A., Duncan S.H., Holtrop G., Anderson S.E., Lobley G.E., Flint H.J. Impact of pH on lactate formation and utilization by human fecal microbial communities. Appl. Environ. Microbiol. 2007;73:6526–6533. doi: 10.1128/AEM.00508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D., Mader E., Lee T.K., Woebken D., Wang Y., Zhu D., Palatinszky M., Schintlmeister A., Schmid M.C., Hanson B.T., Shterzer N., Mizrahi I., Rauch I., Decker T., Bocklitz T., Popp J., Gibson C.M., Fowler P.W., Huang W.E., Wagner M. Tracking heavy water incorporation for identifying and sorting active microbial cells. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E194. doi: 10.1073/pnas.1420406112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., Bai Y., Bisanz J.E., Bittinger K., Brejnrod A., Brislawn C.J., Brown C.T., Callahan B.J., Caraballo-Rodríguez A.M., Chase J., Cope E.K., Da Silva R., Diener C., Dorrestein P.C., Douglas G.M., Durall D.M., Duvallet C., Edwardson C.F., Ernst M., Estaki M., Fouquier J., Gauglitz J.M., Gibbons S.M., Gibson D.L., Gonzalez A., Gorlick K., Guo J., Hillmann B., Holmes S., Holste H., Huttenhower C., Huttley G.A., Janssen S., Jarmusch A.K., Jiang L., Kaehler B.D., Kang K.B., Keefe C.R., Keim P., Kelley S.T., Knights D., Koester I., Kosciolek T., Kreps J., Langille M.G.I., Lee J., Ley R., Liu Y.-X., Loftfield E., Lozupone C., Maher M., Marotz C., Martin B.D., McDonald D., McIver L.J., Melnik A.V., Metcalf J.L., Morgan S.C., Morton J.T., Naimey A.T., Navas-Molina J.A., Nothias L.F., Orchanian S.B., Pearson T., Peoples S.L., Petras D., Preuss M.L., Pruesse E., Rasmussen L.B., Rivers A., Robeson M.S., Rosenthal P., Segata N., Shaffer M., Shiffer A., Sinha R., Song S.J., Spear J.R., Swafford A.D., Thompson L.R., Torres P.J., Trinh P., Tripathi A., Turnbaugh P.J., Ul-Hasan S., van der Hooft J.J.J., Vargas F., Vázquez-Baeza Y., Vogtmann E., von Hippel M., Walters W., Wan Y., Wang M., Warren J., Weber K.C., Williamson C.H.D., Willis A.D., Xu Z.Z., Zaneveld J.R., Zhang Y., Zhu Q., Knight R., Caporaso J.G. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoluzzi C., Serpa Vieira B., de Paula Dorigam J.C., Menconi A., Sokale A., Doranalli K., Applegate T.J. Bacillus subtilis DSM 32315 supplementation attenuates the effects of Clostridium perfringens challenge on the growth performance and intestinal microbiota of broiler chickens. Microorganisms. 2019;7:71. doi: 10.3390/microorganisms7030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calik A., Omara I.I., White M.B., Evans N.P., Karnezos T.P., Dalloul R.A. Dietary non-drug feed additive as an alternative for antibiotic growth promoters for broilers during a necrotic enteritis challenge. Microorganisms. 2019;7:257. doi: 10.3390/microorganisms7080257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly D.L., D'Inca R., Auclair E., Drider D. Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: a microbiologist's perspective. Front. Microbiol. 2015;6:1336. doi: 10.3389/fmicb.2015.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M.C., Gueimonde M., Hernandez M., Sanz Y., Salminen S. Adhesion of selected Bifidobacterium strains to human intestinal mucus and the role of adhesion in enteropathogen exclusion. J. Food Prot. 2005;68:2672–2678. doi: 10.4315/0362-028x-68.12.2672. [DOI] [PubMed] [Google Scholar]
- Danzeisen J.L., Kim H.B., Isaacson R.E., Tu Z.J., Johnson T.J. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS One. 2011;6:e27949. doi: 10.1371/journal.pone.0027949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N.K., Calik A., White M.B., Kimminau E.A., Dalloul R.A. Effect of probiotics and multi component feed additives on microbiota, gut barrier and immune responses in broiler chickens during subclinical necrotic enteritis. Front. Vet. Sci. 2020;7:572142. doi: 10.3389/fvets.2020.572142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N.K., Calik A., White M.B., Young M., Dalloul R.A. Necrotic enteritis in broiler chickens: the role of tight junctions and mucosal immune responses in alleviating the effect of the disease. Microorganisms. 2019;7:231. doi: 10.3390/microorganisms7080231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasina Y.O., Newman M.M., Stough J.M., Liles M.R. Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poult. Sci. 2016;95:247–260. doi: 10.3382/ps/pev329. [DOI] [PubMed] [Google Scholar]
- Gaucher M.L., Quessy S., Letellier A., Arsenault J., Boulianne M. Impact of a drug-free program on broiler chicken growth performances, gut health, Clostridium perfringens and Campylobacter jejuni occurrences at the farm level. Poult. Sci. 2015;94:1791–1801. doi: 10.3382/ps/pev142. [DOI] [PubMed] [Google Scholar]
- Gerritsen J., Smidt H., Rijkers G.T., de Vos W.M. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209–240. doi: 10.1007/s12263-011-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau A.L., Ahern P.P., Griffin N.W., Goodman A.L., Gordon J.I. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y., Verstegen M.W.A., Tamminga S., Williams B.A. The role of the commensal gut microbial community in broiler chickens. World’s Poult. Sci. J. 2005;61:95–104. [Google Scholar]
- Li Z., Wang W., Liu D., Guo Y. Effects of Lactobacillus acidophilus on gut microbiota composition in broilers challenged with Clostridium perfringens. PLoS One. 2017;12:e0188634. doi: 10.1371/journal.pone.0188634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke A., Böhm J., Zebeli Q., Metzler-Zebeli B.U. Dietary deoxynivalenol and oral lipopolysaccharide challenge differently affect intestinal innate immune response and barrier function in broiler chickens. J. Anim. Sci. 2018;96:5134–5143. doi: 10.1093/jas/sky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Wang W., Zhang H., Wang J., Zhang W., Gao J., Wu S., Qi G. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci. Rep. 2018;8:15358. doi: 10.1038/s41598-018-33762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M'Sadeq S.A., Wu S., Swick R.A., Choct M. Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim. Nutr. 2015;1:1–11. doi: 10.1016/j.aninu.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada A.E., Needham D.M., Fuhrman J.A. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016;18:1403–1414. doi: 10.1111/1462-2920.13023. [DOI] [PubMed] [Google Scholar]
- Parks D.H., Tyson G.W., Hugenholtz P., Beiko R.G. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda-Quiroga C., Borda-Molina D., Chaves-Moreno D., Ruiz R., Atxaerandio R., Camarinha-Silva A., García-Rodríguez A. Microbial and functional profile of the ceca from laying hens affected by feeding prebiotics, probiotics, and synbiotics. Microorganisms. 2019;7:123. doi: 10.3390/microorganisms7050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J.F., Sivendra R., Barnum D.A. The use of bacitracin in the prevention and treatment of experimentally-induced necrotic enteritis in the chicken. Can. Vet. J. 1978;19:181–183. [PMC free article] [PubMed] [Google Scholar]
- Rho J.-h., Wright D.P., Christie D.L., Clinch K., Furneaux R.H., Roberton A.M. A novel mechanism for desulfation of mucin: identification and cloning of a mucin-desulfating glycosidase (sulfoglycosidase) from Prevotella strain RS2. J. Bacteriol. 2005;187:1543–1551. doi: 10.1128/JB.187.5.1543-1551.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzi M.M., Abdelrahman W., Mohnl M., Dalloul R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014;93:2772–2778. doi: 10.3382/ps.2014-04207. [DOI] [PubMed] [Google Scholar]
- Servin A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 2004;28:405–440. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Servin A.L., Coconnier M.H. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract. Res. Clin. Gastroenterol. 2003;17:741–754. doi: 10.1016/s1521-6918(03)00052-0. [DOI] [PubMed] [Google Scholar]
- Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet. Res. 2012;43:74. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.A. Experiences with drug-free broiler production. Poult. Sci. 2011;90:2670–2678. doi: 10.3382/ps.2010-01032. [DOI] [PubMed] [Google Scholar]
- Sokale A.O., Menconi A., Mathis G.F., Lumpkins B., Sims M.D., Whelan R.A., Doranalli K. Effect of Bacillus subtilis DSM 32315 on the intestinal structural integrity and growth performance of broiler chickens under necrotic enteritis challenge. Poult. Sci. 2019;98:5392–5400. doi: 10.3382/ps/pez368. [DOI] [PubMed] [Google Scholar]
- Stanley D., Hughes R.J., Geier M.S., Moore R.J. Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: Challenges presented for the identification of performance enhancing probiotic bacteria. Front. Microbiol. 2016;7:187. doi: 10.3389/fmicb.2016.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D., Wu S.-B., Rodgers N., Swick R.A., Moore R.J. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS One. 2014;9:e104739. doi: 10.1371/journal.pone.0104739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Der Wielen P.W., Biesterveld S., Notermans S., Hofstra H., Urlings B.A., van Knapen F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl. Environ. Microbiol. 2000;66:2536–2540. doi: 10.1128/aem.66.6.2536-2540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Ni X., Qing X., Liu L., Lai J., Khalique A., Li G., Pan K., Jing B., Zeng D. Probiotic enhanced intestinal immunity in broilers against subclinical necrotic enteritis. Front. Immunol. 2017;8:1592. doi: 10.3389/fimmu.2017.01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber N., Liou D., Dommer J., MacMenamin P., Quiñones M., Misner I., Oler A.J., Wan J., Kim L., Coakley McCarthy M., Ezeji S., Noble K., Hurt D.E. Nephele: a cloud platform for simplified, standardized and reproducible microbiome data analysis. Bioinformatics. 2017;34:1411–1413. doi: 10.1093/bioinformatics/btx617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Morrison M., Yu Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013;92:671–683. doi: 10.3382/ps.2012-02822. [DOI] [PubMed] [Google Scholar]
- Whelan R.A., Doranalli K., Rinttilä T., Vienola K., Jurgens G., Apajalahti J. The impact of Bacillus subtilis DSM 32315 on the pathology, performance, and intestinal microbiome of broiler chickens in a necrotic enteritis challenge. Poult. Sci. 2019;98:3450–3463. doi: 10.3382/ps/pey500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.-B., Stanley D., Rodgers N., Swick R.A., Moore R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014;169:188–197. doi: 10.1016/j.vetmic.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Yadav S., Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W.-Y., Lee Y., Lu H., Chou C.-H., Wang C. Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS One. 2019;14:e0205784. doi: 10.1371/journal.pone.0205784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegani M., Korver D.R. Factors affecting intestinal health in poultry. Poult. Sci. 2008;87:2052–2063. doi: 10.3382/ps.2008-00091. [DOI] [PMC free article] [PubMed] [Google Scholar]