Abstract
The effect of supplementation of different enzymatic associations in the feed of broiler chickens formulated with corn dried at 80°C or 110°C on growth performance and carcass yield was evaluated. In addition, the influence of the different enzymatic associations on the cecal microbiota was studied. One-day-old male broiler chicks (1,320) were distributed in a completely randomized design in a 2 × 5 factorial arrangement (6 replicates; 22 birds/replicate). The treatments were 2 corn drying temperatures (80°C and 110°C) and 5 diets. The diets consisted of a positive control (PC), a negative control (NC) with a reduction of 100 kcal/kg of apparent metabolizable energy, and 3 enzyme combinations added to the NC diet: amylase, amylase + xylanase, and amylase + xylanase + protease. The feed conversion ratio (FCR) from 1 to 7 d of chickens fed diets formulated with corn dried at 80°C was better (P = 0.045) than that of chickens fed diets dried at 110°C. Regardless of the enzymatic association, the supplementation improved body weight gain (P = 0.01) of the NC group to the same level as the PC group. The FCR of the NC was similar to that of the PC only when the 3 enzymes were included from 1 to 21 d (P = 0.001) and regardless of the enzymatic association for the period from 1 to 42 d (P = 0.007). Regarding cecal microbiota, the alpha diversity was similar among the groups (P > 0.05). The beta-diversity analysis showed that the microbiota of the birds receiving the combination of the 3 enzymes was similar to that of birds fed the PC diet (P = 0.18; R = 0.074), with a similar effect observed for the predicted metabolic functions (Linear discriminant analysis effect size). In conclusion, chickens fed diets formulated with corn dried at 80°C had better FCR during the prestarter phase. The enzymatic supplementation improved the FCR of the birds, which may partially be explained by the modulation of the cecal microbiota.
Key words: amylase, cecal microbiota, drying, protease, xylanase
Introduction
Corn is an ingredient of high nutritional value for poultry nutrition, contributing approximately 65% of the metabolizable energy and 20% of the protein in the diets of broilers (Cowieson, 2005). Although corn is highly digestible by poultry, the temperature used to dry the grains after harvest (Odjo et al., 2015) and the presence of antinutritional factors (Knudsen, 2014) may directly influence the use of the nutrients by the birds.
The drying temperature applied by the industry, often above 100°C, may lead to physical-chemical and functional alterations of the starch (Bhuiyan et al., 2010) and protein (Malumba et al., 2008) in the corn. These alterations are able to interfere with the digestive processes of the animals and to reduce the digestibility of the diet (Huart et al., 2018). The addition of exogenous enzymes to poultry diets may attenuate these effects, eliminating antinutritional factors (Alabi et al., 2019) and complementing the action of endogenous enzymes (Stefanello et al., 2019). These mechanisms of action allow the use of unconventional ingredients and reduce cost and nutrient waste (Abd El-Hack et al., 2018; Alagawany et al., 2018), with beneficial effects on nutrient digestibility (Whiting et al., 2017; Alabi et al., 2019) and intestinal health (Yan et al., 2017; Kiarie et al., 2019).
The combined use of enzymes such as amylase, xylanase, and protease, despite being substrate-dependent, has shown additive or synergistic effects in the animals (Amerah et al., 2017; Singh et al., 2017, 2019). The hydrolysis of the cellular wall by xylanases allows the access of endogenous enzymes to the intracellular content (Ravindran, 2013), and the action of protease may increase the digestibility of starch and fat (Cowieson and Roos, 2016).
The effect of exogenous enzymes on the digestibility of nutrients can also reduce the possibility of the interaction between pathogenic microorganisms and available substrates, benefiting the overall health of the bird (Huyghebaert et al., 2011; Bedford and Cowieson, 2012). This action modulates the microbiota, which participates in several physiological mechanisms on the host (Rychlik, 2020), improving the intestinal barrier, favoring the formation of beneficial molecules and the extraction of energy from the diet, and modulating the immune system (Rinttilä and Apajalahti, 2013; Saleh et al., 2018; Cowieson and Kluenter, 2019). Therefore, it was hypothesized that the drying temperature of the corn may change its nutritional quality and influence the performance of broiler chickens, but enzymatic supplementation minimizes these negative effects by, in part, modulating the intestinal microbiota. The objective of this work was to study the effects of the supplementation with different enzymatic associations of diets formulated with corn dried at 80°C or 110°C on the performance and carcass and cuts yield. In addition, the influence of different enzymatic associations on the diversity, composition, and predicted function of the cecal microbiota was evaluated.
Material and methods
Housing, Birds, and Treatments
The experiment was conducted at the Poultry Research Center at the Western State Paraná University, Mal. C. Rondon, Paraná, Brazil, and was approved by the Institutional Animal Care and Use Committee (protocol # 06/19). A total of 1,320 one-day-old male broiler chickens (Cobb 500) (Cascavel, Brazil) were allocated randomly into ten treatments. Each pen containing 22 birds was considered as an experimental unit, totaling 6 replicate pens per treatment. The feeding program was divided into 2 phases (1–21 and 22–42 d) with feed formulated based on corn and soybean meal according to the nutritional recommendations of Rostagno et al. (2011; Table 1). The birds were placed in new litter (pine shavings) and had ad libitum access to water and feed in mash form throughout the experimental period. The environmental conditions (lighting program, temperature, relative humidity, and ventilation rates) were controlled according to the breeder guidelines by using electric heaters, exhaust fans, and pad cooling. Lighting was kept on continuously for the first 3 d, followed by a lighting program of 18 h light and 6 h dark until the end of the experiment. The rearing temperature was maintained at 33°C ± 1.0°C for the first week of the experiment and then reduced by 2°C per week until reaching 23°C. The climate control system provided a minimum ventilation rate until 14 d of age, and a ventilation flow rate of 0.5 m/s and 2.0 m/s from 15–21 d and from 22–42 d of age, respectively.
Table 1.
Composition of the experimental diets (g/kg, as fed-basis).
| Item | 1–21 d |
22–42 d |
||
|---|---|---|---|---|
| PC1 | NC1 | PC | NC | |
| Ingredients | ||||
| Corn | 544.13 | 578.75 | 662.48 | 680.55 |
| Soybean meal (46% CP) | 380.60 | 374.70 | 269.10 | 266.20 |
| Soybean oil | 32.00 | 3.11 | 28.30 | 13.10 |
| Limestone | 10.40 | 10.46 | 11.00 | 10.98 |
| Monocalcium phosphate | 15.61 | 15.57 | 9.51 | 9.52 |
| NaCl | 4.82 | 4.81 | 4.49 | 4.48 |
| Lysine sulfate (50.7% Lys) | 2.48 | 2.66 | 4.03 | 4.10 |
| DL-Methionine (99% Met) | 2.93 | 2.90 | 2.81 | 2.79 |
| L-Threonine (99% Thr) | 0.41 | 0.41 | 0.97 | 0.97 |
| L-Valine (93.5% Val) | 0.02 | 0.03 | 0.71 | 0.71 |
| Choline chloride (60% Chol) | 0.60 | 0.60 | 0.60 | 0.60 |
| Vitamin-mineral premix2 | 2.50 | 2.50 | 2.50 | 2.50 |
| Mycotoxin absorbent3 | 2.50 | 2.50 | 2.50 | 2.50 |
| Inert4 | 1.00 | 1.00 | 1.00 | 1.00 |
| Calculated composition | ||||
| Metabolizable energy (kcal/kg) | 2,983 | 2,833 | 3,125 | 2,975 |
| Crude protein | 220.0 | 220.0 | 187.5 | 187.5 |
| Calcium | 8.53 | 8.53 | 6.85 | 6.85 |
| Available phosphorus | 4.18 | 4.18 | 3.20 | 3.20 |
| Dig Lysine | 12.19 | 12.19 | 10.44 | 10.44 |
| Dig Methionine + Cystine | 8.79 | 8.79 | 7.62 | 7.62 |
| Dig Threonine | 7.93 | 7.93 | 6.78 | 6.78 |
| Dig Tryptophan | 2.46 | 2.46 | 1.88 | 1.88 |
| Dig Valine | 9.39 | 9.39 | 8.14 | 8.14 |
| Chloride | 3.40 | 3.40 | 3.21 | 3.21 |
| Sodium | 2.10 | 2.10 | 1.97 | 1.97 |
| Potassium | 8.54 | 8.54 | 7.60 | 7.60 |
PC, positive control (meeting the nutritional requirements of broilers without the enzyme inclusion); NC, negative control (reduction of 100 kcal/kg).
Vitamins and minerals provided per kg of feed: vitamin A, 20,000 UI; vitamin D3 7,500 UI; vitamin E 50 mg; vitamin K3 5.25 mg; vitamin B1 4.5 mg; vitamin B2 13.75 mg; vitamin B6 6.25 mg; vitamin B12 37.5 mg; pantothenic acid 27.5 mg; niacin 87.5 mg; folic acid 2.25 mg; biotin, 0.16 mg; selenium, 0.75 mg; copper, 20 mg; iron, 112.5 mg; manganese, 200 mg; cobalt, 5 mg; iodine, 3 mg; zinc, 275 mg.
Bentonite and activated carbon.
The different enzyme combinations were added in replacement for inert (Kaolin) amylase (RONOZYME HiStarch, 80 KNU/kg); amylase + xylanase (RONOZYME WX, 100 FXU/kg); and amylase + xylanase + protease (RONOZYME ProAct, 15,000 PROT/kg).
The experiment consisted of a completely randomized design in a 2 × 5 factorial scheme (2 drying temperatures [80°C and 110°C] and 5 diets). The diets consisted of positive control (PC–meeting the nutritional requirements of broilers for each phase and without enzyme inclusion), a negative control (NC–reduction of 100 kcal/kg of apparent metabolizable energy and without enzyme inclusion), and 3 enzyme combinations added to the NC: amylase (RONOZYME HiStarch, 80 KNU/kg) (DSM Nutritional Products, São Paulo, Brazil), amylase + xylanase (RONOZYME WX, 100 FXU/kg) (DSM Nutritional Products, São Paulo, Brazil) and amylase + xylanase + protease (RONOZYME ProAct, 15,000 PROT/kg) (DSM Nutritional Products, São Paulo, Brazil).
Exogenous Enzymes
The RONOZYME HiStarch is produced from the submerged fermentation of Bacillus licheniformis containing 600 KNU−1. One KNU is the amount of enzyme that releases, from a two-step reaction, 6 mol of nitrophenol per min from 1.86 mM of ethylidene-G7-p-nitrophenyl-maltoheptaose at pH 7.0 and 37°C. The RONOZYME WX is an endo-1,4-beta-xylanase produced from a genetically modified strain of Aspergillus oryzae. The enzyme activity of xylanase in units of xylanase (FXU) is defined as the amount of enzyme that releases 7.8 μmol of reducing sugars (xylose equivalents) of azo wheat arabinoxylan per min at pH 6.0 and 50°C. The RONOZYME ProAct is manufactured from the fermentation of B. licheniformis containing Nocardiopsis prasina with a transcribed gene, being considered a monocomponent protease with 75,000 unit of protease−1. The enzyme activity of protease in protease unit (PROT) is defined as the amount of enzyme needed to degrade 1 μmol of p-nitroanilide of substrate 1 μmol (Suc-Ala-Ala-Pro-Fen-N-succinyl Ala-Ala-Pro-Fen-p-nitroanilide) per minute at pH 9.0 and 37°C.
Drying Process of Corn Grain
The corn grains were harvested with 23% moisture and dried at 80°C or 110°C using a mixed-flow grain dryer. The drying process was concluded when the moisture reached 14% with the water content of the grain being determined throughout the duration of the process using an electronic meter, model 919FOB (MOTOMCO, Porto Alegre, Brazil). The average time of the drying process was 4 h when the grains were dried at 110°C and 6 h at 80°C.
Sample Collection and Analysis Performed
The birds and feed were weighed on day 7, 21, and 42 to determine body weight gain (BWG), feed intake, and feed conversion ratio (FCR) for each treatment. Mortality was recorded daily to correct the productive performance according to Sakomura and Rostagno (2016). On day 21, 2 birds within the average weight (±5%) per experimental unit were euthanized, and the ceca was taken for microbiota analysis. The cecal content was squeezed into a 50-mL tube and immediately frozen at −20°C for further processing. Carcass yield was evaluated on day 42.
Cecal Microbiota
The cecal microbiota was analyzed taking into consideration the dietary treatments only, regardless of the corn-drying temperature. Therefore, each sample used for microbiota analysis consisted of a pool of 4 birds (2 samples from birds fed diets containing corn dried at 80°C and 2 samples from birds fed diets containing corn dried at 110°C) and 6 replicates/treatment. The cecal content was sent to Neoprospecta Microbiome Technologies where the bacterial DNA was isolated and submitted to high-throughput sequencing of the 16S rRNA V3/V4 region with a proprietary protocol (Neoprospecta Microbiome Technologies, Brazil). The amplification of the 16S rRNA V3/V4 region was carried out using the 341F (CCTACGGGRSGCAGCAG) (Wang and Qian, 2009) and 806R (GGACTACHVGGGTWTCTAAT) (Caporaso et al., 2012) primers. The 16S rRNA libraries were sequenced using the MiSeq Sequencing System (Illumina Inc., Foster City, CA) using the V2 kit with 300 cycles and single-end sequencing.
For the bioinformatic analysis, the sequences were processed and analyzed using a Quantitative Insights Into Microbial Ecology 2 (Bolyen et al., 2019) v 2019.7 pipeline. The raw sequences were uploaded to the NCBI Sequence Read Archive under the project number PRJNA613081. Briefly, the sequences were demultiplexed and the Amplicon Sequence Variant table created using DADA2 (Callahan et al., 2016). Before downstream analysis, sequences assigned as chloroplast, mitochondria, and low-abundance Amplicon Sequence Variants, containing less than 0.01% of the total reads in the data set, were removed. All samples were rarefied to even sequencing depth based on the lowest read depth of samples to 34,136 sequences per sample.
Alpha diversity was measured with the Chao1 (richness) and Shannon diversity indices. Beta diversity was evaluated with the weighted phylogeny-based UniFrac (Lozupone and Knight, 2005) distance metric and visualized using a Principal Coordinate Analysis plot. The R-value generated by the Analysis of Similarity (ANOSIM) was used to indicate the similarity of comparison between group pairs. The value of R ranges from −1 to +1: The pairs are more similar when the R index is closer to 0, and the pairs are different from each other when the R index is close to 1. To estimate the metabolic pathways affected by enzyme supplementation, Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) (Douglas et al., 2019) was calculated using the default PICRUSt2 pipeline.
Carcass Yield
Two additional birds per experimental unit within ±5% of average weight were selected, individually weighed, and slaughtered by electronarcosis followed by exsanguination to determined carcass and cuts yields. After the removal of the head, feet, neck, and abdominal fat, the carcass was weighed, and the carcass yield calculated as the proportion of the carcass to the live weight before slaughter. The cuts yield (breast, wings, and legs) was calculated as the proportion of the weight of the eviscerated carcass. The percentage of abdominal fat was determined using the weight of the fat present around cloaca, gizzard, proventriculus, and adjacent abdominal muscles in relation to the weight of the live bird.
Statistical Analysis
All data sets were tested for normality using the Shapiro-Wilk test. The performance and carcass data were subjected to 2-way ANOVA, which assessed individual effects of diets, drying temperature, and their interaction. When a main effect of diet was observed, the means were compared using Tukey's test. The SAS University Edition statistical software (2017) (SAS Inst., Inc., Cary, NC) was used, considering P ≤ 0.05. ANOSIM test within the PRIMER 7 software package (PRIMER-E Ltd., Luton, UK) was used to analyze significant differences in microbial communities between groups. The Kruskal-Wallis test was performed (JMP Pro 11, SAS software Inc. or Prism v.8.2.1; Graphpad Software Inc.) and adjusted for multiple comparisons using Benjamini and Hochberg's False Discovery Rate (Benjamini and Hochberg, 1995) at each taxonomic level, and a P-value of ≤0.05 was considered statistically significant. Post hoc Dunn's multiple comparison test was used to determine the group differences in bacterial taxa.
Results
Growth Performance and Carcass Yield
According to the growth performance results, there was no interaction (P > 0.05) between diet and drying temperature in any of the periods evaluated (Table 2). From 1 to 7 d of age, a main effect of drying temperature was observed, wherein birds fed diets containing corn dried at 80°C showed better FCR (P = 0.045) than the birds fed diets formulated with corn dried at 110°C. There was a main effect of diet on BWG and FCR from 1 to 21 d (P = 0.01 and P = 0.001, respectively) and from 1 to 42 d of age (P = 0.013 and P = 0.007, respectively). In both phases, worse BWG and FCR were observed in birds fed the NC (nutrition-reduced) diet compared with the birds fed the PC diet. In addition, the inclusion of amylase to the NC diet, alone or in combination with xylanase and protease, improved the BWG to the same level as the PC group in both phases. From 1 to 21 d, the FCR of the NC was similar to that of the PC group only when the 3 enzymes were included. However, from 1 to 42 d of age, enzyme supplementation, regardless of the association, improved the FCR of the NC similar to that of the PC group (Table 2).
Table 2.
Performance of broilers fed diets containing corn dried at 2 temperatures and different enzymatic association.
| Treatments | Day 1–7 |
Day 1–21 |
Day 1-42 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| BWG (g) | FI (g) | FCR | BWG (g) | FI (g) | FCR | BWG (g) | FI (g) | FCR | |
| Positive control (PC)1 | 118.1 | 142.1 | 1.201 | 813.8a | 1,089.8 | 1.342b | 2,886.2a | 4,492.5 | 1.556b |
| Negative control (NC)1 | 115.2 | 143.1 | 1.241 | 753.1b | 1,080.3 | 1.436a | 2,742.8b | 4,471.0 | 1.631a |
| NC + Amylase2 | 114.8 | 143.4 | 1.249 | 766.3a,b | 1,076.9 | 1.407a | 2,803.5a,b | 4,403.0 | 1.571b |
| NC + Amylase + Xylanase2 | 117.2 | 148.2 | 1.265 | 769.8a,b | 1,083.1 | 1.408a | 2,825.6a,b | 4,421.6 | 1.565b |
| NC + Amylase + Xylanase + Protease2 | 116.8 | 144.4 | 1.234 | 776.2a,b | 1,070.9 | 1.380a,b | 2,787.2a,b | 4,423.2 | 1.588a,b |
| Average of treatments with enzymes | 116.3 | 145.3 | 1.249 | 770.8 | 1,076.9 | 1.398 | 2,805.4 | 4,415.9 | 1.575 |
| Temperature (°C) | |||||||||
| 80 | 116.0 | 141.0 | 1.215B | 772.6 | 1,074.0 | 1.391 | 2805.6 | 4446.4 | 1.586 |
| 110 | 116.8 | 147.5 | 1.261A | 779.1 | 1,086.4 | 1.398 | 2812.6 | 4438.0 | 1.579 |
| SEM | 0.814 | 1.742 | 0.011 | 5.644 | 5.728 | 0.008 | 13.281 | 20.595 | 0.007 |
| P value | |||||||||
| Treatment | 0.718 | 0.841 | 0.473 | 0.010 | 0.888 | 0.001 | 0.013 | 0.648 | 0.007 |
| Temperature | 0.655 | 0.074 | 0.045 | 0.547 | 0.302 | 0.625 | 0.782 | 0.844 | 0.608 |
| Treatment x Temperature | 0.852 | 0.805 | 0.345 | 0.863 | 0.653 | 0.332 | 0.801 | 0.678 | 0.972 |
Means followed by distinct small and capital letters in the same column are different (P ≤ 0.05) by Tukey's and F's test, respectively.
Abbreviations: BWG, body weight gain; FCR, feed conversion ratio; FI, feed intake.
PC, positive control (meeting the nutritional requirements of broilers without the enzyme inclusion); NC, negative control (reduction of 100 kcal/kg).
Amylase (RONOZYME HiStarch, 80 KNU/kg), xylanase (RONOZYME WX, 100 FXU/kg), and protease (RONOZYME ProAct, 15,000 PROT/kg).
There was no interaction or main effect (P > 0.05) of diet or drying temperature on the carcass and cut (breast, legs, and wings) yield and abdominal fat percentage. The mean averages for carcass, breast, leg, and wing yield were 69.5, 41.4, 21.2, and 10.2%, respectively, and the mean abdominal fat was 1.7% (data not shown).
Cecal Microbiota
Microbial Diversity
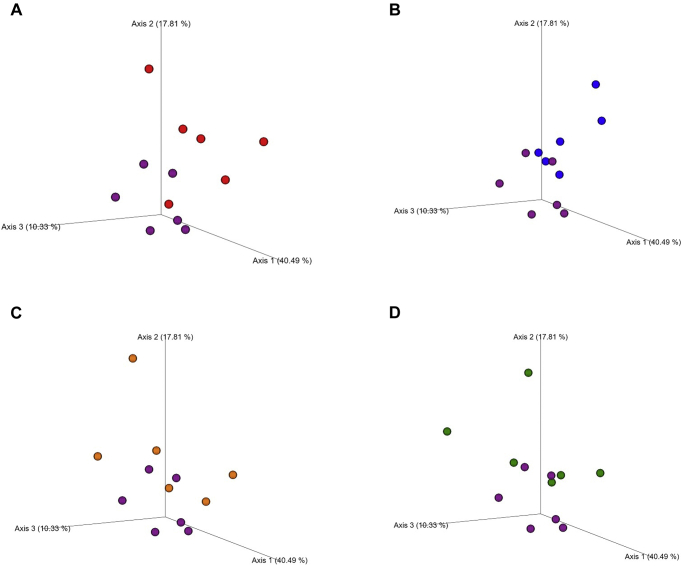
The bacterial alpha diversity as estimated by the Shannon index and Chao index was similar among the groups (P = 0.505 and P = 0.599, respectively) (Table 3). According to the beta diversity and considering the weighted UniFrac explanation (which considers the phylogenetic distances between bacteria present in the sample in addition to their abundance), there was no difference (P = 0.08; R = 0.092) regarding separation of the bacterial communities considering all the dietary groups. However, the ANOSIM pairwise analysis showed a difference between the bacterial communities when comparing the PC vs. NC (P = 0.041; R = 0.241); PC vs. NC + amylase (P = 0.035; R = 0.354), and PC vs. NC + amylase + xylanase (P = 0.056; R = 0.185) groups. The ANOSIM analysis showed that there was no difference in the bacterial community when comparing the PC vs. NC + amylase + xylanase + protease (P = 0.180; R = 0.074), suggesting that the supplementation of the 3 enzymes normalized the cecal microbiota community (Table 4; Figure 1).
Table 3.
Median of microbial diversity (Shannon index) and richness estimator (Chao1 index) calculated based on an equal number of sequences (34,136 sequences/sample) of cecal samples of broiler chickens fed diets with different enzymatic associations.
| Treatments | Shannon index | Chao1 index |
|---|---|---|
| Positive control (PC)1 | 7.05 | 256.5 |
| Negative control (NC)1 | 6.36 | 199.0 |
| NC + Amylase2 | 7.04 | 261.5 |
| NC + Amylase + Xylanase2 | 6.62 | 226.0 |
| NC + Amylase + Xylanase + Protease2 | 6.29 | 221.5 |
| P value | 0.505 | 0.599 |
PC, positive control (meeting the nutritional requirements of broilers without the enzyme inclusion); NC, negative control (reduction of 100 kcal/kg).
Amylase (RONOZYME HiStarch, 80 KNU/kg), xylanase (RONOZYME WX, 100 FXU/kg), and protease (RONOZYME ProAct, 15,000 PROT/kg).
Table 4.
Global test and pairwise tests in beta diversity analysis (weighted Unifrac) of cecal samples of broiler chickens fed diets with different enzymatic associations.
| Item | R significance1 | P value |
|---|---|---|
| Global test | 0.092 | 0.080 |
| Pairwise test | ||
| Positive control (PC) vs. negative control (NC)2 | 0.241 | 0.041 |
| PC vs. NC + Amylase3 | 0.354 | 0.035 |
| PC vs. NC + Amylase + Xylanase3 | 0.185 | 0.056 |
| PC vs. NC + Amylase + Xylanase + Protease3 | 0.074 | 0.180 |
R value ranges from -1 to 1: the pairs are more similar when the R index is closer to 0 and the pairs are different from each other when the R index is close to 1.
PC, positive control (meeting the nutritional requirements of broilers without the enzyme inclusion); NC, negative control (reduction of 100 kcal/kg).
Amylase (RONOZYME HiStarch, 80 KNU/kg), xylanase (RONOZYME WX, 100 FXU/kg), and protease (RONOZYME ProAct, 15,000 PROT/kg).
Figure 1.
Beta-diversity of the microbial communities in the ceca of broilers at 21 d of age based on weighted UniFrac distances (Each spot represents one sample, and each sample is a pool of cecal content from 4 birds.). (A) Positive control (purple spots) vs. negative control (red spots); (B) positive control (purple spots) vs. negative control + amylase (blue spots); (C) positive control (purple spots) vs. negative control + amylase + xylanase (orange spots); (D) positive control (purple spots) vs. negative control + amylase + xylanase + protease (green spots).
Microbial composition
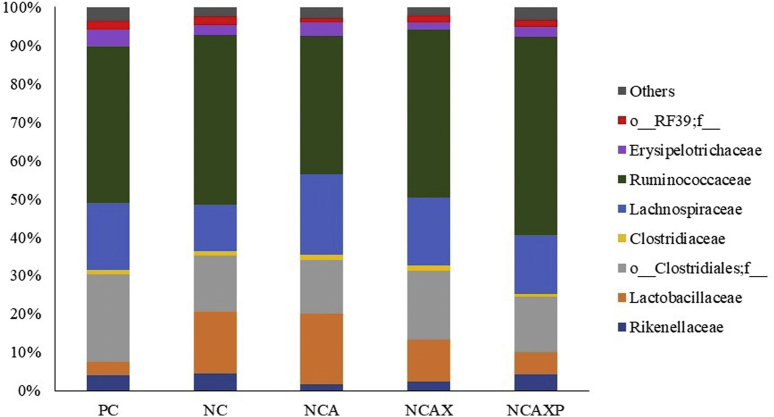
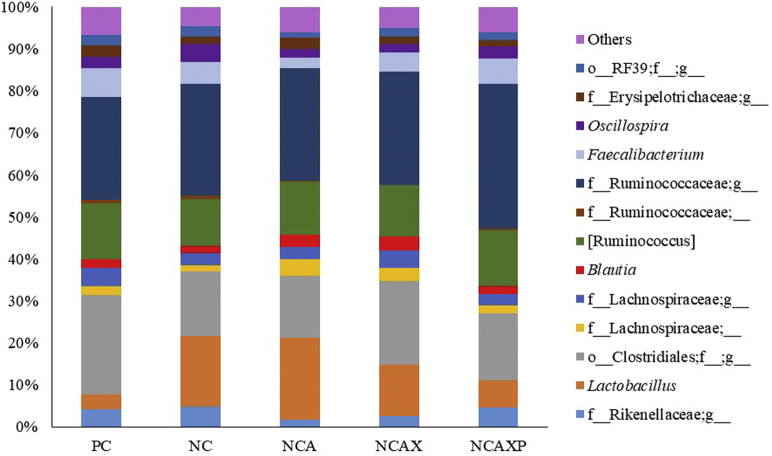
Firmicutes was the most abundant phylum (93.3%), followed by Bacteroidetes (3.9%), with no statistical difference between groups. The relative abundance of the main classes and orders observed was also not different between groups (data not shown). Numerically, the most abundant families were Ruminococcaceae (40.8%), an unidentified family within the order Clostridiales (o__Clostridiales;f__) (15.9%) and Lachnospiraceae (15.8%; Figure 2). The microbiota of the birds fed the NC diet supplemented with amylase or amylase + xylanase showed an increase (P = 0.002) in the frequency of f__Lachnospiraceae;__ when compared with that of the birds fed the NC diet without enzyme supplementation (3.59% for the NC + amylase group, 2.68% for the NC + amylase + xylanase group, and 1.29% for the NC group). In addition, there was a reduction (P = 0.019) in the frequency of the genus f__Ruminococcaceae;__ in birds fed the NC + amylase + xylanase vs. the PC group (Figure 3).
Figure 2.
Predominance of bacterial families present in the cecal microbiota of broilers at 21 d of age fed diets containing different enzymatic association. Abbreviations: NC, negative control; NCA, negative control + amylase; NCAX, negative control + amylase + xylanase; NCAXP, negative control + amylase + xylanase + protease; PC, positive control.
Figure 3.
Predominance of bacterial genera present in the cecal microbiota of broilers at 21 d of age fed diets containing different enzymatic association. The nonitalicized groups represent bacteria that were identified to order or family level. Abbreviations: NC, negative control; NCA, negative control + amylase; NCAX, negative control + amylase + xylanase; NCAXP, negative control + amylase + xylanase + protease; PC, positive control.
Predicted function
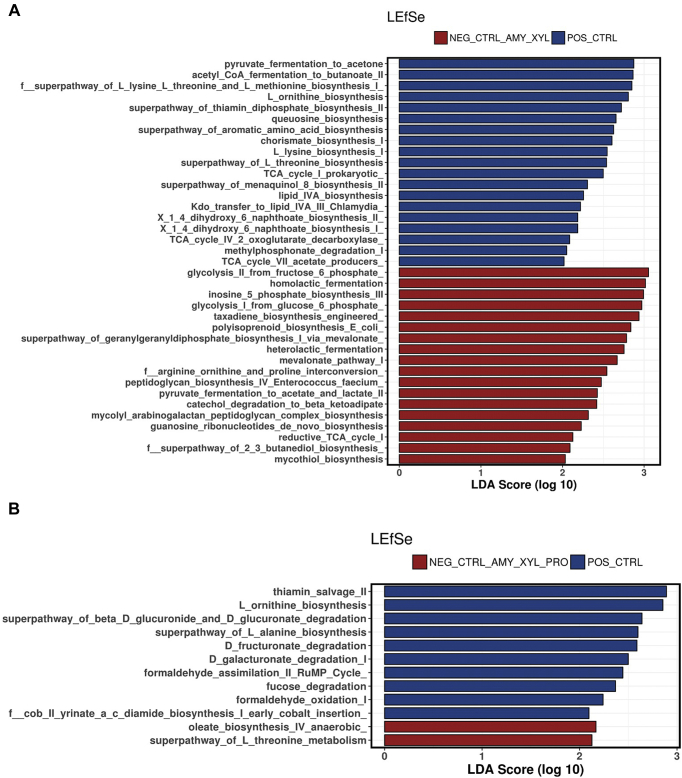
To analyze the differences in the predicted functions of the microbiota, the results of the PICRUSt analysis were compared between groups using the Linear discriminant analysis Effect Size. A total of 58 different metabolic pathways were different when comparing the PC vs. NC groups, where 21 were upregulated in the NC group, and 63 metabolic functions were changed when comparing the PC vs. NC + amylase of which 23 were upregulated in the NC + amylase group (Supplementary Data). However, a more similar microbiota in terms of predicted functions was observed when the NC group was supplemented with amylase + xylanase or amylase + xylanase + protease. A total of 37 and 12 metabolic pathways were changed when comparing the PC vs. NC + amylase + xylanase or NC + amylase + xylanase + protease, respectively (Figures 4A and 4B). These results show that the inclusion of the 3 enzymes in the NC diet reduced the variations in the predicted functions and produced a microbiota more similar to that of the PC group.
Figure 4.
Predicted functions of the cecal microbiota of broiler at 21 d of age identified by linear discriminant analysis coupled with effect size (LEfSe) using default parameters. (A) Positive control (blue) and negative control + amylase + xylanase (red); (B) positive control (blue) and negative control + amylase + xylanase + protease (red).
Discussion
The temperature used in the drying process of corn is of paramount importance because of its influence on the nutritional quality of the grain. High drying temperatures can lead to structural changes in the protein and starch granules making them inaccessible to the endogenous digestive enzymes of animals, with reduced digestibility of these nutrients (Odjo et al., 2017; Huart et al., 2018). The worse FCR observed in the present study in chickens from 1 to 7 d fed diets formulated with corn dried at 110°C may indicate a lower digestibility of nutrients during the prestarter phase. Although there is evidence that the drying temperature of 100°C causes changes in the quality of the grain (Bhuiyan et al., 2010), the temperature used in the present study may not have been high enough to affect the quality of the corn sufficiently to impair the performance of the animals in later phases. On the other hand, the synergistic effect from 1 to 21 d of the combination of the 3 enzymes evaluated (amylase, xylanase, and protease) was evident. The addition of the 3 enzymes to the NC diet improved the FCR by 3.9% compared with that of the nonsupplemented group of birds, this being equivalent to that of the PC group. Considering the entire experimental period (1–42 d), the benefits of enzymatic supplementation were observed either with amylase alone or in association with xylanase and protease, which restored the performance of the birds fed the NC diets.
The benefits of enzymatic supplementation are more evident during the early stages of the life of chickens because of different physiological needs throughout the life of the birds (Olukosi et al., 2007). Young broiler chickens (up to 21 d) are known to have a lower digestive enzyme activity (Uni et al., 1998). Zou et al. (2013) observed a greater effect of exogenous enzymes (β-mannanase, α-galactosidase, and xylanase + β-glucanase) on the activity of endogenous enzymes (trypsin and chymotrypsin) in 21- vs. 42-day-old broilers. Therefore, the inclusion of exogenous enzymes to the diets during the initial period may compensate for the lower secretion of endogenous enzymes, favoring the digestive process, increasing the availability of nutrients, and enhancing the growth performance of the animals.
Although it remains unclear how a complex enzyme combination improves chicken growth, several mechanisms have been proposed. Besides the physiological limitation due to the low production of α-amylase (Noy and Sklan, 1995) in young chickens, starch cannot be completely digested along the gastrointestinal tract (Cowieson et al., 2010) because of the high feed intake observed in modern broilers, which increases the feed passage rate and reduces the time available for the hydrolysis of starch (Weurding et al., 2001; Svihus, 2014). Therefore, the dietary inclusion of α-amylase increases the digestion of starch (Stefanello et al., 2015) and the expression of glucose transporter genes in the intestine (Yuan et al., 2017), showing the capacity of this enzyme in improving starch digestion and glucose absorption. Xylanase hydrolyzes the cellular wall of plants, reduces the antinutritional effects of nonstarch polysaccharides (molecules that are not digested by the normal digestive processes of nonruminant animals), and favors the action of other enzymes, such as amylase and protease. The hydrolysis of the cell wall enhances the accessibility of endogenous enzymes to the intracellular content of the ingredients used in feed formulation (Oryschak et al., 2002).
The influence of exogenous protease on the digestibility of dietary protein is well known (Ding et al., 2016; Stefanello et al., 2016); however, the “extra-proteinaceous” effects of proteases is of interest to the poultry industry (Cowieson and Roos, 2016). Among the potential effects, its indirect action on the usage of starch and fat has to be emphasized. Cowieson et al. (2019) reported the beneficial effects of exogenous protease beyond improvements in ileal amino acid digestibility. According to these authors, the addition of protease to a diet with reduced crude protein and digestible amino acid concentration resulted in a better ileal digestibility of starch (3.8%) and increased jejunal expression of starch digestion–related genes (sucrase-isomaltase). Kalmendal and Tauson (2012) observed enhanced ileal digestibility of fat (2.08%) with the use of exogenous protease. Therefore, the indirect effect of protease on the protein and starch present in the ingredients and the fact that the hydrolysis of the protein facilitates the formation of micelles result in an increase in the digestibility of starch and fat and consequently more energy available for growth (Fru-Nji et al., 2011; Cowieson et al., 2019).
The combination of amylase, xylanase, and protease has been shown to increase the digestibility of nutrients and improve the performance of animals (Amerah et al., 2017; Singh et al., 2019). Cowieson and Ravindran (2008) observed that the addition of these 3 enzymes to diets based on corn and soybean meal improved the metabolizable energy and nitrogen retention by 3 and 11.7%, respectively. However, as shown in the present study, the beneficial use of this enzymatic complex was also due to modulation of the intestinal microbiota.
The reduction in energy of 100 kcal/kg reduced the performance of the birds and changed the cecal microbiota compared with the birds fed the control diet. Metabolic studies related to obesity have reported that caloric restriction in mice leads to changes in the intestinal microbiota that have an impact on the metabolism and nutritional status of the host and is used as a strategy to mitigate obesity (Zheng et al., 2018; Zeb et al., 2020). Indeed, the usage of nutrients and the extraction of energy from the diet proceed by the integration of biochemical processes and the intestinal microbiota (Stanley et al., 2014). In the present study, the diversity, composition, and predicted function of the cecal microbiota at 21 d of age are consistent with the FCR of the birds in this period. The supplementation of the energy-reduced diet with amylase, xylanase, and protease reduced the variation in the cecal microbiota, modulating its diversity and predicted metabolic functions similar to those of the group of birds fed the PC diet. Therefore, it is possible to assume that the 3 enzymes acted on their specific substrates in the superior gastrointestinal tract, improved the use of nutrients, changed the nutritional composition of the cecal content, and favored the development of a beneficial microbiota. Changes in the cecal microbiota can affect the microbial population dynamics in the gut and thereby influence functions (Yin et al., 2018). Torok et al. (2011) reported that favoring the proliferation of the commensal microbiota and suppressing pathogenic bacteria are partially responsible for the positive effects of exogenous enzymes. The evidence from this study can be useful in understanding how the manipulation of the gut microbiota by the dietary inclusion of a combination of exogenous enzymes can led to better growth performance.
The intestinal microbiota is involved in important mechanisms, such as maintenance of overall intestinal health, modulation of the immune system, exclusion of pathogenic microorganisms (Clavijo and Flórez, 2018; Willson et al., 2018; Kogut, 2019), and modulation of metabolic processes to improve the usage of nutrients in the diet (Greiner and Bäckhed, 2011; Stanley et al., 2012; Rubio et al., 2015). The products released in the intestine by the action of dietary enzymes such as xylanase may present prebiotic effects (Ravn et al., 2017), which can be used by beneficial bacteria in the lower intestine (Lee et al., 2017). In addition, dietary amylase and protease can increase the digestibility of starch and protein and reduce the amounts of these undigestible components that reach the ceca, which could otherwise be used by pathogenic bacteria (Flores et al., 2016). Nevertheless, the formation of metabolites that could negatively affect the health of the animals is minimized (Apajalahti and Vienola, 2016; Cowieson and Roos, 2016). According to Kogut (2019), a stable and healthy microbiota increases the function of the intestinal barrier and discourages the colonization of unfavorable microbial communities, allowing for better broiler performance.
In summary, drying corn at 110°C negatively influenced the growth performance of broiler chickens during the prestarter phase. Dietary inclusion of amylase, xylanase, and protease counteracted the loss in performance of broilers fed energy-reduced diets and promoted a microbiota with composition and functions similar to those of birds fed the nonrestricted energy diet. Therefore, the modulation of the microbiota may explain, in part, the mechanism of action by which exogenous enzymes improve the growth performance of animals. Even though it was not evaluated herein, the effect of the enzymes on the intestinal microbiota may also be dependent on the age of the birds.
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq–Brasília, DF, Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES–Brasília, DF, Brazil), and DSM Nutritional Products (São Paulo, SP, Brazil).
Disclosures
L.V.T. is employed by DSM Nutritional Products who manufacture the enzymes used in this study. All other authors have no conflict of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.psj.2021.01.035.
Supplementary data
References
- Abd El-Hack M.E., Alagawany M., Arif M., Emam M., Saeed M., Arain M.A., Siyal F.A., Patra A., Elnesr S.S., Khan R.U. The uses of microbial phytase as a feed additive in poultry nutrition-a review. Ann. Anim. Sci. 2018;18:639–658. [Google Scholar]
- Alabi O.O., Shoyombo A.J., Akpor O.B., Oluba O.M., Adeyonu A.G. Exogenous enzymes and the digestibility of nutrients by broilers: a mini review. Int. J. Poult. Sci. 2019;18:404–409. [Google Scholar]
- Alagawany M., Elnesr S.S., Farag M.R. The role of exogenous enzymes in promoting growth and improving nutrient digestibility in poultry. Iran. J. Vet. Res. 2018;19:157–184. [PMC free article] [PubMed] [Google Scholar]
- Amerah A.M., Romero L.F., Awati A., Ravindran V. Effect of exogenous xylanase, amylase, and protease as single or combined activities on nutrient digestibility and growth performance of broilers fed corn/soy diets. Poult. Sci. 2017;96:807–816. doi: 10.3382/ps/pew297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apajalahti J., Vienola K. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed Sci. Technol. 2016;221:323–330. [Google Scholar]
- Bedford M.R., Cowieson A.J. Exogenous enzymes and their effects on intestinal microbiology. Anim. Feed Sci. Technol. 2012;173:76–85. [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., Bai Y., Bisanz J.E., Bittinger K., Brejnrod A., Brislawn C.J., Brown C.T., Callahan B.J., Caraballo-Rodríguez A.M., Chase J., Cope E.K., Da Silva R., Diener C., Dorrestein P.C., Douglas G.M., Durall D.M., Duvallet C., Edwardson C.F., Ernst M., Estaki M., Fouquier J., Gauglitz J.M., Gibbons S.M., Gibson D.L., Gonzalez A., Gorlick K., Guo J., Hillmann B., Holmes S., Holste H., Huttenhower C., Huttley G.A., Janssen S., Jarmusch A.K., Jiang L., Kaehler B.D., Kang K.B., Keefe C.R., Keim P., Kelley S.T., Knights D., Koester I., Kosciolek T., Kreps J., Langille M.G.I., Lee J., Ley R., Liu Y.X., Loftfield E., Lozupone C., Maher M., Marotz C., Martin B.D., McDonald D., McIver L.J., Melnik A.V., Metcalf J.L., Morgan S.C., Morton J.T., Naimey A.T., Navas-Molina J.A., Nothias L.F., Orchanian S.B., Pearson T., Peoples S.L., Petras D., Preuss M.L., Pruesse E., Rasmussen L.B., Rivers A., 2nd Robeson M.S., Rosenthal P., Segata N., Shaffer M., Shiffer A., Sinha R., Song S.J., Spear J.R., Swafford A.D., Thompson L.R., Torres P.J., Trinh P., Tripathi A., Turnbaugh P.J., Ul-Hasan S., van der Hooft J.J.J., Vargas F., Vázquez-Baez Y., Vogtmann E., von Hippel M., Walters W., Wan Y., Wang M., Warren J., Weber K.C., Williamson C.H.D., Willis A.D., Xu Z.Z., Zaneveld J.R., Zhang Y., Zhu Q., Knight R., Caporaso J.G. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan M.M., Islam A.F., Iji P.A. Variation in nutrient composition and structure of high-moisture maize dried at different temperatures. S. Afr. J. Anim. Sci. 2010;40:190–197. [Google Scholar]
- Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Huntley J., Fierer N., Owens S.M., Betley J., Fraser L., Bauer M., Gormley N., Gilbert J.A., Smith G., Knight R. Ultra high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo V., Flórez M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 2018;97:1006–1021. doi: 10.3382/ps/pex359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowieson A.J. Factors that affect the nutritional value of maize for broilers. Anim. Feed Sci. Technol. 2005;119:293–305. [Google Scholar]
- Cowieson A.J., Bedford M.R., Ravindran V. Interactions between xylanase and glucanase in maize-soy-based diets for broilers. Br. Poult. Sci. 2010;51:246–257. doi: 10.1080/00071661003789347. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Kluenter A.M. Contribution of exogenous enzymes to potentiate the removal of antibiotic growth promoters in poultry production. Anim. Feed Sci. Technol. 2019;250:81–92. [Google Scholar]
- Cowieson A.J., Ravindran V. Effect of exogenous enzymes in maize-based diets varying in nutrient density for young broilers: growth performance and digestibility of energy, minerals and amino acids. Br. Poult. Sci. 2008;49:37–44. doi: 10.1080/00071660701812989. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Roos F.F. Toward optimal value creation through the application of exogenous mono-component protease in the diets of non-ruminants. Anim. Feed Sci. Technol. 2016;221:331–340. [Google Scholar]
- Cowieson A.J., Toghyani M., Kheravii S.K., Wu S.–B., Romero F., Choct M. A mono-component microbial protease improves performance, net energy, and digestibility of amino acids and starch, and upregulates jejunal expression of genes responsible for peptide transport in broilers fed corn/wheat-based diets supplemented with xylanase and phytase. Poult. Sci. 2019;98:1321–1332. doi: 10.3382/ps/pey456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X.M., Li D.D., Li Z.R., Wang J.P., Zeng Q.F., Bai S.P., Su Z.W., Zhang K.Y. Effects of dietary crude protein levels and exogenous protease on performance, nutrient digestibility, trypsin activity and intestinal morphology in broilers. Livest. Sci. 2016;193:26–31. [Google Scholar]
- Douglas G. M. Maffei V. J. Zaneveld J. Yurgel S. N. Brown J. R. Taylor C. M. Huttenhower C. Langille M. G. I. PICRUSt2: an improved and extensible approach for metagenome inference. 10.1101/672295, accessed Mar. 15, 2021 [DOI]
- Flores C., Williams M., Pieniazek J., Dersjant-Li Y., Awati A., Lee J.T. Direct-fed microbial and its combination with xylanase, amylase, and protease enzymes in comparison with AGPs on broiler growth performance and foot-pad lesion development. J. Appl. Poult. Res. 2016;25:328–337. [Google Scholar]
- Fru-Nji F., Kluenter A.–M., Fischer M., Pontoppidan K. A feed serine protease improves broiler performance and increases protein and energy digestibility. J. Poult. Sci. 2011;48:239–246. [Google Scholar]
- Greiner T., Bäckhed F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol. Metab. 2011;22:117–123. doi: 10.1016/j.tem.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Huart F., Malumba P., Odo S., Al-Izzi W., Béra F., Beckers Y. In vitro and in vivo assessment of the effect of initial moisture content and drying temperature on the feeding value of maize grain. Br. Poult. Sci. 2018;59:452–462. doi: 10.1080/00071668.2018.1477253. [DOI] [PubMed] [Google Scholar]
- Huyghebaert G., Ducatelle R., Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Kalmendal R., Tauson R. Effects of a xylanase and protease, individually or in combination, and an ionophore coccidiostat on performance, nutrient utilization, and intestinal morphology in broiler chickens fed a wheat-soybean meal-based diet. Poult. Sci. 2012;91:1387–1393. doi: 10.3382/ps.2011-02064. [DOI] [PubMed] [Google Scholar]
- Kiarie E.G., Leung H., Kakhki R.A.M., Patterson R., Barta J.R. Utility of feed enzymes and yeast derivatives in ameliorating deleterious effects of coccidiosis on intestinal health and function in broiler chickens. Front. Vet. Sci. 2019;6:1–13. doi: 10.3389/fvets.2019.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K.E.B. Fiber and nonstarch polyssacharide content and variation in common crops used in broiler diets. Poult. Sci. 2014;93:2380–2393. doi: 10.3382/ps.2014-03902. [DOI] [PubMed] [Google Scholar]
- Kogut M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Sci. Technol. 2019;250:32–40. [Google Scholar]
- Lee S.A., Apajalahti J., Vienola K., González-Ortiz G., Fontes C.M.G.A., Bedford M.R. Age and dietary xylanase supplementation affects ileal sugar residues and short chain fatty acid concentration in the ileum and caecum of broiler chickens. Anim. Feed Sci. Technol. 2017;234:29–42. [Google Scholar]
- Lozupone C., Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumba P., Vanderghem C., Deroanne C., Béra F. Influence of drying temperature on the solubility, the purity of isolates and the electrophoretic patterns of corn proteins. Food Chem. 2008;111:564–572. [Google Scholar]
- Noy Y., Sklan D. Digestion and absorption in the young chick. Poult. Sci. 1995;74:366–373. doi: 10.3382/ps.0740366. [DOI] [PubMed] [Google Scholar]
- Odjo S., Huart F., Berá F., Jacquet N., Richel A., Blecker C., Malumba P. Influence of corn variety, drying temperature, and moisture content at harvest on the saccharides released during an in vitro pepsin-pancreatin digestion. Starch/Stärke. 2017;69:1–5. [Google Scholar]
- Odjo S.D.P., Malumba P.K., Beckers Y., Béra F. Impact of drying and heat treatment on the feeding value of corn. Biotechnol. Agron. Soc. Environ. 2015;19:301–312. [Google Scholar]
- Olukosi O.A., Cowieson A.J., Adeola O. Age-related influence of a cocktail of xylanase, amylase, and protease or phytase individually or in combination in broilers. Poult. Sci. 2007;86:77–86. doi: 10.1093/ps/86.1.77. [DOI] [PubMed] [Google Scholar]
- Oryschak M.A., Simmins P.H., Zijlstra R.T. Effect of dietary particle size and carbohydrase and/or phytase supplementation on nitrogen and phosphorus excretion of grower pigs. Can. J. Anim. Sci. 2002;82:533–540. [Google Scholar]
- Ravn J.L., Thøgersen J.C., Eklöf J., Pettersson D., Ducatelle R., van Immerseel F., Pedersen N.R. GH11 xylanase increases prebiotic oligosaccharides from wheat bran favouring butyrate-producing bacteria in vitro. Anim. Feed Sci. Technol. 2017;226:113–123. [Google Scholar]
- Ravindran V. Feed enzymes: the science, practice, and metabolic realities. J. Appl. Poult. Res. 2013;22:628–636. [Google Scholar]
- Rinttilä T., Apajalahti J. Intestinal microbiota and metabolites – implications for broiler chicken health and performance. J. Appl. Poult. Res. 2013;22:647–658. [Google Scholar]
- Rychlik I. Composition and function of chicken gut microbiota. Animals. 2020;10:1–20. doi: 10.3390/ani10010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostagno H.S., Albino L.F.T., Donzele J.L., Gomes P.C., Oliveira R.F., Lopes D.C., Ferreira A.S., Barreto S.L.T., Euclides R.F. 3rd ed. UFV; Viçosa, MG, Brazil: 2011. Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais. [Google Scholar]
- Rubio L.A., Peinado M.J., Ruiz R., Suárez-Pereira E., Ortiz Mellet C.O., García Fernández J.M. Correlations between changes in intestinal microbiota composition and performance parameters in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2015;99:418–423. doi: 10.1111/jpn.12256. [DOI] [PubMed] [Google Scholar]
- Sakomura N.K., Rostagno H.S. 2nd ed. Jaboticabal; Brasil: Funep: 2016. Métodos de pesquisa em nutrição de monogástricos; p. 262. [Google Scholar]
- Saleh A., El-Far A.H., Abdel-Latif M.A., Emam M.A., Ghanem R., Abd El-Hamid H. Exogenous dietary enzyme formulations improve growth performance of broiler chickens fed a low-energy diet targeting the intestinal nutrient transporter genes. PLoS ONE. 2018;13:e0198085. doi: 10.1371/journal.pone.0198085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc . SAS Inst. Inc; Cary, NC: 2017. SAS User`s Guide: Statistics. Version 9.3 Edition. [Google Scholar]
- Singh A.K., Diaz Berrocoso J.F., Dersjant-Li Y., Awati A., Jha R. Effect of a combination of xylanase, amylase and protease on growth performance of broilers fed low and high fiber diets. Anim. Feed Sci. Technol. 2017;232:16–20. [Google Scholar]
- Singh A.K., Tiwari U.P., Berrocoso J.D., Dersjant-Li Y., Awati A., Jha R. Effects of a combination of xylanase, amylase and protease, and probiotics on major nutrients including amino acids and non-starch polysaccharides utilization in broilers fed different level of fibers. Poult. Sci. 2019;98:5571–5581. doi: 10.3382/ps/pez310. [DOI] [PubMed] [Google Scholar]
- Stanley D., Denman S.E., Hughes R.J., Geier M.S., Crowley T.M., Chen H., Haring V.R., Moore R.J. Intestinal microbiota associated with differential feed conversion efficiency in chickens. Appl. Microbiol. Biotechnol. 2012;96:1361–1369. doi: 10.1007/s00253-011-3847-5. [DOI] [PubMed] [Google Scholar]
- Stanley D., Hughes R.J., Moore R.J. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- Stefanello C., Vieira S.L., Rios H.V., Simões C.T., Sorbara J.O.B. Energy and nutrient utilisation of broilers fed soybean meal from two different Brazilian production areas with an exogenous protease. Anim. Feed Sci. Technol. 2016;221:267–273. [Google Scholar]
- Stefanello C., Vieira S.L., Santiago G.O., Kindlein L., Sorbara J.O.B., Cowieson A.J. Starch digestibility, energy utilization, and growth performance of broilers fed corn-soybean basal diets supplemented with enzymes. Poult. Sci. 2015;94:2472–2479. doi: 10.3382/ps/pev244. [DOI] [PubMed] [Google Scholar]
- Stefanello C., Vieira S.L., Soster P., Dos Santos B.M., Dalmoro Y.K., Favero A., Cowieson A.J. Utilization of corn-based diets supplemented with an exogenous α-amylase for broilers. Poult. Sci. 2019;98:5862–5869. doi: 10.3382/ps/pez290. [DOI] [PubMed] [Google Scholar]
- Svihus B. Starch digestion capacity of poultry. Poult. Sci. 2014;93:2394–2399. doi: 10.3382/ps.2014-03905. [DOI] [PubMed] [Google Scholar]
- Torok V.A., Hughes R.J., Mikkelsen L.L., Perez-Maldonado R., Balding K., MacAlpine R., Percy N.J., Ophel-Keller K. Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl. Environ. Microbiol. 2011;77:5868–5878. doi: 10.1128/AEM.00165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uni Z., Ganot S., Sklan D. Posthatch development of mucosal function in the broiler small intestine. Poult. Sci. 1998;77:75–82. doi: 10.1093/ps/77.1.75. [DOI] [PubMed] [Google Scholar]
- Wang Y., Qian P. Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS One. 2009;4:e7401. doi: 10.1371/journal.pone.0007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weurding R.E., Veldman A., Veen W.A.G., van der Aar P.J., Verstegen M.W.A. In vitro starch digestion correlates well with rate and extent of starch digestion in broiler chickens. J. Nutr. 2001;131:2336–2342. doi: 10.1093/jn/131.9.2336. [DOI] [PubMed] [Google Scholar]
- Whiting I.M., Pirgozliev V., Rose S.P., Wilson J., Amerah A.M., Ivanova S.G., Staykova G.P., Oluwatosin O.O., Oso A.O. Nutrient availability of different batches of wheat distillers dried grains with solubles with and without exogenous enzymes for broiler chickens. Poult. Sci. 2017;96:574–580. doi: 10.3382/ps/pew262. [DOI] [PubMed] [Google Scholar]
- Willson N., Nattrass G.S., Hughes R.J., Moore R.J., Stanley D., Hynd P.I., Forder R.E.A. Correlations between intestinal innate immune genes and cecal microbiota highlight potential for probiotic development for immune modulation in poultry. Appl. Microbiol. Biotechnol. 2018;102:9317–9329. doi: 10.1007/s00253-018-9281-1. [DOI] [PubMed] [Google Scholar]
- Yan F., Dibner J.J., Knight C.D., Vazquez-Anon M. Effect of carbohydrase and protease on growth performance and gut healthof young broilers fed diets containing rye, wheat, and feather meal. Poult. Sci. 2017;96:817–828. doi: 10.3382/ps/pew300. [DOI] [PubMed] [Google Scholar]
- Yin D., Yin X., Wang X., Lei Z., Wang M., Guo Y., Aggrey S.E., Nie W., Yuan J. Supplementation of amylase combined with glucoamylase or protease changes intestinal microbiota diversity and benefits for broilers fed a diet of newly harvested corn. J. Anim. Sci. Biotechnol. 2018;9:1–13. doi: 10.1186/s40104-018-0238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Wang X., Yin D., Wang M., Yin X., Lei Z., Guo Y. Effect of different amylases on the utilization of cornstarch in broiler chickens. Poult. Sci. 2017;96:1139–1148. doi: 10.3382/ps/pew323. [DOI] [PubMed] [Google Scholar]
- Zeb F., Wu X., Chen L., Fatima S., Ijaz-ul-Haq, Chen A., Xu C., Jianglei R., Feng Q., Li M. Time restricted feeding are associated with changes in human gut microbiota related to nutrients intake. Nutrition. 2020;78:110797. doi: 10.1016/j.nut.2020.110797. [DOI] [PubMed] [Google Scholar]
- Zheng X., Zhou K., Zhang Y., Han X., Zhao A., Liu J., Qu C., Ge K., Huang F., Hernandez B., Yu H., Panee J., Chen T., Jia W., Jia W. Food withdrawal alters the gut microbiota and metabolome in mice. FASEB J. 2018;32:4878–4888. doi: 10.1096/fj.201700614R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Zheng P., Zhang K., Ding X., Bai S. Effects of exogenous enzymes and dietary energy on performance and digestive physiology of broilers. J. Anim. Sci. Biotechnol. 2013;4:1–9. doi: 10.1186/2049-1891-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.