Abstract
Background
This review presents the surgical indications, surgical procedures, and results in the treatment of asymptomatic and ruptured abdominal aortic aneurysms (AAA).
Methods
An updated search of the literature on screening, diagnosis, treatment, and follow-up of AAA, based on the German clinical practice guideline published in 2018.
Results
Surgery is indicated in men with an asymptomatic AAA ≥ 5.5 cm and in women, ≥ 5.0 cm. The indication in men is based on four randomized trials, while in women the data are not conclusive. The majority of patients with AAA (around 80%) meanwhile receive endovascular treatment (endovascular aortic repair, EVAR). Open surgery (open aneurysm repair, OAR) is reserved for patients with longer life expectancy and lower morbidity. The pooled 30-day mortality is 1.16% (95% confidence interval [0.92; 1.39]) following EVAR, 3.27% [2.7; 3.83] after OAR. Women have higher operative/interventional mortality than men (odds ratio 1.67%). The mortality for ruptured AAA is extremely high: around 80% of women and 70% of men die after AAA rupture. Ruptured AAA should, if possible, be treated via the endovascular approach, ideally with the patient under local anesthesia. Treatment at specialized centers guarantees the required expertise and infrastructure. Long-term periodic monitoring by mean of imaging (duplex sonography, plus computed tomography if needed) is essential, particularly following EVAR, to detect and (if appropriate) treat endoleaks, to document stable diameter of the eliminated aneurysmal sac, and to determine whether reintervention is necessary (long-term reintervention rate circa 18%).
Conclusion
Vascular surgery now offers a high degree of safety in the treatment of patients with asymptomatic AAA. Endovascular intervention is preferred.
An abdominal aortic aneurysm (AAA) is an arterial wall weakness in the abdominal section of the aorta, resulting in changes in flow and pressure conditions in this area which ultimately lead to expansion and bulging of the weakened vessel wall.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. The CME questions on this article can be found at http://daebl.de/RY95. Answers must be submitted by 26 November 2021.
Participation is possible at cme.aerztebatt.de
The prevalence of AAA is influenced by age and gender. A CT colonography study found that the prevalence of AAA—defined as an AAA diameter ≥ 30 mm—was 1.3% among male participants aged 55 to 64.9 years, 9.1% in the age group from 65.0 to 74.9 years, 16.8% in the age group 75.0 to 84.9 years, and 22.0% in the age group >85 years. The corresponding prevalence rates for women were 0.4%, 2.0%, 3.9%, and 6.2%, respectively (1). With increasing AAA diameter, the risk of rupture also increases: from approx. 0.3% per year for diameters ≤ 3.9 cm to 6.5% for diameters of 5.0 to 5.9 cm (2). Given the high mortality rate associated with this risk, prophylactic surgical repair of AAA should be recommended to symptom-free patients starting from a defined size of the aneurysm.
Under the leadership of the German Society for Vascular Surgery and Vascular Medicine (DGG e. V.), a clinical practice (S3) guideline of the Association of the Scientific Medical Societies in Germany (AWMF) was issued in 2018. This guideline addresses screening, diagnosis, therapy and follow-up of abdominal aortic aneurysms (3). This review article provides an update on the aspects of surgical repair. Please refer to the guideline for information about perioperative management and conservative treatment.
Method
This review is based on a literature search of the PubMed (Medline) database. Articles published in the last two years (January 2018 to March 2020) were searched using the following search terms: “abdominal aortic aneurysm [Title]“, “abdominal aortic aneurysm AND diameter threshold“, “abdominal aortic aneurysm AND outcome“, “abdominal aortic aneurysm AND mortality“, and “ruptured abdominal aortic aneurysm [Title]“. The method of this review follows the method of the 2018 clinical practice (S3) guideline (3). For the years before 2018, the systematic literature search of the 2018 guideline was relied on. All newly published guidelines, systematic reviews/meta-analyses, randomized trials, and registry studies were included.
Indication for surgery in asymptomatic patients
The indication for prophylactic elective surgery was very similar in all three guidelines on the management of AAA published in the last two years (3– 5). In the DGG guideline, it reads (3):
“Patients with an infrarenal or juxtarenal AAA ≥ 5.5 cm are strongly recommended to undergo elective interventions.
Patients with an infrarenal or juxtarenal AAA of 5.0 cm to 5.4 cm can be considered for elective intervention.
Invasive interventions should be considered in women when the maximum AAA diameter has reached 5.0 cm.
If the AAA growth rate is >10 mm/year, a conventional, open aneurysm repair (OAR [vascular graft via laparotomy]) or endovascular intervention (EVAR, interventional placement of a stent graft) is indicated, regardless of the AAA diameter.“
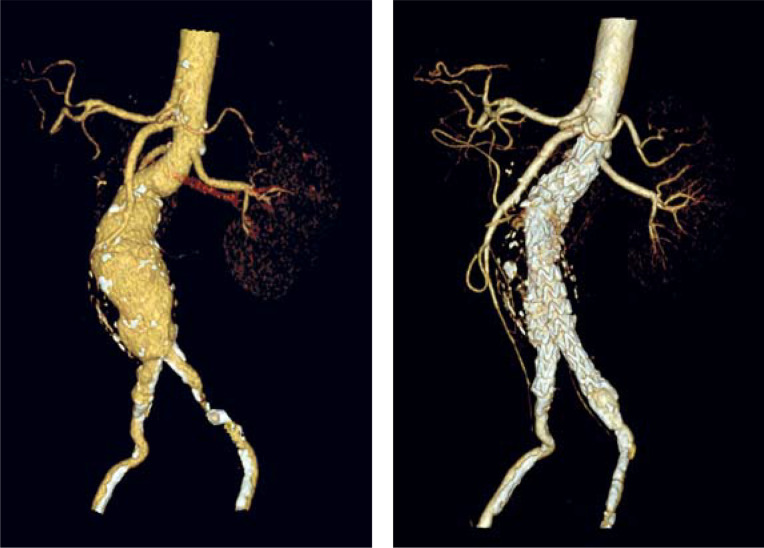
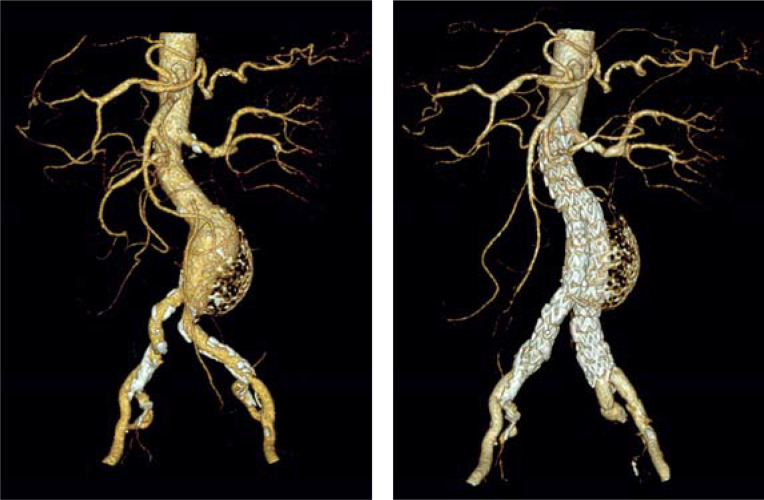
Neither the guidelines of the Society for Vascular Surgery (SVS) (4) nor those of the European Society for Vascular Surgery (ESVS) (5) state for men that an intervention can already be “considered” starting from an AAA diameter of 5.0 cm. It should be noted that these recommendations exclusively apply to the fusiform AAA (Figure 1). Due to the lack of data, no evidence-based statements can be made with regard to the indication for surgery in patients with eccentric saccular AAA. Saccular aneurysms are rare (5). In an analysis of 206 ruptured AAAs, saccular aneurysms were observed in 4.4% of cases (6). In general, however, the indication for interventional treatment is established earlier in patients with saccular AAA (Figure 2) compared to fusiform AAA because of the higher risk of rupture.
Figure 1:
Fusiform abdominal aortic aneurysm in an 85-year-old patient:
a) before; b) after endovascular repair
Figure 2:
Eccentric saccular abdominal aortic aneurysm in a 66-year-old patient: a) before; b) after endovascular repair
Yet, de facto these indication parameters are often not considered, possibly due to the fact that once an AAA has been detected patients are reluctant to wait until the intervention threshold has been reached, but also for economic reasons.
In an analysis of 44 089 patients with intact AAA (iAAA) in eleven countries (7), the percentages of men who underwent surgery for a small AAA<5,5 cm ranged from 6% (Iceland) and 16% (Sweden) up to 40% (USA) and 43% (Germany). Another cross-national study also reported significant differences in the indication for surgery. In this study, the rates of ruptured AAA repairs did not differ significantly between countries. The rate was also not influenced by the fact that the indication for repair of iAAA was established at an earlier or later point in the various countries. Consequently, repairing smaller iAAAs did not lower the rate of ruptured AAAs which were scheduled for treatment (8).
Selection of the surgical technique for intact abdominal aortic aneurysm
The German clinical practice (S3) guideline recommends for patients with acceptable periprocedural risk both EVAR and OAR to the same extent—provided the pathoanatomical feasibility of EVAR (3). Frequently, the decision is determined by patient preferences, depending on whether the patient prioritizes an early survival benefit (then EVAR) or the absence of later complications and follow-up examinations (then OAR) (box). Results of three new meta-analyses reported in the literature are listed in Table 1.
BOX. Cancer risk associated with endovascular repair.
Patients treated with endovascular aneurysm repair (EVAR) are exposed to a significantly higher radiation dose compared to patients treated with open repair (OAR). On the one hand, this is due to intraoperative fluoroscopic visualization of the endograft, and, on the other hand, it is a result of the subsequent CT scans which are regularly performed to monitor stent-graft positioning and aneurysm size. Using the English Hospital Episode Statistics (HES) from 2005 to 2013, Markar et al. (40) explored whether the higher exposure to radiation increases the cancer risk in patients treated with EVAR. They analyzed data from 14 150 patients with EVAR and 24 465 patients with OAR. Follow-up duration was up to seven years. In this cohort, EVAR was associated with an increased risk of postoperative abdominal tumor disease (hazard ratio [HR] 1.14; 95% confidence intervals: [1.03; 1.27]) and of cancer overall (HR 1.09; [1.02; 1.17]). With regard to the lung cancer risk, no statistically significant differences between EVAR and OAR were found (HR 1.04; [0.92; 1.18]). Follow-up with CT surveillance did not increase the tumor risk in the EVAR group. The authors concluded that the radiation exposure during the intervention may increase the radiation-related cancer risk and regarded intra-operative exposure to radiation and fluoroscopy time as important quality parameters for EVAR. However, the authors did not provide information about the overall mortality in this patient population.
Table 1. Endovascular repair (EVAR) versus open repair (OAR) of the intact abdominal aortic aneurysm (elective procedure), results of the meta-analyses.
| Authors | Data source | Results |
| Bulder et al., 2019 (9) | 51 studies/189 022 patients | 30-day mortality after EVAR with 1.16%, 95% CI [0.92; 1.39] significantly lower than after OAR with 3.27% [2.71; 3.83] (p<0.001); no difference in long-term survival up to 10 years post-operatively |
| Li et al., 2019 (10) | 54 studies/203 246 patients | Higher long-term mortality after 5–9 years with EVAR (27.3%) versus OAR (24.7%) (p = 0.03); higher rate of reintervention (OR 2.12 [1.67; 2.69]) and higher secondary rupture rate (OR 4.84 [2.63; 8.89]) with EVAR |
| Antoniou et al., 2020 (11) | Synthesis of 7 randomized trials; 2 983 patients | 30-day case mortality for EVAR with 1.2% significantly lower compared to OAR with 3.1% (OR 0.36 [0.20; 0.66]), p = 0.001; over the long term, aneurysm-related mortality, rates of reintervention and rupture higher after EVAR compared to OAR. |
CI, confidence interval; OR, odds ratio
The SVS guideline (4) emphasizes the endovascular approach—provided it is technically and pathoanatomically feasible—as the single standard of care and considers OAR only as an alternative surgical procedure for special situations, as it is associated with higher perioperative morbidity and mortality. The ESVS guideline (5) recommends OAR for patients with longer life expectancy because of its slightly better long-term outcomes compared to EVAR. Conversely, the guideline’s view on EVAR is that it offers clear advantages for patients with limited life expectancy or with an above-average operative risk. We recommend to use the DIGG risk score of the German Institute for Vascular Health Research (DIGG) to preoperatively estimate the operative risk (12).
Gender differences in the management of the intact abdominal aortic aneurysm
Liu et al. (13) found in a meta-analysis (32 cohort studies, 74 969 patients) in women who underwent EVAR a significantly higher 30-day mortality compared to men (odds ratio 1.67; 95% confidence interval [1.50; 1.87]; p<0.001). Likewise, the rates of further complications, such as ischemia of the lower extremities as well as renal and cardiac complications after EVAR were higher in women compared to men. In addition, the long-term survival was found reduced in women (hazard ratio 1.23; [1.09; 1.38]; p = 0.001). By contrast, no significant gender differences were identified in respect to early and late re-interventions or endoleaks. The authors mentioned as reasons for the more unfavorable outcomes after EVAR in women compared to men the relatively larger AAA diameter in women at the time of intervention if the indication for intervention was based on an AAA diameter of 5.5 cm as in men (the aortic diameters in women are smaller), as well as the older age among women compared to men and the less favorable anatomical situation with smaller access vessels for endovascular intervention and a more hostile aneurysmal neck anatomy. Thus, these findings confirm the results of an earlier meta-analysis (14) which also showed that the morphology of AAA in women is less favorable for EVAR that that in men. Furthermore, the 30-day mortality rate is almost twice as high in women as it is in men, both after EVAR and after OAR.
As a consequence it has been suggested to set the limit indicator for intervention in women at a smaller AAA diameter, to develop better endografts and, given the increased risk among women, to preferentially treat female patients in specialized centers with adequate experience in both EVAR and open repair.
Complex abdominal aortic aneurysms
Being technically challenging, the repair of complex AAAs is associated with an increased rate of complications. It is distinguished between juxtarenal, pararenal and suprarenal AAAs. Doonan et al. (15) compared the outcomes achieved with EVAR versus OAR in patients with complex AAAs in a meta-analysis of 15 non-randomized studies including 5121 patients (1506 EVAR, 3615 OAR). EVAR was associated with a significantly lower 30-day case fatality rate compared to OAR (odds Ratio [OR] 0.50; [0.34; 0.74]; p<0.001) and a lower rate of acute post-operative renal failure (OR 0.50; [0.28; 0.89]; p = 0.02), but with a higher rate of spinal cord ischemia. Here, however, the absolute differences were small (EVAR 1.23%; OAR 0.31%). Likewise, the rate of intestinal ischemia and the length of hospital stay was shorter after EVAR compared to OAR. The limited available long-term outcome data showed no significant differences between the two approaches; however, the rate of reintervention was higher after EVAR. The available evidence indicates that patients with comorbidity may benefit from the endovascular strategy.
Symptomatic abdominal aortic aneurysm
A symptomatic AAA is defined as an AAA which causes symptoms such as pain without having developed a gap in the aortic wall. Diagnostic investigations show no retroperitoneal hematoma. In patients with symptomatic AAA, the perioperative risk is higher compared to patients with asymptomatic AAA.
Soden et al. (16) identified 4495 asymptomatic AAAs in their search of the database of the National Surgical Quality Improvement Program (NSQIP) and compared these cases with 455 cases of symptomatic AAA. They found no significant difference between the two groups with regard to AAA diameter; however, in a multivariable model, the postoperative 30-day mortality after data adjustment was twice as high among patients with symptomatic AAA compared to asymptomatic patients (OR 2,1; [1.3; 3,5]). While this applied to both OAR and EVAR, the hospital mortality rate of symptomatic AAA was lower with EVAR compared to OAR (3.8% vs 7.7%). In a Dutch registry, too, a significantly higher 30-day mortality rate was observed among patients treated for symptomatic AAA compared asymptomatic AAA. This analysis also found better outcomes after EVAR compared to OAR (17).
Ruptured abdominal aortic aneurysm – prognosis
A ruptured abdominal aortic aneurysm (rAAA) is a surgical emergency requiring immediate treatment. A population-based survey (10 724 persons, of these 24.4% women) in Sweden covering the period from 2001 to 2015 shows the problem of treatment delay analyzed by gender (18). Women with rAAA were with a mean age of 79.7 years significantly older than men (mean age 76.1 years). The mortality rates for all patients with rAAA (with and without hospital admission) were 81.1% and 68.5% for women and men, respectively. Of all patients with rAAA admitted to hospital, only about half (52.7%) were treated in the hospital and women significantly less frequently than men (table 2). The mortality among women treated for rAAA remained significantly higher up to one year after the intervention compared to that of men. However, the postoperative complication rates of women and men did not differ significantly (35.1% vs 33.1%).
Table 2. Gender-specific prognosis of patients with ruptured abdominal aortic aneurysm after hospital admission (Sweden 2001 – 2015, population-based study [18]).
| Parameter | Women n (%) | Men n (%) | p |
| Hospital admissions (rAAA) | 2 032 | 6 466 | – |
| Treated – OAR – EVAR |
820 (40.4) 691 (84.3) 129 (15.7) |
3 660 (56.6) 3 067 (83.8) 593 (16.2) |
<0.001 – – |
| 30-day mortality | 325 (39.6) | 1 107 (30.2) | <0.001 |
| 90-day mortality | 367 (44.8) | 1 274 (34.8) | <0.001 |
| 1-year mortality | 426 (52.0) | 1 496 (40.9) | <0.001 |
EVAR, endovascular aneurysm repair; OAR, open aneurysm repair; rAAA, ruptured abdominal aortic aneurysm
A comparative analysis of rAAA mortality in England and the USA confirms the large number of patients with rAAA who only received non-corrective treatment after hospital admission. In England, this applied to 41.6% of patients, in the USA to 19.6% (p<0.0001) (19). The rate of patients with rAAA in whom the indication for intervention was established in the hospital was significantly higher, both in England and the USA, if the patients were admitted to an academic teaching hospital. This finding supports the approach to admit these patients to tertiary care hospitals. In this comparative study, the hospitals were also classified by bed capacity. Both in the USA and in England, the best outcomes were achieved in the hospitals with the highest capacity. The same observation was made by Fierro et al. (20) in Catalonia where 69.2% of the patients admitted to a tertiary care hospital underwent an intervention compared to only 29.5% of patients admitted to local hospitals.
Selection of the surgical technique for ruptured abdominal aortic aneurysms
Both the SVS (4) and ESVS (5) guidelines strongly recommend endovascular treatment of ruptured AAA, if technically feasible; the levels of evidence of this recommendation are set as C and B, respectively. Whether endovascular repair or open repair is the better approach to treat a ruptured aneurysm was recently evaluated by Kontopodis et al. (21) in a meta-analysis of 136 studies (267 259 patients; EVAR: 58 273, OAR: 208 986). Among these studies were 109 comparative investigations, including four randomized trials. In the meta-regression model, the pooled perioperative mortality accounted for 0.245 [0.234; 0.257] and 0.378 [0.364; 0.392] after EVAR and OAR, respectively. Thus, EVAR was associated with significantly lower perioperative mortality (OR 0.54 [0.51; 0.57], p<0.001). In addition, with OAR, but not with EVAR a significant inverse association between hospital mortality and hospital case load was found, with better outcomes in high-volume hospitals. Overall, the outcomes had improved over the period from 2002 to 2019, both after EVAR and OAR, and the difference between EVAR and OAR had increased in favor of EVAR.
Varkevisser et al. (22) also investigated whether the 5-year survival rates after endovascular and open repair of rAAA had changed over the last 14 years. They found a change for EVAR, but not for OAR. In the late cohort (interventions performed between 2014 and 2018), EVAR was associated with a significantly higher survival rate compared to OAR—a result that supports the use of EVAR in patients with rAAA. Additional results on the management of rAAA are listed in Table 3.
Table 3. Results after endovascular repair (EVAR) and open repair (OAR) of the ruptured abdominal aortic aneurysm.
| Authors | Data source | Results |
| IMPROVE Trial Investigators 2014 BMJ (23) |
Randomized trial (n = 613) |
30-day mortality: EVAR 35.4%, 95% CI: [112; 316], OAR 37.4% [111; 297]; p = 0.62; women benefit significantly more from EVAR than men (p = 0.02). 30-day mortality: women EVAR 37%, OAR 57%; men EVAR 35%, OAR 32% |
| IMPROVE Trial Investigators 2017 BMJ (24) |
Randomized trial | After three years, 48% of patients in the EVAR group and 56% in the OAR group had died (p = 0.053). Mortality after seven years about 60% in both groups. |
| Wang et al.2019 (25) | VQI cohort, n = 3 719 (2 922 men; 797 women) |
30-day mortality women 32.8%; men 25.5% (p<0.001); in both men and women, oar is associated with higher mortality compared to evar (or 1.69 [1.29; 2.22]; p<0.001) |
| Melillo et al. 2020 (26) | NSQIP database (n = 3 806) |
30-day mortality: men EVAR 17.7%, OAR 28.2% (p<0.001)) women evar 22.4%; oar 32.8% (p<0.001) |
| D’Oria et al. 2020 (27) | VQI database, EVAR n = 2 389; OAR n = 1 868 |
OAR is associated with significantly increased hospital mortality compared to EVAR (OR 2.08 [1.76; 2.45]; p<0.001). the survival benefit of evar is maintained over a period of five years postoperatively. |
| Varkevisser et al. 2020 (22) | VQI cohort; N = 1 177 matched pairs |
5-year survival after EVAR 63%, after OAR 54% (HR 0.69, [0.60; 0.79]; p<0.001) |
| Kontopodis et al. 2020 (21) | Meta-analysis; 136 studies, 267 259 patients |
EVAR is associated with significantly lower perioperative mortality compared to OAR (OR 0.54 [0.51; 0.57], p<0.001). |
HR, hazard ratio; CI, confidence interval; NSQIP, National Surgical Quality Improvement Program (USA); OR, odds ratio; VQI, Vascular Quality Initiative (USA)
In the randomized IMPROVE study, patients with rAAA who were treated with EVAR under local anesthesia had a significantly lower 30-day mortality rate compared to patients treated with EVAR under general anesthesia (28). In the meantime, these results have been confirmed in registry studies (29– 31). EVAR should be performed in patients with rAAA under local anesthesia, if possible.
Treatment in centers,minimum volume
In Germany, there are no minimum case volume requirements for elective AAA treatment in place. So far, no generally binding minimum volumes have been defined in the literature (32). When analyzing 22 227 elective OARs (German DRG data [DRG, diagnose-related case groups]), Nimptsch and Mansky (33) calculated a limit of at least 18 OARs per year needed to reduce hospital mortality associated with open treatment below the average. Likewise, Trenner et al. (34) found in their analysis of DRG data of the period from 2012 to 2016 a significant correlation between high annual procedure numbers and low hospital mortality. They regarded the call for a minimum volume of 30 AAAs/year as useful and feasible.
Follow-up after endovascular aneurysm repair
The German clinical practice (S3) guideline (3) and other guidelines (4, 5) recommend long-term (lifelong) periodic imaging surveillance for patients with EVAR, preferably using Duplex ultrasonography as the most cost-effective strategy (35). It is intended to monitor endoleaks, to document the stability or shrinking of the excluded aneurysm sac and to plan re-interventions where necessary.
The extent to which these recommendations are actually implemented in daily practice and the consequences of non-compliance was assessed by Grima et al. (36) in a systematic review (7 studies, 14 311 patients). They concluded that approximately 42% of patients did not comply with the recommendations. There was no difference in overall mortality after five years between the compliance and non-compliance patient groups. However, long-term imaging surveillance is indispensable, especially after EVAR. Given the large number of patients who do not comply with the recommended monitoring intervals after EVAR, there is a demand for personalizing the follow-up programs to a greater extent. Based on the data of the EVAR 1 and EVAR 2 trials (38, 39), Grootes et al. (37) designed a Cox regression model addressing this issue. They arrived at the conclusion that only patients showing an aneurysm growth of more than 1 mm per year required intensive follow-up. Five years after the intervention, aneurysm growth of more the 1 mm per year was observed in 85% of patients who either had experienced aneurysm rupture or required a rupture-preventing intervention within the next two years (37). Conversely, in the presence of aneurysm growth of less than 1 mm/year, about half of all cases could be categorized as low-risk and the number of further follow-ups could be reduced.
Key Messages.
Patients with an asymptomatic infrarenal abdominal aortic aneurysm (AAA) ≥ 5.5 cm are strongly recommended to undergo elective AAA repair. In women, invasive treatment should already be considered if the aortic diameter is 5.0 cm.
For patients with acceptable periprocedural risk, EVAR or OAR are equally recommendable, assuming anatomical feasibility for EVAR. Patients with longer life expectancy benefit from OAR, while limited life expectancy of the patient or increased operative risk would support EVAR.
Patients with complex AAA or with symptomatic AAA should preferably be offered EVAR.
These patients should only be treated in high-volume centers with constant intervention readiness and adequate experience in OAR and EVAR.
The results of the last five years show superiority of the endovascular repair over the open repair of the ruptured AAA. In these patients, EVAR should ideally be performed under local anesthesia.
In Germany, no minimum annual case volume requirements for AAA repairs are in place. Nevertheless, published data clearly show that AAA repair outcomes significantly improve with increasing case volume of the hospital.
Questions on the article in issue 22–23/2020: Endovascular and Open Repair of Abdominal Aortic Aneurysm.
The submission deadline is 26 November 2021. Only one answer is possible per question. Please select the answer that is most appropriate.
Question no. 1
What is the prevalence of abdominal aortic aneurysm (AAA) – defined by a AAA diameter ≥ 30 mm – in the age group 65 to 75 years?
22% in men and 3.9% in women
9.1% in men and 6.2% in women
16.8% in men and 0.4% in women
22% in men and 6.2% in women
9.1% in men and 2.0% in women
Question no. 2
What statement applies to the saccular abdominal aortic aneurysm (AAA)?
It is less common than the fusiform AAA.
It is associated with a lower risk of rupture compared to the fusiform AAA.
The decision to operate should be made with caution.
High-quality evidence is available with regard to the indication to operate a saccular AAA.
The saccular AAA should be regarded as physiological in men.
Question no. 3
Starting from what maximum aortic diameter, one should consider a prophylactic elective intervention to treat an abdominal aortic aneurysm in women according to the DGG guideline?
from a maximum aortic diameter of 6.0 cm
from a maximum aortic diameter of 4.5 cm
from a maximum aortic diameter of 5.5 cm
from a maximum aortic diameter of 5.0 cm
from a maximum aortic diameter of 5.7 cm
Question no. 4
The use of which score is recommended by the authors to preoperatively estimate the surgical risk associated with EVAR and OAR?
ASCVD risk score
MICA risk score
HRQoL score
QoL score
DIGG risk score
Question no. 5
What minimum volume requirements are in place for centers providing elective AAA repair in Germany?
30 per year
There is no minimum volume requirement.
20 per year
70 per year
15 per year
Question no. 6
With regard to endovascular repair (EVAR), which were the areas where gender differences were found?
Early re-interventions and long-term survival
Ischemia of the lower extremities and late re-interventions
Renal complications and endoleaks
Ischemia of the lower extremities and 30-day mortality
Late re-intervention and cardiac complications
Question no. 7
According to a population-based survey in Sweden, what is the mortality rate in patients (with or without hospitalization) with ruptured abdominal aortic aneurysm (rAAA)?
Approx. 50% in women and approx. 80% in men
approx. 80% in women and approx. 70% in men
approx. 30% in women and approx. 10% in men
approx. 25% in women and approx. 50% in men
approx. 70% in women and approx. 90% in men
Question no. 8.
Which type of intervention is recommended as the treatment of choice for ruptured abdominal aortic aneurysm (rAAA)?
Endovascular repair (EVAR) under regional anesthesia
Endovascular repair (EVAR) under local anesthesia
Endovascular repair (EVAR) under general anesthesia
Open surgery (OAR) under local anesthesia
Open surgery (OAR) under general anesthesia
Question no. 9
In Germany, what percentage of men with intact abdominal aortic aneurysm (iAAA) with a diameter <5.5 cm were surgically treated according to an international study?
7%
14%
23%
43%
65%
Question no. 10
What type of follow-up is recommended by the German clinical practice (S3) guideline for patients after endovascular repair (EVAR) of an aneurysm?
Imaging surveillance in the first three years after the intervention
Imaging surveillance for one to two years after the intervention
Lifelong periodic surveillance using Duplex ultrasonography
Abdominal CT scan at least every six months
Abdominal CT scan at least once a year
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest
Prof. Schmitz-Rixen is the Managing Director of the Deutsche Insitut für Gefäßmedizinische Gesundheitsforschung (DIGG gGmbH) where a topic-related registry is kept. He received study support (third party funding) from Medtronic.
Prof. Böckler received consulting fees, congress fees and reimbursement of travel expenses from W. L. Gore & Ass. and Medtronic. He received lecture fees from W. L. Gore & Ass., Medtronic and Abbott. He received study support (third party funding) from W. L. Gore & Ass., Medtronic, Cook Medical, and Siemens. He received fees for expert opinions related to the topic from various district courts.
Prof. Grundmann received fees for expert opinions related to the topic from courts.
Prof. Vogl declares no conflict of interest.
References
- 1.Khashram M, Jones GT, Roake JA. Prevalence of abdominal aortic aneurysm (AAA) in a population undergoing computed tomography colonography in Canterbury, New Zealand. Eur J Vasc Endovasc Surg. 2015;50:199–205. doi: 10.1016/j.ejvs.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Brown LC, Powell JT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance UK Small Aneurysm Trial Participants. Ann Surg. 1999;230:289–297. doi: 10.1097/00000658-199909000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Debus ES, Heidemann F, Gross-Fengels W, et al. S3-Leitlinie zu Screening, Diagnostik, Therapie und Nachsorge des Bauchaortenaneurysmas. AWMF-Registernummer 004-14. Stand 07.07.2018. doi: 10.1007/s00104-020-01116-8. [DOI] [PubMed] [Google Scholar]
- 4.Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77. doi: 10.1016/j.jvs.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 5.Wanhainen A, Verzini F, Van Herzeele I, et al. Editor‘s choice - European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57:8–93. doi: 10.1016/j.ejvs.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Kristmundsson T, Dias N, Resch T, Sonesson B. Morphology of small abdominal aortic aneurysms should be considered before continued ultrasound surveillance. Ann Vasc Surg. 2016;31:18–22. doi: 10.1016/j.avsg.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Beck AW, Sedrakyan A, Mao J, et al. Variations in abdominal aortic aneurysm care: a report from the International Consortium of Vascular Registries. Circulation. 2016;134:1948–1958. doi: 10.1161/CIRCULATIONAHA.116.024870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grima MJ, Behrendt CA, Vidal-Diez A, et al. Assessment of correlation between mean size of infrarenal abdominal aortic aneurysm at time of intact repair against repair and rupture rate in nine countries. Eur J Vasc Endovasc Surg. 2020;59:890–897. doi: 10.1016/j.ejvs.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Bulder RMA, Bastiaannet E, Hamming JF, Lindeman JHN. Meta-analysis of long-term survival after elective endovascular or open repair of abdominal aortic aneurysm. Br J Surg. 2019;106:523–533. doi: 10.1002/bjs.11123. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Khan S, Salata K, et al. A systematic review and meta-analysis of the long-term outcomes of endovascular versus open repair of abdominal aortic aneurysm. J Vasc Surg. 2019;70:954–969. doi: 10.1016/j.jvs.2019.01.076. [DOI] [PubMed] [Google Scholar]
- 11.Antoniou GA, Antoniou SA, Torella F. Editor‘s choice - endovascular vs. open repair for abdominal aortic aneurysm: systematic review and meta-analysis of updated peri-operative and long term data of randomised controlled trials. Eur J Vasc Endovasc Surg. 2020;59:385–397. doi: 10.1016/j.ejvs.2019.11.030. [DOI] [PubMed] [Google Scholar]
- 12.Steffen M, Schmitz-Rixen T, Jung G, Böckler D, Grundmann RT. das Deutsche Institut für Gefäßmedizinische Gesundheitsforschung gGmbH (DIGG): Der DIGG-Risikoscore. Ein Vorhersagemodell der perioperativen Sterblichkeit bei elektiver Versorgung des intakten Bauchaortenaneurysmas im DIGG-Register. Chirurg. 2019;90:913–920. doi: 10.1007/s00104-019-0968-3. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Yang Y, Zhao J, et al. Systematic review and meta-analysis of sex differences in outcomes after endovascular aneurysm repair for infrarenal abdominal aortic aneurysm. J Vasc Surg. 2020;71:283–296. doi: 10.1016/j.jvs.2019.06.105. [DOI] [PubMed] [Google Scholar]
- 14.Ulug P, Sweeting MJ, von Allmen RS, Thompson SG, Powell JT. SWAN Collaborators: Morphological suitability for endovascular repair, non-intervention rates, and operative mortality in women and men assessed for intact abdominal aortic aneurysm repair: systematic reviews with meta-analysis. Lancet. 2017;389:2482–2491. doi: 10.1016/S0140-6736(17)30639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doonan RJ, Girsowicz E, Dubois L, Gill HL. A systematic review and meta-analysis of endovascular juxtarenal aortic aneurysm repair demonstrates lower perioperative mortality compared with open repair. J Vasc Surg. 2019;70:2054–2064e3. doi: 10.1016/j.jvs.2019.04.464. [DOI] [PubMed] [Google Scholar]
- 16.Soden PA, Zettervall SL, Ultee KH, et al. Outcomes for symptomatic abdominal aortic aneurysms in the American College of Surgeons National Surgical Quality Improvement Program. J Vasc Surg. 2016;64:297–305. doi: 10.1016/j.jvs.2016.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lijftogt N, Vahl AC, Wilschut ED, et al. Dutch Society of Vascular Surgery, the Steering Committee of the Dutch Surgical Aneurysm Audit, and the Dutch Institute for Clinical Auditing: Adjusted hospital outcomes of abdominal aortic aneurysm surgery reported in the Dutch surgical aneurysm audit. Eur J Vasc Endovasc Surg. 2017;53:520–532. doi: 10.1016/j.ejvs.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Zommorodi S, Bottai M, Hultgren R. Sex differences in repair rates and outcomes of patients with ruptured abdominal aortic aneurysm. Br J Surg. 2019;106:1480–1487. doi: 10.1002/bjs.11258. [DOI] [PubMed] [Google Scholar]
- 19.Karthikesalingam A, Holt PJ, Vidal-Diez A, et al. Mortality from ruptured abdominal aortic aneurysms: clinical lessons from a comparison of outcomes in England and the USA. Lancet. 2014;383:963–969. doi: 10.1016/S0140-6736(14)60109-4. [DOI] [PubMed] [Google Scholar]
- 20.Fierro A, Mestres G, Díaz MA, Tripodi P, Yugueros X, Riambau V. Influence of on-call vascular surgery team and off-hour effect on survival after ruptured abdominal aortic aneurysm. Ann Vasc Surg. 2020;64:80–87. doi: 10.1016/j.avsg.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Kontopodis N, Galanakis N, Antoniou SA, et al. Meta-analysis and meta-regression analysis of outcomes of endovascular and open repair for ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2020;59:399–410. doi: 10.1016/j.ejvs.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Varkevisser RRB, Swerdlow NJ, de Guerre LEVM, et al. Society for Vascular Surgery Vascular Quality Initiative: Five-year survival following endovascular repair of ruptured abdominal aortic aneurysms is improving. J Vasc Surg. 2020;72:105–113e4. doi: 10.1016/j.jvs.2019.10.074. [DOI] [PubMed] [Google Scholar]
- 23.Powell JT, Sweeting MJ, Thompson, et al. IMPROVE Trial Investigators, Endovascular or open repair strategy for ruptured abdominal aortic aneurysm: 30 day outcomes from IMPROVE randomised trial. BMJ. 2014;348 doi: 10.1136/bmj.f7661. [DOI] [PubMed] [Google Scholar]
- 24.IMPROVE Trial Investigators. Comparative clinical effectiveness and cost effectiveness of endovascular strategy v open repair for ruptured abdominal aortic aneurysm: three year results of the IMPROVE randomised trial. BMJ. 2017;359 doi: 10.1136/bmj.j4859. j4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang LJ, Locham S, Dakour-Aridi H, Lillemoe KD, Clary B, Malas MB. Sex disparity in outcomes of ruptured abdominal aortic aneurysm repair driven by in-hospital treatment delays. Ann Surg. 2019;270:630–638. doi: 10.1097/SLA.0000000000003482. [DOI] [PubMed] [Google Scholar]
- 26.Melillo AM, Trani JL, Gaughan JP, Carpenter JP, Lombardi JV. Assessing trends, morbidity, and mortality in ruptured abdominal aortic aneurysm repair with 9 years of data from the National Surgical Quality Improvement Program. J Vasc Surg. 2020;71:423–431. doi: 10.1016/j.jvs.2019.04.462. [DOI] [PubMed] [Google Scholar]
- 27.D’Oria M, Hanson KT, Schermerhorn M, et al. Short term and long term outcomes after endovascular or open repair for ruptured infrarenal abdominal aortic aneurysms in the Vascular Quality Initiative. Eur J Vasc Endovasc Surg. 2020;59:703–716. doi: 10.1016/j.ejvs.2019.12.032. [DOI] [PubMed] [Google Scholar]
- 28.Powell JT, Hinchliffe RJ, Thompson MM, et al. IMPROVE trial investigators, Observations from the IMPROVE trial concerning the clinical care of patients with ruptured abdominal aortic aneurysm. Br J Surg. 2014;101:216–224; discussion 224. doi: 10.1002/bjs.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faizer R, Weinhandl E, El Hag S, et al. Decreased mortality with local versus general anesthesia in endovascular aneurysm repair for ruptured abdominal aortic aneurysm in the Vascular Quality Initiative database. J Vasc Surg. 2019;70:92–101. doi: 10.1016/j.jvs.2018.10.090. [DOI] [PubMed] [Google Scholar]
- 30.Bennett KM, McAninch C, Scarborough JE. Locoregional anesthesia is associated with lower 30-day mortality than general anesthesia in patients undergoing endovascular repair of ruptured abdominal aortic aneurysm. J Vasc Surg. 2019;70:1862–1867. doi: 10.1016/j.jvs.2019.01.077. [DOI] [PubMed] [Google Scholar]
- 31.Mouton R, Rogers CA, Harris RA, Hinchliffe RJ. Local anaesthesia for endovascular repair of ruptured abdominal aortic aneurysm. Br J Surg. 2019;106:74–81. doi: 10.1002/bjs.10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grundmann RT, Thomsen J. Fallvolumen und Ergebnis (“Volume-Outcome-Beziehung“) - Bauchaortenaneurysma Versorgungsqualität in der operativen Medizin. In: Debus ES, Grundmann RT, editors. Springer. Berlin: 2020. pp. 153–165. [Google Scholar]
- 33.Nimptsch U, Mansky T. Hospital volume and mortality for 25 types of inpatient treatment in German hospitals: observational study using complete national data from 2009 to 2014. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016184. e016184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trenner M, Salvermoser M, Busch A, Schmid V, Eckstein HH, Kühnl A. The effect of minimum procedure number on hospital mortality in abdominal aortic aneurysm repair—a secondary analysis of German Health Registry (DRG) data. Dtsch Arztebl Int. 2020;117:820–827. doi: 10.3238/arztebl.2020.0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brazzelli M, Hernández R, Sharma P, et al. Contrast-enhanced ultrasound and/or colour duplex ultrasound for surveillance after endovascular abdominal aortic aneurysm repair: a systematic review and economic evaluation. Health Technol Assess. 2018;22:1–220. doi: 10.3310/hta22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grima MJ, Boufi M, Law M, et al. Editor‘s Choice - The implications of non-compliance to endovascular aneurysm repair surveillance: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2018;55:492–502. doi: 10.1016/j.ejvs.2017.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grootes I, Barrett JK, Ulug P, et al. Predicting risk of rupture and rupture-preventing reinterventions following endovascular abdominal aortic aneurysm repair. Br J Surg. 2018;105:1294–1304. doi: 10.1002/bjs.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel R, Sweeting MJ, Powell JT, Greenhalgh RM EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366–2374. doi: 10.1016/S0140-6736(16)31135-7. [DOI] [PubMed] [Google Scholar]
- 39.Sweeting MJ, Patel R, Powell JT, Greenhalgh RM EVAR Trial Investigators. Endovascular repair of abdominal aortic aneurysm in patients physically ineligible for open repair: very long-term follow-up in the EVAR-2 randomized controlled trial. Ann Surg. 2017;266:713–719. doi: 10.1097/SLA.0000000000002392. [DOI] [PubMed] [Google Scholar]
- 40.Markar SR, Vidal-Diez A, Sounderajah V, et al. A population-based cohort study examining the risk of abdominal cancer after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2019;69:1776–1785. doi: 10.1016/j.jvs.2018.09.058. [DOI] [PubMed] [Google Scholar]