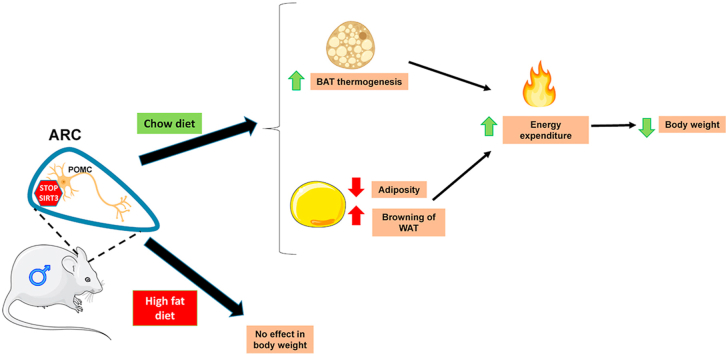
Abstract
Sirtuin 3 (SIRT3) is one of the seven mammalian sirtuin homologs of the yeast Sir2 gene that has emerged as an important player in the regulation of energy metabolism in peripheral tissues. However, its role in the hypothalamus has not been explored. Herein, we show that the genetic inhibition of SIRT3 in the hypothalamic arcuate nucleus (ARC) induced a negative energy balance and improvement of several metabolic parameters. These effects are specific for POMC neurons, because ablation of SIRT3 in POMC, but not in AgRP neurons, decreased body weight and adiposity, increased energy expenditure and brown adipose tissue (BAT) activity, and induced browning in white adipose tissue (WAT). Notably, the depletion of SIRT3 in POMC neurons caused these effects in male mice fed a chow diet but failed to affect energy balance in males fed a high fat diet and females under both type of diets. Overall, we provide the first evidence pointing for a key role of SIRT3 in POMC neurons in the regulation of energy balance.
Keywords: Sirtuins, Hypothalamus, brown adipose tissue, Energy balance, Adiposity, Browning
Graphical abstract
1. Introduction
Communication between the brain and internal organs is essential to maintain energy homeostasis. Among several brain areas implicated in the interaction with the periphery, the hypothalamus plays a prominent role orchestrating the regulation of energy homeostasis. The hypothalamus is composed by different interconnected nuclei where the arcuate nucleus (ARC) stands out as the area best positioned to receive and respond to homeostatic signals from the rest of the body. The ARC integrates information from peripheral tissues, with a subsequent coordinated neuronal response to a wide variety of nutrients and hormones to modulate energy balance [[1], [2]]. The ARC is mainly composed by two antagonist neuronal populations, NPY/AgRP neurons that induce a positive energy balance and POMC neurons that induce a negative energy balance [3,4]. Both neuronal types regulate food intake, energy expenditure and nutrient partitioning.
Some of the seven mammalian sirtuin homologs of the yeast Sir2 gene belong to the key factors regulating energy balance in the CNS [[5], [6], [7]]. Sirtuins are a family of enzymes that remove acetyl groups from lysine residues on specific protein substrates. SIRT1 is the most intensively studied sirtuin, which regulates multiple mechanisms controlling feeding and energy expenditure [[8], [9], [10], [11]]. On the other hand, SIRT3 is known as an oxidative stress relief factor in mitochondria [[12], [13], [14]], but also for its implication in functions such as regulation of mitochondrial biogenesis and induction of fatty acid oxidation [12]. SIRT3 has been studied in peripheral organs and is increased in white adipose tissue (WAT), skeletal muscle, and liver after calorie restriction [[15], [16], [17]]. SIRT3 null mice were more sensitive to a high caloric diet and had increased insulin resistance and glucose intolerance, steatohepatitis, hyperlipidemias, and exhibited increased circulating inflammatory cytokines with age, compared to wild-type mice fed the same diet [18]. Assessment of the contribution of SIRT3 in each individual tissue indicated that it plays different roles in a tissue-specific manner. Overexpression of SIRT3 in the liver of a mouse model of non alcoholic fatty liver disease improved hepatic function [19]. Similarly, SIRT3 overexpresion in the intestine improves glucose homeostasis and insulin resistance in diet induced obese (DIO) mice by enhancing enterocyte oxidative metabolism [20]. However, mice lacking SIRT3 in muscle and liver do not show apparent metabolic dysregulation [21]. In the brain, it has been shown that SIRT3 modulates adaptive responses of cortical neurons to physiological challenges and resistance to degeneration [13]. Moreover, studies of non-neuronal cells suggest that SIRT3 mediates adaptations of mitochondria to increased energy demand [14,15]. However, despite the pivotal role of the hypothalamus in the regulation of energy balance [22], the potential actions of SIRT3 in hypothalamic neurons remains completely unknown. Here, we aim to investigate the role of SIRT3 at hypothalamic level.
2. Material and methods
2.1. Animal care
8- to 10-week-old (250–300 g) male Sprague Dawley rats (University of Santiago de Compostela), POMC-IRES-Cre male and female mice (on C57BL/6J background), age 8–10 weeks old, and AgRP-IRES-cre male and female mice (on C57BL/6J background), age 8–10 weeks old from Jackson Laboratory, were housed under conditions of controlled temperature (23 °C) and illumination (12-h light/12-h dark cycle). The animals were allowed ad libitum access to water and low-fat (LF) diet (10% Kcal fat) or a high fat diet (HFD; 60% Kcal fat) (reference #: D12492 and D12450B resp., Research Diets, NJ, US). The care of all animals was within institutional animal care committee guidelines, and all procedures were reviewed and approved by the Ethics Committee of the USC, in accordance with EU normative for the use of experimental animals. To test the effect of acute metabolic changes in ARC SIRT3 levels, 10-week-old male rats were deprived of food for 48 h or refed for 24 h after fasting while the control group was fed ad libitum.
Body weights and food intake were monitored weekly after lentivirus injection. Whole body composition was measured using nuclear magnetic resonance imaging (Whole Body Composition Analyzer; EchoMRI, Houston, TX) as previously described [23]. Heat production was visualized using a high-resolution infrared camera (FLIRS systems) as previously described [23,24]. Animals were killed by decapitation, and the tissues were removed rapidly, frozen immediately on dry ice, and kept at −80 °C until analysis.
2.2. Stereotaxic microinjection of lentiviral expression vectors
Ad libitum fed rats were anesthetized by an intraperitoneal injection of ketamine (100 mg/kg body weight)/xylazine (15 mg/kg body weight) and subjected to stereotaxic surgery. Lentiviral vectors (1 μl per hemisphere) encoding a shSIRT3 that silence SIRT3 expression (shSIRT3) (1.0 × 106 PFU/mL, Sigma-Aldrich) or GFP controls (1.0 × 106 PFU/mL, Sigma-Aldrich) were injected bilaterally into the ARC AP: 2.85 mm, L: ±0.3 mm, and V: 10.2 mm, with a 25-gauge needle (Hamilton) [[21], [22], [23]]. GFP fluorescence was used as a visual marker of effective transduction of the lentivirus at the injection site. Dissection of the ARC was performed by micropunches under the microscope, as previously shown [[25], [26], [27], [28]].
POMC-IRES-Cre- or AgRP-IRES-Cre C57BL/6 mice of both sexes were fed with chow or HFD for 15 consecutive weeks. Mice were anesthetized by an intraperitoneal injection of Ketamine/Xylazine cocktail (ketamine 15 mg/kg BW/xylazine 3 mg/kg BW) and placed in a stereotaxic frame (Kopf Instruments). AAV8-hSyn-shSIRT3-DIO-GFP (1.17 × 1013 PFU/ml, Vector Builder) or AAV8-hSyn-DIO-GFP (1.1 × 1013 PFU/ml, Vector Builder) under cell-type specific cre promotorers, were injected in the ARC. The ARC was targeted bilaterally using a 32-gauge needle connected to a 1-ml syringe (Neuro-Syringe, Hamilton) and AAV were delivered at a rate of 0.1 μl/min for 5 min (0,5 μl/injection site) according to the following coordinates: 1.5 mm posterior to the bregma, ± 0.2 mm lateral to midline, and −6 mm below the surface of the skull as previously reported [27,28].
2.3. Glucose and insulin tolerance test
We performed glucose (GTT) and insulin tolerance tests (ITT) in rats and mice by injecting d-glucose (2 mg/g) or insulin (0.50 IU/kg) intraperitoneally (ip) after 6 h fasting. Blood samples were collected immediately before and 15, 30, 60 and 120 min after glucose or insulin administration. Blood glucose was measured using a Glucometer (Arkray) from the tail vein [27,28]. Insulin levels were determined by a ELISA kit using reagents and methods provided by Millipore Corporation.
2.4. Western blot analysis
Western blot was performed as previously described [[26], [27], [28]]. Briefly, protein lysates from ARC (14 μg) WAT and BAT (20 μg) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, electrotransferred on a polyvinylidene difluoride membrane, and probed with various primary antibodies (Supplementary Table 1). For protein detection, we used horseradish-peroxidase–conjugated secondary antibodies (Dako Denmark, Glostrup, Denmark) and chemiluminescence (Pierce ECL Western Blotting Substrate; Thermo Scientific, Waltham, MA). Then, the membranes were exposed to radiograph film (Super RX, Fuji Medical X-Ray Film; Fujifilm, Tokyo, Japan) and developed with developer and fixing liquids (AGFA, Mortsel, Belgium) under appropriate dark room conditions. The protein levels were normalized to β-actin, GAPDH or α-tubulin for each sample.
2.5. Hematoxylin/eosin staining
WAT and BAT samples were fixed in 10% formalin buffer for 24 h, and then dehydrated and embedded in paraffin by a standard procedure. Sections of 3 μm thickness were prepared with a microtome and stained using a standard Hematoxylin/Eosin Alcoholic (BioOptica) procedure according to the manufacturer's instructions [23,26,28].
2.6. Immunofluorescence
Pomc-Cre and AgRP-Cre mice were anesthetized with ketamine-xylazine and perfused intracardially with saline (0.9% NaCl) followed by 10% buffered formalin. Fixed brains were immersed in 30% sucrose and 0.01% sodium azide in PBS at 4 °C for 2 days. Next, 3 sets of coronal sections (40-μm-thick) were cut in a freezing microtome Leica CM1850 UV and stored at −20 °C in cryo-protectant.
One set of free-floating sections from each animal was washed three times in TBS 0.1 M for 10 min each and incubated in blocking solution (2% donkey serum + 0.3% Triton X-100) in TBS 0.1 M for 60 min. Then, sections were incubated in rabbit anti-POMC (1/200; Phoenix pharmaceuticals, Cat# H-029-30), goat anti-AgRP (1/5000; Phoenix pharmaceuticals, Cat# H-003-57) or chicken anti-GFP (1/1000, Invitrogen A10262 Cat# 10,524,234) in blocking solution for 24 h at 4 °C. After incubation in the primary antibody, sections were rinsed with TBS 0.1 M three times for 10 min each and then incubated in the secondary antibody: Cy3 donkey anti-rabbit (Jackson ImmunoResearch Labs Cat#711-165-162); Cy3 donkey anti-goat (Jackson ImmunoResearch Labs Cat#705-165-147) and goat anti-chicken Alexa 488 (abcam Cat# ab150169) for 60 min at room temperature. Sections were then washed and coverslipped with Fluorogel coverslip mounting solution [27,28].
2.7. Statistical analysis and data presentation
Data are expressed as mean ± SEM. Protein data was expressed relative to (%) to the levels in control (GFP-treated) rats/mice. Error bars represent SEM. Statistical significance was determined by student's t-test when two groups were compared or one- or two-way analysis of variance (ANOVA) with post-hoc two-tailed Bonferroni test when more than two groups were compared. P < 0.05 was considered significant.
3. Results
3.1. SIRT3 levels are regulated by nutrient availability
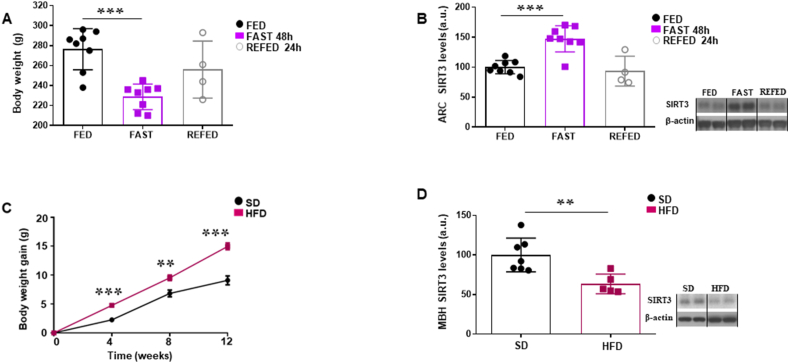
SIRT3 is expressed in POMC and AgRP neurons [29], specifically located in the ARC and essential in the control of energy balance. In order to investigate regulation of ARC SIRT3 levels by nutritional status, we measured SIRT3 in rats fed ad libitum, fasted and refed conditions. Rats fasted during 48 h exhibited a loss of body weight, whereas the refeeding during 24 h led to a partial recovery (Fig. 1A). SIRT3 levels were increased in the ARC of fasted rats, which recovered to baseline after refeeding (Fig. 1B). Next, we sought to investigate if the SIRT3 levels were also regulated after chronic metabolic changes such as diet-induced obesity. We found that SIRT3 levels in the medio-basal hypothalamus (MBH) of obese mice were decreased compared to control mice (Fig. 1C and D). Therefore, our results suggest that hypothalamic SIRT3 might play a metabolic role.
Fig. 1.
(A) Body weight of rats under different nutritional status (fed, fast 48 h, refed 24 h) under standard diet. (B) Protein levels of SIRT3 in the ARC of rats under different nutritional status (fed, fast 48 h, refed 24 h). (C) Body weight gain of DIO mice. (D) Protein levels of SIRT3 in the MBH of DIO mice. Values are mean ± SEM of 8 animals per group. *P ≤ 0.05, **P ≤ 0.01 vs controls (Paired t-test and one-way ANOVA).
3.2. Genetic inhibition of SIRT3 in the ARC decreases nutrient intake and weight gain
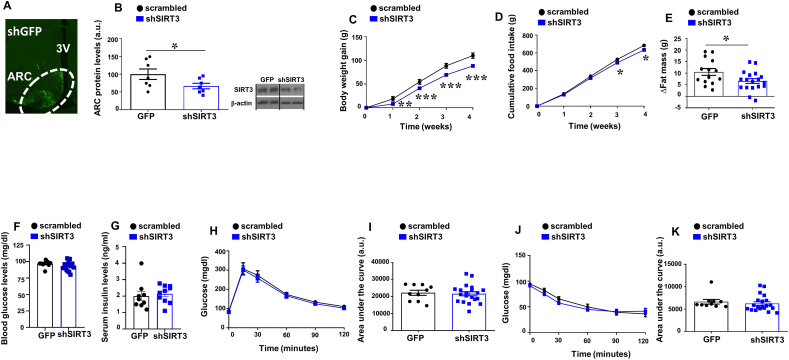
We next employed virogenetic approaches to inhibit SIRT3 in the ARC of adult rats. For this purpose, we injected lentiviruses encoding scrambled or shSIRT3 together with GFP into the ARC. The infection efficiency was assessed by the expression of GFP in the ARC (Fig. 2A) and by protein levels of SIRT3 in the ARC that were significantly decreased in shSIRT3 treated animals compared to shGFP injected controls (Fig. 2B). Inhibition of ARC SIRT3 led to significantly less weight gain than scrambled injected controls after 4 weeks on a chow diet (Fig. 2C). Accordingly, the genetic knockdown of SIRT3 in ARC also decreased nutrient intake (Fig. 2D). Body composition revealed that shSIRT3 injected rats accrued less fat mass compared to rats injected with shGFP (Fig. 2E).
Fig. 2.
(A) GFP expression in the hypothalamic ARC after stereotaxic injection of shSIRT3 lentivirus (B) Protein levels of SIRT3 in the ARC of male rats stereotaxically injected with scrambled or shSIRT3 lentiviruses. (C) Effect on body weight (D) and food intake and (E) Fat mass. Fasting levels of glucose (F) and insulin (G) glucose tolerance test (H) and area under the curve (I) insulin tolerance test (J) and area under the curve (K) in male rats fed chow diet that received shSIRT3 or GFP scrambled lentiviruses in the ARC. β-actin was used to normalize protein levels. Dividing lines indicate spliced bands from the same gel. Values are mean ± SEM of 7–18 animals per group. *P ≤ 0.05, **P ≤ 0.01 vs controls (Paired t-test and two way ANOVA).
SIRT3 is known to be involved in the regulation of insulin resistance and glucose homeostasis in peripheral tissues. Specifically, SIRT3 KO mice exihibit an aggravation of insulin resitance by impairment of muscle glucose uptake [30,31]. Therefore, we tested whether the observed actions of hypothalamic SIRT3 on body weight could be expanded to the control of glucose homeostasis and insulin sensitivity. First, we measured glucose and insulin levels after 6 h of fasting. Our results showed that SIRT3 inhibition in the ARC does not affect basal glucose levels (Fig. 2F) or basal insulin levels (Fig. 2G). A more in-depth investigation of glucose homeostasis, using GTT (Fig. 2H and I) and ITT (Fig. 2J and K), confirmed that SIRT3 knockdown in the ARC does not affect glucose tolerance or insulin sensitivity.
3.3. Genetic inhibition of SIRT3 in the ARC is associated with lipid mobilization, stimulated BAT thermogenesis and browning of WAT
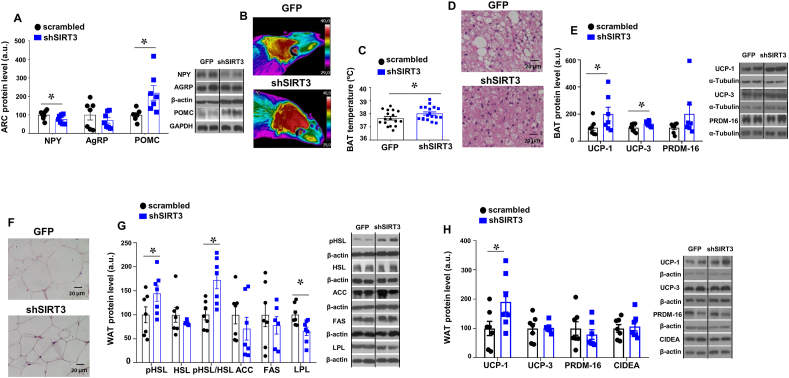
Consistent with the observed decrease in body weight and food intake after the genetic inhibition of SIRT3 in the ARC, we found increased protein levels of POMC and decreased protein levels of NPY in this brain area (Fig. 3A). We also found that the inhibition of SIRT3 in the ARC significantly increased the BAT interscapular temperature and decreased the size of the lipid droplets in the BAT (Fig. 3B–D). Consistent with these data, we found increased protein levels of the thermogenic markers uncoupling protein −1 (UCP1) and −3 (UCP-3) levels in BAT of shSIRT3 treated rats (Fig. 3E). Furthermore, shSIRT3 injected rats showed a diminished size of white adipocytes compared to shGFP treated rats (Fig. 3F). In the white adipose tissue, we also assessed the different components of lipid metabolism, and found a decrease in LPL (that may suggest a reduction in fatty acid uptake) and a significant increase in the phosphorylated levels of hormone sensitive lipase (pHSL), a surrogate marker of fatty acid lipolysis (Fig. 3G). However no changes were observed in other enzymes of the fatty acid biosynthesis namely acetyl coenzyme A carboxylase (ACC) and fatty acid synthase (FAS). (Fig. 3G).
Fig. 3.
(A) Protein levels of NPY, AgRP and POMC in the hypothalamic ARC of male rats stereotaxically injected with scrambled or shSIRT3 lentiviruses in the ARC (B) Representative infrared thermal images (C) BAT temperature (D) representative pictures of BAT histology and (E) BAT protein levels. (F) Representative pictures of WAT histology and (G) WAT protein levels of pHSL, HSL, ACC, FAS and LPL (H) WAT protein levels of UCP-1, UCP-3, PRDM16 and CIDEA in male rats fed chow diet injected with shSIRT3 or GFP scrambled lentiviruses into the ARC. α-tubulin, GAPDH or β-actin were used as loading control. Dividing lines indicate spliced bands from the same gel. Values are mean ± SEM of 7–18 animals per group. *P ≤ 0.05, **P ≤ 0.01 vs controls (Paired t-test).
Emerging evidence in the last years have demonstrated that activation of beige/brite adipocytes in the WAT, a process known as browning, has impact on total energy balance [32,33]. According to the decreased body weight after shSIRT3 injection in the ARC we found that, in addition to the increased thermogenesis in BAT, the protein levels of the thermogenic marker UCP1 was significantly increased in WAT, although no changes were detected in the other thermogenic markers studied (Fig. 3H). These data suggest that, in addition to elevated BAT activity, the browning of WAT might be also an important mechanism to reduce body weight following SIRT3 knockdown.
3.4. SIRT3 is expressed in AgRP and POMC neurons
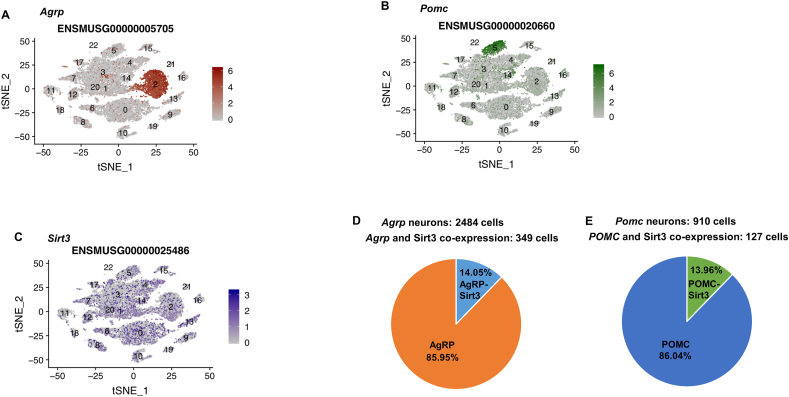
Taking advantage of the public dataset of ARC single cell transcriptomics [34] we analyzed SIRT3 expression levels across various cell clusters in this brain region, including the well-defined subsets of AgRP and POMC neurons (Fig. 4A and B). SIRT3 was found to be ubiquitously expressed in the 22 cell clusters within the ARC (Fig. 4C). We observed an enrichment in AgRP and POMC clusters, where the degree of colocalization was 14.05% and 13.96% respectively (Fig. 4D and E).
Fig. 4.
FACS sorting and single-cell RNA sequencing of AgRP neurons (A) and POMC neurons (B) and sirt3 expression in the ARC (C) % Sirt3 co-expression in AgRP neurons (D) % Sirt3 co-expression in POMC neurons (E) from (GEODatabase repository, GEO Accession: GSE92707).
3.5. Genetic inhibition of SIRT3 in AgRP neurons does not regulate energy balance
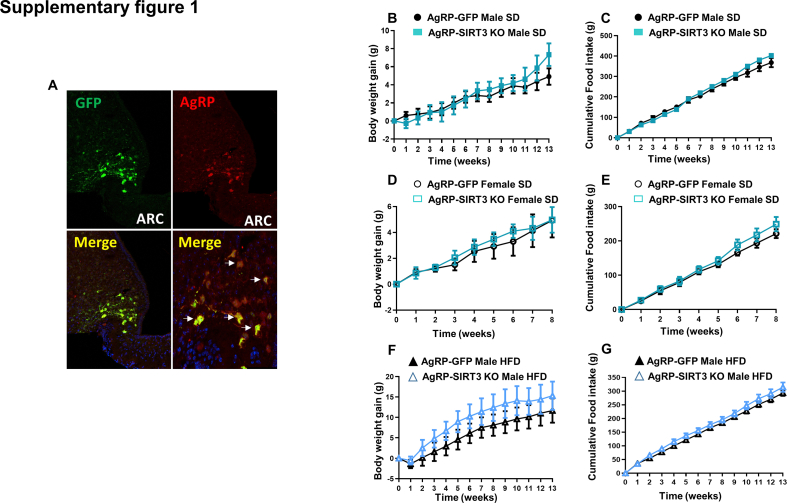
To shed more light about which neuronal population within the ARC is required for the metabolic actions elicited by SIRT3 inhibition, we generated mice with selective ablation of SIRT3 in AgRP neurons by injecting AAV8-hSyn-shSIRT3-DIO-GFP into the ARC of male and female AgRP-IRES-Cre mice fed a chow diet. The specificity of the infection was demonstrated because GFP staining was restricted to AgRP neurons (Supplementary Figure 1A). Male and female AgRP-SIRT3 KO mice showed no differences in food intake and body weight when compared with their control littermates (Supplementary Fig. 1B-1E). In addition, the deletion of SIRT3 in mice fed a HFD did not alter body weight and feeding (Supplementary Fig. 1F and 1G). Thus, these data determine that the effects of ARC SIRT3 inhibition over the control of energy balance are independent of AgRP neurons.
3.6. Genetic inhibition of SIRT3 in POMC neurons decreases weight gain and increases energy expenditure
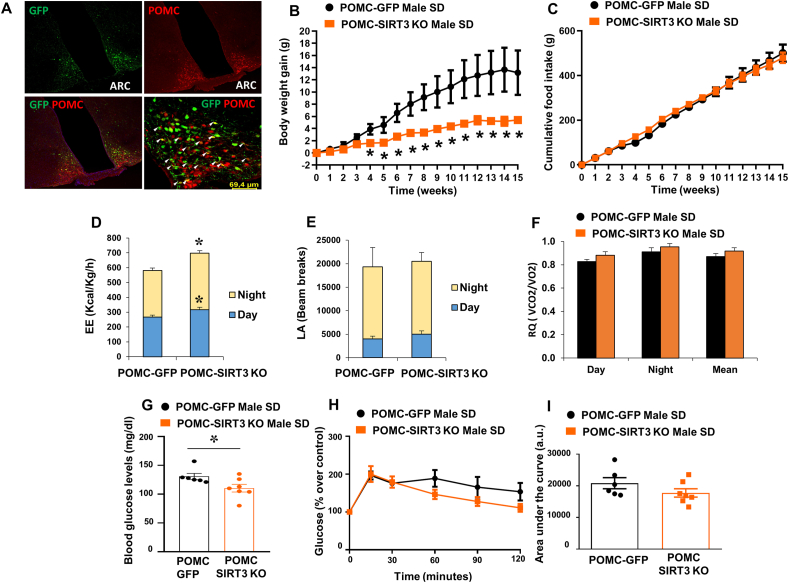
To ascertain if SIRT3 inhibition requires POMC neurons for the metabolic actions elicited in the ARC, we next generated mice with selective ablation of SIRT3 in POMC neurons, by injecting AAV8-hSyn-shSIRT3-DIO-GFP into the ARC of POMC-IRES-Cre mice. Our results showed the specificity of the infection because GFP staining was restricted to POMC neurons (Fig. 5A). POMC-SIRT3 KO mice fed a chow diet showed reduced body weight without changes in food intake (Fig. 5B and C). Consitently with these data, POMC-SIRT3 KO mice exhibited increased energy expenditure, without changes in locomotor activity or respiratory quotient (Fig. 5D–F). In order to ascertain the posible role of SIRT3 in POMC neurons on glucose homeostasis, we first measured basal glucose after 6 h of fasting. Our results showed that SIRT3 inhibition in the POMC reduced basal glucose levels (Fig. 5G). However, we did not find any change in the glucose tolerance between groups (Fig. 5H and I). These results confirm that SIRT3 inhibition in POMC neurons, but not AgRP neurons, regulate energy balance in the ARC.
Fig. 5.
(A) Immunofluorescence picture showing that GFP infection was restricted to POMC neurons. (B) Effect on body weight (C) food intake (D) energy expenditure (E) locomotor activity (F) respiratory quotient (G) Basal blood glucose levels (H) glucose tolerance test and (I) area under the curve in male mice fed chow diet that received AAV8-hSyn-shSIRT3-DIO-GFP or AAV8-hSyn-DIO-GFP scrambled viruses in the POMC neurons. Values are mean ± SEM of 6–7 animals per group. *P ≤ 0.05, **P ≤ 0.01 vs controls (Paired t-test and two way ANOVA).
3.7. Genetic inhibition of SIRT3 in the POMC neurons increases lipid mobilization, BAT thermogenesis and browning of WAT
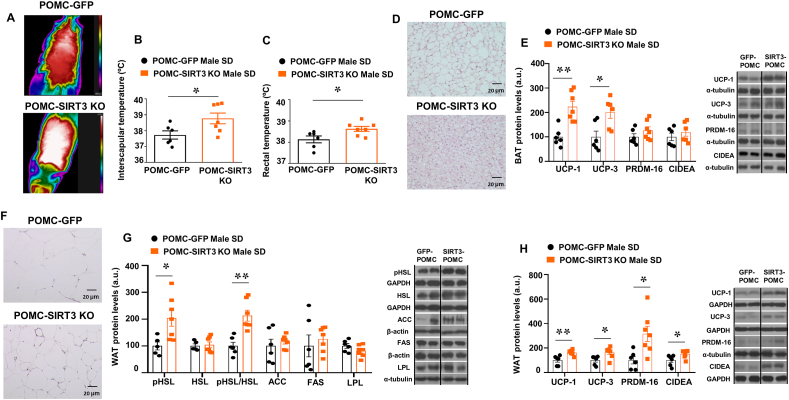
In agreement with the increased energy expenditure in the POMC-SIRT3 KO mice, they displayed a higher body and interscapular surface temperature adjacent to BAT (Fig. 6A–C), less lipid droplets in the BAT (Fig. 6D) and up-regulated protein levels of BAT UCP1 and UCP-3 (Fig. 6E). POMC SIRT3 KO mice also showed reduced white adipocyte size (Fig. 6F) and increased expression of lipid oxidation markers, as reflected by a significant increase in the phosphorylated levels of HSL, while no changes in other enzymes of the fatty acid metabolism, namely LPL, ACC or in FAS (Fig. 6G). In addition to the effects in BAT, deletion of SIRT3 in POMC neurons significantly increased protein levels of the thermogenic marker UCP1, UCP3, PRDM16 and CIDEA in WAT (Fig. 6H). These data suggest that lipid oxidation, BAT thermogenesis and the browning of WAT participate in the reduction of body weight of POMC SIRT3 KO mice.
Fig. 6.
(A) Representative infrared thermal images (B) BAT temperature (C) rectal temperature (D) representative pictures of BAT histology and (E) BAT protein levels. (F) representative pictures of WAT histology and (G) WAT protein levels of pHSL, HSL, ACC, FAS and LPL and (H) WAT protein levels of UCP-1, UCP-3, PRDM16 and CIDEA in male mice fed chow diet injected with AAV8-hSyn-shSIRT3-DIO-GFP or AAV8-hSyn-DIO-GFP scrambled viruses in POMC neurons. α-tubulin, GAPDH or β-actin were used as loading control. Dividing lines indicate spliced bands from the same gel. Values are mean ± SEM of 6–7 animals per group. *P ≤ 0.05, **P ≤ 0.01 vs controls (Paired t-test).
3.8. Genetic inhibition of SIRT3 in the POMC does not affect body weight in female mice
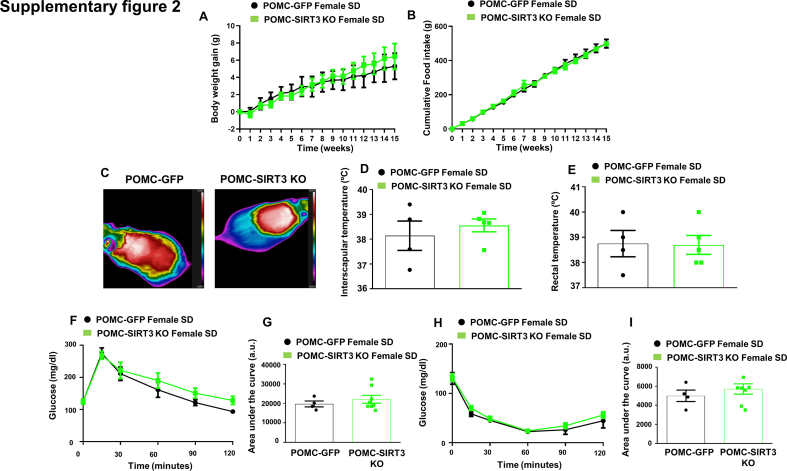
Since there is evidence for sex differences in obesity and body fat distribution, possibly related to differences in how body metabolism is regulated by the central nervous system, we also injected AAV8-hSyn-shSIRT3-DIO-GFP in the ARC of POMC-IRES-Cre female mice fed a chow diet to delete SIRT3 in POMC neurons. Notably, our results claim for a sex-specific role on the actions of SIRT3 in POMC neurons since no effect on body weight, food intake, BAT thermogenesis, glucose or insulin sensitivity were detected in female SIRT3 POMC KO mice compared to their control littermates injected with the scrambled GFP virus (Supplementary Figure 2).
3.9. Genetic inhibition of SIRT3 in the POMC neurons does not regulate energy balance in DIO animals
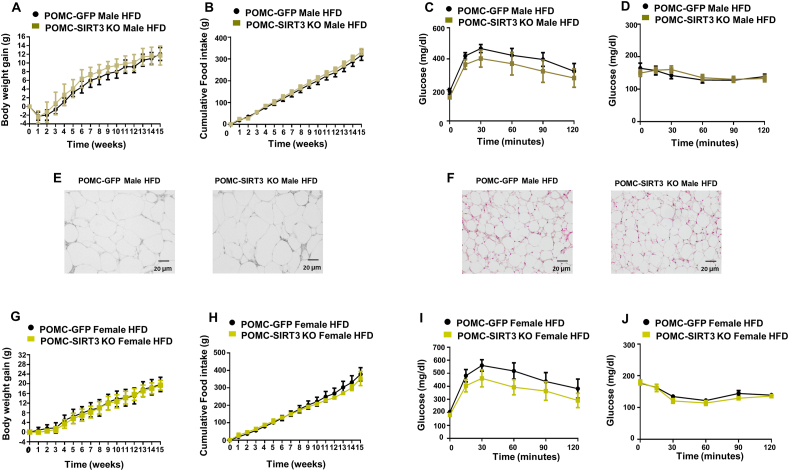
To shed more light on the involvement of SIRT3 in obesity, and taking into account that SIRT3 levels are decreased in the MBH of DIO mice, we challenged male and female SIRT3 POMC KO mice with 60% HFD for 15 weeks. However, in contrast with our expectations, no changes in body weight, food intake, adipocyte size, BAT lipid content, glucose tolerance or insulin sensitivity were found after injection of AAV8-hSyn-shSIRT3-DIO-GFP in the ARC of male and female POMC-IRES-Cre mice under HFD (Fig. 7A-J). Therefore, our results suggest that the effect of SIRT3 ablation in POMC neurons is blunted by DIO.
Fig. 7.
(A) Effect on body weight (B) food intake (C) glucose tolerance test and (D) insulin tolerance test (E) representative pictures of WAT histology and (F) representative pictures of BAT histology in male mice fed HFD that received AAV8-hSyn-shSIRT3-DIO-GFP or AAV8-hSyn-DIO-GFP scrambled viruses in the POMC neurons. (G) Effect on body weight (H) food intake (I) glucose tolerance test and (J) insulin tolerance test in female mice fed HFD that received AAV8-hSyn-shSIRT3-DIO-GFP or AAV8-hSyn-DIO-GFP scrambled viruses in the POMC neurons. Values are mean ± SEM of 6–7 animals per group.
4. Discussion
Recent studies have identified sirtuins as potential therapeutic targets for the treatment of metabolic diseases such as obesity or type II diabetes [6,35]. SIRT3, which is a nicotinamide adenine dinucleotide-dependent enzyme that exerts post-translational modifications of its target proteins, was originally identified as a mitochondrial and oxidative stress regulator [6]. This knowledge mainly arose from studies on global or conditional knock-in or knock-out mouse models focusing on the role of SIRT3 in peripheral tissues [[18], [19], [20]]. However, the potential metabolic role of SIRT3 in the CNS has remained largely unexplored. In this work, we find for the first time that SIRT3 levels in ARC are regulated by nutrient availability and that its genetic inhibition in this hypothalamic area reduces body weight, adiposity, food intake, and stimulates BAT thermogenic activity and the browning of WAT. Interestingly, these effects rely on POMC neurons, because the deletion of SIRT3 in this neuronal population, but not in AgRP neurons, exert the same metabolic actions as displayed by the inhibition of SIRT3 in the whole ARC.
We found that energy deficiency elevated the protein levels of SIRT3 in the ARC, which were restored after refeeding. Moreover the levels decreased in a state of positive energy balance, such as diet-induced obesity. This regulation is similar to what has been found in peripheral tissues such as skeletal muscle, BAT or liver [[15], [16], [17], [18], [19]] or for other sirtuins in the hypothalamus [36,37]. At the molecular level, these actions involve the upregulation of POMC, the precursor of melanocortins, that not only control feeding behavior and adiposity [38,39], but also play a key role in the control of body temperature [40,41].
In our study we showed that SIRT3 in POMC neurons modulates body weight and energy expenditure by altering BAT and WAT metabolism. More specifically, silencing SIRT3 in POMC neurons stimulates BAT thermogenesis by triggering the level of some well-known molecular markers such as UCP-1 and UCP-3. Furthermore, the capacity of UCP-1 to increase the browning of WAT, provides an additional mechanism by which downregulation of SIRT3 in POMC neurons may control energy balance and reduce body weight gain. This central regulation of thermogenesis implies a novel pathway by which SIRT3 regulates body weight gain and metabolic parameters, since previous reports focused on SIRT3-mediated regulation of thermogenesis by direct actions in brown adipocytes [16,42]. Interestingly, the metabolic role of SIRT3 seems to be specific to POMC neurons since changes in body weight or energy expenditure occurred when the virogenetic inhibition SIRT3 was targeted to this neuronal population but not to AgRP neurons. We also identified that SIRT3 in POMC neurons regulates adipocyte lipid metabolism as showed by the findings that the expression of fatty acid mobilization markers was augmented after silencing SIRT3 in POMC neurons, as represented by the higher phosphorylated levels of HSL protein. These changes in lipid metabolism together with the increased thermogenic capacity of WAT, likely contribute to the reduction of adiposity and consequently body weight. Moreover, we discard any effect of SIRT3 in POMC neurons on the regulation of glucose homeostasis or insulin sensitivity. These data were unexpected because the deletion of SIRT3 in the ARC or POMC neurons leads to a loss of body weight and adiposity, two parameters that strongly influence glucose tolerance and insulin sensitivity. In this sense, it was recently reported that the over-expression of SIRT3 specifically in the intestine of DIO, but not lean mice, enhances glucose tolerance and reduces insulin resistance in absence of changes in body weight and adiposity [20]. Therefore, it seems that the effects of SIRT3 on glucose homeostasis occur in a tissue-specific manner.
Besides, given that gender strongly influence obesity and multiple metabolic pathways [43], we conducted experiments in females to investigate whether the deletion of SIRT3 could have a similar effect in both sexes. Our results suggest a clear sexual dimorphism in the action of hypothalamic SIRT3 in energy balance, because the reduced weight gain and increased thermogenic effect after the depletion of SIRT3 in POMC neurons of male mice were not seen in females. Indeed, this data are important for further research to determine the biological mechanism by which SIRT3 regulates body weight. The divergent actions of SIRT3 in our animal models is in concordance with the aforementioned heterogeneity of POMC and AgRP neurons [34,44].
Finally, we also did not find any metabolic effect of hypothalamic SIRT3 ablation in DIO animals, because the inhibition of this enzyme in POMC neurons of DIO mice did not produce any significant alterations in body weight, food intake or glucose homeostasis. Future studies will be necessary to address the molecular pathways involved in this diet-induced resistance, but it is well established that in obese states the signalling of a large number of pathways are disrupted causing resistance to hormones such as leptin and insulin [2,45].
In conclusion, we provide the first experimental evidence implicating hypothalamic SIRT3 in the regulation of energy balance, by modulating body weight, adiposity, non-shivering thermogenesis in BAT and browning in WAT. Importantly these effects are sex and nutrient dependent and rely on the action of SIRT3 in POMC neurons.
Authors contributions
M.Q., R.H.B., D. B and F.L.Y-L. performed in vivo experiments and western blots and collected and analyzed the data and made figures. A.S., V.H. and AG G-V. processed the histological samples and took fluorescent images. J.F., M.S., V.P., M.C., M.L., C. D., R.N. and O. A-M designed the experiments, discussed and edited the manuscript. M.Q., R.N. and O. A-M, wrote the manuscript.
Declaration of competing interest
There are no conflicts of interest to declare.
Acknowledgements
This work has been supported by grants from FEDER/Ministerio de Ciencia, Innovación y Universidades-Agencia Estatal de Investigación (CD: BFU2017-87721; RN: RTI2018-099413-B-I00; Xunta de Galicia (RN: 2015-CP080 and 2016-PG057) and ED431G 2019/02, Fundación BBVA (RN), Fundación Atresmedia (RN), European Foundation for the Study of Diabetes (RN), “la Caixa” Foundation (ID 100010434; under the agreements LCF/PR/HR19/52160016 to RN/MC and LCF/PR/HR19/52160022 to ML), Helse Vest RHF (JF: Western Norway Regional Health Authority) and Xunta de Galicia (MQ: 2018-PG013). The research leading to these results has also received funding from the European Community's H2020 Framework Programme under the following grants: ERC Synergy Grant-2019-WATCH- 810331 to RN, VP and MS and ERC Consolidator Grant MITOSENSING-725004 to MC. CIBERobn is an initiative of the Instituto de Salud Carlos III (ISCIII) of Spain which is supported by FEDER funds. M.Q. is a recipient of a Postdoctoral contract from Galician Government (Xunta de Galicia ED481B2018/004). OA-M was funded by a research contract Miguel Servet (CP20/00146) from the ISCIII.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.101945.
Contributor Information
Mar Quiñones, Email: mar.quinones@usc.es.
Omar Al-Massadi, Email: omar.al-massadi.iglesias@sergas.es.
Ruben Nogueiras, Email: ruben.nogueiras@usc.es.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
(A) Immunofluorescence picture showing that GFP infection was restricted to AgRP neurons. (B) Effect on body weight (C) food intake in male mice fed chow diet that received AAV8-hSyn-shSIRT3-DIO-GFP or AAV8-hSyn-DIO-GFP scrambled viruses in the AgRP neurons. (D) Effect on body weight (E) food intake in female mice fed chow diet that received AAV8-hSyn-shSIRT3-DIO-GFP or AAV8-hSyn-DIO-GFP scrambled viruses in the AgRP neurons. (F) Effect on body weight (G) food intake in male mice fed HFD diet that received AAV8-hSyn-shSIRT3-DIO-GFP or AAV8-hSyn-DIO-GFP scrambled viruses in the AgRP neurons. Values are mean ± SEM of 5–7 animals per group
Supplementary Fig. 2.
(A) Effect on body weight (B) food intake and (C) Representative infrared thermal images (D) BAT temperature (E) rectal temperature (F) glucose tolerance test and (G) area under the curve (H) insulin tolerance test and (I) area under the curve in female mice fed chow diet that received AAV8-hSyn-shSIRT3-DIO-GFP or AAV8-hSyn-DIO-GFP scrambled viruses in the POMC neurons. Values are mean ± SEM of 4–7 animals per group
References
- 1.Belsham D.D., Dalvi P.S. Insulin signalling in hypothalamic neurones. J. Neuroendocrinol. 2020 doi: 10.1111/jne.12919. [DOI] [PubMed] [Google Scholar]
- 2.Cui H., Lopez M., Rahmouni K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 2017;13(6):338–351. doi: 10.1038/nrendo.2016.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeo G.S., Heisler L.K. Unraveling the brain regulation of appetite: lessons from genetics. Nat. Neurosci. 2012;15(10):1343–1349. doi: 10.1038/nn.3211. [DOI] [PubMed] [Google Scholar]
- 4.Andermann M.L., Lowell B.B. Toward a wiring diagram understanding of appetite control. Neuron. 2017;95(4):757–778. doi: 10.1016/j.neuron.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onyango P., Celic I., McCaffery J.M., Boeke J.D., Feinberg A.P. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc. Natl. Acad. Sci. U. S. A. 2002;99(21):13653–13658. doi: 10.1073/pnas.222538099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nogueiras R., Habegger K.M., Chaudhary N., Finan B., Banks A.S., Dietrich M.O. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol. Rev. 2012;92(3):1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes P., Outeiro T.F., Cavadas C. Emerging role of sirtuin 2 in the regulation of mammalian metabolism. Trends Pharmacol. Sci. 2015;36(11):756–768. doi: 10.1016/j.tips.2015.08.001. Nov. [DOI] [PubMed] [Google Scholar]
- 8.Ramadori G., Fujikawa T., Fukuda M., Anderson J., Morgan D.A., Mostoslavsky R. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metabol. 2010;12(1):78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich M.O., Antunes C., Geliang G., Liu Z.W., Borok E., Nie Y. Agrp neurons mediate Sirt1's action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J. Neurosci. 2010;30(35):11815–11825. doi: 10.1523/JNEUROSCI.2234-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfluger P.T., Herranz D., Velasco-Miguel S., Serrano M., Tschöp M.H. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl. Acad. Sci. U. S. A. 2008;105(28):9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppari R. Metabolic actions of hypothalamic SIRT1. Trends Endocrinol. Metabol. 2012;23(4):179–185. doi: 10.1016/j.tem.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He W., Newman J.C., Wang M.Z., Ho L., Verdin E. Mitochondrial sirtuins: regulators of protein acylation and metabolism. Trends Endocrinol. Metabol. 2012;23(9):467–476. doi: 10.1016/j.tem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Cheng A., Yang Y., Zhou Y., Maharana C., Lu D., Peng W. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metabol. 2016;23(1):128–142. doi: 10.1016/j.cmet.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kincaid B., Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front. Aging Neurosci. 2013;5:48. doi: 10.3389/fnagi.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palacios O.M., Carmona J.J., Michan S., Chen K.Y., Manabe Y., Ward J.L., 3rd Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (N Y) 2009;1(9):771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi T., Wang F., Stieren E., Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J. Biol. Chem. 2005;280(14):13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- 17.Hirschey M.D., Shimazu T., Goetzman E., Jing E., Schwer B., Lombard D.B. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285):121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirschey M.D., Shimazu T., Jing E., Grueter C.A., Collins A.M., Aouizerat B. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell. 2011;44(2):177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He J., Hu B., Shi X., Weidert E.R., Lu P., Xu M. Activation of the aryl hydrocarbon receptor sensitizes mice to nonalcoholic steatohepatitis by deactivating mitochondrial sirtuin deacetylase Sirt3. Mol. Cell Biol. 2013;33(10):2047–2055. doi: 10.1128/MCB.01658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramachandran D., Clara R., Fedele S., Hu J., Lackzo E., Huang J.Y. Intestinal SIRT3 overexpression in mice improves whole body glucose homeostasis independent of body weight. Mol Metab. 2017;6(10):1264–1273. doi: 10.1016/j.molmet.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Marcos P.J., Jeninga E.H., Canto C., Harach T., de Boer V.C., Andreux P. Muscle or liver-specific Sirt3 deficiency induces hyperacetylation of mitochondrial proteins without affecting global metabolic homeostasis. Sci. Rep. 2012;2:425. doi: 10.1038/srep00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Tilve D. Novel hypothalamic mechanisms in the pathophysiological control of body weight and metabolism. Endocrinology. 2017;158(5):1085–1094. doi: 10.1210/en.2016-1944. [DOI] [PubMed] [Google Scholar]
- 23.Folgueira C., Beiroa D., Callon A., Al-Massadi O., Barja-Fernandez S., Senra A. Uroguanylin action in the brain reduces weight gain in obese mice via different efferent autonomic pathways. Diabetes. 2016;65(2):421–432. doi: 10.2337/db15-0889. [DOI] [PubMed] [Google Scholar]
- 24.Al-Massadi O., Porteiro B., Kuhlow D., Kohler M., Gonzalez-Rellan M.J., Garcia-Lavandeira M. Pharmacological and genetic manipulation of p53 in Brown fat at adult but not embryonic stages regulates thermogenesis and body weight in male mice. Endocrinology. 2016;157(7):2735–2749. doi: 10.1210/en.2016-1209. [DOI] [PubMed] [Google Scholar]
- 25.Quinones M., Al-Massadi O., Gallego R., Ferno J., Dieguez C., Lopez M. Hypothalamic CaMKKbeta mediates glucagon anorectic effect and its diet-induced resistance. Mol Metab. 2015;4(12):961–970. doi: 10.1016/j.molmet.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imbernon M., Sanchez-Rebordelo E., Romero-Pico A., Kallo I., Chee M.J., Porteiro B. Hypothalamic kappa opioid receptor mediates both diet-induced and melanin concentrating hormone-induced liver damage through inflammation and endoplasmic reticulum stress. Hepatology. 2016;64(4):1086–1104. doi: 10.1002/hep.28716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Massadi O., Quinones M., Clasadonte J., Hernandez-Bautista R., Romero-Pico A., Folgueira C. MCH regulates SIRT1/FoxO 1 and reduces POMC neuronal activity to induce hyperphagia, adiposity, and glucose intolerance. Diabetes. 2019;68(12):2210–2222. doi: 10.2337/db19-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quinones M., Al-Massadi O., Folgueira C., Bremser S., Gallego R., Torres-Leal L. p53 in AgRP neurons is required for protection against diet-induced obesity via JNK1. Nat. Commun. 2018;9(1):3432. doi: 10.1038/s41467-018-05711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henry F.E., Sugino K., Tozer A., Branco T., Sternson S.M. Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. Elife. 2015;4 doi: 10.7554/eLife.09800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lantier L., Williams A.S., Williams I.M., Yang K.K., Bracy D.P., Goelzer M. SIRT3 is crucial for maintaining skeletal muscle insulin action and protects against severe insulin resistance in high-fat-fed mice. Diabetes. 2015;64(9):3081–3092. doi: 10.2337/db14-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin L., Chen K., Abdel Khalek W., Ward J.L., 3rd, Yang H., Chabi B. Regulation of skeletal muscle oxidative capacity and muscle mass by SIRT3. PloS One. 2014;9(1) doi: 10.1371/journal.pone.0085636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nedergaard J., Cannon B. The browning of white adipose tissue: some burning issues. Cell Metabol. 2014;20(3):396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Fisher F.M., Kleiner S., Douris N., Fox E.C., Mepani R.J., Verdeguer F. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26(3):271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell J.N., Macosko E.Z., Fenselau H., Pers T.H., Lyubetskaya A., Tenen D. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 2017;20(3):484–496. doi: 10.1038/nn.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppari R., Ramadori G., Elmquist J.K. The role of transcriptional regulators in central control of appetite and body weight. Nat. Clin. Pract. Endocrinol. Metabol. 2009;5(3):160–166. doi: 10.1038/ncpendmet1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Velasquez D.A., Martinez G., Romero A., Vazquez M.J., Boit K.D., Dopeso-Reyes I.G. The central Sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes. 2011;60(4):1177–1185. doi: 10.2337/db10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang Q., Gao Y., Liu Q., Yang X., Wu T., Huang C. Sirt6 in pro-opiomelanocortin neurons controls energy metabolism by modulating leptin signaling. Mol Metab. 2020;37:100994. doi: 10.1016/j.molmet.2020.100994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joly-Amado A., Cansell C., Denis R.G., Delbes A.S., Castel J., Martinez S. The hypothalamic arcuate nucleus and the control of peripheral substrates. Best Pract. Res. Clin. Endocrinol. Metabol. 2014;28(5):725–737. doi: 10.1016/j.beem.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Krashes M.J., Lowell B.B., Garfield A.S. Melanocortin-4 receptor-regulated energy homeostasis. Nat. Neurosci. 2016;19(2):206–219. doi: 10.1038/nn.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butler A.A., Cone R.D. The melanocortin receptors: lessons from knockout models. Neuropeptides. 2002;36(2–3):77–84. doi: 10.1054/npep.2002.0890. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda T., Masaki T., Kakuma T., Yoshimatsu H. Hypothalamic melanocortin system regulates sympathetic nerve activity in brown adipose tissue. Exp. Biol. Med. 2004;229(3):235–239. doi: 10.1177/153537020422900303. [DOI] [PubMed] [Google Scholar]
- 42.Giralt A., Hondares E., Villena J.A., Ribas F., Diaz-Delfin J., Giralt M. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha controls transcription of the Sirt3 gene, an essential component of the thermogenic brown adipocyte phenotype. J. Biol. Chem. 2011;286(19):16958–16966. doi: 10.1074/jbc.M110.202390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez M., Tena-Sempere M. Estrogens and the control of energy homeostasis: a brain perspective. Trends Endocrinol. Metabol. 2015;26(8):411–421. doi: 10.1016/j.tem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Lam B.Y.H., Cimino I., Polex-Wolf J., Nicole Kohnke S., Rimmington D., Iyemere V. Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing. Mol Metab. 2017;6(5):383–392. doi: 10.1016/j.molmet.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konner A.C., Bruning J.C. Selective insulin and leptin resistance in metabolic disorders. Cell Metabol. 2012;16(2):144–152. doi: 10.1016/j.cmet.2012.07.004. [DOI] [PubMed] [Google Scholar]