Abstract
We present here the most comprehensive analysis to date of neuroaesthetic processing by reporting the results of voxel-based meta-analyses of 93 neuroimaging studies of positive-valence aesthetic appraisal across four sensory modalities. The results demonstrate that the most concordant area of activation across all four modalities is the right anterior insula, an area typically associated with visceral perception, especially of negative valence (disgust, pain, etc.). We argue that aesthetic processing is, at its core, the appraisal of the valence of perceived objects. This appraisal is in no way limited to artworks but is instead applicable to all types of perceived objects. Therefore, one way to naturalize aesthetics is to argue that such a system evolved first for the appraisal of objects of survival advantage, such as food sources, and was later co-opted in humans for the experience of artworks for the satisfaction of social needs.
Keywords: Aesthetics, Arts, Neuroaesthetics, Brain, fMRI, Meta-analysis, ALE, Orbitofrontal, Anterior insula, Anterior cingulate, Pleasure, Valence, Pleasantness, Hedonics, Music, Preference, Visceral, Interoceptive, Exteroceptive
Introduction
The notion of “the aesthetic” is a concept from the philosophy of art of the 18th century according to which the perception of beauty in sublime artworks occurs by means of a special process distinct from the appraisal of ordinary objects, for example food items (Goldman, 2001; Guyer, 2005). Hence, our appreciation of a painting is presumed to be cognitively distinct from our appreciation of an apple. This is due in part to our “disinterested” approach to the painting, in other words our emotional detachment from the painting due to its lack of practical use. The field of “neuroaesthetics” has adopted this distinction between art and non-art objects by seeking to identify brain areas that mediate the aesthetic appreciation of artworks, generally works from the domain of visual art (Zeki, 1999; Di Dio and Gallese, 2009; Skov, 2009; but see Brown and Dissanayake, 2009, and Vartanian, 2009).
However, studies from neuroscience and evolutionary biology challenge this separation of art from non-art, and instead call for a naturalization of aesthetics, in other words a revised conception of aesthetic processing that is more biological and adaptive in scope. Human neuroimaging studies have convincingly shown that the brain areas that mediate aesthetic responses to artworks overlap those that mediate the appraisal of objects of evolutionary importance, such as the desirability of food items or the attractiveness of potential mates. Hence, it is likely that artworks have co-opted the neural systems that subserve these kinds of adaptive assessments rather than having evolved a distinct type of neural processing. In addition, while “the aesthetic” of Enlightenment philosophy places an exclusive focus on positive-valence assessments – and thus beauty – aesthetic processing is best thought of as a binary phenomenon, with both positive and negative counterparts. Thus, negative-valenced emotions such as dislike and disgust are just as much aesthetic emotions as are awe and ecstasy. Aesthetic processing, at its core, can thus be equated with object-appraisal processes, resulting in emotions that sit along the spectrum from transcendence to repulsion. When seen in this way, aesthetic emotions become major factors in guiding motivation and decision making. We would thus expect neural pathways for object appraisal to involve areas not only for object perception but those for homeostatic processing, emotion, motivation, and motor control as well.
An important step towards naturalizing aesthetics is to ground aesthetic emotions in theories of emotion more generally. The standard model of emotion in psychology and biology – the basic emotion theory – offers little insight into aesthetics since it contains no primary emotion for positive-valenced appraisals such as pleasure, although it does include the negative-valenced counterpart of disgust (Ekman, 1992). The only positive-valenced emotion in the basic emotion theory is happiness, an emotion that is often conflated with pleasure. An influential alternative to the basic emotion theory, that put forward by Ortony et al. (1988), makes clear that aesthetic pleasure is an object-related emotion, whereas happiness is an outcome-related emotion. Hence, aesthetic emotions such as pleasure and repulsion are qualitatively distinct from outcome-related emotions such as happiness and disappointment.
This distinction between objects and outcomes figures prominently in neuroscience as well. Rushworth et al. (2008) presented a neural model of decision-making based on the notion that the appraisal of objects (what they refer to as “stimuli”) is most strongly associated with the orbitofrontal cortex (OFC), whereas the appraisal of outcomes (what they refer to as “actions”) is most strongly associated with the anterior cingulate cortex (ACC), and that these two appraisals can be doubly dissociated through lesions in animals. This is consistent with neuroanatomical studies showing that the OFC is a form of higher-level sensory cortex receiving input from “what” sensory pathways involved in object processing (Rolls, 2005), whereas the ACC is a premotor area involved in predicting and monitoring outcomes in relation to motivational intentions (Carter and van Veen, 2007). Hence, the object/outcome dichotomy makes important predictions about aesthetic processing, suggesting that aesthetic emotions should be primarily associated with object appraisal mechanisms in the OFC.
There is, in fact, an abundant neuroimaging literature in humans (most of it distinct from the neuroaesthetic banner) showing that the OFC is reliably activated during tasks that require people to make appraisals of the quality of objects, both art and non-art objects (Kringelbach, 2005; Wallis, 2007). This would suggest that the OFC is a prime candidate for being the “aesthetic center” of the human brain. But the situation is more complicated than that. First, the OFC plays a prominent role in general sensory processing, being a secondary sensory cortex for both olfaction and gustation (Kringelbach, 2004; Rolls, 2004). Second, it serves a role in polysensory convergence, not least in the interaction between olfaction and gustation during “flavor” processing. Third, the OFC processes emotions of both valences, and there is no clear understanding of how positive- and negative-valenced appraisals are represented in the OFC.
Our goal in this study was to apply quantitative meta-analysis techniques to a comprehensive corpus of human neuroimaging studies of aesthetic processing across multiple sensory modalities and across both non-art and art objects. We were interested in seeing if there was indeed a supramodal brain area that is active during the process of object appraisal. The OFC was clearly our strongest candidate for such an area. Therefore, one of the major questions we wanted to address was whether regions of OFC activation across sensory modalities were overlapping or instead sensory-specific.
Methods
inclusion criteria for papers
Meta-analysis of 93 published neuroimaging studies was performed using “activation likelihood estimation” (ALE) analysis. The studies are summarized in Supplementary Tables 1 and 2. They included papers that performed ROI-based and correlational analyses in addition to standard activation analyses. Database searches were carried out by the first three authors using search terms such as “aesthetics”, “aesthetics + fMRI”, “aesthetics + orbitofrontal” and “aesthetics + insula”. In addition, extensive use was made of the Web of Knowledge database in order to find articles citing ones we already had. All three authors had to agree on the suitability of a paper for it to be included in a meta-analysis.
Our inclusion criteria for articles were: 1) that whole-brain analyses were performed, using either functional magnetic resonance imaging (fMRI) or positron emission tomography (PET) (thereby excluding electrophysiology-based studies; ROI-based analyses were taken from whole-brain studies); 2) that the papers provided either Talairach or Montreal Neurological Institute (MNI) coordinates for their activation foci (thereby excluding papers that reported activations using neuroanatomical labels alone); 3) that the subjects were healthy individuals and not part of clinical populations (thereby excluding studies on, for example, depressed patients or individuals with feeding or body-perception disorders, such obese individuals or anorexics); 4) that the tasks involved some type of aesthetic evaluation of the presented stimuli, including ratings of pleasantness, attractiveness, and liking (thereby excluding studies of general sensory processing, decision making alone, or studies in which no explicit aesthetic appraisal was made by subjects); and 5) that task appraisals were of positive valence (thereby excluding studies of disgust, pain, noxious quality, unpleasantness, and the like). While the issue of negative valence is of central relevance to our approach to aesthetics, there were not enough papers across the five major sensory modalities to justify doing a parallel set of meta-analyses for negative-valence processing at the present time. Some of the studies that were included in the meta-analyses performed comparisons between positive- and negative-valence assessments, but we only selected the positive-valence tasks from those papers.
To elaborate further on our exclusion criteria, we excluded papers that did not place a central focus on aesthetic evaluation. This included studies that were primarily devoted to decision making, studies that were focused on learning and conditioning, studies that used rewarding stimuli as primes for other cognitive tasks, studies that used aesthetic-connoting words only, studies that had people state preferences among two choices, and studies that focused on reward processing in neuroeconomic experiments. In addition, we excluded studies of valence or arousal processing in which no ratings of pleasantness or attractiveness were made by subjects. This exclusion covered many studies of picture processing, including those of erotic stimuli.
While the distinction between “liking” and “wanting” is one that is frequently discussed in the neural literature on reward processing (Berridge and Kringelbach, 2009; Berridge et al., 2009), we do not consider this distinction here, as most of the studies looked at ratings of pleasantness or attractiveness. Hence, the focus was more on hedonic value (liking) than incentive value (wanting) or preference.
Activation likelihood estimation (ALE) analysis
Four parallel ALE meta-analyses were performed for four major sensory modalities, respectively: 1) vision (56 papers, 242 foci across the whole brain); 2) audition (8 papers, 95 foci); 3) gustation (16 papers, 136 activation foci); and 4) olfaction (13 papers, 109 foci). These are summarized in Supplementary Table 2. The vision category had a number of subcategories, including artworks, faces, general pictures, erotic images, food images, and images of loved ones (such as infants and romantic partners). For the purposes of this meta-analysis, these subcategories were combined. While we intended to include touch as a fifth sensory-specific analysis, there was an insufficient number of papers dealing with positive-valenced evaluation in order to do so (see Francis et al., 1999; Rolls et al., 2003).
Coordinates for activation foci from conditional contrasts were taken from the original publications. No deactivations were examined in our meta-analyses. ALE meta-analyses were performed using GingerALE 2.0.1a3 (brainmap.org). MNI coordinates were automatically converted to Talairach coordinates. The ALE statistic, first described by Turkeltaub et al. (2002), was computed according to the modified procedure of Eickhoff et al. (2009), which is based on creating activation maps for each experiment, and then running a random effects analysis across all experiments, with significance determined by a non-parametric permutation test thresholded at p < 0.05 using the “false discovery rate” correction for multiple comparisons and a cluster threshold of k = 10 voxels. Compared to the original ALE procedure, this modified procedure indicates convergence of brain activation across experiments rather than foci. The ALE results were registered onto a Talairach-normalized template brain using Mango (ric.uthscsa.edu/mango). The logical analysis presented in Fig. 3 was performed using Mango.
Fig. 3.
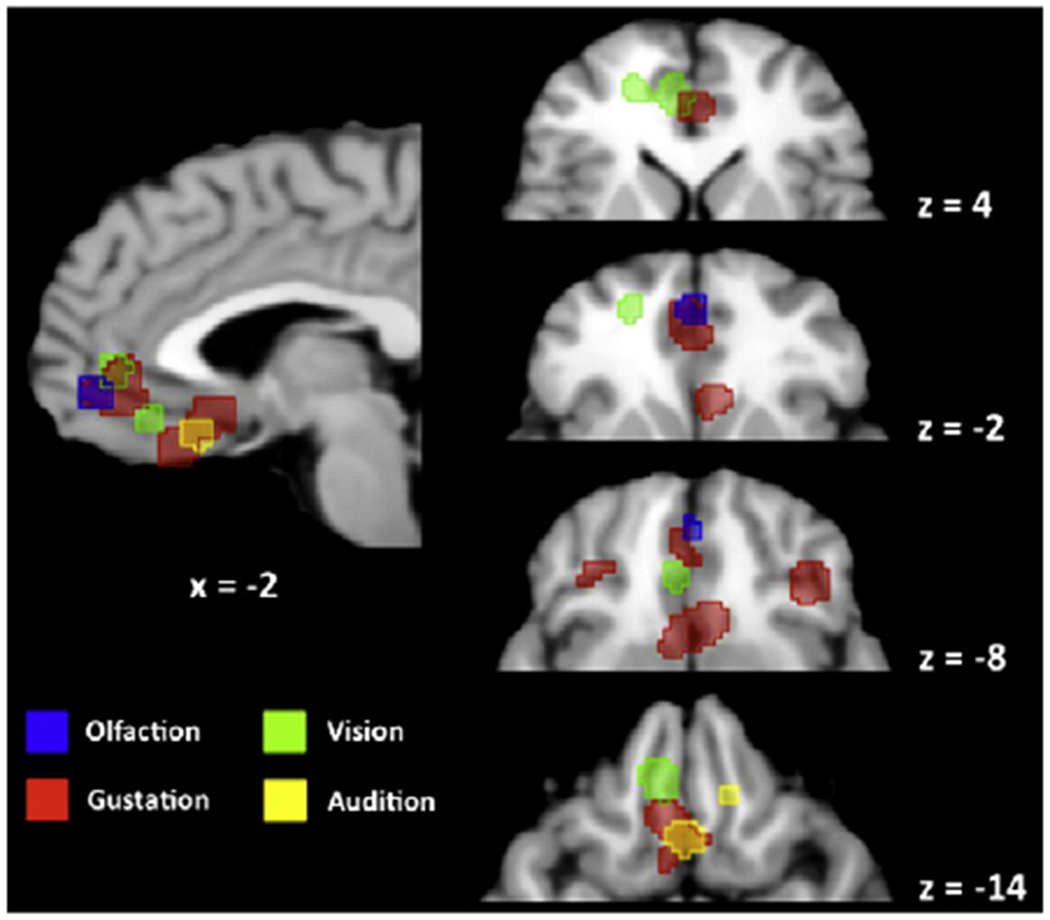
Logical analysis of ALE foci in the orbitofrontal cortex. ALE foci are displayed for the individual-level ALE analyses for the four sensory modalities in the orbitofrontal cortex and ventral cingulate region, demonstrating the relationship among the modalities. For the four axial slices at right, only the anterior portion of each slice is shown. Talairach z coordinates are shown below the sagittal slice and to the right of each axial slice. The right side of a slice is the right side of the brain. The color coding of the sensory modalities is shown at the bottom left. An overall pattern of adjacency among modalities is observed. However, in the pregenual ACC, gustation overlaps with vision (z = 4) and olfaction (z = −2). In medial OFC, gustation overlaps with audition (z = −14). For simplicity, structures outside of the ventral frontal cortex have been masked in all the slices.
A conjunction analysis was carried out to identify voxels engaged by aesthetic processing independent of stimulus modality. To identify convergence among the four analyses, the FDR-thresholded ALE maps obtained from the individual analyses were binarized. The resulting images – indicating voxels that were significantly associated with aesthetic processing in the visual, auditory, gustatory or olfactory domains – were then summed up, producing a probabilistic map indicating how many of the modality-specific analyses overlapped in each voxel of the reference space. A series of such conjunctions was carried out: a 2-of-4 overlap (i.e., a conjunction where any 2 of the 4 individual meta-analyses overlapped); a 3-of-4 overlap (where any 3 of the 4 meta-analyses overlapped); and a 4-of-4 overlap (where all 4 meta-analyses overlapped).
Results
Four sensory-specific ALE meta-analyses (i.e., vision, audition, gustation, and olfaction) were carried out, as shown in Fig. 1 (the Talairach coordinates of the ALE foci are reported in Supplementary Tables 3–6). As predicted, all analyses showed activation in the orbitofrontal cortex. Additional common areas of activation included the anterior cingulate cortex, anterior insula, and ventral region of the basal ganglia. Modality-specific brain areas in the individual analyses included the following patterns. 1) For vision: the left inferior parietal lobule (Brodmann area [BA] 40), fusiform gyri bilaterally (BA 19/37), inferior frontal gyri bilaterally (BA 44), hypothalamus, and caudate nuclei and amygdala bilaterally. 2) For audition: the supplementary motor area (BA 6), midbrain in the region of the periaqueductal gray, and posterior cerebellum. 3) For gustation, olfaction and vision: the left anterior insula and pregenual anterior cingulate cortex (ventral BA 32).
Fig. 1.
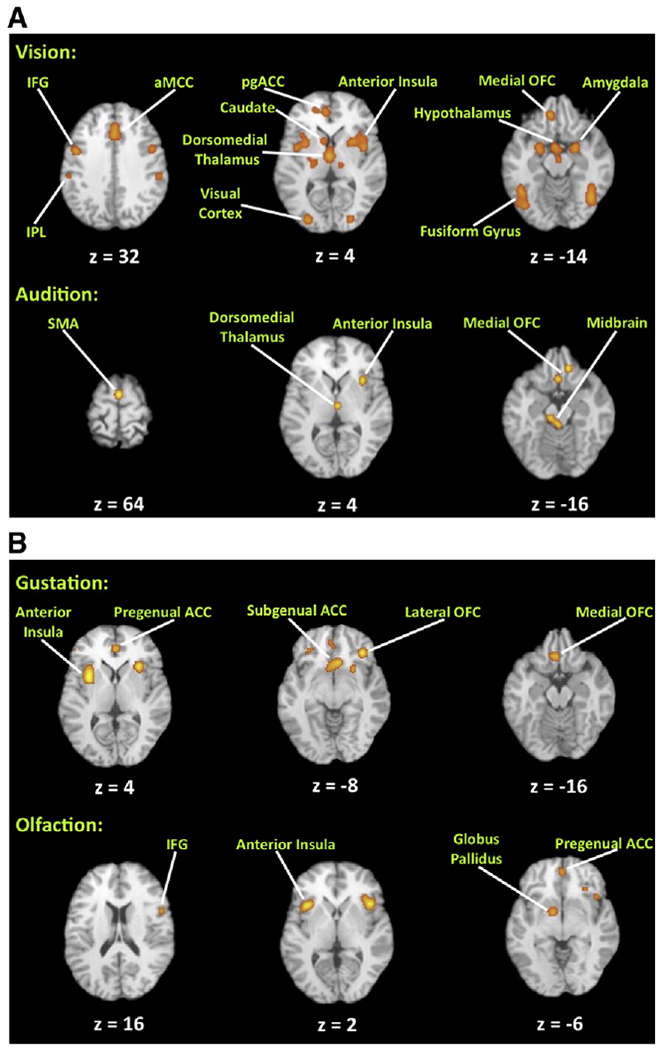
Principal ALE foci for the four sensory-specific meta-analyses. Principal sites of activation are labeled. Talairach z coordinates are shown below each slice. The right side of a slice is the right side of the brain. The threshold is p<0.05, corrected for multiple comparisons using the false discovery rate. Abbreviations (from left to right): IFG: inferior frontal gyrus; IPL, inferior parietal lobule; pgACC, pregenual anterior cingulate cortex; OFC, orbitofrontal cortex; SMA, supplementary motor area; ACC, anterior cingulate cortex.
The principal aim of the study was to examine zones of polysensory convergence for aesthetic processing. To do this, we binarized the four individual FDR-corrected maps and computed a probabilistic map of each voxel’s engagement across the four modalities. Fig. 2A shows voxels that were concordantly engaged by aesthetic processing in any two of the four modalities (“2-of-4” analysis). The regions identified in this analysis define a general limbic network associated with aesthetic appraisal and emotion, including the anterior midcingulate cortex, bilateral anterior insula, left nucleus accumbens, and medial orbitofrontal cortex (Table 1).
Fig. 2.
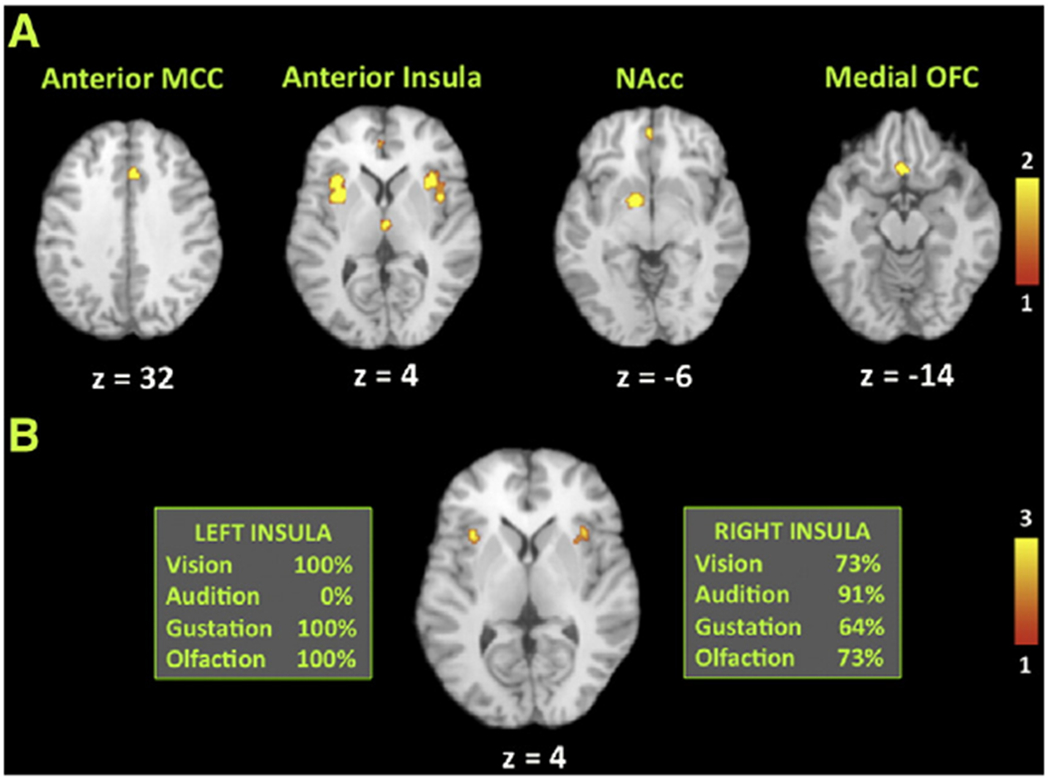
Principal ALE foci for the conjunction analyses. These analyses represent probabilistic maps indicating where binarized FDR-corrected ALE analyses for the four sensory-specific meta-analyses overlap, as registered onto a Talairach-normalized template brain. The right side of a slice is the right side of the brain. A) “2-of-4”, analysis, showing where any two individual meta-analyses overlap. The principal sites of overlap are labeled above each slice. Talairach z coordinates are shown below each slice. The color bar indicates the number of sensory-specific analyses in which overlap occurs. B) “3-of-4” analysis, showing where any three individual meta-analyses overlap. This analysis reveals foci exclusively in the bilateral anterior insula. The boxes to the left and right of the slice indicate the percentage of studies in each meta-analysis showing activation in this region. All four sensory modalities show activation in the right anterior insula. The color bar indicates the number of sensory-specific analyses in which overlap occurs. Abbreviations (from left to right): MCC, midcingulate cortex; NAcc, nucleus accumbens.
Table 1.
Principal ALE foci for the conjunction analyses. The table shows the principal ALE clusters derived from the “2-of-4” (top) and “3-of-4” (bottom) conjunction analyses. The columns labeled as x, y, and z are the Talairach coordinates for the maximum or sub-maxima of each cluster. After several anatomical names in the “region” column is the Brodmann area in parentheses.
| Region | x | y | z | z score |
|---|---|---|---|---|
| 2-of-4 analysis | ||||
| Left anterior insula | −32 | 16 | 6 | 3.46 |
| −32 | 14 | 0 | 2.60 | |
| −28 | 0 | 4 | 1.48 | |
| −26 | 2 | 6 | 1.41 | |
| Ventral basal ganglia/nucleus accumbens | −12 | 2 | −6 | 3.33 |
| −10 | 4 | −8 | 3.22 | |
| Right anterior insula | 42 | 12 | −4 | 3.20 |
| 34 | 20 | 4 | 3.09 | |
| 34 | 14 | 12 | 2.65 | |
| 32 | 14 | 2 | 2.56 | |
| Pregenual anterior cingulate cortex (32) | −2 | 44 | 0 | 3.07 |
| Anterior midcingulate cortex (32) | 2 | 22 | 32 | 2.71 |
| Dorsomedial nucleus of the thalamus | 4 | −16 | 8 | 2.35 |
| Ventral basal ganglia | 26 | 8 | −4 | 2.01 |
| Medial orbitofrontal cortex (25/11) | −2 | 22 | −12 | 1.25 |
| −4 | 20 | −14 | 1.16 | |
| 0 | 14 | −14 | 0.24 | |
| 3-of-4 analysis | ||||
| Left anterior insula | −32 | 16 | 6 | 3.46 |
| Right anterior insula | 34 | 18 | 4 | 2.78 |
| 32 | 14 | 2 | 2.56 | |
| 34 | 16 | 0 | 2.14 | |
No voxel in the brain was concordantly engaged in all four modalities (“4-of-4” analysis). However, the analysis revealed that the anterior insula bilaterally was the most concordant region of activation in three of the four analyses (“3-of-4” analysis; Fig. 2B). In a follow-up assessment, we examined which of the four analyses contributed to these clusters of convergence (see boxes in Fig. 2B). While three of the four analyses contributed to the cluster in the left anterior insula (all except audition), all four analyses contributed to the cluster in the right anterior insula. That is, while no voxel in the brain showed overlap among all four analyses, we found a region in the right anterior insula where all four analyses became significant, with three of the four FDR-thresholded ALE maps overlapping in each voxel of this region. The right anterior insula therefore emerged as the strongest candidate for a supramodal area for positive-valence aesthetic processing.
Contrary to our expectations, there were no OFC voxels in the 3-of-4 analysis. In other words, the OFC showed a more sensory-specific than supramodal pattern of activation. This is demonstrated in Fig. 3 as a logical analysis of the four sensory-specific ALE analyses. Some overlap was seen among modalities, as suggested in the 2-of-4 conjunction analysis. There was overlap between gustation and olfaction and between gustation and vision in the pregenual ACC (ventral BA 32), although this area is not typically found in published work on flavor processing. There was also overlap between audition and gustation in the posterior part of the gyrus rectus. Overall, the OFC did not satisfy the criterion of being a supramodal aesthetic area, in that no single area showed concordance across all four sensory modalities or even among subsets of three.
Finally, we were interested in whether studies devoted to artworks would show the same activation pattern as those involving non-artworks. Because of the small number of studies devoted to artworks and because these covered only two sensory modalities (audition and vision), we simply performed a qualitative analysis of these papers to see if they showed activation in the anterior insula. Four of the six studies of musical pleasantness did (Blood and Zatorre, 2001; Brown et al., 2004; Koelsch et al., 2006; Menon and Levitin, 2005), while only one of the five studies of visual artworks did (Di Dio et al., 2007). The reasons for this discrepancy are unclear. Music might simply be a more emotive/hedonic stimulus than photographs of visual artworks, and the insula might be tracking this difference.
Discussion
The combined results of the four sensory-specific meta-analyses and the two conjunction analyses point to the right anterior insula as being the most concordant area of activation across all of the studies of positive-valence aesthetic processing. The OFC did not have any significant voxels in the 3-of-4 conjunction analysis. Instead, we observed a pattern of adjacency among modalities. Hence, an overall picture of aesthetic processing emerged of sensory-specific regions of the OFC being co-activated with a supramodal area located in the anterior insula.
Before discussing the activation profiles in detail, it is important to point out limitations of this study vis-à-vis our objective of naturalizing aesthetics. While we argued in the Introduction that standard models of aesthetics place an unbalanced focus on positive-valenced appraisals, and thereby ignore the other half of aesthetic processing, our own meta-analyses were unable to rectify that situation, due to an insufficient number of articles devoted to negative-valenced aesthetic processing across the four sensory modalities. Article limitations likewise prevented us from doing a quantitative comparison between artworks and non-artworks. However, the burden of proof is on artwork-centered aesthetic models to demonstrate neural specificity for artworks. A comparison of our supramodal findings against individual studies of artwork processing does not support such a view of neural specificity for artworks.
Anterior insula
The anterior insula‘s status as the most concordant brain area for positive-valenced aesthetic appraisal was an unexpected finding of this work, given that much of the literature dealing with the insula highlights its importance for negative-valenced emotions such as disgust, sadness, and pain. Moreover, aside from emotional processing, the insula has well-established roles in the sensory processing of taste, touch, vestibular function, and visceral perception. Finally, the anterior insula is situated directly medial to the frontal operculum (and is often confounded with it) and thus a brain area involved in vocalization, language syntax, and imitation (Ackermann and Riecker, 2004; Dronkers, 1996). Hence, the insula is a complex region with a multitude of functions and unclear anatomical demarcations from surrounding regions. How can all the functions attributed to it be disentangled?
The most strongly activated region corresponds with the anterior insula proper, a paralimbic region associated with interoceptive awareness/insight (Critchley, 2005; Critchley et al., 2004), a sense of the “feeling self” (Craig, 2009), core affect (Barrett et al., 2007), the subjective experience of emotions such as pain, sadness, anxiety and disgust (Liotti et al., 2000), and the capacity to predictively anticipate the impact of emotional events on the body’s responses (Bechara and Damasio, 2005; Singer et al., 2009). Two ALE meta-analyses of the insula have attempted to specify functional sub-domains within this structure. The ALE foci of our 3-of-4 conjunction analysis mapped onto the anterior-dorsal part of the insula that Kurth et al. (2010) demonstrated as being a functional integration zone within this structure – representing such processes as emotion, empathy, interoception, olfaction, and gustation – and the zone that Mutschler et al. (2009) associated with the “sense of agency” in hand movement.
The major input to the anterior insula is from the dorsal posterior insula, which is considered to be the primary interoceptive cortex, receiving input from the nucleus of the solitary tract via the ventral posteromedial nucleus of the thalamus. The posterior insula is located next to the secondary somatosensory cortex (S2), and processes tactile information, including somatic pain. Rostral to that, the primary gustatory cortex is located in the anterior insula (de Araujo and Simon, 2009; de Araujo et al., 2003; Small et al., 1999), as corroborated by our gustatory ALE meta-analysis. Electrophysiological studies in the Rhesus monkey have shown that the gustatory cortex responds not only to the chemical properties of tastants but to the viscosity, fat texture, grittiness, and temperature of foodstuffs in the mouth (Verhagen et al., 2004). Human neuroimaging studies have shown that viewing pictures of appetizing foods can lead to activation close to this region (Simmons et al., 2005). According to de Araujo and Simon (2009), the gustatory cortex is a “multisensory system dedicated to evaluating the biological significance of intra-oral stimuli” (p. S34). This is in good agreement with our findings, except that the anterior insula might be processing the biological significance of all stimuli, not just intra-oral stimuli (Kurth et al., 2010). Given that aesthetic preference is often considered to be a matter of “taste” (Korsmeyer, 1999, 2001), these results about the insular cortex demonstrate that aesthetic processing literally maps onto the taste center of the human brain, an area associated with subjective awareness of interoceptive state more generally. This part of the brain, along with the anterior cingulate cortex, has shown a strong evolutionary expansion in humans, and contains a unique and recently-evolved type of projection neuron, called the von Economo neuron, that is only found in higher primates (Allman et al., 2005).
While bilateral activation of the anterior insula was seen in most of the analyses of this study, the right anterior insula emerged as a slightly more concordant area of activation than the left, in part due to the presence of the right but not left insula in the auditory analysis. Laterality effects have been described for the anterior insula. For example, Craig (2003) argued that the right anterior insula contains a meta-representation of the state of the body that is associated with the subjective awareness of the “feeling self”, based on input from visceral afferent pathways leading to interoceptive representations in the posterior insula. In addition, the right anterior insula is associated with sympathetic autonomic activity whereas the left anterior insula is associated with parasympathetic activity (Craig, 2005). By this reasoning, aesthetic processing should most likely be associated with the right insula due to its arousing nature.
How can we reconcile our findings with the large literature on negative-valence processing in the insula? One proposal is laterality. Craig (2005) argued that the right insula is more associated with negative valence and the left insula with positive valence, a proposal that is not borne out in our meta-analyses, with their bilateral activations for positive aesthetic evaluation. Another possibility could relate to the antero-posterior dimension, with the anterior part of the insular cortex being positive and the posterior part negative. Tsukiura and Cabeza (2011) analyzed the neural basis of the “beauty-as-good” intuition by having subjects perform attractiveness ratings for faces as well as “action goodness” ratings for short sentences describing hypothetical actions of individuals. Parametric analysis of neural areas whose activity varied systematically with these two ratings showed that a part of the right insular cortex decreased in activity with increasing ratings of both attractiveness and moral goodness. However, this area was in the posterior insula, being 33 mm more posterior than the centers of mass our anterior insula foci (y = 18 compared with y= −15 in their study). So, the posterior insula might process negative valence, and the anterior insula positive valence.
In the dataset of Tsukiura and Cabeza (2011), no brain area showed a U-shaped response for attractiveness or goodness ratings that would be suggestive of bivalent functioning. However, Viinikainen et al. (2010) showed exactly such a profile of responsiveness for both the right and left anterior insula, in a region anterior to our conjunction foci. In that study, subjects viewed pictures from the International Affective Picture System (IAPS) database and had to make ratings of their valence (pleasantness) along a 9-point scale. A handful of regions, including a very anterior part of the insula bilaterally, showed an inverted-U pattern, being strongest for neutral stimuli and being equally strong for either positive or negative stimuli (though less so than for neutral stimuli). This supports the contention that the insula is a bivalent region. Viinikainen et al. found this pattern comparably for the right and left insula, arguing against a strong laterality effect for valence.
Overall, our findings of concordant activation in the right anterior insula for positive-valence aesthetic processing across four sensory modalities suggest that interoceptive processing (whether conscious or unconscious) is key to the assignment of valence to perceived objects. It probably does so by referencing the visceral/motivational state of the individual in the presence of such objects. This part of the right insula might correspond to a sub-component of a bivalent brain region that is more responsive to positive than negative valence. The greater concordance of the right hemisphere compared to the left might reflect the functional specialization of this part of the insula for meta-representations of subjective bodily awareness (Craig, 2003). Finally, it should be pointed out that the insula is strongly responsive to autonomic parameters such as heart rate and blood pressure (Critchley, 2005; Critchley et al., 2004), and so it is likely that autonomic changes that are induced by the perception of attractive stimuli are contributing to the observed activations in the insula.
Orbitofrontal cortex
The OFC receives inputs from the five major sensory pathways (through what are referred to as “what” sensory pathways for vision and audition) as well as from the visceral afferent system, and is thus one of the most important brain areas underlying multisensory integration, not least in the form of gustatory/olfactory coupling for “flavor” sensation (Rolls, 2004, 2005). But in addition, the OFC plays an important role in monitoring and learning about the reward value of stimuli (Kringelbach, 2005; Wallis, 2007), as reflected in subjective assessments of pleasantness, and as shown in these meta-analyses.
There is an extensive literature implicating the OFC in reward processing across all sensory modalities, which is why we predicted that the OFC would be the most likely candidate for a supramodal aesthetic area. Our major finding was that, while several OFC regions appeared in each sensory-specific analysis, none of them was present in the conjunction, thereby indicating an absence of convergence. In addition, we observed surprisingly little convergence of ALE foci across the individual gustation and olfaction analyses, despite strong expectations in the literature for such a convergence for flavor processing. Most of the overlap for these two modalities was seen in the ventral part of the cingulate cortex (the pregenual ACC) in BA 32. Overall, the strongest pattern seen for the OFC across our analyses was adjacency between modalities, rather than overlap, consistent with there being sensory-specific zones in the OFC.
To a first approximation, the OFC shows striking similarities to the anterior insula as “a gateway to subjective conscious experience” (Kringelbach, 2005:699), especially the experience of emotion. Both areas receive extensive sensory and visceral inputs, and are important for polysensory convergence. Both connect with visceromotor areas such as the ACC and hypothalamus. In addition, both are responsive to the reward value of stimuli, and are important brain areas for emotion. Where they seem to differ is that the OFC is more strongly associated with learning and memory processes than the insula, most likely via its connections with the dorsolateral prefrontal cortex. The OFC is able to track the reward value of a stimulus as this reward value changes as a function of conditioning in stimulus-reward reversal experiments. The OFC might not only represent the value of an object but keep this value in working memory to influence decision making and behavior. So, while both the insula and OFC respond to primary reinforcers, the OFC might be more important in creating responsiveness to secondary reinforcers, for example when reward contingencies change. Overall, the OFC and insula are reciprocally connected with one another and show parallel functions when it comes to reward and emotion. But the OFC might be more linked to conditioned learning and working memory processes than the insula, and may thus serve as a brain area for storing the reward history of stimuli, as is important for decision making (although see Singer et al. (2009) for the neuroeconomic implications of the anterior insula).
The OFC, like the anterior insula, is a bivalent brain region. One hypothesis about valence processing (Kringelbach, 2005) is that the medial OFC is linked to positive valence (rewards), and the lateral OFC (closest to the anterior insula) to negative valence (punishers). While our meta-analyses did not include negative valence, several of the studies within them did perform comparisons between valences, and some were supportive of a mediolateral distinction for valence. For example, Small et al. (2001) had subjects eat chocolate to beyond satiety. As chocolate eating changed from being rewarding to aversive, activity switched from medial OFC to lateral OFC. Likewise, a medial-positive lateral-negative relationship has been found in studies of valence processing for gustation (Small et al., 2003) and olfaction (Anderson et al., 2003). However, the study of O’Doherty et al. (2001) showed rather opposite results. They had subjects consume solutions of either glucose (pleasant) or salt (aversive), and found more lateral OFC activity for glucose, and more medial activity for salt. Finally, Zald et al. (2002) found medial OFC activation for the consumption of both pleasant (sugar) and aversive (quinine) solutions, with no mediolateral differences across valence. Further work is needed to resolve the important issue of valence coding in both the OFC and insula.
It is reasonable to speculate that, to the extent that polysensory convergence of reward processing does occur in the OFC, it most likely evolved in the service of perceiving the quality of food sources, including their gustatory, olfactory, visual, and textural (somatosensory) features (Kringelbach, 2004; Rozin, 1999). This is strikingly similar to the reasoning that has been applied to the anterior insula/gustatory cortex (de Araujo and Simon, 2009), highlighting yet another deep similarity between the OFC and anterior insula. It also suggests that the most primordial aesthetic appraisal that animals engage in relates to foraging and the search for nutritional sources, and that all other appraisals – from attractive mates to transcendent artworks – have co-opted an ancestral system of food appraisal (see discussion below).
A functional connectivity model
An important way to naturalize aesthetic processing is to see it as the appraisal of valence of perceived objects, and to argue that this appraisal process comes about through a comparison between subjective awareness of current homeostatic state – as mediated by the anterior insula – and exteroceptive perception of objects in the environment, as mediated by the sensory pathways leading up to the OFC. The basic model of aesthetic processing that we propose involves an interaction between interoceptive and exteroceptive processing via recurrent connectivity between anterior insula and OFC, respectively, as shown in Fig. 4. We call this a “core circuit for aesthetic processing”, and is comprised of areas contained in our 2-of-4 conjunction analysis. See Barrett et al. (2007) for a similar model. This circuit is in no way restricted to aesthetic processing, but may be related to all cognitive processes that involve viscerality (Bechara and Damasio, 2005), as shown by the observation that it is active when people evaluate the truth or falsity of religious propositions (Harris et al., 2009). We propose that recurrent connectivity between the anterior insula and the OFC can mediate what Craig (2009) calls “homeostatic emotions” and thus the assignment of valence to objects as a function of current homeostatic state.
Fig. 4.
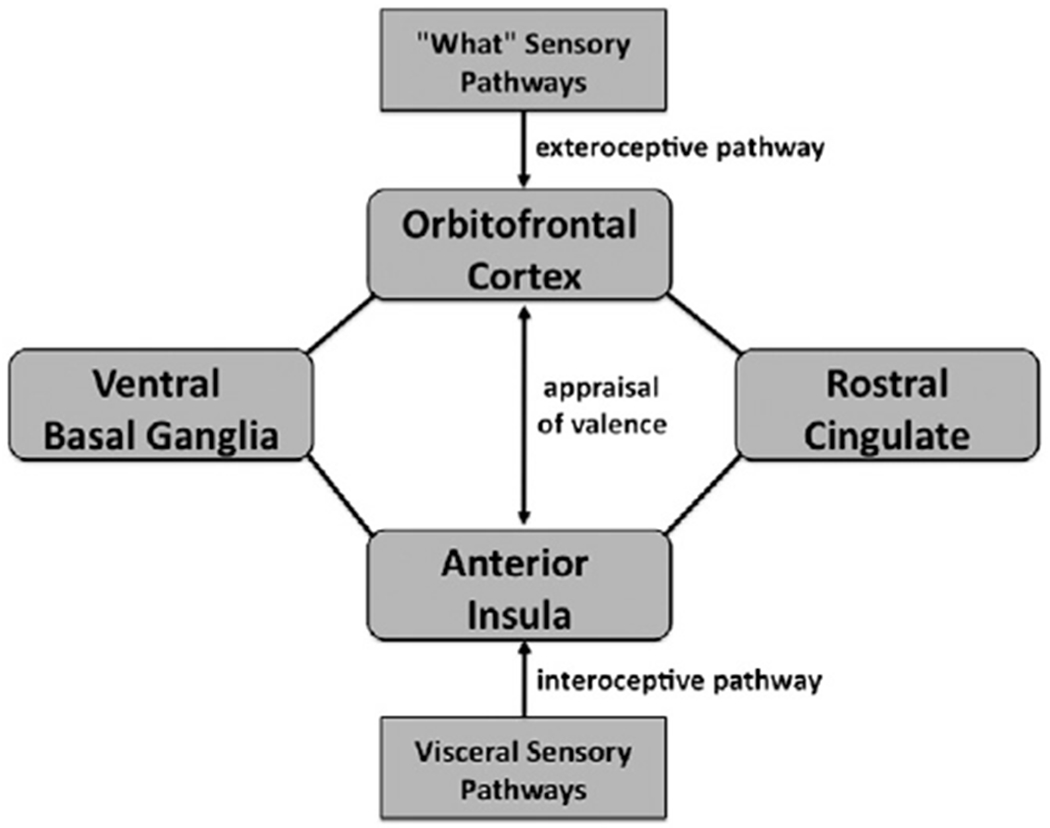
The core aesthetic network of the human brain. This is presented as a connectivity model in which aesthetic appraisal is seen as a comparison between exteroceptive information passing through the OFC and interoceptive information passing through the anterior insula. The appraisal of an object‘s valence is assumed to involve recurrent connectivity between these two areas. Also shown in the figure are the rostral/emotional part of the cingulate cortex (BA 32) and the ventral basal ganglia, the latter being one of the “hedonic hotspots” of the brain. This diagram is not meant to represent connectivity in any comprehensive manner. Key limbic areas missing from this diagram include the amygdala, hippocampus, hypothalamus, and basal forebrain. Also missing are general sensory projections to the insula, and visceral afferent projections to the OFC.
In this model, the OFC is seen as an extension of “what” sensory pathways that process object-recognition information, whereas the anterior insula contains a meta-representation of bodily responses from inputs coming from the interoceptive cortex in the posterior insula. Another important part of this circuit is the ACC. The rostral part of the ACC is reciprocally connected with both the anterior insula and OFC, and is co-activated with both of them in many imaging experiments, as is clearly shown in all of our meta-analyses. Taylor et al. (2009) used fMRI to examine resting-state connectivity between the various divisions of the insula and cingulate cortex. One of their major findings was strong resting-state connectivity between the anterior insula and the most rostral part of the cingulate (BA 32; pregenual anterior cingulate). This interconnected system is proposed to be involved in “emotional salience monitoring” and to operate across all sensory modalities (Taylor et al., 2009). Finally, we include the ventral basal ganglia in this circuit, which are considered to be among the “hedonic hotspots” of the brain (Berridge et al., 2009). Unlike areas such as the OFC and insula, which seem to be bivalent, the ventral basal ganglia are overwhelmingly associated with reward processing, pleasure, and thus positive valence. There is good anatomical evidence that the ventral basal ganglia are connected with the OFC and perhaps anterior insula as well (Haber and Knutson, 2010).
Naturalizing aesthetics: what needs do the arts satisfy?
We have argued that aesthetic processing is, at its core, the appraisal of valence of objects, and that this is a general cognitive process that applies to both non-art and art objects. We suggest that this processing is rooted in a comparison between subjective interoceptive state and exteroceptive perception, whose goal is to determine whether perceived objects will satisfy or oppose our homeostatic needs. The presence of anterior insula and OFC activations in the analysis of artworks raises the important question about what kind of “need” is being satisfied by artworks. Activation of reward circuits in response to artworks might explain how we come to feel pleasure in response to art, but it does not explain the deeper question of why we do. Why are artworks aesthetic objects?
While the homeostatic need to appraise food quality can probably account for polysensory convergence for aesthetic processing in the OFC and insula, it cannot account for why music and other artworks activate this same circuitry. For that, we need to switch the focus to social needs and the emotions associated with them. Principal among these are the needs to find mates and to maintain the integrity of social relations within family groups and communities, the latter including parent–child interactions and affiliative interactions among non-kin members of a social group (Dissanayake, 2008, 2009). Evolutionary scenarios for the arts invoke the mechanisms of sexual selection and group selection to explain these capacities (Brown, 2000; Brown and Dissanayake, 2009; Miller, 2000; Voland and Grammer, 2003). So, while the rewarding properties of the arts derive most likely from social needs rather than homeostatic needs, similar neural circuitry seems to be involved in responding to attractive faces and alluring music as to desirable foods. The system of social needs seems to have co-opted the basic circuitry used for homeostatic needs, as evidenced by the everyday observation that artworks lead to strong bodily responses and can be as desirable and motivating as homeostatic need satisfaction (Gabrielsson, 2001).
In conclusion, an important way to naturalize aesthetics is to argue that such a system evolved first for the appraisal of appetitive objects of biological importance, including food sources and suitable mates, and was later co-opted for artworks such as songs and paintings. The larger evolutionary mystery of Enlightenment aesthetics is why artworks are rewarding to begin with and therefore why they activate the same reward circuitry as food sources that relate to homeostatic needs. The invocation of “social needs” provides some insight into the evolution of aesthetics. Interestingly, the paralimbic structures highlighted in the present study are critically involved in social cognition and empathy. This, therefore, may provide a neural basis for shared aesthetic experience, which is so central to the social functionality of the arts in human life, including their prominent role in religion.
Supplementary Material
Acknowledgments
We are grateful to Ivan de Araujo and Ellen Dissanayake for critical comments on an earlier version of the manuscript. This work was funded by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) to SB and ML, the Grammy Foundation to SB, and the Canada Foundation for Innovation (CFI) to ML. SBE was supported by the Human Brain Project (R01-MH074457-01A1), the Helmholtz Alliance on Systems Biology (Human Brain Model), and the DFG (IRTG 1328).
Footnotes
Supplementary materials related to this article can be found online at doi:10.1016/j.neuroimage.2011.06.012.
References
- Ackermann H, Riecker A, 2004. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang. 89, 320–328. [DOI] [PubMed] [Google Scholar]
- Allman JM, Watson KK, Tetresault NA, Hakeem AY, 2005. Intuition and autism: a possible role for Von Economo neurons. Trends Cogn. Sci. 9, 367–373. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JDE, Sobel N, 2003. Dissociated neural representations of intensity and valence in human olfaction. Nat. Neurosci. 6, 196–202. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ, 2007. The experience of emotion. Annu. Rev. Psychol. 58, 373–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, 2005. The somatic marker hypothesis: a neural theory of economic decision. Games Econ. Behav. 52, 336–372. [Google Scholar]
- Berridge KC, Kringelbach ML, 2009. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology 199, 457–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW, 2009. Dissecting components of reward: “liking”, “wanting”, and learning. Curr. Opin. Pharmacol. 9, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ., 2001. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc. Natl. Acad. Sci. U. S. A. 98, 11818–11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, 2000. Evolutionary models of music: from sexual selection to group selection. In: Tonneau F, Thompson NS (Eds.), Perspectives in Ethology. Behavior, Evolution and Culture., 13. Plenum Publishers, New York, pp. 231–281. [Google Scholar]
- Brown S, Dissanayake E, 2009. The arts are more than aesthetics: neuroaesthetics as narrow aesthetics. In: Skov M, Vartanian O (Eds.), Neuroaesthetics. Baywood, Amityville, NY, pp. 43–57. [Google Scholar]
- Brown S, Martinez MJ, Parsons LM, 2004. Passive music listening spontaneously engages limbic and paralimbic systems. Neuroreport 15, 2033–2037. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V, 2007. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn. Affect. Behav. Neurosci. 7, 367–379. [DOI] [PubMed] [Google Scholar]
- Craig AD, 2003. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 13, 500–505. [DOI] [PubMed] [Google Scholar]
- Craig AD, 2005. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn. Sci. 9, 566–571. [DOI] [PubMed] [Google Scholar]
- Craig AD, 2009. How do you feel — now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70. [DOI] [PubMed] [Google Scholar]
- Critchley HD, 2005. Neural mechanisms of autonomic, affective, and cognitive integration. J. Comp. Neurol. 493, 154–166. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ, 2004. Neural systems supporting interoceptive awareness. Nat. Neurosci. 7, 189–195. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Simon SA, 2009. The gustatory cortex and multisensory integration. Int. J. Obes. 33, 534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IET, Rolls ET, Kringelbach ML, McGlone F, Phillips N, 2003. Tasteolfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur. J. Neurosci. 18, 2059–2068. [DOI] [PubMed] [Google Scholar]
- Di Dio C, Gallese V, 2009. Neuroaesthetics: a review. Curr. Opin. Neurobiol. 19, 1–6. [DOI] [PubMed] [Google Scholar]
- Di Dio C, Macaluso E, Rizzolatti G, 2007. The golden beauty: brain response to classical and Renaissance sculptures. PLoS One 2, e1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake E, 2008. If music is the food oflove, what about survival and reproductive success? Musicae Scientiae 169–195 Special Issue. [Google Scholar]
- Dissanayake E, 2009. The artification hypothesis and its relevance to cognitive science, evolutionary aesthetics, and neuroaesthetics. Cogn. Semiot. 5, 148–173. [Google Scholar]
- Dronkers NF, 1996. A new brain region for coordinating speech articulation. Nature 384, 159–161. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT, 2009. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30, 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, 1992. An argument for basic emotions. Cogn. Emot. 6, 169–200. [Google Scholar]
- Francis S, Rolls ET, Bowtell R, McGlone F, O'Doherty J, Browning A, Clare S, Smith E, 1999. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport 10, 453–459. [DOI] [PubMed] [Google Scholar]
- Gabrielsson A, 2001. Emotions in strong experiences with music. In: Juslin PN, Sloboda JA (Eds.), Music and Emotion. Oxford University Press, Oxford, pp. 431 –449. [Google Scholar]
- Goldman A, 2001. The aesthetic. In: Gaut B, Lopes DM (Eds.), The Routeledge Companion to Aesthetics. Routledge, London, pp. 181 –192. [Google Scholar]
- Guyer P, 2005. Values ofBeauty: Historical Essays in Aesthetics. Cambridge University Press, Cambridge. [Google Scholar]
- Haber SN, Knutson B, 2010. The reward circuit: linking primate anatomy and human imaging. Psychopharmacology 35, 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S, Kaplan JT, Curiel A, Bookheimer SY, Iacoboni M, Cohen MS, 2009. The neural correlates of religious and nonreligious belief. PLoS One 4, e0007272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S, Fritz T, Cramon DY, Muller K, Friederici AD, 2006. Investigating emotion with music: an fMRI study. Hum. Brain Mapp. 27, 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer C, 1999. Making Sense of Taste: Food and Philosophy. Cornell University Press, Ithica. [Google Scholar]
- Korsmeyer C, 2001. Taste. In: Gaut B, Lopes DM (Eds.), The Routeledge Companion to Aesthetics. Routledge, London, pp. 193–202. [Google Scholar]
- Kringelbach ML, 2004. Food for thought: hedonic experience beyond homeostasis in the human brain. Neuroscience 126, 807–819. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, 2005. The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci. 6, 691–702. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB, 2010. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 214, 519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT, 2000. Differential limbic—cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol. Psychiatry 48, 30–42. [DOI] [PubMed] [Google Scholar]
- Menon V, Levitin DJ, 2005. The rewards of music listening: response and physiological connectivity of the mesolimbic system. Neuroimage 28, 175–184. [DOI] [PubMed] [Google Scholar]
- Miller GF, 2000. The Mating Mind: How Sexual Selection Shaped the Evolution of Human Nature. Doubleday, New York. [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, Ball T, 2009. Functional organization of the human anterior insular cortex. Neurosci. Lett. 457, 66–70. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, 2001. Representation of pleasant and aversive taste in the human brain. J. Neurophysiol. 85, 1315–1321. [DOI] [PubMed] [Google Scholar]
- Ortony A, Clore GL, Collins A, 1988. The Cognitive Structure of Emotion. Cambridge University Press, Cambridge. [Google Scholar]
- Rolls ET, 2004. Convergence of sensory systems in the orbitofrontal cortex inprimates and brain design for emotion. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 281A, 1212–1225. [DOI] [PubMed] [Google Scholar]
- Rolls ET, 2005. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol. Behav. 85, 45–56. [DOI] [PubMed] [Google Scholar]
- Rolls ET, O‘Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F, 2003. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb. Cortex 13, 308–317. [DOI] [PubMed] [Google Scholar]
- Rozin P, 1999. Preadaptation and the puzzle and properties of pleasure. In: Kahneman D, Diener E, Schwarz N (Eds.), Well-Being: The Foundations of Hedonic Psychology. Russell Sage, New York, pp. 109–113. [Google Scholar]
- Rushworth MFS, Behrens TEJ, Rudebeck PH, Walton ME, 2008. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behavior. Trends Cogn. Sci. 11, 168–176. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Martin A, Barsalou LW, 2005. Pictures of appetizing foods activate gustatory cortices for taste reward. Cereb. Cortex 15, 1602–1608. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K, 2009. A common role of the insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 13, 334–340. [DOI] [PubMed] [Google Scholar]
- Skov M, 2009. Neuroaesthetic problems: a framework for neuroaesthetic research. In:Skov M, Vartanian O (Eds.), Neuroaesthetics. Baywood, Amityville, NY, pp. 9–26. [Google Scholar]
- Small DM, Zald DH, Jones-Gotman M, Zatorre RJ, Pardo JV, Frey S, Petrides M, 1999. Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport 10, 7–14. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M, 2001. Changes in brain activity related to eating chocolate. Brain 124, 1720–1733. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T, 2003. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron 39, 701–711. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD, 2009. Two systems ofresting state connectivity between the insula and cingulate cortex. Hum. Brain Mapp. 30, 2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiura T, Cabeza R, 2011. Shared brain activity for aesthetic and moral judgments: implications of the beauty-is-good stereotype. Soc. Cogn. Affect. Neurosci. 6, 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA, 2002. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16, 765–780. [DOI] [PubMed] [Google Scholar]
- Vartanian O, 2009. Conscious experience of pleasure in art. In: Skov M, Vartanian O (Eds.), Neuroaesthetics. Baywood, Amityville, NY, pp. 261–273. [Google Scholar]
- Verhagen JV, Kadohisa M, Rolls ET, 2004. Primate insular/opercular taste cortex: neuronal representations of the viscosity, fat texture, grittiness, temperature, and taste of foods. J. Neurophysiol. 92, 1685–1699. [DOI] [PubMed] [Google Scholar]
- Viinikainen M, Jääskeläinen IP, Alexandrov Y, Balk MH, Autti T, Sams M, 2010. Nonlinear relationship between emotional valence and brain activity: evidence of separate negative and positive valence dimensions. Hum. Brain Mapp. 31, 1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voland E, Grammer K (Eds.), 2003. Evolutionary Aesthetics. Springer-Verlag, Berlin. [Google Scholar]
- Wallis JD, 2007. Orbitofrontal cortex and its contribution to decision-making. Annu. Rev. Neurosci. 30, 31–56. [DOI] [PubMed] [Google Scholar]
- Zald DH, Hagen MC, Pardo JV, 2002. Neural correlates of tasting concentrated quinine and sugar solutions. J. Neurophysiol. 87, 1068–1075. [DOI] [PubMed] [Google Scholar]
- Zeki S, 1999. Inner Vision: An Exploration of Art and the Brain. Oxford University Press, Oxford. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


