Abstract
The main reason for the emergency implantation of venoarterial extracorporeal membrane oxygenation (VA-ECMO) is the restoration of adequate systemic perfusion, while protecting the failing heart and promoting myocardial recovery are equally important goals. Following initial haemodynamic stabilization and often the urgent revascularization of the culprit lesion, the clinical focus is then directed towards the most efficient strategy for cardioprotection. Frequent echocardiography measurements may help to estimate the degree of unwanted left ventricular (LV) overloading during VA-ECMO. Additionally, the estimation of high LV filling pressures by Doppler echocardiography or their (in-)direct measurement using a dedicated surgical left atrial pressure line and conventional pulmonary artery catheter in a wedge position or a pigtail catheter in the left ventricle can be performed. Mechanical overload of the left ventricle is the major adverse effect and an obvious mechanistic and prognostic challenge of contemporary ECMO care. Many efforts are under way to overcome this phenomenon by LV unloading, which was effectively achieved by the current combined approach using an axial decompression device, while novel technical developments and approaches are tested and urgently anticipated. The aim of this report is to introduce in depth pathophysiological background, current concepts, and future perspectives in LV unloading strategies.
Keywords: Cardiogenic shock, Venoarterial ECMO, Cardioprotection, Cardiac arrest, Oxygenation, Unloading
Introduction and pathophysiological considerations
A well-recognized and accepted indication for venoarterial extracorporeal membrane oxygenation (VA-ECMO) is severe cardiogenic shock and refractory cardiac arrest resistant to conventional treatment.
The main reason for the emergency implantation of VA-ECMO is the restoration of adequate systemic perfusion, while protecting the failing heart and promoting myocardial recovery are equally important goals. The most frequent approach to establish emergency VA-ECMO is peripheral, bifemoral cannulation using a long venous cannula that goes through the inferior vena cava to the right atrium and a short arterial cannula implanted retrogradely via the femoral to the iliac artery. Following initial haemodynamic stabilization and often the urgent revascularization of the culprit lesion, the clinical focus is then directed towards the most efficient strategy for cardioprotection. Optimal organ perfusion is mediated by the total cardiac output, i.e. the sum of extracorporeal and native systemic blood flow, which aids in protecting peripheral organ function, but adverse effects may arise from altered myocardial loading conditions, i.e. mechanical stress and strain. These forces act proportionally to the degree of extracorporeal membrane oxygenation (ECMO) support flow and can potentially create deleterious myocardial mechanical overload, which further jeopardizes the already injured myocardium and may negatively affect cardiac recovery.1,2
From a pathophysiological perspective, ECMO has an impact on several compartments of the circulation. Comparison of ECMO and other mechanical circulatory support devices including main characteristics, advantages and complications are outlined in Tables 1 and 2.
Table 1.
Mechanical circulatory support (MCS) devices—characteristics
| VA-ECMO | Impella family |
TandemHeart | ProtekDuo | |||||
|---|---|---|---|---|---|---|---|---|
| Impella 2.5 | Impella CP | Impella 5.0/LD | Impella 5.5 | Impella RP | ||||
| Access | Percutaneous, femorala (arterial, venous) | Percutaneous, femorala | Percutaneous, femorala | Surgical, axillary/femoral or ascending aorta | Surgical, axillary/femoral or ascending aorta | Percutaneous, femoral vein | Percutaneous, transseptal | Right internal jugular |
| Mechanism | RA → aorta (oxygenation) | LV → aorta | LV → aorta | LV → aorta | LV → aorta | LA → aorta | RA → PA (±oxygenation) | |
| Output (max) | 5.0–7.0 L/min | 2.5 L/min | 3.7 L/min | 5.0 L/min | 5.5 L/min | 4.6 L/min | 5 L/min | 4.5 L/min |
| Cannula size | 15–23 arterial 19–28 venous | 12F | 14F | 21F | 21F | 22F | 15–17 arterial 21 venous | 16–29F |
| Cardiac power | ↑↑↑ | ↑ | ↑↑ | ↑↑↑ | ↑↑↑ | ↑↑ | ↑↑ | ↑↑ |
| Afterload | ↑↑↑ | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ |
| Coronary perfusion | — | ↑ | ↑ | ↑↑ | ↑↑ | — | — | — |
LV, left ventricle; PA, pulmonary artery; RA, right atrium.
Occassionally axillary.
Table 2.
Advantages and complications of different MCS techniques
| VA-ECMO | IMPELLA |
ECpella |
|||
|---|---|---|---|---|---|
| 2.5/CP | 5.0/5.5 | ECMO + Impella 2.5/CP | ECMO + Impella 5.0 | ||
| Insertion | Percutaneous (surgical) | Percutaneous | Surgical | Percutaneous (surgical) | Percutaneous surgical |
| Support level | Full biventricular support | Partial LV support | Full LV support | Full biventricular support, unloading | Full biventricular support, unloading |
| Coronary perfusion | — | ↑ | ↑↑ | ↑ | ↑ |
| Peripheral tissue perfusion | ↑↑ | ↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ |
| Oxygenation | Yes | No | No | Yes | Yes |
| Mobilization ambulation | ↓a | ↓ or ↑a | ↑↑ | ↓ | ↓ |
| Recovery/bridge to | ↑ | ↑ | ↑↑ | ↑↑ | ↑↑ |
| Vascular complications | ↑↑ | ↑ | ↑ | ↑↑ | ↑↑ |
| Bleedingb | ↑↑ | ↑ | ↑ | ↑↑↑ | ↑↑↑ |
| Infectious complications | ↑↑ | ↓ | ↓ | ↑↑ | ↑↑ |
| Haemolysis | ↑ | ↑ | ↓ | ↑↑ | ↑↑ |
Depending on access. Axillary access will allow mobilization/ambulation both in ECMO and with Impella 2.5/CP, but is rarely used.
Considering best practices in access management and anticoagulation.
Venous system
VA-ECMO implantation initially leads to blood redistribution to the ECMO circuit, but after that, in contrast to intraoperative heart–lung machine (HLM) circulation, the venous system is only partially further unloaded by ECMO. This occurs because the venous system is the only capacitance vessel in the circulation, and in addition, sufficient venous filling is a prerequisite for proper ECMO function. Thus, ECMO cannot fully unload the right heart.
Left ventricle
As outlined above, peripheral VA-ECMO represents a closed extracorporeal circulatory system in contrast to the HLM, which features an open reservoir that allows the right heart to continue at least residual ejection feeding to the left atrium and ventricle. Moreover, it should be noted that the areas supplied by the bronchial arteries are dominantly drained via pulmonary veins towards the left atrium and the left ventricle. This would theoretically also occur even if pulmonary artery flow was completely abolished and aortic regurgitation was absent. In the situation where ECMO needs to be modified to augment arterial pressure to a degree that prevents the acutely diseased left ventricle from ejecting, the deleterious consequences are progressively rising left ventricular (LV) diastolic pressure that compromises endomyocardial perfusion, increases LV wall tension, elevates pulmonary venous pressure (triggering hydrostatic pulmonary oedema) and, last but not least, leads to blood stasis cumulating in a significant and potentially deadly risk of extensive thrombosis in the left ventricle, aortic root, and the small circulation.
Coronary arteries
In peripheral VA-ECMO, the coronary circulation is the most remote arterial vascular bed from a circulatory viewpoint. If the precarious state of ‘no forward flow through the aortic valve’ is impending, the coronary arteries are solely fed oxygenated blood from ECMO, but coronary circulation is impeded by coexisting high diastolic ventricular pressures. If there is residual forward blood flow through the aortic valve, this blood originates from the lungs, which are typically compromised by severe pulmonary oedema, and this forward flow of hypoxaemic blood dominantly feeds the first branches of the arterial tree, namely, the coronary arteries. Unfortunately, this proximal aortic root hypoxaemia cannot be reliably detected by any clinical oxygenation monitoring, including right-hand pulse oximetry, unless direct sampling from the diagnostic or guiding catheters during coronary angiography or percutaneous intervention is performed.
This type of acute left heart disease with increased cardiac oxygen demand due to high wall tension, myocardial oxygen wasting, and catecholamine-driven metabolic activation combined with compromised coronary microcirculation and coronary hypoxaemia has not been sufficiently studied clinically but may well in part explain the disappointing results of ECMO support in cardiogenic shock.
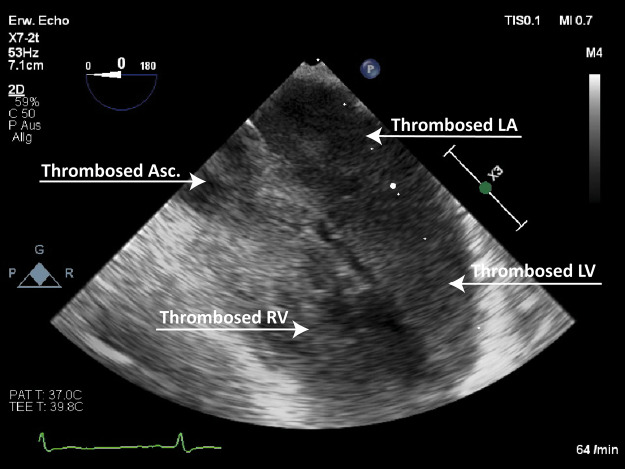
From a pathophysiological perspective, it should be stressed in this context that in peripheral VA-ECMO, the right ventricular preload tends to be reduced due to central venous blood drainage. However, a relatively well-preserved right ventricular ejection may still play a pivotal role in LV overload.3 In contrast, the significant increase in LV afterload is largely mediated by retrograde aortic extracorporeal blood flow and maintained by direct and continuous reinfusion into the iliac artery in a retrograde fashion. As a consequence, especially in a severely compromised left ventricle, the dilatation and cessation of contractility may ensue, with the virtually persistent closure of the aortic valve and resulting impending cavity and aortic root thrombosis (Figure 1). Moreover, it is well known that the presence of pulsatile vs. non-pulsatile blood flow has a broad range of clinically important consequences, and ‘non-pulsatility’ is defined as a pulse pressure of less than 15 mmHg.4
Figure 1:
Extensive LV/small circulation thrombosis Source: University Hospital Basel, Basel, Switzerland
In this kind of typical clinical scenario, the conditions of LV (over)load are usually directly evaluated in terms of a need for an additional unloading strategy. In daily practice, the decision to perform an unloading intervention is rather complex and depends on various aspects, such as patient-specific requirements, procedural benefits, risks and limitations. Importantly, it should be generally recognized that in cases of severe cardiogenic shock and significant LV overload during VA-ECMO, additional interventions are often justified (Figure 2.). Obviously, conservative measures and optimal VA-ECMO management are compulsory in this setting and may relieve cardiac mechanical overload to some degree. However, they may be significantly less effective than appropriate additional invasive strategies for LV unloading during VA-ECMO.
Figure 2:
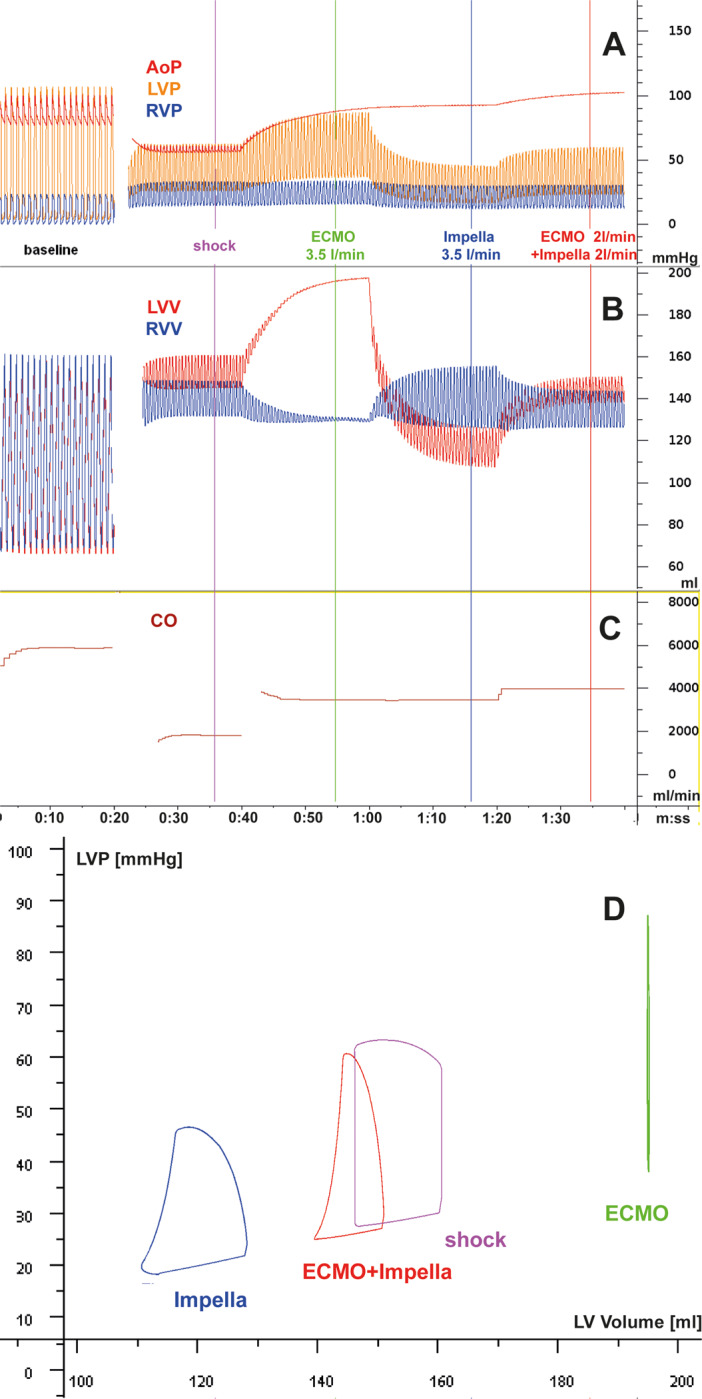
The differential haemodynamic effects of cardiogenic shock in acute myocardial infarction with mechanical support by ECMO, Impella, and their combination is modelled based on the Basel Virtual Patient, a multi-organ computational patient simulator at the University Hospital of Basel. Panels A–C shows parameters over time, and Panel D shows the left ventricular pressure/volume loops at the time points indicated by coloured bars in panels A–C, with violet: unsupported cardiogenic shock (CS); green: CS with ECMO support at 3.5 L/min; blue: CS with Impella support at 3.5 L/min; red: CS with ECMO at 2 L/min plus Impella at 2 L/min. Starting with a normal cardiac function at baseline (heart rate, 60/min), a myocardial infarction-triggered shock state is induced by partial loss of contractile left ventricular myocardium [reduced overall left ventricular (LV) systolic contractility], impaired LV diastolic function (delayed relaxation and increased stiffness), resulting in acute heart failure and triggering adrenergic activation with tachycardia (120/min), systemic and pulmonary vasoconstriction and venous pooling, while right ventricular systolic and diastolic function are not affected. AoP, aortic pressure; CO, combined cardiac output from heart and devices; LVP, left ventricular pressure; LVV, left ventricular volume; RVP, right ventricular pressure; RVV, right ventricular volume. (A–C) In the absence of RV failure, ECMO and Impella are similarly capable of delivering a cardiac output to the systemic circulation, but their effects on the left ventricle and the pulmonary circulation differ: in ECMO, residual forward flow through the pulmonary artery and the bronchial artery system and eventually some backflow from minor aortic regurgitation may lead to increased LV loading, increased LV pressures, and LV wall tension in ECMO. In Impella, the LV is consistently unloaded, wall tension is decreased and forward flow in the small circulation is maintained. When aortic pressure exceeds the maximum pressure that can be developed by the infarcted LV, the entire cardiac output is delivered by the respective device(s). Despite venous drainage in ECMO, high LV filling pressures in ECMO can occur and are compatible with the occurrence of pulmonary oedema in these patients, in contrast to the patients treated by ECpella or Impella alone. The combination of the two pumps, even with lower output from each individual device, reverses the LV loading observed by the ECMO. (D) ECMO support typically leads to a right shift of the PV loop (i.e. loading). Impella support consistently leads to a left shift of the PV loop indicating reduced diastolic and systolic wall tension and reduced myocardial oxygen consumption as predicted by pressure loop area. Source: University Hospital Basel, Basel, Switzerland
Clinical aspects of left ventricular unloading
Clinical decisions should generally be guided by carefully considering potential therapeutic benefits and risks in every individual case, including the intended goal and expected length of the bridging strategy with VA-ECMO.
Left ventricular overload at any time during VA-ECMO may occur in up to 70% of patients; however, urgent decompression is undertaken in only approximately 10% of patients, whereas an additional 20% might need an unloading intervention at a later stage.5,6
The appropriateness of unloading and the effort to avoid low- to non-pulsatile states has to be strongly individualized. Notably, clinical studies and literature analyses suggest improved outcomes when adjunctive unloading strategies are deployed.6,7
In routine clinical practice, patients under high-flow VA-ECMO support (>4 L/min) who exhibit a dilated left ventricle with nearly absent native left ventricular contractility should always be considered at a very high risk for significant LV overload.2,5 Moreover, the right–left ventricular interaction is of paramount importance, as sustained right ventricular contractility may actually contribute to mechanical overload, which negatively impacts the failing LV myocardium. In this setting, despite right ventricular drainage, it may still be capable of ejecting enough blood via the left atrium into the failing left ventricle, which is facing an increased afterload mediated by the retrogradely directed extracorporeal flow in the aorta towards the left ventricle.3 Therefore, paradoxically, preserved right ventricular function is a critical additional risk factor for significantly overloading a failing left ventricle under VA-ECMO support.3
Again, paradoxically and in contrast to what was stated above, following LV unloading initiation, appropriate right ventricular ejection and flow generation is needed to facilitate actual unloading and to avoid ‘over-unloading’. This right–left and left–right interaction is truly very dynamic, and the balance has to be maintained continuously.
Generally, frequent echocardiography measurements may help to estimate the degree of unwanted LV overloading during VA-ECMO simultaneously with the occurrence and aggravation of pulmonary oedema on serial chest X-rays or decreasing pulmonary compliance. Additionally, the estimation of high LV filling pressures by Doppler echocardiography or their (in-)direct measurement using a dedicated surgical left atrial pressure line and conventional pulmonary artery catheter in a wedge position or a pigtail catheter in the left ventricle can be performed.
Conservative and pharmacological management to optimize LV (un)loading
Conservative measures alone or in combination may be effective enough to unload the left ventricle during VA-ECMO support in mild and moderate cases. Both a reduction in the circulating volume and vasodilation, if possible, i.e. pre- and afterload reduction, are encouraged to optimize LV overload during extracorporeal support. Furthermore, the initiation of inodilators, e.g. dobutamine, milrinone, or levosimendan, may ameliorate LV overload, but potentially at the expense of inadvertent cellular effects, e.g. increased oxygen demand and intracellular calcium overload, which negatively affect recovering cardiomyocytes and frequently lead to various (supra-)ventricular arrhythmias. In mechanically ventilated patients, an adequate level of positive end-expiratory pressure, e.g. >8–10 cm H2O, theoretically supports LV mechanics and avoids pulmonary oedema, atelectasis and, in the longer run, potentially concomitant acute lung injury and related sequelae.8
Invasive measures to optimize LV (un)loading
Anecdotally, a large-bore LV pigtail catheter connected to the venous line of the VA-ECMO circuit has been used to drain/unload the left ventricle,9 but it is not being used routinely to the best of the authors’ knowledge. In contrast, the combination of VA-ECMO with an intra-aortic balloon pump (IABP) has long been advocated for LV unloading, and several studies have suggested that it might help to reduce capillary wedge pressure and pulmonary oedema and might even promote survival7,10,11; however, its routine use during VA-ECMO is not unequivocally agreed upon. In this context, it should be noted that on theoretical grounds, only up to an approximately 5 mmHg reduction in LV filling pressures may be expected from the adjunctive use of an IABP.2
Other invasive measures are considered more powerful adjunctive unloading strategies, as quantified in simulation experiments, but they may require more invasive procedures or even surgery.5 Among those measures, the use of an Impella device, commonly referred to as the ECpella (or ECMella) strategy, can yield very powerful LV unloading effects and is characterized by acceptable invasiveness (see below). Moreover, indirect and direct venting of the left ventricle has been described, e.g. atrial transseptal catheterization, atrial septostomy, pulmonary artery drainage/venting, transaortic venting and direct left atrial and LV venting. All the latter techniques have shown to have different unloading potentials and are characterized by procedural peculiarities that require sufficient operational and critical care management experience.2,5,12
The evidence for the effectiveness of all of the above techniques (IABP, tandem heart, transseptal venting, etc.) is rather limited and variable.12 For reports on the TandemHeart and atrial septostomy, mostly case series have been reported, whereas a comprehensive meta-analysis performed by Cheng et al.13 did not prove that the use of IABPs in association with VA-ECMO is associated with a significant change in survival outcomes. Therefore, it can be concluded that the actual indication, its timing, the effect on clinical outcomes, and differences between the available interventions remain unclear so far.12 Apparently, the use of a transaortic drainage device represents one of the most frequently adopted tools to achieve effective LV unloading; another promising percutaneous technique of indirect LV unloading might be the catheter/cannula-based draining of blood directly from the pulmonary artery, similar to how VA-ECMO actually drains central venous blood within the right atrium.14,15
Because of inherent difficulties in measuring the detailed haemodynamic effects of unloading clinically, several experimental and only limited clinical studies have compared the above adjunctive measures to evaluate their unloading effects on the left ventricle: the Impella® acts by generating continuous blood flow from the LV cavity into the aorta and thereby decompressing the left ventricle, leading to an inherent cessation of the isovolumetric periods within the pressure–volume tracings, i.e. the loops change into a triangular shape and shift leftwards with decreasing LV volume and pressure. Even more importantly, in relation to the flow generated by the Impella device, e.g. a decreasing trend in LV end-diastolic volume and LV end-diastolic pressure and also slightly decreasing trend in pulmonary capillary wedge pressure is being observed.2,16–19 Comparably, a small clinical series confirmed these findings in six patients on VA-ECMO who received LV unloading with an adjunctive Impella device.20
ECpella for left ventricular unloading/venting
Several clinical studies have evaluated the role of the Impella® in unloading/venting in VA-ECMO-treated patients with cardiogenic shock. Pappalardo et al.21 described for the first time a large series of patients treated with the combination of VA-ECMO and the Impella compared with patients treated with VA-ECMO only. Retrospectively collected data from 157 patients were evaluated: 123 with VA-ECMO support and 34 with concomitant VA-ECMO and the Impella® were compared with a propensity-matching analysis approach. Forty-two patients undergoing VA-ECMO alone (control group) were compared with 21 patients treated with VA-ECMO and the Impella. Patients in the combination group had significantly lower hospital mortality (47% vs. 80%, P < 0.001) and a higher rate of successful bridging to either recovery or further therapy (68% vs. 28%, P < 0.001) than patients in the VA-ECMO treatment group. A higher need for continuous renal replacement therapy (CVVHD) (48% vs. 19%, P = 0.02) and increased haemolysis (76% vs. 33%, P = 0.004) were reported, and bleeding complications were comparable at approximately 30%.
Similarly, a USA study by Patel et al.22 compared 36 VA-ECMO patients and 30 VA-ECMO + Impella patients, referred to as the ECpella cohort. Thirty-day all-cause mortality was significantly lower in the ECpella cohort (57% vs. 78%; hazard ratio 0.51 (0.28–0.94), log rank P = 0.02). Importantly, no difference between secondary outcomes (duration of support, stroke, major bleeding, haemolysis, inotropic score, and cardiac recovery) was observed, except for the inotrope score, which was greater in the VA-ECMO group by day 2 (11 vs. 0; P = 0.001). In a study by Akanni et al.23 with 29 patients on ‘ECVAD’, i.e. ECpella and isolated ECMO therapy (n = 196), showed comparable favourable results, with the only difference being an increased rate of haemolysis in the ECpella group (45 vs. 17%, P = 0.002). In summary, these studies suggest that concomitant treatment with VA-ECMO and the Impella may improve outcomes in patients with cardiogenic shock compared with VA-ECMO treatment alone.
A recent meta-analysis by Grajeda Silvestri et al.24 further confirmed the above statement and included three studies involving a total of 448 patients, 26% female, with a mean age of 57 years. VA-ECMO was performed in 355 out of 448 (79%) patients, while ‘ECVAD’ (ECMO combined with the Impella) was performed in 93 out of 448 (21%). Death occurred in 49 out of 93 (52.6%) patients on ECVAD and 226 out of 355 (63.6%) on ECMO [relative risk (RR): 0.76, 95% confidence interval (CI) (0.62–0.94), P = 0.01]. This positive effect on mortality was outweighed by the increased occurrence of haemolysis in 46 (49.4%) patients in the ECVAD group vs. 67 (18%) in the ECMO group [RR: 2.64, 95% CI (1.97–3.55), P < 0.01). Bleeding was comparable in patients on ECVAD (45.2 vs. 38%) and patients in the ECMO group, who also needed CVVHD (33.3 vs. 25%). In this meta-analysis, the use of the Impella as an unloading strategy in patients with VA-ECMO apparently decreased mortality and increased the rate of haemolysis while having a neutral bleeding risk and similar rates of acute kidney injury requiring CVVHD. Similarly, in a large recent multicenter propensity matched analysis, LV unloading was associated with lower mortality in cardiogenic schok patients treated with VA-ECMO, despite higher complication rates.25
Early use of combined mechanical support with Impella and VA-ECMO
In contrast to an approach of unloading the left ventricle in an already overloaded state, the early use of combined mechanical support to simultaneously enhance organ perfusion and protect the left ventricle with an adjunctive unloading intervention seems to be very promising. A German registry study26 analysed 69 consecutive patients suffering from severe refractory cardiogenic shock with a 70% predicted mortality in whom Impella pumps and VA-ECMO were combined early (duration of combined support: median 94 h; interquartile range, 49–150 h). Early mechanical circulatory support (MCS) escalation stabilized the patients rapidly and reduced the number and doses of catecholamines (P < 0.05 vs. baseline), while haemodynamics improved. Importantly, an improvement in microcirculation followed by lactate normalization was also noted. Survival rates reached 60% on support, 49% at 30 days and 40% at the 6-month follow-up. In a multivariate analysis, the duration of shock-to-first device and lactate levels after 12 h of MCS independently predicted survival. Interestingly, right ventricular failure predisposed patients to treatment futility. Therefore, the early and consequent combination of the Impella and VA-ECMO may enable even more effective clinical stabilization and rescue high-risk patients with refractory cardiogenic shock (Figure 3). Surprisingly, the combined approach with both the axial device as a first step, or vice versa, VA-ECMO first and the Impella pump added subsequently, seems to be an increasingly accepted approach for immediate patient stabilization in the most severe forms of cardiogenic shock.27
Figure 3:
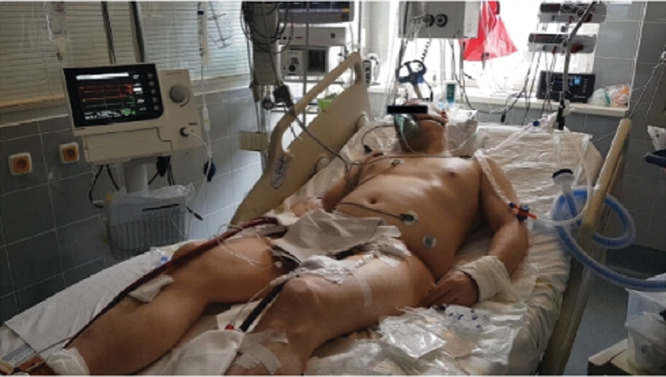
A patient in cardiogenic shock supported by a combined VA ECMO and Impella CP approach, venous cannula and Impella catheter in the left groin, and arterial cannula in the right groin. Source: General University Hospital, Prague.
Complications
As stated above, this highly invasive approach of implanting VA-ECMO and the Impella in combination bears an inherent risk of major complications, mainly those that are vascular in nature as well as bleeding, haemolysis and acute renal injury. Haemolysis remains among the major adverse drawbacks of the combined Impella–VA-ECMO approach, with the rate of haemolysis reported as up to 50–70% of cases in a larger series.21,23,24
A typical clinical scenario for using combined VA-ECMO and the Impella approach
A 70-year-old male was admitted directly to the catheterization lab with an anterior ST-elevation myocardial infarction and cardiogenic shock, and his blood pressure was 70/40 mmHg, which was obtunded and agitated. Coronary angiography revealed a proximal left anterior descending (LAD) artery thrombotic preocclusion with large intraluminal thrombi. During preparation for percutaneous coronary intervention (PCI), his haemodynamic status deteriorated progressively, and cardiac arrest occurred due to ventricular fibrillation (VF). Following three unsuccessful defibrillations, VA-ECMO was emergently implanted with cardiac massage. After VA-ECMO was initiated with a support flow over 4 L/min, PCI was performed, resulting in optimal LAD reperfusion; however, refractory VF persisted, and the left ventricle was dilated and non-pulsatile. After another six unsuccessful defibrillations, an Impella CP was implanted, and the left ventricle was unloaded with 1.5 L/min, which resulted in the immediate restoration of sinus rhythm following another defibrillation. However, the left ventricle remained in a state of sustained non-pulsatility for the next 48 h, after which a weaning trial was initiated.
This case clearly demonstrates the power of an adjunctive LV unloading intervention in clinical practice for an acutely overloaded ischaemic myocardium. The figure illustrates how the patient was stabilized in the Coronary Care Unit after combining VA-ECMO and the Impella.
Future perspectives
Mechanical overload of the left ventricle is the major adverse effect and an obvious mechanistic and prognostic challenge of contemporary ECMO care. Many efforts in current research aim to overcome this phenomenon by LV unloading, which was effectively achieved by the current combined approach using an axial decompression device, while novel technical developments and approaches are tested and urgently anticipated.
In this context, the reliable identification of individual patients who would truly benefit from a tailored adjunct LV unloading intervention remains challenging, as the majority of VA-ECMO patients currently do not undergo adjunctive unloading. Large clinical studies are desperately needed in this regard. These analyses should concurrently study different common clinical scenarios with the individualized need for a certain degree of LV unloading based on theoretical considerations.2,7
Interestingly, novel electrocardiogram-synchronized ECMO systems have been introduced that operate in a pulsatile mode and thereby aim to unload the left ventricle during specific phases of the cardiac cycle, while others have proposed novel cannula designs featuring an intrinsic unloading capacity.28,29
Acknowledgements
This manuscript is one of eight manuscripts published as a Supplement to address patient management and mechanical circulatory support in the ICU.
Funding
This work has been supported by the Abiomed Europe GmbH to cover publication costs as well as professional language editing of each manuscript. No individual fees were paid to the authors in the generation of this publication. This paper was published as part of a supplement financially supported by Abiomed GmbH.
Conflict of interest: J.B. reports personal fees from Abiomed, personal fees from Getinge, during the conduct of the study; personal fees from Novartis, personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, outside the submitted work. P.H. reports personal fees from Abiomed, outside the submitted work. D.W.D. reports personal fees from Getinge and Fresenius and research cooperation with Getinge and Xenios NovaLung.
References
- 1. Ostadal P, Mlcek M, Kruger A, Hala P, Lacko S, Mates M, Vondrakova D, Svoboda T, Hrachovina M, Janotka M, Psotova H, Strunina S, Kittnar O, Neuzil P. Increasing venoarterial extracorporeal membrane oxygenation flow negatively affects left ventricular performance in a porcine model of cardiogenic shock. J Transl Med 2015;13:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donker DW, Brodie D, Henriques JPS, Broomé M. Left ventricular unloading during veno-arterial ECMO: a simulation study. ASAIO J 2019;65:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Donker DW, Sallisalmi M, Broomé M. Right–left ventricular interaction in left-sided heart failure with and without venoarterial extracorporeal membrane oxygenation support—a simulation study. ASAIO J 2020;doi:10.1097/MAT.0000000000001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ündar A, Frazier OH, Fraser CD Jr. Defining pulsatile perfusion: quantification in terms of energy equivalent pressure. Artif Organs 1999;23:712–716. [DOI] [PubMed] [Google Scholar]
- 5. Donker DW, Brodie D, Henriques JPS, Broomé M. Left ventricular unloading during veno-arterial ECMO: a review of percutaneous and surgical unloading interventions. Perfusion 2019;34:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Truby LK, Takeda K, Mauro C, Yuzefpolskaya M, Garan AR, Kirtane AJ, Topkara VK, Abrams D, Brodie D, Colombo PC, Naka Y, Takayama H. Incidence and implications of left ventricular distention during venoarterial extracorporeal membrane oxygenation support. ASAIO J 2017;63:257–265. [DOI] [PubMed] [Google Scholar]
- 7. Meuwese CL, Koudstaal S, Braithwaite S, Hermens JAJ, Donker DW. Left ventricular unloading during extracorporeal membrane oxygenation. J Am Coll Cardiol 2019;73:3034–3035. [DOI] [PubMed] [Google Scholar]
- 8. Meuwese CL, Ramjankhan FZ, Braithwaite SA, de Jonge N, de Jong M, Buijsrogge MP, Janssen JGD, Klöpping C, Kirkels JH, Donker DW. Extracorporeal life support in cardiogenic shock: indications and management in current practice. Neth Heart J 2018;26:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barbone A, Malvindi PG, Ferrara P, Tarelli G. Left ventricle unloading by percutaneous pigtail during extracorporeal membrane oxygenation. Interact Cardiovasc Thorac Surg 2011;13:293–295. [DOI] [PubMed] [Google Scholar]
- 10. Petroni T, Harrois A, Amour J, Lebreton G, Brechot N, Tanaka S, Luyt CE, Trouillet JL, Chastre J, Leprince P, Duranteau J, Combes A. Intra-aortic balloon pump effects on macrocirculation and microcirculation in cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Crit Care Med 2014;42:2075–2082. [DOI] [PubMed] [Google Scholar]
- 11. Bréchot N, Demondion P, Santi F, Lebreton G, Pham T, Dalakidis A, Gambotti L, Luyt CE, Schmidt M, Hekimian G, Cluzel P, Chastre J, Leprince P, Combes A. Intra-aortic balloon pump protects against hydrostatic pulmonary oedema during peripheral venoarterial-extracorporeal membrane oxygenation. Eur Heart J Acute Cardiovasc Care 2018;7:62–69. [DOI] [PubMed] [Google Scholar]
- 12. Meani P, Gelsomino S, Natour E, Johnson DM, Rocca HPBL, Pappalardo F, Bidar E, Makhoul M, Raffa G, Heuts S, Lozekoot P, Kats S, Sluijpers N, Schreurs R, Delnoij T, Montalti A, Sels JW, van de Poll M, Roekaerts P, Poels T, Korver E, Babar Z, Maessen J, Lorusso R. Modalities and effects of left ventricle unloading on extracorporeal life support: a review of the current literature. Eur J Heart Fail 2017;19:84–91. [DOI] [PubMed] [Google Scholar]
- 13. Cheng R, Hachamovitch R, Makkar R, Ramzy D, Moriguchi JD, Arabia FA, Esmailian F, Azarbal B. Lack of survival benefit found with use of intraaortic balloon pump in extracorporeal membrane oxygenation: a pooled experience of 1517 patients. J Invasive Cardiol 2015;27:453–458. [PubMed] [Google Scholar]
- 14. Jensen PB, Kann SH, Veien KT, Møller-Helgestad OK, Dahl JS, Rud CS, Jensen MK, Jensen LO, Schmidt H, Møller JE. Single-centre experience with the Impella CP, 5.0 and RP in 109 consecutive patients with profound cardiogenic shock. Eur Heart J Acute Cardiovasc Care 2018;7:53–61. [DOI] [PubMed] [Google Scholar]
- 15. Tepper S, Masood MF, Baltazar Garcia M, Pisani M, Ewald GA, Lasala JM, Bach RG, Singh J, Balsara KR, Itoh A. Left ventricular unloading by impella device versus surgical vent during extracorporeal life support. Ann Thorac Surg 2017;104:861–867. [DOI] [PubMed] [Google Scholar]
- 16. Weil BR, Konecny F, Suzuki G, Iyer V, Canty JM Jr. Comparative hemodynamic effects of contemporary percutaneous mechanical circulatory support devices in a porcine model of acute myocardial infarction. JACC Cardiovasc Interv 2016;9:2292–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saku K, Kakino T, Arimura T, Sunagawa G, Nishikawa T, Sakamoto T, Kishi T, Tsutsui H, Sunagawa K. Left ventricular mechanical unloading by total support of impella in myocardial infarction reduces infarct size, preserves left ventricular function, and prevents subsequent heart failure in dogs. Circ Heart Fail 2018;11:e004397. [DOI] [PubMed] [Google Scholar]
- 18. Watanabe S, Fish K, Kovacic JC, Bikou O, Leonardson L, Nomoto K, Aguero J, Kapur NK, Hajjar RJ, Ishikawa K. Left ventricular unloading using an impella cp improves coronary flow and infarct zone perfusion in ischemic heart failure. J Am Heart Assoc 2018;7:e004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meani P, Mlcek M, Kowalewski M, Raffa GM, Popkova M, Pilato M, Arcadipane A, Belohlavek J, Lorusso R. Trans-aortic or pulmonary artery drainage for left ventricular unloading and veno-arterial extracorporeal life support: a porcine cardiogenic shock model. Semin Thorac Cardiovasc Surg 2020;doi:10.1053/j.semtcvs.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 20. Lim HS. The effect of Impella CP on cardiopulmonary physiology during venoarterial extracorporeal membrane oxygenation support. Artif Organs 2017;41:1109–1112. [DOI] [PubMed] [Google Scholar]
- 21. Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G, Greco T, Lembo R, Müllerleile K, Colombo A, Sydow K, De Bonis M, Wagner F, Reichenspurner H, Blankenberg S, Zangrillo A, Westermann D. Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 2017;19:404–412. [DOI] [PubMed] [Google Scholar]
- 22. Patel SM, Lipinski J, Al-Kindi SG, Patel T, Saric P, Li J, Nadeem F, Ladas T, Alaiti A, Phillips A, Medalion B, Deo S, Elgudin Y, Costa MA, Osman MN, Attizzani GF, Oliveira GH, Sareyyupoglu B, Bezerra HG. Simultaneous venoarterial extracorporeal membrane oxygenation and percutaneous left ventricular decompression therapy with impella is associated with improved outcomes in refractory cardiogenic shock. ASAIO J 2019;65:21–28. [DOI] [PubMed] [Google Scholar]
- 23. Akanni OJ, Takeda K, Truby LK, Kurlansky PA, Chiuzan C, Han J, Topkara VK, Yuzefpolskaya M, Colombo PC, Karmpaliotis D, Moses JW, Naka Y, Garan AR, Kirtane AJ, Takayama H. EC-VAD: combined use of extracorporeal membrane oxygenation and percutaneous microaxial pump left ventricular assist device. ASAIO J 2019;65:219–226. [DOI] [PubMed] [Google Scholar]
- 24. Grajeda Silvestri ER, Pino JE, Donath E, Torres P, Chait R, Ghumman W. Impella to unload the left ventricle in patients undergoing venoarterial extracorporeal membrane oxygenation for cardiogenic shock: a systematic review and meta-analysis. J Card Surg 2020;35:1237–1242. [DOI] [PubMed] [Google Scholar]
- 25. Schrage B, Becher PM, Bernhardt A, Bezerra H, Blankenberg S, Brunner S, Colson P, Cudemus Deseda G, Dabboura S, Eckner D, Eden M, Eitel I, Frank D, Frey N, Funamoto M, Goßling A, Graf T, Hagl C, Kirchhof P, Kupka D, Landmesser U, Lipinski J, Lopes M, Majunke N, Maniuc O, McGrath D, Möbius-Winkler S, Morrow DA, Mourad M, Noel C, Nordbeck P, Orban M, Pappalardo F, Patel SM, Pauschinger M, Pazzanese V, Reichenspurner H, Sandri M, Schulze PC, Schwinger RHG, Sinning JM, Aksoy A, Skurk C, Szczanowicz L, Thiele H, Tietz F, Varshney A, Wechsler L, Westermann D. Left ventricular unloading is associated with lower mortality in cardiogenic shock patients treated with veno-arterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation 2020;142:2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tongers J, Sieweke JT, Kühn C, Napp LC, Flierl U, Röntgen P, Schmitto JD, Sedding DG, Haverich A, Bauersachs J, Schäfer A. Early escalation of mechanical circulatory support stabilizes and potentially rescues patients in refractory cardiogenic shock. Circ Heart Fail 2020;13:e005853. [DOI] [PubMed] [Google Scholar]
- 27. Garan AR, Takeda K, Salna M, Vandenberge J, Doshi D, Karmpaliotis D, Kirtane AJ, Takayama H, Kurlansky P. Prospective comparison of a percutaneous ventricular assist device and venoarterial extracorporeal membrane oxygenation for patients with cardiogenic shock following acute myocardial infarction. J Am Heart Assoc 2019;8:e012171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ostadal P, Mlcek M, Gorhan H, Simundic I, Strunina S, Hrachovina M, Krüger A, Vondrakova D, Janotka M, Hala P, Mates M, Ostadal M, Leiter JC, Kittnar O, Neuzil P. Electrocardiogram-synchronized pulsatile extracorporeal life support preserves left ventricular function and coronary flow in a porcine model of cardiogenic shock. PLoS One 2018;13:e0196321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ježek F, Strunina S, Carlson BE, Hozman J. A simulation study of left ventricular decompression using a double lumen arterial cannula prototype during a veno-arterial extracorporeal membrane oxygenation. Int J Artif Organs 2019;42:748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]