Abstract
The rationale for mechanical circulatory support (MCS) in cardiogenic shock is to restore cardiac output in selected patients when critically low or in case of refractory cardiac arrest. Furthermore, an MCS device that moves blood from either the left atrium or the left ventricle to the systemic circulation will potentially unload the ventricle. These devices are used alone or in combination with venoarterial extracorporeal membrane oxygenation (VA-ECMO). If a left-sided Impella device is used, it should be run at the highest possible performance level during treatment while avoiding suction events. When combined with VA-ECMO, the Impella device should be run at a lower performance level, ensuring sufficient left ventricular emptying but avoiding suction. Continuous monitoring is pivotal and patients managed outside the catheterization laboratory should be monitored with an arterial line, a central venous catheter, frequent use of pulmonary artery catheters and regular imaging by transthoracic echocardiogram.
Keywords: Mechanical circulatory support, Unloading, Patient monitoring
Introduction
The rationale for mechanical circulatory support (MCS) in cardiogenic shock (CS) is to restore cardiac output in selected patients when critically low, or in case of refractory cardiac arrest. There are no randomized clinical trials addressing benefits or optimal timing or selection of CS but recent data demonstrate benefit of venoarterial extracorporeal membrane oxygenation (VA-ECMO) in patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation.1 Any MCS device that moves blood from either the left atrium or the left ventricle to the systemic circulation will potentially unload the ventricle, with corresponding reductions in left ventricular (LV) volumes and LV end-diastolic pressure, causing a decrease in pressure–volume area and reducing LV wall stress, thus leading to a reduction in LV workload and myocardial oxygen consumption.2–4 In the failing left ventricle, this has intuitive benefits, especially but not solely in the case of ischaemic LV failure. In agreement with this concept, preclinical studies have suggested that LV unloading reduces infarct size in models of ST-elevation myocardial infarction (STEMI).5,6 The exact mechanism is unknown, but translational data suggest that the mechanisms of reduced infarct size and lowered myocardial oxygen consumption are linked to activation of cardioprotective signalling, improved mitochondrial integrity and increased collateral coronary blood flow.7 Previous studies have also suggested a dose–response relationship, with a direct association between the degree of unloading and reduction in myocardial oxygen consumption and infarct size in STEMI models.4 The concept of unloading has also increased the use of Impella devices in combination with VA-ECMO to unload the left ventricle and avoid the deleterious effects of excessive afterload induced by VA-ECMO on the left ventricle and pulmonary circulation in some cases.8 In the event of severely depressed myocardial function with critically low cardiac output in CS, an improvement in end-organ perfusion is pivotal to avoid multiorgan failure and improve survival. In contrast to left-sided Impella devices, a potential therapeutic unloading effect has not been demonstrated for the Impella RP.
Pump flow setting in left ventricular support
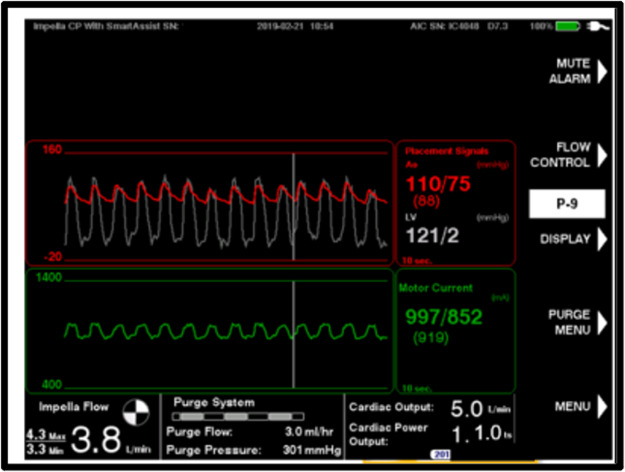
Thus, based on preclinical data, LV unloading is potentially associated with several advantages, and when a transvalvular pump is implanted, it seems intuitive to unload the left ventricle and augment systemic perfusion as much as possible. If a left-sided Impella device (2.5, CP, 5.0 or 5.5) is used, we suggest that it be run at the highest possible performance level during treatment. However, this must be accompanied by continuous surveillance for signs of suction events. Especially in non-dilated and hypertrophic hearts with small LV cavities, cases of right ventricular failure and hypovolaemia come with a risk of excessive unloading accompanied by LV emptying and suction. The consequence of suction events will be a decrease in pump flow, increased erythrocyte shear stress and haemolysis, and likely an increased risk of arrhythmia. In this situation, pump flow should be reduced until the underlying cause has been optimally resolved. In the newest versions of the Impella devices, there are several new metrics in Automated Impella Controller to aid positioning and weaning. The traditional fluid-filled pressure tracer has been replaced by an optical pressure sensor that senses aortic pressure. A second sensor has been incorporated into the microaxial motor. As the electric current running through the motor is very sensitive to the pressure difference between the ventricle and aorta, the motor current can estimate the aortic pressure signal and differential pressure signal. Based on these pressure waveforms, the Automated Impella Controller will provide estimates of left ventricular end-diastolic pressure, mean arterial pressure, and cardiac power output (Figure 1).
Figure 1.
A screenshot of automated Impella controller from the optical Impella CP demonstrating simultaneous estimated aortic and left ventricular pressure, estimated cardiac output and cardiac power output.
In rare cases, especially with the more powerful Impella 5.0 and Impella 5.5, high pump flow may cause interatrial right-to-left shunting across a persistent foramen ovale or atrial defects. Thus, in the event of unexpectedly poor oxygenation, especially when the patient is unresponsive to oxygen therapy and there is no pulmonary congestion, intracardiac shunting should be suspected, and transoesophageal echocardiography should be performed. In the case of shunting, the performance level should be reduced until clinically important shunting ceases or device closure of the defect is performed.
Pump flow settings during ECpella treatment
When combined with VA-ECMO, the Impella device should be run at a low performance level guided by echocardiography with a focus on LV distention and mitral and aortic regurgitation to ensure LV emptying but avoid suction. Usually, performance level P2 is adequate to achieve LV emptying, as the majority of flow is delivered by the VA-ECMO system and the Impella’s initial role is LV venting. With improvement in right heart function, VA-ECMO flow can usually be reduced gradually over hours or days with a concomitant increase in Impella flow until the patient is weaned off VA-ECMO.
Pump flow settings with Impella RP
The Impella RP should be run at the support level to meet the patient’s demand while avoiding suction. Signs of pulmonary congestion should be monitored, as, at least in theory, high Impella RP support with reduced LV function could cause overflow and pulmonary congestion. The use of pulmonary artery catheters with ongoing RP has not been thoroughly evaluated, and evaluation should also rely on clinical evaluation, oxygen demand, chest X-ray, etc. In clinical practice, this is rarely encountered, but especially in the case of BIPELLA therapy combining Impella RP and CP, careful balancing of the pumps is recommended.
Monitoring of pump flow settings
Patients requiring management with Impella support extended outside the catheterization laboratory should, at minimum, be monitored with an arterial line, a central venous catheter, frequent use of pulmonary artery catheters and regular imaging by transthoracic echocardiogram.
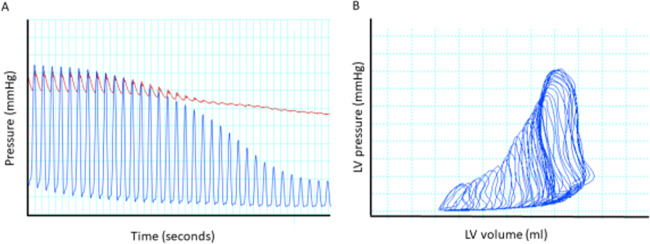
At the arterial line, attention should be paid to pulse pressure and mean arterial pressure. A non-pulsatile arterial signal suggests uncoupling between LV and systemic pressure. A change from pulsatility to non-pulsatility should trigger echocardiography to ensure proper positioning of the device, but non-pulsatility may suggest that the left ventricle is well unloaded. Unless, the flow delivered by the device is inadequate to meet the patient’s demand (SvO2 < 55% and high lactate), no action is required in this situation to restore arterial pulsatility, as this will increase myocardial oxygen consumption, and the uncoupling of left ventricle and aortic pressure suggests that the left ventricle is well unloaded (Figure 2).9 This scenario is very different from VA-ECMO support, as, in the event that pulsatility is lost, inotropes and LV venting should be considered.
Figure 2.
(A) Simultaneous pressure recording of left ventricle (blue) and aortic (red) pressure in a 70 kg Landrace pig with experimentally induced ischaemic cardiogenic shock supported by Impella CP and low-dose norepinephrine. Preload reduction was performed by inferior vena cava occlusion with resultant uncoupling of left ventricle and aortic pressure but maintenance of aortic pressure. (B) Corresponding pressure–volume trace from the left ventricle, demonstrating a marked reduction in pressure–volume area despite maintenance of aortic pressure. Adapted from ref.8
From pulmonary arterial traces, the goal is to find a balance of adequate pressure to allow LV filling without suction events and keep filling pressures as low as possible to avoid pulmonary congestion. Usually, a pulmonary artery capillary wedge pressure of 12–18 mmHg will produce a good balance. To some degree, this information can also be gained from the newest version of devices with optical sensors that allow estimation of LV end-diastolic pressure, which is shown directly on the console. In addition, Impella Connect facilitates the remote monitoring of pump performance and haemodynamic parameters to optimize 24/7 patient support and pump management.
Focused echocardiography also has a pivotal role in the assessment of pump flow settings. Imaging should focus on placement of the device, left and right ventricular size and function, volume status including assessment of the inferior vena cava, and valve regurgitation. This assessment should usually be performed at least once daily and cannot be replaced by other modalities or haemodynamic monitoring. Assessment of end-systolic volume and device placement is of great importance to detect early signs of suction; therefore, echocardiograms in this situation are more qualitative than quantitative, and the echocardiographer needs to be familiar with the function and imaging features of the device. In addition, echocardiography should be performed quickly in the event of any unexplained acute event: a drop in Impella flow, an alarm, acute pulmonary oedema, or hypotension.
Key messages
Left-sided transvalvular pumps can lead to left ventricular unloading that, in preclinical models, appears to provide benefits beyond augmentation of flow, especially in terms of myocardial recovery. This has not been demonstrated for right-sided devices.
Left-sided devices should be set at the highest performance level possible without suction during treatment.
The Impella RP should be run at the support level to meet the patient’s demand while avoiding suction.
Unloading is monitored by interpretation of pulse pressure and pulmonary artery wedge pressure and by frequent imaging (echocardiography).
Acknowledgements
This manuscript is one of eight manuscripts published as a Supplement to address patient management and mechanical circulatory support in the ICU.
Funding
This work has been supported by the Abiomed Europe GmbH to cover publication costs as well as professional language editing of each manuscript. No individual fees were paid to the authors in the generation of this publication. This paper was published as part of a supplement financially supported by Abiomed GmbH.
Conflict of interest: J.E.M. reports grants and personal fees from Abiomed, personal fees from Novartis, personal fees from Orion Pharma, personal fees from Boeinger Ingleheim, outside the submitted work; C.D. reports personal fees from ABIOMED, personal fees from ABBOTT, personal fees from BOSTON, outside the submitted work; L.B. reports grants; from Abbott, grants from Boston, personal fees and non-financial support from Abiomed, non-financial support from Biosensor, outside the submitted work; F.P. personal fees from Abiomed, during the conduct of the study; C.H. has nothing to disclose.
References
- 1. Yannopoulos D, Bartos J, Raveendran G, Walser E, Connett J, Murray TA, Collins G, Zhang L, Kalra R, Kosmopoulos M, John R, Shaffer A, Frascone RJ, Wesley K, Conterato M, Biros M, Tolar J, Aufderheide TP. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet 2020;396:1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kapur NK, Alkhouli MA, DeMartini TJ, Faraz H, George ZH, Goodwin MJ, Hernandez-Montfort JA, Iyer VS, Josephy N, Kalra S, Kaki A, Karas RH, Kimmelstiel CD, Koenig GC, Lau E, Lotun K, Madder RD, Mannino SF, Meraj PM, Moreland JA, Moses JW, Kim RL, Schreiber TL, Udelson JE, Witzke C, Wohns DHW, O’Neill WW. Unloading the left ventricle before reperfusion in patients with anterior ST-segment-elevation myocardial infarction. Circulation 2019;139:337–346. [DOI] [PubMed] [Google Scholar]
- 3. Møller-Helgestad OK, Hyldebrandt JA, Banke A, Rud CS, Udesen NLJ, Linde L, Okkels-Jensen L, Schmidt H, Ravn HB, Møller JE. Impella CP or VA-ECMO in profound cardiogenic shock: left ventricular unloading and organ perfusion in a large animal model. EuroIntervention 2019;14:e1585–e1592. [DOI] [PubMed] [Google Scholar]
- 4. Saku K, Kakino T, Arimura T, Sunagawa G, Nishikawa T, Sakamoto T, Kishi T, Tsutsui H, Sunagawa K. Left ventricular mechanical unloading by total support of Impella in myocardial infarction reduces infarct size, preserves left ventricular function, and prevents subsequent heart failure in dogs. Circulation 2018;11:e004397. [DOI] [PubMed] [Google Scholar]
- 5. Kapur NK, Paruchuri V, Urbano-Morales JA, Mackey EE, Daly GH, Qiao X, Pandian N, Perides G, Karas RH. Mechanically unloading the left ventricle before coronary reperfusion reduces left ventricular wall stress and myocardial infarct size. Circulation 2013;128:328–336. [DOI] [PubMed] [Google Scholar]
- 6. Kapur NK, Qiao X, Paruchuri V, Morine KJ, Syed W, Dow S, Shah N, Pandian N, Karas RH. Mechanical pre-conditioning with acute circulatory support before reperfusion limits infarct size in acute myocardial infarction. JACC 2015;3:873–882. [DOI] [PubMed] [Google Scholar]
- 7. Swain L, Reyelt L, Bhave S, Qiao X, Thomas CJ, Zweck E, Crowley P, Boggins C, Esposito M, Chin M, Karas RH, O’Neill W, Kapur NK. Transvalvular ventricular unloading before reperfusion in acute myocardial infarction. J Am Coll Cardiol 2020;76:684–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schrage B, Burkhoff D, Rübsamen N, Becher PM, Schwarzl M, Bernhardt A, Grahn H, Lubos E, Söffker G, Clemmensen P, Reichenspurner H, Blankenberg S, Westermann D. Unloading of the left ventricle during venoarterial extracorporeal membrane oxygenation therapy in cardiogenic shock. JACC 2018;6:1035–1043. [DOI] [PubMed] [Google Scholar]
- 9. Udesen NLJ, Helgestad OKL, Banke ABS, Frederiksen PH, Josiassen J, Jensen LO, Schmidt H, Edelman ER, Chang BY, Ravn HB, Møller JE. Impact of concomitant vasoactive treatment and mechanical left ventricular unloading in a porcine model of profound cardiogenic shock. Crit Care 2020;24:95–95. [DOI] [PMC free article] [PubMed] [Google Scholar]