Abstract
Cardiogenic shock (CS) is a clinical entity that includes a wide spectrum of different scenarios. Mechanical circulatory support (MCS) plays a fundamental role in the contemporary treatment of CS, and device selection is a key element in determining optimal treatment in this complex population. Cardiac support with mechanical devices should allow reduction and complete weaning from inotropes. Persistence of elevated left ventricular (LV) filling pressures, pulmonary congestion, metabolic decompensation, and end-organ damage during current MCS are criteria for MCS escalation. Precise diagnosis of the underlying cause of right ventricular (RV) failure is fundamental for undertaking the correct escalation strategy. In the setting of both MCS escalation and de-escalation, it is important to select a strategy in relation to long-term perspectives (bridge-to-recovery, bridge-to-LV assist device, or bridge-to-heart transplantation). Small retrospective studies have demonstrated that the BiPella approach is feasible, reduces cardiac filling pressures and improves cardiac output across a range of causes of CS. Simultaneous LV and RV device implantation and lower RV afterload may be associated with better outcomes in biventricular CS, but prospective studies are still required.
Keywords: Mechanical circulatory support, Cardiogenic shock, Ventricular dysfunction, Unloading
Introduction
Cardiogenic shock (CS) is a clinical entity that includes a wide spectrum of different scenarios. Each patient presents with peculiar characteristics; the haemodynamic status rapidly changes, and survival in this complex population suffers from many variables. In this context, physicians should move towards patient-centred care with a specific patient-tailored approach.
Mechanical circulatory support (MCS) plays a fundamental role in the contemporary treatment of CS, and device selection is a key element in determining optimal treatment in this complex population. Contemporary critical care cardiology is moving towards the strategic use of different MCS devices, each characterized by specific nominal flow, potential complications, and expected durations. A simultaneous or consecutive combination of MCS, with correct timing and indications for implantation, escalation and de-escalation, customized for each patient and clinical scenario, can improve outcomes. Table 1 summarizes haemodynamic effects of different MCS devices. Previous studies demonstrated a temporal relationship between the early use of percutaneous MCS and improved clinical outcomes in CS patients.1–3
Table 1.
Haemodynamic effects of different mechanical circulatory support devices
| IABP | Impella |
VA-ECMO | ||
|---|---|---|---|---|
| 2.5/CP | 5.0/5.5 | |||
| LV flow | ↑ | ↓ | ↓ | ↓ |
| CO | ↑ | ↑↑ | ↑↑↑ | ↑↑↑ |
| MAP | ↑ | ↑↑ | ↑↑ | ↑↑ |
| PCWP | = or ↓ | ↓ | ↓↓ | = or ↑ |
| LV afterload | ↓ | ↓ | ↓ | ↑↑↑ |
| CVP | = or ↓ | = or ↓ | = or ↓ | ↓ |
| MVO2 | ↓ | ↓↓ | ↓↓ | = or ↑ |
| Coronary perfusion | ↑ | ↑ | ↑ | = |
| Peripheral tissue perfusion | ↑ | ↑↑ | ↑↑↑ | ↑↑↑ |
↑, increased; ↓, reduced; CO, cardiac output; CVP, central venous pressure; IABP, intra-aortic balloon pump; LV, left ventricle; MAP, mean arterial pressure; MVO2, myocardial oxygen consumption; PCWP, pulmonary capillary wedge pressure; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
Likewise, a multidisciplinary shock team that merges different profiles of physicians provides a timely diagnosis and delivers the most appropriate care, reducing mortality in CS.4 All patients with CS should be cared for in an intensive care unit with the availability of comprehensive haemodynamic invasive and non-invasive monitoring. The use of pulmonary artery catheters is strongly advised, not to support diagnosis but rather for advanced and comprehensive management. Continuous monitoring should focus on pump performance, cardiac output, blood pressure, cardiac filling pressures, and residual ejection of the left ventricle; these, together with shock parameters (lactates, metabolic acidosis, mixed venous oxygen saturation), should drive pump speed settings and detect haemodynamic status changes to guide prompt therapeutic action by physicians.
Escalation
Criteria and timing
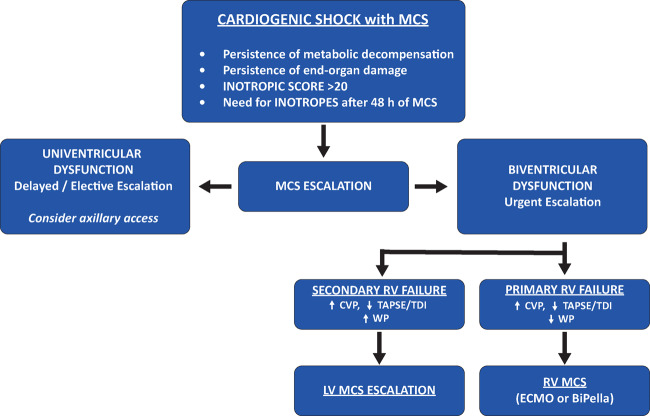
As the dynamics of myocardial dysfunction is extremely complex in CS due to the interplay between primary and secondary myocardial dysfunction and, eventually, the occurrence of right ventricular (RV) failure, clinical sensitivity should trigger the need for escalation of MCS and selection of the most appropriate configuration (ECpella, BiPella, Impella 5.0/5.5, intrathoracic ventricular assist device). Aortic regurgitation should be approached at this stage.
First, adequate cardiac support with mechanical devices should allow reduction and complete weaning from inotropes. Despite the positive effect on myocardial contractility and temporary improvements in haemodynamics, administration of inotropes is burdened by increased myocardial oxygen consumption and arrhythmic risk, which might be associated with increased mortality.5,6 The current European Society of Cardiology (ESC) Guidelines on Acute and Chronic Heart Failure state that inotropes may be considered as initial therapy if there is a need to maintain systolic blood pressure in the presence of persistent hypoperfusion (Class IIb); however, rather than combining several inotropes, device therapy has to be considered when there is an inadequate response.7
In the setting of CS with MCS, inotrope cut-off values are extremely valuable for clinical purposes: an inotropic score >20 on MCS should warrant evaluation for escalation.8 Similarly, the requirement of inotropes for more than 48 h should trigger a full haemodynamic re-evaluation.
Moreover, persistence of elevated left ventricular (LV) filling pressures, pulmonary congestion, metabolic decompensation, and end-organ damage during current MCS are criteria for MCS escalation.
Complete clinical, haemodynamic, and echocardiographic evaluations should be repeated at least every 24 h, and prompt MCS escalation should be considered when previous criteria are matched, given the evidence that the early use of appropriate MCS correlates with better clinical outcomes.1–3
Uni/biventricular escalation
Multiparametric evaluation is fundamental to make the correct diagnosis of persistent CS (uni- or biventricular dysfunction) and, consequently, to select the appropriate timing and MCS escalation strategy.
Univentricular dysfunction (LV dysfunction with preserved RV contractility) allows delayed or elective MCS escalation (a few to 24 h). Usually, first-line MCS in patients with residual LV function is represented by femoral percutaneous devices [Impella 2.5/CP or intra-aortic balloon pump (IABP)] due to their easier and faster implantation. When MCS escalation is needed, the transition from femoral percutaneous MCS (Impella 2.5/CP or IABP) to an upper body approach with a more powerful pump (Impella 5.0/5.5) is advised.
The Impella 5.0 device is designed for surgical transaxillary insertion (21-F pump motor with a 9-F catheter) and requires time to assess the technical feasibility (first, the sizing of axillary arteries) and to organize the availability of an operating room and vascular surgeons. The following items are considered absolute contraindications for performing the procedure: a vessel artery diameter <6 mm, the presence of heavy calcifications, obstruction or dissection, pre-existing upper extremity ischaemia, previous arterial axillary open cannulations or surgical access/scars, infraclavicular infections, arteriovenous fistulae for dialysis, and a patent internal mammary artery graft; the right axillary artery is preferably used, unless contraindicated due to anatomical, clinical, or pathological reasons.9
To date, there is no robust evidence regarding the Impella 5.0 device in randomized clinical trials. However, observational studies have demonstrated the efficacy and safety of this device in the context of CS. The main advantages of the Impella 5.0 device with respect to other percutaneous LV assist devices (LVADs) are as follows:
Impella 5.5/5.0 provide full cardiac support, even in the absence of residual LV function, due to the nominal flow of 5.5 or 5 L/min respectively.
Impella 5.0 is associated with a lower rate of vascular complications than other types of MCS such as venoarterial extracorporeal membrane oxygenation (VA-ECMO)10 and is characterized by greater stability of the device position due to surgical insertion and fixation9; the patient can be safely mobilized out of bed, favouring the recovery of his or her general physical status.
The longer duration of support allows time to evaluate myocardial recovery, and if not present, Impella 5.5 and 5.0 represent a valuable bridge-to-decision device towards long-term therapies such as LVAD implantation or heart transplantation (discussed in the next section long-term strategies).
Biventricular dysfunction with concomitant right heart failure development, on the contrary, requires urgent decision-making and MCS escalation (hours).
Precise diagnosis of the underlying cause of RV failure is fundamental for undertaking the correct escalation strategy. RV failure should be detected with both haemodynamic and echocardiographic monitoring, as increased central venous pressure [right atrial pressure (RAP) > 16 mmHg] and reduced RV function echocardiographic (tricuspid annular plane systolic excursion and tissue Doppler S′ wave) and/or invasive (low pulmonary artery pulsatility index < 1.85 or high RAP/pulmonary capillary wedge pressure ratio > 0.59) parameters.11
First, the most common differential diagnosis of RV failure must be ruled out: pulmonary complications (pneumothorax, pleural effusion, atelectasis), pulmonary embolism, RV outflow tamponade, LV pump displacement or suction, and the persistence of metabolic decompensation (acidosis, hypercapnia).
Once these mechanisms are excluded, to select the correct MCS escalation strategy, the definition of the underlying cause of RV failure is mandatory: haemodynamic monitoring is necessary for differential diagnosis between primary and secondary RV failure.
Secondary RV failure is characterized by increased LV filling pressure and requires LV MCS escalation with upgrade of LV unloading by increasing pump flow or with a more powerful pump (see univentricular dysfunction).
Instead, primary RV failure is characterized by normal LV filling pressure values and requires escalation with RV MCS support. The biventricular MCS strategies are VA-ECMO support, the addition of an RV assist device with ProtekDuo cannula and BiPELLA support.12
When upgrading with VA-ECMO, concomitant LV unloading with a transaortic pump (ECpella strategy) is strongly recommended. Previous studies demonstrated a reduction in mortality with LV unloading with Impella while on VA-ECMO support.3,13,14
A second more recent approach for biventricular dysfunction is represented by the concomitant use of LV Impella (CP/5.0/5.5) and RV Impella (RP).15 Small retrospective studies have demonstrated that the BiPella approach is feasible, reduces cardiac filling pressures and improves cardiac output across a range of causes of CS. Simultaneous LV and RV device implantation and lower RV afterload may be associated with better outcomes in biventricular CS, but prospective studies are still required16–18 (Figure 1).
Figure 1.
Mechanical circulatory support escalation. CVP, central venous pressure; ECMO, extracorporeal membrane oxygenation; LV, left ventricular; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion; WP, wedge pressure.
De-escalation
VA-ECMO is still considered the standard of MCS in patients with profound CS (INTERMACS 1 patient) or refractory cardiac arrest (eCPR).
Despite its efficacy as rescue therapy in refractory CS patients, VA-ECMO support is associated with a substantial burden of complications, and these patients often require definitive heart replacement therapy after resuscitation due to the lack of myocardial recovery. This is further evidenced by the fact that ECMO provides full haemodynamic support for patients but increases the afterload to the left ventricle with end-diastolic pressure and volume augmentation, jeopardizing myocardial recovery.19 Furthermore, previous studies have reported that in the setting of acute de novo CS treated with VA-ECMO, the duration of VA-ECMO is strongly correlated with the risk of hospital mortality.20
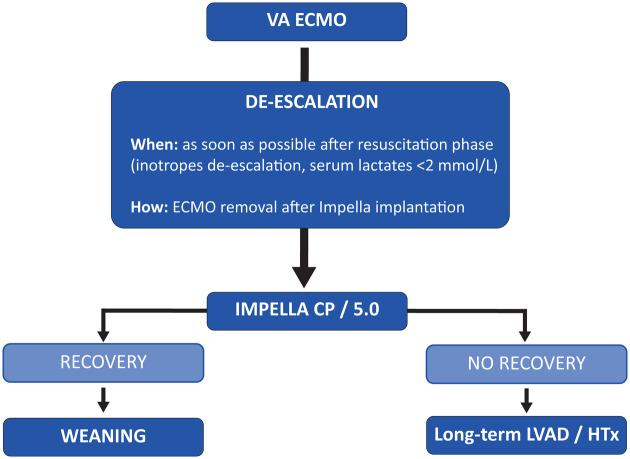
From this perspective, the need for a device that allows for rapid weaning from ECMO and a longer period of support as a bridge to recovery or heart replacement therapies should be acknowledged.
Minimally invasive LVAD therapy using the Impella 5.0 or 5.5 device via axillary surgical access has been demonstrated to be the most attractive strategy for MCS de-escalation in this population. The rationale of this two-step strategy is to overcome the resuscitation phase and optimize the haemodynamic status with a short VA-ECMO support period and then to implant the Impella 5.0 device for ECMO weaning and mid-term support.
Criteria and timing
At the time of implantation, VA-ECMO flow parameters must be set with the primary goal of reverting circulatory and metabolic alterations as measured by clinical and haemodynamic parameters, and LV unloading with percutaneous MCS must be strategically pursued to prevent the complications associated with LV stagnation in akinetic LV ventricles and to facilitate myocardial salvage.
Once haemodynamic and metabolic stabilization is achieved (significant reduction in serum lactates and end-organ damage), due to the lack of consistent myocardial recovery, Impella 5.0 should be implanted and set at maximum flow. In the next hours, VA-ECMO flow must be progressively decreased. Invasive haemodynamic monitoring and echocardiographic parameters must drive ECMO de-escalation and appropriate ECMO removal timing.
In a recent case series, we first showed that all nine enrolled patients could be effectively weaned from VA-ECMO with axillary Impella 5.0 after a median time of 22 h after implantation21 (Figure 2).
Figure 2.
Mechanical circulatory support de-escalation. HTx, heart transplantation; LVAD, left ventricular assist device; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
Special scenarios
Special scenarios occur when the patient presents contraindications to Impella device, like intraventricular thrombus, severe aortic disease or mechanical aortic prosthesis. Alternative therapeutic strategies with different devices must be considered:
TandemHeart (CardiacAssist, Inc., Pittsburgh, USA). In the presence of intraventricular thrombus or mechanical aortic prosthesis, the TandemHeart device should be considered for both escalation therapy and weaning from VA-ECMO support. TandemHeart requires a venous cannula to be advanced in the left atrium after transeptal puncture and provides left chambers unloading; oxygenated blood is drawn from the left atrium and returned via an external centrifugal pump and an arterial cannula in the femoral artery to the lower abdominal aorta. However, TandemHeart requires fluoroscopic guidance for transseptal puncture, making it less adequate for the urgent setting.22
Paracorporeal LVAD/biventricular assist device (BiVAD). In VA-ECMO supported patients with persistent CS and concomitant severe aortic valve regurgitation, aortic valve replacement with a tissue valve combined with paracorporeal LVAD/BiVAD positionement should be considered in order to effectively wean the patient from VA-ECMO support.
Long-term strategies
In the setting of both MCS escalation and de-escalation, it is important to select a strategy in relation to long-term perspectives (bridge-to-recovery, bridge-to-LVAD, or bridge-to-heart transplantation).
In this setting, MCS with the most powerful pump, Impella 5.0/5.5, with an upper body approach is advised. The main therapeutic advantages of the Impella 5.0/5.5 device with respect to other percutaneous LVADs are as follows (Table 2):
Table 2.
Midterm MCS with Impella 5.0/5.5
| Axillary Impella 5.0/5.5 | |
|---|---|
| Strategies |
|
| Advantages |
|
Resolution of intercurrent clinical conditions. In the complex and frail population affected by CS, the longer duration of MCS allows the resolution of intercurrent clinical conditions such as infection and organ damage.
Early extubation and mobilization. Axillary support allows patient extubation, oral feeding and mobilization with physical therapy while receiving maximal haemodynamic support, which has been demonstrated to aid weaning from temporary MCS and bridge-to-LVAD and is associated with a better prognosis at discharge.23
Bridge-to-long-term therapies. The implantation of a full support midterm LVAD (Impella 5.0/5.5) allows the evaluation of RV function and pulmonary vascular resistance; this is particularly valuable in light of the dismal results of direct INTERMACS 1 implantation of LVAD or heart transplantation. Previous studies have shown that VA-ECMO support before LVAD implantation is a strong independent predictor of post-LVAD RV failure24,25; bridging with IMPELLA 5.0 allows, thorough evaluation and optimization of the right ventricle, a real ‘LVAD test’ in terms of haemodynamics, thus improving outcomes after long-term LVAD implantation.21
Neurological evaluation and patient consent: an important percentage of these subjects experience cardiac arrest and subsequent anoxic brain injury. Indeed, Bernhardt et al.26 demonstrated that Impella 5.0 support represented a valuable bridge-to-decision option in patients treated with ECMO, allowing neurological recovery in approximately two-thirds of patients. Furthermore, during this period, given the abrupt onset of the disease, patients and relatives can be adequately informed about the therapeutic program and provide their consent, and they have time to realize and accept the new condition.
Outlook
For historical reasons, escalating therapy by adding mechanical devices in CS for the condition of insufficient response to inotropic and vasopressor support is a frequent approach chosen.
The absence of randomized or cohort-based evidence that any (beta-adrenergic or phosphodiesterase inhibiting) inotrope saves lives in CS after half a century of their use raised concerns regarding this approach. Potentially, such a stepwise approach only prolongs the duration of cardiac and multiorgan damage in CS.
As the core of CS is severe organ damage to the heart and as heart recovery is the key to long-term survival, the more rational approach of avoiding any pharmacological or mechanical stress to the diseased heart by primary implantation of a mechanical support device and full resting of the heart pharmacologically and mechanical unloading merits strong scrutiny in the future and is the objective of ongoing clinical trials. While IABP is only marginally effective haemodynamically and ECMO is haemodynamically powerful but has a large complication rate and a limited cardiac recovery rate in CS for reasons discussed in a companion paper in this supplement, the Impella family, characterized by being haemodynamically powerful but associated with a low rate of complications, has opened the door to a potentially new era in CS therapy.
Acknowledgements
This manuscript is one of eight manuscripts published as a Supplement to address patient management and mechanical circulatory support in the intensive care unit.
Funding
This work has been supported by the Abiomed Europe GmbH to cover publication costs as well as professional language editing of each manuscript. No individual fees were paid to the authors in the generation of this publication. This paper was published as part of a supplement financially supported by Abiomed GmbH.
Conflict of interest: C.D. reports personal fees from ABIOMED, personal fees from ABBOTT, personal fees from BOSTON outside the submitted work; F.P. reports personal fees from Abiomed during the conduct of the study. All other authors have declared no conflict of interest.
References
- 1. Basir MB, Schreiber TL, Grines CL, Dixon SR, Moses JW, Maini BS, Khandelwal AK, Ohman EM, O'Neill WW. Effect of early initiation of mechanical circulatory support on survival in cardiogenic shock. Am J Cardiol 2017;119:845–851. [DOI] [PubMed] [Google Scholar]
- 2. Pieri M, Sorrentino T, Oppizzi M, Melisurgo G, Lembo R, Colombo A, Zangrillo A, Pappalardo F. The role of different mechanical circulatory support devices and their timing of implantation on myocardial damage and mid-term recovery in acute myocardial infarction related cardiogenic shock. J Interv Cardiol 2018;31:717–724. [DOI] [PubMed] [Google Scholar]
- 3. Tongers J, Sieweke JT, Kühn C, Napp LC, Flierl U, Röntgen P, Schmitto JD, Sedding DG, Haverich A, Bauersachs J, Schäfer A. Early escalation of mechanical circulatory support stabilizes and potentially rescues patients in refractory cardiogenic shock. Circ Heart Fail 2020;13:e005853. [DOI] [PubMed] [Google Scholar]
- 4. Tehrani BN, Truesdell AG, Sherwood MW, Desai S, Tran HA, Epps KC, Singh R, Psotka M, Shah P, Cooper LB, Rosner C, Raja A, Barnett SD, Saulino P, deFilippi CR, Gurbel PA, Murphy CE, O’Connor CM. Standardized team-based care for cardiogenic shock. J Am Coll Cardiol 2019;73:1659–1669. [DOI] [PubMed] [Google Scholar]
- 5. Thiele H, Ohman EM, Desch S, Eitel I, de Waha S. Management of cardiogenic shock. Eur Heart J 2015;36:1223–1230. [DOI] [PubMed] [Google Scholar]
- 6. den Uil CA, Lagrand WK, van der Ent M, Jewbali LS, Cheng JM, Spronk PE, Simoons ML. Impaired microcirculation predicts poor outcome of patients with acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2010;31:3032–3039. [DOI] [PubMed] [Google Scholar]
- 7. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European society of cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 8. Na SJ, Chung CR, Cho YH, Jeon K, Suh GY, Ahn JH, Carriere KC, Park TK, Lee GY, Lee JM, Song YB, Hahn JY, Choi JH, Choi SH, Gwon HC, Yang JH. Vasoactive inotropic score as a predictor of mortality in adult patients with cardiogenic shock: medical therapy versus ECMO. Rev Esp Cardiol (Engl Ed) 2019;72:40–47. [DOI] [PubMed] [Google Scholar]
- 9. Bertoglio L, Katsarou M, Scandroglio M, Bertoldi L, Chiesa R, Pappalardo F. Surgical transaxillary placement of the Impella 5.0 ventricular assist device. J Card Surg 2019;34:92–98. [DOI] [PubMed] [Google Scholar]
- 10. Karami M, den Uil CA, Ouweneel DM, Scholte NT, Engström AE, Akin S, Lagrand WK, Vlaar AP, Jewbali LS, Henriques JP. Mechanical circulatory support in cardiogenic shock from acute myocardial infarction: Impella CP/5.0 versus ECMO. Eur Heart J Acute Cardiovasc Care 2020;9:164–172. [DOI] [PubMed] [Google Scholar]
- 11.Interagency Registry for Mechanically Assisted Circulatory Support. Appendix A—adverse event definitions. UAB School of Medicine. https://www.uab.edu/medicine/intermacs/intermacs-documents (02 April 2016).
- 12. Ruhparwar A, Zubarevich A, Osswald A, Raake PW, Kreusser MM, Grossekettler L, Karck M, Schmack B. ECPELLA 2.0-minimally invasive biventricular groin-free full mechanical circulatory support with Impella 5.0/5.5 pump and ProtekDuo cannula as a bridge-to-bridge concept: a first-in-man method description. J Card Surg 2020;35:195–199. [DOI] [PubMed] [Google Scholar]
- 13. Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G, Greco T, Lembo R, Müllerleile K, Colombo A, Sydow K, De Bonis M, Wagner F, Reichenspurner H, Blankenberg S, Zangrillo A, Westermann D. Concomitant implantation of Impella(®) on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 2017;19:404–412. [DOI] [PubMed] [Google Scholar]
- 14. Meani P, Pappalardo F. The step forward for VA ECMO: left ventricular unloading! J Thorac Dis 2017;9:4149–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hunziker P, Hunziker L. Percutaneous biventricular cardiac assist device in cardiogenic shock. Eur Heart J 2013;34:1620–1620. [DOI] [PubMed] [Google Scholar]
- 16. Pappalardo F, Scandroglio AM, Latib A. Full percutaneous biventricular support with two Impella pumps: the Bi-Pella approach. ESC Heart Fail 2018;5:368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pieri M, Pappalardo F. Impella RP in the treatment of right ventricular failure: what we know and where we go. J Cardiothorac Vasc Anesth 2018;32:2339–2343. [DOI] [PubMed] [Google Scholar]
- 18. Kuchibhotla S, Esposito ML, Breton C, Pedicini R, Mullin A, O'Kelly R, Anderson M, Morris DL, Batsides G, Ramzy D, Grise M, Pham DT, Kapur NK. Acute biventricular mechanical circulatory support for cardiogenic shock. J Am Heart Assoc 2017;6:e006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Truby LK, Takeda K, Mauro C, Yuzefpolskaya M, Garan AR, Kirtane AJ, Topkara VK, Abrams D, Brodie D, Colombo PC, Naka Y, Takayama H. Incidence and implications of left ventricular distention during venoarterial extracorporeal membrane oxygenation support. ASAIO J 2017;63:257–265. [DOI] [PubMed] [Google Scholar]
- 20. Tarzia V, Bortolussi G, Bianco R, Buratto E, Bejko J, Carrozzini M, De Franceschi M, Gregori D, Fichera D, Zanella F, Bottio T, Gerosa G. Extracorporeal life support in cardiogenic shock: impact of acute versus chronic etiology on outcome. J Thorac Cardiovasc Surg 2015;150:333–340. [DOI] [PubMed] [Google Scholar]
- 21. Bertoldi LF, Pappalardo F, Lubos E, Grahn H, Rybczinski M, Barten MJ, Legros T, Bertoglio L, Schrage B, Westermann D, Lapenna E, Reichenspurner H, Bernhardt AM. Bridging INTERMACS 1 patients from VA-ECMO to LVAD via Impella 5.0: de-escalate and ambulate. J Crit Care 2020;57:259–263. [DOI] [PubMed] [Google Scholar]
- 22. Thiele H, Smalling RW, Schuler GC. Percutaneous left ventricular assist devices in acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2007;28:2057–2063. [DOI] [PubMed] [Google Scholar]
- 23. Esposito ML, Jablonski J, Kras A, Krasney S, Kapur NK. Maximum level of mobility with axillary deployment of the Impella 5.0 is associated with improved survival. Int J Artif Organs 2018;41:236–239. [DOI] [PubMed] [Google Scholar]
- 24. Shah P, Smith S, Haft JW, Desai SS, Burton NA, Romano MA, Aaronson KD, Pagani FD, Cowger JA. Clinical outcomes of advanced heart failure patients with cardiogenic shock treated with temporary circulatory support before durable LVAD implant. ASAIO J 2016;62:20–27. [DOI] [PubMed] [Google Scholar]
- 25. Soliman OII, Akin S, Muslem R, Boersma E, Manintveld OC, Krabatsch T, Gummert JF, de By T, Bogers A, Zijlstra F, Mohacsi P, Caliskan K. Derivation and validation of a novel right-sided heart failure model after implantation of continuous flow left ventricular assist devices: the EUROMACS (European Registry for Patients with Mechanical Circulatory Support) right-sided heart failure risk score. Circulation 2018;137:891–906. [DOI] [PubMed] [Google Scholar]
- 26. Bernhardt AM, Zipfel S, Reiter B, Hakmi S, Castro L, Söffker G, Kluge S, Lubos E, Rybczinski M, Grahn H, Schrage B, Becher PM, Barten MJ, Westermann D, Blankenberg S, Reichenspurner H. Impella 5.0 therapy as a bridge-to-decision option for patients on extracorporeal life support with unclear neurological outcomes. Eur J Cardiothorac Surg 2019;56:1031–1036. [DOI] [PubMed] [Google Scholar]