Abstract
The clinical management of patients on Impella support requires multimodality monitoring and imaging. Upon intensive care unit admission, echocardiography is essential to ensure correct pump positioning/guide repositioning, to monitor acute myocardial infarction/device-related cardiac complications and to evaluate baseline left and right ventricular function. Over time, the echocardiographic assessment of myocardial viability has become an essential target for guiding mechanical circulatory support escalation and long-term strategies. The recognition and grading of any valvular dysfunction and damage in Impella patients are challenging, as the device interferes with the colour Doppler signal, and the loading conditions of the left ventricle are modified by the pump. Valvular disease in such patients is often secondary, and correct identification is pivotal for future therapeutic strategies. The emerging use of newer techniques, including speckle-tracking echocardiography, is of increasing interest in the imaging of critically ill patients.
Keywords: Mechanical circulatory support, Echocardiography, Radiography, Positioning, Repositioning
Introduction
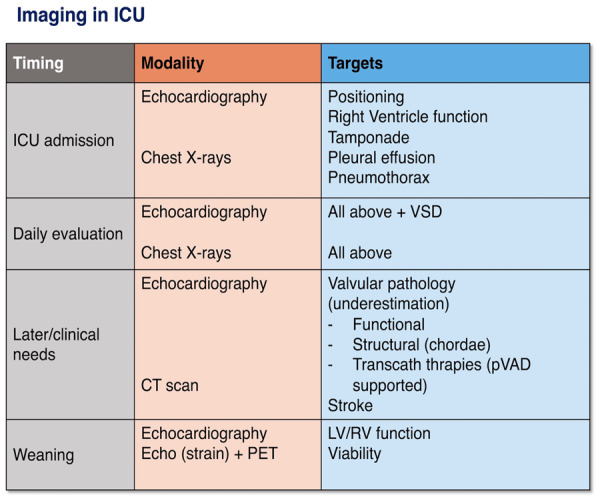
The clinical management of patients on Impella support requires multimodality monitoring and imaging. While haemodynamic monitoring using pulmonary artery catheterization (PAC) has a well-defined role, integration with imaging is essential. Chest radiography (CXR) and echocardiography are the main imaging modalities used in the daily evaluation of Impella patients. The objectives and the timeline of imaging techniques are summarized in Figure 1.
Figure 1.
Timelines and objectives of imaging techniques.
Timeline of patient evaluation
Upon intensive care unit (ICU) admission, echocardiography is essential to ensure correct pump positioning/guide repositioning, to monitor acute myocardial infarction (AMI)/device-related cardiac complications (tamponade, mitral regurgitation, and mechanical complications) and to evaluate baseline left and right ventricular (LV/RV) function. Later in the clinical course, the main objectives of imaging include the evaluation of cardiac recovery, myocardial viability, and valve function as well as the surveillance of AMI/device-related complications. Daily imaging is recommended and should be synthesized with the clinical course, inodilator therapy, mechanical ventilation, and pump parameters.
Plain chest radiography and transthoracic echocardiography to evaluate pump positioning
The metallic housing of the pump and inlet/outlet areas are intensely radiopaque and readily visualized using plain CXR. The Impella position is grossly identified by the inlet area and pigtail catheter projected over the LV contour, whereas the pump outlet is located in the ascending aorta, just above the aortic root.1 Determining the optimal pump position, however, cannot be judged on the basis of CXR alone, as no accurate data regarding the cannula direction, interference with the mitral valve apparatus or the distance of the inlet area from the aortic valve annulus can be obtained. A ratio-based tool using plain supine CXR can be useful to evaluate the position of the intracardiac device. The aortic valve position can be identified caudal to the carina at a distance of 0.25 ± 0.05 times the thoracic width for male patients and 0.28 ± 0.05 times the thoracic width for female patients.2 CXR is additionally useful to identify complications, including pleural effusion, pneumothorax, pulmonary atelectasis, and/or consolidation.
Transthoracic echocardiography (TTE) is the imaging technique of choice, as it is non-invasive, highly informative and readily performed at the bedside. However, up to 30% of ICU patients do not have an adequate acoustic window due to lung pathology or distention during mechanical ventilation and forced decubitus.3 Transoesophageal echocardiography (TOE) provides similar information with better imaging quality from the mid-oesophageal aortic valve long-axis view. The use of TOE must be carefully considered, as patients are usually on antiplatelet and anticoagulant therapy and therefore are at increased risk of oropharyngeal and oesophageal bleeding.
Using TTE, from a parasternal long-axis (PLAX) view from the apex of the left ventricle to the aortic valve, the distal portion of the pump can be distinguished from the thin line of the pigtail catheter and by the intense bright artefact of the inflow cage (referred to as a teardrop). The cannula appears as two parallel echogenic lines (railroad track) crossing the aortic valve. The hyperechogenic outlet area is then visualized in the ascending aorta (Figure 2). Identification of the transition area between the pigtail catheter and inlet area can be challenging and relies on optimal image quality. Correct positioning occurs when the inlet area is located 35 mm from the aortic valve plane, at the middle of the LV cavity, with the distal tip of the pump oriented towards the LV apex, and with no contact with the mitral valve leaflets or subvalvular apparatus. Alternatively, a distance of 40 mm from the aortic annulus and a bright artefact of the transition zone between the pigtail catheter and inlet area (teardrop) can be used. The curvature of the Impella should be located just below the aortic valve.
Figure 2.
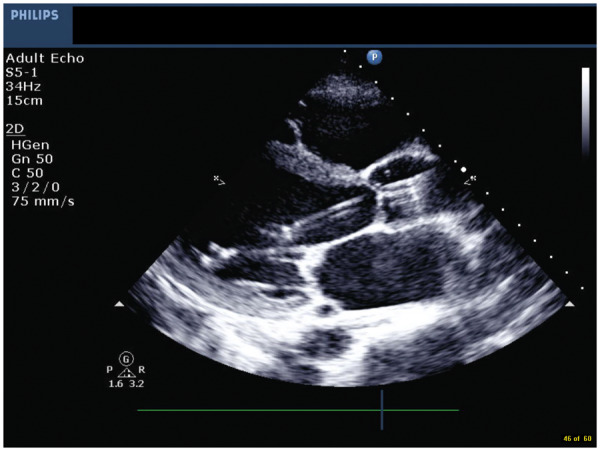
Parasternal long-axis view showing correct Impella positioning with aliasing in the ascending aorta.
The most frequent suboptimal positions can be readily identified with a combination of PLAX and three- and five-chamber views, where the pump impacts the mitral valve leaflets or subvalvular apparatus, or it can be directed towards the posterior ventricular wall. In all these cases, the pump can cause mitral regurgitation due to restricted leaflet motion, can damage the chordae tendineae or the papillary muscles, and can induce clinically significant haemolysis as a consequence of modifications of the blood flow characteristics at the inlet area, and it may often result in suction problems and ventricular arrhythmias. In most cases, repositioning of the Impella device cannot be performed in the ICU under echocardiographic guidance and requires transport of the patient to the catheterization laboratory.
Evaluation of right ventricular function
Right ventricular failure (RVF) can occur in patients on Impella support as a primary or secondary phenomenon. Correct diagnosis and management strategies rely on detailed evaluation of the clinical setting, as well as echocardiographic and haemodynamic parameters. In primary RVF, ischaemic involvement of the right ventricle, pulmonary hypertension, and post-cardiotomy RV dysfunction are the most frequent conditions. In secondary RV failure, Impella support may induce or unmask RV dysfunction (secondary RVF) by increasing cardiac output and modifying ventricular interdependence and/or the position of the interventricular septum (IVS). Moreover, insufficient LV unloading, through the upstream transmission of elevated end-diastolic LV pressure, can increase the mean pulmonary arterial pressure and hence RV afterload. The treatment of these two clinical situations differs, and timely diagnosis is essential for a good outcome. The evaluation of RV function requires a multiparametric approach (Figure 3).
Figure 3.
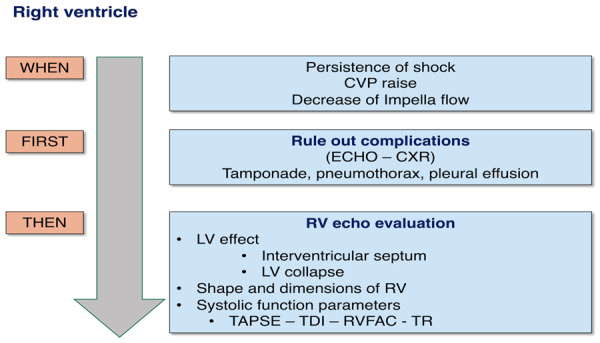
Algorithm for evaluation of the right ventricle.
Troubleshooting right ventricular dysfunction in the context of Impella support
RV dysfunction should be suspected in cases of the persistence of shock despite optimal Impella support, abrupt or progressive increases in central venous pressure, decreases in Impella flow and suction alarms, elevation of hepatic biomarkers, liver enlargement, abdominal distension, and new onset of gastrointestinal dysfunction.
The first step is to rule out complications that can induce secondary RV failure. Cardiac tamponade, pleural effusion, or massive pulmonary atelectasis can be easily identified through CXR and bedside TTE and then effectively managed. The second step is evaluation of the IVS. In conditions of optimal support, the septum is midline. Both leftward and rightward IVS shifts can subtend or generate RVF. Indeed, a rightward IVS shift indicates insufficient unloading or further deterioration of LV function, causing secondary RV dysfunction. A leftward shift can be an index of inadequate LV filling as a consequence of RVF, RV pressure overload or excessive pump suction and is able to induce RVF by itself.
Evaluation of right ventricular systolic function on Impella
The assessment of RV function integrates several indices and changes over time. The complex three-dimensional structure of the right ventricle prevents the use of geometric assumptions analogous to that of left ventricle, hampering the calculation of ejection fraction. RV shape and dimensions must be evaluated on admission to the ICU and over time. The development of new or worsening tricuspid regurgitation can signify acute RV dilation. Although the right ventricle: LV diameter ratio is a validated index of RV dysfunction, it is unreliable when the LV diameter is artefactually changed by the action of the pump. Longitudinal RV function can be measured through tricuspid annulus plane systolic excursion (TAPSE) and the S′ wave of tissue Doppler imaging of the lateral tricuspid annulus; however, severe tricuspid regurgitation can overestimate TAPSE; additionally, RV long-axis function can remain impaired years following cardiac surgical operations, and interpretation in the context of pulmonary hypertension is challenging. Fractional area change is another index of RV systolic function, but it relies on good-quality images due to the need for optimal visualization of endocardial borders. The pulmonary circulation can be comprehensively evaluated through echocardiography,4 and in patients not monitored with PAC, a basic evaluation should include at least pulmonary artery systolic pressure estimation, which can be derived from the velocity of the tricuspid regurgitation jet on the basis of the Bernoulli equation, taking into account the right atrial pressure.
Left ventricular evaluation: adequacy of unloading and myocardial viability and recovery
Cardiac unloading can be defined as the reduction of total mechanical power expenditure of the ventricle, which correlates with the reduction in myocardial oxygen consumption and haemodynamic forces that lead to ventricular remodelling.5
Preclinical studies have demonstrated that unloading of the LV with an Impella device protects the heart from cell death in AMI by reducing wall stress and cardiac oxygen consumption and by increasing coronary blood flow.6–8
Moreover, the magnitude of these beneficial effects is directly correlated with the degree of LV unloading.9 Routine echocardiographic evaluation must guide pump and medical management to provide adequate LV unloading and to determine the optimal timing for mechanical circulatory support (MCS) weaning. However, in Impella-supported patients, the most common Doppler-based measurements may be affected by the mechanical noise of the device and continuous flow. Furthermore, in patients with severe LV dysfunction, biplane ejection fraction [LV ejection fraction (LVEF)] measurement alone is insufficient for detecting myocardial recovery and guiding weaning strategies. In clinical practice, adequate Impella LV unloading is defined as the highest effective P-value speed without suction and a weaning process that is guided by multimodality evaluations with both invasive and echocardiographic monitoring. In this regard, it is worth clarifying that the operator can set the Impella flow by selecting a performance level of the pump from P1 to P9, with an incremental generated flow. Indeed, these levels correlate with an increase of the number of the revolution-per-minute of the pump and a subsequent flow increase. The interaction with the native heart pulsatility is responsible for the variability from a minimum to a maximal flow at any given P-level.
Newer technologies and future perspectives: speckle-tracking echocardiography
Two-dimensional speckle-tracking echocardiography (STE) is a technique, independent from the angle of insonation that provides the measurement of strain, the dimensionless physical quantity of fractional myocardial shortening.
Speckle-tracking echocardiography has an expanding role in clinical practice. The technology is validated and reproducible within an acceptable range and uses bright speckles in echocardiographic grayscale images to evaluate tissue deformation. Because these speckles are not subject to mechanical noise, STE can be a reliable approach for evaluating heart function in the setting of mechanical LV unloading. Furthermore, the assessment of global longitudinal strain (GLS) from speckle-tracking analysis is a feasible alternative to LVEF for the assessment of LV function.
GLS is a simple parameter derived from post-processing analysis of apical images of the left ventricle that expresses longitudinal shortening as a percentage (change in length as a proportion to baseline length). GLS normally varies with age, sex, and LV loading conditions but in adults GLS <16% is considered abnormal, >18% normal and 16–18% borderline.
The superior sensitivity of LV strain analysis over LV ejection fraction for detecting subtle changes in LV function has been reported.10,11
Different aspects of strain can be displayed differently: waveforms can be used to illustrate contraction delay and temporal dispersion in different myocardial segments, while parametric display (‘bull's-eye plot’) illustrates spatial dispersion and offers an intuitive visual overview of the global and regional LV myocardial function in a single diagram.
However, reliable evaluation of the GLS strictly depends on a good image quality that may be difficult to obtain in the setting of mechanical ventilation and forced decubitus; a suboptimal regional tracking in more than two myocardial segments in a single view is sufficient to make unreliable the GLS calculation.
Despite STE being not widely validated in the ICU setting, certain features suggest potential utility in Impella-supported patients. GLS has several limitations: a reliable evaluation strictly depends on a good image quality, difficult to obtain in the setting of mechanical ventilation and forced decubitus; moreover, a suboptimal regional tracking in more than two myocardial segments in a single view is sufficient to make unreliable the GLS calculation.
Evaluation of left ventricular unloading
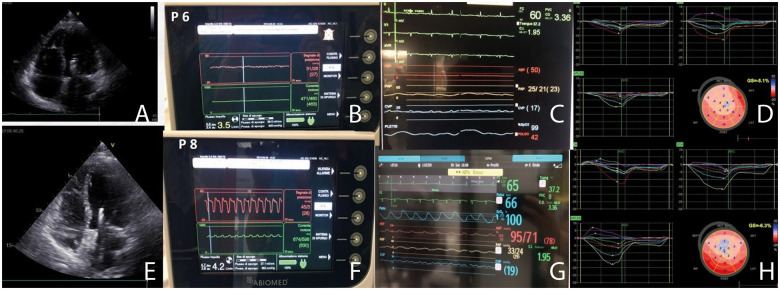
Hammoudi et al.12 demonstrated that STE strain analysis exhibited significant linear relationships with LV stroke work in post-myocardial infarction pigs with acute LV unloading using Impella, suggesting its utility in noninvasively assessing the degree of LV unloading at the bedside. STE analysis can be combined with invasive haemodynamic monitoring to detect optimal LV unloading. Impella speed might be adjusted with the aim of providing optimal unloading (evaluation of pulmonary and filling pressures) and improving myocardial contractility (increases in GLS). A practical example is shown in Figure 4.
Figure 4.
Speckle-tracking echocardiography combined with haemodynamic monitoring for evaluating the degree of left ventricular unloading. (A–D) Impella monitoring, haemodynamic status and strain analysis at P6 Impella speed. (E–H) Increased unloading at P8 provides better haemodynamic status and myocardial contractility. Changes in myocardial contractility between P6 and P8 unloading were not detectable with left ventricular ejection fraction measurement.
Monitoring myocardial recovery
A general definition of ‘myocardial recovery’ is the improvement of the parameters of ventricular function, usually evaluated with echocardiographic examination.
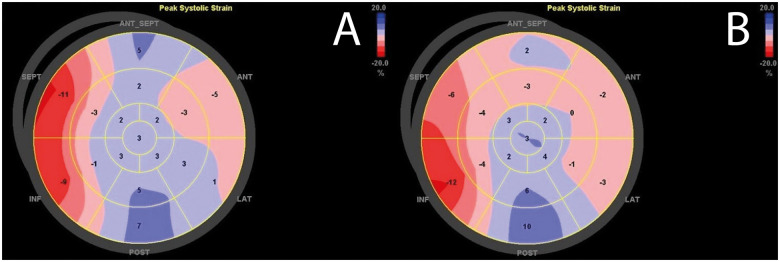
LV strain analysis has superior sensitivity over LVEF in detecting subtle changes in LV function and thus myocardial recovery (Figure 5). GLS is, however, highly dependent on pre- and afterload, and to date, the magnitude of changes in GLS that should be considered clinically relevant due to interstudy variability in the ICU setting remains unknown.
Figure 5.
Left ventricular systolic strain displayed as bull’s eye plots at day 1 (A) and day 3 (B) on Impella support. Bull’s eye plots show partial myocardial recovery with particularly improved peak systolic strain of antero-septal and inferior medio-basal segments.
Myocardial viability
Myocardial viability has a pathophysiological and clinical definition. At a cellular level, viable cardiomyocytes are defined by the presence of cellular, metabolic and microscopic contractile function. On a clinical ground, viability is defined by a dysfunctional myocardium at rest, but able to recover after flow restoration or unloading.13
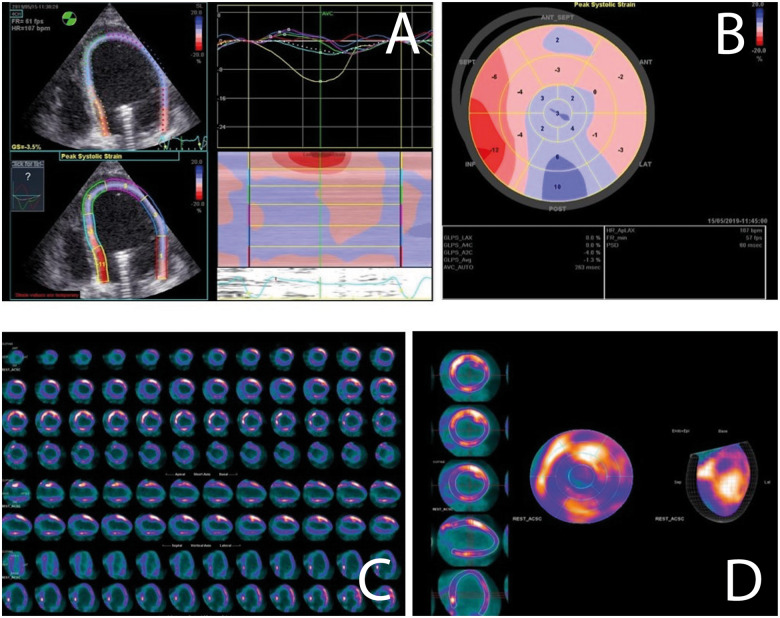
If early myocardial recovery does not occur in Impella-supported patients, it is necessary to assess myocardial viability to guide MCS escalation and longer-term strategies. Here, viability assessment is challenging due to imaging practicalities (often unstable and mechanically ventilated patients with high risks or impossible mobilization) and device interference (for example, with cardiac magnetic resonance). Here, two imaging techniques are available (Figure 6).
Figure 6.
Cardiac viability assessment with different imaging techniques in the same patient while on Impella support. (A and B) Speckle tracking echocardiography analysis presented as waveform (A) and bull’s eye plot (B). (C and D) Cardiac positron emission tomography analysis similarly presented as short- and long-axis slices (C) and bull’s eye plot (D).
Echocardiography
This offers real-time assessment of cardiac function at the bedside, making it the easiest modality for evaluating Impella-supported patients. Doppler analysis alone is insufficient for assessing myocardial viability. LV long-axis function during dobutamine-stress echocardiography has been shown to provide important information.14,15 However, further studies are needed to validate dobutamine stress echocardiography and myocardial viability assessments in the setting of Impella-supported patients.
Cardiac positron emission tomography
Nuclear imaging can be considered for viability assessments of patients on MCS because it is not affected by device interference. Specifically, [18F]fluorodeoxyglucose (FDG)-positron emission tomography (PET) is the clinical gold standard for myocardial viability and identifies viable myocardial tissue via the noninvasive visualization of glucose metabolism; FDG-PET is able to distinguish between hibernating myocardium (viable myocardium) and scar tissue.16 Observational studies suggest that FDG-PET has the greatest sensitivity for predicting global LV functional recovery following revascularization compared with different viability tools (single-photon emission computed tomography, dobutamine stress echocardiography and cardiac magnetic resonance imaging).17,18 No studies about FDG-PET in Impella-supported patients have been published, but the feasibility and future potential are highlighted by expert opinions. A real-life case of FDG-PET in a patient on Impella support is shown in Figure 6.
Evaluation of valvular disease
The evaluation of aortic and mitral valvular diseases while on Impella support is challenging, as the device interferes with the colour Doppler signal, and LV loading conditions are modified by the pump. Multiple mechanisms can affect valve function in this clinical scenario (primary or device-related valvular disease); however, prompt recognition and management of valve dysfunction is pivotal for safe and successful weaning from MCS.
Aortic stenosis
Patients with severe aortic stenosis (AS) and cardiogenic shock have a poor prognosis. Percutaneous balloon aortic valvuloplasty (BAV) can be lifesaving as a bridge to further definitive treatment, such as Transcatheter Aortic Valve Replacement or surgical Aortic Valve Replacement (TAVR or sAVR, according to guidelines). BAV in these critically ill patients is, however, associated with high periprocedural and in-hospital mortality. Severe AS is considered a relative contraindication for the use of Impella given concerns about worsening haemodynamics with the introduction of the catheter through a severely stenotic aortic valve orifice and the possibility of increased cerebrovascular events due to calcified aortic leaflets. Despite these concerns, its use has been proven feasible, and it has demonstrated promising results in selected patients with severe AS. The concomitant use of Impella support during BAV may improve the safety and tolerability of the procedure by providing stable cardiac output and permitting longer balloon inflation times.19,20 The cVAD registry provides the largest series to date of patients with severe AS undergoing Impella-assisted BAV.21 A total of 116 patients underwent BAV with the Impella device placed electively in most cases prior to BAV, whereas it was placed emergently in 27% of the cases.
Aortic regurgitation
Primary severe aortic regurgitation (AR) is a contraindication for Impella placement. Although rare, new AR is one of the potential complications of Impella implantation and can occur due to mechanical impedance of leaflet opposition or iatrogenic injury to the valve (leaflet perforation or commissural avulsion). AR must be excluded if clinical and haemodynamic improvement is not observed despite appropriate ventricular support. An echocardiographic diagnosis of AR in this setting can be challenging due to artefacts on colour Doppler generated by the device; the use of TOE is recommended. AR from defective leaflet coaptation will improve once the device is removed or repositioned, while iatrogenic injury may require surgical correction.22,23
Functional mitral regurgitation
Functional mitral regurgitation (fMR) is relatively common in ischaemic cardiogenic shock, and it may negatively affect the attainment of adequate LV unloading or hinder Impella weaning. The echocardiographic quantification of fMR while on Impella support is challenging due to LV unloading. A ‘weaning trial’ by reducing the Impella speed (P2–P3) may unmask severe fMR. Severe fMR often represents the underlying cause of weaning failure. A few case reports have demonstrated the safety and feasibility of the MitraClip procedure while on Impella support.24 However, in patients with failure to wean and severe fMR with a lack of LV recovery, long-term left ventricular assist device implantation should be considered.
Primary mitral regurgitation
Impella device positioning may, in rare cases, be complicated by mitral apparatus damage, leading to organic MR. When MR occurs secondary to Impella placement, this may be due to either direct mitral damage [including chordal rupture with leaflet flail (most common) or perforation]. The management of device-related MR may be percutaneous (MitraClip) or surgical in relation to the anatomical characteristics of the lesion.25–27
Functional mitral stenosis
Although rare, Impella mispositioning may cause functional mitral stenosis by dislocating the shaft of the device lying on the anterior mitral leaflet. Frequent echocardiographic surveillance of correct device positioning is fundamental, especially in patients who show a poor response to therapy; repositioning is mandatory when the Impella device inappropriately interferes with the mitral valve apparatus.28
Integration between haemodynamic data and imaging
Haemodynamic data provided by the PAC and by the new Impella console SmartAssist have to be integrated with imaging to obtain a thorough evaluation of the patients’ clinical status.
Specifically, the evaluation of RV function, degree of unloading and weaning form Impella take advantage from an integrated approach.
No single echocardiographic parameter is able per se to provide a complete RV evaluation, whose function is better interpreted with measured and derived haemodynamic and echocardiographic data.
Echocardiography-derived parameters are complementary to LV end-diastolic pressure to define the degree and the adequacy of cardiac unloading.
Finally, the weaning from Impella is based on the evidence of haemodynamic recovery, with the onset of a sustained systemic arterial and pulmonary pulsatility, stability of native cardiac output and wedge pressure; these phenomena are often concomitant with imaging data suggesting improvement of LV/RV systolic function.
Conclusion
Echocardiography is of unique importance for the clinical management of patients on Impella support, for monitoring the possible complications and for the weaning process. Standard two-dimensional echocardiography is currently the basis for clinical conclusions, whereas the role of new imaging techniques still needs to be validated in further studies.
Acknowledgements
This manuscript is one of eight manuscripts published as a Supplement to address patient management and mechanical circulatory support in the ICU.
Funding
This work has been supported by the Abiomed Europe GmbH to cover publication costs as well as professional language editing of each manuscript. No individual fees were paid to the authors in the generation of this publication. This paper was published as part of a supplement financially supported by Abiomed GmbH.
Conflict of interest: A.M. reported personal fees from Abiomed, during the study period. L.F.B., and C.H. have nothing to disclose. J.M. reports grants and personal fees from Abiomed, personal fees from Novartis, personal fees from Orion Pharma, personal fees from Boehringer Ingelheim, outside the submitted work. F.P. reports personal fees from Abiomed, during the conduct of the study.
References
- 1. Kligerman S, Horowitz M, Jacobs K, Weihe E. Imaging of cardiac support devices. In: Travis H Mellnick, eds. Imaging the ICU Patients or Hospitalized Patient. 1st ed. Philadelphia: Elsevier; 2020. pp. 151–166. [Google Scholar]
- 2. Ouweneel DM, Sjauw KD, Wiegerinck EMA, Hirsch A, Baan J, de Mol BAJM, Lagrand WK, Planken RN, Henriques JPS. Assessment of cardiac device position on supine chest radiograph in the ICU. Crit Care Med 2016;44:e957–e963. [DOI] [PubMed] [Google Scholar]
- 3. Price S, Canivet JL. Echocardiography for advanced extracorporeal support. In: Zamorano J, Bax J, Knuuti J, Sechtem U, Lancellotti P, Badano L, eds. ESC Textbook of Cardiovascular Imaging. 2nd ed. Oxford, UK: Oxford University Press; 2015. pp. 432–444. [Google Scholar]
- 4. Bossone E, D’Andrea A, D’Alto M, Citro R, Argiento P, Ferrara F, Cittadini A, Rubenfire M, Naeije R. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr 2013;26:1–14. [DOI] [PubMed] [Google Scholar]
- 5. Uriel N, Sayer G, Annamalai S, Kapur NK, Burkhoff D. Mechanical unloading in heart failure. J Am Coll Cardiol 2018;72:569–580. [DOI] [PubMed] [Google Scholar]
- 6. Kapur NK, Paruchuri V, Urbano-Morales JA, Mackey EE, Daly GH, Qiao X, Pandian N, Perides G, Karas RH. Mechanically unloading the left ventricle before coronary reperfusion reduces left ventricular wall stress and myocardial infarct size. Circulation 2013;128:328–336. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe S, Fish K, Kovacic JC, Bikou O, Leonardson L, Nomoto K, Aguero J, Kapur NK, Hajjar RJ, Ishikawa K. Left ventricular unloading using an Impella CP improves coronary flow and infarct zone perfusion in ischemic heart failure. J Am Heart Assoc 2018;7:e006462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Esposito ML, Zhang Y, Qiao X, Reyelt L, Paruchuri V, Schnitzler GR, Morine KJ, Annamalai SK, Bogins C, Natov PS, Pedicini R, Breton C, Mullin A, Mackey EE, Patel A, Rowin E, Jaffe IZ, Karas RH, Kapur NK. Left ventricular unloading before reperfusion promotes functional recovery after acute myocardial infarction. J Am Coll Cardiol 2018;72:501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soucy KG, Bartoli CR, Phillips D, Giridharan GA, Sobieski MA, Wead WB, Dowling RD, Wu ZJ, Prabhu SD, Slaughter MS, Koenig SC. Continuous-flow left ventricular assist device support improves myocardial supply: demand in chronic heart failure. Ann Biomed Eng 2017;45:1475–1486. [DOI] [PubMed] [Google Scholar]
- 10. Potter E, Marwick TH. Assessment of left ventricular function by echocardiography. JACC Cardiovasc Imaging 2018;11:260–274. [DOI] [PubMed] [Google Scholar]
- 11. Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J 2016;37:1196–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hammoudi N, Watanabe S, Bikou O, Ceccaldi A, Fish K, Yamada KP, Miyashita S, Lebreton G, Hajjar RJ, Ishikawa K. Speckle-tracking echocardiographic strain analysis reliably estimates degree of acute LV unloading during mechanical LV support by Impella. J Cardiovasc Transl Res 2019;12:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia MJ, Kwong RY, Scherrer-Crosbie M, Taub CC, Blankstein R, Lima J, Bonow RO, Eshtehardi P, Bois JP; American Heart Association Council on Cardiovascular Radiology and Intervention and Council on Clinical Cardiology. State of the art: imaging for myocardial viability: a scientific statement from the American Heart Association. Circ Cardiovasc Imaging 2020;13:e000053. [DOI] [PubMed] [Google Scholar]
- 14. Duncan AM, Francis DP, Gibson DG, Henein MY. Differentiation of ischemic from nonischemic cardiomyopathy during dobutamine stress by left ventricular long-axis function. Circulation 2003;108:1214–1220. [DOI] [PubMed] [Google Scholar]
- 15. Duncan AM, O'Sullivan CA, Gibson DG, Henein MY. Electromechanical interrelations during dobutamine stress in normal subjects and patients with coronary artery disease: comparison of changes in activation and inotropic state. Heart 2001;85:411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen K, Miller EJ, Sadeghi MM. PET-based imaging of ischemic heart disease. PET Clin 2019;14:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Underwood SR, Bax JJ, Vom Dahl J, Henein MY, Knuuti J, van Rossum AC, Schwarz ER, Vanoverschelde JL, van der Wall EE, Wijns W; Study Group of the European Society of Cardiology. Imaging techniques for the assessment of myocardial hibernation. Report of a study group of the European society of cardiology. Eur Heart J 2004;25:815–836. [DOI] [PubMed] [Google Scholar]
- 18. Schinkel AFL, Bax JJ, Delgado V, Poldermans D, Rahimtoola SH. Clinical relevance of hibernating myocardium in ischemic left ventricular dysfunction. Am J Med 2010;123:978–986. [DOI] [PubMed] [Google Scholar]
- 19. Singh V, Mendirichaga R, Inglessis-Azuaje I, Palacios IF, O'Neill WW. The role of Impella for hemodynamic support in patients with aortic stenosis. Curr Treat Options Cardiovasc Med 2018;20:44. [DOI] [PubMed] [Google Scholar]
- 20. Martinez CA, Singh V, Londoño JC, Cohen MG, Alfonso CE, O'Neill WW, Heldman AW. Percutaneous retrograde left ventricular assist support for interventions in patients with aortic stenosis and left ventricular dysfunction. Catheter Cardiovasc Interv 2012;80:1201–1209. [DOI] [PubMed] [Google Scholar]
- 21. Singh V, Yadav PK, Eng MH, Macedo FY, Silva GV, Mendirichaga R, Badiye AP, Sakhuja R, Elmariah S, Inglessis I, Alfonso CE, Schreiber TL, Cohen M, Palacios I, O'Neill WW. Outcomes of hemodynamic support with Impella in very high-risk patients undergoing balloon aortic valvuloplasty: results from the global cVAD registry. Int J Cardiol 2017;240:120–125. [DOI] [PubMed] [Google Scholar]
- 22. Hong E, Naseem T. Color Doppler artifact masking iatrogenic aortic valve injury related to an Impella device. J Cardiothorac Vasc Anesth 2019;33:1584–1587. [DOI] [PubMed] [Google Scholar]
- 23. Sharma S, Briasoulis A, Afonso L. Persistent aortic insufficiency secondary to Impella device implantation. Int J Cardiol 2014;176:e9–e10. [DOI] [PubMed] [Google Scholar]
- 24. Foerst J, Cardenas A, Swank G. Safety of mitraclip implant in the unstable patient. JACC Cardiovasc Interv 2016;9:e71–e72. [DOI] [PubMed] [Google Scholar]
- 25. Khalid N, Shlofmitz E, Case BC, Waksman R. Chordae tendineae rupture and iatrogenic severe mitral regurgitation related to Impella. EuroIntervention 2021; 16(15):e1262–e1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhatia N, Richardson TD, Coffin ST, Keebler ME. Acute mitral regurgitation after removal of an Impella device. Am J Cardiol 2017;119:1290–1291. [DOI] [PubMed] [Google Scholar]
- 27. Eftekhari A, Eiskjær H, Terkelsen CJ, Nielsen SL, Christiansen EH, Poulsen SH. Perforation of the anterior mitral leaflet after Impella LP 5.0 therapy in cardiogenic shock. Am J Cardiol 2016;117:1539–1541. [DOI] [PubMed] [Google Scholar]
- 28. Toggweiler S, Jamshidi P, Erne P. Functional mitral stenosis: a rare complication of the Impella assist device. Eur J Echocardiogr 2008;9:412–413. [DOI] [PubMed] [Google Scholar]