Abstract
Background
The rate of intravenous thrombolysis for acute ischaemic stroke remains low in China. We investigated whether the implementation of a citywide Acute Stroke Care Map (ASCaM) is associated with an improvement of acute stroke care quality in a Chinese urban area.
Methods
The ASCaM comprises 10 improvement strategies and has been implemented through a network consisting of 20 tertiary hospitals. We identified 7827 patients with ischaemic stroke admitted from April to October 2017, and 506 patients underwent thrombolysis were finally included for analysis.
Results
Compared with ‘pre-ASCaM period’, we observed an increased rate of administration of tissue plasminogen activator within 4.5 hours (65.4% vs 54.5%; adjusted OR, 1.724; 95% CI 1.21 to 2.45; p=0.003) during ‘ASCaM period’. In multivariate analysis models, ‘ASCaM period’ was associated with a significant reduction in onset-to-door time (114.1±55.7 vs 135.7±58.4 min, p=0.0002) and onset-to-needle time (ONT) (169.2±58.1 vs 195.6±59.3 min, p<0.0001). Yet no change was found in door-to-needle time. Clinical outcomes such as symptomatic intracranial haemorrhage, favourable functional outcome (modified Rankin Scale ≤2) and in-hospital mortality remained unchanged.
Conclusion
The implementation of ASCaM was significantly associated with increased rates of intravenous thrombolysis and shorter ONT. The ASCaM may, in proof-of-principle, serve as a model to reduce treatment delay and increase thrombolysis rates in Chinese urban areas and possibly other highly populated Asian regions.
Keywords: stroke, thrombolysis
Introduction
Stroke is the leading cause of mortality and disability in China.1 Despite being the most effective medical therapy to improve outcomes in acute ischaemic stroke (AIS), the use of thrombolytic therapy of intravenous tissue plasminogen activator (tPA) was merely 1.6% in China.2 Moreover, the rate of intravenous tPA is also low in most Asian countries.3
Implementation of various bundles of improvement strategies has been proven to be effective in reducing pretreatment time, increasing rates of thrombolysis and endovascular thrombectomy and improving clinical outcomes for patients with AIS. Meanwhile, substantial progress in stroke care has been made in China during the past decade by implementing multiple levels of initiatives, interventions and strategies.4 5 However, the effectiveness of implementing customised improvement strategies has largely remained to be answered in highly populated Chinese stroke care systems.
Recently, with the support of Stroke Prevention Project Committee of Chinese National Health Commission (SPPCCNHC), a number of cities, such as Shenzhen, Yantai, Hangzhou, Suzhou, Qingdao and Shenyang, have implemented their Acute Stroke Care Maps (ASCaMs). The map, which integrates prehospital emergency medical services (EMS) and in-hospital strategies, labels eligible hospitals and aims primarily to provide quality thrombolytic and endovascular service in a two-tier stroke care system (primary and advanced stroke centres) for Chinese patients with AIS.
In this study, we tested the hypothesis that citywide ASCaM in Shenyang is effective in increasing thrombolysis rates with improved clinical outcomes in these patients.
Materials and methods
Study design and management
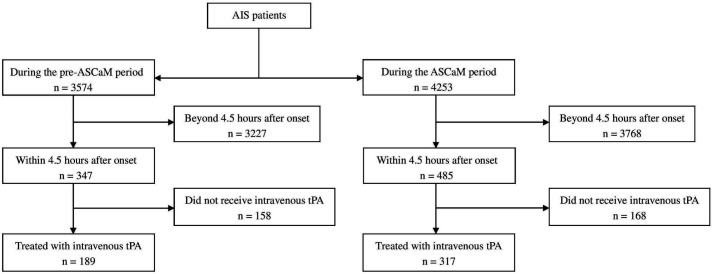
The present study is a prospective, multicentre, hospital-based registry of consecutive ischaemic stroke patients treated with intravenous tPA, investigating the effectiveness of the implementation of ASCaM in patients with AIS in Shenyang. Patients were eligible for enrolment if they were diagnosed with AIS on admission and treated with intravenous tPA within 4.5 hours after onset (figure 1). Information on patient characteristics, including age, sex, lifestyle factors, stroke classification, medical history, medication, education and points of National Institute of Health Stroke Scale (NIHSS), use of tPA, complications and pre-tPA time consumption was simultaneously collected when patients were admitted to the hospitals. Clinical outcomes and prognosis such as occurrence of symptomatic intracerebral haemorrhage (sICH), modified Rankin Scale (mRS) and death were all identified and assessed by stroke neurologists. Accuracy and completeness of variables were monitored and checked by dedicated centre coordinators. A full list of hospital-based registries is displayed in online supplemental materials.
Figure 1.
Flowchart of the included study population. AIS, acute ischaemic stroke; ASCaM, Acute Stroke Care Map; tPA, tissue plasminogen activator.
svn-2020-000332supp001.pdf (92.7KB, pdf)
Hospitals were eligible if they first had established stroke units or organised stroke care teams and then obtained more than 80 points in a self-rated questionnaire survey assessing stroke infrastructure, facilities and servicing abilities of the centre for treating acute stroke patients (online supplemental table 1). Predominantly based on proved hospital-level characteristics associated with good clinical practice and outcomes in previous studies,6–8 we designed and developed the questionnaire and inspection procedures. Eventually after the survey and inspection from the expert review panel, 20 tertiary hospitals were included in Shenyang ASCaM, representing the capacity of the city to deliver quality thrombolytic service for AIS.
We designed the study in a before-and-after manner (approximately 3 months each) to facilitate the implementation of improvement strategies for AIS care into clinical practice in participating sites in Shenyang. The before-and-after study design means that all hospitals were observed under pre-existing (pre-ASCaM) conditions according to the usual management of patients with AIS and then received intervention (ASCaM) with 10 predefined strategies (table 1) by obtaining implementation training from stroke experts. To primarily prove the improving efficacy of ASCaM, the duration for the pre-ASCaM period and ASCaM period were each defined at approximately 3 months. The outcomes were analysed by two independent statisticians blinded to intervention (Liqiang Zheng and Jianfeng Luo). Although the training of whole bundle of strategies were conducted during pre-ASCaM period, we assigned every site principle investigators to ensure the strategies were not applied immediately and treatment decisions were not influenced until the actual implementation of the ASCaM. Strategies, protocols and behaviours both at institution-level and doctor-level were requested to be remained unchanged throughout the observed pre-ASCaM period.
Table 1.
Strategies implemented among Shenyang ASCaM hospitals (with selected references)
| Strategies | Description | References |
| EMS prenotification | Ambulance staff prenotifies hospital stroke neurologists regarding medical history and abnormalities. | 10 27 28 39 |
| Advanced ED preparation | Preparation in advance of intravenous lines, catheters, infusion/infiltration pump, electrocardiographic monitoring or DSA suite if needed. | * |
| Dedicated stroke neurologists 24/7 availability | Assign dedicated stroke fellows or at least neurology residents in ED with 24/7 availability, and neurointerventionists as conditioned. | 11 |
| Rapid stroke triage/notification | Rapid stroke triage protocol and stroke team notification must be applied. | 10 39 |
| Staff accompany | Thrombolysis-indicated patients must be accompanied by ED staffs (generally stroke nurses) all way through before the actual administration of intravenous tPA. | * |
| Immediate neuroimaging interpretation | Brain imaging was read and interpreted by ED neurologist on the spot once yielded. | 10 12 39 |
| First-line neurologist decision | Thrombolysis decision is made by the first-line neurologists and confirmed by stroke fellow by phone or in person. | 10 |
| First priority for thrombolysis indicated patients | Hospital-wide first priority such as access to neuroimaging and laboratory facilities for thrombolysis-indicated patients must be strictly applied. | 13 |
| Stroke toolkits 24/7 availability | Stroke toolkits including assessment scales, written inform and consent form and tPA are 24/7 available in ED. | 10 39 |
| Laboratory and neuroimaging in nearest location | Laboratory and neuroimaging facilities were required to be renovated or relocated to the nearest possible location within the radius of ED. | 10 12 27 40 |
*Strategies adapted to local healthcare system.
DSA, digital subtraction angiography; ED, emergency department; EMS, emergency medical services; tPA, tissue plasminogen activator.
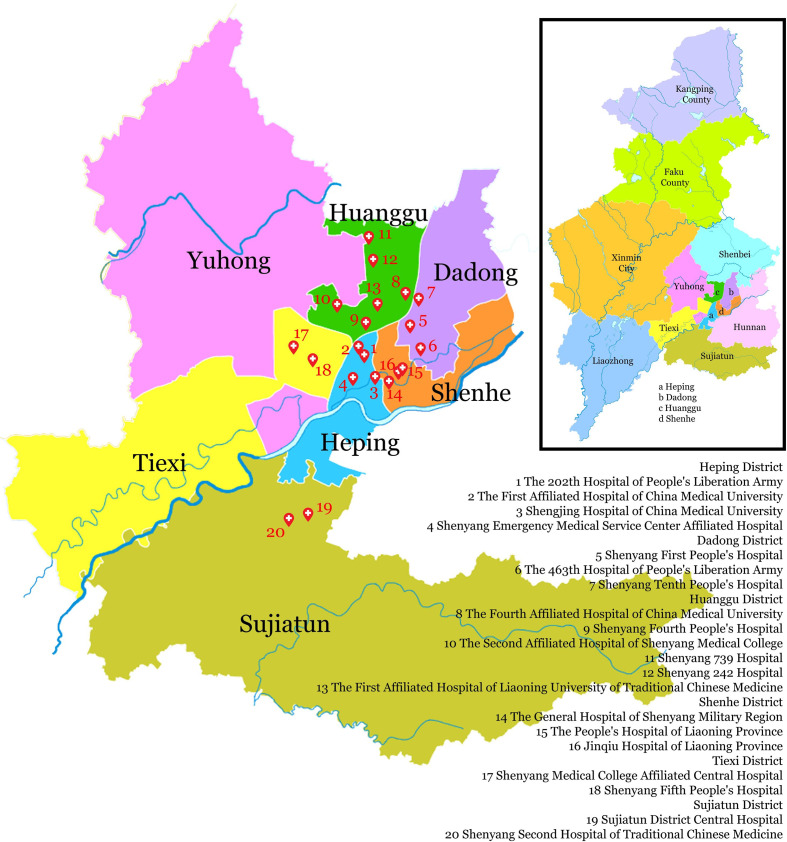
On 15 July 2017, the Greater Shenyang ASCaM was officially implemented in these 20 hospitals (figure 2). The publicity of Shenyang ASCaM was conducted by local mainstream mass media to convey the information of the distribution of ASCaM hospitals. The implementation of citywide ASCaMs is a national scheme, and there have been 30 cities with ASCaM in place.4 Shenyang is the sixth city doing do.
Figure 2.
Greater Shenyang Acute Stroke Care Map. The geographical locations of all 20 participating hospitals are displayed. The inset image is the greater Shenyang map illustrating individual administrative divisions. The figure was modified from https://en.wikipedia.org/wiki/Shenyang under licensing of CC0 1.0 universal public domain dedication.
Definitions of outcomes
The primary outcome was the difference of intravenous tPA use in patients within 4.5 hours after onset between the pre-ASCaM (8 April 2017 to 15 July 2017) and the ASCaM period (16 July 2017 to 13 October 2017). Secondary outcomes included Onset-to-Door Time (ODT), door-to-needle time (DNT), onset-to-needle time (ONT), sICH, favourable outcome (mRS ≤2) and in-hospital mortality between these two periods.
In this study, we defined sICH as per European Cooperative Acute Stroke Study II criteria, that is, any intracranial haemorrhage associated with at least a 4-point increase of NIHSS. We defined a favourable outcome as a mRS ≤2 points at discharge.
The proportion of intravenous tPA within 4.5 hours after onset was calculated by the number of patients actually received intravenous tPA within 4.5 hours, divided by the number of patients present within 4.5 hours after onset (figure 1).
Statistical analyses
The quantitative variables are expressed as the mean and SD, and the categorical variables are expressed as frequencies (%). Student’s t-test, Pearson’s χ2 test and Fisher’s exact test were used to compare the differences between the pre-ASCaM (control) and ASCaM periods (intervention). Unadjusted and adjusted logistic regression models or linear regression models were used to analyse the impact of ASCaM on proportion of tPA use, ODT, DNT, ONT and clinical outcomes. For the adjusted model, we adjusted patient-level factors, including age, sex, medical history (including atrial fibrillation/atrial flutter, prosthetic heart valve, previous stroke or transient ischaemic attack, coronary artery disease/prior myocardial infarction, carotid stenosis, peripheral vascular disease, hypertension, dyslipidaemia, seizure and current smoking), stroke severity (NIHSS score). All covariates adjusted in multivariable model were prespecified and prospectively collected in individual centres. To correct the hospital-level heterogeneity, we considered the contribution of thrombolytic cases from every individual hospitals as a confounder and conducted Mantel-Haenszel Common OR Estimate analysis for the primary outcome of thrombolytic rates within 4.5 hours. P<0.05 was considered statistically significant. All statistical analyses were conducted with SAS software (V.9.3).
Results
During the pre-ASCaM period (8 April 2017 to 15 July 2017), there were 3574 patients with AIS, 347 of whom within 4.5 hours after onset (9.7%) were enrolled, while in the ASCaM era (16 July 2017 to 13 October 2017), there were 4253 patients with AIS, 485 of whom within 4.5 hours after onset (11.4%) enrolled. There were 189 patients who received intravenous tPA during the pre-ASCaM period and 317 patients who received intravenous tPA during the ASCaM period (figure 1). The demographics and clinical characteristics of the tPA-treated populations are shown in table 2. Of all the analysed tPA-treated patients, the mean age was 64 and 64.4, respectively, sex was equally distributed, and the mean NIHSS score at admission did not show a difference between the pre-periods and ASCaM periods. There were no significant differences in patient baseline characteristics between pre-cohorts and ASCaM cohorts except for the population of age >80 years old, carotid stenosis and dyslipidaemia. For the baseline comparison with regard to age and admission NIHSS, there were incomplete data collection for 2 and 8 patients, respectively.
Table 2.
Demographic and baseline characteristics of acute ischaemic stroke patients treated with tPA within 4.5 hours after onset
| Pre-ASCaM (N=189) |
ASCaM (N=317) |
P value | |
| Age (year) | |||
| N (n missed) | 189 (0) | 315 (2) | |
| Mean±SD | 64.0±9.9 | 64.4±10.2 | 0.6408 |
| Admission NIHSS | |||
| N (n missed) | 189 (0) | 309(8) | |
| Mean±SD | 8.2±6.2 | 7.5±5.3 | 0.1996 |
| Age >80, n (%) | 15 (7.9%) | 12 (3.8%) | 0.0445 |
| Male, n (%) | 131 (69.3%) | 210 (66.5%) | 0.5071 |
| Previous stroke/TIA, n (%) | 48 (25.4%) | 77 (24.3%) | 0.7801 |
| Carotid stenosis, n (%) | 78 (41.3%) | 82 (25.9%) | <0.001 |
| HTN, n (%) | 111 (58.7%) | 202 (63.7%) | 0.2634 |
| DM, n (%) | 53 (28.0%) | 84 (26.5%) | 0.7054 |
| Dyslipidaemia, n (%) | 122 (64.6%) | 243 (76.7%) | 0.0033 |
| CAD/MI, n (%) | 39 (20.6%) | 64 (20.2%) | 0.9041 |
| AF/AFL, n (%) | 21 (11.1%) | 35 (11.0%) | 0.9806 |
| CHF, n (%) | 5 (2.6%) | 8 (2.5%) | 0.9332 |
| Prosthetic heart valve, n (%) | 0 | 3 (0.9%) | 0.2966 |
| Seizure, n (%) | 2 (1.1%) | 0 | 0.1391 |
| Peripheral vascular disease, n (%) | 21 (11.1%) | 39 (12.3%) | 0.6883 |
| Smoking, n (%) | 89 (47.1%) | 153 (48.4%) | 0.7726 |
AF/AFL, atrial fibrillation/atrial flutter; ASCaM, Acute Stroke Care Map; CAD, coronary artery disease; CHF, congestive heart failure; DM, diabetes mellitus; HTN, hypertension; MI, myocardial infarction; NIHSS, National Institute of Health Stroke Scale; TIA, transient ischaemic attack.
For the primary outcome, we detected an increased rate of tPA administration within 4.5 hours (54.5% vs 65.4%; OR, 1.724; 95% CI 1.21 to 2.45; p=0.003) in ASCaM period. In the multivariate analysis model of examining ODT, DNT, ONT and favourable outcome (mRS ≤2), data were adjusted for variables of statistical significance in univariate comparisons (p<0.1 for selection, see table 2), and patients-level factors previously reported to be associated with these parameters and used in Get With The Guidelines-Stroke analyses6 7 9 (table 3).
Table 3.
Comparison of key performance measures and clinical outcomes, Pre-ASCaM versus ASCaM period
| Pre-ASCaM (N=189) |
ASCaM (N=317) |
P value | Unadjusted OR (95 % CI) or β | P value | Adjusted OR (95 % CI) or β | P value | |
| tPA within 4.5 hours, % | 54.5% (189/347) | 65.4% (317/485) | 0.0015 | 1.235 (1.08 to 1.41) | 0.0015 | 1.724 (1.21 to 2.45)* | 0.003* |
| ODT (Mean±SD) | 135.7±58.4 | 114.1±55.7 | <0.001 | −22.02922 | <0.0001 | −20.59164 | 0.0002 |
| DNT (Mean±SD) | 57.1±30.6 | 56.1±25.3 | 0.6838 | −0.91595 | 0.7188 | −0.24447 | 0.9260 |
| ONT (Mean±SD) | 195.6±59.3 | 169.2±58.1 | <0.001 | −26.74615 | <0.0001 | −24.89451 | <0.0001 |
| Favourable outcome (mRS ≤2), n (%) | 151 (79.9%) | 270 (85.2%) | 0.1244 | 0.677 (0.422 to 1.087) | 0.1064 | 0.761 (0.444 to 1.306) | 0.3222 |
| sICH, n (%) | 6 (3.2%) | 10 (3.2%) | 0.9901 | 1.003 (0.359 to 2.806) | 0.9950 | ||
| In-hospital mortality | 6 (3.2%) | 8 (2.5%) | 0.6659 | 1.262 (0.431 to 3.696) | 0.6708 |
For multivariate analysis, data were adjusted for patient-level factors, including age, sex, medical history (including atrial fibrillation or atrial flutter, prosthetic heart valve, previous stroke or transient ischemic attack, coronary artery disease or prior myocardial infarction, carotid stenosis, peripheral vascular disease, hypertension, dyslipidaemia, seizure and current smoking), stroke severity (NIHSS score).
*Data were adjusted for the contribution of thrombolytic cases from every individual hospitals.
ASCaM, Acute Stroke Care Map; DNT, door-to-needle time; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale; ODT, onset-to-door time; ONT, onset-to-needle time; sICH, symptomatic intracerebral haemorrhage.
Compared with the mean pre-ASCaM ODT of 135.7 min, the implementation of ASCaM was associated with a significantly decreased ODT of 114.1 min (β, −20.59164; p, 0.0002). Likewise, the ONT showed a significant reduction before and after the implementation of ASCaM (β, −24.89451; p<0.0001). Unfortunately, the mean DNT, with 57.1 min and 56.1 min, respectively, remained identical during the pre-periods and ASCaM periods (β, −0.24447; p, 0.9260).
With respect to clinical outcomes, the proportions of sICH occurred before and after the ASCaM were identical to both 3.2% (OR, 1.003; 95% CI 0.359 to 2.806; p, 0.9950). The favourable outcome during the ASCaM period slightly increased to 85.2% from 79.9% of the pre-ASCaM period but failed to reach statistical significance in the multiple logistic regression model (OR, 0.761; 95% CI 0.444 to 1.306; p, 0.3222). Similarly, although the in-hospital mortality decreased from 3.2% to 2.5% after the implementation of the ASCaM, no difference was revealed (OR, 1.262; 95% CI 0.431 to 3.696; p, 0.6708).
Discussion
In this study, we found that in a Chinese urban city with an 8.1 million population, the rate of intravenous tPA within 4.5 hours after onset was substantially increased by 10.9% in only 3 months through the implementation of a regional map integrating a bundle of strategies among 20 hospitals. The networking ASCaM meanwhile, serving as a guide for both patients to seek medical service and EMS to dispatch, also reduced the ONT at the same period of time.
Applying evidence-based strategies in multiple sites means system reconfiguration and will be subject to challenges. There have been a number of studies exploring effective interventions in attempts to improve the AIS care quality on hospital-levels, region-levels and nation-levels.5 9–22 Specifically, to increase the rate of intravenous thrombolysis, the GWTG project in the USA is a successful example of a stroke quality improvement programme on the national level.9 The southeast Texas experience focused on periodically informing chief executive officers regarding the rates of tPA administration, and the intervention resulted in an increase of 21.1% of thrombolysis rate among participating hospitals.21 In Kaiser Permanente stroke centres in North California, based on a modified Helsinki model, they additionally managed every patient with AIS, either EMS-delivered or self-walked in, with a dedicated telestroke neurologist. As a result, cases of tPA administration increased from 34 to 62 per month.17 HASTE (Hurry Acute Stroke Treatment and Evaluation) study is a successful example of staggered implementation of improvement strategies in a single centre.23 From June 2012 to June 2015, the DNT was significantly reduced from 53 to 35 min via measures such as moving patients directly with EMS stretcher, registering an incoming severe stroke patients as unknown and administrating tPA in CT (computer tomography) suite. The PRACTISE (PRomoting ACute Thrombolysis in Ischemic StrokE) study using the breakthrough approach to implement improvement strategies also demonstrated a 1.5-fold higher rate of thrombolysis after intervention.20
The difference between these studies and ours mainly lies in that we pre-evaluated the eligibility of participating hospitals with a self-rated questionnaire survey containing evidence-based hospital-level factors, and principle investigators (stroke champions) and administrators were educated and trained before the actual implementation of the 10 predefined strategies. For instance, in our study, individual hospitals were stratified prior to the inclusion of the ASCaM in terms of the admission of patients with AIS and thrombolysis cases per year. We considered admission of patients with AIS less than 100 per year and accumulated tPA cases less than 10 or annual tPA cases less than 5 implies some obstacles to conducting thrombolysis (online supplemental table 1), although these may also be properties indicating substantial room for improvement. These hospitals scored less on the self-rated questionnaire as a result.
Compared with the gradual and consistent reduction of DNT for AIS during the past two decades, prehospital time, which constitutes the largest component of the overall pretreatment time course, has largely remained unchanged.24 In return, the efforts to increase thrombolysis rates within the therapeutic window have been counteracted and diminished.25 Lachkhem et al 26 defined the timeframe of AIS into four stages, that is, onset recognition to intention to seek medical service; intention to actual medical contract; contact to hospital admission and admission to treatment. The Shenyang ASCaM may have reduced the prehospital delay by raising public awareness of symptom recognition.
Prehospital notification is a key factor in reducing pretreatment time and increasing thrombolysis rates.27 For instance, the prehospital notification system (implemented with smartphone applications) employed in Hangzhou city has significantly reduced the DNT and ONT for stroke thrombolysis.28
Referral from another hospital, following the first consultation in primary care, is the second most frequent factor responsible for prehospital delay for intravenous thrombolysis.29 In this study, we publicised the ASCaM through mainstream local mass media. Awareness of hospital capability might make patients with AIS go directly to thrombolysis-eligible hospitals and EMS staff easier to dispatch, thus reducing the likelihood of referral from other hospitals. The increase in thrombolysis rates was by nature a result of the shortened ODT. In the next stage of the trial, we will collect the referral and referral times for each patient before admission. Collectively in this pilot stage, we presume increased awareness of symptom recognition, reduced referral from prior hospital and coordination between EMS and hospitals were major factors reducing ODT.
Intravenous tPA in Chinese patients with AIS remains low.2 Given a much higher stroke incidence of 601.9/100 000 population in Shenyang30 compared with the national average of 246.8/100 000,1 we chose, in the pilot stage, intravenous tPA administration among patients within 4.5 hours after onset as primary outcome because we were more concerned with substantially promoting this evidence-based and guideline-recommended therapy. In addition, pre-hospital optimisation such as ODT was considered as one of secondary outcomes mainly because 9 out of 10 interventions employed in the study were for in hospital. Not surprisingly, the failure to observe any improvement in clinical outcomes such as sICH, in-hospital mortality and favourable outcome may be due to inadequate time and population of the observation.
In this pilot study, we did not consider hospital-level factors in multiple regression analysis when assessing thrombolysis rates and clinical outcomes. However, we did account for these factors in the pre-evaluation procedures when assessing individual hospitals for eligibility. These factors were very important aspects in the scalable self-assessment questionnaire (see online supplemental table 1). Moreover, because the study is a before-and-after design, we made the assumption that in a consecutive 6 months time, these factors would remain stable in individual hospitals and would not serve as confounding factors influencing AIS outcomes. Finally, we conducted Mantel-Haenszel Common OR Estimate analysis to correct hospital-level heterogeneity.
The arrival mode (EMS transport or private vehicle) can be another factor to confound the comparison of thrombolysis rates and clinical outcomes.31 However, according to a recent cross-sectional study conducted in Hubei Province in China, only 15.4% of patients with AIS used EMS.32 Similar observations of not more than 20% stroke patients using EMS were confirmed in three other Chinese cities.33 Taken together in this study, we assumed that the arrival model made no significant difference in thrombolysis rates and clinical outcomes in Chinese patients with AIS.
Although nine out of ten of our improvement strategies were pertaining to in-hospital improvement, we failed to observe a reduction of DNT. Apart from inadequate observation time at this pilot stage, we presumed not taking measures to monitor hospitals’ or doctors’ adherence to predefined strategies may be part of the reason we had not achieved improvement in DNT, as well as clinical outcomes. Further study will use hospital-level survey and clinician-level log to monitor their fidelity. Recent studies have reported multifaceted quality improvement intervention increased doctors’ adherence to evidence-based therapies for patients with AIS. We are going to use similar all-or-none and composite endpoints to measure hospital-level adherence to our implemented strategies.5 34 35 In addition, we cannot exclude the possibility that some participating sites might have already applied some but not all our predefined strategies before the actual introduction of the training. We speculated this incomplete transition may partially accounted for the insignificant improvement of DNT as well as clinical outcomes.
One limitation of the before-and-after study design is that any observed changes may have been subject to general trends or interventions targeting public health or individual behaviour changing.36 To our knowledge, we observed no public health campaign targeting the improvement of stroke care introduced in Greater Shenyang area at the same time as the study.
In summary, the implementation of Shenyang ASCaM increased the administration of intravenous tPA in patients with AIS and simultaneously reduced the ONT. We emphasised a hubless system focusing on pre-hospital and in-hospital coordination, as well as the reconfiguration of existing infrastructure and human resources. We also pre-evaluated the quality of individual hospitals for their ability to deliver thrombolysis therapy with a self-rated questionnaire. Moreover, we implement the ASCaMs with the endorsement and supervision of local and national authorities such as SPPCCNHC. Effectiveness of bundle care integrating proven strategies targeting acute stroke care has remained to be elucidated in heavy-burden Chinese medical systems. In addition, unprecedented national construction of ASCaM has been implemented across China and its effectiveness is yet to be answered. In present study, the Shenyang ASCaM integrating both implementation of local public awareness and quality improving strategies increases the rate of intravenous tPA for patients with AIS within 4.5 hours in only 3 months. Since Shenyang is a typical Chinese metropolitan city with 8.1 million residents, an ethnically homogenous population and a tertiary medical hierarchy, the model may be complementary to China’s current construction of the national network of stroke centre certification, and rapidly improves stroke care service for the time being in China. On the other hand, since the rate of intravenous tPA remains low in most Asian countries, who shares similar demographic characteristics with Shenyang, and the rate of intravenous tPA has been frequently examined as an effect of improvement in low-income and low-middle-income countries in Asia,3 37 38 the model might also be applied to other highly populated Asian cities. Future studies in these cities will further evaluate the association between ASCaMs and clinical outcomes in a larger population.
Acknowledgments
We thank Professor Longde Wang, Mr Baohua Chao and Lei Cao from Stroke Prevention Project Committee of Chinese National Health Commission (SPPCCNHC), and Dr Mei Li from China Stroke Data Center for constructive suggestions. We also thank Wiley Editing Services for English editing. We thank Dr Honghao Li for the production of Figure 2 and Dr Bin Li from Shenyang Health Commission for the provision of local demographical and epidemiological information.
Footnotes
Contributors: Study conception and design: YS, QF, BX and YY. Acquisition, analysis or interpretation of data: YS, JL, WZ, LZ, CD, YZ, LR, YX, HZ and BX. Manuscript preparation: YS, CD, WZ and YZ. Critical revision of the manuscript for important intellectual content: YS, BY, WZ, JL, LZ, YX, HZ, MP, LR and WL. Study supervision: YS and BX.
Funding: This work was supported by grants from China Cardiovascular Association (2017-CCA-VG-048) and Shenyang Committee of Science and Technology (18-014-4-51) to Dr YS.
Map disclaimer: The depiction of boundaries on the map(s) in this article does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. The map(s) are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the ethical review committee of Shenyang First People’s Hospital. Individual ethics approvals were obtained from the institutional review boards when required. As a routine observational quality registry, written patient inform consent was waived in all participating hospitals. All patient-related information was de-identificated when subject to statistical analysis.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Data were entered and managed by dedicated stroke neurologists or nurses either using electronic databases or Microsoft Excel spreadsheets in respective centers. Accuracy and completeness of variables were monitored and checked by dedicated center coordinators. A full list of hospital-based registries is displayed in online Supplementary Materials.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1. Wang W, Jiang B, Sun H, et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation 2017;135:759–71. 10.1161/CIRCULATIONAHA.116.025250 [DOI] [PubMed] [Google Scholar]
- 2. Liu L, Wang D, Wong KSL, et al. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke 2011;42:3651–4. 10.1161/STROKEAHA.111.635755 [DOI] [PubMed] [Google Scholar]
- 3. Wasay M, Khatri IA, Kaul S. Stroke in South Asian countries. Nat Rev Neurol 2014;10:135–43. 10.1038/nrneurol.2014.13 [DOI] [PubMed] [Google Scholar]
- 4. Liu L, Liu J, Wang Y, et al. Substantial improvement of stroke care in China. Stroke 2018;49:3085–91. 10.1161/STROKEAHA.118.022618 [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Li Z, Zhao X, et al. Effect of a multifaceted quality improvement intervention on hospital personnel adherence to performance measures in patients with acute ischemic stroke in China: a randomized clinical trial. JAMA 2018;320:245–54. 10.1001/jama.2018.8802 [DOI] [PubMed] [Google Scholar]
- 6. Fonarow GC, Smith EE, Saver JL, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation 2011;123:750–8. 10.1161/CIRCULATIONAHA.110.974675 [DOI] [PubMed] [Google Scholar]
- 7. Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013;309:2480–8. 10.1001/jama.2013.6959 [DOI] [PubMed] [Google Scholar]
- 8. Alberts MJ, Latchaw RE, Selman WR, et al. Recommendations for comprehensive stroke centers: a consensus statement from the brain attack coalition. Stroke 2005;36:1597–616. 10.1161/01.STR.0000170622.07210.b4 [DOI] [PubMed] [Google Scholar]
- 9. Fonarow GC, Zhao X, Smith EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA 2014;311:1632–40. 10.1001/jama.2014.3203 [DOI] [PubMed] [Google Scholar]
- 10. Xian Y, Xu H, Lytle B, et al. Use of strategies to improve Door-to-Needle times with tissue-type plasminogen activator in acute ischemic stroke in clinical practice: findings from target: stroke. Circ Cardiovasc Qual Outcomes 2017;10. 10.1161/CIRCOUTCOMES.116.003227 [DOI] [PubMed] [Google Scholar]
- 11. Paul CL, Levi CR, D'Este CA, et al. Thrombolysis ImPlementation in Stroke (TIPS): evaluating the effectiveness of a strategy to increase the adoption of best evidence practice--protocol for a cluster randomised controlled trial in acute stroke care. Implement Sci 2014;9:38. 10.1186/1748-5908-9-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meretoja A, Strbian D, Mustanoja S, et al. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology 2012;79:306–13. 10.1212/WNL.0b013e31825d6011 [DOI] [PubMed] [Google Scholar]
- 13. Meretoja A, Weir L, Ugalde M, et al. Helsinki model cut stroke thrombolysis delays to 25 minutes in Melbourne in only 4 months. Neurology 2013;81:1071–6. 10.1212/WNL.0b013e3182a4a4d2 [DOI] [PubMed] [Google Scholar]
- 14. Xian Y, Smith EE, Zhao X, et al. Strategies used by hospitals to improve speed of tissue-type plasminogen activator treatment in acute ischemic stroke. Stroke 2014;45:1387–95. 10.1161/STROKEAHA.113.003898 [DOI] [PubMed] [Google Scholar]
- 15. Busby L, Owada K, Dhungana S, et al. Code fast: a quality improvement initiative to reduce door-to-needle times. J Neurointerv Surg 2016;8:661–4. 10.1136/neurintsurg-2015-011806 [DOI] [PubMed] [Google Scholar]
- 16. LaMonte MP, Bahouth MN, Magder LS, et al. A regional system of stroke care provides thrombolytic outcomes comparable with the NINDS stroke trial. Ann Emerg Med 2009;54:319–27. 10.1016/j.annemergmed.2008.09.022 [DOI] [PubMed] [Google Scholar]
- 17. Nguyen-Huynh MN, Klingman JG, Avins AL, et al. Novel Telestroke program improves thrombolysis for acute stroke across 21 hospitals of an integrated healthcare system. Stroke 2018;49:133–9. 10.1161/STROKEAHA.117.018413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamal N, Holodinsky JK, Stephenson C, et al. Improving door-to-needle times for acute ischemic stroke: effect of rapid patient registration, moving directly to computed tomography, and giving alteplase at the computed tomography scanner. Circ Cardiovasc Qual Outcomes 2017;10:e003242. 10.1161/CIRCOUTCOMES.116.003242 [DOI] [PubMed] [Google Scholar]
- 19. Fischer U, Aguiar de Sousa D, Norrving B, et al. Status and perspectives of acute stroke care in Europe. Stroke 2018;49:2281–2. 10.1161/STROKEAHA.118.022992 [DOI] [PubMed] [Google Scholar]
- 20. Dirks M, Niessen LW, van Wijngaarden JDH, et al. Promoting thrombolysis in acute ischemic stroke. Stroke 2011;42:1325–30. 10.1161/STROKEAHA.110.596940 [DOI] [PubMed] [Google Scholar]
- 21. Damani RH, Anand S, Asgarisabet P, et al. Regional intervention of stroke care to increase thrombolytic therapy for acute ischemic stroke. Stroke 2018;49:2008–10. 10.1161/STROKEAHA.118.021109 [DOI] [PubMed] [Google Scholar]
- 22. Scott PA, Meurer WJ, Frederiksen SM, et al. A multilevel intervention to increase community hospital use of alteplase for acute stroke (instinct): a cluster-randomised controlled trial. Lancet Neurol 2013;12:139–48. 10.1016/S1474-4422(12)70311-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamal N, Holodinsky JK, Stephenson C, et al. Improving Door-to-Needle times for acute ischemic stroke: effect of rapid patient registration, moving directly to computed tomography, and giving alteplase at the computed tomography scanner. Circ Cardiovasc Qual Outcomes 2017;10. 10.1161/CIRCOUTCOMES.116.003242 [DOI] [PubMed] [Google Scholar]
- 24. Tong D, Reeves MJ, Hernandez AF, et al. Times from symptom onset to hospital arrival in the Get with the Guidelines--Stroke Program 2002 to 2009: temporal trends and implications. Stroke 2012;43:1912–7. 10.1161/STROKEAHA.111.644963 [DOI] [PubMed] [Google Scholar]
- 25. Bouckaert M, Lemmens R, Thijs V. Reducing prehospital delay in acute stroke. Nat Rev Neurol 2009;5:477–83. 10.1038/nrneurol.2009.116 [DOI] [PubMed] [Google Scholar]
- 26. Lachkhem Y, Rican S, Minvielle Étienne. Understanding delays in acute stroke care: a systematic review of reviews. Eur J Public Health 2018;28:426–33. 10.1093/eurpub/cky066 [DOI] [PubMed] [Google Scholar]
- 27. Fassbender K, Balucani C, Walter S, et al. Streamlining of prehospital stroke management: the golden hour. Lancet Neurol 2013;12:585–96. 10.1016/S1474-4422(13)70100-5 [DOI] [PubMed] [Google Scholar]
- 28. Zhang S, Zhang J, Zhang M, et al. Prehospital notification procedure improves stroke outcome by shortening onset to needle time in Chinese urban area. Aging Dis 2018;9:426–34. 10.14336/AD.2017.0601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pulvers JN, Watson JDG. If time is brain where is the improvement in prehospital time after stroke? Front Neurol 2017;8:617. 10.3389/fneur.2017.00617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun Z, Zheng L, Detrano R, et al. An epidemiological survey of stroke among rural Chinese adults results from the Liaoning Province. Int J Stroke 2013;8:701–6. 10.1111/j.1747-4949.2012.00897.x [DOI] [PubMed] [Google Scholar]
- 31. El Khoury R, Jung R, Nanda A, et al. Overview of key factors in improving access to acute stroke care. Neurology 2012;79:S26–34. 10.1212/WNL.0b013e3182695a2a [DOI] [PubMed] [Google Scholar]
- 32. Yin X, Yang T, Gong Y, et al. Determinants of emergency medical services utilization among acute ischemic stroke patients in Hubei Province in China. Stroke 2016;47:891–4. 10.1161/STROKEAHA.115.011877 [DOI] [PubMed] [Google Scholar]
- 33. Jiang B, Ru X, Sun H, et al. Pre-Hospital delay and its associated factors in first-ever stroke registered in communities from three cities in China. Sci Rep 2016;6:29795. 10.1038/srep29795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Machline-Carrion MJ, Santucci EV, Damiani LP, et al. Effect of a quality improvement intervention on adherence to therapies for patients with acute ischemic stroke and transient ischemic attack: a cluster randomized clinical trial. JAMA Neurol 2019. 10.1001/jamaneurol.2019.1012. [Epub ahead of print: 06 May 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cadilhac DA, Andrew NE, Kilkenny MF, et al. Improving quality and outcomes of stroke care in hospitals: protocol and statistical analysis plan for the Stroke123 implementation study. Int J Stroke 2018;13:96–106. 10.1177/1747493017730741 [DOI] [PubMed] [Google Scholar]
- 36. Sedgwick P. Before and after study designs. BMJ 2014;349:g5074. 10.1136/bmj.g5074 [DOI] [PubMed] [Google Scholar]
- 37. Pandian JD, Sudhan P. Stroke epidemiology and stroke care services in India. J Stroke 2013;15:128–34. 10.5853/jos.2013.15.3.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suwanwela NC. Stroke epidemiology in Thailand. J Stroke 2014;16:1–7. 10.5853/jos.2014.16.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fonarow GC, Smith EE, Saver JL, et al. Improving door-to-needle times in acute ischemic stroke: the design and rationale for the American heart Association/American stroke association's target: stroke initiative. Stroke 2011;42:2983–9. 10.1161/STROKEAHA.111.621342 [DOI] [PubMed] [Google Scholar]
- 40. Lindsberg PJ, Häppölä O, Kallela M, et al. Door to thrombolysis: ER reorganization and reduced delays to acute stroke treatment. Neurology 2006;67:334–6. 10.1212/01.wnl.0000224759.44743.7d [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2020-000332supp001.pdf (92.7KB, pdf)