Abstract
Objective
To investigate the effects of DL-3-N-butylphthalide (NBP) via intranasal delivery after ischaemic stroke in mice.
Methods
C57BL/6 mice were divided into three groups: sham, stroke with vehicle and stroke with NBP treatment. Ischaemic stroke was induced by permanent ligation of right middle cerebral artery with 7 min common carotid artery occlusion. NBP (100 mg/kg) or vehicle was intranasally administered at 1 hour after stroke and repeated once a day until sacrifice. Bromodeoxyuridine (BrdU) (50 mg/kg/day) was given from the third day until sacrifice. Sensorimotor function was tested during 1–21 days after stroke. Local cerebral blood flow in the ischaemic and peri-infarct regions was measured using laser Doppler flowmetry before, during and 3 days after ischaemia. Expressions of vascular endothelial growth factor (VEGF) and endothelial nitric oxide synthase as well as regenerative marker BrdU in the peri-infarct region were analysed by western blotting and immunohistochemical methods.
Results
Compared with the vehicle group, NBP treatment significantly increased the VEGF expression in the poststroke brain. Stroke mice that received NBP showed significantly less vascular damage after stroke and more new neurons and blood vessels in the peri-infarct region at 21 days after stroke. In the adhesive removal test, the sensorimotor function of stroke mice treated with NBP performed significantly better at 1, 3 and 7 days after stroke compared with vehicle controls.
Conclusion
Daily intranasal NBP treatment provides protective and neurogenic/angiogenic effects in the poststroke brain, accompanied with functional improvements after a focal ischaemic stroke in mice.
Keywords: stroke, blood flow, brain, drug
Introduction
Stroke is one of the most common causes of death and disability.1 Ischaemic stroke accounts for 85% of all strokes, which is the second leading cause of death worldwide after coronary heart disease.2 Ischaemic stroke occurs when cerebral blood flow is blocked followed by tissue damage and infarct formation in the brain. Intravenous thrombolysis and mechanical thrombectomy are Food and Drug Administration-approved treatments with limited time window for acute cerebral ischaemia.3 There is currently no approved treatment involving regenerative therapeutics for patients who had chronic stroke. Butyl phthalein was originally extracted from celery seeds. DL-3-N-butylphthalide (NBP) was synthesised and approved by the National Medical Products Administration in China since 2002 for clinical treatments of patients who had a stroke. Basic and clinical research demonstrated protective effects of NBP after cerebral ischaemia, via the mechanisms of suppressing oxidative stress injury, mitochondria damage, neuronal apoptosis and neuroinflammation.4 More investigations have shown beneficial effects of NBP for ischaemic stroke,5–7 Alzheimer’s disease,8–10 Parkinson’s disease11–13 and spinal cord injury.14–16 NBP also reduces oxidative stress-related damage, inhibits platelet aggregation, regulates energy metabolic homeostasis, improves microcirculation and reduces neurological deficits.17–23 Using a focal ischaemic stroke model of mice, the current study tested the effect of chronic intranasal NBP delivery as a potential regenerative treatment for long-term recovery after ischaemic stroke.
Materials and methods
Animals
Male healthy C57BL/6 mice, 8–10 weeks old with a weight range of 25±5 g, were purchased from Jackson Laboratory and kept in the animal facility at Emory University. Animals were housed five per cage with free access to food and water. Room temperature ranged between 21°C and 24°C and had a relative humidity of 40%–70%.
Drug preparation
NBP was from Hebei Shiyao Group Enbipu Pharmaceutical. The drug was freshly prepared each time before administration and was prepared by diluting in vegetable oil (Kroger, Ohio). Bromodeoxyuridine (BrdU) (0.5% stock solution) was prepared in 0.9% sterile saline.
Mouse model of focal cerebral ischaemia of the sensorimotor cortex
A reproducible model of focal cerebral ischaemia targeting the right sensorimotor cortex in mice was established according to a previous publication.5 Briefly, mice were anaesthetised by 2% isoflurane. The distal middle cerebral artery (MCA) of the right side was permanently ligated, and the common carotid artery (CCA) on both sides was transiently blocked for 7 min. The sham operation group had the same procedures as the stroke group except that distal MCA or CCA was not ligated. Body temperature was maintained at 37°C.
Drug administration
Mice were randomly divided into a sham-operated group (sham), a vegetable oil vehicle group (stroke+oil) and an intranasal NBP group (stroke+NBP). The same volume (25 μL) of the vegetable oil vehicle or NBP (100 mg/kg) diluted in oil was administered to both nasals starting 1 hour after the stroke surgery to mimic acute on-site treatment conditions. After the first, daily intranasal administration of NBP was performed for a total of either 3 or 21 days. The dosage of NBP was selected based on our previous study on ischaemic stroke.5 Animals were sacrificed 3 or 21 days after stroke.
Body weight monitoring
The body weight of the control and NBP groups was measured and recorded every day and compared after stroke. The weight of both the vehicle group and the NBP group was plotted as before, during and after the stroke. All data are recorded at a fixed time point of the day in a double-blinded manner.
Antibodies for protein expression analyses
Immunohistochemical staining and/or western blotting used specific antibodies against vascular endothelial growth factor (VEGF) (Santa Cruz Biotechnology, California; Cat #Sc7269), endothelial nitric oxide synthase (eNOS) (Cell Signaling Technology, Massachusetts; Cat #32027), β-actin (Abcam, UK; Cat #ab49900), Glut-1 (Abcam; Cat #ab40084), NeuN (MilliporeSigma, Massachusetts; Cat #ABN78A4), BrdU (Abcam; Cat #ab6326), secondary antibody (Jackson ImmunoResearch Laboratories, Pennsylvania), OCT (Sakura Finetek, Japan) and fish gelatin (Sigma, Massachusetts). The 3M surgical adhesive (Fisher Scientific, New Hampshire) was used in the adhesive removal test.
Frozen microtome (Leica Microsystems, Germany) and fluorescence confocal microscope BX61 (Olympus, Japan) were used in brain sectioning and image experiments.
Western blot analysis
The cerebral cortical tissue of the infarcted region was dissected and placed into EP (Eppendorf tubes) tubes. Protein was extracted and the amount was quantified by bicinchoninic acid (BCA)method. A total of 40 μg protein for each sample was electrophoresed in a 10% sodium dodecyl sulfate (SDS)-PAGE gel, transferred to a Polyvinylidene fluoride (PVDF) membrane, and incubated with 5% Bovine Serum Albumin (BSA) solution in Tris-buffered saline (TBS) at room temperature for 1 hour and with primary antibodies in TBS at 4°C overnight. Secondary antibodies were added for 1 hour and washed in Tris-buffered saline, 0.1% Tween 20 (TBST) three times for 10 min each, for 5-bromo-4-chloro-3-indolyl phosphate (BCIP)- nitroblue tetrazolium (NBT)-based visualisation on a PVDF membrane.
Immunohistochemical staining
At the indicated times, mice were euthanised by carbon dioxide. Brains were immediately placed in dry ice, embedded in OCT embedding agent and stored at −80°C. A frozen microtome was then used to cut 10-micron section slices and stored at −80°C. Before the staining with primary antibodies, brain tissue sections were dried at room temperature, fixed with 10% formalin solution, washed three times in PBS for 10 min each, treated with 0.2% Triton X-100 in PBS, washed another three times with PBS for 10 min each and incubated with 1% fish gel for 1 hour at room temperature. Primary antibodies were diluted, mixed in PBS and incubated overnight at 4°C. The sections were washed three times with PBS for 10 min each and incubated with secondary antibodies in PBS at 37°C in the dark for 1 hour, washed another three times with PBS for 10 min each and mounted for fluorescence imaging. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining of cell death was performed using a TUNEL assay kit (Invitrogen, Carlsbad, California, USA) according to the manufacturer’s protocol.
Local cerebral blood flow measurement
Doppler cerebral blood flow metre (PeriFlux System 5000-PF5010 Laser Doppler Perfusion Monitoring (LDPM) unit, Sweden) was used to measure local cerebral blood flow (LCBF).24 The flow was recorded over the skull at the same location before, during and 3 days after ischaemia.
Animal behaviour test
The adhesive removal test was performed in double-blinded manner. Time is recorded when the mouse perceives the sticker attached onto the foot and removes it, as a measure of sensorimotor function.25 At 1, 3, 7, 14 and 21 days after stroke, functional recovery outcomes were measured.
Statistical methods
GraphPad Prism V.7.0 statistical software was used for the analyses. All data were presented in mean value or mean±SD for each group. Comparison between two groups was performed using Student’s t-test. Comparisons among the three groups were performed using one-way analysis of variance followed with Bonferroni correction. A threshold of p<0.05 was considered statistically significant.
Results
Effects of NBP on LCBF and regulatory gene expression in the peri-infarct region
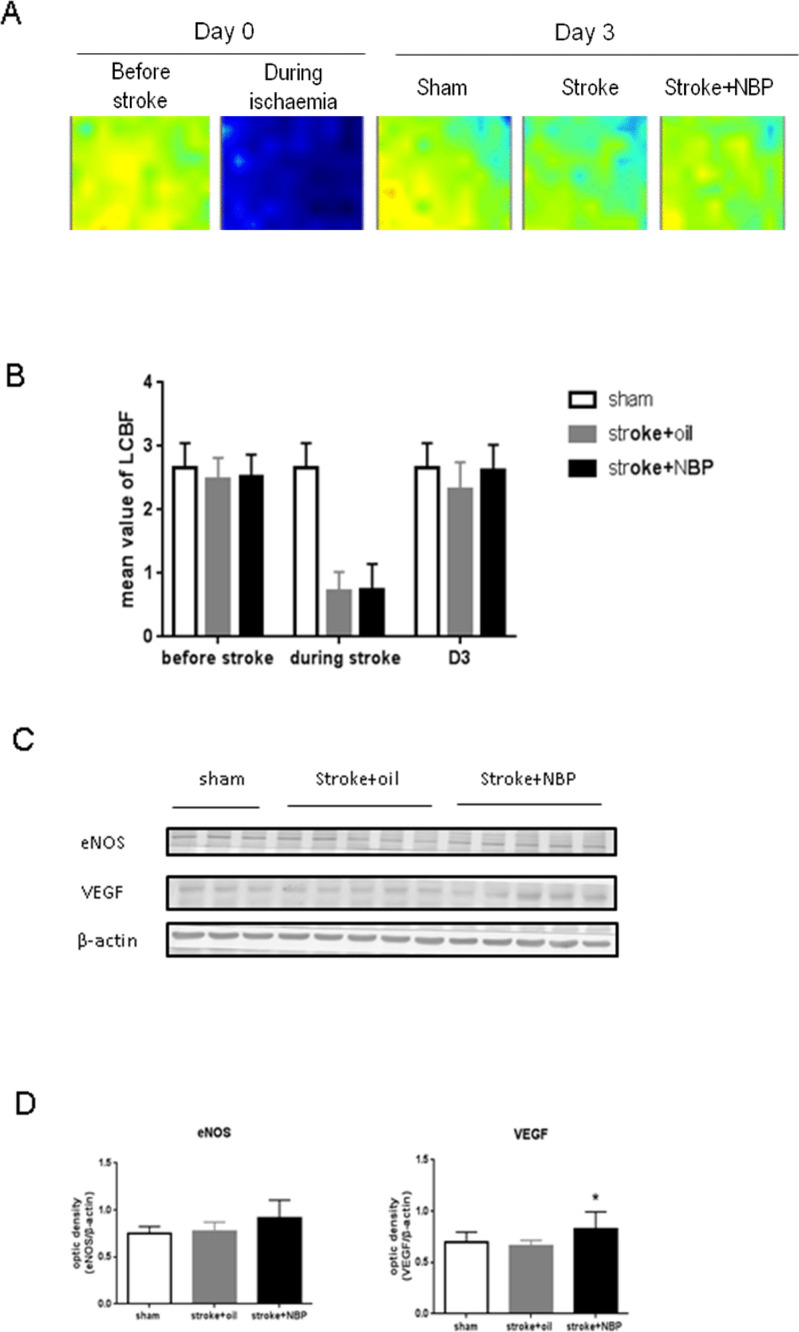
Stroke animals were given daily intranasal treatment of NBP from 1 hour after stroke until sacrifice (figure 1). Compared with the vehicle group, the LCBF was similarly reduced during ischaemic surgery and recovered at 3 days after stroke (figure 2A, B). NBP treatment showed a trend of better LCBF recovery but the effect was not significant (figure 2B). The observation was consistent with our measurement on the eNOS level in the peri-infarct region, showing no significant differences among all groups, although the NBP group displayed a trend towards a higher level of eNOS (figure 2C, D). On the other hand, the NBP treatment significantly increased VEGF at 3 days after stroke, suggesting that NBP might have an impact on poststroke regeneration (figure 2C, D).
Figure 1.
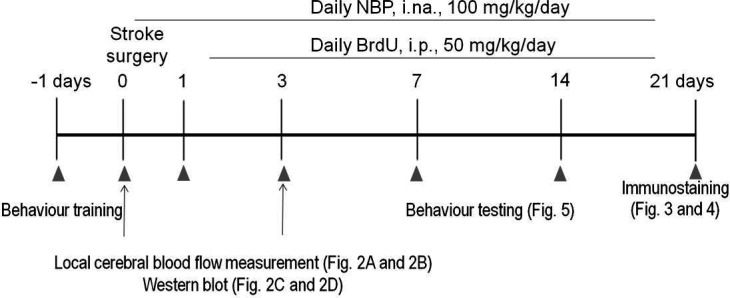
Experimental paradigm. The timeline of the experimental study. Stroke mice were treated with NBP or vehicle for 3 days or until sacrifice. Mice were trained before stroke surgery and then were tested for behaviour on different days. BrdU, bromodeoxyuridine; i.na., intranasal; i.p., intraperitoneal; NBP, DL-3-N-butylphthalide.
Figure 2.
LCBF measurement and expression of eNOS and VEGF in the peri-infarct region. (A, B) The blood flow over the ischaemic and peri-infarct (penumbra) regions was measured before, during and 3 days after the stroke surgery. (C) Western blot band was shown. (D) At 3 days after stroke, NBP increased VEGF protein expression. n=3–5 per group. Mean±SD. *P<0.05 compared with stroke group. D3, day 3; eNOS, endothelial nitric oxide synthase; LCBF, local cerebral blood flow; NBP, DL-3-N-butylphthalide; VEGF, vascular endothelial growth factor.
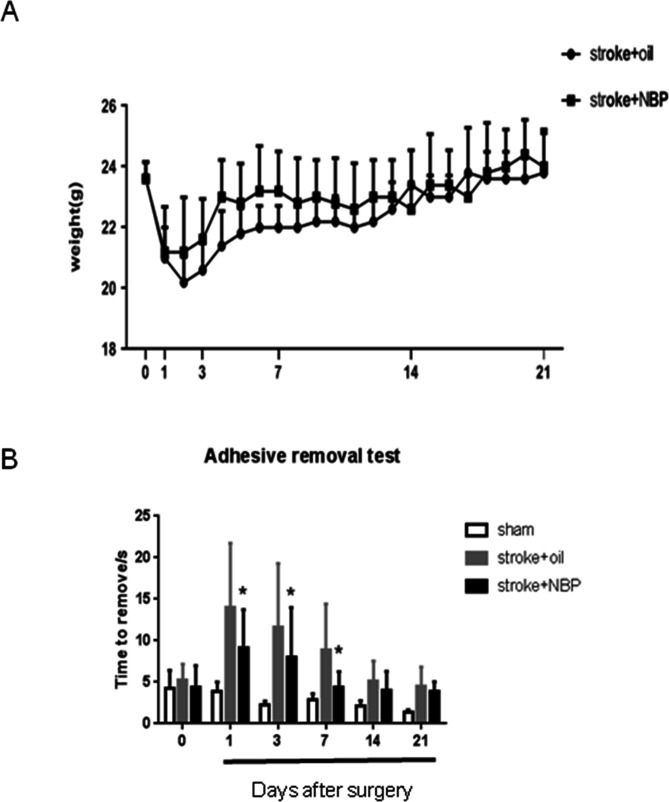
Effects of NBP on body weight and sensorimotor functional recovery after stroke
The body weight of mice gradually recovered after stroke. There was in general no statistical difference between the vehicle and the NBP-treated groups, although the NBP group tended to have less body weight loss during the early days after stroke (figure 3A). The adhesive removal test measured the sensorimotor function associated with the stroke-damaged right sensorimotor cortex. At 1, 3 and 7 days after stroke, compared with the vehicle group, stroke mice that received intranasal NBP treatments performed significantly better in the removal of the adhesive. A spontaneous recovery was observed at 14 days after stroke in all stroke groups (figure 3B).
Figure 3.
Body weight in the stroke mice and behaviour recovery after ischaemic stroke in mice. (A) The body weight of experimental mice was measured at different days after stroke. Mean value was used. (B) At different days after ischaemic stroke, the time for the mice to remove the sticker was recorded. n=10 per group. Mean±SD. *P<0.05 compared with stroke group. NBP, DL-3-N-butylphthalide.
Protective and regenerative effects of NBP in the poststroke brain
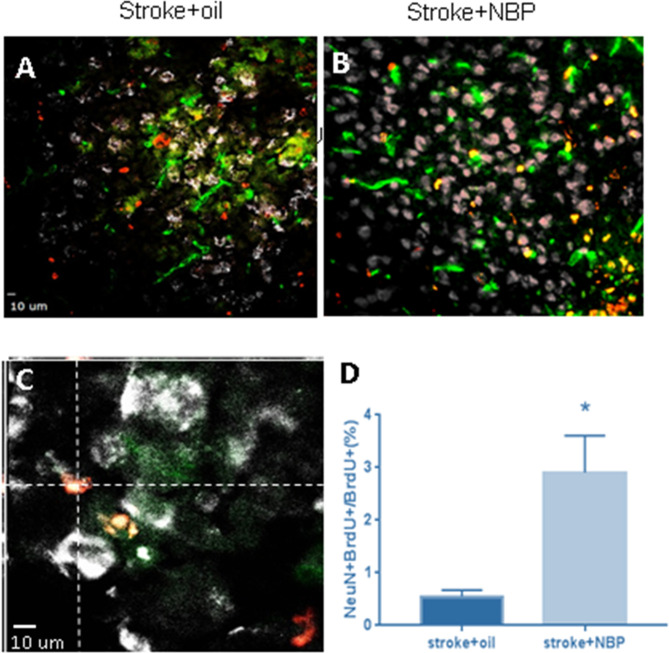
At different days after stroke, mice were sacrificed for the analysis of vascular damage and neurovasculature regeneration in the peri-infarct region. Three days after stroke, immunohistochemical staining reveals many Glut-1-positive vascular cells that were also TUNEL-positive, suggesting damaged vessels in the poststroke brain (figure 4A). Stroke animals that received NBP treatment, however, displayed significantly less TUNEL-positive vessels, suggesting a vascular protective effect (figure 4A). At 21 days after stroke, immunostaining showed significantly increased Glut-1 and BrdU double-positive cells in mice that received NBP treatments compared with stroke control mice (figure 4B, C). Meanwhile, there was also a marked increase in NeuN and BrdU double-positive cells with NBP treatment (figure 5A, B). These data demonstrated that NBP provides neuroprotective and proregenerative effects in the poststroke brain.
Figure 4.
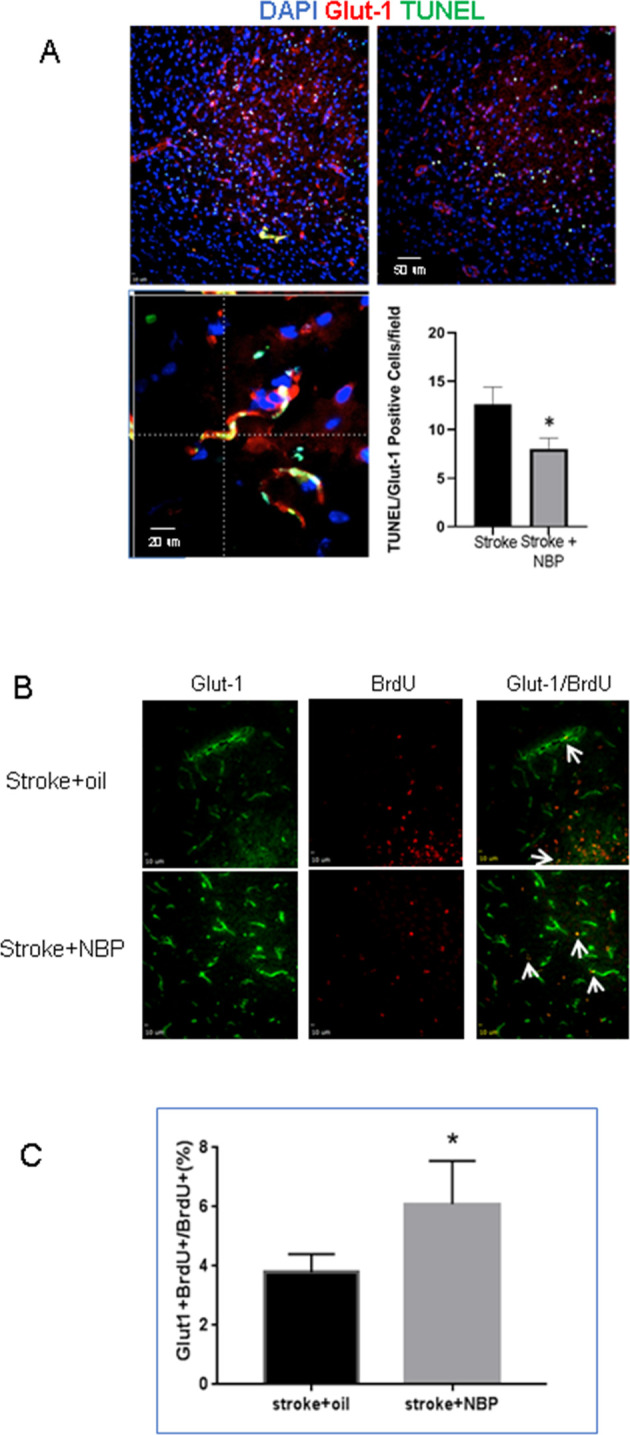
NBP effects on vascular damage and regeneration in the peri-infarct region of the poststroke brain. (A) Three days after stroke, TUNEL (green) and Glut-1 (red) staining revealed vascular damage in the peri-infarct region. Blue colour of Hoechst staining shows all cells. The bar graph quantified the counting of TUNEL and Glut-1 double-positive cells, which were much less in the stroke+NBP group. n=4 per group. *P<0.05 versus stroke only controls. (B) At 21 days after stroke, Glut-1 (green) and BrdU (red) double staining was used to detect newly formed vessels. NBP treatment increased the colabelled Glut-1+/BrdU+ cells (arrows). (C) Quantified cell counting of Glut-1+/BrdU+ cells (per cent of total cells). n=4 per group. Mean±SD. *P<0.05 compared with stroke group. BrdU, bromodeoxyuridine; NBP, DL-3-N-butylphthalide. DAPI, 4′,6-diamidino-2-phenylindole; TUNEL, Terminal deoxynucleotidyl transferase dUTP nick end labeling.
Figure 5.
NBP increased neurogenesis in the poststroke brain. Immunohistochemical staining of NeuN and BrdU cells for newly formed neurons in the peri-infarct region 21 days after stroke. (A, B) Representative images of NeuN (green) and BrdU (red) positive cells. (C) A confocal image showing colabelling of NeuN and BrdU staining. (D) Summarised data in the bar graph show NBP treatment increased the colabelled NeuN+/BrdU+ cells. n=4 per group. Mean±SD. *P<0.05 compared with stroke group. BrdU, bromodeoxyuridine; NBP, DL-3-N-butylphthalide.
Discussion
Intranasal drug delivery is a non-invasive and efficient method that allows bypassing the blood–brain barrier and entry into the brain. It is believed that drugs applied to the nasal mucosa are able to enter the brain regions through the olfactory nerve pathway, olfactory epithelium pathway and bloodstream, making it suitable for neurological treatments.26–28 We demonstrated that intranasal NBP treatment after brain injury improves neural regeneration.26 NBP increased the endogenous neural progenitor cell (NPC) migration to the ischaemic striatum and other injured brain regions.29 A mechanism study revealed that NBP increased the dentate gyrus NPC via regulation of PKA/BDNF/CREB and STAT3 signalling pathways to promote hippocampal neurogenesis.30 In a mouse model of Alzheimer’s disease, NBP activated BDNF/TrkB/CREB/Akt pathway and promoted neural regeneration.9
The current study reveals a vascular protective effect at 3 days after stroke by intranasally delivering NBP. Although this early protection did not translate to a significant increase in LCBF at the same time, it may be beneficial for LCBF and regeneration at later time points. Promoting neurovascular regeneration has been considered as an effective strategy for tissue repair after ischaemic stroke.31 32 VEGF is an angiogenic factor that stimulates blood vessel growth by inducing endothelial cell proliferation, migration and neovascularisation.33 34 The observed VEGF increase is consistent with other reports that NBP increased expressions of VEGF and basic fibroblast growth factor.23 35 36 Endothelial eNOS plays an important role in vascular reconstruction and vasodilatation in stroke brains. In the present study, the acute eNOS levels after stroke were not significantly changed, which was consistent with the LCBF data. On the other hand, eNOS can promote endothelial cell proliferation and migration, smooth muscle cell differentiation, arteriogenesis, collateriogenesis, and angiogenesis.37 38 It will be interesting to test whether NBP shows a chronic regulation on eNOS and the long-term changes in LCBF in the postischaemic brain.
The present investigation shows that repeated intranasal administrations of NBP starting from the acute phase of ischaemia increase the expression of VEGF, promote neurovascular regeneration in the peri-infarct region and improve sensorimotor recovery after stroke. Further studies may explore long-term changes and regulatory factors such as eNOS in regenerative and functional benefits of NBP treatments.39 40
Footnotes
Contributors: MQ: performed the experiments, data analysis and manuscript writing. JZ, YiZ, JS: performed the experiments and data analysis. LL: conception and experimental design. LW: conception and experimental design, data analysis and manuscript approval. YoZ: conception and financial support.
Funding: This study was funded by the National Natural Science Foundation of China (81371355 to YZ, 81500989 to YZ, 81671191 to YZ and 81820108012 to LL).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: All animal experiments were approved by the Institutional Animal Care and Use Committee of Emory University. The protocol met the standards of the National Institute of Health.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. All data related to the study have been reported in the paper. Additional data are available upon request.
References
- 1. Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: global burden of disease study. Lancet 1997;349:1269–76. 10.1016/S0140-6736(96)07493-4 [DOI] [PubMed] [Google Scholar]
- 2. Mikulik R, Wahlgren N. Treatment of acute stroke: an update. J Intern Med 2015;278:145–65. 10.1111/joim.12387 [DOI] [PubMed] [Google Scholar]
- 3. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart Association/American stroke association. Stroke 2018;49:e46–110. 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 4. Gallo S, Sala V, Gatti S, et al. Cellular and molecular mechanisms of HGF/Met in the cardiovascular system. Clin Sci 2015;129:1173–93. 10.1042/CS20150502 [DOI] [PubMed] [Google Scholar]
- 5. Li J, Li Y, Ogle M, et al. DL-3-n-butylphthalide prevents neuronal cell death after focal cerebral ischemia in mice via the JNK pathway. Brain Res 2010;1359:216–26. 10.1016/j.brainres.2010.08.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yan H, Yan Z, Niu X, et al. Dl-3-n-butylphthalide can improve the cognitive function of patients with acute ischemic stroke: a prospective intervention study. Neurol Res 2017;39:337–43. 10.1080/01616412.2016.1268775 [DOI] [PubMed] [Google Scholar]
- 7. Li F, Ma Q, Zhao H, et al. L-3-n-Butylphthalide reduces ischemic stroke injury and increases M2 microglial polarization. Metab Brain Dis 2018;33:1995–2003. 10.1007/s11011-018-0307-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang C-Y, Xu Y, Wang X, et al. Dl-3-n-Butylphthalide inhibits NLRP3 inflammasome and mitigates Alzheimer's-like pathology via Nrf2-TXNIP-TrX axis. Antioxid Redox Signal 2019;30:1411–31. 10.1089/ars.2017.7440 [DOI] [PubMed] [Google Scholar]
- 9. Lei H, Zhang Y, Huang L, et al. L-3-n-Butylphthalide regulates proliferation, migration, and differentiation of neural stem cell in vitro and promotes neurogenesis in APP/PS1 mouse model by regulating BDNF/TrkB/CREB/Akt pathway. Neurotox Res 2018;34:477–88. 10.1007/s12640-018-9905-3 [DOI] [PubMed] [Google Scholar]
- 10. Peng Y, Sun J, Hon S, et al. L-3-n-butylphthalide improves cognitive impairment and reduces amyloid-beta in a transgenic model of Alzheimer's disease. J Neurosci 2010;30:8180–9. 10.1523/JNEUROSCI.0340-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang J-Z, Chen Y-Z, Su M, et al. DL-3-n-Butylphthalide prevents oxidative damage and reduces mitochondrial dysfunction in an MPP(+)-induced cellular model of Parkinson's disease. Neurosci Lett 2010;475:89–94. 10.1016/j.neulet.2010.03.053 [DOI] [PubMed] [Google Scholar]
- 12. Xiong N, Huang J, Chen C, et al. Dl-3-n-butylphthalide, a natural antioxidant, protects dopamine neurons in rotenone models for Parkinson's disease. Neurobiol Aging 2012;33:1777–91. 10.1016/j.neurobiolaging.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 13. Chen Y, Wu T, Li H, et al. Dl-3-n-Butylphthalide exerts dopaminergic Neuroprotection Through Inhibition of Neuroinflammation. Front Aging Neurosci 2019;11:44. 10.3389/fnagi.2019.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng B, Zhou Y, Zhang H, et al. Dl-3-n-butylphthalide prevents the disruption of blood-spinal cord barrier via inhibiting endoplasmic reticulum stress following spinal cord injury. Int J Biol Sci 2017;13:1520–31. 10.7150/ijbs.21107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He Z, Zhou Y, Lin L, et al. Dl-3-n-butylphthalide attenuates acute inflammatory activation in rats with spinal cord injury by inhibiting microglial TLR4/NF-κB signalling. J Cell Mol Med 2017;21:3010–22. 10.1111/jcmm.13212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. He Z, Zhou Y, Huang Y, et al. Dl-3-n-butylphthalide improves functional recovery in rats with spinal cord injury by inhibiting endoplasmic reticulum stress-induced apoptosis. Am J Transl Res 2017;9:1075–87. [PMC free article] [PubMed] [Google Scholar]
- 17. Peng Y, Zeng X, Feng Y, et al. Antiplatelet and antithrombotic activity of L-3-n-butylphthalide in rats. J Cardiovasc Pharmacol 2004;43:876–81. 10.1097/00005344-200406000-00018 [DOI] [PubMed] [Google Scholar]
- 18. Liu C-L, Liao S-J, Zeng J-S, et al. dl-3n-butylphthalide prevents stroke via improvement of cerebral microvessels in RHRSP. J Neurol Sci 2007;260:106–13. 10.1016/j.jns.2007.04.025 [DOI] [PubMed] [Google Scholar]
- 19. Li Z, Lu J, Ma L, et al. dl-3-n-butylphthalide for alleviation of neurological deficit after combined extracranial-intracranial revascularization for moyamoya disease: a propensity score-matched analysis. J Neurosurg 2019;132:421–33. 10.3171/2018.10.JNS182152 [DOI] [PubMed] [Google Scholar]
- 20. Cui L-Y, Zhu Y-C, Gao S, et al. Ninety-day administration of dl-3-n-butylphthalide for acute ischemic stroke: a randomized, double-blind trial. Chin Med J 2013;126:3405–10. [PubMed] [Google Scholar]
- 21. Jia J, Wei C, Liang J, et al. The effects of DL-3-n-butylphthalide in patients with vascular cognitive impairment without dementia caused by subcortical ischemic small vessel disease: a multicentre, randomized, double-blind, placebo-controlled trial. Alzheimers Dement 2016;12:89–99. 10.1016/j.jalz.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 22. Xue L-X, Zhang T, Zhao Y-W, et al. Efficacy and safety comparison of DL-3-n-butylphthalide and Cerebrolysin: effects on neurological and behavioral outcomes in acute ischemic stroke. Exp Ther Med 2016;11:2015–20. 10.3892/etm.2016.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang S-C, Luo C-J, Zhang K-H, et al. Effects of dl-3-n-butylphthalide on serum VEGF and bFGF levels in acute cerebral infarction. Eur Rev Med Pharmacol Sci 2017;21:4431–6. [PubMed] [Google Scholar]
- 24. Stern MD, Lappe DL, Bowen PD, et al. Continuous measurement of tissue blood flow by laser-Doppler spectroscopy. Am J Physiol 1977;232:H441–8. 10.1152/ajpheart.1977.232.4.H441 [DOI] [PubMed] [Google Scholar]
- 25. Bouet V, Boulouard M, Toutain J, et al. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nat Protoc 2009;4:1560–4. 10.1038/nprot.2009.125 [DOI] [PubMed] [Google Scholar]
- 26. Zhao Y, Lee JH, Chen D, et al. DL-3-n-butylphthalide induced neuroprotection, regenerative repair, functional recovery and psychological benefits following traumatic brain injury in mice. Neurochem Int 2017;111:82–92. 10.1016/j.neuint.2017.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wei ZZ, Zhang JY, Taylor TM, et al. Neuroprotective and regenerative roles of intranasal Wnt-3a administration after focal ischemic stroke in mice. J Cereb Blood Flow Metab 2018;38:404–21. 10.1177/0271678X17702669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manickavasagam D, Lin L, Oyewumi MO. Nose-to-brain co-delivery of repurposed simvastatin and BDNF synergistically attenuates LPS-induced neuroinflammation. Nanomedicine 2020;23:102107. 10.1016/j.nano.2019.102107 [DOI] [PubMed] [Google Scholar]
- 29. Sun Y, Cheng X, Wang H, et al. dl-3-n-butylphthalide promotes neuroplasticity and motor recovery in stroke rats. Behav Brain Res 2017;329:67–74. 10.1016/j.bbr.2017.04.039 [DOI] [PubMed] [Google Scholar]
- 30. Yang L-C, Li J, Xu S-F, et al. L-3-n-butylphthalide promotes neurogenesis and neuroplasticity in cerebral ischemic rats. CNS Neurosci Ther 2015;21:733–41. 10.1111/cns.12438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol 2009;117:481–96. 10.1007/s00401-009-0483-6 [DOI] [PubMed] [Google Scholar]
- 32. Guo S, Kim WJ, Lok J, et al. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc Natl Acad Sci U S A 2008;105:7582–7. 10.1073/pnas.0801105105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin K, Zhu Y, Sun Y, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A 2002;99:11946–50. 10.1073/pnas.182296499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Breier G, Albrecht U, Sterrer S, et al. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 1992;114:521–32. [DOI] [PubMed] [Google Scholar]
- 35. Qin C, Zhou P, Wang L, et al. Dl-3-N-butylphthalide attenuates ischemic reperfusion injury by improving the function of cerebral artery and circulation. J Cereb Blood Flow Metab 2019;39:2011–21. 10.1177/0271678X18776833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao Y, Liu D, Li J, et al. L-NBP, a multiple growth factor activator, attenuates ischemic neuronal impairments possibly through promoting neuritogenesis. Neurochem Int 2019;124:94–105. 10.1016/j.neuint.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 37. Jiang T, Yu J-T, Zhu X-C, et al. Angiotensin-(1-7) induces cerebral ischaemic tolerance by promoting brain angiogenesis in a Mas/eNOS-dependent pathway. Br J Pharmacol 2014;171:4222–32. 10.1111/bph.12770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen J, Cui X, Zacharek A, et al. eNOS mediates TO90317 treatment-induced angiogenesis and functional outcome after stroke in mice. Stroke 2009;40:2532–8. 10.1161/STROKEAHA.108.545095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou P-T, Wang L-P, Qu M-J, et al. Dl-3-N-butylphthalide promotes angiogenesis and upregulates sonic hedgehog expression after cerebral ischemia in rats. CNS Neurosci Ther 2019;25:748–58. 10.1111/cns.13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li Z, Wang C, Jiang Y, et al. Rationale and design of patient-centered retrospective observation of Guideline-Recommended execution for stroke sufferers in China: China PROGRESS. Stroke Vasc Neurol 2019;4:165–70. 10.1136/svn-2019-000233 [DOI] [PMC free article] [PubMed] [Google Scholar]